SUMMARY
Interferon-induced proteins with tetratricopeptide repeats (IFIT) inhibit infection of many viruses by recognizing their RNA, but the mechanisms regulating their remain unclear. We report a crystal structure of cap 0 (m7GpppN)-RNA-bound to human IFIT1 in complex with the C-terminal domain of human IFIT3. Structural, biochemical, and genetic studies suggest that IFIT3 binding to IFIT1 has dual regulatory functions: (a) extending the half-life of IFIT1, which increases its steady-state levels in cells and (b) allosterically regulating the IFIT1 RNA-binding channel, which enhances the specificity of recognition for cap 0 but not cap 1 (m7GpppNm) or 5′-ppp RNA. Mouse Ifit3 lacks this key C-terminal domain and does not bind mouse Ifit1. The IFIT3 interaction with IFIT1 was important for restricting infection of viruses lacking 2′-O methylation in their RNA cap structures. Our experiments establish differences in regulation of IFIT1 orthologs and define targets for modulating the activity of human IFIT proteins.
INTRODUCTION
To restrict viral infection, host cells must recognize invasion and elicit a rapid inhibitory response. Pathogen-associated molecular patterns (PAMPs) in viruses include single-stranded and double-stranded nucleic acids that are detected by pattern recognition receptors (PRRs) such as Toll-like receptors (TLR3, TLR7, TLR8, and TLR9), RIG-I-like receptors (RIG-I and MDA5), and DNA sensors (cGAS, DAI, and IFI16) (Cai et al., 2014; Kawai and Akira, 2006; Keating et al., 2011; Leung and Amarasinghe, 2016). PRR binding of viral PAMPs triggers signaling cascades that induce the expression of immunomodulatory and antiviral genes including type I interferons (IFN). Type I IFNs signal in autocrine and paracrine manners to induce hundreds of interferon-stimulated genes (ISGs), including IFN-induced proteins with tetratricopeptide repeat (IFIT) genes (Diamond and Farzan, 2013).
IFIT family members regulate immune responses and restrict viral infections through a variety of mechanisms, including the restriction of viral RNA translation (Diamond and Farzan, 2013; Zhou et al., 2013). There is substantial species variation in the IFIT locus; for example, the human IFIT locus encodes five family members (IFIT1, IFIT1B, IFIT2, IFIT3, and IFIT5), whereas the mouse locus encodes six genes including Ifit1, Ifit2, and Ifit3 and several less well characterized paralogs (Ifit1b, Ifit1c, and Ifit3b). IFIT proteins are structurally related and contain tandem copies of helix-turn-helix tetratricopeptide repeat (TPR) motifs (Abbas et al., 2017; Abbas et al., 2013). However, IFIT orthologs vary in sequence, numbers, and organization of TPR motifs (Diamond and Farzan, 2013; Zhou et al., 2013) (Fig S1A and B). A recent study highlights the functional differences of IFIT1 gene orthologs; human IFIT1 and mouse Ifit1 varied in their ability to inhibit viruses with distinct mRNA cap structures (Daugherty et al., 2016).
Several IFIT family members recognize PAMPs on the 5′ end of viral RNA (Daffis et al., 2010; Hyde and Diamond, 2015; Hyde et al., 2014; Kumar et al., 2014). Canonical eukaryotic capping of mRNA involves modification of RNA 5′ through a series of enzymatic steps. The 5′ cap structure contains an inverted N-7 methylated guanosine linked to the mRNA moiety by a triphosphate bridge (m7GpppN, cap 0 structure). In higher eukaryotes, additional methylation of mRNA occurs at the 2′-O position of the first ribose sugar (m7GpppNm, cap 1 structure) via a nuclear 2′-O methyltransferase enzyme. Because some IFIT family members (e.g., mouse Ifit1) directly recognize cap 0 RNA, host 2′-O methylation serves to distinguish self and non-self RNA (Daffis et al., 2010; Werner et al., 2011; Zust et al., 2011).
Although human IFIT1 inhibits infection of viruses by reducing cap-dependent protein translation (Habjan et al., 2013; Hyde et al., 2014; Pichlmair et al., 2011), mouse Ifit1 selectively inhibits viruses lacking 2′-O-methylation of their mRNA 5′ caps (Daffis et al., 2010; Daugherty et al., 2016). IFIT1 and Ifit1 also may sense and sequester uncapped 5′-ppp viral RNA (Habjan et al., 2013; Pichlmair et al., 2011). Studies with Ifit1−/− mouse fibroblasts and myeloid cells show enhanced replication of vesicular stomatitis virus (VSV) despite normal production of type I IFN and other inflammatory cytokines. In vivo, Ifit1−/− mice are more vulnerable to infection with VSV, with higher virus-induced mortality observed (Pichlmair et al., 2011). This result has not been corroborated, as subsequent experiments reveal no difference in mortality between Ifit1−/− and WT mice after infection with VSV (Fensterl et al., 2012) or other negative strand RNA viruses (Pinto et al., 2015) that display 5′-ppp moieties on their genomic RNA.
IFIT family members also engage in protein-protein interactions, including interactions with IFIT proteins and other host defense molecules (Habjan et al., 2013; Pichlmair et al., 2011). For example, IFIT2 and IFIT3 interactions with IFIT1 reportedly enhance the antiviral activity of IFIT1 (Habjan et al., 2013; Pichlmair et al., 2011). Consistent with this observation, gene silencing of IFIT1, IFIT2, or IFIT3 results in increased replication of Rift valley fever (RVFV), VSV, and influenza A (IAV) viruses (Fensterl et al., 2012; Schmeisser et al., 2010), whereas ectopic expression of IFIT1, IFIT2, or IFIT3 independently has less inhibitory effect (Pichlmair et al., 2011). Although IFIT-IFIT complexes are hypothesized as necessary for optimal antiviral effects, their regulatory mechanisms remain unclear.
In vitro studies suggest that IFIT3 has antiviral and/or immunomodulatory activity. Ectopic expression of human IFIT3 in human A549 cells results in decreased VSV and encephalomyocarditis (EMCV) infection and reciprocally, gene silencing results in modestly increased viral titers (Schmeisser et al., 2010). Analogous results were seen with porcine reproductive and respiratory syndrome and swine influenza viruses after ectopic expression of porcine IFIT3 (Li et al., 2015; Zhang et al., 2013). Silencing of IFIT3 in human A549 cells also results in increased dengue virus infection, and this phenotype is associated with greater cell death (Hsu et al., 2013). IFIT3 also may regulate innate immune responses, as its ectopic expression enhanced IRF3-mediated gene induction (Liu et al., 2011) via an interaction with TBK1 and MAVS on mitochondria, and silencing of human IFIT3 results in diminished STAT1 phosphorylation in human astrocytoma cells (Imaizumi et al., 2016). To date, no studies have described an antiviral effect of mouse Ifit3, which is shorter than other species and lacks 87 amino acids in the C-terminus relative to human IFIT3.
Despite their postulated contribution to the host cell antiviral response, the molecular basis and functional consequences of IFIT-IFIT interactions remain undetermined. Here, we have reported a 2.55 Å heterotrimeric crystal structure of 5′-cap 0 RNA-bound to human IFIT1 in complex with C-terminal residues of IFIT3, which provides insight into a regulatory role for IFIT-IFIT interactions. Our structural studies, combined with biochemical, genetic, and biophysical experiments, reveal how IFIT3 binding has dual regulatory functions for IFIT1. Binding of the C-terminal domain of IFIT3 enhanced IFIT1 protein stability and modulated the IFIT1 RNA-binding pocket conformation, which resulted in preferential recognition of cap 0 RNA compared to IFIT1 alone. To validate the functional significance of IFIT1-IFIT3 interaction, we have shown that the infectivity of viruses lacking 2′-O methylation of their 5′ RNA caps was inhibited by IFIT1 only when IFIT3 was co-expressed. Collectively, these results define how IFIT-IFIT interactions enable selective recognition of non-self RNA to inhibit viral infections and help to explain evolutionary differences in IFIT gene structure and function across species.
RESULTS
Human IFIT3 binds to human IFIT1
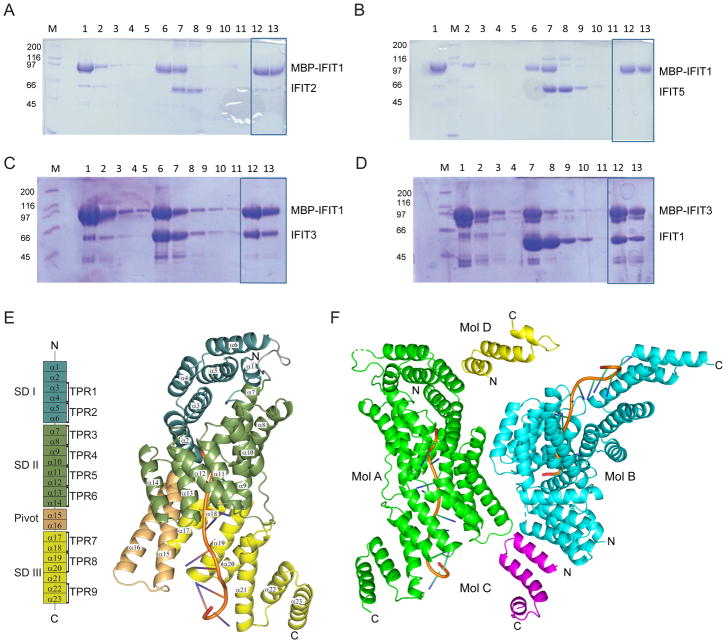
Prior mass spectrometry studies have identified a multimeric complex of human IFIT proteins that may regulate the cap recognition and the antiviral activity of IFIT1 (Habjan et al., 2013). We performed biochemical studies to define which IFIT proteins directly bind to IFIT1. We generated a series of constructs including IFIT2, IFIT3, and IFIT5 and evaluated binding to maltose binding protein (MBP)-tagged IFIT1 via in vitro co-precipitation assays. Only IFIT3, and not IFIT2 or IFIT5, bound strongly to MBP-IFIT1 (Fig 1A–D and Fig S1C–D). Reversal of the MBP tag did not affect the IFIT3-IFIT1 interaction, as MBP-IFIT3 bound to native IFIT1 (Fig 1C–D). Subsequently, we engineered N-terminal and C-terminal truncated versions of IFIT3 (IFIT3 1–403 (IFIT3ΔCTD) and IFIT3 403–490 (IFIT3CTD)) and tested for interaction with MBP-tagged IFIT1. Because a minimal IFIT3CTD, but not IFIT3ΔCTD, retained binding to IFIT1 (Fig S1E–F), the C-terminal 87 amino acids of IFIT3 are necessary and sufficient for IFIT1-IFIT3 interaction.
Figure 1. Structure of the RNA-bound IFIT1-IFIT3CTD complex.
Co-precipitation assays between IFIT1 and (A) IFIT2, (B) IFIT5, and (C–D) IFIT3. MBP-tagged IFIT proteins were incubated with amylose resin (lane 1) and unbound MBP-tagged protein was washed (lanes 2–5). Prebound MBP-tagged IFIT (lane 6) was incubated with untagged IFIT proteins (lane 7) prior to washes (lane 8–11) and final beads (lane 12). Bound protein was eluted with maltose-containing buffer (lane 13). Final beads and eluted samples (boxed) were assessed for binding. Data shown are representative of at least two independent experiments. (E) Structure and domain organization of IFIT1 bound to 5′ cap 0 RNA (left), including location of TPR regions. Cartoon representation of IFIT1 colored by subdomain (right). (F) Cartoon representation of IFIT1-FIT3-ap 0 RNA heterotrimeric complex structure: asymmetric unit shows IFIT1 molecule A (green), IFIT1 molecule B (cyan) bound to IFIT3CTD molecule C (magenta), IFIT3CTD molecule D (yellow), and 5′ cap 0 RNA (orange). See also Figure S1 and S2.
To assess for species-specific variation in IFIT1-IFIT3 interactions, we evaluated the reciprocal binding of human (IFIT1 and IFIT3) with murine (Ifit1 and Ifit3) proteins because the human IFIT3CTD is absent in the murine Ifit3 ortholog (Fig S1A). In co-precipitation assays, IFIT3 failed to interact with Ifit1 (Fig S1G), and Ifit3 did not bind Ifit1 (Fig S1H) or IFIT1 (Fig S1I). However, a chimeric construct Ifit3-IFIT3CTD bound to IFIT1 (Fig S1J). These results indicate species-specific differences in IFIT-IFIT interactions; the C-terminal domain of human IFIT3 interacts with human IFIT1 whereas the mouse Ifit3 ortholog lacks the C-terminus and does not bind appreciably to either IFIT1 or Ifit1.
The C-terminal domain of IFIT3 interacts with IFIT1 bound to RNA
To define the molecular determinants of IFIT1-RNA and IFIT1-IFIT3 binding, an X-ray crystal structure of cap 0 RNA-bound IFIT1-IFIT3CTD was solved at 2.55 Å resolution in the P1 space group (Fig 1 and Table 1). The overall IFIT1 structure in our complex was similar to the previously described IFIT1 and RNA-bound IFIT1 structures (PDB 4HOU and PDB 5W5H). IFIT1 is comprised of 23 α-helices, which form subdomain (SD)I (α1-α6), SDII (α7-α14), Pivot (α15-α16), and SDIII (α17-α23). The subdomains arrange to form a central RNA-binding channel accommodating a single-stranded A-form RNA with 10 nucleotides and a 5′ cap 0 structure (Fig 1E). In the crystal structure, we also observed two heterotrimeric complexes, each consisting of cap 0 RNA-bound IFIT1-IFIT3CTD complexes (Fig 1F). IFIT3CTD residues 417–460 form three α-helices and three potential interaction interfaces with IFIT1, which we termed Interfaces 1, 2, and 3 (Fig S2). Interactions along Interface 1 are predominantly hydrophobic between IFIT3CTD (molecule C) and IFIT1 molecule B helices of SDI (Fig S2A) with a buried surface area 290 Å2. In contrast, Interface 2 consists of three hydrogen bonds and hydrophobic interactions between IFIT3CTD and IFIT1 Pivot helices (α15-α16) of molecule A and results in a buried surface area 173 Å2 (Fig S2B). Analysis of symmetry mates suggested an alternative interface between IFIT3CTD (molecule C) and symmetry mate molecule B’ (Fig S2C). This symmetry related Interface 3 results in the largest buried surface area of approximately 1,045 Å2 and is comprised of 5 hydrogen bonds and numerous hydrophobic interactions between IFIT3CTD and IFIT1 helices (α20-α23) of SDIII (Fig S2C).
Table 1.
Data Collection, Structure Solution, and Refinement Statistics
| Data Collection | ||
|---|---|---|
| Space Group | P1 | |
| Unit cell parameters | a, b, c (Å) | 51.96, 80.50, 88.06 |
| α, β, γ (°) | 80, 80, 90 | |
| Resolution range (Å) | 50.00 - 2.55 (2.59 – 2.55) | |
| Unique reflections | 45147 (1762) | |
| Redundancy | 3.4 (2.9) | |
| Completeness (%) | 90.9 (75.6) | |
| Wilson B factor | 18.2 | |
| I/Iσ | 13.59 (3.19) | |
| CC1/2 last shell | (0.87) | |
|
| ||
| Phase Determination, Structure Solution, and Refinement | ||
|
| ||
| Figure of merit | 0.8036 | |
| Resolution (Å) | 50.00 - 2.55 (2.59 – 2.55) | |
| No. of reflections | 45147/2330 | |
| 1762/89 | ||
| Completeness (%) | 90.9 (75.6) | |
| Total No. of non-hydrogen atoms | 3745 | |
| Rwork/Rfree (%) | 18.0/23.8 | |
| RMS deviations | Bond lengths (Å) | 0.014 |
| Bond angles (°) | 1.719 | |
| B-factors (Å2) | Protein | 39.2 (chain A) |
| Protein | 37.7 (chain B) | |
| Protein | 48.9 (chain C) | |
| Protein | 45.2 (chain D) | |
| Protein | 62.4 (chain E) | |
| Protein | 60.7 (chain F) | |
| Water | 34.3 | |
| Ramachandran plot outliers (%) | 0.00% | |
| Average B, all atoms (Å2) | 40.3 | |
| Molprobity Clashscore, all atoms | 4.43 (99th percentile) | |
| Molprobity Score | 1.89 (90th percentile) | |
Values in parentheses are for the shell with the highest resolution.
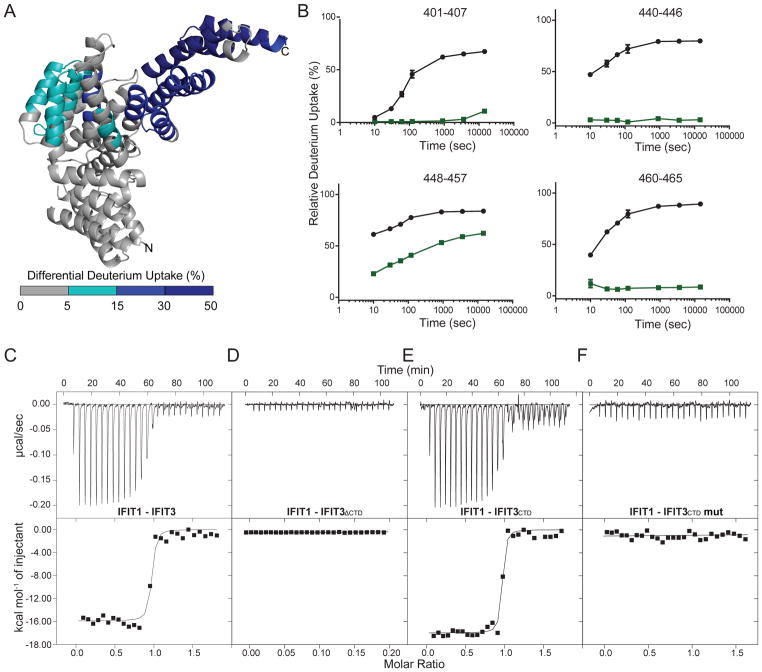
To validate the interaction interface between IFIT1 and IFIT3, we performed Hydrogen-Deuterium Exchange with Mass Spectrometry (HDX-MS). A series of samples, including IFIT1, IFIT3, and IFIT1-IFIT3 with and without cap 0 RNA were evaluated for structural perturbations upon complex formation, as determined by differential hydrogen-deuterium exchange rates. In each experiment, we used IFIT1 or IFIT3 alone as a comparison to generate differential exchange rates upon binding. Upon IFIT3 binding, differential deuterium uptake of IFIT1 was observed with protection of the SDII helices (α11–14~ 5–15%) and SDIII helices (α17–23; ~ 25–70%) (Fig 2A). The C-terminal peptides of Interface 3 (residues 401–465) of IFIT1 were protected from deuterium uptake by as much as 70% (Fig 2B). In contrast, we observed minimal perturbation of HDX rates for Interfaces 1 and 2 (Fig S3A; peptides 48–83 and 70–96, and 302–323, respectively). Notably, IFIT3 C-terminal peptides (residues 419–458) were protected (20–60%) from deuterium uptake when bound to IFIT1, further confirming the IFIT3CTD critical binding site. All other regions of IFIT1 and IFIT1-RNA complexes bound to IFIT3 showed no difference in hydrogen-deuterium exchange (Fig S3A).
Figure 2. Biophysical validation of the IFIT1-IFIT3 interface using hydrogen-deuterium exchange-mass spectrometry (HDX-MS) and isothermal titration calorimetry (ITC).
(A) The molecular interface between IFIT1 and IFIT3 is defined by HDX-MS. Differences in deuterium uptake induced by IFIT3 binding are displayed as a color gradient (see legend) and highlighted in the cartoon representation of IFIT1. (B) Comparison of deuterium uptake kinetic curves of IFIT1 (black) and IFIT1-IFIT3 (green). Representative ITC raw data and binding isotherm for (C) IFIT1 and IFIT3. Measured values are KD = 1.5 ± 2.0 nM, ΔH = − 1.3 ± 0.02 × 104 cal/mol, ΔS = − 2.19 cal/mol/deg, and n (no. of sites) = 0.93 ± 0.01. (D) IFIT1 and IFIT3ΔCTD. KD = not determined. (E) IFIT1 and IFIT3CTD. Measured values are KD = 1.4 ± 3.1 nM, ΔH = − 1.7 ± 0.06 × 104 cal/mol, ΔS = − 2.08 cal/mol/deg., and n (number of sites) = 0.97 ± 0.03. (F) IFIT1 and IFIT3CTD K426A-E439A-L445A-S451A-I453A-F457A mutant (IFIT3 CTD mut). KD = not determined. All experiments were performed at least four independent times. See also Figure S3 and S4.
To corroborate these findings, we determined the dissociation constant for complex formation for full-length IFIT1 bound to IFIT3, IFIT3CTD, and IFIT3 lacking C-terminal residues 403–490 (IFIT3ΔCTD). Isothermal titration calorimetry defined a high affinity interaction between IFIT1 and IFIT3 (KD: 1.5 ± 2.0 nM) and 1:1 binding stoichiometry (n = 0.93 ± 0.01) (Fig 2C). IFIT3ΔCTD did not bind to IFIT1, whereas IFIT3CTD bound with similar affinity as full-length IFIT3 (Fig 2D–E).
We next analyzed interface contributions computationally using HyPare analysis (Fig S4A–B), which calculates association and disassociation rates between IFIT1 and IFIT3 based on structural analysis. These results identified several amino acids (K426, E439, L445, S451, I453, and F457A) with the highest likelihood of affecting IFIT3 binding to IFIT1. To test these predictions, we generated a mutant IFIT1 protein with all six residues changed; this mutant protein folded correctly, as determined by circular dichroism spectroscopy (Fig S4C), but failed to bind IFIT3, as predicted (Fig 2F). Collectively, these results suggest that IFIT3CTD interacts with IFIT1, and Interface 3 is required for this interaction.
RNA binding induces conformational changes in IFIT1
We next utilized HDX-MS with IFIT1 alone or in complex with cap 0 RNA to identify regions of differential hydrogen-deuterium exchange and define the RNA binding channel on IFIT1 in solution (Fig 1E). SDII, Pivot, and SDIII helices form a positively charged, RNA-binding channel in IFIT1 and were protected (>30%) from deuterium uptake in solution (Fig 3A and Fig S3B); these regions interact with the triphosphate bridge and RNA strand via electrostatic interactions. This channel is occupied by cap 0 single-stranded RNA with a total of ten nucleotides spanning the groove and the extended C-terminal region of SDIII. In contrast, the N-7 methyl guanosine binding pocket was comprised of mostly hydrophobic interactions (Fig S4D).
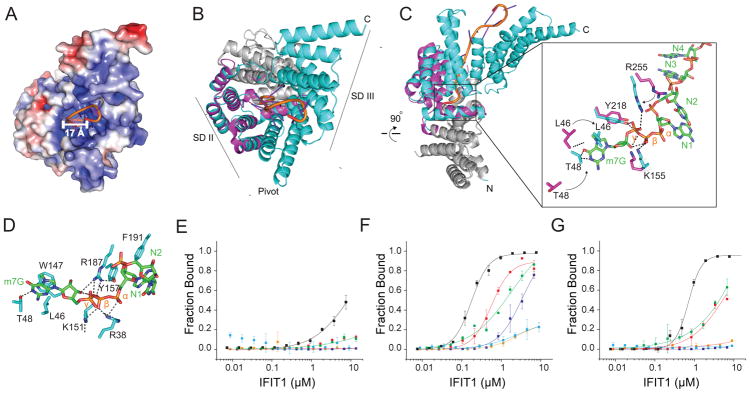
Figure 3. Molecular determinants of RNA recognition by IFIT1.
(A) Surface electrostatic potential representation of IFIT1 (−10 kTe−1; red to +10 kTe−1; blue) with cap 0 RNA (cartoon). Diameter of the IFIT1 binding channel is indicated by the white bar. (B) Structural alignment of IFIT1 (PDB 4HOU; magenta) and cap 0 RNA bound-IFIT1 (cyan) (RMSD = 0.8 Å). (C) SDII residues that undergo conformational change upon cap 0 RNA binding are shown as stick representation. (D) IFIT1-cap 0 RNA direct binding residues are shown as stick representation. (E–G) Filter binding assays measuring IFIT1 (black), IFIT1 R187A-Y218A (purple), IFIT1 L46A-T48A (red), IFIT1 Y157A-F191A (green), IFIT1 R38A-K151A (orange), and IFIT1 W147A (blue) binding to (E) 5′-ppp, (F) cap 0, or (G) cap 1 RNA. Data shown are representative of at least two independent experiments. See also Figure S4.
Comparison of our IFIT1-IFIT3-cap 0 RNA structure to a crystal structure of the N-terminal residues (8–277) of IFIT1 (Abbas et al., 2013) revealed an overall similar structure (Root Mean Square Difference (RMSD = 0.8 Å)). However, a number of conformational changes were observed. In particular, helices in the SDII region rearranged to make direct contact with the RNA and formed a more compact structure (Fig 3B). In the IFIT1-IFIT3-cap 0 RNA complex structure, triphosphate binding residues R255, Y218, and K155 shifted more than 4 Å to make electrostatic interactions with RNA (Fig 3C). In addition, the SDII helices, which form the cap-binding pocket, rearrangeed to fit the N7-methyl guanosine cap (Fig 3C), with flexible loop residues L46 and T48 moving ~6 Å (Fig 3C). Additional residues along the RNA-binding channel did not undergo conformation changes, but nonetheless contacted the cap 0 RNA. For example, W147 makes π–π stacking interactions with N7-methyl guanosine moiety, and R38, K151, and Y157 coordinate the triphosphate bridge to the ribose of the guanosine nucleoside (Fig 3D).
IFIT3 modulates RNA recognition by IFIT1
To determine the functional significance of the channel residues of IFIT1 for RNA binding, we tested structure-guided IFIT1 mutants for their ability to recognize 5′-ppp, cap 0, and cap 1 RNA in filter-binding assays (Fig 3E–G). Initially, we determined the binding affinity of wild-type (WT) IFIT1 for differentially capped versions of 10 nucleotide single-stranded RNA. WT IFIT1 bound cap 0 RNA (KD = 175 ± 8 nM) more avidly than cap 1 RNA (KD = 710 ± 4.4 nM) or 5′-ppp RNA (KD = 8,500 ± 500 nM). Alanine mutations in cap-binding loop residues L46A and T48A resulted in minor changes for cap 0 and cap 1 RNA recognition (6 to 7-fold reduction, P < 0.07), whereas an alanine substitution at the m7G-binding residue W147 abrogated binding to cap 0 and cap 1 RNA (2,000 to 3,000-fold reduction, P < 0.05). Alanine mutations of R38, K151, and R187, which surround the triphosphate bridge, also disrupted binding (2,000 to 3,000-fold reduction, P < 0.05) of cap 0 and cap 1 RNA. In contrast, mutation of other residues (e.g., Y157 and F191) within the binding channel had minimal impact on binding. These results suggest that IFIT1 interactions with both the guanosine cap and triphosphate bridge are critical for RNA binding. To address whether IFIT1 interacts with IFIT3 is an RNA-independent manner, we tested the ability of IFIT3 to bind to an IFIT1 mutant (R38A and K151A) that is unable to bind cap 0 RNA (Fig 3D and (Kumar et al., 2014)). The IFIT1 RNA binding mutant (R38A and K151A) bound to IFIT3 with an affinity (2.1 ± 6 nM) similar to that of WT IFIT1 (Fig S4E).
To begin to define the regulatory role of IFIT3 in IFIT1 RNA recognition, we compared our structure to the published 5′-ppp RNA bound to IFIT5 (Abbas et al., 2013), cap 0 RNA-bound dimeric IFIT1, and cap 0 RNA bound monomeric IFIT1 L457E and L464E (Abbas et al., 2017). Although the secondary structures of IFIT1 and IFIT5, dimeric IFIT1, and IFIT1 L457E and L464E are similar (RMSD = 1.47 Å, 0.45 Å, and 0.55 Å, respectively), differences between IFIT5-5′-ppp RNA and IFIT1-IFIT3-cap 0 RNA were apparent. IFIT5 and IFIT1 SDIII helices diverge up to ~22.5 Å, and IFIT1 SDII and Pivot subdomains create a more closed channel around cap 0 RNA (Fig 4A). Similarly, structural comparisons of IFIT1-IFIT3-cap 0 RNA and dimeric IFIT1-cap 0 RNA structures revealed conformational changes of IFIT1 SDIII helices to form a more compact channel upon IFIT3 binding (Fig S5A). In contrast, the IFIT1 SDIII helices move from the channel compared to monomeric IFIT1 L457E and L464E-cap 0 RNA structure; these conformational changes suggest that the oligomeric state of IFIT1 and IFIT3 modulates IFIT1 interaction with the RNA (Fig S5B).
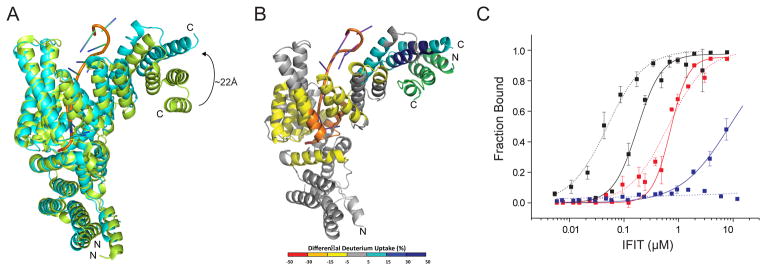
Figure 4. IFIT1-RNA interaction is modulated by IFIT3 binding.
(A) Comparison of 5′-ppp RNA-bound IFIT5 (green) (PDB 4HOR) and IFIT1 bound to cap 0 RNA and IFIT3CTD (cyan; (primary PDB validation is provided), current structure). (B) Structural changes on IFIT1 upon IFIT3 binding as defined by HDX-MS. Differences in deuterium uptake induced by IFIT3 binding to IFIT1-cap 0 RNA complex are displayed as a color gradient (see legend) and highlighted in the cartoon representation of IFIT1. Data shown are representative of two independent experiments. Multiple peptides were identified by MS/MS, and only the ten highest abundant peptides were used in the analysis. (C) Quantitative filter binding assay for IFIT1 (solid) and IFIT1-IFIT3CTD (dotted) and cap 0 RNA (black), cap1 RNA (red), and 5′-ppp RNA (blue). The results are the average of at least three independent experiments. Error bars represent standard error of the mean (SEM). See also Figure S5.
To gain insight into the regulatory role of IFIT3 for IFIT1, HDX-MS analysis was performed for IFIT1-cap 0 RNA and IFIT1-IFIT3-cap 0 RNA complexes. As expected, the IFIT1 SDIII interface was protected by IFIT3 binding. The helices along the IFIT1-RNA binding channel showed an increased rate of deuterium uptake (~5–30%) as a result of IFIT3 binding (Fig 4B), which suggests an additional allosteric impact of IFIT3. To define the functional role of IFIT3 in the allosteric control of IFIT1, RNA-binding experiments were performed for the IFIT1-IFIT3 complex and IFIT1 alone. These experiments demonstrated an increase in binding affinity for cap 0 RNA for the IFIT1-IFIT3 complexes (KD = 49.1 ± 5.7 nM) compared to free IFIT1 (KD = 175 ± 8.3 nM) (Fig 4C). In comparison, there was no major impact of IFIT3 binding on IFIT1-cap 1 RNA recognition, as IFIT1 and IFIT1-IFIT3 bound cap 1 RNA with KD values of 710 ± 4.4 nM and 641 ± 4.9 nM, respectively. Under similar experimental conditions, IFIT1 weakly bound 5′-ppp RNA (KD = 8,500 ± 500 nM) whereas, no appreciable 5′ ppp RNA binding was observed after IFIT1-IFIT3 complex formation. This suggests that IFIT3 binding allosterically regulates the IFIT1 RNA-binding channel and leads to enhanced and preferential recognition of cap 0 RNA.
Selective restriction of viruses lacking 2′-O methylation requires IFIT1 and IFIT3 co-expression
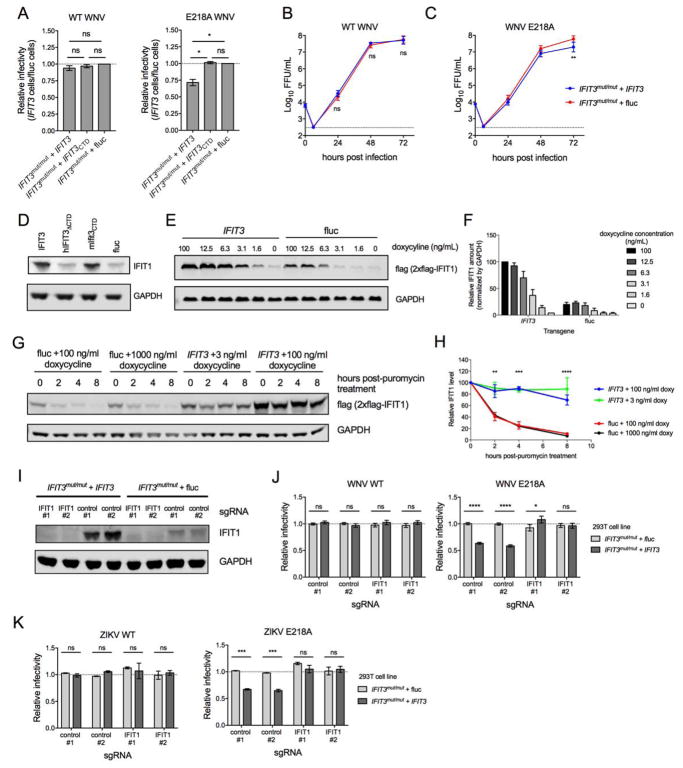
We next explored the impact of IFIT3 on IFIT1 binding to cap 0 RNA on infection of isogenic WT and mutant viruses containing or lacking 2′-O methylation of their genomic RNA. To first investigate the antiviral effects of IFIT3, we generated 293T cells lacking IFIT3 by CRISPR/Cas9 gene editing (Fig S6A, herein called IFIT3mut/mut), and then trans-complemented them with IFIT3, IFIT3ΔCTD, or a control gene, firefly luciferase (fluc) (Fig S6A–B). Subsequently, we infected the 293T-IFIT3mut/mut + IFIT3, + IFIT3ΔCTD, and + fluc cells with cDNA clone-derived WNV WT (New York 1999 strain) or WNV NS5 E218A, a mutant lacking 2′-O methyltransferase activity, that produces a genomic viral RNA with a cap 0 structure (Daffis et al., 2010). Following infection, we measured the percentage of WNV-infected cells at 24 h post-infection (hpi) by flow cytometry. Notably, WNV WT infection in 293T-IFIT3mut/mut + IFIT3, 293T-IFIT3mut/mut + IFIT3ΔCTD, and 293T-IFIT3mut/mut + fluc cells was not significantly different (P > 0.2), whereas WNV NS5 E218A infection in 293T-IFIT3mut/mut + IFIT3 cells was reduced relative to 293T-IFIT3mut/mut + IFIT3ΔCTD or 293T-IFIT3mut/mut + fluc cells (P < 0.02 for both comparisons; Fig 5A and Fig S6C). Virus production in the supernatants of 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells revealed a similar pattern: growth of WNV NS5 E218A but not WNV WT was reduced in 293T-IFIT3 compared to 293T-fluc cells (Fig 5B–C). These data suggest that IFIT3 specifically restricts viruses lacking 2′-O methylation of their RNA caps, and that the antiviral effect depends on the C-terminal tail. One limitation in interpreting these studies is that we independently observed differential expression of IFIT1 in IFIT3mut/mut cells trans-complemented with IFIT3 or luciferase after exogenous IFN-β treatment (Fig 5D). An increase in IFIT1 expression in IFIT3-expressing cells depended on the presence of the C-terminal domain of IFIT3, as IFIT3mut/mut + IFIT3ΔCTD cells had similar IFIT1 amounts as the fluc control, and IFIT3mut/mut cells trans-complemented with chimeric mouse IFIT3 having the human C-terminal domain appended resulted in similar IFIT1 amounts as IFIT3mut/mut + IFIT3 cells (Fig 5D). Thus, the antiviral effect of IFIT3 on WNV NS5 E218A could be multifactorial, whereby IFIT3 modulates the cap recognition specificity of IFIT1 (see Fig 4C) and independently affects IFIT1 mRNA induction or protein stability and overall expression. To address whether the effect on IFIT1 by IFIT3 was due to transcriptional activation, we transduced IFIT3 or fluc into 293T cells expressing a flag-tagged version of IFIT1 under a heterologous doxycycline-inducible promoter (293T-IFIT1-doxy cells). At baseline (0 ng/ml of doxycycline, low but equivalent amounts of IFIT1 were detected in IFIT3- and fluc-expressing cells (Fig 5E–F). Following doxycycline treatment, IFIT1 amounts were higher in IFIT3-expressing cells relative to fluc cells, indicating that the IFIT3-mediated increase in IFIT1 expression was independent of endogenous IFIT1 gene transcription. To test whether IFIT1 protein stability was enhanced by IFIT3, we performed pulse-chase studies in 293T-IFIT1-doxy cells + IFIT3 or + fluc with doxycycline, followed by puromycin treatment to halt new translation. In the absence of IFIT3, IFIT1 degraded rapidly (t1/2 of 1.9 hours) (Fig 5G–H). However, in the presence of IFIT3 expression, the half-life of IFIT1 was prolonged substantially (t1/2 of 25.9 hours; for IFIT3 + 100 ng/mL doxy vs fluc + 100 ng/mL, P = 0.002 at 2 h, P < 0.0001 at 4 and 8 h; additionally, all IFIT3 vs fluc comparisons for all doses were significantly different at 2, 4, and 8 h, P < 0.05). Thus, IFIT3 binding stabilizes IFIT1, which correlates with the greater steady-state levels of IFIT1 observed.
Figure 5. Effect of IFIT3 expression on infection of WNV WT and WNV NS5-E218A.
IFIT3 gene edited (IFIT3mut/mut) 293T cells were trans-complemented with IFIT3 (IFIT3mut/mut + IFIT3), IFIT3 lacking the C-terminal tail (IFIT3mut/mut + IFIT3CTD), or firefly luciferase (IFIT3mut/mut + fluc). (A) 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells were infected at an MOI of 5 with WNV WT or isogenic WNV NS5-E218A lacking 2′-O methylation of the cap structure of genomic RNA. Infection was measured 24 hpi by flow cytometry by staining for intracellular WNV E protein. The fraction of infected 293T-IFIT3mut/mut + IFIT3 cells relative to infected 293T-IFIT3mut/mut + fluc are shown for both viruses. Data are the mean of three independent experiments, and error bars represent SEM. (B–C) 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells were infected with WNV WT or WNV NS5-E218A at an MOI of 0.001. Supernatant was collected at the indicated times after infection and analyzed by focus-forming assay. Data are the mean titers from six independent experiments, and errors bars represent SEM. (D) IFIT3mut/mut + IFIT3, IFIT3mut/mut + hIFIT3ΔCTD, IFIT3mut/mut + mIfit3CTD, and IFIT3mut/mut + fluc cells were stimulated with IFN-β and assessed for IFIT1 expression by Immunoblotting. One representative experiment of three is shown. (E–F) 293T cells expressing human IFIT1-flag under an inducible promoter (293T-IFIT1-doxy) were trans-complemented with IFIT3 or fluc and analyzed for IFIT1 expression (anti-flag tag) by Immunoblotting. (E) One of two representative Immunoblots is shown. (F) IFIT1 amounts were normalized to GAPDH in IFIT3 or fluc-transduced cells treated with doxycycline. Error bars represent SEM from two independent experiments. (G–H) 293T-IFIT1-doxy cells trans-complemented with IFIT3 or fluc were stimulated with doxycycline for 16 h at indicated concentrations, subsequently treated with 50 μM puromycin to arrest translation, and analyzed for IFIT1 (anti-flag tag) at the indicated time post-puromycin treatment. (G) One of three representative Immunoblots is shown. (H) IFIT1 protein amounts were quantified and normalized to the 0 h post-puromycin treatment. Error bars represent SEM from three independent experiments. The statistical significance shown is for the comparison of IFIT3 + 100 ng/ml doxy vs fluc + 100 ng/ml doxy; comparisons between other doses yielded similar results. (I-K) CRISPR-Cas9 gene editing was used to abolish IFIT1 expression from 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells using two different IFIT1-targeting sgRNAs or as a control, scrambled sgRNAs. (I) IFIT1 expression following IFN-β stimulation was determined by Immunoblot. (J–K) 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells that received IFIT1 or scrambled sgRNAs were infected with WNV WT or WNV NS5-E218A at an MOI of 5 (J) or WT or NS5-E218A ZIKV (Cambodia, 2010; strain FSS13025) at an MOI or 1 (K) and scored for infectivity at 24 (J) or 30 (K) hpi by flow cytometry. Infectivity was normalized to the fraction of infected 293T-IFIT3mut/mut + fluc cells that received scrambled sgRNAs. The mean relative infectivity from three (K) and five (J) independent experiments is shown, and error bars represent the SEM. In this Figure, statistical significance was determined using an ANOVA followed by Tukey’s (A, B, and C) or Sidak’s (H, and J–K) multiple comparisons tests (ns, not significant; *, P > 0.05; ***, P <0.001; ****, P < 0.0001). See also Figure S6.
To determine whether the antiviral effect of IFIT3 required IFIT1 expression, we abrogated IFIT1 expression completely in 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells using CRISPR/Cas9 gene editing and two IFIT1-specific single-guide RNAs (sgRNA) or scrambled sgRNAs as controls (Fig 5I, IFIT1 and control lanes). We infected these cells with WNV WT or WNV NS5 E218A and measured the percentage of WNV-infected cells at 24 hpi by flow cytometry. Notably, WNV WT infected 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc similarly, regardless of whether IFIT1 was expressed (Fig 5J, left panel). WNV NS5 E218A showed reduced infectivity of 293T-IFIT3mut/mut + IFIT3 cells compared to 293T-IFIT3mut/mut + fluc cells when scrambled sgRNAs were used. However, infection of WNV NS5 E218A was similar in 293T-IFIT3mut/mut + IFIT3 cells and 293T-IFIT3mut/mut + fluc cells when IFIT1 was gene-edited and not expressed (Fig 5J, right panel, and Fig S6D). Similar results were observed following infection of 293T-IFIT3mut/mut + IFIT3 and 293T-IFIT3mut/mut + fluc cells with ZIKV NS5 E218A (Fig 5K). These data suggest that IFIT3-dependent restriction of viruses lacking 2′-O methylation of their 5′ RNA caps requires expression of IFIT1.
Inhibitory effect of IFIT1 against viruses lacking 2′-O methylation is augmented by IFIT3
To further assess whether IFIT1-mediated restriction of viruses lacking 2′-O methylation is enhanced by co-expression of IFIT3, we introduced IFIT3 or fluc expression plasmids into the 293T cells that inducibly express IFIT1 (see Fig 5E). One day after transfection, cells were treated with doxycycline to induce IFIT1 expression, and 16 h later infected with WNV WT or WNV NS5 E218A. Doxycycline-induced expression of IFIT1 failed to restrict infectivity of WNV WT, and ectopic expression of IFIT3 did not modulate this (Fig 6A). Thus, increases in IFIT1 expression mediated by IFIT3 (see Fig 5E–H) were insufficient to inhibit infection of WNV displaying cap 1 (2′-O methylated) mRNA structures. In contrast, IFIT1 restricted WNV NS5 E218A infection in a doxycycline dose-dependent manner (Fig 6B). Moreover, relative to fluc-transfected cells, IFIT3-expressing cells required less doxycycline and IFIT1 induction (Fig 5E) to restrict infection of WNV NS5-E218A (Fig 6B and Fig S6E). Thus, IFIT1 restriction of WNV NS5-E218A is enhanced by the expression of IFIT3.
Figure 6. Effect of IFIT3 on IFIT1-mediated restriction of viruses with cap 0 and cap 1 RNA.
(A, B and E) 293T-IFIT1-doxy cells were transfected with plasmids encoding fluc, IFIT3, or the indicated IFIT3 mutants, and treated with doxycycline prior to infection with (A) WNV WT or (B and E) WNV NS5 E218A. Infection and transfection data were analyzed by flow cytometry 24 hpi (A and B, left panels; and also see Fig S6E) to determine the percentage of transfected cells that were positive for intracellular WNV E protein. Data are representative of three independent experiments, and error bars represent SEM. (A and B, right panels, and E) Data are normalized to infectivity of doxycycline-untreated cells for each independent transfection (IFIT3, IFIT3 mutants, or fluc) experiment. Errors bars represent the SEM, and data was pooled from three to six independent experiments for statistical analysis (below). (C–D) 293T-IFIT1-doxy cells were trans-complemented with IFIT3 or fluc and infected with (C) VEEV-TC83 (MOI of 1, 16 h) or (D) VSV (MOI of 1, 6 h). Left panels show the mean percentage of infected cells from one representative experiment, and error bars indicate the SEM from triplicate replicates. Right panels show data normalized to infectivity of doxycycline-untreated cells. Errors bars represent the SEM, and data was pooled from three independent experiments. In this Figure, statistical significance was determined using a t-test (A) or an ANOVA followed by Sidak’s (B, C, and D) or Dunnett’s (E) multiple comparisons tests (ns, not significant; *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001). See also Figure S6.
To corroborate and extend these findings, we assessed the effect of IFIT1 and IFIT3 co-expression on Venezuelan equine encephalitis virus (VEEV) strain TC83, a positive-stranded RNA alphavirus that naturally has a cap 0 RNA but lacks a secondary structure element in the 5′ untranslated region that functions to antagonize Ifit1 restriction (Hyde et al., 2014). Doxycycline-induced expression of IFIT1 failed to restrict infectivity of VEEV-TC83 in cells trans-complemented with fluc (Fig 6C). Analogously, IFIT3 expression in the absence of IFIT1 induction had little effect on VEEV-TC83 infection (Fig 6C, 0 ng/ml doxycycline treatment). However, co-expression of IFIT3 with doxycycline-induced IFIT1 resulted in markedly reduced VEEV-TC83 infection (Fig 6C and Fig S6F). In comparison, vesicular stomatitis virus (VSV), a negative-sense RNA virus with a 5′-ppp genomic RNA and a cap 1 mRNA (Rhodes and Banerjee, 1975), also was restricted by IFIT1 in an IFIT3-dependent manner (Fig 6D), although this inhibition was less (~50% reduction) compared to VEEV-TC83 or WNV E218A (~90–98% reduction) (Fig 6B and C). These data confirm a role for IFIT3 in enhancing IFIT1-mediated restriction of viruses lacking 2′-O methylation as well as establishing a partial effect on a negative strand RNA virus. This latter effect might reflect the enhanced expression of IFIT1 in IFIT3-expressing cells.
Our structural data suggested that the C-terminal tail of IFIT3 (residues 403–490) was required for IFIT1-IFIT3 binding, with IFIT3 residues K426, E439, L445, S451, I453, and F457 mediating part of the physical interaction. Accordingly, we tested the functional effect of C-terminal tail mutations on IFIT3-dependent enhancement of IFIT1 restriction of WNV NS5 E218A. When the C-terminal tail of IFIT3 was deleted (IFIT3ΔCTD), IFIT3 no longer enhanced IFIT1 restriction of WNV NS5 E218A (Fig 6E). When alanine mutations were introduced at residues K426, E439, L445, S451, I453, and F457 of IFIT3, expression was maintained but modulation of IFIT1-mediated viral restriction was lost (Fig 6E and Fig S6G). However, when mutations at K426 and E439 alone were introduced, IFIT1-mediated viral restriction was retained (Fig 6E). Remarkably, transfection of the IFIT3 tail alone (IFIT3CTD) enhanced IFIT1 function similar to full-length IFIT3 (Fig 6E). These data confirm a functional role for residues in the C-terminal tail of IFIT3 in regulating IFIT1-mediated restriction of WNV E218A, a virus lacking 2′-O methylation on its genomic RNA.
DISCUSSION
In the present study, we evaluated the molecular mechanisms and the corresponding functional impacts of the interaction between IFIT1 and IFIT3. We report an X-ray crystal structure of cap 0 RNA-bound IFIT1 in complex with IFIT3CTD. The IFIT3CTD observed in this structure is a region of the protein that is absent in the murine ortholog, Ifit3. The high-affinity interaction of the C-terminal domain of IFIT3 enhances IFIT1 protein stability in cells and allosterically modulates the IFIT1 RNA-binding channel and SDIII, resulting in preferential recognition of cap 0 RNA. We determined the functional importance of an IFIT1-IFIT3 interaction in the context of restriction of WNV, ZIKV, and VEEV that lack 2′-O methylation of the cap structures on their genomic RNA. Our results also reveal key differences between human and mouse IFIT1 and IFIT3 orthologs, which impact their ability to preferentially recognize RNA moieties and inhibit viruses lacking 2′-O methyltransferase activity. These findings have implications for how differences in IFIT protein family members across species restrict viral infections.
The IFIT family of proteins functions as antiviral molecules by virtue of their recognition of non-self RNA (5′-ppp and cap 0 RNA), inhibition of cap-dependent and cap-independent translation, and modulation of immune responses (Diamond et al., 2013). Although the IFIT protein family shares conserved features throughout mammalian phylogeny, gene duplication and sequence variation occurs within individual mammalian species, perhaps due to evolutionary pressures by viral pathogens (Daugherty et al., 2016). Indeed, recent studies of mammalian IFIT1 and IFIT3 proteins have suggested functional differences among orthologs and paralogs with regard to their RNA-binding specificity and antiviral restriction. Differences in the specificity of IFIT1 and Ifit1 for specific RNA moieties may be due to their descent from distinct paralogs (Daugherty et al., 2016). Mouse Ifit1 discriminates between cap 0 and cap 1 mRNA, and specifically inhibits viral mutants lacking 2′-O methylation of their mRNAs. Although human IFIT1B is a nonfunctional allele, it is more closely related to primate and rabbit IFIT1B orthologs that have RNA binding specificity similar to mouse Ifit1 (Daugherty et al., 2016). In comparison, human IFIT1 in isolation appears to lack discrimination between cap 0 and cap 1 RNA. Our data represents an explanation for this apparent paradox of why human IFIT1 alleles (IFIT1 and IFIT1B) lacked the ability to recognize and inhibit viruses with cap 0 RNA structures (Daugherty et al., 2016): human IFIT1 requires interaction with the C-terminal domain of IFIT3 to alter its RNA ligand specificity and bind viral RNA lacking 2′-O methylation. Studies are planned to assess whether the C-terminal domain of IFIT3 analogously binds IFIT1B and alters its RNA binding properties.
Although structural studies have been performed with individual IFIT proteins and RNA, these experiments did not evaluate the effect of IFIT-IFIT and IFIT-heterologous protein interactions, which have been described biochemically (Habjan et al., 2013; Pichlmair et al., 2011). We found that the C-terminal region of IFIT3 binds IFIT1 and allosterically modulates the structure of the IFIT1 RNA-binding channel. In the structure, we observed conformational changes within the RNA binding pocket that were supported by our HDX-MS data. In addition to changes in the specific side chains in the RNA binding channel, we also noted several structural shifts when comparing our IFIT1-IFIT3-RNA complex to a published IFIT1-RNA complex (Abbas et al., 2017). Collectively, these data support a model, where interaction between IFIT1 and IFIT3 resulted in an increase in affinity of IFIT1 binding to cap 0 but not cap 1 or 5′-ppp RNA compared to IFIT1 alone, supporting a role for enhanced specificity. The binding of the C-terminal region of IFIT3 enhanced the selective restriction by human IFIT1 of cap 0-expressing WNV NS5 E218A but not cap 1-expressing WNV WT. Although our biochemical and functional data establish a role for IFIT3-mediated regulation of IFIT1 binding of cap 0 RNA with antiviral consequences, IFIT3-dependent effects on IFIT1 expression likely also contribute to the IFIT1-dependent antiviral phenotype. Experiments with non-native doxycycline-inducible promoter suggested that IFIT3-dependent regulation of IFIT1 did not occur transcriptionally. Rather, pulse-chase studies indicate that binding of the C-terminal region of IFIT3 prolonged the half-life of IFIT1, which resulted in increased steady-state levels. Additional studies are needed to determine precisely how IFIT3 binding modulates IFIT1 stability, for example, whether it prevents ubiquitination and proteasomal degradation of IFIT1.
Previous studies have shown that ectopic expression of IFIT3 can restrict infection of multiple viruses including PRRSV and IAV (Li et al., 2015; Schmeisser et al., 2010; Zhang et al., 2013). The antiviral effect of IFIT3 was believed to be indirect through interactions with other IFIT proteins or host defense molecules, as direct binding of IFIT3 to RNA has not been described (Habjan et al., 2013; Kumar et al., 2014; Pichlmair et al., 2011). For example, IFIT3 reportedly enhances IFN-β promoter activity (Li et al., 2015; Zhang et al., 2013) and promotes cell survival following infection (Hsu et al., 2013). IFIT3 may bind to MAVS and TBK-1, resulting in the induction of IRF3- and NF-κB-responsive genes (Li et al., 2015; Liu et al., 2011). We also observed an antiviral effect of IFIT3 upon ectopic expression, but this occurred only for flavivirus mutants lacking 2′-O methylation of their 5′ caps. The antiviral effect of IFIT3 in this context was mediated via IFIT1, as gene editing of IFIT1 abolished the inhibitory activity of ectopically expressed IFIT3. These data support a model in which IFIT3 can have an indirect antiviral role, through forming a complex and enhancing the ability of IFIT1 to bind and sequester cap 0 RNA to restrict virus replication. IFIT3-IFIT1 interactions mediated a partial antiviral effect on VSV, which has both cap 1 mRNA and genomic negative-sense 5′-ppp RNA; these, results are consistent with prior studies (Daugherty et al., 2016; Pichlmair et al., 2011). While further studies are needed to determine whether VSV infection is inhibited by IFIT1-IFIT3 by virtue of IFIT1 recognition of VSV mRNA or genomic RNA, WNV-WT, which has a cap 1 mRNA, was not inhibited under the same conditions. However, when cellular IFIT1 expression was increased substantially through transfection of an expression IFIT1 plasmid under the control of an EF1a promoter, infection of WNV WT was inhibited, and this effect did not require IFIT3 co-expression (data not shown). These results are consistent with earlier in vitro studies suggesting that IFIT1 can globally inhibit cap-dependent translation (Hui et al., 2003). Beyond this, it remains to be determined whether the other antiviral functions of IFIT3, which are mediated through MAVS and TBK-1, also require interactions with other IFIT proteins.
The C terminal tail of IFIT3 (residues 403–490) was necessary and sufficient to mediate the IFIT1-IFIT3 interaction and enhance IFIT1-mediated virus restriction. This observation has implications for the functional variability of IFIT orthologs, such as murine Ifit3, which lack the C terminal tail. This finding is consistent with our observation that Ifit3 did not bind to murine Ifit1 or human IFIT1, although appending residues 403–490 of human IFIT3 to the end of murine Ifit3 resulted in efficient binding of murine Ifit3 to human IFIT1. This data helps to explain the species-specificity of IFIT1-IFIT2-IFIT3 complex formation, which was not observed for mouse Ifit1-Ifit3 (Habjan et al., 2013). Moreover, this observation is consistent with a study of porcine IFIT3, which showed the C terminal tail was required for its antiviral effects against PRRSV. (Li et al., 2015); however, that study did not explore the IFIT1-dependence of this phenotype.
The lack of murine Ifit1-Ifit3 interaction raises questions as to how murine Ifit1 mediates specificity of RNA cap binding and the function of Ifit3. If murine Ifit3 does not participate in IFIT-IFIT protein interactions, what is the role of Ifit3 and its nearly identical paralog, Ifit3b, in host defense? Although the N-terminal region of human IFIT3 reportedly interacts with TBK1 and MAVS to regulate IRF3 activation (Liu et al., 2011) and is conserved among gene orthologs, a corresponding function of murine Ifit3 has not yet been ascribed. These questions, coupled with our results, suggest that IFIT proteins may have multiple aspects of control, with some of the regulation dependent on IFIT-IFIT interactions for preferential recognition of non-self RNA. Future studies that assess IFIT-IFIT and IFIT-protein interactions may define additional regulatory mechanisms for control of host-defense pathways and virus infections.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Request for data or reagents should be directed and will be fulfilled by the lead author, Gaya K. Amarasinghe; gamarasinghe@wustl.edu; 314-286-0619
EXPERIMENTAL MODEL AND SUBJECT DETAILS
IFIT protein expression constructs
The coding regions of IFIT1 (NCBI accession number NP_001539.3), IFIT2 (NCBI accession number NP_001538.4), IFIT3 (NCBI accession number NP_001540.2), IFIT5 (NCBI accession number NP_036552.1), ifit1(NCBI accession number NP_032357.2), and ifit3 (NCBI accession number NP_034631.1) were codon optimized for expression in E. coli (Genscript) and used as templates to subclone the coding regions into a modified pET15b vector (Novagen). Mutations were generated by overlap PCR method and verified by sequencing.
Protein Expression and Purification
IFIT1-Ifit1 and IFIT3-Ifit3 proteins were expressed in BL21(DE3) E. coli cells (Novagen), cultured in Luria Broth media at 37°C, induced at an OD600 (optical density at 600 nm) of 0.6 with 0.5 mM IPTG, and grown for 12–15 h at 18°C. Cells were resuspended in lysis buffer containing 25 mM sodium phosphate (pH 7.5), 250 mM NaCl, 5 mM 2-mercaptoethanol, lysed using an EmulsiFlex-C5 homogenizer (Avestin) and clarified by centrifugation at 47,000 × g at 4°C for 40 min. Proteins were purified using sequential affinity and ion-exchange chromatographic columns (GE Healthcare). Following TEV protease digestion of the maltose binding protein (MBP) tag, the resulting sample was purified further by ion-exchange and size exclusion chromatography. Protein purity was assessed by Coomassie staining of SDS-PAGE and mass spectrometry.
RNA for structural studies
RNA was purchased from TriLink Biotechnologies (San Diego, CA) with 5′ m7Gppp AUA GGC GGC G 3′ sequence.
IFIT1-IFIT3-cap 0 RNA complex formation
Purified IFIT1 and IFIT3 were mixed in a 1:1.5 ratio followed by size exclusion chromatography. The complex was verified by SDS-PAGE and concentrated to 16 mg/mL. Complex and cap 0 RNA (10 nucleotide) were mixed 1:1.5 prior to crystal growth. Crystals were grown using the vapor diffusion hanging drop method. Crystals were vitrified in a solution containing the crystallization condition and 25% glycerol by plunge freezing into liquid nitrogen.
Data Collection and Structure Determination
Crystals were screened at Advanced Photon Source Beamline 19ID. Table 1 shows data collection and refinement statistics. Diffraction images were processed by HKL3000 (Otwinowski and Minor, 1997). PDB ID 4HOR was used as a search model, and IFIT1 residues were built by Buccaneer (Cowtan, 2006), manual building in COOT (Emsley and Cowtan, 2004), and refinement with REFMAC5 (Collaborative Computational Project, Number 4, 1994). The structure quality was assessed by MolProbity (Davis et al., 2007). Structure figures were prepared using PyMOL. Protein-protein interactions were analyzed using LigPlot+ (Laskowski and Swindells, 2011).
Isothermal titration calorimetry
Binding assays were performed on a VP-isothermal titration calorimeter (VP-ITC) (MicroCal). Protein samples were dialyzed against buffer (10 mM HEPES pH 7.5, 150 mM NaCl, and 2 mM Tris (2-carboxyethyl) phosphine (TCEP)) for 12 h at 4°C. Titrations were set up with 50–100 μM protein solution (IFIT3) in the syringe and 4–10 μM protein solution (IFIT1 or mutant IFIT1) in the cell. A reference power of 3 μcal/s was used, and the resulting isothermal titration calorimetry data were processed and fit to a one-site binding model to determine n (number of binding sites) and KD (dissociation constant) using ORIGIN 7.0 software.
Hydrogen deuterium exchange mass spectrometry (HDX-MS)
IFIT1, IFIT3, IFIT1-cap 0 RNA, IFIT1-IFIT3, and IFIT1-IFIT3-cap 0 RNA samples were buffer-exchanged with PBS pH 7.4. Deuterium labeling was initiated by diluting samples (50 μM, 2 μl) 10-fold with D2O buffer, or H2O buffer for samples measured for no-deuterium control. At different time intervals (10, 30, 60, 120, 360, 900, 3600, and 14400 s), the labeling reaction was quenched by rapidly adjusting the pH to 2.5 with 30 μl of quench buffer (3 M urea, 1% trifluoroacetic acid, H2O) at 4°C. Subsequently, the protein mixture was immediately injected into a custom-built HDX device and passed through a column containing immobilized pepsin (2 mm × 20 mm) at a flow rate of 100 μl/min in 0.1% formic acid, and the resulting peptic peptides were captured on a ZORBAX Eclipse XDB C8 column (2.1 mm × 15 mm, Agilent) for desalting (3 min). The C8 column was then switched in-line with a Hypersil Gold C18 column (2.1 mm × 50 mm, Thermo Fisher), and a linear gradient (4% to 40% acetonitrile, 0.1 % formic acid, 50 μ/min flow rate, over 5 min) was used to separate the peptides and direct them to a LTQ-FTICR mass spectrometer (Thermo Fisher) equipped with an electrospray ionization source. Valves, columns, and tubing for protein digestion and peptide separation were submerged in an ice-water bath to minimize back-exchange.
The resulting data were processed and peptides identified by exact mass analysis and LC–MS/MS using Mascot (Matrix Science). Raw HDX spectra and peptide sets also were submitted to HDX Workbench (Pascal et al., 2012) for calculation and data visualization in a fully automated fashion. Peptides for each run were assessed based on relative representation and statistical validation. Only the top 6 peptides from each MS scan were used in the final analysis. Deuterium uptake at each time point was calculated by subtracting the centroid of the isotopic distribution of the undeuterated peptide from the deuterated peptide. The relative deuterium uptake was plotted versus the labeling time to afford kinetic curves. For comparison between apo states and complex samples, differences in deuterium uptake following all incubation time points were calculated. Absolute differences in perturbation values larger than 5% D were considered significant. The 15-min time point was mapped onto the protein three-dimensional (3D) structure for data visualization.
Filter binding assays
RNA (cap 0, cap 1, and 5′-ppp) were radiolabeled with 32P-α-GTP using capping reaction kit (Cellscript) and purified by 12% urea-PAGE. Labeled RNAs (5 nM) were heated at 95°C for 5 minutes, annealed on ice, mixed with increasing concentrations of IFIT1, IFIT3, or IFIT1-IFIT3 complex in a 96-well plate and incubated for 15 min. Samples were applied to a 96-well dot blot apparatus (Whatman) with nitrocellulose and nylon membranes (Bio-Rad). The amounts of 32P-labeled RNAs present on nitrocellulose and nylon membranes were quantified by phosphoimager (GE Healthcare). Binding was calculated as the fraction of RNA bound to the nitrocellulose membrane compared to the sum of RNA bound to nitrocellulose and nylon membranes and plotted versus IFIT concentration using ORIGIN 7.0 software.
Circular dichroism studies
Wild type and mutant IFIT protein samples were prepared in 25 mM sodium phosphate (pH 7), 150 mM NaCl, 2 mM tris(2-carboxyethyl)phosphine (TCEP) at 10 μM. CD spectra were acquired in triplicate using a Chirascan CD spectrometer (Applied Photophysics). Wavelength scans from 200 to 280 nm were performed to monitor the change in molar ellipticity of each protein at 4°C and at a 40 nm/min scan rate.
Co-immunoprecipitation assays
Amylose resin was pre-equilibrated with buffer (10 mM HEPES pH 7.5, 150 mM NaCl, and 2 mM tris(2-carboxyethyl)phosphine (TCEP)) prior to the addition of purified MBP-tagged proteins. The resin was incubated for 10 min followed by washes and subsequent resuspension. Purified untagged proteins were applied to the resin and incubated for 20 min prior to washes and final resuspension and elution with buffer containing 1% (w/v) maltose. Samples were taken at each step and visualized by Coomassie staining of SDS-PAGE.
Cells
All cell lines were maintained at 37°C in the presence of 5% CO2. HEK-293T cells were passaged in Dulbecco’s Modified Eagle Medium (DMEM) (Invitrogen) supplemented with 10% fetal bovine serum (FBS) (Omega Scientific). Flp-In 293 T-REx cell lines were passaged in DMEM supplemented with 10% Tet System Approved FBS (Clontech Laboratories), 15 μg/mL blasticidin, and 200 μg/mL hygromycin B. Vero cells were passaged in DMEM supplemented with 5% FBS.
To create IFIT3 gene-edited cells lines, the sgRNA 5′-ACACCTAGATGGTAACAACG-3′ was cloned into the plasmid pSpCas9(BB)-2A-Puro (PX459) V2.0 (Addgene #62988). This plasmid was transfected into 293T cells using the FugeneHD transfection reagent, and 24 h later cells were treated with puromycin (2.5 ug/mL) for a three-day drug selection. A clonal cell line subsequently was isolated by limiting dilution and analyzed for IFIT3 loss-of-expression deletions by Sanger sequencing. For construction of 293T-IFIT3, 293T- IFIT3ΔCTD, and 293T-fluc cell lines in the background of the IFIT3mut/mut cell line, genes for IFIT3, IFIT3 lacking C-terminal residues 403–490 (IFIT3ΔCTD), or firefly luciferase (fluc) were cloned into the pFCIV lentivirus expression vector which expresses a GFP marker from an IRES promoter (Hope Center, Washington University). Lentiviruses were produced by transfecting HEK-293T cells with psPAX2 (Addgene #12260), pMD2.G (Addgene #12259), and pFCIV plasmids. The 293T IFIT3mut/mut cell line was trans-complemented after transduction with IFIT3, IFIT3ΔCTD, or fluc lentiviruses and then sorted based on GFP expression before analysis of IFIT3 expression by Immunoblot and flow cytometry. CRISPR-based gene-editing of IFIT1 from the 293T-IFIT3 and 293T-fluc cell lines was performed by cloning sgRNAs targeting IFIT1 or scrambled control sgRNA into pLentiCRISPR V2.0 (Addgene # 52961). sgRNA sequences used were 5′-ATGACAACCAAGCAAATGTG-3′ (IFIT1 sgRNA #1), 5′-CACTCCATTCTA-TAGCGGAA-3′ (IFIT1 sgRNA #2), 5′-ACGGAGGCTAAGCGTCGCAA-3′ (scrambled sgRNA #1), 5′-CGCTTCCGCGGCCCGTTCAA-3′ (scrambled sgRNA #2). Lentiviruses were produced by co-transfection of HEK-293T cells with a pLentiCRISPR plasmid and the packaging plasmids psPAX2 and pMD2.G. 293T-IFIT3 and 293T-fluc cells were transduced with lentiviruses and selected with puromycin for six days before downstream analysis.
A 293T cell line was constructed to inducibly express IFIT1 with an N-terminal 2× Flag tag under the control of a tetracycline inducible CMV promoter using the Flp-In 293 T-Rex system (Invitrogen) according to the manufacturer’s instructions. To create 293T-IFIT1-doxy cells that stably expressed IFIT3 or fluc, cells were transduced using the lentiviruses described above (pFCIV + IFIT3 or + fluc) and sorted based on GFP expression before analysis of IFIT3 or fluc expression by flow cytometry.
Virus production and infection
WNV-WT and WNV-NS5-E218A were propagated in BHK21-15 cells as previously described (Daffis et al., 2010; Zhou et al., 2007). ZIKV-WT and ZIKV-NS5-E218A were generated from an infectious cDNA clone of a Cambodian strain FSS13025 (2010) as previously described (Shan et al., 2016) and propagated in Vero cells. Viral titers were determined by focus-forming assay using Vero cells as previously described (Brien et al., 2013). VSV Indiana strain was propagated in BHK21 cells and titered by plaque assay on Vero cells. VEEV-TC83 was produced from an infectious cDNA clone in BHK21-15 cells and titered on Vero cells by focus forming assay, as previously described (Hyde et al., 2014).
For viral growth analysis, multistep growth curves were performed using a multiplicity of infection (MOI) of 0.01. For flow cytometric analysis, cells were infected with WNV (MOI 5, 24 h) ZIKV (MOI 1, 30 h), VSV (MOI 1, 6 h), or VEEV-TC83 (MOI 1, 16 h). Following infection, cells were fixed with 1% paraformaldehyde for 10 min at room temperature, permeabilized with 0.1% saponin in HBSS with 10 mM HEPES, and stained for viral antigen using virus-specific antibodies human WNV-E16 (Oliphant et al., 2005), mouse E60 (Oliphant et al., 2006), mouse ZV-2 (Zhao et al., 2016), mouse anti-VSV-G (Kerafast), or mouse anti-VEEV-3B4.C4 (Diamond laboratory, unpublished). Cells were then stained with Alexa Fluor 647-conjugated goat anti-human IgG or anti-mouse IgG.
For co-transfection and WNV infection experiments, 293T-IFIT1 cells were transfected with an pFCIV expression vector containing fluc with an N-terminal HA tag, IFIT3, IFIT3ΔCTD, IFIT3CTD, IFIT3 K426A-E439A-L445A-S451A-I453A-F457A, or IFIT3 K426A-E439A. One-day post-transfection, cells were treated with doxycycline at the indicated dose for 16 h before infection with WNV-WT or WNV-NS5-E218A (MOI 5, 24 h). Following fixation and permeabilization, cells were co-stained with human WNV-E16, mouse anti-IFIT3 (OTI1G1, Origene), or HA-tagged fluc (mouse anti-HA, R&D Systems). IFIT3CTD lacks the epitope recognized by anti-IFIT3 OTI1G1 and instead was monitored for GFP expression as a marker of transfection (rabbit polyclonal anti-GFP, Abcam). Cells then were stained with Alexa Fluor 647-conjugated goat anti-human IgG and Alexa Fluor 488-conjugated goat anti-mouse or anti-rabbit before analysis by flow cytometry.
Pulse-chase studies
Pulse-chase experiments were performed by stimulating 293T-IFIT1-doxy + IFIT3 and 293T-IFIT1-doxy + fluc cells with doxycycline for 16 hours, followed by treatment with 50 μM puromycin to arrest translation. At 0, 2, 4, and 8 h post-puromycin treatment, cell lysates were collected and analyzed for IFIT1 expression by Western blot using an anti-flag tag antibody.
Immunoblotting
Cells were disrupted using Radioimmunoprecipitation assay (RIPA) lysis and extraction buffer (Sigma-Aldrich) supplemented with protease inhibitor cocktail (Sigma-Aldrich). Immunoblots were performed using a rabbit polyclonal anti-IFIT1 (Thermo Fisher), rabbit anti-GAPDH (Cell Signaling Technology), rabbit polyclonal anti-IFIT3 (Fensterl et al., 2008), or mouse monoclonal anti-FLAG M2 (Sigma-Aldrich), followed by IRDye 680RD goat anti-rabbit or IRDye 800CW goat anti-mouse (LiCor). Blots were imaged using a LiCor Odyssey infrared imaging system.
Statistical analyses
Statistical analyses were performed using Prism software Version 7.0 for Mac OS X (GraphPad Software). For flow cytometry experiments, relative infectivity values were compared by student’s t-test when comparing two samples or, for comparisons of more than two samples, by two-way ANOVA followed by a Sidak’s, Dunnett’s, or Tukey’s correction for multiple comparisons where indicated. For Immunoblot quantification, relative protein amounts were compared by two-way ANOVA followed by a Sidak’s correction for multiple comparisons. Growth curves were analyzed by comparing log10 focus-forming unit (FFU)/ml values by two-way ANOVA followed by Tukey’s multiple comparisons test. Protein half-life was analyzed using single-phase exponential-decay curves.
KEY RESOURCES TABLE
The table highlights the genetically modified organisms and strains, cell lines, reagents, software, and source data essential to reproduce results presented in the manuscript. Depending on the nature of the study, this may include standard laboratory materials (i.e., food chow for metabolism studies), but the Table is not meant to be comprehensive list of all materials and resources used (e.g., essential chemicals such as SDS, sucrose, or standard culture media don’t need to be listed in the Table). Items in the Table must also be reported in the Method Details section within the context of their use. The number of primers and RNA sequences that may be listed in the Table is restricted to no more than ten each. If there are more than ten primers or RNA sequences to report, please provide this information as a supplementary document and reference this file (e.g., See Table S1 for XX) in the Key Resources Table.
Please note that ALL references cited in the Key Resources Table must be included in the References list. Please report the information as follows:
REAGENT or RESOURCE: Provide full descriptive name of the item so that it can be identified and linked with its description in the manuscript (e.g., provide version number for software, host source for antibody, strain name). In the Experimental Models section, please include all models used in the paper and describe each line/strain as: model organism: name used for strain/line in paper: genotype. (i.e., Mouse: OXTRfl/fl: B6.129(SJL)-Oxtrtm1.1Wsy/J). In the Biological Samples section, please list all samples obtained from commercial sources or biological repositories. Please note that software mentioned in the Methods Details or Data and Software Availability section needs to be also included in the table. See the sample Table at the end of this document for examples of how to report reagents.
SOURCE: Report the company, manufacturer, or individual that provided the item or where the item can obtained (e.g., stock center or repository). For materials distributed by Addgene, please cite the article describing the plasmid and include “Addgene” as part of the identifier. If an item is from another lab, please include the name of the principal investigator and a citation if it has been previously published. If the material is being reported for the first time in the current paper, please indicate as “this paper.” For software, please provide the company name if it is commercially available or cite the paper in which it has been initially described.
-
IDENTIFIER: Include catalog numbers (entered in the column as “Cat#” followed by the number, e.g., Cat#3879S). Where available, please include unique entities such as RRIDs, Model Organism Database numbers, accession numbers, and PDB or CAS IDs. For antibodies, if applicable and available, please also include the lot number or clone identity. For software or data resources, please include the URL where the resource can be downloaded. Please ensure accuracy of the identifiers, as they are essential for generation of hyperlinks to external sources when available. Please see the Elsevier list of Data Repositories with automated bidirectional linking for details. When listing more than one identifier for the same item, use semicolons to separate them (e.g. Cat#3879S; RRID: AB_2255011). If an identifier is not available, please enter “N/A” in the column.
A NOTE ABOUT RRIDs: We highly recommend using RRIDs as the identifier (in particular for antibodies and organisms, but also for software tools and databases). For more details on how to obtain or generate an RRID for existing or newly generated resources, please visit the RII or search for RRIDs.
Please use the empty table that follows to organize the information in the sections defined by the subheading, skipping sections not relevant to your study. Please do not add subheadings. To add a row, place the cursor at the end of the row above where you would like to add the row, just outside the right border of the table. Then press the ENTER key to add the row. Please delete empty rows. Each entry must be on a separate row; do not list multiple items in a single table cell. Please see the sample table at the end of this document for examples of how reagents should be cited.
KEY RESOURCES TABLE.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit monoclonal anti-Snail | Cell Signaling Technology | Cat#3879S; RRID: AB_2255011 |
| Mouse monoclonal anti-Tubulin (clone DM1A) | Sigma-Aldrich | Cat#T9026; RRID: AB_477593 |
| Rabbit polyclonal anti-BMAL1 | This paper | N/A |
| Bacterial and Virus Strains | ||
| pAAV-hSyn-DIO-hM3D(Gq)-mCherry | Krashes et al., 2011 | Addgene AAV5; 44361-AAV5 |
| AAV5-EF1a-DIO-hChR2(H134R)-EYFP | Hope Center Viral Vectors Core | N/A |
| Cowpox virus Brighton Red | BEI Resources | NR-88 |
| Zika-SMGC-1, GENBANK: KX266255 | Isolated from patient (Wang et al., 2016) | N/A |
| Staphylococcus aureus | ATCC | ATCC 29213 |
| Streptococcus pyogenes: M1 serotype strain: strain SF370; M1 GAS | ATCC | ATCC 700294 |
| Biological Samples | ||
| Healthy adult BA9 brain tissue | University of Maryland Brain & Tissue Bank; http://medschool.umaryland.edu/btbank/ | Cat#UMB1455 |
| Human hippocampal brain blocks | New York Brain Bank | http://nybb.hs.columbia.edu/ |
| Patient-derived xenografts (PDX) | Children’s Oncology Group Cell Culture and Xenograft Repository | http://cogcell.org/ |
| Chemicals, Peptides, and Recombinant Proteins | ||
| MK-2206 AKT inhibitor | Selleck Chemicals | S1078; CAS: 1032350-13-2 |
| SB-505124 | Sigma-Aldrich | S4696; CAS: 694433-59-5 (free base) |
| Picrotoxin | Sigma-Aldrich | P1675; CAS: 124-87-8 |
| Human TGF-β | R&D | 240-B; GenPept: P01137 |
| Activated S6K1 | Millipore | Cat#14-486 |
| GST-BMAL1 | Novus | Cat#H00000406-P01 |
| Critical Commercial Assays | ||
| EasyTag EXPRESS 35S Protein Labeling Kit | Perkin-Elmer | NEG772014MC |
| CaspaseGlo 3/7 | Promega | G8090 |
| TruSeq ChIP Sample Prep Kit | Illumina | IP-202-1012 |
| Deposited Data | ||
| Raw and analyzed data | This paper | GEO: GSE63473 |
| B-RAF RBD (apo) structure | This paper | PDB: 5J17 |
| Human reference genome NCBI build 37, GRCh37 | Genome Reference Consortium | http://www.ncbi.nlm.nih.gov/projects/genome/assembly/grc/human/ |
| Nanog STILT inference | This paper; Mendeley Data | http://dx.doi.org/10.17632/wx6s4mj7s8.2 |
| Affinity-based mass spectrometry performed with 57 genes | This paper; and Mendeley Data | Table S8; http://dx.doi.org/10.17632/5hvpvspw82.1 |
| Experimental Models: Cell Lines | ||
| Hamster: CHO cells | ATCC | CRL-11268 |
| D. melanogaster: Cell line S2: S2-DRSC | Laboratory of Norbert Perrimon | FlyBase: FBtc0000181 |
| Human: Passage 40 H9 ES cells | MSKCC stem cell core facility | N/A |
| Human: HUES 8 hESC line (NIH approval number NIHhESC-09-0021) | HSCI iPS Core | hES Cell Line: HUES-8 |
| Experimental Models: Organisms/Strains | ||
| C. elegans: Strain BC4011: srl-1(s2500) II; dpy-18(e364) III; unc-46(e177)rol-3(s1040) V. | Caenorhabditis Genetics Center | WB Strain: BC4011; WormBase: WBVar00241916 |
| D. melanogaster: RNAi of Sxl: y[1] sc[*] v[1]; P{TRiP.HMS00609}attP2 | Bloomington Drosophila Stock Center | BDSC:34393; FlyBase: FBtp0064874 |
| S. cerevisiae: Strain background: W303 | ATCC | ATTC: 208353 |
| Mouse: R6/2: B6CBA-Tg(HDexon1)62Gpb/3J | The Jackson Laboratory | JAX: 006494 |
| Mouse: OXTRfl/fl: B6.129(SJL)-Oxtrtm1.1Wsy/J | The Jackson Laboratory | RRID: IMSR_JAX:008471 |
| Zebrafish: Tg(Shha:GFP)t10: t10Tg | Neumann and Nuesslein-Volhard, 2000 | ZFIN: ZDB-GENO-060207-1 |
| Arabidopsis: 35S::PIF4-YFP, BZR1-CFP | Wang et al., 2012 | N/A |
| Arabidopsis: JYB1021.2: pS24(AT5G58010)::cS24:GFP(−G):NOS #1 | NASC | NASC ID: N70450 |
| Oligonucleotides | ||
| siRNA targeting sequence: PIP5K I alpha #1: ACACAGUACUCAGUUGAUA | This paper | N/A |
| Primers for XX, see Table SX | This paper | N/A |
| Primer: GFP/YFP/CFP Forward: GCACGACTTCTTCAAGTCCGCCATGCC | This paper | N/A |
| Morpholino: MO-pax2a GGTCTGCTTTGCAGTGAATATCCAT | Gene Tools | ZFIN: ZDB-MRPHLNO-061106-5 |
| ACTB (hs01060665_g1) | Life Technologies | Cat#4331182 |
| RNA sequence: hnRNPA1_ligand: UAGGGACUUAGGGUUCUCUCUAGGGACUUAGGGUUCUCUCUAGGGA | This paper | N/A |
| Recombinant DNA | ||
| pLVX-Tight-Puro (TetOn) | Clonetech | Cat#632162 |
| Plasmid: GFP-Nito | This paper | N/A |
| cDNA GH111110 | Drosophila Genomics Resource Center | DGRC:5666; FlyBase:FBcl0130415 |
| AAV2/1-hsyn-GCaMP6-WPRE | Chen et al., 2013 | N/A |
| Mouse raptor: pLKO mouse shRNA 1 raptor | Thoreen et al., 2009 | Addgene Plasmid #21339 |
| Software and Algorithms | ||
| Bowtie2 | Langmead and Salzberg, 2012 | http://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Samtools | Li et al., 2009 | http://samtools.sourceforge.net/ |
| Weighted Maximal Information Component Analysis v0.9 | Rau et al., 2013 | https://github.com/ChristophRau/wMICA |
| ICS algorithm | This paper; Mendeley Data | http://dx.doi.org/10.17632/5hvpvspw82.1 |
| Other | ||
| Sequence data, analyses, and resources related to the ultra-deep sequencing of the AML31 tumor, relapse, and matched normal. | This paper | http://aml31.genome.wustl.edu |
| Resource website for the AML31 publication | This paper | https://github.com/chrisamiller/aml31SuppSite |
Supplementary Material
Acknowledgments
This work was supported by grants from the NIH (R01 AI10497 and U19 AI109680 (to M.S.D.), U19 AI083019 (to M.S.D. and G.K.A.), GM103422 (to M.L.G), AI120943 and AI109945 (to G.K.A).
Footnotes
AUTHOR CONTRIBUTIONS.
B.J., L.A.V., W.X., J.A., M.L.G., D.W.L., M.S.D., and G.K.A. designed the experiments. B.J., L.A.V., W.X., and J.A. performed the experiments. C.S., P.Y.S., R.Z., and J.P.W. contributed key reagents. B.J., L.A.V., W.X., D.W.L, M.S.D., and G.K.A. analyzed the data. B.J. and L.A.V. wrote the first draft of the paper with M.S.D., and G.K.A providing major editorial comments. All authors participated in editing the final version of the manuscript.
DECLARATION OF COMPETING INTERESTS
The authors declare no competing interests.
References
- Abbas YM, Laudenbach BT, Martinez-Montero S, Cencic R, Habjan M, Pichlmair A, Damha MJ, Pelletier J, Nagar B. Structure of human IFIT1 with capped RNA reveals adaptable mRNA binding and mechanisms for sensing N1 and N2 ribose 2′-O methylations. Proc Natl Acad Sci U S A. 2017;114:E2106–E2115. doi: 10.1073/pnas.1612444114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas YM, Pichlmair A, Górna MW, Superti-Furga G, Nagar B. Structural basis for viral 5′-PPP-RNA recognition by human IFIT proteins. Nature. 2013;494:60–64. doi: 10.1038/nature11783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brien JD, Lazear HM, Diamond MS. Propagation, quantification, detection, and storage of West Nile virus. Curr Protoc Microbiol. 2013;31:15D 13 11–15D 13 18. doi: 10.1002/9780471729259.mc15d03s31. [DOI] [PubMed] [Google Scholar]
- Cai X, Chiu YH, Chen ZJ. The cGAS-cGAMP-STING pathway of cytosolic DNA sensing and signaling. Mol Cell. 2014;54:289–296. doi: 10.1016/j.molcel.2014.03.040. [DOI] [PubMed] [Google Scholar]
- Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H, et al. 2′-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daugherty MD, Schaller AM, Geballe AP, Malik HS. Evolution-guided functional analyses reveal diverse antiviral specificities encoded by IFIT1 genes in mammals. Elife. 2016:5. doi: 10.7554/eLife.14228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis IW, Leaver-Fay A, Chen VB, Block JN, Kapral GJ, Wang X, Murray LW, Arendall WB, 3rd, Snoeyink J, Richardson JS, et al. MolProbity: all-atom contacts and structure validation for proteins and nucleic acids. Nucleic Acids Res. 2007;35:W375–383. doi: 10.1093/nar/gkm216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MS, Farzan M. The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol. 2013;13:46–57. doi: 10.1038/nri3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Fensterl V, Wetzel JL, Ramachandran S, Ogino T, Stohlman SA, Bergmann CC, Diamond MS, Virgin HW, Sen GC. Interferon-Induced Ifit2/ISG54 Protects Mice from Lethal VSV Neuropathogenesis. PLoS Pathogens. 2012:8. doi: 10.1371/journal.ppat.1002712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fensterl V, White CL, Yamashita M, Sen GC. Novel characteristics of the function and induction of murine p56 family proteins. J Virol. 2008;82:11045–11053. doi: 10.1128/JVI.01593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habjan M, Hubel P, Lacerda L, Benda C, Holze C, Eberl CH, Mann A, Kindler E, Gil-Cruz C, Ziebuhr J, et al. Sequestration by IFIT1 Impairs Translation of 2′O-unmethylated Capped RNA. PLoS Pathogens. 2013:9. doi: 10.1371/journal.ppat.1003663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YL, Shi SF, Wu WL, Ho LJ, Lai JH. Protective roles of interferon-induced protein with tetratricopeptide repeats 3 (IFIT3) in dengue virus infection of human lung epithelial cells. PLoS One. 2013;8:e79518. doi: 10.1371/journal.pone.0079518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui DJ, Bhasker CR, Merrick WC, Sen GC. Viral stress-inducible protein p56 inhibits translation by blocking the interaction of eIF3 with the ternary complex eIF2.GTP.Met-tRNAi. J Biol Chem. 2003;278:39477–39482. doi: 10.1074/jbc.M305038200. [DOI] [PubMed] [Google Scholar]
- Hyde JL, Diamond MS. Innate immune restriction and antagonism of viral RNA lacking 2′-O methylation. Virology. 2015;479:66–74. doi: 10.1016/j.virol.2015.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JL, Gardner CL, Kimura T, White JP, Liu G, Trobaugh DW, Huang C, Tonelli M, Paessler S, Takeda K, et al. A Viral RNA Structural Element Alters Host Recognition of Nonself RNA. Science. 2014;343:783–787. doi: 10.1126/science.1248465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi T, Yoshida H, Hayakari R, Xing F, Wang L, Matsumiya T, Tanji K, Kawaguchi S, Murakami M, Tanaka H. Interferon-stimulated gene (ISG) 60, as well as ISG56 and ISG54, positively regulates TLR3/IFN-beta/STAT1 axis in U373MG human astrocytoma cells. Neurosci Res. 2016;105:35–41. doi: 10.1016/j.neures.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- Keating SE, Baran M, Bowie AG. Cytosolic DNA sensors regulating type I interferon induction. Trends Immunol. 2011;32:574–581. doi: 10.1016/j.it.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Kumar P, Sweeney TR, Skabkin MA, Skabkina OV, Hellen CU, Pestova TV. Inhibition of translation by IFIT family members is determined by their ability to interact selectively with the 5′-terminal regions of cap0-, cap1- and 5′ppp- mRNAs. Nucleic Acids Res. 2014;42:3228–3245. doi: 10.1093/nar/gkt1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski RA, Swindells MB. LigPlot+: multiple ligand-protein interaction diagrams for drug discovery. J Chem Inf Model. 2011;51:2778–2786. doi: 10.1021/ci200227u. [DOI] [PubMed] [Google Scholar]
- Leung DW, Amarasinghe GK. When your cap matters: structural insights into self vs non-self recognition of 5′ RNA by immunomodulatory host proteins. Curr Opin Struct Biol. 2016;36:133–141. doi: 10.1016/j.sbi.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wen Z, Zhou H, Wu S, Jia G, Qian W, Jin M. Porcine interferon-induced protein with tetratricopeptide repeats 3, poIFIT3, inhibits swine influenza virus replication and potentiates IFN-beta production. Dev Comp Immunol. 2015;50:49–57. doi: 10.1016/j.dci.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Liu XY, Chen W, Wei B, Shan YF, Wang C. IFN-induced TPR protein IFIT3 potentiates antiviral signaling by bridging MAVS and TBK1. J Immunol. 2011;187:2559–2568. doi: 10.4049/jimmunol.1100963. [DOI] [PubMed] [Google Scholar]
- Oliphant T, Engle M, Nybakken GE, Doane C, Johnson S, Huang L, Gorlatov S, Mehlhop E, Marri A, Chung KM, et al. Development of a humanized monoclonal antibody with therapeutic potential against West Nile virus. Nat Med. 2005;11:522–530. doi: 10.1038/nm1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant T, Nybakken GE, Engle M, Xu Q, Nelson CA, Sukupolvi-Petty S, Marri A, Lachmi BE, Olshevsky U, Fremont DH, et al. Antibody recognition and neutralization determinants on domains I and II of West Nile Virus envelope protein. J Virol. 2006;80:12149–12159. doi: 10.1128/JVI.01732-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- Pascal BD, Willis S, Lauer JL, Landgraf RR, West GM, Marciano D, Novick S, Goswami D, Chalmers MJ, Griffin PR. HDX workbench: software for the analysis of H/D exchange MS data. J Am Soc Mass Spectrom. 2012;23:1512–1521. doi: 10.1007/s13361-012-0419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichlmair A, Lassnig C, Eberle CA, Gorna MW, Baumann CL, Burkard TR, Burckstummer T, Stefanovic A, Krieger S, Bennett KL, et al. IFIT1 is an antiviral protein that recognizes 5′-triphosphate RNA. Nat Immunol. 2011;12:624–630. doi: 10.1038/ni.2048. [DOI] [PubMed] [Google Scholar]
- Pinto AK, Williams GD, Szretter KJ, White JP, Proenca-Modena JL, Liu G, Olejnik J, Brien JD, Ebihara H, Muhlberger E, et al. Human and Murine IFIT1 Proteins Do Not Restrict Infection of Negative-Sense RNA Viruses of the Orthomyxoviridae, Bunyaviridae, and Filoviridae Families. J Virol. 2015;89:9465–9476. doi: 10.1128/JVI.00996-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes DP, Banerjee AK. 5′-terminal sequence of vesicular stomatitis virus mRNA's synthesized in vitro. J Virol. 1975;17:33–42. doi: 10.1128/jvi.17.1.33-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser H, Mejido J, Balinsky CA, Morrow AN, Clark CR, Zhao T, Zoon KC. Identification of alpha interferon-induced genes associated with antiviral activity in Daudi cells and characterization of IFIT3 as a novel antiviral gene. J Virol. 2010;84:10671–10680. doi: 10.1128/JVI.00818-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan C, Xie X, Muruato AE, Rossi SL, Roundy CM, Azar SR, Yang Y, Tesh RB, Bourne N, Barrett AD, et al. An Infectious cDNA Clone of Zika Virus to Study Viral Virulence, Mosquito Transmission, and Antiviral Inhibitors. Cell Host Microbe. 2016;19:891–900. doi: 10.1016/j.chom.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner M, Purta E, Kaminska KH, Cymerman IA, Campbell DA, Mittra B, Zamudio JR, Sturm NR, Jaworski J, Bujnicki JM. 2′-O-ribose methylation of cap2 in human: function and evolution in a horizontally mobile family. Nucleic Acids Res. 2011;39:4756–4768. doi: 10.1093/nar/gkr038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Liu J, Bai J, Du Y, Wang X, Liu X, Jiang P. Poly(I:C) inhibits porcine reproductive and respiratory syndrome virus replication in MARC-145 cells via activation of IFIT3. Antiviral Res. 2013;99:197–206. doi: 10.1016/j.antiviral.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Michal JJ, Zhang L, Ding B, Lunney JK, Liu B, Jiang Z. Interferon Induced IFIT Family Genes in Host Antiviral Defense. International Journal of Biological Sciences. 2013;9:200–208. doi: 10.7150/ijbs.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard KA, Shi PY, Li H. Structure and function of flavivirus NS5 methyltransferase. J Virol. 2007;81:3891–3903. doi: 10.1128/JVI.02704-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zust R, Cervantes-Barragan L, Habjan M, Maier R, Neuman BW, Ziebuhr J, Szretter KJ, Baker SC, Barchet W, Diamond MS, et al. Ribose 2′-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.