Abstract
Introduction
Nicotine dependence (ND) is a chronic, relapsing mental disorder characterized by compulsive cigarette seeking and smoking. Although the cerebellum plays an increasingly implicated role in ND, the exact cerebellar alterations in ND remain unclear. Identifying the localization of these cerebellar abnormalities in ND may help to further understand the role of the cerebellum in ND. Thus, we investigated the structural and functional alterations in the cerebellum in a large sample of smokers using the spatially unbiased infratentorial template (SUIT).
Methods
High-resolution structural magnetic resonance imaging (MRI) data were acquired from 85 smokers and 41 nonsmokers. We applied voxel-based morphometry (VBM) and the SUIT cerebellar atlas to compare the cerebellar gray matter (GM) volume between smokers and nonsmokers. Using resting-state functional MRI data, we also performed seed-based functional connectivity (FC) analysis to examine the functional correlates of the GM volume changes.
Results
Both VBM and lobular analyses revealed smaller GM volume in the bilateral Crus I in smokers. The GM volume of the left Crus I was inversely correlated with the severity of nicotine dependence as assessed by Fagerström Test for Nicotine Dependence (r = −.268, p = .013). We also found reduced FC between the bilateral Crus I and brain regions involved in the default mode network and motor system, as well as the frontal and temporal cortex in smokers.
Conclusions
Our results indicate that decreased cerebellar GM volume and corticocerebellar FC are associated with ND, and these may underlie the core ND phenotypes, including automatized smoking behavior, cognitive, and emotional deficits.
Implications
As smoking remains a worldwide public health problem, identifying the related neural alterations may help to understand the pathophysiology of ND. Based on previous findings in the cerebellum, we investigated the localization of the GM differences and related FC changes in ND subjects. Our findings highlight altered corticocerebellar circuits in ND, suggesting an association between the cerebellum and the phenotypes of ND.
Introduction
Nicotine dependence (ND) is a chronic, relapsing mental disorder characterized by compulsive cigarette seeking and smoking. The neurological basis of ND is predominantly related to dopaminergic neural circuitry comprising the prefrontal cortex, midbrain, insula, and striatum.1,2 Recent research has raised awareness of the involvement of the cerebellum in ND. The cerebellum has multiple nicotinic acetylcholine receptor (nAChR) subtypes, including the homomeric α7 and α4β2 subtypes, which are sensitive to nicotine-induced sensitization.3,4 Repeated nicotine exposure can upregulate the number of nAChRs, resulting in alterations in synaptic plasticity.5,6 Further, the excitotoxic actions of nicotine can cause cell loss in the cerebellum, particularly of Purkinje and granular neurons.7,8 More recently, a role for the cerebellum in addiction was highlighted by Miquel et al, with evidence that drug-induced functional and structural changes in the cerebellum were central to the transition from a pattern of recreational drug taking to a compulsive behavioral phenotype.9
The structural and functional abnormalities of the cerebellum in ND are supported by several neuroimaging studies. For example, functional magnetic resonance imaging (fMRI) data indicate that the cerebellar activation along with cortical activation in ND is associated with working memory performance,10 response inhibition11 and cue-induced craving.12,13 According to cerebellar networks theory, interactions with the cerebral cortex may represent neural substrates for cerebellar involvement in cue-induced craving and addiction.14 We previously reported that the cerebellum was an important hub of the brain network related to smoking relapse, with cerebellar functional connectivity (FC) independently predicting smoking relapse with 75.4% accuracy.15 However, the specific alterations in the cerebellar networks in ND remain unclear, although one study reported evidence of altered FC strength in different cerebellar subregions when using posterior cingulate cortex (PCC) as a seed.16 An increasing number of studies have also employed structural MRI to investigate anatomical alterations in the brain in ND, with the majority reporting a reduction in cerebellar gray matter (GM) in smokers when compared to nonsmokers.17–21 Recently, a meta-analysis of voxel-based morphometry (VBM) studies examining structural changes in the brains in ND also showed a decrease in GM in the right cerebellum.22
Although previous studies provide valuable evidence for abnormal structure and function in the cerebellum, the majority have examined the cerebellum as a whole and only considered its role in movement. In fact, the cerebellum is anatomically divided into 3 lobes and 10 lobules (lobules I–X): the anterior lobe (lobules I–V), the posterior lobe (lobules VI–IX), and the flocculonodular lobe (lobule X). Each of these lobules has been linked to specific corticocerebellar functional loops, supporting its own function, such as emotion, cognition, and movement.23,24 Thus, identifying the specific cerebellar localization of structural changes and the related functional circuits may be beneficial for elucidating the role of the cerebellum in ND.
At present, the reported changes in specific cerebellar lobules in ND are inconsistent. For instance, decreased cerebellar GM was frequently observed in the Crus I, lobule VII, and lobule VIII18–20 but less so in the Crus II, lobule V, and lobule VI.17,21 These contrasting findings may relate to the small sample sizes in those studies or that changes were typically examined at the whole-brain level. Indeed, the small size and functional heterogeneity of the cerebellum make it a technical challenge for normalization. Commonly used whole-brain normalization algorithms, such as SPM segmentation, result in poor alignment of regions within the cerebellar cortex, which reduces statistical power.25 However, using a cerebellum-only template, the spatially unbiased infratentorial template (SUIT) method was reported to significantly improve the overlap of cerebellar subregions across individuals.25,26 Thus, in the present study, we used the SUIT to investigate the regional structural alterations in the cerebellum with a relatively large sample of male smokers. By selecting the regions with significant difference in VBM analysis as seeds, we further performed a seed-based, resting-state FC analysis to assess for dysfunction in cerebellum-related network.
Methods
Participants
A total of 126 healthy volunteers, including 85 smokers (aged 22–54 years) and 41 nonsmokers (aged 26–56 years), were recruited by posted flyers and online advertisements (Table 1). All participants were male, Han Chinese, and right-handed. Smokers were defined as individuals who smoked more than 10 cigarettes per day in the last 1 year and met the DSM-IV criteria of nicotine dependence. Nonsmokers were defined as individuals who had smoked fewer than 20 cigarettes in their lifetime and none in the past 10 years. Exclusion criteria for all participants were as follows: (1) a history of neurological or psychiatric diseases; (2) systemic diseases (i.e., diabetes and hypertension); (3) current use of psychotropic medications or concurrent substance abuse, such as alcohol and heroin; (4) MRI contraindications like claustrophobia and metal implants. All aspects of the research protocol were reviewed and approved by the Institutional Review Boards of the Second Affiliated Hospital of Zhejiang University School of Medicine. All subjects provided signed informed consents prior to study participation.
Table 1.
Characteristics of smokers and nonsmokers
| Control (n = 41) | Smoker (n = 85) | t | p | |
|---|---|---|---|---|
| Age (years) | 38.46 ± 8.60 | 38.24 ± 6.81 | −0.161 | .872 |
| Education (years) | 15.37 ± 4.67 | 14.01 ± 2.94 | −1.702 | .094 |
| Smoking initiation (years) | – | 20.87 ± 5.07 | – | – |
| Smoking years | – | 17.36 ± 6.58 | – | – |
| Cigarettes/day | – | 23.46 ± 9.53 | – | – |
| Pack-years | – | 20.63 ± 12.28 | – | – |
| FTND | – | 5.18 ± 2.18 | – | – |
Abbreviations: Pack-years, cigarettes/day × smoking years/20; FTND, Fagerström test for nicotine dependence.
Demographic and smoking data were obtained from all participants by a questionnaire prior to scanning. ND severity was assessed using Fagerström Test for Nicotine Dependence (FTND).27 Exhaled carbon monoxide (CO) was measured to confirm participants’ smoking status (smoker ≥ 10ppm, nonsmoker ≤ 6ppm). Smokers smoked their last cigarette approximately ten minutes before resting-state scanning. Four smokers were excluded because of excessive head motion, resulting in a total number of 81 smokers and 41 nonsmokers for resting-state fMRI analysis. Imaging data from a subgroup of these subjects’ were previously published.28
Image Acquisition
All scans were acquired on a 3.0 T GE SIGNA scanner with a birdcage head coil. Conventional T1- and T2-weighted images were performed to rule out structural abnormalities. Resting-state functional scans consisted of 185 echo-planar imaging (EPI) volumes with the following parameters: 30 slices (thickness/gap = 4/1 mm), repetition time (TR) = 2000 ms, echo time (TE) = 30 ms, matrix = 64 × 64, field of view (FOV) = 240 × 240 mm2, and flip angle = 80°. During fMRI scanning (370s), participants were instructed to lie still, keep eyes closed, and not to fall asleep. Ear plugs and foam padding were used to reduce scanner noise and head motion. Additionally, a set of high-resolution anatomical T1-wighted images were obtained using 3D fast-spoiled gradient echo (FSPGR) sequence with following parameters: 136 sagittal slices (thickness/gap = 1.2/0 mm), TR = 5.06 ms, TE = 1.12 ms, matrix = 256 × 256, FOV = 240 × 216 mm2, and flip angle = 15°. All MRI data were visually inspected for image artifacts and anatomical abnormalities by an experienced neuroradiologist.
Image Processing
Lobular Volume analysis
Lobular volumes were calculated using the SUIT (http://www.diedrichsenlab.org/imaging/suit.htm)26 implemented in SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The origin of the image was set to the anterior commissure, followed by segmentation into GM and white matter (WM) using the “isolate” function in SUIT. Meanwhile, a classification map was generated to exclude any GM included outside the cerebellum. The segmented GM images were then normalized and resliced to the SUIT template with DARTEL. Finally, the number of voxels in each lobule in the resliced images was summarized. This process resulted in 28 volumetric GM measurements, including the 10 bilateral lobules (I–X right and I–X left; lobules I–IV were combined into one measure, and lobule VII wass divided into VIIb, Crus I and Crus II; lobule VIII is divided into VIIIa and VIIIb) and the vermis lobules VI–X.
Voxel-Based Morphometry Analysis
Structural images were processed using the SUIT toolbox to optimize the VBM procedure, per the lobular volume analysis. After isolation, normalization, and reslicing, the modulated images were then smoothed with a 4-mm full-width half-maximum (FWHM) Gaussian kernel in SPM8. To compare with previous whole-brain studies, we also performed whole-brain analysis using DARTEL (see Supplementary Materials).
Seed-Based Functional Connectivity Analysis
Resting BOLD data were preprocessed with DPARSFA (Data Processing Assistant for Resting-State fMRI Advanced Edition, http://www.restfmri.net/forum/DPARSF), which is based on SPM8. The first 10 volumes were discarded to reduce magnetization disequilibrium, followed by slice-timing correction and head motion correction. Exclusion criteria on head motion was exceeding more than 2 mm/degree (four smokers were excluded). After segmentation of T1 images, resting images were coregistered to T1 images and then registered to the standard Montreal Neurological institute (MNI) template and resampled into 3 × 3 × 3 mm3 cubic voxels. Spatial smoothing was then performed with an isotropic 6-mm FWHM kernel. Finally, linear detrending and temporal band-pass filtering (0.01–0.08 Hz) were performed to remove low- and high-frequency noise. To remove any residual effects of motion and other nonneuronal factors, nuisance covariates, including six head motion parameters and signals of white matter and cerebrospinal fluid, were regressed out. We also calculated the mean framewise displacement (FD)29 of each participant. Group comparison showed no FD difference between smokers (0.22 ± 0.08, mean ± standard deviation) and nonsmokers (0.20 ± 0.08) (t = 1.445, p = .151). Given the potential for spurious signal changes from head micromovements, we removed frames with FD > 0.5mm (“scrubbing”).30
Cerebellar regions showing significant group effects in GM volume, if any, were used as a seed to investigate FC differences between smokers and nonsmokers. A correlation coefficient map for each seed was obtained by correlating the average time course from the seed with every voxel’s time course, which was then transformed into z-value using Fisher’s z transformation. To avoid any influence related to the selection of seeds, we also analyzed the FC maps with regions of interest (ROIs) defined as a sphere (3 mm radius) around the peak voxels of the significant GM clusters (see Supplementary Materials).
Statistical Analysis
Group differences in demographic and smoking data were analyzed using SPSS 19.0. Age and education were compared between smokers and nonsmokers using independent-sample t tests. All tests were two-tailed, and results were considered significant at p<.05.
Lobular Volume Analysis
Lobular volumes were corrected for total cerebellar volume (individual lobular volumes / total cerebellar volume), and independent-sample t tests were performed to identify group differences.
Voxel-Based Morphometry Analysis
Regional differences in GM between groups were assessed using the general linear model (GLM) in SPM8. Smoothed GM images were entered into a voxel-wise two-sample t test analysis with age, education, and total cerebellar volume as covariates. The threshold was set at a voxelwise p < .05 false discovery rate (FDR) corrected for multiple comparisons in SPM. Signals from the significant clusters were extracted and their correlations with smoking data (i.e., pack-years and FTND scores) were calculated.
Seed-Based FC Analysis
The VBM analyses revealed that GM volumes of the left Crus I and the right Crus I were significantly smaller in smokers. For each seed, a GLM was used to examine the differences between smokers and nonsmokers with age, education, and total cerebellar volume as covariates. Multiple comparisons were corrected at a threshold of α <.05 determined by the AFNI 3dClustSim Program. The following parameters were used: single voxel p = .005, cluster size = 27 voxels (729 mm3), FWHM = 6 mm, and cluster connection radius r = 5 mm. Signals from the significant clusters were extracted and their correlations with smoking data (i.e., pack-years and FTND scores) were calculated.
Results
Cerebellar Lobular Volumes
Lobular volume analysis revealed that the right Crus I was significant smaller in smokers (11.15% ± 0.25% of cerebellar volume) compared to nonsmokers (11.26% ± 0.25% of cerebellar volume, p = .018). Further, there was a trend toward a smaller left Crus I in smokers (7.21% ± 0.20% of cerebellar volume) compared to nonsmokers (7.27% ± 0.15% of cerebellar volume, p = .058).
Voxel-Based Morphometry
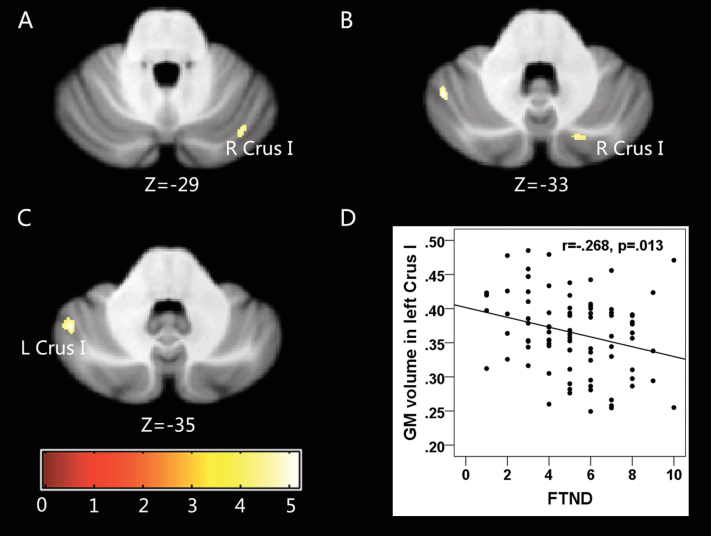
As for lobular analysis, smokers showed significantly smaller left Crus I (Figure 1C) and right Crus I volume(Figure 1A and B; Table 2). Correlation analysis revealed a negative correlation between GM volume of the left Crus I and FTND scores (r = −.268, p = .013; Figure 1D).
Figure 1.
Statistical parametric map showing the significant differences in gray matter (GM) volume between smokers and nonsmokers (region of interest for resting-state FC). (A and B) Smokers had smaller GM volume (versus nonsmokers) in the right Crus I. (C) Smokers had smaller GMvolume (versus nonsmokers) in the left Crus I. The results were corrected for multiple comparisons (voxelwise p < .05, false discovery rate [FDR] corrected). (D) Scatter plot of gray matter volume extracted from the left Crus I and Fagerström Test for Nicotine Dependence (FTND) scores in smokers (r = −.268, p = .013).
Table 2.
Cerebellar areas where there were significant differences in gray matter volume between smokers and nonsmokers
| Location | L/R | Cluster Size | MNI Coordinate | T value | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| smoker < nonsmoker | ||||||
| Cerebellum_Crus I | L | 128 | −45 | −56 | −33 | 5.174 |
| Cerebellum_Crus I | R | 31 | 18 | −76 | -34 | 4.637 |
| Cerebellum_Crus I | R | 47 | 37 | −72 | −30 | 5.099 |
Abbreviation: MNI, Montreal Neurological institute.
Seed-Based FC
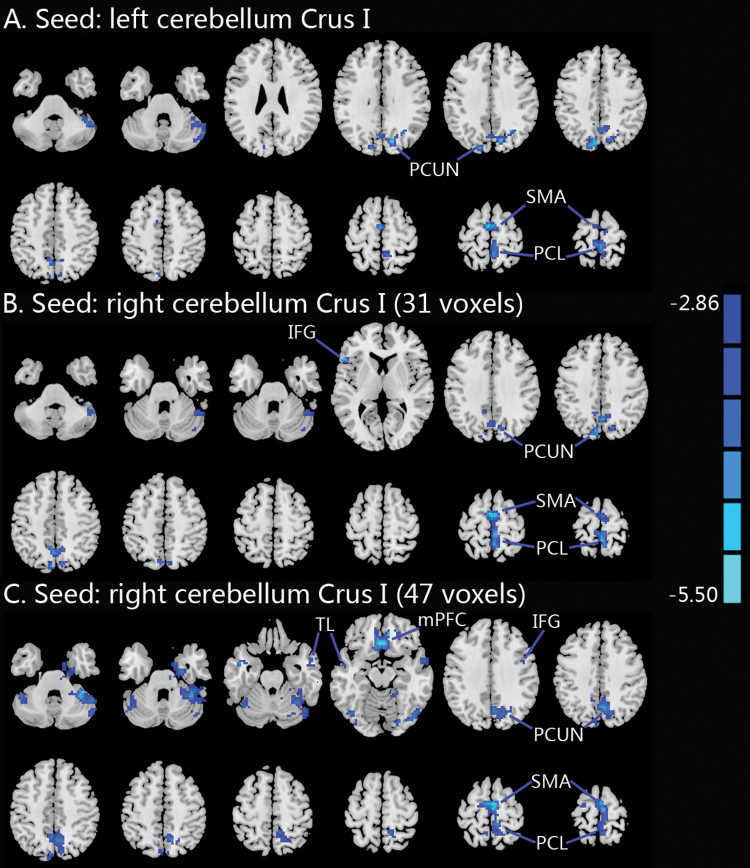
The results of the seed-based FC analyses are shown in Figure 2 and Table 3. Smokers showed significantly lower FC between the bilateral Crus I and brain regions involved in the default mode network (DMN), such as the medial prefrontal cortex (mPFC) and the precuneus. Smokers also showed significantly lower FC between the bilateral Crus I and brain regions associated with motor planning, such as the paracentral lobule and the supplementary motor area (SMA). Lower FC was also found between the right Crus I and temporal lobe and inferior frontal gyrus in smokers. Using the right cerebellar Crus I (47 voxels) as a seed, the FC with the cluster including the bilateral SMA, bilateral precuneus, and right paracentral lobule was negatively correlated with cigarettes per day (r = −.234, p = .035).
Figure 2.
Group differences in seed-based resting-state FC. (A) Compared to non-smokers, smokers showed decreased FC between the left Crus I and bilateral PCL, bilateral SMA and bilateral PCUN. (B) Compared to nonsmokers, smokers showed decreased FC between the right Crus I (31 voxels) and bilateral PCL, bilateral SMA, bilateral PCUN, and left IFG. (C) Compared to nonsmokers, smokers showed decreased FC between the right Crus I (47 voxels) and bilateral mPFC, bilateral PCL, bilateral SMA, bilateral TL, right PCUN, and right IFG. A voxelwise threshold of p < .005 and a spatial extent of 27 contiguous voxels were determined via Monte Carlo simulation to provide a corrected threshold of p < .05. IFG, inferior frontal gyrus; mPFC, medial prefrontal cortex; FC, functional connectivity; PCL, paracentral lobule; PCUN, precuneus; SMA, supplementary motor area; TL, temporal lobe.
Table 3.
The locations of the regions showing significantly lower FC with the three cerebellar clusters showing GM volume reduction in smokers compared to nonsmokers
| Seed | Voxels | Brain regions | MNI coordinate | Peak t |
|---|---|---|---|---|
| A. left cerebellum Crus I | 151 | Bilateral PCL | −3 −33 75 | −4.3255 |
| 100 | Bilateral SMA | −6 −9 69 | −4.1147 | |
| 271 | Bilateral PCUN | −9 −78 42 | −4.2543 | |
| B. right cerebellum Crus I (31 voxels) | 261 | Bilateral PCL | 3 −9 66 | −4.5143 |
| Bilateral SMA | ||||
| 205 | Bilateral PCUN | −9 −78 42 | −4.0356 | |
| 33 | Left IFG | −54 24 6 | −3.9711 | |
| C. right cerebellum Crus I (47 voxels) | 240 | Bilateral mPFC | 3 27 −15 | −5.3152 |
| 644 | Bilateral SMA | 3 −9 66 | −5.1613 | |
| Bilateral PCUN | ||||
| Right PCL | ||||
| 72 | Right TL | 60 3 −18 | −4.1837 | |
| 37 | Left TL | −45 0 −24 | −4.4238 | |
| 42 | Right IFG | 36 9 30 | −4.1729 |
Abbreviations: IFG, inferior frontal gyrus; FC, functional connectivity; mPFC, medial prefrontal cortex; PCL, paracentral lobule; PCUN, precuneus; SMA, supplementary motor area; TL, temporal lobe.
Discussion
In the present study, we investigated the localization of cerebellar structural differences between smokers and nonsmokers. Both VBM and lobular analyses showed that smokers had smaller GM volume in the bilateral Crus I than nonsmokers. In smokers, there was a negative correlation of GM volume with ND severity for a cluster in the left Crus I. Further, seed-based FC analysis showed significantly lower FC between the bilateral Crus I and various brain regions related to the DMN and motor planning in smokers.
VBM has been widely used to examine the structural differences in ND for two decades. Although some studies17–21 reported GM reduction in the cerebellum of smokers, the localization of the abnormalities was inconsistent. We owed it to the image-processing methods and the small sample size. Therefore, we tested the effects of nicotine on cerebellar GM volume in a large cohort using a spatially unbiased algorithm. We found smaller GM volume in the bilateral Crus I, which is consistent with two previous studies, one20 in a large sample of 80 smokers and the other18 using SUIT. The present study also extended these findings to adult male smokers with the entire range of nicotine dependence severity.
Although the cerebellum is classically considered important for coordinating movement, maintaining posture, and equilibrium,9 growing evidence suggests a role in a wide range of other functions, including regulation of cognition and emotion, attention processing, and decision-making, many of which are affected in addicts.31 As such, the cerebellum has been suggested to form part of the addiction circuitry.32 The Crus I is located in the posterior lobe of the cerebellum and is important for cognitive function.33 Altered neural activity in the Crus I has also been reported in smokers. For example, in a positron emission tomography (PET) study,34 increased regional cerebral blood flow (rCBF) in the Crus I was associated with monetary and nonmonetary reinforcement in smokers, but not in non-smokers. rCBF in the bilateral Crus I was also reported to be increased by cigarette smoking in smokers after overnight abstinence.35 These studies suggest a role for the Crus I in processing reinforcement and regulating emotion. Although it is clear that chronic smoking can cause cognitive impairment,36 whether the GM reduction in the Crus I is associated with cognitive decline in smokers needs to be studied further.
We also found that GM volume in the left Crus I was inversely correlated with FTND scores, which is consistent with a previous report.18 The stronger correlation in that study may be a result of the smaller sample size (33 smokers) and the narrow FTND score range (4–8). We also observed a volume reduction in the bilateral cerebellum in male smokers, similar to that previously reported.19,20 These findings contrast with a report of reduced GM volume in the right cerebellum of both sexes. It might be caused by gender effects, as a gender-specific analysis20 showed larger and bilateral volume reduction in male smokers.
The Crus I is anatomically and functionally connected to the prefrontal, temporal, and parietal regions of the cerebral cortex, while resting-state FC data suggest that the Crus I is functionally connected to the ventral attention, frontoparietal, and DMN regions.23,37 Among these networks, alterations in the DMN are commonly reported in ND. The DMN is involved in self-referential mental activity, emotional processing, and recollection of prior experiences.38 Previous neuroimaging studies consistently reported abnormal DMN activation and disrupted integration of the DMN with other brain regions in nicotine-dependent individuals across a range of cognitive paradigms, including working memory,39 attention bias,40 and inhibition control.41 Resting-state and pharmacological fMRI studies have also demonstrated increased BOLD signal in the DMN in deprived smokers and decreased BOLD signal in the DMN after acute nicotine administration.42,43 Further, in a case report, damage to the PCC, a core region of the DMN, completely disrupted addiction to cigarette smoking.44 Thus, the DMN is thought to play an important role in ND, particularly in withdrawal symptoms, such as craving and difficulty concentrating.45 In the present study, we found a significant reduction in FC between the bilateral Crus I and the DMN. Our findings are consistent with previous studies showing Crus I involvement in the spontaneous brain activity of the DMN.23,37 Further, our findings are similar to those of Wetherill et al, who identified the core nodes of the DMN using the PCC as a seed region and found lower FC strength between the PCC and the cerebellum (Crus I/II) in satiated smokers.16 Overall, these data suggest that reduced FC strength between the DMN and the Crus I may underlie the cognitive and emotional deficits associated with ND.
In addition, we observed decreased FC between the bilateral Crus I and the paracentral lobule and SMA, structures involved in automatized behavior and motor planning. Drug-taking skills were suggested to constitute the core of drug acquisition and consumption behavior, which becomes highly automated after repeated practice.46 Recently, Yalachkov et al proposed a model for the involvement of motor mechanisms in addiction.47 Further, a number of studies using the cue reactivity paradigm have reported preferential activations in the SMA, cerebellum, motor, and premotor cortex for drug-related cues in addicts in comparison to neutral cues.48–50 The responses in these regions were also correlated with the severity of ND,50 and the degree of automaticity of the behavioral responses toward smoking-related cues.49 A recent study51 of resting-state regional homogeneity (ReHo) in heavy smokers also found increased ReHo predominately in the paracentral lobule, SMA, and cerebellum. Given these findings, we speculate that FC between the Crus I and the motor system may be important for automatized smoking behavior.
Limitations
Because of the cross-sectional design, we were unable to determine the casual relationship between cigarette smoking and cerebellar alterations. Another limitation was that only male smokers were recruited in our study. Given the gender effect on ND,52 the findings may not be extended in female smokers. Further, as the images in our study were acquired just 10 minutes after the last cigarette, it is possible that the FC findings may be attributed to acute rather than chronic smoking.
Conclusions
In conclusion, by simultaneously using VBM and lobular analyses of the cerebellum in a large cohort of cigarette smokers, we confirmed a significant reduction in cerebellar GM volume in the bilateral Crus I. We also found that the GM volume in the left Crus I was related to FTND, a stable and heritable measure of nicotine dependence severity. Finally, we observed reduced FC between the bilateral Crus I and the DMN and motor system in smokers. Although we did not examine the specific mechanisms, these findings contribute to our knowledge of cerebellar morphology and its FC with the cerebral cortex in ND.
Supplementary Material
Supplementary data is available at Nicotine & Tobacco Research online.
Funding
This work was supported by the National Natural Science Foundation of China (81171310), Medical and Health Scientific Research Fund Project of Zhejiang Province (2017KY080) and the Scientific Research Project of Zhejiang Province (2011C23094).
Declaration of Interests
None declared.
Supplementary Material
Acknowledgments
We would like to thank all the smokers and non-smokers who participated in our study. YY is supported by the Intramural Research Program of the National Institute on Drug Abuse, the National Institutes of Health.
References
- 1. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159(10):1642–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Paterson D, Nordberg A. Neuronal nicotinic receptors in the human brain. Prog Neurobiol. 2000;61(1):75–111. [DOI] [PubMed] [Google Scholar]
- 4. Turner JR, Kellar KJ. Nicotinic cholinergic receptors in the rat cerebellum: multiple heteromeric subtypes. J Neurosci. 2005;25(40):9258–9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mukhin AG, Kimes AS, Chefer SI et al. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F-FA-85380. J Nucl Med. 2008;49(10):1628–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Court JA, Lloyd S, Thomas N et al. Dopamine and nicotinic receptor binding and the levels of dopamine and homovanillic acid in human brain related to tobacco use. Neuroscience. 1998;87(1):63–78. [DOI] [PubMed] [Google Scholar]
- 7. Chen WJ, Edwards RB, Romero RD, Parnell SE, Monk RJ. Long-term nicotine exposure reduces Purkinje cell number in the adult rat cerebellar vermis. Neurotoxicol Teratol. 2003;25(3):329–334. [DOI] [PubMed] [Google Scholar]
- 8. Fonnum F, Lock EA. Cerebellum as a target for toxic substances. Toxicol Lett. 2000;112-113:9–16. [DOI] [PubMed] [Google Scholar]
- 9. Miquel M, Toledo R, García LI, Coria-Avila GA, Manzo J. Why should we keep the cerebellum in mind when thinking about addiction?Curr Drug Abuse Rev. 2009;2(1):26–40. [DOI] [PubMed] [Google Scholar]
- 10. Kumari V, Gray JA, ffytche DH et al. Cognitive effects of nicotine in humans: an fMRI study. Neuroimage. 2003;19(3):1002–1013. [DOI] [PubMed] [Google Scholar]
- 11. Weywadt CR, Kiehl KA, Claus ED. Neural correlates of response inhibition in current and former smokers. Behav Brain Res. 2017; 319: 207–218. [DOI] [PubMed] [Google Scholar]
- 12. Engelmann JM, Versace F, Robinson JD et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage. 2012;60(1):252–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs - a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci. 2011;33(7):1318–1326. [DOI] [PubMed] [Google Scholar]
- 14. Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17(5):241–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shen Z, Huang P, Wang C, Qian W, Yang Y, Zhang M. Increased network centrality as markers of relapse risk in nicotine-dependent individuals treated with varenicline. Prog Neuropsychopharmacol Biol Psychiatry. 2017; 75: 142–147. [DOI] [PubMed] [Google Scholar]
- 16. Wetherill RR, Fang Z, Jagannathan K, Childress AR, Rao H, Franklin TR. Cannabis, cigarettes, and their co-occurring use: Disentangling differences in default mode network functional connectivity. Drug Alcohol Depend. 2015;153:116–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brody AL, Mandelkern MA, Jarvik ME et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry. 2004;55(1):77–84. [DOI] [PubMed] [Google Scholar]
- 18. Kuhn S, Romanowski A, Schilling C et al. Brain grey matter deficits in smokers: focus on the cerebellum. Brain Struct Funct. 2012; 217 (2): 517–522. [DOI] [PubMed] [Google Scholar]
- 19. Yu R, Zhao L, Lu L. Regional grey and white matter changes in heavy male smokers. PLoS One. 2011; 6(11): e27440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franklin TR, Wetherill RR, Jagannathan K et al. The effects of chronic cigarette smoking on gray matter volume: influence of sex. PLoS One. 2014; 9(8): e104102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wetherill RR, Jagannathan K, Hager N, Childress AR, Rao H, Franklin TR. Cannabis, Cigarettes, and Their Co-Occurring Use: Disentangling Differences in Gray Matter Volume. Int J Neuropsychopharmacol. 2015; 18(10): pyv061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sutherland MT, Riedel MC, Flannery JS et al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016;12(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(5):2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. 2010;46(7):831–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Diedrichsen J. A spatially unbiased atlas template of the human cerebellum. Neuroimage. 2006; 33(1): 127–138. [DOI] [PubMed] [Google Scholar]
- 26. Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. [DOI] [PubMed] [Google Scholar]
- 27. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 28. Shen Z, Huang P, Qian W et al. Severity of dependence modulates smokers’ functional connectivity in the reward circuit: a preliminary study. Psychopharmacology (Berl). 2016;233(11):2129–2137. [DOI] [PubMed] [Google Scholar]
- 29. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014; 84: 320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Moulton EA, Elman I, Becerra LR, Goldstein RZ, Borsook D. The cerebellum and addiction: insights gained from neuroimaging research. Addict Biol. 2014;19(3):317–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miquel M, Vazquez-Sanroman D, Carbo-Gas M et al. Have we been ignoring the elephant in the room? Seven arguments for considering the cerebellum as part of addiction circuitry. Neurosci Biobehav Rev. 2016;60:1–11. [DOI] [PubMed] [Google Scholar]
- 33. Strick PL, Dum RP, Fiez JA. Cerebellum and nonmotor function. Annu Rev Neurosci. 2009;32:413–434. [DOI] [PubMed] [Google Scholar]
- 34. Martin-Sölch C, Magyar S, Künig G, Missimer J, Schultz W, Leenders KL. Changes in brain activation associated with reward processing in smokers and nonsmokers. A positron emission tomography study. Exp Brain Res. 2001;139(3):278–286. [DOI] [PubMed] [Google Scholar]
- 35. Zubieta JK, Heitzeg MM, Xu Y et al. Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry. 2005;162(3):567–577. [DOI] [PubMed] [Google Scholar]
- 36. Richards M, Jarvis MJ, Thompson N, Wadsworth ME. Cigarette smoking and cognitive decline in midlife: evidence from a prospective birth cohort study. Am J Public Health. 2003;93(6):994–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeo BT, Krienen FM, Sepulcre J et al. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106(3):1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Raichle ME. The brain’s default mode network. Annu Rev Neurosci. 2015;38:433–447. [DOI] [PubMed] [Google Scholar]
- 39. Jacobsen LK, Mencl WE, Constable RT, Westerveld M, Pugh KR. Impact of smoking abstinence on working memory neurocircuitry in adolescent daily tobacco smokers. Psychopharmacology (Berl). 2007;193(4):557–566. [DOI] [PubMed] [Google Scholar]
- 40. Luijten M, Veltman DJ, van den Brink W et al. Neurobiological substrate of smoking-related attentional bias. Neuroimage. 2011;54(3):2374–2381. [DOI] [PubMed] [Google Scholar]
- 41. de Ruiter MB, Oosterlaan J, Veltman DJ, van den Brink W, Goudriaan AE. Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend. 2012;121(1-2):81–89. [DOI] [PubMed] [Google Scholar]
- 42. Sutherland MT, McHugh MJ, Pariyadath V, Stein EA. Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage. 2012;62(4):2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sutherland MT, Ray KL, Riedel MC, Yanes JA, Stein EA, Laird AR. Neurobiological impact of nicotinic acetylcholine receptor agonists: an activation likelihood estimation meta-analysis of pharmacologic neuroimaging studies. Biol Psychiatry. 2015;78(10):711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jarraya B, Brugières P, Tani N et al. Disruption of cigarette smoking addiction after posterior cingulate damage. J Neurosurg. 2010;113(6):1219–1221. [DOI] [PubMed] [Google Scholar]
- 45. Li Y, Yuan K, Bi Y et al. Neural correlates of 12-h abstinence-induced craving in young adult smokers: a resting-state study. Brain Imaging Behav. 2016;11(3):677–684. [DOI] [PubMed] [Google Scholar]
- 46. Jasinska AJ, Stein EA, Kaiser J, Naumer MJ, Yalachkov Y. Factors modulating neural reactivity to drug cues in addiction: a survey of human neuroimaging studies. Neurosci Biobehav Rev. 2014;38:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yalachkov Y, Kaiser J, Naumer MJ. Sensory and motor aspects of addiction. Behav Brain Res. 2010;207(2):215–222. [DOI] [PubMed] [Google Scholar]
- 48. McClernon FJ, Kozink RV, Lutz AM, Rose JE. 24-h smoking abstinence potentiates fMRI-BOLD activation to smoking cues in cerebral cortex and dorsal striatum. Psychopharmacology (Berl). 2009;204(1):25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yalachkov Y, Kaiser J, Naumer MJ. Brain regions related to tool use and action knowledge reflect nicotine dependence. J Neurosci. 2009;29(15):4922–4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Smolka MN, Bühler M, Klein S et al. Severity of nicotine dependence modulates cue-induced brain activity in regions involved in motor preparation and imagery. Psychopharmacology (Berl). 2006;184(3-4):577–588. [DOI] [PubMed] [Google Scholar]
- 51. Wu G, Yang S, Zhu L, Lin F. Altered spontaneous brain activity in heavy smokers revealed by regional homogeneity. Psychopharmacology (Berl). 2015;232(14):2481–2489. [DOI] [PubMed] [Google Scholar]
- 52. Wetherill RR, Jagannathan K, Shin J, Franklin TR. Sex differences in resting state neural networks of nicotine-dependent cigarette smokers. Addict Behav. 2014;39(4):789–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.