Abstract
Background
Two genetic variants in apolipoprotein L1 (APOL1) are associated with increased risk of focal segmental glomerulosclerosis as well as other glomerular phenotypes. These risk variants are common in individuals of African ancestry but absent in other racial groups. Yet, the majority of individuals with two APOL1 risk alleles [high-risk (HR) genotype] do not have renal disease. It is critical to identify environmental and secondary genetic influences that, when combined with these alleles, lead to kidney disease. In a recent study of black children with glomerular disease enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) and Chronic Kidney Disease in Children Study (n = 104), we found that subjects with an HR genotype had a 4.6-fold increase in the odds of preterm birth as compared to those with a low risk (LR) genotype [odds ratio 4.6 (CI 1.4–15.5)]. There are known racial disparities in preterm birth, which itself is a known risk factor for chronic kidney disease and focal segmental glomerulosclerosis. Thus, we questioned whether an HR APOL1 genotype is associated with prematurity in the general African American population.
Methods
We analyzed two publically available genetic datasets of preterm birth in African Americans, including 867 infants and 519 mothers from the Gene Environment Association Studies (GENEVA) study of preterm delivery and 960 mothers from the Boston Medical Center genome-wide association study of preterm birth. We performed multivariable analyses testing for association between HR APOL1 and birth outcomes.
Results
In both studies, there was no association between HR APOL1 in mothers and prematurity, gestational age or birthweight. Additionally, in the GENEVA study, we saw no association between infant HR APOL1 and prematurity, gestational age or birthweight.
Conclusion
From these data, we conclude that the previously observed association between HR APOL1 and prematurity is specific to those with glomerular disease, suggesting prematurity may act as an additional risk factor in APOL1-associated renal disease.
Keywords: CKD, epidemiology, ethnicity, risk allele, pediatrics, prematurity
INTRODUCTION
Less than 10 000 years ago, as a result of positive selection, two specific genetic variants in apolipoprotein L1 (APOL1), termed G1and G2, became common in Africans [1, 2]. As a result, 13% of all African Americans harbor two copies of G1 and/or G2 [3]. This is called a high-risk(HR) APOL1 genotype because individuals with two copies of these alleles have a 10–20 times increased risk of focal segmental glomerulosclerosis (FSGS) and a 2–3 times increased risk of developing chronic kidney disease (CKD) in general [4].
Yet, despite the greatly increased odds of kidney disease conferred by an HR APOL1 genotype, it exhibits incomplete penetrance of the renal disease phenotype. This has led to a search for factors that modify the penetrance of APOL1's HR genotype. Previously discovered modifiers include HIV [5, 6] and other genetic variants [7].
In a recent study of black children with proteinuric disease enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) and the Chronic Kidney Disease in Children Study (CKiD) (n = 104), we found that children with an HR genotype had 4.6-fold increased odds of reported preterm birth as compared to those with a low-risk (LR) genotype [odds ratio (OR) 4.6 (CI 1.4–15.5)] [8]. These results raised the question of whether prematurity acts as an additional risk factor that increases the risk of an African American with an HR APOL1 genotype developing proteinuric disease. If indeed prematurity is a risk-modifying factor for APOL1-associated glomerular disease, preterm infants with HR APOL1 may be particularly vulnerable to subsequent kidney disease.
At the same time, there is a known increased prevalence of premature birth in African Americans [9, 10], and preterm children are at a higher risk of CKD (thought to be mediated in part through low birthweight) [11–13]. In humans, there is also high expression of ApoL1 in the placenta [14, 15] and within the vasculature [16]. Furthermore, a recently created mouse model expressing transgenic APOL1 (wild-type and with the G2 risk variant; under the nephrin promoter) displays a pregnancy phenotype [17]. In this report, Bruggeman et al. [17] observe that pregnant female mice expressing transgenic APOL1 have placental expression of APOL1 and develop preeclampsia/eclampsia and fetal and neonatal demise. This phenotype was worse with expression of the G2 variants. It is thus possible that an HR APOL1 genotype could itself be associated with prematurity.
Disentangling these relationships between black race, prematurity, kidney disease and risk genotypes could increase our understanding of the biologic mechanisms underlying HR APOL1's harm and its clinical consequences. To this end, we used existing genotype and clinical data from African Americans in two general population cohorts of mothers and infants to evaluate the association between the maternal or infant APOL1 genotype and birth outcomes as well as the same associations with joint mother–infant APOL1 status. In this way, we describe whether either maternal or infant genotype status was associated with preterm birth or birthweight.
MATERIALS AND METHODS
Description of parent studies
Genotype and phenotype data from two independent case–control genome-wide association studies (GWASs) of preterm birth and other birth-related outcomes were downloaded from the Database of Genotypes and Phenotypes (dbGaP).
BMC
The GWAS of Preterm Birth (‘BMC’) (dbGaP Study ID: phs000332) was a matched case–control study of preterm birth in 1028 African American mothers that sought to determine genetic factors associated with preterm birth. Recruitment of BMC subjects began in 1998 and included mothers who delivered babies at the Boston Medical Center, excluding pregnancies involving (i) multiple gestations (i.e. twins, triplets), (ii) in vitro fertilization, (iii) chromosomal abnormalities or major birth defects, (iv) congenital or acquired uterine lesions or known history of incompetent cervix or (v) preterm birth due to maternal trauma. Control mothers were defined as mothers with no gestational complications who delivered normal birthweight (>2500 g) infants at 38–42 weeks of gestation. Cases and controls were 1:1 frequency matched based on maternal age (±5 years), parity and year of delivery.
GENEVA
As part of the Gene Environment Association Studies (GENEVA) initiative, a GWAS of prematurity using 2141 African American infants and mothers was designed to identify novel genetic factors contributing to preterm birth (dbGaP Study ID: phs000353). A subaim of this study was designed to specifically address prematurity and its complications in African Americans. To achieve this, term and preterm mothers and infants were recruited from multiple sites and centers across the USA, typically as part of ongoing studies of preterm birth. In total, subjects were recruited from six primary sites. Cases and controls were not matched and case–control status was not balanced across sites. Enrollment criteria details at each GENEVA site are presented in Supplementary data, Table S1.
Genotyping and imputation
Details about the genotyping platforms and quality filtering used by the GENEVA and BMC studies are provided in Supplementary data, Note 1. In GENEVA, both single-nucleotide polymorphisms (SNPs) that make up the G1 haplotype (rs73885319 and rs60910145) were directly genotyped, while genotypes for the 6 base pair deletion (G2; rs71785313) were imputed. In BMC, rs73885319 was directly genotyped, while rs60910145 and G2 were imputed. Details on imputation methods and quality metrics are presented in Supplementary data, Note 2 and Tables S2 and S3.
Inclusion criteria for APOL1 study
Details on inclusion criteria for GENEVA and BMC participants as a function of genotype quality, relatedness and African ancestry are presented in Supplementary data, Notes 1 and 3. In GENEVA, three sets of subjects were identified for analysis: (i) unrelated mothers, (ii) unrelated infants and (iii) unrelated mother–infant pairs. Of these, we excluded any subjects for whom data were missing on preterm birth, gestational age, birthweight, maternal age at birth or type of delivery (vaginal versus cesarean). For BMC, only maternal DNA was collected; thus our cohort consisted of unrelated mothers of African ancestry. Mothers were excluded from analyses when data were missing on any of the following clinical variables: preterm birth, gestational age, birthweight, type of delivery, parity, maternal age, body mass index (BMI), preeclampsia, eclampsia, chronic hypertension (HTN), HELLP (hemolysis, elevated liver enzymes and low platelet count) syndrome, smoking status in the second and third trimester and second-hand smoke exposure.
Classifying APOL1 risk status
Subjects were categorized with zero (G0G0), one (G1G0 or G2G0) or two (G1G1, G2G2 or G1G2) risk alleles. Subjects were classified as high risk (HR) if they harbored two risk alleles and low risk (LR)if they had zero or one risk allele. Primary analyses were performed using the recessive model (LR versus HR). We explored an additive model for two major outcomes.
Defining clinical outcomes
For both studies, preterm birth was defined as <37 weeks of gestation and birthweight was measured in grams. Gestational age was measured in weeks for both studies. For singletons (nontwins, triplets, etc.), birthweight and gestational age were used to define small for gestational age (SGA) as a birthweight less than the 10th percentile for a given gestational age (Supplementary data, Table S4) [18]. In GENEVA, preeclampsia, eclampsia and HTN were measured as a single binary trait indicating whether or not the mother was diagnosed with any of the three conditions. In BMC, these conditions were recorded separately as preeclampsia (no, mild or severe), eclampsia (no, yes), chronic HTN (no, yes) and HELLP syndrome (no, yes).
Statistical analyses
Our main motivation in pursuing this analysis was to determine whether an HR APOL1 genotype of mother or infant is associated with prematurity or birthweight-related outcomes. Analyses of BMC participants solely evaluated the association between maternal HR APOL1 and the outcome of interest. In GENEVA, genotype data were obtained for both mothers and infants. Thus, for each GENEVA outcome, two main analyses were performed: (i) association between maternal HR APOL1 and the outcome and (ii) association between infant HR APOL1 and the outcome. Additionally, we analyzed the association of combinations of maternal and infant APOL1 risk genotypes with a subset of outcomes for mother–infant pairs in the GENEVA study. This allowed us to investigate not only whether an HR APOL1 genotype was associated with these outcomes, but also how maternal, infant and combined APOL1 risk status might each contribute to prematurity or birthweight-related outcomes.
Univariate associations to test for association between outcomes and HR APOL1 genotypes were evaluated using chi-squared tests for binary outcomes (preterm birth and SGA) and Wilcoxon rank-sum tests for continuous outcomes (gestational age and birthweight) due to nonnormal distributions. Multivariable regression analyses were used to test for associations between outcomes and HR APOL1 while adjusting for potential confounders (covariates included for each multivariable regression are listed in Supplementary data, Tables S5–S7). Logistic regression and ordered logistic regression were used for binary and ordinal outcomes, respectively. Linear regression was used for continuous outcomes. In both BMC and GENEVA, the distribution of gestational age was nonnormal due to study design. Therefore, model assumptions of normally distributed residuals and constant variance were not met. To correct for this, we analyzed term and preterm infants separately. Similarly, to meet model assumptions, regression analyses of birthweight were adjusted for gestational age and log-transformed, as necessary.
Although the original BMC study was a matched-pair design, matching was not preserved in this secondary analysis. Apart from analyses described in Supplementary data, Note 5, all analyses of both cohorts were performed using unpaired data.
Significance of association was evaluated using a significance threshold of P < 0.05. All statistical analyses were performed using R version 3.2.0 [19]. Figures were generated using base R and the package ‘ggplot2’ [20].
RESULTS
Overview
In GENEVA, 519 unrelated mothers and 867 unrelated infants of genotype-based African ancestry met our inclusion criteria (Supplementary data, Table S1). There were 412 mother-infant pairs that were analyzed jointly (Supplementary data, Note 7). In BMC, 960 unrelated African American mothers had high-quality genetic data and met inclusion criteria. Characteristics of each cohort, stratified by APOL1 status, are summarized in Table 1 and in Supplementary data, Figure S1.
Table 1.
BMC and GENEVA preterm birth cohorts by APOL1 risk status
| BMC |
GENEVA |
|||||
|---|---|---|---|---|---|---|
| Mothers (n = 960; 13% HR APOL1) |
Infants (n = 867; 14% HR APOL1) |
Mothers (n = 519; 14% HR APOL1) |
||||
| LR APOL1a (n = 840) |
HR APOL1a (n = 120) |
LR APOL1 (n = 749) |
HR APOL1 (n = 118) |
LR APOL1 (n = 446) |
HR APOL1 (n = 73) |
|
| Birth characteristics | ||||||
| Preterm | 421 (50) | 63 (53) | 447 (60) | 73 (62) | 145 (33) | 25 (34) |
| Gestational age (weeks) | 37 (35–40) | 37 (34–40) | 32 (26–38) | 30 (26–38) | 38 (35–39) | 38 (35–39) |
| <28 | 58 (7) | 9 (8) | 277 (37) | 46 (39) | 28 (6) | 5 (7) |
| 28–33 | 101 (12) | 16 (13) | 110 (15) | 23 (19) | 58 (13) | 12 (16) |
| 34–36 | 170 (20) | 22 (18) | 60 (8) | 4 (3) | 59 (13) | 8 (11) |
| >36 | 511 (61) | 73 (61) | 302 (40) | 45 (38) | 301 (67) | 48 (66) |
| Birthweight (g) | 2865 (2089–3395) |
2715 (2132–3246) |
1773 (776–3104) |
1190 (741–3164) |
2989 (2363–3370) |
2990 (2504–3250) |
| Small for gestational age | 105 (13) | 12 (10) | 44/355 (12) | 3/61 (5) | 6/72 (8) | 0/15 (0) |
| C-section | 300 (36) | 35 (29) | 317 (42) | 55 (47) | 150 (34) | 49 (33) |
| Maternal characteristics | ||||||
| Age at birth (years) | 28 (22–33) | 28 (24–34) | 25 (21–30) | 24 (22–30) | 25 (21–30) | 26 (21–30) |
| Smoked in 2nd trimester | 115 (14) | 21 (18) | – | – | – | – |
| Second hand smoke during pregnancy | 190 (23) | 32 (27) | – | – | – | – |
| Preeclampsia/eclampsia/HTN | 86/388 (22) | 12/66 (18) | 5/73 (7) | 1/15 (7) | ||
| Preeclam: Mild | 24 (3) | 5 (4) | – | – | – | – |
| Preeclam: Severe | 79 (9) | 11 (9) | – | – | – | – |
| Eclampsia | 2 (<1) | 1 (1) | – | – | – | – |
| Chronic HTN | 49 (6) | 8 (7) | – | – | – | – |
| HELLPb | 10 (1) | 1 (1) | – | – | – | – |
| Body mass index | 26 (22–29) | 25 (22–29) | – | – | – | – |
| Study design | Matched case–control Frequency matched on maternal age, parity and birth year | Unmatched case–control | ||||
| Study site | Boston, MA, USA | Multiple sites, USA | ||||
| Study population ethnicity | African American and Haitian | African American | ||||
Data presented as median (IQR) for continuous traits and number (%) for binary traits. Data on preeclampsia/eclampsia/HTN and SGA were missing for some GENEVA subjects. Numbers of subjects with nonmissing data for these variables are indicated in the table.
aLR APOL1 indicates subjects with a low-risk APOL1 genotype; HR APOL1 indicates subjects with a high-risk APOL1 genotype.
bHELLP stands for hemolysis elevated liver enzymes and low platelet count.
Imputation
Quality of imputation was assessed through a number of measures that ultimately gave us confidence in our imputation allele accuracy for G2. In addition, we performed this should make it a better sentence sensitivity analyses in order to determine whether our conclusions could be significantly altered due to systematic misclassification of G2 as wild-type. No results from this sensitivity analysis raised concern about our power to detect a true association, even with a misclassification rate of up to 10%. See Supplementary data, Note 2 for details.
Prematurity
Infants
In GENEVA, the HR APOL1 frequency was similar between preterm (14%) and term infants (13%) [OR 1.1 (95% CI 0.7–1.6)]. Unconditional multivariable logistic regression of preterm birth adjusting for maternal age, delivery type and ancestry showed no association between prematurity and infant APOL1 risk status [OR 1.0 (95% CI 0.7–1.5)] (Table 2 and Supplementary data, Table S5).
Table 2.
Multivariable linear and logistic regression analyses of birth outcomes and maternal characteristics versus APOL1 risk status
| BMC |
GENEVA |
|||||
|---|---|---|---|---|---|---|
| Mothers (n = 960) |
Infants (n = 867) |
Mothers (n = 519) |
||||
| Effect size (HR versus LR) |
95% CI | Effect size (HR versus LR) |
95% CI | Effect size (HR versus LR) |
95% CI | |
| Preterm | OR = 1.0 | 0.7–1.5 | OR = 1.0 | 0.7–1.5 | OR = 1.1 | 0.6–1.9 |
| Gestational age in preterm group (weeks) | β = −0.3 | −1.2–0.7 | β = −0.5 | −1.4–0.4 | β = −0.3 | −1.9–1.4 |
| Log birthweight (g)a | exp(β) = 1.0 | 0.96–1.03 | exp(β) = 1.02 | 0.99–1.06 | exp(β) = 1.0 | 0.96–1.04 |
| Small for gestational age | OR = 0.7 | 0.4–1.4 | OR = 0.4 (n = 416)b | 0.1–1.2 | – | – |
ORs of HR versus LR APOL1 and their 95% CIs are presented for logistic regression models of binary outcomes. Estimated regression coefficients for HR APOL1 and their associated P-values are reported for linear regression models of continuous outcomes. Parameter estimates for all dependent variables from each multivariable model are provided in Supplementary data, Tables S5–S7.
aTo meet model assumptions of normality and constant variance, birthweight was log transformed. Effects of risk alleles on birthweight [exp(β)] are multiplicative and can be interpreted as fold change.
bSmall for gestational age was only explored for infants who were not multiples (twins, triplets, etc.). For BMC, not being a multiple was one of the inclusion criteria. For GENEVA infants, only 416 of 867 subjects were known not to be multiples. For GENEVA mothers, the number of subjects who were known not to have had a multiple pregnancy was so small (n = 87)results were not reported.
Mothers
In BMC, the frequency of HR APOL1 was 12% among mothers of term infants and 13% among mothers of preterm infants [OR 1.1 (95% CI 0.8–1.6)]. Similarly, in GENEVA, the frequency of HR APOL1 was 14% among mothers of term infants and 15% among mothers of preterm infants [OR 1.08 (95% CI 0.6–1.8)]. For each cohort, an unconditional multivariable logistic regression model of preterm birth by APOL1 genotype was fit, adjusting for relevant covariates as available (see Supplementary data, Tables S6 and S7 for covariates included in all multivariable models of BMC and GENEVA mothers, respectively). In these models, APOL1 risk genotype was not significantly associated with prematurity in either BMC [OR 1.0 (95% CI 0.7–1.5)] or GENEVA [OR 1.1 (95% CI 0.6–1.9)] cohorts (Table 2 and Supplementary data, Tables S6 and S7).
Gestational age
Infants
A univariate Wilcoxon rank-sum test showed no association between gestational age and infant APOL1 risk status (P = 0.54). Additionally, infant APOL1 risk status was not associated with gestational age in multivariable linear regression stratified by preterm status and adjusted for relevant covariates as available (see Methods) [β = −0.5 (95% CI−1.4–0.4) among preterm infants; β = 0.2 (95% CI −0.1–0.5) among term infants] (Table 2 and Supplementary data, Table S5).
Mothers
In univariate analyses, gestational age in weeks did not differ significantly as a function of mother's APOL1 genotype status in either cohort (Wilcoxon rank-sum test: P = 0.98 in BMC and P = 0.59 in GENEVA). Multivariable linear regression models of gestational age were fit stratified by preterm status (see Methods). No significant associations were observed between maternal APOL1 risk status and gestational age in these analyses (Table 2 and Supplementary data, Tables S6 and S7).
Birthweight
Infants
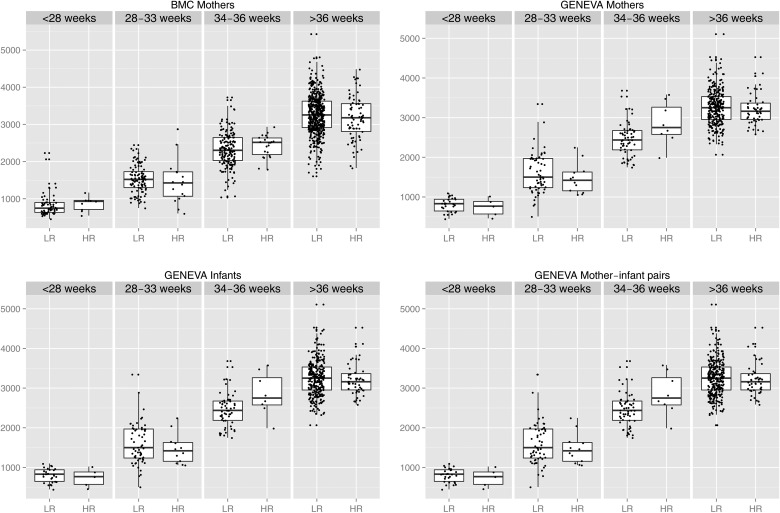
The median birthweight was higher in LR APOL1 (1773 g) than HR APOL1 (1190 g) GENEVA infants (Table 1), but the difference was not statistically significant (P = 0.70). A linear regression model was fit with log birthweight as the dependent variable and HR APOL1, gestational age, maternal age, delivery type and ancestry as independent variables. In this multivariable model, birthweight was not associated with infant APOL1 genotype [exp(β) = 1.01 (95% CI 0.99–1.06), where exp(β) is the fold change in birthweight in HR versus LR subjects—see Methods]. Within discrete gestational age ranges, birthweight was not associated with APOL1 risk genotype for GENEVA infants (Figure 1).
FIGURE 1.
Birthweight in grams (y-axis) versus APOL1 risk group (x-axis) stratified by weeks of gestation. There is no evidence that for a given gestational age range, birthweight is lower in infants with HR APOL1 or infants whose mother carries HR APOL1.
Mothers
In BMC, the median birthweight of infants of LR and HR genotype mothers was 2865 and 2715 g, respectively (Table 1) (P = 0.41). Likewise, in GENEVA, the median birthweight was essentially the same in infants of LR and HR mothers; 2989 versus 2990 g, respectively (Table 1) (P = 0.67). Additionally, in multivariable linear regression, birthweight was not associated with maternal APOL1 risk status in BMC [β = −18 (95% CI −98–62)] or GENEVA [β = 18 (95% CI −80–116)] cohorts (Table 2 and Supplementary data, Tables S5 and S6). Within discrete gestational age ranges, birthweight was not associated with APOL1 risk genotype of BMC or GENEVA mothers (Figure 1).
Small for gestational age
In both studies, a binary indicator of SGA was calculated for singletons.
Infants
SGA could be calculated for 355 LR APOL1 infants and 61 HR APOL1 infants. The percent of infants that were SGA was 12% (44/355) and 5% (3/61) among LR and HR APOL1 infants, respectively (Table 1) [OR 0.37 (95% CI = 0.1–1.2)]. There was no association between infant APOL1 risk status and SGA, when adjusting for the maternal age, delivery type and ancestry [OR 0.4 (95% CI 0.1–1.2)] (Table 2 and Supplementary data, Table S7).
Mothers
In BMC, the frequency of SGA was similar in infants of HR and LR mothers [OR 0.8 (95% CI 0.4–1.5)] (Table 1). In BMC mothers, a multivariable logistic regression model showed no association of SGA and HR genotype [OR 0.7 (95% CI 0.4–1.4)] (Table 2 and Supplementary data, Table S5). Due to missing data on multiple births in the GENEVA cohort, SGA could only be calculated for 72 LR and 15 HR GENEVA mothers. Of these, 6/72 (8%) LR mothers had infants that were SGA, while none (0/15) of the HR mothers had infants that were SGA (Table 1).
Additive risk inheritance model
Under an additive model of risk inheritance, where APOL1 risk was coded as 0, 1 or 2 risk alleles, there was no association between risk alleles and either preterm status or birthweight (Supplementary data, Note 8 and Tables S13–S15).
Additional analyses
We performed several additional analyses to address outstanding questions. We investigated a potential role for APOL1 risk genotypes in gestational complications, such as preeclampsia, eclampsia and chronic hypertension. There were no significant associations (see Supplementary data, Note 4). To gain potential increased efficiency, we used a matched study design to examine APOL1 genotypes and preterm birth and did not find any significant associations (Supplementary data, Note 5). To evaluate potential bias due to six different recruitment sites within the GENEVA study, we performed each of the multivariable analyses presented above using the subset of subjects solely recruited from the GENEVA site with the largest numbers of both cases and controls (Site 4). Results were consistent with findings from analyses of subjects from all six sites and are presented in Supplementary data, Note 6. Finally, to investigate potentially synergistic effects of particular combinations of mother and infant APOL1 risk status (e.g. both mother and infant HR, mother LR and infant HR, etc.), we tested for associations of all possible combinations of mother–infant APOL1 risk genotypes with preterm birth, gestational age and birthweight (Supplementary data, Note 7 and Tables S11 and S12). We found no significant association between the combined mother–infant APOL1 risk genotypes and any of these outcomes.
DISCUSSION
This study was motivated by our previous discovery of significantly increased odds of preterm birth in children with glomerular disease and an HR APOL1 genotype. By studying a population cohort of mothers and infants, we aimed to determine whether an HR APOL1 genotype itself, whether in the mother, infant or potentially both, was associated with prematurity and other abnormal birth outcomes. In this way we could continue to elucidate the role of an HR APOL1 genotype in the complex interplay between prematurity, kidney disease and black race.
To this end, we analyzed publically available genetic and clinical data from >2000 African American mothers and infants from two population-based cohorts studying preterm birth. We did not find any evidence of a direct association of HR APOL1 genotypes with preterm birth, birthweight or other abnormal birth outcomes.
The results of this study add to our understanding of the relationships between high-risk APOL1 genotypes, prematurity and kidney disease. They suggest that the association between preterm birth and the HR APOL1 genotype is specific to subjects with glomerular disease rather than evidence of direct effects of HR genotype on preterm birth. Given this, we propose two conceptual frameworks that could explain these observations.
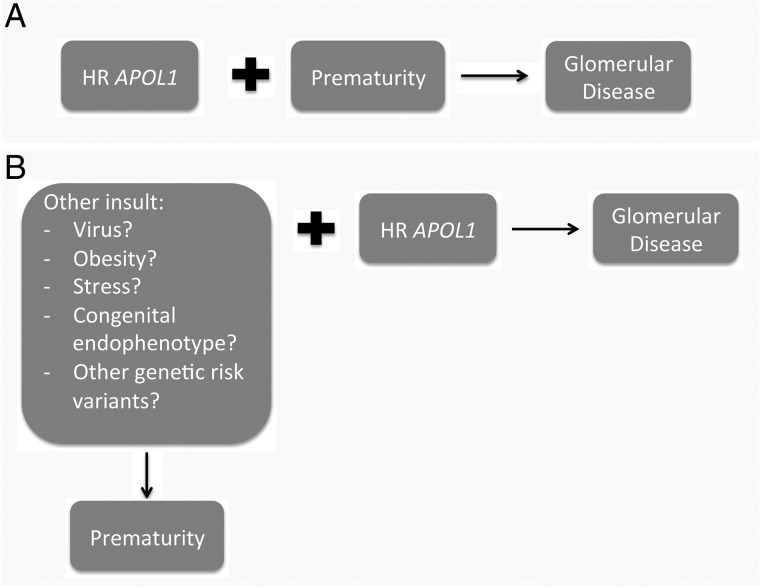
One possibility is that prematurity could act as an additional risk factor to an HR APOL1 genotype and increase susceptibility to glomerular disease (Figure 2A). In a second scenario, other stresses leading to increased risk of prematurity could themselves be additional risk factors in the process leading to APOL1-associated nephropathy (Figure 2B). Such stresses, which in this scenario would also directly affect the kidney, could include maternal health conditions prior to conception, socioeconomic factors, congenital endophenotypes or other genetic susceptibility alleles. Indeed, as reviewed by Freedman and Skorecki [21], a number of studies have sought to identify gene–gene and gene–environment interactions with an HR APOL1 genotype that modify its phenotypic manifestation of progressive kidney disease. The same pursuit may be fruitful when considering the preterm phenotype. Further characterization of the molecular pathways leading to prematurity and underlying APOL1-associated nephropathies should help us understand whether there are shared or complementary pathomechanisms converging ultimately upon damage to the glomerular filtration barrier.
FIGURE 2.
Proposed conceptual frameworks in which APOL1 risk alleles are associated with prematurity in individuals with glomerular disease. (A) Prematurity increases the penetrance of HR APOL1, leading to glomerular disease. (B) A third factor increases both the risk of prematurity and the penetrance of HR APOL1, leading to a concurrent increase in susceptibility to prematurity and glomerular disease.
There were some limitations to this study, which are particularly important to discuss here given that we are reporting a lack of association between the HR APOL1 genotype status and birth outcomes. First, while the sample size and point estimates of the associations between HR genotype and both preterm status and birthweight give us confidence in the stability of our results, we had 80% power to detect a 1.7–1.9 increased odds of prematurity and a 2.0–2.1 increased odds of SGA status (Supplementary data, Table S16). Thus, we would be underpowered to detect modestly different odds of preterm birth or SGA status by risk genotype. Second, we did not have a large number of observations for maternal outcomes such as eclampsia/preeclampsia nor many maternal–infant pairs who both harbored the HR genotype. Consequently, we are underpowered to detect associations with these particular exposures and outcomes (Supplementary data, Note 9). The maternal studies primarily consisted of mothers of moderately preterm (gestational age >32 weeks) infants (Supplementary data, Figure S1). Consequently, signals involving maternal HR APOL1 specific to preterm births <32 weeks may be undetectable. Finally, both GENEVA and BMC were large cohorts that aimed to understand the genetic determinants of preterm birth and consequently collected relevant clinical and genetic data useful for our APOL1 specific question. However, we are cognizant that our study was a secondary analysis of these existing datasets. A study prospectively designed to specifically describe the association between APOL1 genotype and birth outcomes would mitigate many of the general challenges of secondary data analysis.
Even in the absence of association with clinical outcomes, the HR APOL1 genotype may still be associated with intermediate biologic phenotypes that impact birth outcomes. It would be particularly interesting to determine if the HR APOL1 genotype is associated with abnormalities of the highly vascular placenta, given that APOL1 is highly expressed within it [14, 15] and is also localized to the vascular endothelium of the kidney in disease states [16].
The results of this study suggest that an important next step would be to determine if preterm babies harboring the HR APOL1 genotype have an increased lifetime risk of glomerular disease. The answer to this question could have important clinical implications, including decisions surrounding whether preterm black children should be APOL1 genotyped or whether HR preterm babies should be more routinely screened with blood pressure measurements and urinalyses. It may also contribute to ongoing conversations regarding decisions surrounding living donor transplantation [22, 23]. Ultimately, clarifying these relationships should allow us to improve our ability to identify African American children or mothers at highest risk for poor outcomes (whether prematurity, CKD or both) and devise interventions that either reduce their incidence or improve the care of those children already affected.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
Supplementary Material
ACKNOWLEDGEMENTS
M.G.S. is supported by the Charles Woodson Clinical Research Fund, K08-DK100662, RO1DK108805 and is a Carl W. Gottschalk Research Scholar of American Society of Nephrology Foundation for Kidney Research. We thank Megha Trivedi for submitting a dbGaP data access request, as well as Michael Cotten (Duke University), Jeff Murray (University of Iowa), Kelli Ryckman (University of Iowa; Epidemiology) and Grier Page (University of Alabama Birmingham) for additional assistance in obtaining and interpreting data. We thank Sayantan Das (University of Michigan) for his assistance with imputation using Minimac3. This study makes use of data from two dbGaP studies (study accessions phs000353 and phs000332). Full lists of investigators who contributed to the generation of these datasets can be found on the dbGaP website (http://www.ncbi.nlm.nih.gov/gap). Findings in this paper were presented in a poster at the June 2015 ‘ApoL1 and Kidney Disease’ Conference in Hyattsville, MD, USA.
CONFLICT OF INTEREST STATEMENT
None declared.
REFERENCES
- 1. Genovese G, Friedman DJ, Ross MD. et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010; 329: 841–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tzur S, Rosset S, Shemer R. et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet 2010; 128: 345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman DJ, Kozlitina J, Genovese G. et al. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol 2011; 22: 2098–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kruzel-Davila E, Wasser WG, Aviram S. et al. APOL1 nephropathy: from gene to mechanisms of kidney injury. Nephrol Dial Transplant 2016; 31: 349–358 [DOI] [PubMed] [Google Scholar]
- 5. Kasembeli AN, Duarte R, Ramsay M. et al. APOL1 risk variants are strongly associated with HIV-associated nephropathy in Black South Africans. J Am Soc Nephrol 2015; 26: 2882–2890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kopp JB, Nelson GW, Sampath K. et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol 2011; 22: 2129–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Divers J, Palmer ND, Lu L. et al. Gene-gene interactions in APOL1-associated nephropathy. Nephrol Dial Transplant 2014; 29: 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ng DK, Robertson CC, Woroniecki RP. et al. APOL1-associated glomerular disease among African American children: a collaboration of the Chronic Kidney Disease in Children (CKiD) and Nephrotic Syndrome Study Network (NEPTUNE) cohorts. Nephrol Dial Transplant 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shiono PH, Klebanoff MA. Ethnic differences in preterm and very preterm delivery. Am J Public Health 1986; 76: 1317–1321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kessel SS, Villar J, Berendes HW. et al. The changing pattern of low birth weight in the United States—1970 to 1980. JAMA 1984; 251: 1978–1982 [PubMed] [Google Scholar]
- 11. Ruggajo P, Skrunes R, Svarstad E. et al. Familial factors, low birth weight, and development of ESRD: a nationwide registry study. Am J Kidney Dis 2016; 67: 601–608 [DOI] [PubMed] [Google Scholar]
- 12. Frey HA, Klebanoff MA. The epidemiology, etiology, and costs of preterm birth. Semin Fetal Neonatal Med 2016; 21: 68–73 [DOI] [PubMed] [Google Scholar]
- 13. Greenbaum LA, Munoz A, Schneider MF. et al. The association between abnormal birth history and growth in children with CKD. Clin J Am Soc Nephrol 2011; 6: 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Duchateau PN, Pullinger CR, Cho MH. et al. Apolipoprotein L gene family: tissue-specific expression, splicing, promoter regions; discovery of a new gene. J Lipid Res 2001; 42: 620–630 [PubMed] [Google Scholar]
- 15. Page NM, Butlin DJ, Lomthaisong K. et al. The human apolipoprotein L gene cluster: identification, classification, and sites of distribution. Genomics 2001; 74: 71–78 [DOI] [PubMed] [Google Scholar]
- 16. Madhavan SM, O'Toole JF, Konieczkowski M. et al. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol 2011; 22: 2119–2128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bruggeman LA, Wu Z, Luo L. et al. Mice transgenic for APOL1-G0 or APOL1-G2 develop preeclampsia but not kidney disease. J Am Soc Nephrol (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alkalay AL, Graham JM Jr, Pomerance JJ. Evaluation of neonates born with intrauterine growth retardation: review and practice guidelines. J Perinatol 1998; 18: 142–151 [PubMed] [Google Scholar]
- 19. Gonzales PA, Pisitkun T, Hoffert JD. et al. Large-scale proteomics and phosphoproteomics of urinary exosomes. J Am Soc Nephrol 2009; 20: 363–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ling XB, Sigdel TK, Lau K. et al. Integrative urinary peptidomics in renal transplantation identifies biomarkers for acute rejection. J Am Soc Nephrol 2010; 21: 646–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Freedman BI, Skorecki K. Gene-gene and gene-environment interactions in apolipoprotein L1 gene-associated nephropathy. Clin J Am Soc Nephrol 2014; 9: 2006–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Freedman BI, Julian BA, Pastan SO. et al. Apolipoprotein L1 gene variants in deceased organ donors are associated with renal allograft failure. Am J Transplant 2015; 15: 1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ojo A, Knoll GA. APOL1 genotyping of African American deceased organ donors: not just yet. Am J Transplant 2015; 15: 1457–1458 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.