Abstract
Cerenkov luminescence (CL) is blue glow light produced by charged subatomic particles travelling faster than the phase velocity of light in a dielectric medium such as water or tissue. CL was first discovered in 1934, but for biomedical research it was only recognized in 2009 after advances in optical camera sensors brought the required high sensitivity. Recently, applications of CL from clinical radionuclides have been rapidly expanding to include not only preclinical and clinical biomedical imaging but also an approach to therapy. Cerenkov Luminescence Imaging (CLI) utilizes CL generated from clinically relevant radionuclides alongside optical imaging instrumentation. CLI is advantageous over traditional nuclear imaging methods in terms of infrastructure cost, resolution and imaging time. Furthermore, CLI is a truly multimodal imaging method where the same agent can be detected by two independent modalities, with optical (CL) imaging and with positron emission tomography (PET) imaging. CL has been combined with small molecules, biomolecules and nanoparticles to improve diagnosis and therapy in cancer research. Here, we cover the fundamental breakthroughs and recent advances in reagents and instrumentation methods for CLI as well as therapeutic application of CL.
Introduction
Cerenkov luminescence (CL), or Cerenkov radiation (CR), was first described by the Russian scientist Pavel Cerenkov in 1934. He observed feeble blue light by accident when he placed a sulfuric acid solution above radium salts.1 He came to the conclusion that the observed visible radiation was emitted by solvent interacting with charged subatomic particles from the radium moving faster than the speed of light through the solution.2 Further theoretical studies were done by Ilya Frank and Igor Tamm famously described mathematically the dependence of the light on nuclide energy, medium refractive index, and cone angle of light emitted. In 1958, Cerenkov, Frank and Tamm were awarded the Nobel Prize in Physics for their discovery and explanation of the Cerenkov effect. The Cerenkov phenomenon has been applied for photomultiplier tube scintillation counting, detection of cosmic subatomic particles and the estimation of fuel rod activity in nuclear power plants.3 However, it was not until 2009 that CL was applied for biomedical imaging research when Robertson et al. first described the new imaging tool, Cerenkov Luminescence Imaging (CLI).4 Robertson et al. discovered that the radiotracer 2-deoxy-2-(18F)fluoro-D-glucose (18F-FDG) could be used for optical imaging applications.5–7 Therefore, CLI provides a unique multimodal imaging system with positron emission tomography (PET) imaging where medical radiotracers can be imaged by two independent modalities (Fig. 1a and 1b) and if necessary merged for quantitative tomographic information and optical identification. This system could be advantageous over traditional clinical nuclear imaging modalities in terms of cost effectiveness, short imaging time and broad applicability of radionuclides and experimental conditions8, though at a loss for tomographic capabilities and limited quantification without the PET data. In this review, we will describe the basic principles of CLI and recent advances in the imaging reagents and instrumentation methods for CLI.
Figure1.
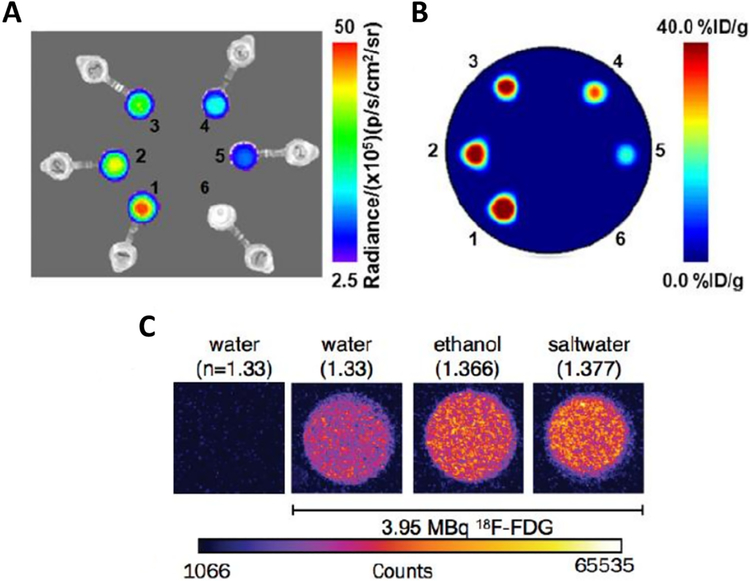
Cerenkov luminescence images (A) and PET images (B) of six samples of 89Zr activity in water. 1–6 corresponded to activity concentrations of 40.3, 32.6, 27.4, 20.4, 13.3, and 0.00 kBq/mL. (C) Images of equal activities of F-18 samples (3.952 MBq, 20L) diluted in 2 mL of water (H2O, refractive index: n = 1.3359), ethanol (C2H5OH, n = 1.366), saltwater (H2O and saturating NaCl, n = 1.377) along with a control sample of water without radionuclide. (A) and (B) adapted with permission from Ruggiero et al.7 (C) Adapted with permission from Thorek et al.3
Physics behind Cerenkov Imaging
Charged beta (β) particles, such as positively charged positrons and negatively charged electrons released by radioactive decay, interact with the surrounding dielectric water molecules in tissues. The randomly oriented water molecules align with the rapidly passing charged particles, polarizing the atoms in the vicinity and creating a coherent wavefront.8 Described by the Huygens’s principle the wavefront emits photons in the direction of the charged particle when the medium relaxes. This phenomenon is called Cerenkov radiation (CR) or Cerenkov luminescence (CL), where the threshold speed is the phase velocity in a medium. In general the relativistic β-particle velocity is calculated using equation 1,
| (1) |
Where c is the speed of light in a vacuum, E is the particle energy and E0 is the mass of the β-particle at rest. The number of photons produced can be calculated using the Frank-Tamm equation 2,
| (2) |
Here θ is the fine structure constant (1/137), λ is the wavelength, n is the refractive index, and φ is the fractional velocity of the β-particle relative to the speed of light in a vacuum. The photons propagate at a forward angle (θ) in the direction of the charged particle travels. The relationship between the Cerenkov cone angle and the kinetic energy of the charged particle is described by equation 3,
| (3) |
Where β is the kinetic energy of the charged particle, n is the refractive index and ω is the specific frequency. Therefore the Cerenkov threshold is described in the following relationship in equation 4,
| (4) |
CR is generated only when the velocity of the charged particle is equal or excess to the phase velocity of light in the medium, and the Cerenkov energy threshold decreases in a dielectric medium with increasing refractive index (n) leading to increased photon fluxes observed (Fig. 1c).
Cerenkov Imaging Agents
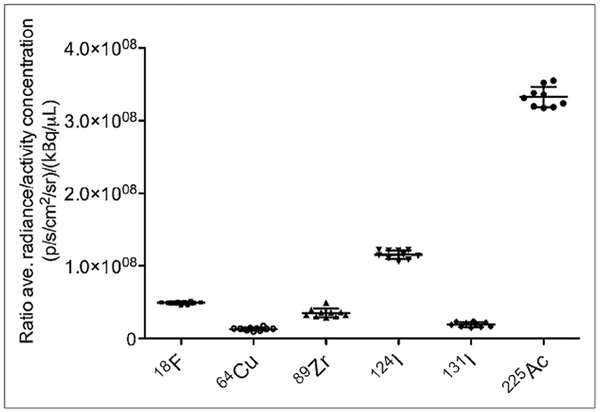
Cerenkov luminescence Imaging (CLI) was first recognized by Robertson et al. in 2009 who showed CL from the positron emitting 18F-FDG in vivo could be imaged with widely used optical instrumentation.4 Following this study, some radionuclides were found to produce CL in preclinical studies including 18F, 64Cu, 89Zr, 90Y, 68Ga, 124I, 131I, 177Lu, and 225Ac (Fig. 2).9,10 Therefore, CLI offers a multimodal system where the reagent can be detected by two independent modalities; optical imaging (CLI) and positron emission tomography (PET) imaging.8
Figure2.
Plot of ratio of average radiance (p/s/cm2/sr)/ activity con-centration (mCi/mL) vs radionuclide. The figure adapted with permission from Ruggiero et al.7
CLI utilizes the same small animal imaging system consisting of a CCD camera and an animal isolation chamber used for bioluminescence imaging.3 2-deoxy-2-[18F]fluoro-D-glucose (18F-FDG) is the most commonly used small molecule PET radiotracer, which selectively accumulates in tumors by upregulated glucose uptake and metabolism due to the Warburg effect.11,12 CLI with 18F-FDG has been shown to strongly correlate with PET imaging.13 Macromolecules such as peptides and antibodies have been studied for CLI. Liu et al. showed that a 90Y labeled dual targeting peptide for RGD (targeting αvβ3 integrin) and bombesin (targeting gastrin receptor) could visualize PC3 tumors in mice while blocking studies abolished the CLI signal confirming specificity.13 Furthermore, cancer targeting radiolabeled Herceptin antibody by 124I has been used as a Her2/neu positive imaging agent in a mouse bearing NIH3T6.7 tumor cells by both CLI and PET.14
Nanoparticles have garnered attention in biomedical applications because of their unique and tunable chemical, physical and biological properties. Secondary Cerenkov emission fluorescence imaging (SCIFI), or Cerenkov radiation energy transfer (CRET), both describe an imaging method that converts the UV/blue-weighted light from CL to longer wavelengths using the emissions of select nanoparticles. Nanoparticles are more suitable for SCIFI, or CRET, due to their higher optical cross sections than small fluorescence molecules.8 Dothager et al. demonstrated that CR from 64Cu and 18F can be spectrally coupled to quantum nanoparticles (Qtracker705) to emit a red-shifted fluorescence in vitro and in vivo.15 Thorek et al. also showed that the quantum dot 605 (QD605) can be excited by 18F-FDG.16
While paring CL with nanoparticles provides advantages in vivo imaging, the systems cannot be easily modulated and are always activated regardless of their biological context. Therefore, developing smart activatable imaging systems that can be stimulated by biological, chemical or physical processes were needed. Photoactivation using CL was reported by Ran et al.17 They utilized CL released from 18F-FDG to uncage 1-(4,5-dimethoxy-2-nitrophenyl) ethyl ester-luciferin that can be cleaved by near UV light. The transfected luciferase in a tumor catalyzes the bioluminescence process. CL was required to activate the caged-luciferin and the resulting bioluminescence was monitored in a tumor bearing mouse. This study successfully showed CL delivered to the tumor by 18F-FDG could activate the caged fluorophore. Another approach in developing smart activatable imaging agents uses enzyme expression in a tumor to drive signal conversion over time. Thorek et al. developed the CL excited smart nanoparticle that can be turned on by matrix metallopeptidase-2 enzyme, where the fluorochrome FAM (fluorescein) was quenched by a gold nanoparticle. Only upon peptide cleavage and release of FAM from the nanoparticle would Cerenkov mediated excitation occur.16
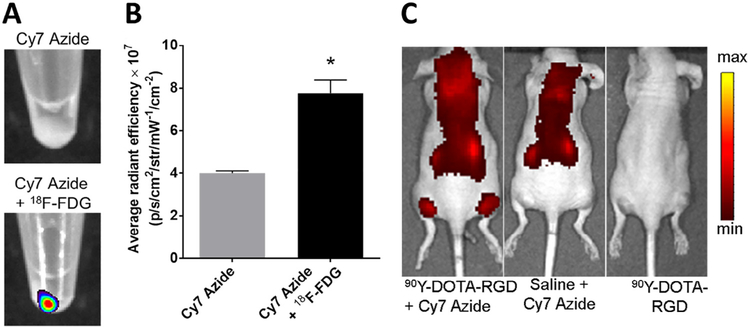
The major limitations of CL are the several magnitude lower signal intensity relative to fluorescence, the prolonged scanning time potentially lowering image quality if the subject moves, and the limited duration of imaging based on the radionuclide half-life. Das et al. addressed these limitations by developing a CL activated “sticky tag” that can convert the weak CL signal into a stronger fluorescence signal that could be measured later after radionuclide decay. The sticky tag converts CL to fluorescence when CL activates the formation of nitrenes from an azide that subsequently fix locally a fluorescence probe in tissue allowing a CL radiotracer to “write” where both sticky tag and radionuclide travel. This CL-mediated photoactivation of a Cy7 azide was demonstrated both in vitro and in vivo (Fig. 3a-c) and could be applied for intraoperative imaging. They also developed a CL-mediated delivery of anticancer drug doxorubicin showing therapeutic response in vitro.18
Figure3.
(A) Fluorescence images of HT1080 cell pellets incubated with 18F-FDG and Cy7 Azide or Cy7 Azide alone.(B) Quantitative analysis of the HT1080 cell pellet fluorescence signal after incubation with Cy7 Azide alone or to-gether with 18F-FDG. (C) Fluorescence images of mice injected with Cy7 Azide 7 hours after 90Y-DOTA-RGD or saline injection, or 90Y-DOTARGD alone. (A), (B), and (C) adapted with permission from Das et al.15
Instrumentation
Visualizing CL has come a long way since the first reports of blue light present in Madame Curie’s laboratory (probably radioluminescence as well as Cerenkov light)19 and Pavel Alekseyevich Cherenkov’s discovery that the origin of the light was specific to radionuclides in dielectric media with a refractive index greater than air. CL has been used almost exclusively in the fields of astronomy and physics to determine particle energy until the first CL images emerged in 2009 of 18F-FDG in a tumor bearing mouse.4 Initially the signal was thought to be from the incident 511keV gammas from 10MBq 18F-FDG striking the detector, but this could easily be dismissed by placing a piece of paper in front of the subject, which abolished the signal, indicating the signal was primarily from visible photons attributed to CL and not high energy photons (gammas) or particles hitting the camera.
CLI in the biomedical field was made possible through instrumentation advances in Charge Coupled Device (CCD) cameras where back-thinned, back-illuminated, and cooled CCDs drastically increased sensitivity and reduced thermal noise in the sensor, limiting bleed over between pixels and enabled photon counting. In addition the system required light tight enclosure to block out ambient light as well as optics with f=0.95 apertures that do not restrict incident light to the CCD. Photon counting is extremely important in CLI given the fluence rates are on the order of 1.32 and 33.9 photons per 18F and 68Ga positron emission respectively.20 These low photon fluxes are several orders of magnitude lower than traditional fluorescence or even bioluminescence.21,22 Despite these low fluency rates, activity administered typically ranges from kbq to GBq meaning tens of thousands to billions of photons are generated each second by these administered radionuclides. The expansion of CLI is enabled by a plethora of CCD camera vendors not only in the pre-clinical field with the ubiquity of the IVIS imaging system, but also custom made and commercial CCD setups have allowed translation of CLI into the clinic.23–28
Optical imaging has served as a preferred modality in both the pre-clinical and clinical theaters as a method to determine tumor burden, margins, and aid in intraoperative removal. The majority of optical imaging has been limited to traditional dyes like isosulfan blue and fluorescent probes such as fluorescein29, introduced in 1947, and subsequently indocyanine green. Dyes have advanced rapidly in the pre-clinical space but have faced obstacles translating to the clinic based upon high molar amounts of dye needed and narrow operation range of clinical instruments. CLI on the other hand emits across a spectrum, enabling a wide configuration of cameras to record Cerenkov and when paired with a select fluorophores, secondary Cerenkov induce fluorescence imaging (SCIFI) can further convert CL from the UV16, absorbed by most tissues8, to wavelengths above 500nm, which is more favorable for tissue imaging due to reduced absorbance and better penetration. In addition, SCIFI was found to have nearly six-fold greater signal to noise ratios than fluorescence, making SCIFI a highly specific optical imaging technique.
The majority of pre-clinical optical work is conducted with the commercial IVIS imaging system (Perkin Elmer) due to their wide availability but other prototype imaging devices using stand-alone CCD systems have been developed as well.3,23 Previously IVIS imaging systems were used for fluorescence and bioluminescence studies, and were easily adapted for work with radiotracers such as 18F-FDG. This imaging system utilizes a cooled CCD installed inside a light tight temperature controlled enclosure. Early versions utilized a back-thinned, back–illuminated, cooled grade 1 CCD with a 2.7 × 2.7 cm area and capable of detecting as few as 100 photons/s/cm2/sr. The optics utilized a fast f/0.95 lens directly mounted to the CCD with an interchangeable filter wheel containing 4 emission filters and capable of imaging a field of view (FOV) between 100–625cm2. Newer models have expanded the emission filter set to cover the 400–860nm spectrum in 20nm steps and a focused FOV from 16–506cm2. In addition, newer models incorporate three-dimensional imaging as well as an integrated computed tomography (CT) system for optical-CT dual modality imaging. While current limitations in CLI technology cite low intensity of the light, requiring higher activity amounts or longer image acquisitions, CLI can be done for pre-clinical specimens within 2–5 minutes per image. Low energy nuclides, such as 177Lu and trace activity levels can require longer acquisition times compared to high energy emitters like 90Y or 68Ga. Another drawback of CLI specifically to the use of traditional CCD cameras is the UV, or blue weighted distribution of CL. Traditional CCD cameras were optimized for high quantum efficiency (>50%) between 500 and 800nm, while the majority of CL emission is weighted below 500nm. Back thinned CCD cameras expand this efficiency range further into the blue region. Conversion of UV-weighted CL into this region through SCIFI had two fold advantages for CLI; photons emitted from red shifted CL are emitted into a more sensitive region of the CCD16 and in a window with less tissue attenuation.8
The first Cerenkov cameras allow detection of a radiotracer primarily at the surface or located in lymph nodes just below the skin. Measurement of CL deeper in a body cavity however, requires an alternative approach either with surgery to expose the potential area or excision. Imaging of exposed and excised tumors were shown in a preclinical model using 89Zr-DFO-Trastuzumab, where a HER2/neu positive tumor on the mouse was targeted and imaged using PET and CLI for time activity concentration as well as mean SUV and radiance correlation.30 Simulating a clinical intraoperative scenario, CLI was used on an IVIS optical imaging system to identify a HER2/neu positive tumor and confirm resection from the region of interest. Subsequent studies have advanced the strong correlation of CL radiance and PET uptake in both preclinical and clinical models and have been recently extensively reviewed.26 For tumors or CLI imaging of tissue much further inside the body, the use of a fiber bundle to bridge light emitted from any internalized source into the connected CCD and focusing optics is needed. The first demonstration of a Cerenkov endoscope utilized a fiber bundle and microimaging lens that allowed detection of activity down to 1.2μCi per 300μL volume.31 The spatial resolution at 5cm from the source could resolve 2.4mm and 1.6mm cylindric holes from a PET/CT phantom. CL imaging was achieved with a 108mm fiber bundle using 10μm individual fibers attached distally with a f/1.4 12mm microimaging lens and a separate f/1.4 lens attached to the CCD camera. CL images were acquired over 5 minutes intervals.
The discovery of CLI for use in pre-clinical imaging has opened the door to researchers to conduct topographic imaging with radiotracers at rates faster than currently available to PET and SPECT systems. Despite this speed, CLI still requires acquisitions on the order of several minutes and in complete darkness making real time and tomographic32–34 imaging difficult. Further advances in preclinical instrumentation need to focus on maximizing light generated from a radiotracer source while simultaneously minimizing loss of light from absorbance in the optical path to detector wavelength sensitivity. Solving these CLI shortcomings can accelerate the clinical translation from in vivo models.
Clinical Cerenkov Luminescence Imaging
Not long after the initial rediscovery of CLI for preclinical imaging in 2009, first in human imaging was performed in 2013 using a prototype CCD-camera based system. In the first published account of CLI, one patient undergoing radioablation therapy for Grave’s disease with 550MBq of 131-Iodine (131I) was imaged.24 A cooled electron multiplying CCD (EMCCD) (512×512 pixel), with was used with a f/1.4 8mm C mount lens at a binning = 1 for 16μm pixel resolution. When cooled to −80OC and set at a gain of 400, patient CLI was achieved within 2 minutes. The resulting image was corrected for gamma strikes with a 4 pixel median box filter algorithm. As with pre-clinical images, the patient was placed in a hospital basement room without lights and the door was covered with a thick black cloth for the imaging session except for a bright light image to provide anatomical reference. The first clinical CLI study however did not provide quantitation of the thyroid but demonstrated the feasibility of optical imaging of patients with a radiotracer.
In a more rigorous example of clinical CLI, quantitation was implemented and shown across several cancer patients with different cancers (lymphoma, head and neck cancer) and compared to the clinical positron emission tomography (PET) images to correlate PET positive uptake with Cerenkov luminescence.23 Similar instrumentation was used for clinical CLI with a cooled CCD coupled with a 50mm f/0.8 lens and a 605nm long pass filter to select tissue penetrating CL. Linearity was performed using the same isotope in vitro allowing quantitative image analysis for patients imaged. The patients were injected with a mean of 444MBq (12mCi) of the radiotracer 18F-FDG, imaged on a PET/CT to define negative and positive nodes, and subsequently underwent CLI in a darkened and light-isolated room. Positive nodes by PET were found to have significantly higher (p=0.02) CL intensity than negative nodes and CLI intensity correlated with PET MBq/mL max values. Lymph nodes with less than 2 μCi activity could be identified with CLI
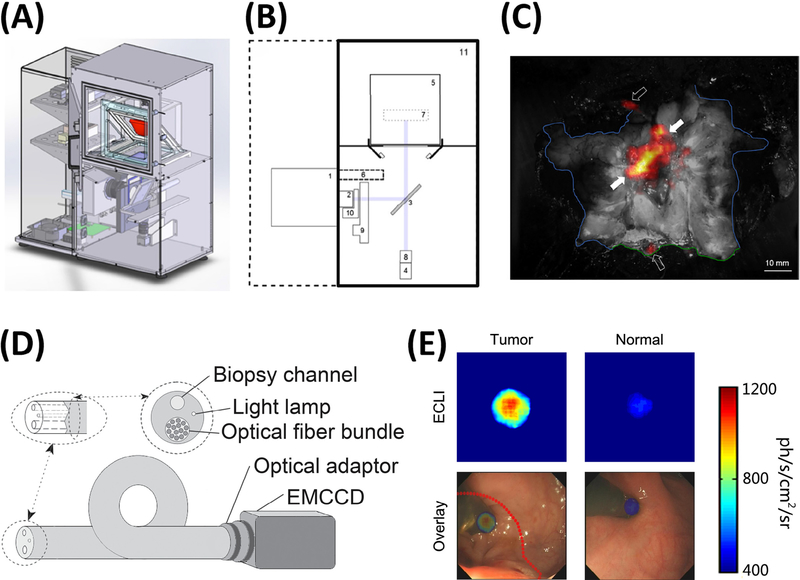
A common technique in intraoperative imaging to evaluate tumor margin is to remove a tumor specimen and subsequently image the resected area to visually confirm the complete removal of a tumor. While this has mostly been done using fluorescence imaging, alternatively, specimens identified through CLI could be imaged, removed and confirmed ex vivo. An early example used [90Y-DOTA0,Tyr3]-octreotide (90Y-DOTATOC) for the initial identification and margin identification and then the subsequent CL imaging of the tumor post resection to confirm removal.25 Unfortunately, this work did not image the patient’s brain post tumor resection to confirm accurate removal as a best practice for CLI guided tumor resection but clearly shows the CLI technique can be applied to nearly any clinical radiotracer whether for imaging alone or for therapy as well. Subsequent studies have shown a specimen analyzer under development for ex vivo imaging of CL in the operating theater (Fig. 4a-b). CLI was compared to traditional radiographic, γ-probe, and histology. Using 18F-FDG as the probe for sentinel lymph node mapping and biopsy CLI was found to confirm location of hot lymph nodes during breast conserving surgery and identified more hot nodes than through the use of the γ-probe.26,27 A commercial device allowed the imaging of excised tissue during breast conserving surgery (Fig. 4c), and other CLI clinical trials were described recently.26
Figure4.
Cerenkov luminescence instrumentation for clinical CLI. CAD drawing of a CLI specimen analyzer (A), where the tissue to be images resides in holder (red). Schematic of clinical CLI specimen analyzer (B) showing CCD (1), focusing lens (2), reflex mirror (3), CMOS reference camera (4), specimen holder region (5), lead shield for CCD (6), focal region (7), reference camera lens (8), filter wheel for spectral analysis (9), and LED light source for white light images (10). Merged white light and CLI image during breast conserving surgery shows activity containing regions during lymph node mapping (C) with blue line as feature margin of specimen. Endoscope schematic outlining the necessary components to produce a Cerenkov endoscope (D) containing a fiber bundle, light lamp, and biopsy channel attached to an EMCCD. Resulting images during Cerenkov endoscopy (E) with a patient receiving 18FDG in a human pilot trial where Cerenkov images (top row) show greater accumulation of 18FDG in the tumor, and CLI image overlaid with optical image (bottom row) to aid in tumor removal and fiducial marker identification. (A-C) Adapted with permission from Grootendorst et al.23 (D-E) Adapted with permission from Hu et al.25
Beyond superficial imaging of lymph nodes and thyroid uptake, a clinical endoscope CLI (ECLI) system (Fig. 4d) was tested in patients bearing 18F-FDG positive colorectal tumors.28 The ECLI system utilized an EMCCD camera, clinical flexible fiberscope and an optical adapter and was able to detect a 1.35-fold and significant (p=0.01) increase in luminescence signal with 18F-FDG positive colorectal lesions compared to normal tissue in the same patient (Fig 4e). The endoscope prepared was able to detect as little as 5μCi in phantom studies and showed very good correlation (r2=0.9779) of signal intensity with activity titration. This endoscope was inserted through the working channel of a clinical colonoscope and used in contact mode only. The authors mention however that despite the linearity and ability to detect the lesions in patients, the total acquired signal of the endoscope was approximately nine times lower than traditional CLI setups. The reduction in signal was likely due to reduced light from the distal focusing optic and loss in the fiber bundle.
Non-Radioactive Cerenkov Imaging
Beyond radionuclide administration, external beams can provide sufficient energy to produce CL in tissue. Bombardment of tissue by a LINAC beam can produce a focused source of Cerenkov photons that is independent of any radionuclide administration. One implementation is Cerenkov excited luminescence scanned imaging (CELSI) where an intensified CCD camera is used to observe Cerenkov photons produced within the LINAC beam path in tissue.35 Under this configuration, imaging of ~1mm inclusions at 20mm below the surface could be achieved at a feasible radiation dose of 0.06cGy. This imaging modality has been used to excite PtG4, an oxygen sensitive phosophorescent probe, enabling cerenkov mediated imaging of oxygen stores in lymph nodes. Furthermore, it has been explored as a means to estimate the surface dose in patients undergoing local or whole body irradiation.36–38
Recently proton therapy has been identified as another imaging source that produces CL.39 Compared to traditional radionuclides that require a 0.262MeV energy for CL photon production in water, protons are much heavier and require over 482MeV directly.19 However, CL has been observed from 60MeV proton beams due to coulombic ionization, fast electrons, generated from a prompt gamma, and a slow component due to the emission of a positron from a radioactive species.39 A CCD camera used in conjunction with a proton beam could not only to aid in dose estimation, but could be also used for imaging the area undergoing therapy. Initially it was shown that visible light can be generated in water and in an acrylic block from a proton beam, and in addition the combination with fluorescein in water increased the luminescence observed.40 This was attributed to CL, however a recent report shows the emission profile to be different from CL41 raising the question if CL is generated or if material-specific phenomena are driving the light emission. Nonetheless, the application of optical imaging to proton beam imaging could greatly aid dose deposition and estimation in the clinical setting.
Therapeutic Applications of CL
In addition to biomedical imaging, CL has been applied to photodynamic therapy (PDT) for the treatment of cancer. PDT utilizes light to activate photosensitizer to generate singlet oxygens and radical species that kill cancerous cells. In the traditional PDT, red or infrared light from external light source is used for photoactivation. However, since it is difficult to deliver the light to deep tumor site from outside, placing an optical fiber into the patient’s body is required. To circumvent this invasive approach, CL from radiotracers has been used to break the depth limitation of PDT. Kotagiri et al. combined the PDT nanoparticles with CL from radionuclides, 18F-FDG and 64Cu, for Cerenkov radiation induced therapy (CRIT).42,43 Titanium dioxide was activated by CL to generate radical species that kill cancerous cells. The nanoparticles were targeted to tumors by transferrin coating to the surface and the CL induced radical formation regressed tumors completely, though co-injection of PDT nanoparticles with nonradioactive material did not show any significant effect on tumor growth. Furthermore, Hartl et al. reported that CR from 90Y induced PDT using clinically relevant porphyrin photosensitizers.44 Therefore, CR is a viable method to deliver light into deep tumor sites for the activation of photosensitizers without invasive steps.
Conclusions
The Cerenkov effect has become an area of great interest in both basic and applied sciences. In the medical imaging field, CL from clinical radiotracers provides an easy to use system that can take advantage of traditional PET imaging radiotracers in addition to others used in radiotherapy. CLI has advanced rapidly from a standalone pre-clinical system to several clinical trials and clinical instruments in the past 8 years. PET and CL imaging techniques serve as compatible imaging methods using identical radiotracers and could provide physicians options to use PET for initial identification and subsequently CLI for removal of tissue in the operating theater.45 The instrumentation available now both for preclinical and clinical imaging put CLI in a position to accelerate basic diagnostic methods at a fraction of time and cost of current nuclear imaging modalities. Further development of smart activatable reagents and instrumentation methods for CLI will accelerate preclinical and clinical radiotracer research that can respond to local biological features. The availability of Cerenkov endoscopes will enable researchers and surgeons alike to go beyond the surface with CLI and image deep into tissues of interest. In less than a decade, CLI has emerged from a fledgling imaging technology to clinical trials providing critical information to surgeons in the operating theater.
Acknowledgements
This work was supported by the US National Institutes of Health grant R01CA183953 (JG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bolotovskii BM. Vavilov – Cherenkov radiation: its discovery and application. Physics-Uspekhi. 2009;52(11):1099–1110. doi:10.3367/UFNe.0179.200911c.1161. [Google Scholar]
- 2.Das S, Grimm J, Thorek DLJ. Cerenkov Imaging. Vol 124 1st ed. Elsevier Inc.; 2014. doi:10.1016/B978-0-12-411638-2.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thorek DL, Robertson R, Bacchus WA, et al. Cerenkov imaging - a new modality for molecular imaging. Am J Nucl Med Mol Imaging. 2012;2(2):163–173. http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3477724&tool=pmcentrez&rendertype=abstract. [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson R Optical imaging of Cerenkov light generation from positron-emitting radiotracers Optical imaging of Cerenkov light generation from positron-emitting radiotracers. PhysMed Biol. 2009;54:N355–N365. doi:10.1088/00319155/54/16/N01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beattie BJ, Thorek DLJ, Schmidtlein CR, Pentlow KS, Humm JL, Hielscher AH. Quantitative Modeling of Cerenkov Light Production Efficiency from Medical Radionuclides. PLoS One. 2012;7(2):e31402. doi:10.1371/journal.pone.0031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boschi F, Calderan L, Ambrosio DD, et al. In vivo 18 F-FDG tumour uptake measurements in small animals using Cerenkov radiation. Eur J Nucl Med Mol Imaging. 2011;38:120–127. doi:10.1007/s00259-010-1630-y. [DOI] [PubMed] [Google Scholar]
- 7.Imaging G, Avenue OS. In vivo Cerenkov luminescence imaging : a new. Phil Trans R Soc A. 2011;369:4605–4619. doi:10.1098/rsta.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaffer TM, Pratt EC, Grimm J. Utilizing the power of Cerenkov light with nanotechnology. Nat Nanotechnol. 2017;12(2):106–117. doi:10.1038/nnano.2016.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratt EC, Shaffer TM, Grimm J. Nanoparticles and radiotracers : advances toward radionanomedicine. WIREs Nanomed Nanobiotechnol. 2016;8(6):872–890. doi:10.1002/wnan.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ruggiero A, Holland JP, Lewis JS, Grimm J. Cerenkov Luminescence Imaging of Medical Isotopes. J Nucl Med. 2010;51(7):1123–1130. doi:10.2967/jnumed.110.076521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liberti MV, Locasale JW. The Warburg Effect : How Does it Bene fi t Cancer Cells ? Trends Biochem Sci. 2016;41(3):211–218. doi:10.1016/j.tibs.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvaresi EC, Hergenrother PJ. Glucose conjugation for the specific targeting and treatment of cancer. Chem Sci. 2013;4(6):2319–2333. doi:10.1039/C3SC22205E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu H, Ren G, Miao Z, et al. Molecular Optical Imaging with Radioactive Probes. PLoS One. 2010;5(3):e9470. doi:10.1371/journal.pone.0009470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan J, Il G, Park S, et al. Luminescence imaging using radionuclides : a potential application in molecular imaging ☆. Nucl Med Biol. 2011;38(3):321–329. doi:10.1016/j.nucmedbio.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Dothager RS, Goiffon RJ, Jackson E, Harpstrite S. Cerenkov Radiation Energy Transfer ( CRET ) Imaging : A Novel Method for Optical Imaging of PET Isotopes in Biological Systems. 2010;5(10):1–7. doi:10.1371/journal.pone.0013300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorek DLJ, Ogirala A, Beattie BJ, Grimm J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat Med. 2013;19(10):1345–1350. doi:10.1038/nm.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ran C, Zhang Z, Hooker J, Moore A. In vivo photoactivation without “light”: Use of cherenkov radiation to overcome the penetration limit of light. Mol Imaging Biol. 2012;14(2):156–162. doi:10.1007/s11307-011-0489-z. [DOI] [PubMed] [Google Scholar]
- 18.Das S, Haedicke K, Grimm J. Cerenkov-activated sticky tag for in vivo fluorescence imaging. J Nucl Med. 2017;117:198549. doi:10.2967/jnumed.117.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.L’Annunziata MF. Introduction: Radioactivity and Our Well-Being In: Radioactivity. Amsterdam: Elsevier Science B.V.; 2007:1–45. doi:https://doi.org/10.1016/B978-044452715-8.50003-7. [Google Scholar]
- 20.Gill RK, Mitchell GS, Cherry SR. Computed Cerenkov luminescence yields for radionuclides used in biology and medicine. Phys Med Biol. 2015;60(11):4263–4280. doi:10.1088/0031-9155/60/11/4263. [DOI] [PubMed] [Google Scholar]
- 21.Beattie BJ, Thorek DL, Schmidtlein CR, Pentlow KS, Humm JL, Hielscher AH. Quantitative modeling of Cerenkov light production efficiency from medical radionuclides. PLoS One. 2012;7(2):e31402. doi:10.1371/journal.pone.0031402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chin PTK, Welling MM, Meskers SCJ, Valdes Olmos RA, Tanke H, van Leeuwen FWB. Optical imaging as an expansion of nuclear medicine: Cerenkov-based luminescence vs fluorescence-based luminescence. Eur J Nucl Med Mol Imaging. 2013;40(8):1283–1291. doi:10.1007/s00259-013-2408-9. [DOI] [PubMed] [Google Scholar]
- 23.Thorek DL, Riedl CC, Grimm J. Clinical Cerenkov luminescence imaging of (18) F-FDG. J Nucl Med. 2014;55(1):95–98. doi:10.2967/jnumed.113.127266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spinelli AE, Ferdeghini M, Cavedon C, et al. First human Cerenkography. In: Vol (18) SPIE; 2013:3. [DOI] [PubMed] [Google Scholar]
- 25.Spinelli AE, Schiariti MP, Grana CM, Ferrari M, Cremonesi M, Boschi F. Cerenkov and radioluminescence imaging of brain tumor specimens during neurosurgery. In: Vol 21 SPIE; 2016:4. [DOI] [PubMed] [Google Scholar]
- 26.Grootendorst MR, Cariati M, Kothari A, Tuch DS, Purushotham A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin Transl Imaging. 2016;4(5):353–366. doi:10.1007/s40336-016-0183-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grootendorst MR, Cariati M, Pinder SE, et al. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery Using 18F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J Nucl Med. 2017;58(6):891–898. doi:10.2967/jnumed.116.181032. [DOI] [PubMed] [Google Scholar]
- 28.Hu H, Cao X, Kang F, et al. Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: first human results. Eur Radiol. 2015;25(6):1814–1822. doi:10.1007/s00330-014-3574-2. [DOI] [PubMed] [Google Scholar]
- 29.MOORE GE. Fluorescein as an Agent in the Differentiation of Normal and Malignant Tissues. Science (80- ). 1947;106(2745):130–131. doi:10.1126/science.106.2745.130-a. [DOI] [PubMed] [Google Scholar]
- 30.Holland JP, Normand G, Ruggiero A, Lewis JS, Grimm J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol Imaging. 2011;10(3):1–3–186. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3083828/pdf/nihms243512.pdf. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu H, Carpenter CM, Jiang H, et al. Intraoperative imaging of tumors using Cerenkov luminescence endoscopy: a feasibility experimental study. J Nucl Med. 2012;53(10):1579–1584. doi:10.2967/jnumed.111.098541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spinelli AE, Kuo C, Rice BW, et al. Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt Express. 2011;19(13):1260–512618. doi:10.1364/OE.19.012605. [DOI] [PubMed] [Google Scholar]
- 33.Li C, Mitchell GS, Cherry SR. Cerenkov luminescence tomography for small-animal imaging. Opt Lett. 2010;35(7):1109–1111. doi:10.1364/OL.35.001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu Z, Liang J, Yang W, et al. Experimental Cerenkov luminescence tomography of the mouse model with SPECT imaging validation. Opt Express. 2010;18(24):24441–24450. doi:10.1364/OE.18.024441. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R, D’souza A V, Gunn JR, et al. Cherenkov-excited luminescence scanned imaging. Opt Lett. 2015;40(5):827–830. doi:10.1364/OL.40.000827. [DOI] [PubMed] [Google Scholar]
- 36.Andreozzi JM, Zhang R, Glaser AK, Jarvis LA, Pogue BW, Gladstone DJ. Camera selection for real-time in vivo radiation treatment verification systems using Cherenkov imaging. Med Phys. 2015;42(2):994–1004. doi:10.1118/1.4906249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pogue BW. Molecular Imaging through centimeters of tissue: High resolution imaging with Cerenkov excitation In: Frontiers in Optics 2015. OSA Technical Digest (online). San Jose, California: Optical Society of America; 2015:FW5E.1. doi:10.1364/FIO.2015.FW5E.1. [Google Scholar]
- 38.Zhang R, Andreozzi JM, Gladstone DJ, et al. Cherenkoscopy based patient positioning validation and movement tracking during post-lumpectomy whole breast radiation therapy. Phys Med Biol. 2015;60(1):L1–L14. doi:10.1088/0031-9155/60/1/L1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Helo Y, Kacperek A, Rosenberg I, Royle G, Gibson AP. The physics of Cerenkov light production during proton therapy. Phys Med Biol. 2014;59(23):7107–7123. doi:10.1088/0031-9155/59/23/7107. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto S, Toshito T, Okumura S, Komori M. Luminescence imaging of water during proton-beam irradiation for range estimation. Med Phys. 2015;42(11):6498–6506. doi:10.1118/1.4932630. [DOI] [PubMed] [Google Scholar]
- 41.Darafsheh A, Taleei R, Kassaee A, Finlay JC. The visible signal responsible for proton therapy dosimetry using bare optical fibers is not Čerenkov radiation. Med Phys. 2016;43(11):5973–5980. doi:10.1118/1.4964453. [DOI] [PubMed] [Google Scholar]
- 42.Kotagiri N, Sudlow GP, Akers WJ, Achilefu S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat Nanotechnol. 2015;10(4):370–379. doi:10.1038/nnano.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grimm J Therapy from within. Nat Nanotechnol. 2015;10(4):299–300. doi:10.1038/nnano.2015.63. [DOI] [PubMed] [Google Scholar]
- 44.Hartl BA, Hirschberg H, Marcu L, Cherry SR, Clinic M. Radiation Generated from Yttrium-90. 2017;35(2):185–192. doi:10.1615/JEnvironPatholToxicolOncol.2016016903.Activating. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grimm J Cerenkov Luminescence Imaging BT - Imaging and Visualization in The Modern Operating Room: A Comprehensive Guide for Physicians. In: Fong Y, Giulianotti PC, Lewis J, Groot Koerkamp B, Reiner T, eds. New York, NY: Springer New York; 2015:107–120. doi:10.1007/978-1-4939-2326-7_8. [Google Scholar]