Abstract
Duchenne muscular dystrophy (DMD) is a neuromuscular disease that predominantly affects boys as a result of mutation(s) in the dystrophin gene. DMD is characterized by musculoskeletal and cardiopulmonary complications, resulting in shorter life-span. Boys afflicted by DMD typically exhibit symptoms within 3–5 years of age and declining physical functions before attaining puberty. We hypothesized that rapidly deteriorating health of pre-pubertal boys with DMD could be due to diminished anabolic actions of androgens in muscle, and that intervention with an androgen receptor (AR) agonist will reverse musculoskeletal complications and extend survival. While castration of dystrophin and utrophin double mutant (mdx-dm) mice to mimic pre-pubertal nadir androgen condition resulted in premature death, maintenance of androgen levels extended the survival. Non-steroidal selective-AR modulator, GTx-026, which selectively builds muscle and bone was tested in X-linked muscular dystrophy mice (mdx). GTx-026 significantly increased body weight, lean mass and grip strength by 60–80% over vehicle-treated mdx mice. While vehicle-treated castrated mdx mice exhibited cardiopulmonary impairment and fibrosis of heart and lungs, GTx-026 returned cardiopulmonary function and intensity of fibrosis to healthy control levels. GTx-026 elicits its musculoskeletal effects through pathways that are distinct from dystrophin-regulated pathways, making AR agonists ideal candidates for combination approaches. While castration of mdx-dm mice resulted in weaker muscle and shorter survival, GTx-026 treatment increased the muscle mass, function and survival, indicating that androgens are important for extended survival. These preclinical results support the importance of androgens and the need for intervention with AR agonists to treat DMD-affected boys.
Introduction
Duchenne muscular dystrophy (DMD) is an X-linked autosomal recessive disease affecting 1/3500 to 1/5000 males world-wide, with ∼18 000 DMD patients living in the USA (1). DMD is caused by frameshift or nonsense mutations in the gene dystrophin, which result in a dysfunctional or partially functional protein (2–4). The dystrophin protein is part of a sarcolemma protein complex (dystrophin–glycoprotein complex) that protects the muscle cell membrane from physical trauma during muscle contraction (5–7). Although the first clinical observation of DMD was made in 1860s, only in 1986 was the gene underlying DMD cloned and characterized (8). DMD is characterized by progressive musculoskeletal wasting starting at 3–5 years of age, with patients experiencing difficulty in walking and encountering mortality in their 20s and 30s (9–11). Although braces and walkers provide some support, a loss of ambulation during childhood leads to wheelchair confinement, eventual cardiomyopathy, respiratory dysfunction (diaphragm fibrosis) and death (12–14).
Respiratory supportive care in DMD is provided by assisted ventilator and cough-assisting devices, whereas cardiac care is provided by ACE inhibitors and β-blockers (15–17). Glucocorticoids are the standard of care that slows the disease progression due to their anti-inflammatory effects, which in turn strengthen muscles and slows the deterioration of physical function (18). While one drug (Ataluren) has been approved in Europe for the treatment of DMD, recently an antisense oligonucleotide eteplirsen (exon-skipping) that restores the reading frame of dystrophin at exon 51 was approved in the USA (19,20). Since exon 51 is mutated in ∼15% of the patients, the drug is expected to modify the disease condition in this subset of patients.
A direct correlation between higher muscle mass and survival in patients with cancer cachexia (a disease also characterized by skeletal muscle wasting) has prompted evaluation of anabolic agents such as androgens to slow the decline in physical function in DMD (21). Two pilot studies with the steroidal androgen oxandrolone in boys with DMD have demonstrated an improvement in muscle mass and muscle protein synthetic rate (22). However, the steroidal backbone, non-selectivity between muscle and secondary sexual organs, and hepatotoxicity have precluded the extended use of oxandrolone in DMD. The discovery of non-steroidal tissue-selective-androgen receptor modulators (SARMs) offers an alternative to the use of steroidal androgens for improving muscle quality and function (23). The lead SARM, enobosarm (GTx, Inc, Memphis, TN, USA), has been shown to cause a significant improvement in muscle mass and physical function in patients suffering from cancer cachexia (24). Since AR agonists increase lean mass, strength and protein synthesis, targeting the AR may be useful in treating other muscle wasting diseases and supports the use of these androgen receptor (AR) agonists as possible treatments for DMD (25).
Given that androgens are important for the development and maintenance of muscle, bone and secondary sexual organs (26,27), we suggest that the decline in physical function in young boys with DMD is exacerbated by the absence of androgens. Therefore, we hypothesize that treatment with AR agonists will prevent muscle deterioration and protect young DMD patients from the decline in muscle function.
Here we tested this hypothesis using a potent SARM, GTx-026 (GTx Inc.; an analog of enobosarm), in preclinical models of DMD. Of the several SARMs that were developed, the arylpropionamide SARM, GTx-024 (or enobosarm), is clinically the most advanced (23,28). Multiple cancer cachexia phase II and III clinical trials have established the efficacy of GTx-024 for improving muscle mass and physical function (28). The potent anabolic activity of SARMs (muscle and bone beneficial effects) with minimum to no androgenic activity (effects on secondary sexual organs and virilization) confers them advantage over steroidal androgens (29). We used a mouse model (X-linked muscular dystrophy mouse) where mutation in dystrophin causes a frame-shifted dysfunctional protein (mdx) to evaluate the effects of GTx-026 on muscle mass, function, and cardiopulmonary anatomy and physiology in the absence of endogenous androgens. Utrophin (utrn) is an ortholog of dystrophin that partially compensates for the loss of dystrophin and its expression in mdx mice is considered as one of the reasons for the milder phenotype in mdx mice (30–34). Thus, as the phenotype of mdx mice is mild, we also studied double mutant mice [dmd/utrn double mutant (mdx-dm)], where both dystrophin and utrophin (mutation in exon 7) are mutated, which more closely represent the human clinical condition. GTx-026 potently increased body weight, lean mass and grip strength in castrated mdx mice. Echocardiogram studies indicate that the 16-week-old castrated mdx mice had cardiac and pulmonary anatomical and physiological impairments that were significantly improved by GTx-026. In addition, treatment of castrated mdx-dm mice with GTx-026 improved body weight, lean mass, muscle function and survival. Overall, the AR ligands have impressive effects in preclinical models of DMD and these studies could become an impetus for clinical development of an AR agonist for DMD.
Results
Circulating androgens are important for survival
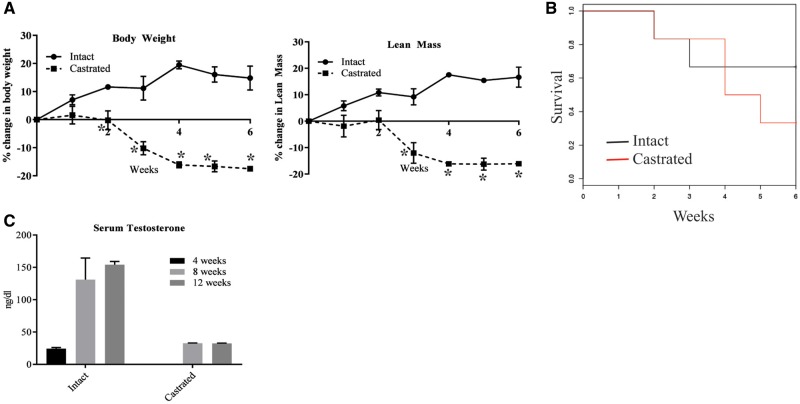
To test our hypothesis that the absence of circulating androgens leads to accelerated deterioration of skeletal muscle in DMD, we sham-operated or castrated mdx-dm mice. Since, mdx mice survive comparably to wild-type mice, we chose mdx-dm mice that live only for 15–20 weeks (35–37). We measured body weight, body composition and monitored their survival. Sham-operated mdx-dm mice modestly gained body weight and lean mass by ∼20% from the initiation of the study and maintained these increases throughout the 6-week study period (Fig. 1A). In contrast, castrated mdx-dm mice lost body weight and lean mass by ∼20% (P < 0.01) (Fig. 1A). Almost 66% of the castrated mdx-dm mice died within 6 weeks of the study initiation, while only 25% of the sham-operated mice died during the same period (Fig. 1B). This indicates that circulating androgens are important for the maintenance of body weight and lean mass and are critical for the survival of mdx-dm mice.
Figure 1.
Circulating androgens improve muscle mass and survival in Dystrophin/utrophin double mutant mice. Dystrophin/utrophin (dmd/utrn) double mutant mice (n = 6 in sham-operated and 8 in castrated group; 4 weeks old) were sham-operated or castrated and weekly body weight (A, left panel) and lean mass (A, right panel) by MRI were measured and their survival monitored (B). (C) Serum testosterone levels were measured in sham-operated or castrated mdx mice aged 4, 8 and 12 weeks (n = 4/time point) using ELISA. Values are represented as average ± SE. *Significance at P < 0.01.
We understood that there are no perfect preclinical models to represent pre-pubertal conditions. Although slowly maturing dog or pig models are available, they are expensive and they lack comprehensive understanding (38). Surge in hormonal levels initiate puberty. We decided to retain the androgen levels in our studies at lower levels for two reasons; to mimic pre-pubertal hormonal condition and to evaluate the effect of SARMs in the absence of endogenous hormonal influence.
We measured serum testosterone in castrated and intact mice at 4, 8 and 12 weeks of age that corresponds to the experimental duration. While intact or sham operated mice had a surge in serum testosterone from 20 to 30 ng/dl at 4 weeks of age to 150–200 ng/dl at 12 weeks of age, the castrated mice exhibited the serum testosterone levels of around 25 ng/dl through the 8-week period (Fig. 1C), which correspond to pre-pubertal range of 7–30 ng/dl.
SARMs potently activate the AR
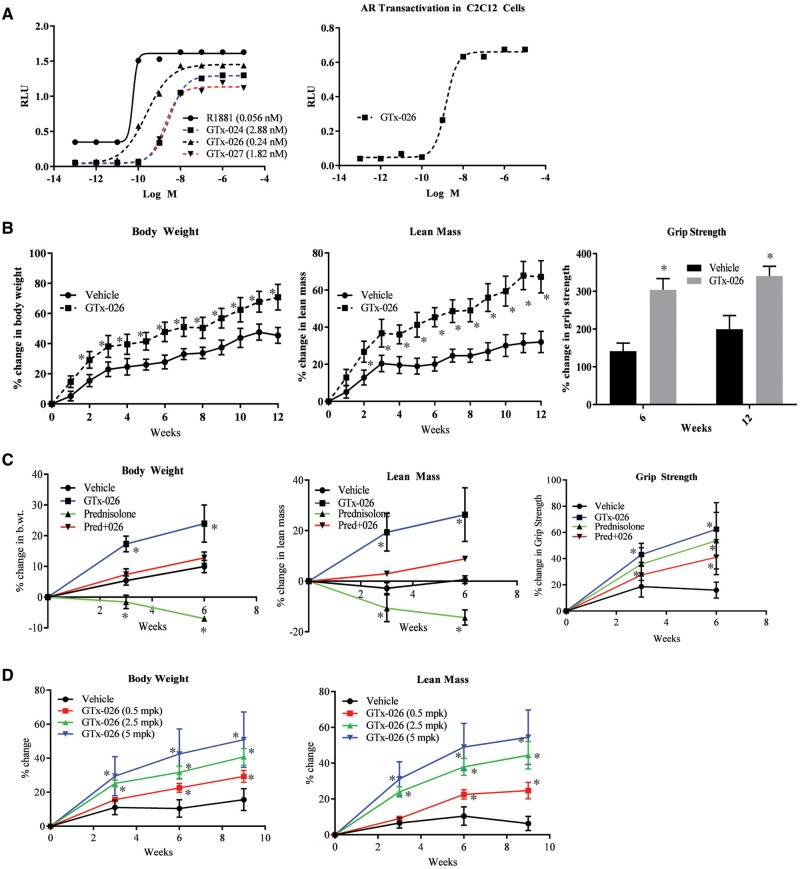
To determine if AR agonists can rescue the detrimental phenotype caused by the absence of androgens, we conducted studies with non-steroidal SARMs that have the ability to maximally activate the AR. We chose these SARMs from the arylpropionamide class and performed transactivation experiments using a tissue culture system to assess their effects on AR transactivation. Several advantages favored the continued use of SARMs in our studies (such as, the non-steroidal backbone, selectivity to muscle and bone, lack of hepatotoxicity etc.). Moreover, DHT, a potent steroidal androgen, can be enzymatically inactivated to weaker androgens or estrogens (39,40). Previous studies have demonstrated beneficial effects of a SARM in mdx mice (25). Here, the three arylpropionamide SARMs that bind to the AR ligand-binding domain (LBD) with Ki between 2 and 10 nM were tested in HEK-293 cells using a luciferase assay. All three SARMs were extremely potent in increasing the AR transactivation in nanomolar concentration, with GTx-026 being the most potent of the three tested (Fig. 2A, left panel).
Figure 2.
Selective androgen receptor modulator (SARM), GTx-026, increases muscle mass and strength in mdx mice. (A). GTx-026 is a potent activator of the androgen receptor (AR). AR transactivation studies were performed in HEK-293 cells (left panel) or C2C12 cells (right panel) by transfecting 25 ng CMV-hAR, 0.25 μg GRE-LUC and 10 or 20 ng CMV-renilla-LUC. Twenty-four hours after transfection, cells were treated with vehicle, R1881 or three SARMs (left panel) or GTx-026 (right panel). Cells were harvested 48 h after transfection and luciferase assay was performed. (B) GTx-026 increases muscle mass and strength in mdx mice. Male mdx mice (4 weeks of age; n = 6/group) were castrated to reduce the circulating androgens and treated with vehicle or 10 mg/kg/day s.c. of GTx-026. Weekly body weight and MRI to measure the muscle mass were recorded. Grip strength was measured at the start of the experiment, and after 6 and 12 weeks. Values are represented as percent change from baseline. *P < 0.001 (body weight and muscle mass) and P < 0.05 (grip strength). (C) GTx-026 improves prednisolone effects on body weight and muscle mass in castrated mdx mice. Castrated mdx mice (n = 5/group) were treated with vehicle, prednisolone (5 mg/kg/day p.o.) or GTx-026 (10 mg/kg/day s.c.) or combination of prednisolone and GTx-026 for 6 weeks. Body weight, body composition and grip strength were measured at the beginning of the study and at weeks 3 and 6. *P < 0.01. (D) Clinically comparable doses of GTx-026 improve body weight and lean mass in mdx mice. Castrated mdx mice (n = 6/group) were treated orally with vehicle or indicated doses of GTx-026. Body weight and composition were measured at the beginning of the study and after 3, 6 and 9 weeks of dosing. *P < 0.001. RLU, relative light units; mpk, milligrams per kilogram.
An intracellular milieu of coactivators and corepressors regulate the potency of nuclear receptor ligands (41). In order to evaluate whether a muscle cell environment will alter the efficacy of GTx-026, a transactivation assay was also performed with GTx-026 in undifferentiated C2C12 myoblasts. GTx-026 was extremely potent in activating the AR in C2C12 cells (Fig. 2A, right panel). This result shows that GTx-026 is potent in a muscle cell environment.
SARMs increased lean mass, function and body weight of wild-type mice
C57BL6/J male mice (6 weeks old; n = 6/group) were castrated to remove the interference of circulating androgens and were treated subcutaneously with vehicle or 10 mg/kg/day GTx-026. After 1 week, GTx-026 treatment caused a greater increase in body weight (67% vs 33% in vehicle-treated mice) and lean mass (64% vs 31% in vehicle-treated mice) compared with vehicle treatment. These differences were maintained for the experimental duration (Supplementary Material, Fig. S1A). Grip strength, which was used as a measure of muscle function, was also increased by GTx-026 (Supplementary Material, Fig. S1A). All these increases were statistically significant at P < 0.001.
GTx-026 increased lean mass, body weight and grip strength in mdx mice
Dystrophin homozygous mutated/utrn heterozygous mutated mice purchased from JAX labs were bred in-house to generate the male Dystrophin mutant/utrn wild-type (mdx) mice used in these studies. Four weeks old male mice were castrated and treated subcutaneously with either vehicle or 10 mg/kg/day GTx-026. Body weight and composition were measured weekly. Grip strength of fore- and hind-limbs was recorded at the beginning of the study and again after 6 and 12 weeks of treatment. After 2 weeks, the body weights of the GTx-026-treated mice were greater than those of the vehicle-treated mice, with this difference increasing in magnitude throughout the duration of the study (62% in GTx-026-treated mice vs 31% in vehicle-treated mice; P < 0.001) (Fig. 2B). GTx-026 treatment also increased lean mass (20% vs 60%; P < 0.001) compared with vehicle treatment. Consistent with the increase in lean mass, grip strength also increased in the GTx-026-treated animals (Fig. 2B). These results were reproduced with the other two SARMs, GTx-024 and GTx-027, indicating that these effects are SARM-dependent (Supplementary Material, Fig. S1B).
Glucocorticoids are the standard of care in DMD. Glucocorticoids act through glucocorticoid receptor (GR) to mediate their anti-inflammatory effects that result in strengthening of muscle. Glucocorticoids and androgens share a common pool of coactivators and bind to several overlapping regulatory cis elements. We tested the effect of combination of a glucocorticoid, prednisolone, and GTx-026 and compared the effects with that produced by individual treatments. Prednisolone at 5 mg/kg oral dose significantly reduced the body weight and lean mass compared with vehicle-treated mice, while GTx-026 increased the body weight and lean mass of castrated mdx mice (Fig. 2C). Mice that were treated with a combination of prednisolone and GTx-026 exhibited body weight and muscle mass that were comparable to that of vehicle-treated mice, i.e. neither increased nor decreased (Fig. 2C). This result indicates that the AR and GR agonists reverses the negative and positive effects, respectively, of the other treatment. Interestingly, both GTx-026 and prednisolone increased grip strength individually, while the combination produced similar effect. This study indicates that combining an AR agonist with a GR agonist may be beneficial to stabilize the muscle mass and body weight that will be reduced by a GR agonist, without affecting the muscle function. However, additive or synergistic effects may not be obtained from the combination treatment.
As patients with DMD exhibit pathologic signs as early as 3–5 years of age when no circulating androgens can be detected, we performed the majority of our experiments in castrated male mice to replicate pre-pubertal androgenic environment. However, since DMD is not reversed by the onset of puberty, we also studied the effects of GTx-026 in sham-operated intact male mice. After 2 weeks of treatment, GTx-026-treated mdx mice had increased lean mass compared with vehicle-treated mdx mice (∼20%) and this difference was maintained for 6 weeks (Supplementary Material, Fig. S2). However, by 8 weeks of treatment (12 weeks of age), the difference in lean mass was no longer apparent, which could be due to the presence of endogenous androgens. Concurrent with this increase in lean mass, grip strength (represented as percent change from baseline) also increased in mice treated with GTx-026 (∼150% vs 50% in vehicle-treated mice) compared with the vehicle-treated mdx mice (Supplementary Material, Fig. S2).
The dose (10 mg/kg/day s.c.) used in the studies was much higher than the clinical doses of 3, 9 and 18 mg of enobosarm (GTx-024) used in cancer cachexia or breast cancer clinical trials. To ensure that lower clinically comparable doses of GTx-026 have the ability to increase muscle mass in mdx mice, we treated castrated mdx mice with 0.5, 2.5 and 5 mg/kg/day of GTx-026 orally. These doses are in the clinical dose range of 3, 9 and 18 mg. GTx-026 significantly and dose-dependently increased body weight and lean mass at all doses (P < 0.001) (Fig. 2D). These studies indicate that clinically relevant doses will be sufficient to provide anatomical and physiological improvements in DMD.
Gastrocnemeous and soleus muscle from GTx-026-treated mice had lower incidence of centrally nucleated cells and fibrosis
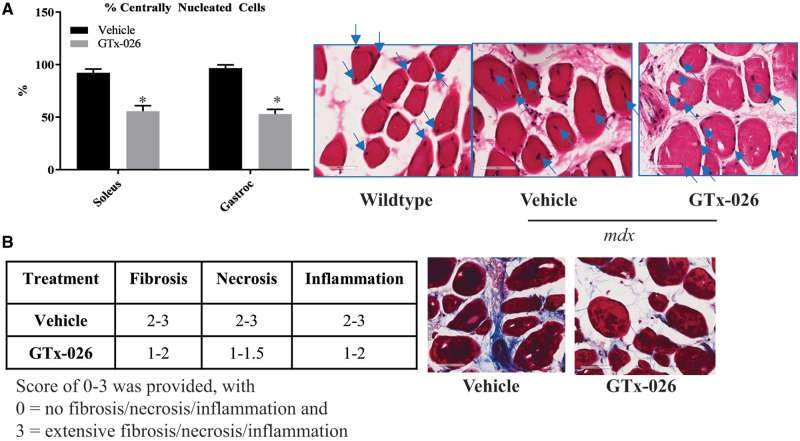
One of the hallmarks of impaired muscle regeneration is the presence of centrally nucleated cells (42). While healthy muscle cells have nuclei in the periphery, muscle from DMD patients and mdx mice have centrally located nuclei. H&E stained soleus and gastracnemeous sections from castrated mdx mice exhibited 90–95% centrally nucleated cells, while the sections from wild-type mice had few to no centrally nucleated cells (P < 0.01) (Fig. 3A). Treatment with GTx-026 partially reduced the number of centrally nucleated cells to 50–55% from ∼90 to 95% in vehicle-treated sections, indicating a potential improvement in regeneration of muscle fibers.
Figure 3.
GTx-026 improves histological markers in mdx mice. (A) Castrated mdx mice treated with vehicle or GTx-026 were sacrificed and gastrocnemius and soleus muscle (n = 4/group) were isolated and stained with hematoxylin and eosin (H&E). Number of centrally nucleated cells (100 cells/section) were counted in 8–10 fields in each section and represented as percent centrally nucleated cells in the bar graph. Blue arrows on the H&E stained images point to nucleus. *P < 0.01. (B) GTx-026 reduces fibrosis, necrosis and inflammation. Gastrocnemius muscle (n = 4/group) from vehicle- or GTx-026-treated castrated mdx mice were fixed and stained to measure fibrosis (trichrome staining), necrosis and inflammation. The intensity of staining was scored between 0 and 3. The range is represented in the table. Representative trichrome staining is shown in the figure. Values are represented as average ± SE.
The extent of fibrosis was evaluated in Mason trichrome-stained soleus and gastrocnemeous sections and a score of 0–3 was provided based on the intensity and extent of staining. Soleus and gastrocnemeous muscle of vehicle-treated castrated mdx mice (n = 4 mice and 2 sections/mouse) had substantially higher fibrosis scores, close to 3 in several cases, while those from mice treated with GTx-026 had a lower fibrosis scores (Fig. 3B). The same trend was observed in the number of necrosed cells (Fig. 3B), while no differences in the number of inflammatory cell infiltration was observed between vehicle- and GTx-026-treated muscles.
Staining for fast and slow fibers in gastrocnemeous muscle showed that majority of the cells and fibers had the characteristics of fast fibers. SARM-treatment did not alter the characteristics of the fiber or cell subtype.
GTx-026 and dystrophin regulate distinct pathways in mdx mice
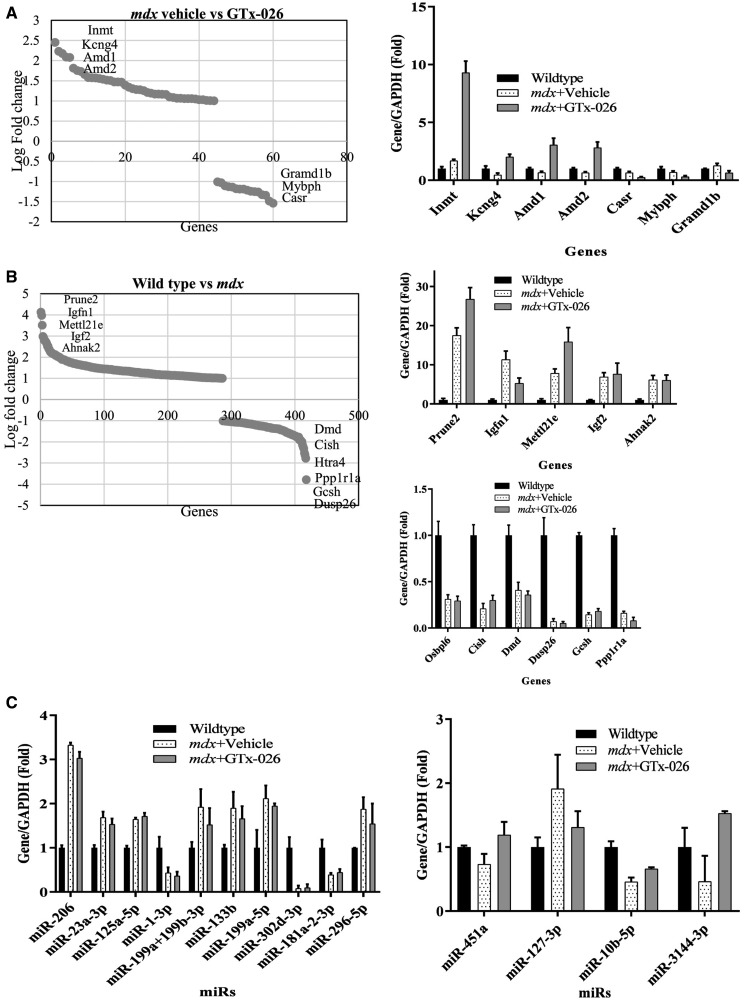
To determine the mechanism of GTx-026 action, RNA sequencing studies were performed in gastrocnemeous muscle from castrated vehicle-treated wild-type mice and castrated vehicle- or GTx-026-treated mdx mice (n = 3/group). Dysfunctional dystrophin altered the expression of 417 genes compared with wild-type mice (Fig. 4B). Most up-regulated genes in mdx mice include prune-2, Igfn-1, Mettl21e, IGF-2 and Ahnak-2 and the most down-regulated genes include Dusp-26, Gcsh, ppp1r1a, htra4 and Dmd. Ingenuity pathway analysis (IPA) of the genes significantly enriched in mdx mice compared with wild-type mice shows that the top canonical signaling pathway altered is neuronal nitric oxide synthase (nNOS) signaling (43) and creatinine biosynthesis, while the top upstream regulators are all myoanabolic pathway markers such as Dmd, Myod1 and Myog. Moreover, IPA showed that the biological functions of genes enriched in mdx mice represent neurological diseases, respiratory diseases, and skeletal and muscular disorder, and developmental disorder. These indicate that there is a good correlation between the expression of these genes and the phenotype of mdx mice.
Figure 4.
GTx-026 increases the expression of genes important for muscle function. (A) Genes that significantly were up-regulated by GTx-026 in castrated mdx mice are represented. Castrated wild-type or mdx mice (n = 3/group) treated with vehicle or 10 mg/kg/day s.c. GTx-026 for 12 weeks were sacrificed, gastrocnemius muscle was isolated, RNA extracted and expression of genes was measured by RNA-sequencing. Bar graph represents the most up- and down- regulated genes. (B) Genes that are significantly regulated in gastrocnemius muscle of castrated mdx compared with castrated wild-type mice are represented. Genes that are most up-regulated are represented in the top bar graph panel and that are most down-regulated are represented in the bottom bar graph panel. (C). miRNAs are differentially regulated in castrated mdx mice and in castrated mdx mice treated with GTx-026. RNA (n = 3/group) isolated from gastrocnemius as described in panels A&B was used to measure genome-wide miRNA expression. MiRNAs that were differentially regulated in mdx mice compared with wild-type, but not reversed by GTx-026 are shown in the left panel and miRNAs that were partially changed by GTx-026 in mdx mice are shown in right panel. Values are represented as average ± SE. In (A) and (B), genes that are ≥2-fold between groups and have P < 0.05 and q < 0.05 are represented.
Genes regulated by GTx-026 in castrated mdx mice were compared with that of castrated vehicle-treated mdx mice (Fig. 4A). Interestingly, only 60 genes were differentially regulated by GTx-026, which is a much smaller subset compared with the genes altered in prostate by AR ligands. Most up-regulated genes include Inmt, Kcng4, Amd1, Amd2, pla1a, while the most down-regulated genes include Casr, Grmd1b, Mybph and MAPK8ip1. IPA analysis indicates that GTx-026 enriched genes that belong to two highly relevant canonical pathways, spermine and spermidine biosynthesis (44). These two pathways play important roles in skeletal muscle hypertrophy and atrophy (45). The genes that are enriched by GTx-026 mediate the biological functions of connective tissue disorder, skeletal muscle disorder and developmental disorder. Interestingly, there was minimum to no overlap between genes regulated by dystrophin mutation and the genes regulated by GTx-026 treatment, indicating that GTx-026 does not function by reversing the pathways de-regulated by dystrophin mutation. This makes AR agonists like GTx-026 a candidate for combination therapy with exon-skipping drugs and others.
MicroRNAs are non-coding RNAs that are 22–24 nucleotides long. miRNAs are critical for the physiology and pathology of multiple tissues. miRNAs play pivotal role in musculoskeletal equilibrium and pathogenesis (46–48). Using Nanostring technology, we measured the miRNAs in the same sample set used for RNA-sequencing (Fig. 4C). Dystrophin mutation modified the expression of 10 miRNAs, which were not reversed by GTx-026 treatment. Alternatively, GTx-026 had modest effect on miRNA expression modifying the expression of only 4 miRNAs (Fig. 4C, right panel).
GTx-026 protects hearts of castrated mdx mice from pathological transformation
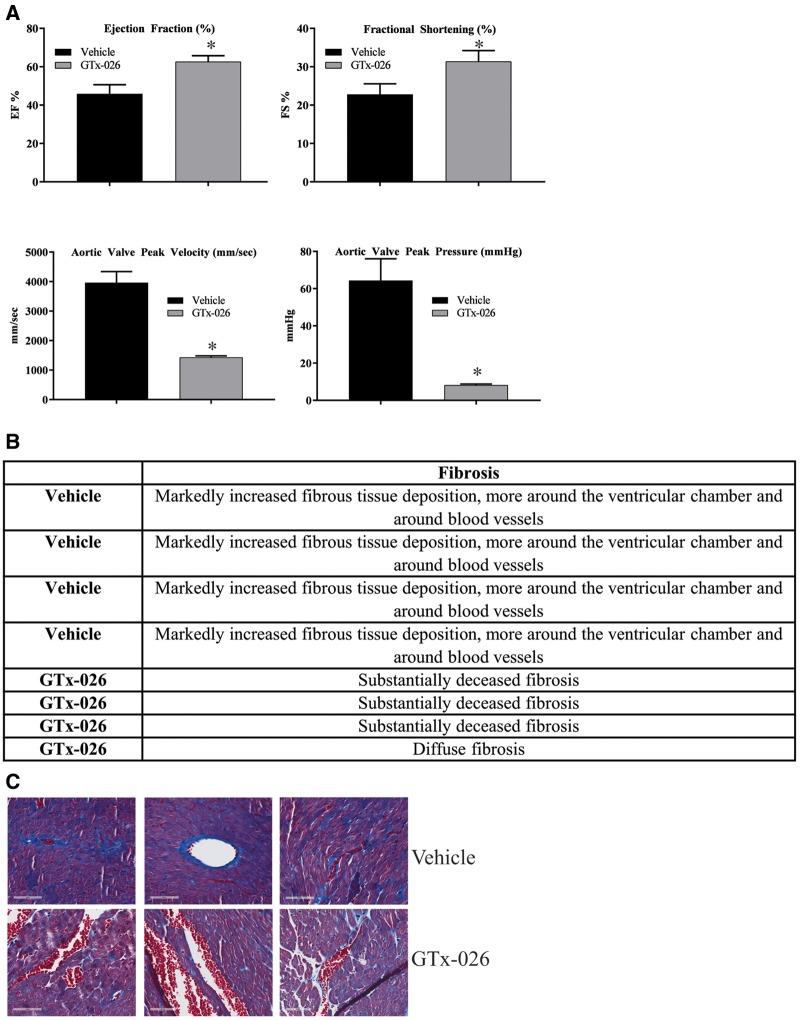
One of the primary causes of death in DMD patients is cardiac and respiratory insufficiencies due to weakening of the respective muscles (14,16,37). Since GTx-026 increased lean mass and muscle function, we expected it to strengthen the cardiac muscle, thereby reversing most, if not all, cardiac pathology. mdx mice (n = 4/group) were castrated and treated with vehicle or GTx-026 for 10 weeks. Body weight, lean mass and grip strength measured at the end of 10 weeks of treatment reproduced the results observed earlier (Supplementary Material, Fig. S3). Echocardiogram was performed to determine the architecture and function of the hearts. Cardiac muscle function worsened in castrated mdx mice compared with reference values for age-matched wild-type mice. These pathological conditions were improved by GTx-026 treatment to the reference values (Fig. 5A). The ejection fraction (EF) in normal healthy adult mice is between 55 and 65% (49). Vehicle-treated castrated mdx mice at 16 weeks of age exhibited an EF of 42–50%, whereas castrated mdx mice treated with GTx-026 had an average EF of 60% (Fig. 5A).
Figure 5.
GTx-026 reverses cardiac abnormalities in mdx mice. (A) mdx mice (n = 4/group) were castrated and treated for 10 weeks with vehicle or 10 mg/kg/day s.c. GTx-026. Echocardiogram was performed to evaluate the cardiac anatomy and physiology. (B) GTx-026 reduces fibrosis in the heart of mdx mice. Heart (n = 4/group) from the animals described in (A) was fixed in formalin, sectioned and stained with mason trichrome to measure the extent of collagen formation. Pathologist report is provided in the table and representative images are shown below. Values are represented as average ± SE. *Significance at P < 0.05.
The fractional shortening (FS) in normal adult mice is around 30% (49). Compared with the normal adult animals’ FS of 30, mdx mice had an average FS of 22.5%. GTx-026 treatment improved the average FS to 30 (Fig. 5A).
While the mdx mice had aortic stenosis, animals treated with GTx-026 did not exhibit aortic stenosis. The aortic valve peak velocity (AVPV) is around 1500 mm/s in normal healthy adult mice (49). While the AVPV of vehicle-treated castrated mdx mice was around 4000 mm/s (Fig. 5A), the AVPV of GTx-026-treated mice was in the range of 1200 mm/s. This could be potentially due to reversal of the aortic valvular stenosis. These results were also reflected in the aortic valve peak pressure (AVPP). While the AVPP of vehicle-treated castrated mdx mice was around 65 mmHg, the AVPP of mdx mice treated with GTx-026 was around 10 mmHg (Fig. 5A). The AVPP of normal healthy adult mice is approximately around 10 mmHg (49). When repeated along with wild-type controls, AVPP of wild-type mice (intact and castrated) and mdx mice (intact and castrated GTx-026 treated) were comparable, while that of castrated vehicle-treated mdx mice were much higher (P < 0.01) (Supplementary Material, Fig. S4).
Histological evaluation of the heart (n = 4) indicated that vehicle-treated castrated mdx mice had markedly higher fibrosis than the GTx-026-treated mdx mice (Fig. 5B and C). This is consistent with the observation of fibrosis in the gastrocnemeous and soleus muscle of mdx mice, and with the echocardiogram results.
Castration of mdx mice significantly increased pulmonary functional insufficiency, which were reversed by GTx-026 treatment
Since lung failure due to lung muscle weakness is one of the primary reasons for death, we extensively evaluated the lung function in castrated mdx mice treated with vehicle or GTx-026 and compared them with wild-type mice.
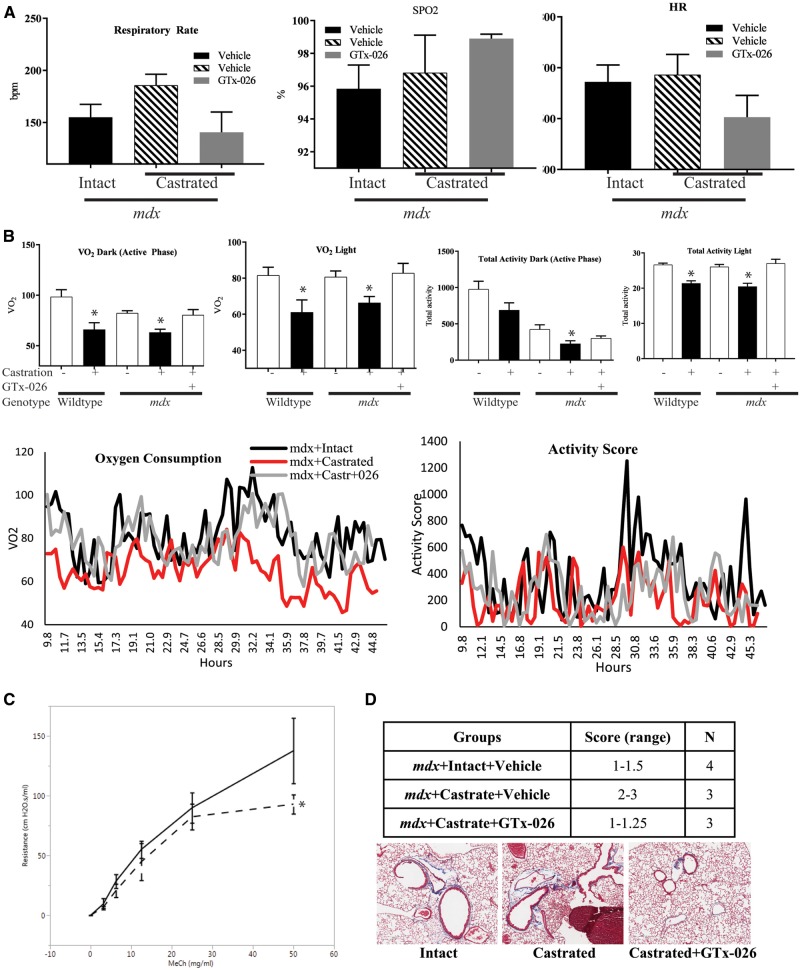
Wild-type or mdx mice (n = 4–5/group) were either sham-operated or castrated, and treated for 10 weeks with vehicle or GTx-026. At the end of the experiment, respiratory rate (RR), heart rate and oxygen content were measured using MouseOx. As shown in Figure 6A, the RR of castrated mdx vehicle-treated mice was higher than that of the sham-operated mdx mice, indicating that the castrated mice required higher breathing rate to maintain the oxygen level in blood. This increase in RR was reversed to the sham-operated mdx mice level by GTx-026. Although the RR in GTx-026-treated castrated mdx mice was lower than the vehicle-treated castrated mdx mice, the saturated oxygen content in the blood was higher in GTx-026-treated castrated mdx mice compared with vehicle-treated castrated mdx mice. These indicate that the compromised lung function in castrated mdx mice has been improved by GTx-026.
Figure 6.
GTx-026 improves the lung function in mdx mice. (A) GTx-026 alters heart and respiratory rates (RRs) and increases blood oxygen levels. Wild-type or mdx mice (n = 4–5/group) were castrated and treated with vehicle or 10 mg/kg/day s.c. GTx-026 for 10 weeks. At the end of the experiment, RR, heart rate (HR) and oxygen content (SPO2) were measured using STARR Life Sciences “MouseOx” with 6.2.1 software version. All recordings were taken over 2–3 minutes with the highest SPO2% recorded for each mouse and the corresponding HR and RR at that particular SPO2% value. (B) Energy expenditure and activity of mice were improved by GTx-026. Wild-type or mdx mice (n = 4–5/group) were either sham operated or castrated and treated with vehicle or 10 mg/kg/day s.c. GTx-026 for 10 weeks. At the end of the experiment, the mice were maintained in Comprehensive Laboratory Activity Monitoring System (CLAMS) for 48 h. Oxygen consumption and activity were measured continuously for 48 h. Averages are provided in the top panels and the continuous measurements are provided in the lower panel. Dark phase is the night time when mice are active. *Significance at P < 0.01 from the respective controls. (C) Lung function is significantly improved by GTx-026 in mdx mice. Wild-type or mdx mice were castrated and treated with vehicle or 10 mg/kg/day s.c. GTx-026 for 12 weeks. Airway resistance to methacholine challenge (MeCh; Sigma-Aldrich) was measured using the FlexiVent FX system (Scireq, Montreal, QC, Canada). Raw data were fitted into a single—compartment model and resistance data were calculated. When no significant difference was observed in baseline values at 0 mg/ml MeCh among groups, airway resistance was normalized and presented as normalized resistance [(values – baseline)/baseline×100%]. N = 4–5/group. Data are represented as average ± SE. *Significance at P < 0.05. (D) Mason trichrome staining for collagen indicates reduced fibrosis in lungs from GTx-026-treated mice. Lungs from animals used in the flexivent study were fixed and stained with mason trichrome stain for collagen as a measure of fibrosis. Stains were scored between 0 and 3 based on the intensity of stain. % SPO2, % of hemoglobin oxygenated; VO2, volume of oxygen; MeCh, methacholine.
We used comprehensive laboratory monitoring system (CLAMS) to measure the oxygen consumption and physical activity of mdx mice. The oxygen consumed in the dark phase (active phase) and light phase (inactive phase) are represented in Figure 6B, upper panel and the continuous monitoring data are represented in Figure 6B lower panel. As shown in the figure, the oxygen consumption (VO2) or energy expenditure was much higher in sham-operated wild-type mice, which decreased substantially upon castration. This was obvious in both the dark (12-h period when lights were turned-off) and light phase (12-h period when lights were on). During the active phase (dark phase), the oxygen consumption of sham-operated vehicle-treated mdx mice was comparable to that of the wild-type castrated mice, indicating that there was a significant reduction in the energy expenditure in mdx mice. Castration of mdx mice resulted in further reduction in oxygen consumption during the active phase, which was reversed to the sham-operated level by GTx-026 treatment. The same phenomenon was observed during the light phase. Since muscle mass is a major component of energy expenditure, we also accounted for the changes in lean mass. After adjusting for changes in lean mass, there were no significant differences in oxygen consumption. These data indicate that aside from changes in lean mass, there are no substantial changes in energy expenditure.
Physical activity of the sham-operated mdx mice was much lower than that of both the sham-operated and castrated wild-type mice in the active phase (Fig. 6B). This was further reduced by castration, indicating that androgens play pivotal role in the physical activity of these mice. The physical activity was only marginally increased by GTx-026 treatment in the active phase, while in the light or resting phase, the physical activity was significantly increased by GTx-026 treatment (P < 0.05). This could mean that AR ligands are not potent enough to overcome the existing DMD pathologies that impede physical activity during waking hours.
To obtain conclusive results on lung function, we performed a ventilator study with flexivent™ in mdx mice castrated and treated with vehicle or GTx-026 for 12 weeks (Fig. 6C). Mice treated with GTx-026 demonstrated a significant decrease in resistance to 50 mg/ml methacholine (MeCh) compared with mice receiving vehicle (93.0 ± 8.0% vs 137.6 ± 27.4%), indicating that GTx-026 treatment improved lung function in mice. No difference was observed in the baseline resistance at 0 mg/ml MeCh between mice treated with GTx-026 and mice receiving vehicle (0.6 ± 0.0 vs 0.6 ± 0.1 cm H2O•s/ml).
To determine if the improvement in lung function is a result of strengthened lung connective tissues, we stained the formalin-fixed lungs from the flexivent study with mason trichrome stain for collagen to determine the extent of fibrosis. Castration of mdx mice increased the intensity of collagen staining compared with sham-operated mdx mice. This increased intensity of staining was reversed to the sham-operated control levels by GTx-026 treatment (Fig. 6D).
These results provide evidence that AR agonists improve cardiopulmonary function and strengthen the heart and lungs in castrated mdx mice.
SARMs increased the lean mass, function and survival of dystrophin/utrn double mutant mice
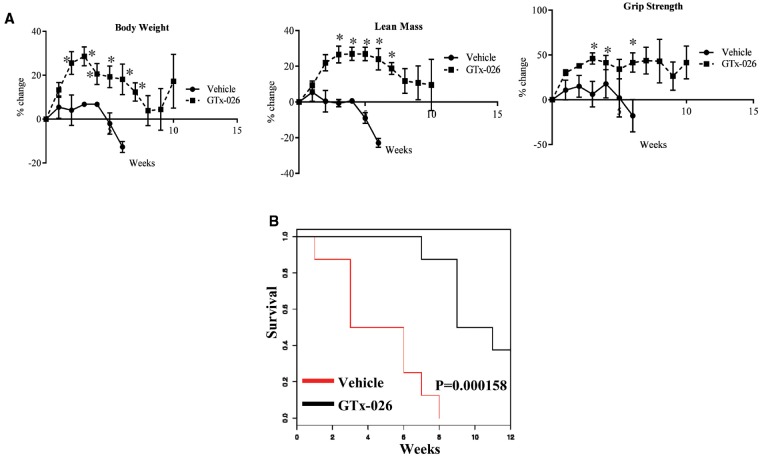
Double-mutant dystrophin (Dmd) and utrophin (Utrn, a homolog of dystrophin and a protein that partially compensates for lack of dystrophin) (i.e. Dmd/utrn mutant) mice present a phenotype more representative of the DMD in humans, including progressive worsening of symptoms, loss of ambulation at ∼12 weeks, and early death by ∼20 weeks. Although previous publications have demonstrated survival of these double mutant mice up to 20 weeks, vehicle-treated castrated double mutant mice in our studies survived only up to 9–12 weeks of age (5–8 weeks after study initiation), indicating that androgens might potentially contribute to the increased survival of these mice (Fig. 1). We determined the ability of GTx-026 to increase the muscle mass and function and subsequently survival in these mdx-dm mice. Body weight, lean mass and grip strength of vehicle-treated castrated double mutant mice all deteriorated quickly (Fig. 7A). Treatment of these mice with GTx-026 increased all these parameters above the baseline and maintained them till the survival or the end of the study. These effects were translated into survival (Fig. 7B). Vehicle-treated castrated mice survived only between 7 and 10 weeks of age (3–7 weeks after study initiation), while SARM-treated castrated mice survived longer with an ∼50–70% increase in survival (P = 0.000158) compared with the vehicle-treated mice.
Figure 7.
GTx-026 increases body weight, muscle mass, function and survival in DMD/utrn double mutant mice. (A) dmd/utrn double mutant (mdx-dm) mice (n = 6–8/group) were castrated and treated with vehicle or 10 mg/kg/day s.c. GTx-026. Weekly body weight, lean mass and grip strength were measured. (B) mdx-dm mice were castrated and treated with vehicle or 10 mg/kg/day s.c. GTx-026. Animals were monitored for survival. N = 6–8 mice. Values are represented as average ± SE. *Significance at P < 0.05.
Discussion
The studies described herein provide evidence in support of using AR agonists to treat DMD. The use of androgens for DMD dates as early as 1950 when testosterone was suggested for the use of muscular dystrophies (50). Subsequently, oxandrolone, a steroidal synthetic androgen was evaluated in a clinical trial in DMD boys (22). Administration of oxandrolone in 5- to 10-year-old boys resulted in an improvement in muscle strength, although statistically insignificant, while muscle strength of untreated boys deteriorated. Evaluation of mechanism of action indicated that oxandrolone increased the fractional synthesis rate of myosin heavy chain (MHC) by 42% and upregulated MHC8 mRNA levels in the RNA-Seq experiment.
The steroidal backbone of oxandrolone and lack of tissue selectivity preclude the prolonged use of steroidal androgens. With the discovery of tissue-selective SARMs, the therapeutic potential of AR ligands can be extended to boys impaired by DMD without concern about the side-effects on secondary sexual organs. Enobosarm (GTX-024) has been evaluated clinically in 23 completed and ongoing clinical trials (24,28). It unequivocally increased muscle mass and has an impeccable safety profile. One of the common side effects of androgen use, elevation of hepatic enzymes, was also only modestly observed with GTx-024. All these justify the advancement of a SARM to treat DMD boys.
Currently, several treatment options are on the horizon for the treatment of DMD. Exon skipping antisense oligonucleotides have the ability to correct the frame-shift mutation so as to synthesize a partially functional protein (51). Although this exhibited early promise, one company faced regulatory set-back in the approval process, while another company won the approval of FDA, despite questions regarding the efficacy. Myostatin inhibitors and utrophin up-regulators are other strategies tested preclinicaly (52,53). Current treatment options for DMD rely on corticosteroids to reduce inflammation. However, prolonged use of corticosteroids results in hyperglycemia, osteoporosis and muscle wasting, which are counterproductive in DMD.
The results presented in this manuscript demonstrate a potential beneficial role of SARMs in DMD. Castrated male mdx mice, which mimic androgen-depleted environment, and intact mdx mice all responded to GTx-026. GTx-026 and other SARMs elicited these beneficial effects at histological and molecular level by reducing the number of centrally nucleated cells, fibrosis, and necrosis and significantly regulating the expression of genes important for muscle hypertrophy.
An interesting result is the reversal of cardiomyopathy in these mice. Although cardiomyopathy has been cited as the primary cause of death in DMD boys, aortic valvular disorder has not been identified pre-clinically in mdx mice. This could be the result of androgen depletion. We found a significant reduction in overall cardiac function with EF numbers comparable to cardiac failure. Although we lack evidence, we speculate that weakening of cardiac muscle could be one of the multifactorial reasons for this cardiomyopathy. GTX-026 restored anatomical, physiological and histological deficiencies to healthy reference control.
As the phenotype of mdx mice is mild, we also studied mdx-dm mice that have a debilitating phenotype similar to DMD boys to evaluate the effect of GTx-026 on survival. Although there is evidence to suggest that these mice survive up to 20 weeks of age, androgen depletion due to castration led to premature death by 10–13 weeks of age. GTx-026 not only improved the body weight, muscle mass and grip strength, but also significantly increased survival.
The major signaling pathway, nNOS, which was found to be deregulated in our studies in the mdx mice, has been shown to be affected in the mdx models, validating our gene expression data (54,55). nNOS pathway was significantly down-regulated in the muscle of mdx mice, which was not reversed by GTx-026. While, GTx-026 failed to reverse the genes altered by dystrophin knockdown, it regulated the expression of genes belonging to spermine and spermidine pathways. Interestingly, spermine oxidase, a gene that alone is sufficient to maintain skeletal muscle and prevent any atrophy was significantly increased by 2–3-fold by GTx-026 in castrated mdx mice (56). Again, genes belonging to the spermidine pathway, a product of spermine oxidase, was also up-regulated by GTx-026. Spermidine has been shown to be important for enhanced cell survival. These results provide a rationale for combining AR-targeted therapeutics with exon-skipping molecules, utrophin up-regulators, or myostatin inhibitors. Since these signaling pathways target distinct proteins, we expect a synergy by combining them.
Most of the muscle-specific miRNAs are modified in the gastrocnemeous muscle of mdx mice. These include miR-206, miR-133 and miR-1 (57). These miRs play important roles in myogenesis and myoblast differentiation. It is counterintuitive to find these miRNAs to be up-regulated in the muscle of castrated mdx mice. It could be a compensatory mechanism to overcome the debilitating phenotype of the loss of dystrophin. In future studies we will knock-down these miRNAs to determine if the loss of their expression will result in a more debilitating phenotype such as seen in mdx-dm.
In addition to the muscle mass and performance increase, AR agonists such as SARMs are also beneficial in building bone (58,59). This could be an added benefit in DMD where the boys are not only suffering from loss of muscle mass and function, but also undergoing extensive bone-remodeling due to either disuse or due to chronic administration of corticosteroids. Although the SARMs’ beneficial action in muscle are negatively regulated by corticosteroid such as prednisolone, the interaction between the two agents in bone needs to be evaluated. Despite these beneficial effects, one limitation that needs to be addressed is the effect of SARMs on secondary sexual organs such as prostate. With broader selectivity between anabolic and androgenic tissues and appropriate dose selection, this limitation can be addressed to some extent.
These studies are the first comprehensive analyses of AR signaling pathway in preclinical models of DMD. With such positive effects on multiple tissues and organs, we expect that AR ligands such as GTx-026 could become the treatment of choice for DMD. These molecules could be used as stand-alone or in combination with other therapies. The magnitude of response observed in lung and heart are compelling enough to argue that these molecules could be very useful to extend survival when administered to DMD patients.
Materials and Methods
All in vitro experiments were performed at least thrice and in vivo experiments were performed with ∼4–10 mice/group, as indicated in the respective figure legend.
AR transactivation
AR transactivation was performed as previously described (60). Briefly, human AR cloned into CMV vector backbone was used for the transactivation study. HEK-293 or undifferentiated C2C12 cells over-expressing the AR were plated at 120 000 cells per well of a 24-well plate in DME containing 5% csFBS. The cells were transfected using Lipofectamine (Invitrogen, Carlsbad, CA, USA) with 0.25 μg GRE-LUC (consensus response element that recognizes AR), 0.02 μg CMV-LUC (renilla luciferase) and 0.025 μg of the AR. The cells were treated 24 h after transfection as indicated in the figures and luciferase assay performed 48 h after transfection. The IC50 were obtained from four parameter logistics curve.
mdx and mdx-dm animal studies
mdx and mdx-dm mice were derived from breeding DMD homozygous/utrn heterozygous mice (Jackson Laboratories, Bar Harbor, ME, USA). Genotyping was performed in accordance with the Jackson Laboratories protocol using the recommended primers. Male mice (4-week old) were used for the experiments. MRI measurements were performed as indicated in the figure using the EchoMRI (Houston, TX, USA). Grip strength measurements (Columbus instruments, Columbus, OH, USA) were recorded at various time-points as described in the respective figure legends with both front and rear paws and the highest value from each animal was considered. The standard operating procedure published on treat NMD website (http://www.treat-nmd.eu/downloads/file/sops/dmd/MDX/DMD_M.2.2.001.pdf) was used for measuring grip strength.
All animal experiments were performed under a protocol approved by the University of Tennessee Health Science Center (UTHSC) animal care and use committee (ACUC). Mice were surgically castrated or sham operated under aseptic environment. Briefly, the mice were anesthetized using isoflurane anesthesia and a small incision was made in the midline of the lower abdomen. Testicular artery was cut to castrate the animals and the wound closed with surgical staples. For sham surgery, the peritoneal cavity was exposed with an incision and closed with surgical staples. Analgesic buprenorphine was provided for pain relief.
Histology
Tissues (gastrocnemeous and soleus muscle) were collected in 10% neutral buffered formalin and stored in 4 °C until further processing. Tissues were fixed, paraffin-embedded, and 5 µm sections were cut for staining. Immunohistochemistry and special staining were performed using standard laboratory methods in an automated tissue processor. Sections were stained with hematoxylin and eosin (H&E) using standard laboratory protocol. Mason trichrome staining for interstitial collagen fiber accumulation, which is indicative of fibrosis, was performed according to published method (61). Slides were evaluated by a pathologist and a score between 0 and 3 for fibrosis, necrosis and inflammation was assigned to each slide. To determine the number of centrally nucleated cells, approximately 100 cells were counted in 8–10 fields from each section. Fast and slow muscle staining were performed in gastrocnemeous muscle (n = 3/group). Fast muscle was stained using anti-troponin-1 fast skeletal muscle antibody (ab134838, Abcam, Cambridge, MA, USA) and slow muscle was stained using anti-slow skeletal MHC antibody (ab11083, Abcam). The slides were double-stained with red color for fast muscle and brown color for slow muscle.
RNA-sequencing
Total RNA (1 μg) was enriched for poly-A + RNA using Ambion Dynabeads mRNA Direct Micro kit and barcoded libraries for sequencing were prepared using the Life Technologies RNAseq V2 kit for Ion Torrent according to the manufacturer’s standard protocol. Libraries were amplified 14 cycles and the quality of each library was checked on an Agilent Bioanalyzer DNA High Sensitivity chip. The libraries were pooled based on the concentration of each sample between 200–350 bp, purified on a Pippin Prep gel, quantified by the Agilent Bioanalyzer and sequenced on an Ion Torrent Proton sequencer. Sequencing was performed by University of Tennessee Health Science Center Molecular Resources Center. The GEO number for the deposition is GSE98473.
Bioinformatics
FASTQ files were retrieved from the Ion Torrent Server at the UTHSC Molecular Resource Center. FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was run in order to trim any bases that had a phred score <20. Once the FASTQ files were trimmed, they were aligned to the Mus musculus nine transcriptome using RNA-STAR (http://www.ncbi.nlm.nih.gov/pubmed/23104886). The alignment was able to match 85% of the raw reads on average. The SAM files were then mined for the total read counts for each transcript. The read counts were normalized to the lowest total number of reads across the experiment to allow comparisons across each sample. Once normalized, the read counts were used to calculate the average fold change and Student's t-test between groupings. Only transcripts that showed a fold change ≥ 1.5 and a P value ≤0.05 were selected. Finally, the false discovery rate (FDR) was calculated using the Benjamini and Hochberg FDR method. Only targets with a q value ≤0.05 were selected for the final transcript list. This list was uploaded to IPA for functional analysis.
CLAMS
Prior to energy expenditure measurements, mice were weighed and total fat and fat-free mass determined non-invasively using an EchoMRI-1100 (EchoMRI™). Mice were then individually housed in a home cage-style Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments) where they had free access to food and water. Animals were maintained in a 12:12 h light:dark cycle. Physical activity and respiratory gas exchange were monitored over a 3-day period and values for each of the measured variables calculated using CLAX software (version 2.2.12; Columbus Instruments). The first 20 data collection cycles were excluded from analysis, as this was the pre-determined CLAMS acclimation phase. Physical activity was calculated as ambulatory movement, with the number of consecutive beam breaks across the X and Y cage axes indicating bouts of activity. Data for the light (rest) and dark (active) phases were separated and analyzed independently.
MiRNA expression analysis
miRNA analysis on RNA prepared from mouse tissue was performed using a panel for ∼800 miRNAs (NanoString Technologies, Seattle, WA, USA). In brief, total RNA was mixed with pairs of capture and reporter probes, hybridized on the nCounter Prep Station and purified complexes were quantified on the nCounter digital analyzer. To account for differences in hybridization and purification, data were normalized to the average counts for all control spikes in each sample and analyzed with nSolver software.
Echocardiography
Using a Vevo 2100 Imaging System (Visualsonics, Toronto, Canada) transthoracic echocardiograms were performed with a 30 MHz transducer (MS 400; Visualsonics). Evaluations included baseline imaging at 6 weeks of age and repeated 70 days post treatment (mice were treated for 10 weeks before subjecting to echocardiogram). Briefly, mice were induced with 3–5% isoflurane and fur removed with depilatory cream (Nair, Church & Dwight Co. Inc., Princeton, NJ, USA), then maintained with 2% isoflurane throughout the two-dimensional and M-mode recording of the LV in parasternal long-axis, short-axis and four chamber views. Images were analyzed post recording using Vevo LAB software (1.7.1, Visualsonics) with a minimum of three cardiac cycles measured for each mouse as recommended by the company. FS (%), EF (%) and AV peak pressure (mmHg) were calculated using standard equations within the software.
Flexivent
Airway resistance to methacholine challenge (MeCh; Sigma-Aldrich) was measured using the FlexiVent FX system (Scireq, Montreal, QC, Canada). Raw data were fitted into a single—compartment model and resistance data were calculated. When no significant difference was observed in baseline values at 0 mg/ml MeCh among groups, airway resistance was normalized and presented as normalized resistance [(values – baseline)/baseline ×100%].
SPO2%
Using MouseOx (STARR Life Sciences, Oakmont, PA, USA) with corresponding mouse thigh sensor saturated oxygen levels were measured in all mice at week 10 after initiation of the experiment (mice were treated for 10 weeks before subjecting to echocardiogram). Fur was removed using depilatory cream (Nair) over the medial thigh as recommended for mice with pigmented coats. All recordings were taken over 2–3 min while under manual restraint by the same experienced lab member. Software version 6.2.1 was used to analyze all recorded measures. The highest SPO2% recorded and the corresponding HR and RR are presented.
Statistics
Statistical analyses were performed using JMP pro or sigmaplot software. If two groups were used in an experiment, then the data were analyzed by t-test and if more than two groups were used, then the data were analyzed by one way ANOVA. Before applying the tests for statistical significance, normality of distribution was tested by the Shapiro Wilk test. Survival curves were generated using R studio using survival library (https://rstudio-pubs-static.s3.amazonaws.com/5588_72eb65bfbe0a4cb7b655d2eee0751584.html). The subjects that died during the experiment were censored in the plot at time of death. A number of animals used are indicated in each figure.
Supplementary Material
Supplementary Material is available at HMG online.
Conflict of Interest statement. R.N. is a consultant to GTx Inc., Memphis, TN, USA.
Funding
The work was partly funded by a research grant from GTx, Inc.
Supplementary Material
References
- 1. Emery A.E. (1991) Population frequencies of inherited neuromuscular diseases—a world survey. Neuromuscul. Disord., 1, 19–29. [DOI] [PubMed] [Google Scholar]
- 2. Rahimov F., Kunkel L.M. (2013) The cell biology of disease: cellular and molecular mechanisms underlying muscular dystrophy. J. Cell. Biol., 201, 499–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Grimm T., Meng G., Liechti-Gallati S., Bettecken T., Muller C.R., Muller B. (1994) On the origin of deletions and point mutations in Duchenne muscular dystrophy: most deletions arise in oogenesis and most point mutations result from events in spermatogenesis. J. Med. Genet., 31, 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nallamilli B.R., Ankala A., Hegde M. (2014) Molecular diagnosis of Duchenne muscular dystrophy. Curr. Protoc. Hum. Genet., 83, 9.25.21–9.25.29. [DOI] [PubMed] [Google Scholar]
- 5. Ervasti J.M., Ohlendieck K., Kahl S.D., Gaver M.G., Campbell K.P. (1990) Deficiency of a glycoprotein component of the dystrophin complex in dystrophic muscle. Nature, 345, 315–319. [DOI] [PubMed] [Google Scholar]
- 6. Crosbie R.H., Heighway J., Venzke D.P., Lee J.C., Campbell K.P. (1997) Sarcospan, the 25-kDa transmembrane component of the dystrophin-glycoprotein complex. J. Biol. Chem., 272, 31221–31224. [DOI] [PubMed] [Google Scholar]
- 7. Ervasti J.M., Campbell K.P. (1991) Membrane organization of the dystrophin-glycoprotein complex. Cell, 66, 1121–1131. [DOI] [PubMed] [Google Scholar]
- 8. Hoffman E.P., Brown R.H. Jr., Kunkel L.M. (1987) Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell, 51, 919–928. [DOI] [PubMed] [Google Scholar]
- 9. Flanigan K.M. (2012) The muscular dystrophies. Semin. Neurol., 32, 255–263. [DOI] [PubMed] [Google Scholar]
- 10. Roland E.H. (2000) Muscular dystrophy. Pediatr. Rev., 21, 233; quiz 238. [DOI] [PubMed] [Google Scholar]
- 11. Messina S., Vita G.L., Sframeli M., Mondello S., Mazzone E., D'Amico A., Berardinelli A., La Rosa M., Bruno C., Distefano M.G.. et al. (2016) Health-related quality of life and functional changes in DMD: a 12-month longitudinal cohort study. Neuromuscul. Disord., 26, 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Petrof B.J., Shrager J.B., Stedman H.H., Kelly A.M., Sweeney H.L. (1993) Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc. Natl. Acad. Sci. USA, 90, 3710–3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frankel K.A., Rosser R.J. (1976) The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum. Pathol., 7, 375–386. [DOI] [PubMed] [Google Scholar]
- 14. Politano L., Nigro V., Nigro G., Petretta V.R., Passamano L., Papparella S., Di Somma S., Comi L.I. (1996) Development of cardiomyopathy in female carriers of Duchenne and Becker muscular dystrophies. JAMA, 275, 1335–1338. [PubMed] [Google Scholar]
- 15. McNally E.M., Kaltman J.R., Benson D.W., Canter C.E., Cripe L.H., Duan D., Finder J.D., Groh W.J., Hoffman E.P., Judge D.P.. et al. (2015) Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation, 131, 1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Raman S.V., Hor K.N., Mazur W., Halnon N.J., Kissel J.T., He X., Tran T., Smart S., McCarthy B., Taylor M.D.. et al. (2015) Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: a randomised, double-blind, placebo-controlled trial. Lancet. Neurol., 14, 153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takeuchi F., Yonemoto N., Nakamura H., Shimizu R., Komaki H., Mori-Yoshimura M., Hayashi Y.K., Nishino I., Kawai M., Kimura E.. et al. (2013) Prednisolone improves walking in Japanese Duchenne muscular dystrophy patients. J. Neurol., 260, 3023–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matthews E., Brassington R., Kuntzer T., Jichi F., Manzur A.Y. (2016) Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev., CD003725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stein C.A. (2016) Eteplirsen approved for duchenne muscular dystrophy: the FDA faces a difficult choice. Mol. Ther., 24, 1884–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dowling J.J. (2016) Eteplirsen therapy for Duchenne muscular dystrophy: skipping to the front of the line. Nat. Rev. Neurol., 12, 675–676. [DOI] [PubMed] [Google Scholar]
- 21. Sjoblom B., Gronberg B.H., Wentzel-Larsen T., Baracos V.E., Hjermstad M.J., Aass N., Bremnes R.M., Flotten O., Bye A., Jordhoy M. (2016) Skeletal muscle radiodensity is prognostic for survival in patients with advanced non-small cell lung cancer. Clin. Nutr., 35(6), 1386–1393. [DOI] [PubMed] [Google Scholar]
- 22. Balagopal P., Olney R., Darmaun D., Mougey E., Dokler M., Sieck G., Hammond D. (2006) Oxandrolone enhances skeletal muscle myosin synthesis and alters global gene expression profile in Duchenne muscular dystrophy. Am. J. Physiol. Endocrinol. Metab., 290, E530–E539. [DOI] [PubMed] [Google Scholar]
- 23. Dalton J.T., Mukherjee A., Zhu Z., Kirkovsky L., Miller D.D. (1998) Discovery of nonsteroidal androgens. Biochem. Biophys. Res. Commun., 244, 1–4. [DOI] [PubMed] [Google Scholar]
- 24. Dubois V., Simitsidellis I., Laurent M.R., Jardi F., Saunders P.T., Vanderschueren D., Claessens F. (2015) Enobosarm (GTx-024) modulates adult skeletal muscle mass independently of the androgen receptor in the satellite cell lineage. Endocrinology, 156, 4522–4533. [DOI] [PubMed] [Google Scholar]
- 25. Cozzoli A., Capogrosso R.F., Sblendorio V.T., Dinardo M.M., Jagerschmidt C., Namour F., Camerino G.M., De Luca A. (2013) GLPG0492, a novel selective androgen receptor modulator, improves muscle performance in the exercised-mdx mouse model of muscular dystrophy. Pharmacol. Res., 72, 9–24. [DOI] [PubMed] [Google Scholar]
- 26. Mohler M.L., Bohl C.E., Jones A., Coss C.C., Narayanan R., He Y., Hwang D.J., Dalton J.T., Miller D.D. (2009) Nonsteroidal selective androgen receptor modulators (SARMs): dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem., 52, 3597–3617. [DOI] [PubMed] [Google Scholar]
- 27. Narayanan R., Mohler M.L., Bohl C.E., Miller D.D., Dalton J.T. (2008) Selective androgen receptor modulators in preclinical and clinical development. Nucl. Recept. Signal, 6, e010.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobs A.S., Boccia R.V., Croot C.C., Gabrail N.Y., Dalton J.T., Hancock M.L., Johnston M.A., Steiner M.S. (2013) Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol., 14, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Narayanan R., Coss C.C., Yepuru M., Kearbey J.D., Miller D.D., Dalton J.T. (2008) Steroidal androgens and nonsteroidal, tissue-selective androgen receptor modulator, S-22, regulate androgen receptor function through distinct genomic and nongenomic signaling pathways. Mol. Endocrinol., 22, 2448–2465. [DOI] [PubMed] [Google Scholar]
- 30. Marshall J.L., Oh J., Chou E., Lee J.A., Holmberg J., Burkin D.J., Crosbie-Watson R.H. (2015) Sarcospan integration into laminin-binding adhesion complexes that ameliorate muscular dystrophy requires utrophin and alpha7 integrin. Hum. Mol. Genet., 24, 2011–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heller K.N., Montgomery C.L., Shontz K.M., Clark K.R., Mendell J.R., Rodino-Klapac L.R. (2015) Human alpha7 integrin gene (ITGA7) delivered by adeno-associated virus extends survival of severely affected dystrophin/utrophin-deficient mice. Hum. Gene Ther., 26, 647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McDonald A.A., Hebert S.L., Kunz M.D., Ralles S.J., McLoon L.K. (2015) Disease course in mdx:utrophin+/- mice: comparison of three mouse models of Duchenne muscular dystrophy. Physiol. Rep., 3(4), pii: e12391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tinsley J.M., Potter A.C., Phelps S.R., Fisher R., Trickett J.I., Davies K.E. (1996) Amelioration of the dystrophic phenotype of mdx mice using a truncated utrophin transgene. Nature, 384, 349–353. [DOI] [PubMed] [Google Scholar]
- 34. Deconinck N., Tinsley J., De Backer F., Fisher R., Kahn D., Phelps S., Davies K., Gillis J.M. (1997) Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat. Med., 3, 1216–1221. [DOI] [PubMed] [Google Scholar]
- 35. Wakefield P.M., Tinsley J.M., Wood M.J., Gilbert R., Karpati G., Davies K.E. (2000) Prevention of the dystrophic phenotype in dystrophin/utrophin-deficient muscle following adenovirus-mediated transfer of a utrophin minigene. Gene Ther., 7, 201–204. [DOI] [PubMed] [Google Scholar]
- 36. Deconinck A.E., Rafael J.A., Skinner J.A., Brown S.C., Potter A.C., Metzinger L., Watt D.J., Dickson J.G., Tinsley J.M., Davies K.E. (1997) Utrophin-dystrophin-deficient mice as a model for Duchenne muscular dystrophy. Cell, 90, 717–727. [DOI] [PubMed] [Google Scholar]
- 37. Grady R.M., Teng H., Nichol M.C., Cunningham J.C., Wilkinson R.S., Sanes J.R. (1997) Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell, 90, 729–738. [DOI] [PubMed] [Google Scholar]
- 38. Yu X., Bao B., Echigoya Y., Yokota T. (2015) Dystrophin-deficient large animal models: translational research and exon skipping. Am. J. Transl. Res., 7, 1314–1331. [PMC free article] [PubMed] [Google Scholar]
- 39. Oliveira A.G., Coelho P.H., Guedes F.D., Mahecha G.A., Hess R.A., Oliveira C.A. (2007) 5alpha-Androstane-3beta,17beta-diol (3beta-diol), an estrogenic metabolite of 5alpha-dihydrotestosterone, is a potent modulator of estrogen receptor ERbeta expression in the ventral prostrate of adult rats. Steroids, 72, 914–922. [DOI] [PubMed] [Google Scholar]
- 40. Duffy D.M., Legro R.S., Chang L., Stanczyk F.Z., Lobo R.A. (1995) Metabolism of dihydrotestosterone to 5 alpha-androstane-3 alpha, 17 beta-diol glucuronide is greater in the peripheral compartment than in the splanchnic compartment. Fertil. Steril., 64, 736–739. [DOI] [PubMed] [Google Scholar]
- 41. Smith C.L., Nawaz Z., O'Malley B.W. (1997) Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol., 11, 657–666. [DOI] [PubMed] [Google Scholar]
- 42. Liu M., Yue Y., Harper S.Q., Grange R.W., Chamberlain J.S., Duan D. (2005) Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol. Ther., 11, 245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tameyasu T., Ogura S., Ogihara K. (2004) The effect of e-, i-, and n-nitric oxide synthase inhibition on colonic motility in normal and muscular dystrophy (mdx) mice. Jpn. J. Physiol., 54, 555–566. [DOI] [PubMed] [Google Scholar]
- 44. Wei C., Li H.Z., Wang Y.H., Peng X., Shao H.J., Li H.X., Bai S.Z., Lu X.X., Wu L.Y., Wang R.. et al. (2016) Exogenous spermine inhibits the proliferation of human pulmonary artery smooth muscle cells caused by chemically-induced hypoxia via the suppression of the ERK1/2- and PI3K/AKT-associated pathways. Int. J. Mol. Med., 37, 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chrisam M., Pirozzi M., Castagnaro S., Blaauw B., Polishchuck R., Cecconi F., Grumati P., Bonaldo P. (2015) Reactivation of autophagy by spermidine ameliorates the myopathic defects of collagen VI-null mice. Autophagy, 11, 2142–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pelosi L., Coggi A., Forcina L., Musaro A. (2015) MicroRNAs modulated by local mIGF-1 expression in mdx dystrophic mice. Front Aging Neurosci., 7, 69.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Roberts T.C., Blomberg K.E., Smith C.I., El Andaloussi S., Wood M.J. (2016) mRNA and microRNA transcriptomics analyses in a murine model of dystrophin loss and therapeutic restoration. Genom. Data, 7, 88–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhou J., Gao J., Zhang X., Liu Y., Gu S., Zhang X., An X., Yan J., Xin Y., Su P. (2015) microRNA-340-5p functions downstream of cardiotrophin-1 to regulate cardiac eccentric hypertrophy and heart failure via target gene dystrophin. Int. Heart J., 56, 454–458. [DOI] [PubMed] [Google Scholar]
- 49. Vinhas M., Araujo A.C., Ribeiro S., Rosario L.B., Belo J.A. (2013) Transthoracic echocardiography reference values in juvenile and adult 129/Sv mice. Cardiovasc. Ultrasound, 11, 12.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Perlstein M.A., Gutterman H. (1950) Testosterone in progressive pseudohypertrophic muscular dystrophy. J. Pediatr., 37, 743–749. [DOI] [PubMed] [Google Scholar]
- 51. Hodgkinson L., Sorbera L., Graul A.I. (2016) Duchenne muscular dystrophy drugs face tough path to approval. Drugs Today (Barc), 52, 199–202. [DOI] [PubMed] [Google Scholar]
- 52. Qiao C., Li J., Jiang J., Zhu X., Wang B., Li J., Xiao X. (2008) Myostatin propeptide gene delivery by adeno-associated virus serotype 8 vectors enhances muscle growth and ameliorates dystrophic phenotypes in mdx mice. Hum. Gene Ther., 19, 241–254. [DOI] [PubMed] [Google Scholar]
- 53. Vianello S., Yu H., Voisin V., Haddad H., He X., Foutz A.S., Sebrie C., Gillet B., Roulot M., Fougerousse F.. et al. (2013) Arginine butyrate: a therapeutic candidate for Duchenne muscular dystrophy. FASEB J., 27, 2256–2269. [DOI] [PubMed] [Google Scholar]
- 54. Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. (1995) Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell, 82, 743–752. [DOI] [PubMed] [Google Scholar]
- 55. Chang W.J., Iannaccone S.T., Lau K.S., Masters B.S., McCabe T.J., McMillan K., Padre R.C., Spencer M.J., Tidball J.G., Stull J.T. (1996) Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc. Natl. Acad. Sci. USA, 93, 9142–9147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bongers K.S., Fox D.K., Kunkel S.D., Stebounova L.V., Murry D.J., Pufall M.A., Ebert S.M., Dyle M.C., Bullard S.A., Dierdorff J.M.. et al. (2015) Spermine oxidase maintains basal skeletal muscle gene expression and fiber size and is strongly repressed by conditions that cause skeletal muscle atrophy. Am. J. Physiol. Endocrinol. Metab., 308, E144–E158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Horak M., Novak J., Bienertova-Vasku J. (2016) Muscle-specific microRNAs in skeletal muscle development. Dev. Biol., 410, 1–13. [DOI] [PubMed] [Google Scholar]
- 58. Kearbey J.D., Gao W., Fisher S.J., Wu D., Miller D.D., Dalton J.T. (2009) Effects of selective androgen receptor modulator (SARM) treatment in osteopenic female rats. Pharm. Res., 26, 2471–2477. [DOI] [PubMed] [Google Scholar]
- 59. Kearbey J.D., Gao W., Narayanan R., Fisher S.J., Wu D., Miller D.D., Dalton J.T. (2007) Selective androgen receptor modulator (SARM) treatment prevents bone loss and reduces body fat in ovariectomized rats. Pharm. Res., 24, 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Narayanan R., Yepuru M., Szafran A.T., Szwarc M., Bohl C.E., Young N.L., Miller D.D., Mancini M.A., Dalton J.T. (2010) Discovery and mechanistic characterization of a novel selective nuclear androgen receptor exporter for the treatment of prostate cancer. Cancer Res., 70, 842–851. [DOI] [PubMed] [Google Scholar]
- 61. Goldner J. (1938) A modification of the masson trichrome technique for routine laboratory purposes. Am. J. Pathol., 14, 237–243. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.