Abstract
Objective:
Macular pigment optical density (MPOD) – a non-invasive indicator of retinal xanthophylls and correlate of brain lutein – has been associated with superior cognitive function among adult populations. Given that lutein accumulation in the brain occurs in early life, it is possible that the cognitive implications of greater MPOD may be evident in childhood.
Methods:
Participants aged 8–9 y (N=56) completed MPOD measurements via heterochromatic flicker photometry (HFP). Academic performance was assessed using the Kaufman Test of Academic and Educational Achievement II (KTEA). Habitual dietary intake of L and Z was measured among a subsample of participants (N=35) using averaged 3-day food records. Stepwise hierarchical regression models were developed to determine the relationship between MPOD and academic achievement tests, following the adjustment of key covariates including sex, aerobic fitness, body composition, and intelligence quotient (IQ).
Results:
The regression analyses revealed that MPOD improved the model, beyond the covariates, for overall academic achievement (ΔR2 = 0.10, P < 0.01), mathematics (ΔR2 = 0.07, P = 0.02), and written language composite standard scores (ΔR2 = 0.15, P < 0.01).
Discussion:
This is the first study to demonstrate that retinal L and Z, measured as MPOD, is positively related to academic achievement in children, even after accounting for the robust effects of IQ and other demographic factors. These findings extend the positive associations observed between MPOD and cognitive abilities to a pediatric population.
Trail registration:
The Fitness Improves Thinking in Kids 2 (FITKids2) trial was registered at www.clinicaltrials.gov as NCT01619826
Keywords: preadolescent children, lutein, zeaxanthin, academic performance, macular pigment optical density
Introduction
Lutein (L), zeaxanthin (Z), and meso-zeaxanthin are thought to be important for retinal health, and more recently they have been investigated for their associations with cognitive function in the elderly (1–6). In non-human primates and older humans, macular pigment optical density (MPOD) has been found to serve as a good proxy for the amount of L and Z in the brain (7, 8), thus allowing MPOD to be used as a biomarker. L has been found to preferentially accumulate in the infant brain, accounting for 59% of total brain carotenoids while constituting only 12% of the infants’ carotenoid intake (9). The relative contribution of L to the total carotenoids found in infant brains is almost two-fold greater than in adults, accounting for 59% vs. 34%, respectively (5, 9), suggesting a selective role of L in early neural development. Given accumulating evidence for L’s relationship with cognitive function in the elderly (1–6), and since L is found at higher relative concentrations in the infant brain than the elderly brain, it is a natural extension to ask whether a relationship between MPOD and cognitive abilities exists during childhood.
Performance on standardized academic achievement tests has demonstrated reliable relationships with many facets of life, including academic and job performance (10). Since the implementation of the No Child Left Behind (NCLB) Act of 2001, schools have been under increasing pressure by federal law to deliver on academic milestones, prompting many schools to alter nutrition during the days before standardized testing to boost short-term performance (11). With the external pressure on schools to provide students with a basic academic skill set, compounded by the known relationships between current academic performance and future success in life, it is crucial to provide evidence-based dietary guidance to support children’s’ abilities for long-term scholastic success. Although a growing body of literature supports the impact of overall diet quality and breakfast consumption on improved academic performance (12, 13), the influence of habitual intake of specific food components on academic success remains largely unknown.
Accordingly, the major aim of this study was to determine whether MPOD was associated with academic performance. A secondary aims was to determine if dietary intake of L and Z are related to MPOD measures. We hypothesized that higher MPOD would be associated with superior performance on standardized academic achievement tests among a sample of preadolescent children (8–9-year-olds). Further, we hypothesized that dietary measures would relate to MPOD.
SUBJECTS AND METHODS
Subjects
Preadolescent children between the ages of 8 and 10 years from the East-Central Illinois community were recruited to participate in this study. Participants were excluded due to the presence of neurological disorders, physical disabilities, and psychoactive medication status. All participants had normal or corrected-to-normal vision. All participants provided written assent and their legal guardians provided written informed consent in accordance with the ethical standards and regulations of the Institutional Review Board (IRB) of the University of Illinois at Urbana-Champaign (Institutional Review Board number 12321).
This study utilized children from 2 different waves of the FITKids randomized controlled trial (NCT01334359), an ongoing physical activity intervention trial. All children (n=49) from the 2015–2016 FITKids enrollment were included at their baseline measurement, prior to any intervention. Seven children from the 2014–2015 FITKids enrollment were included in the analysis at post-intervention. These 7 children had the same examiners as the 2015–2016 FITKids cohort, thus reducing variability stemming from the use of more than two examiners (14), thus a total sample size of 56 was obtained.
Measures
All testing protocols were identical at baseline and post-intervention testing, thus including children from both time points should not be a confounding variable. Testing occurred across two separate days. On the first visit to the laboratory participants completed informed assent/ consent, the Woodcock Johnson Tests of Cognitive Abilities to estimate intelligent quotient (IQ) (15), the Kaufman Test of Academic and Educational Achievement II (KTEA II) to assess scholastic achievement (16), had their height and weight measured, and completed a maximal oxygen consumption test (VO2max) to assess aerobic fitness (17). All cognitive testing took place prior to the cardiorespiratory fitness assessment to avoid any confounding effects of acute physical activity on cognitive performance (18). Concurrently, a legal guardian of the participant completed a preliminary screening, demographic and health history questionnaire, and pubertal timing scale (on behalf of their child) via the Tanner Staging Scales (19). From the information provided by the guardian, socioeconomic status (SES) was determined by creating a trichotomous index based on participation in a school meal-assistance program, maternal and paternal education levels, and the number of parents with full time employment. Before leaving the appointment participants were given food records to complete at home for three days (2 week days and 1 weekend day) before their return to the lab. On the second visit, participants completed an assessment of body composition by DXA as described below. At both visits, participants completed the MPOD assessment, and the average of the two values was used throughout this study.
MPOD
MPOD was measured using customized HFP (cHFP) and a macular densitometer (Macular Metrics Corporation, Rehoboth, MA USA) that was described by Wooten et al. (20) (this version of the device differed in that it did not allow a full spatial profile to be measured). This procedure has been described previously for measurement in adults (21). However, the present study utilized a slight variation of the procedure typically described in adult studies; unimpaired adults receive instruction from a trained examiner, and then they manipulate the radiance of the short-wave component of the test stimulus themselves (method of adjustment) to produce a null flicker zone. In the present study, the psychophysical technique was modified as described previously by Renzi et al. (22) for older adults with mild cognitive impairment. Briefly, instead of the participants manipulating the radiance of the short-wave component themselves to find thresholds, the examiner used the method of limits by manipulating the radiance of the short-wave component of the test stimulus while using simplified instructions. After the null zone was found, the method of constant stimuli was used to further narrow the range of the null zone. The two examiners were initially trained by the same instructor and were required to serve as a participant prior to being an examiner themselves. The examiners then proceeded to test 15 adults on two occasions to ensure reliability equal to that currently in the literature (23). After successful completion of this training protocol, the examiners proceeded to test the children in the present study. This training procedure for the examiners was utilized as it has been demonstrated to improve reliability of the measurement in preadolescent children (14). In the current study, participants completed one MPOD assessment at each of their two laboratory visits and the average of the two values was used for analyses. Reliability of this procedure over two sessions has been previously reported by our group (14).
Body composition assessment
Participants’ height and weight were measured in stocking feet using a stadiometer and a Tanita WB-300 Plus digital scale (Tanita, Tokoyo, Japan). The mean of three measurements of height and weight were used for analyses. BMI was calculated by dividing body mass (kg) by height (m) squared ((kg)/ht(m)2). Next, fat and muscle mass were measured using dual-energy X-ray absorptiometry (DXA) with a Hologic Discovery A bone densitometer (software version 12.7.3; Hologic, Bedford, MA).
Academic achievement assessment
Participants were administered the Kaufman Test of Academic and Educational Achievement II (KTEA II) (16) in their native language, English, for all children. The comprehensive form was administered to determine a comprehensive achievement score. Academic outcomes included composite scores on math (math concepts and application and math computation subtests), reading (letter and word recognition and reading comprehension subtests), reading fluency (word recognition fluency and decoding fluency subtests), written language (written expression and spelling subtests), and the comprehensive achievement scores (reading composite, math composite, written expression subtest, and listening comprehension subtest). All scores reported herein are standard scores generated by using the age norms standard scores produced by KTEA II comprehensive norms (24).
The math concepts and application subtest was an 88-item subtest, which began with easier items including basic math concepts such as comparing numbers and rounding numbers, and progressed to more difficult problems requiring algebra, calculus, and trigonometry. The math computation subtest consisted of 72 items and prompted participants to add, subtract, multiply, and divide whole numbers and fractions. Problems progressed in difficulty by involving exponents, decimals, negatives, and unknown variables. Participants had access to pencil and paper but were not allowed to use a calculator for the math subtests. The letter and word recognition subtest had participants pronounce words of gradually increasing difficulty. The reading comprehension subtest began with the participant reading a word and pointing to its corresponding picture. It progressed in difficulty by having the student perform the action of the word, and then answer literal or inferential questions about passages they had read. For the word recognition fluency subtest the participant read isolated words as quickly as possible for one minute, and in the decoding fluency subtest they pronounced as many nonsense words as possible in one minute. For the written expression subtest the participant completed writing tasks in the context of an age-appropriate storybook format. In the spelling subtest the participant wrote words that the examiner dictated from a steeply-graded word list. In the listening comprehension subtest the participant listened to passages played from a CD and then orally responded to questions asked by the examiner (24).
Diet assessment
Three-day food records (including 2 week days and 1 weekend day) were used to determine dietary intake of L and Z. The records were completed by the child with assistance from the guardian. Both child and guardian received instructions on how to correctly fill out the food records. Additionally, the records contained written instructions for recording food intake, including portion size examples, how to describe food preparation methods, added fats, brand names, and ingredients of mixed dishes and recipes. The three days of intake were entered into Nutrition Data System for Research (NDSR 2014; Nutrition Coordinating Center, Minneapolis, MN, USA) software by trained staff. To investigate nutrient-level intakes the intake properties file from NDSR was utilized. The three days of intake were averaged together, and subsequently, these averages are used in the data analyses. The data for this study were collected between the months of May and September 2015, thus the reported lutein intake may potentially be higher relative to other times of the year due to higher availability and cheaper costs of fruits and vegetables during this time of the year (25).
Statistical analysis
A bivariate correlation was performed between MPOD and dietary intake of L and Z. Participants were eliminated from the analysis if they were two or more standard deviations away from the food record mean. Next, bivariate correlations between MPOD, dietary intake of L and Z, and academic composite scores were calculated. Bivariate correlations between MPOD and demographic and health variables were run to determine what needed to be included in step 1 of the final regression model predicting academic performance.
Following correlational analyses, the relationship between MPOD and academic performance was examined using multiple hierarchical linear regression analyses. First, confounding demographic and health variables determined via the bivariate correlations were included in step 1 of the final regression model predicting academic performance. Additionally, variables of a higher accuracy in their measurement of interest were included in the modeling at step 1. For example, if both BMI and whole body percent fat correlated with the academic measures, then whole body percent fat was included because it is a direct measure of fat mass. Further, in instances where factors known to be related to sex, such as whole body percent fat or VO2max, are significantly related to MPOD but sex is not, then independent t-tests were performed to determine whether these measures varied between sexes. In cases where a difference was observed between sexes in the independent t-test, then sex was entered into step 1 of the model. Following adjustment of step 1 variables, MPOD outcomes were included in step 2 of the regression model. The change in variance in performance explained by MPOD on each academic achievement variable in step 2 was examined. The α level was set at 0.05 and SPSS 22 was used to perform all statistical analyses.
RESULTS
Participant Demographic Information
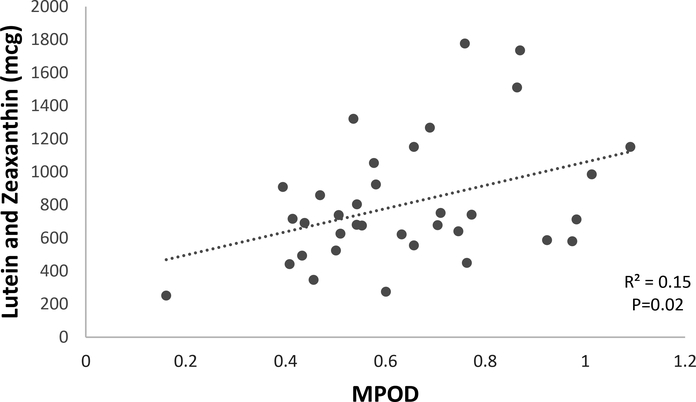
Table 1 presents participant characteristics, KTEA II academic performance standard scores of participants, MPOD, and dietary intake of L and Z. To determine the relationship between the dietary measure of L and Z with the psychophysical measure (i.e., MPOD measured via HFP) a correlation between the two was performed. Figure 1 demonstrates the positive correlation (r = 0.39, P=0.02) between MPOD and dietary intake of L and Z.
Table 1.
Participant characteristics, academic performance, MPOD, and dietary lutein and zeaxanthin intake among preadolescent children1
| Characteristic | Value |
|---|---|
| Age (y) | 8.8 ± 0.1 |
| Sex [n (%)] | |
| Male | 17 (30) |
| Female | 39 (70) |
| IQ | 112.8 ± 1.7 |
| VO2 max (mL*kg−1*min−1) | 43.0 ± 1.1 |
| Fat Free Mass VO2 max (mL*kgFFM−1*min−1) | 61.9 ± 1.0 |
| BMI (kg/m2) | 18.7 ± 0.4 |
| BMI-for-age percentile2 | 70.9 ± 3.5 |
| Underweight, BMI percentile < 5 [n (%)] | 0 (0) |
| Normal weight, BMI percentile ≤5 and <84.9 [n (%)] | 32 (57) |
| Overweight, BMI percentile ≤85 and <94.9 [n (%)] | 14 (25) |
| Obese, BMI percentile > 95 [n (%)] | 10 (18) |
| Whole Body % Fat (%) | 31.3 ± 0.9 |
| SES [n (%)] | |
| Low | 22 (39) |
| Middle | 19 (34) |
| High | 15 (27) |
| Pubertal Timing [n (%)] | |
| Stage 1–2 | 51 (91) |
| Stage 2–3 | 5 (9) |
| Math Composite | 108.3 ± 2.3 |
| Reading Composite | 111.5 ± 2.1 |
| Reading Fluency Composite | 111.1 ± 2.1 |
| Written Language Composite | 106.6 ± 2.5 |
| Achievement Composite | 109.5 ± 2.2 |
| MPOD | 0.64 ± 0.03 |
| Lutein + Zeaxanthin (μg)3 | 806.6 ± 63.0 |
Values are means ± SEM n=56. IQ, intelligence quotient; VO2 max, maximal oxygen uptake; SES, socioeconomic status; MPOD, macular pigment optical density.
Determined by the 2000 Centers for Disease Control and Prevention BMI-for-age growth charts
n=35; n=18 did not return food records and n=3 were cut out due to being outliers (two or more standard deviations away from the food record mean)
Figure 1.
Illustration of the correlation between MPOD and lutein and zeaxanthin intake (n=35; n=18 did not return food records and n=3 were removed as outliers)
Bivariate correlations with Academic Achievement
Bivariate correlations between the achievement composite score and the demographic measures were performed. These correlations revealed that IQ (r = 0.62, P < 0.01), VO2max (r = 0.33, P = 0.01), and fat free mass VO2max (r = 0.26, P = 0.05) were positively correlated with the achievement composite score. BMI (r = −0.37, P < 0.01) and whole body percent fat (r = −0.30, P = 0.03) were negatively correlated with the achievement composite score. Age, sex, pubertal timing, and SES did not significantly correlate with the achievement composite score (r ≤ │0.21│, P ≥ 0.13). Bivariate correlations between MPOD with the KTEA II academic composite scores as well as bivariate correlations between L and Z with the KTEA II academic composite scores are shown in Table 2.
Table 2.
Bivariate correlations between KTEA II performance and lutein and zeaxanthin intake among preadolescent children1
| Carotenoid | Achievement | Reading | Math | Written Language |
Reading Fluency |
|---|---|---|---|---|---|
| MPOD | 0.40** | 0.28* | 0.35** | 0.41** | 0.22 |
| Lutein and Zeaxanthin2 | 0.29 | 0.16 | 0.14 | 0.53** | 0.23 |
All are academic achievement composite standard scores based on age norms.
P<0.05
P<0.01(two-tailed). n=56
n=35; n=18 did not return food records and n=3 were removed as outliers (two or more standard deviations away from the food record mean)
Bivariate correlations between MPOD and demographics revealed that age, sex, pubertal timing, SES, IQ, BMI, whole body percent fat, VO2max, and fat free mass VO2max had no significant correlations with MPOD (r ≤ │0.22│, P ≥ 0.10). Bivariate correlations between L and Z intake and demographics revealed that age, pubertal timing, SES, IQ, BMI, whole body percent fat, VO2max, and fat free mass VO2max had no significant correlations with L and Z intake (r ≤ │0.28│, P ≥ 0.10).
Hierarchical Regressions
The stepwise hierarchical regression models are summarized in Table 3 for the composite scores and Table 4 summarizes the decomposition of their subtests. As IQ, BMI, whole body percent fat, VO2max, and fat free mass VO2max were significantly correlated with the achievement composite score, these factors were considered for entry into step 1 of the model. Sex was entered, despite not being significantly correlated with the academic composite, due to whole body percent fat and fat free mass VO2max differing between genders in independent t-tests [whole body percent fat was significantly different for girls (M=32.8 SE=1.0) and boys (M=27.7, SE=1.5), t(54)= 2.9, p = 0.01; and fat free mass VO2max was significantly different for girls (M=60.5, SE=1.1) and boys (M=64.9, SE=1.9), t(54)= −2.1, p = 0.04]. Subsequent addition of MPOD in step 2 was conducted to determine the contribution to the academic measures following step 1 adjustments. The addition of MPOD did not statistically improve the ΔR2 for the reading or reading fluency composite scores or any of their subtests (letter and word recognition, reading comprehension, word recognition fluency, and decoding fluency), nor did it improve the ΔR2 for the listening comprehension subtest. However, the addition of MPOD resulted in a significant improvement in the model ΔR2 at step 2 for the achievement composite standard scores (ΔR2 = 0.10, P = 0.002), math composite standard scores (ΔR2 = 0.07, P = 0.02), and written language composite standard scores (ΔR2 = 0.15, P = 0.001), as well as the math subtests: math concepts (ΔR2 = 0.05, P = 0.04), and math computation (ΔR2 = 0.09, P = 0.02), and the written language subtests: written expression (ΔR2 = 0.11, P = 0.008), and spelling (ΔR2 = 0.13, P = 0.004).
Table 3.
Summary of regression analyses predicting academic achievement composite standard scores1
| Achievement | Reading | Math | Written Language |
Reading Fluency |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Step and Variable | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 |
| Step 1 | 0.43* | 0.40* | 0.32* | 0.23* | 0.29* | |||||
| Sex | −0.04 | −0.02 | 0.00 | −0.12 | −0.01 | |||||
| IQ | 0.60* | 0.57* | 0.47* | 0.47* | 0.52* | |||||
| Whole body % fat | −0.02 | 0.04 | −0.04 | −0.04 | 0.10 | |||||
| Fat Free Mass VO2 | 0.22 | 0.29* | 0.24 | −0.04 | 0.22 | |||||
| Step 2 | 0.10* | 0.04 | 0.07* | 0.15* | 0.02 | |||||
| MPOD | 0.32* | 0.20 | 0.27* | 0.40* | 0.16 | |||||
IQ, Intelligence quotient. n=56.
P<0.05.
Table 4.
Summary of regression analyses predicting academic achievement subtest standard scores1
| Composite Test | Reading | Math | Written Language | Reading Fluency | Subtest | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Subtest | Letter and Word Recognition |
Reading Comprehension |
Math Concepts | Math Computation |
Written Expression |
Spelling | Word Recognition Fluency |
Decoding Fluency | Listening Comprehension |
|||||||||
| Step and Variable |
β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 | β | ΔR2 |
| Step 1 | 0.31* | 0.33* | 0.38* | 0.20* | 0.16 | 0.19* | 0.25* | 0.24* | 0.29* | |||||||||
| Sex | 0.07 | −0.09 | 0.04 | −0.02 | −0.10 | −0.10 | −0.07 | 0.06 | 0.01 | |||||||||
| IQ | 0.47* | 0.54* | 0.56* | 0.30* | 0.37* | 0.45* | 0.49* | 0.45* | 0.47* | |||||||||
| Whole body % fat | 0.02 | 0.05 | −0.03 | −0.04 | −0.07 | 0.04 | 0.14 | 0.04 | 0.04 | |||||||||
| Fat Free VO2 | 0.25* | 0.26* | 0.15 | 0.29* | −0.09 | 0.05 | 0.24 | 0.17 | 0.26* | |||||||||
| Step 2 | 0.02 | 0.04 | 0.05* | 0.09* | 0.11* | 0.13* | 0.01 | 0.03 | 0.00 | |||||||||
| MPOD | 0.14 | 0.21 | 0.24* | 0.30* | 0.35* | 0.36* | 0.12 | 0.18 | 0.03 | |||||||||
Intelligence quotient.
P<0.05.
DISCUSSION
The aim of the present study was to evaluate the relationships between dietary L and Z intake, MPOD, and academic achievement measures among preadolescent children. The major finding was that children with higher MPOD values have superior performance on academic measures, particularly in math and written language. Given that MPOD was positively related to academic outcomes, even after the adjustment of sex, IQ, whole body percent fat, and fat free mass VO2max, highlights the importance of habitual intake of L and Z, indirectly indicated by MPOD, for improved academic performance. This finding is important because macular L is modifiable and can be manipulated by dietary intake in most of the population (26).
The results of this study demonstrate that the associations of the various academic composite measures with MPOD were fairly consistent, whereas associations between academics and self-reported dietary intake of L and Z were not as consistent. MPOD has been shown to be a stable measure of carotenoids embedded in the retina, and it has been demonstrated to be a better representation of long-term intake of L and Z than serum levels (2). This may explain why MPOD was more consistently related to academic measures than self-reported dietary intake of L and Z. Other factors that may have contributed to the inconsistency of self-report diet records to cognitive measures include potential subject recall bias, as well as possible digestive and absorptive idiosyncrasies among subjects (27).
A positive relationship between MPOD and dietary intake of L and Z was found in this sample. Such a finding is congruent with some, but not all, adult studies (27). This lack of consensus across studies highlights the many factors that likely influence the absorption of L and Z into the blood (28). For example, some tissues may compete for the uptake of L and Z, which may interfere with this relationship (29, 30). As this finding was observed in a subset among those that returned diet records (n=35), further study in larger samples of children is warranted to determine the robustness of this relationship.
L and Z have received considerable attention for their impact on visual health, and more recently on cognitive function in the elderly (1–5). However, the influence of L and Z on cognitive function in preadolescence has received little attention in comparison (31, 32). Although dietary carotenoids have not been investigated directly for their effect on academic performance, studies have been conducted on other dietary factors and their impact on academic performance. Overall diet quality has been demonstrated to impact academic performance in children. A study completed in 5th graders showed a positive association of diet quality and academic performance (12). Within overall diet quality, it was found that students with an increased intake of fruits and vegetables, sources high in carotenoids, was associated with improved academic performance (12). Additionally, dietary fibers, found abundantly in fruits and vegetables, have been related to childhood cognitive function (33). As dietary carotenoids are a hallmark of higher quality diets, our results are consistent with previous studies suggesting a role for diet in childhood cognitive function. The present study contributes to the current literature, as it provides support for the neurocognitive potential of the macular carotenoids specifically, even after adjusting for sex, IQ, whole body percent fat, and fat free mass VO2max, measures that have been previously demonstrated to relate to academic achievement in preadolescence (34–38).
The macular carotenoids L, Z and meso-zeaxanthin, as measured via MPOD, demonstrated a significant relationship with academic performance among preadolescents congruent with our a priori hypothesis based on the preferential accumulation of L in the brain (5, 9). Significant associations have been shown between L and cognition in the elderly. MPOD has been shown to relate to cognition in adults (2, 3, 5). Additionally, supplementation with L has been shown to improve cognitive function in healthy older women (4).
Carotenoids may exert their effects through several mechanisms in the brain to improve cognitive function. We know, for instance, that L (co-localizing with DHA; (39)) is located in areas of the brain that are important in processing information such as the hippocampus and frontal cortex (7–9). In pre-clinical studies, L, specifically, has been shown to protect the hippocampus from hyperglycemic-induced oxidative stress (40),and L in the brain correlates with levels of lysophospholipids (e.g., in frontal cortex and hippocampus; (41) which are known to mediate signaling between and within neurons. Additionally in pre-clinical studies, L itself has been shown to enhance gap junction communications in model mitotic cells (42). There are numerous possible mechanisms that may have driven the associations identified here, but taken together, the implication is that L and Z alters the physiology of the brain in a manner that improves the cognitive function of pre-adolescent children. Fortunately, this can be verified in a future study using a placebo-controlled randomized design.
Of course, for such designs to be optimally interpretable, it is useful to directly measure the results of the intervention: to wit, how much L and Z is actually increased within the CNS following dietary supplementation. This can be accomplished by measuring MPOD. However, our current practice of MPOD measurement via heterochromatic flicker photometry has been shown to only be moderately reliable in children (14), thus improvements in measurement reliability are needed. Further, although the accumulation of L and Z as macular pigments is not completely genetically controlled (43), there are some potential contributing genetic factors that are made clear from supplementation studies demonstrating that some individuals are non-responders to supplementation (25). Measuring the variance explained by such genetic factors would certainly be a useful addition to studies of this type (44). Children in this study had high average MPOD values with relatively low variance. The effects of L and Z on those children with relatively low MPOD can therefore not be inferred from these data. Additional limitations of this study include its cross-sectional design and the limited translatability to other geographical areas. Furthermore, future larger clinical trials are necessary to address other compounding dietary factors that may have a bearing on cognitive function, such as macronutrient intake, testing time after a meal, and composition of previous meal. Lastly, in future studies researchers should record the prescription of all participants’ eyeglasses, as recently it has been demonstrated that the highest quintile of plasma lutein concentrations has been independently associated with a 40% reduced risk of myopia (45). The addition of this information would be helpful to examine the intersection between lutein, myopia, and cognition.
Conclusions
Due to the rapid rise in childhood obesity, links between the detrimental effects of excess fat mass, physical inactivity, and overall diet quality on childhood cognition are becoming clearer (12, 46–48). However, the effect of specific components of food on cognition of children without nutritional deficiencies has not been thoroughly investigated. This study serves the purpose of linking the dietary carotenoids L and Z to academic performance of children. Diet recommendations for increasing foods that are high in these food components have been shown to have other health benefits (49). Thus, improved academic performance among preadolescents may be yet another beneficial aspect of increased intake of foods high in carotenoids. Especially as academic performance influences future educational attainment and income, thus impacting the future health and quality of life of children (10). Additionally, as there is currently no Daily Reference Intake (DRI) for L or Z, studies demonstrating their favorable effects on health contribute to the evidence base supporting consideration of L and Z as important nutrients that can be obtained from food. In the future, this may set the stage for incorporation into public health policy recommendations that might further enable L- and Z-associated benefits among the general public.
Acknowledgements:
We thank Bonnie Hemrick and Jeanine Bensken for their assistance in recruiting and scheduling participants.
Funding: This work was supported by NIH under Grant HD069381 and Abbott Nutrition via a Center for Nutrition, Learning, and Memory (CNLM) grant to the University of Illinois.
List of abbreviations:
- IQ
intelligence quotient
- L
lutein
- MPOD
macular pigment optical density
- SES
socioeconomic status
- VO2max
maximal oxygen consumption
- YAQ
Youth-Adolescent Food-Frequency Questionnaire
- Z
zeaxanthin
Footnotes
Declarations
Ethics approval and consent to participate: All participants provided written assent and their legal guardians provided written informed consent in accordance with the ethical standards and regulations of the Institutional Review Board (IRB) of the University of Illinois at Urbana-Champaign (Institutional Review Board number 12321).
Consent for publication: Not applicable
Availability of data and material: The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
Competing interests: Christopher Moulton is employed by Abbott Nutrition, and Sasha M Barnett was an intern at Abbott after the work for the manuscript was completed. No other authors have any conflicts of interest.
References
- 1.Morris MC, Evans DA, Tangney CC, Bienias JL, Wilson RS. Associations of vegetable and fruit consumption with age-related cognitive change. Neurology. 2006. October 24;67(8):1370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vishwanathan R, Iannaccone A, Scott TM, Kritchevsky SB, Jennings BJ, Carboni G, et al. Macular pigment optical density is related to cognitive function in older people. Age Ageing. 2014. March;43(2):271–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feeney J, Finucane C, Savva GM, Cronin H, Beatty S, Nolan JM, et al. Low macular pigment optical density is associated with lower cognitive performance in a large, population-based sample of older adults. Neurobiol Aging. 2013. 11;34(11):2449–56. [DOI] [PubMed] [Google Scholar]
- 4.Johnson EJ, McDonald K, Caldarella SM, Chung H, Troen AM, Snodderly DM. Cognitive findings of an exploratory trial of docosahexaenoic acid and lutein supplementation in older women. Nutr Neurosci. 2008;11(2):75–83. [DOI] [PubMed] [Google Scholar]
- 5.Johnson EJ, Vishwanathan R, Johnson MA, et al. Relationship between Serum and Brain Carotenoids, alpha-Tocopherol, and Retinol Concentrations and Cognitive Performance in the Oldest Old from the Georgia Centenarian Study. Journal of Aging Research. 2013;2013(Article ID 951786):13 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Snodderly DM. Evidence for protection against age-related macular degeneration by carotenoids and antioxidant vitamins. Am J Clin Nutr. 1995. December;62(6 Suppl):1448S–61S. [DOI] [PubMed] [Google Scholar]
- 7.Vishwanathan R, Neuringer M, Snodderly DM, Schalch W, Johnson EJ. Macular lutein and zeaxanthin are related to brain lutein and zeaxanthin in primates. Nutr Neurosci. 2013;16(1):21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vishwanathan R, Schalch W, Johnson EJ. Macular pigment carotenoids in the retina and occipital cortex are related in humans. Nutr Neurosci. 2016:1–7. [DOI] [PubMed] [Google Scholar]
- 9.Vishwanathan R, Kuchan MJ, Sen S, Johnson EJ. Lutein and preterm infants with decreased concentration of brain carotenoids. Journal of Pediatric Gastroenterology and Nutrition. 2014;59(5):659–665. [DOI] [PubMed] [Google Scholar]
- 10.Kuncel NR, Hezlett SA. Fact and fiction in cognitive ability testing for admissions and hiring decisions. Current Directions in Psychological Science. 2010;19(6):339–45. [Google Scholar]
- 11.Figlio DN, Winicki J. Food for thought: the effects of school accountability plans on school nutrition. Journal of public Economics. 2005;89(2):381–94. [Google Scholar]
- 12.Florence MD, Asbridge M, Veugelers PJ. Diet Quality and Academic Performance*. J Sch Health. 2008;78(4):209–15. [DOI] [PubMed] [Google Scholar]
- 13.Kleinman RE, Hall S, Green H, Korzec-Ramirez D, Patton K, Pagano ME, et al. Diet, breakfast, and academic performance in children. Ann Nutr Metab. 2002;46 Suppl 1:24–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McCorkle SM, Raine LB, Hammond BR, Renzi-Hammond L, Hillman CH, Khan NA. Reliability of Heterochromatic Flicker Photometry in Measuring Macular Pigment Optical Density among Preadolescent Children. Foods. 2015;4(4):594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woodcock R, McGrew K, Mather N, Schrank F. Woodcock-Johnson Tests of Achievement. Riverside Publishing; Itasca, IL: 2001. [Google Scholar]
- 16.Vladescu JC. Test Review: Kaufman Test of Educational Achievement-(KTEA-II). Journal of Psychoeducational Assessment. 2007;25(1):92–100. [Google Scholar]
- 17.Kamijo K, Pontifex MB, O’Leary KC, Scudder MR, Wu C, Castelli DM, et al. The effects of an afterschool physical activity program on working memory in preadolescent children. Developmental science. 2011;14(5):1046–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159(3):1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanner J Growth at adolescence (ed 2) Blackwell Scientific Publications Ltd. 1962. [Google Scholar]
- 20.Wooten BR, Hammond BR, Land RI, Snodderly DM. A Practical Method for Measuring Macular Pigment Optical Density. Investigative Ophthalmology & Visual Science. 1999. October 01;40(11):2481–9. [PubMed] [Google Scholar]
- 21.Stringham JM, Hammond BR, Nolan JM, Wooten BR, Mammen A, Smollon W, et al. The utility of using customized heterochromatic flicker photometry (cHFP) to measure macular pigment in patients with age-related macular degeneration. Exp Eye Res. 2008. 11;87(5):445–53. [DOI] [PubMed] [Google Scholar]
- 22.Renzi LM, Dengler MJ, Puente A, Miller LS, Hammond BR Jr.. Relationships between macular pigment optical density and cognitive function in unimpaired and mildly cognitively impaired older adults. Neurobiol Aging. 2014. 7;35(7):1695–9. [DOI] [PubMed] [Google Scholar]
- 23.Howells O, Eperjesi F, Bartlett H. Measuring macular pigment optical density in vivo: a review of techniques. Graefe’s archive for clinical and experimental ophthalmology. 2011;249(3):315–47. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman AS. K-TEA II: Kaufman Test of Educational Achievement: Comprehensive Form Manual. AGS Pub./American Guidance Service; 2004. [Google Scholar]
- 25.Kamphuis CB, Giskes K, de Bruijn G-J, Wendel-Vos W, Brug J, Van Lenthe FJ. Environmental determinants of fruit and vegetable consumption among adults: a systematic review. Br J Nutr. 2006;96(4):620–635. [PubMed] [Google Scholar]
- 26.Hammond BR Jr, Johnson EJ, Russell RM, Krinsky NI, Yeum K, Edwards RB, et al. Dietary modification of human macular pigment density. Invest Ophthalmol Visual Sci. 1997;38(9):1795–801. [PubMed] [Google Scholar]
- 27.Beatty S, Nolan J, Kavanagh H, O’Donovan O. Macular pigment optical density and its relationship with serum and dietary levels of lutein and zeaxanthin. Arch Biochem Biophys. 2004. October 1;430(1):70–6. [DOI] [PubMed] [Google Scholar]
- 28.Yeum K, Russell RM. Carotenoid bioavailability and bioconversion. Annu Rev Nutr. 2002;22(1):483–504. [DOI] [PubMed] [Google Scholar]
- 29.Hammond BR, Ciulla TA, Snodderly DM. Macular pigment density is reduced in obese subjects. Invest Ophthalmol Vis Sci. 2002;43(1):47–50. [PubMed] [Google Scholar]
- 30.Lademann J, Meinke MC, Sterry W, Darvin ME. Carotenoids in human skin. Exp Dermatol. 2011;20(5):377–82. [DOI] [PubMed] [Google Scholar]
- 31.Mulder KA, Innis SM, Rasmussen BF, Wu BT, Richardson KJ, Hasman D. Plasma lutein concentrations are related to dietary intake, but unrelated to dietary saturated fat or cognition in young children. Journal of nutritional science. 2014;3:e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammond BR. Lutein and cognition in children. Journal of Nutritional Science. 2014;3:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan NA, Raine LB, Drollette ES, Scudder MR, Kramer AF, Hillman CH. Dietary fiber is positively associated with cognitive control among prepubertal children. J Nutr. 2015. January;145(1):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castelli DM, Hillman CH, Buck SM, Erwin HE. Physical fitness and academic achievement in third-and fifth-grade students. Journal of Sport and Exercise Psychology. 2007;29(2):239. [DOI] [PubMed] [Google Scholar]
- 35.Hyde JS, Linn MC. Gender differences in verbal ability: A meta-analysis. Psychol Bull. 1988;104(1):53. [Google Scholar]
- 36.Hyde JS, Fennema E, Lamon SJ. Gender differences in mathematics performance: a meta-analysis. Psychol Bull. 1990;107(2):139. [DOI] [PubMed] [Google Scholar]
- 37.Reilly D, Neumann DL, Andrews G. Sex and sex-role differences in specific cognitive abilities. Intelligence. 2016;54:147–58. [Google Scholar]
- 38.Mayes SD, Calhoun SL, Bixler EO, Zimmerman DN. IQ and neuropsychological predictors of academic achievement. Learning and Individual Differences. 2009;19(2):238–41. [Google Scholar]
- 39.Mohn ES, Matthan NR, Erdman JW, Neuringer M, Kuchan MJ, Johnson EJ. Lutein and DHA Co-localize in Cell Membranes of Brain Regions Controlling Cognition in the Rhesus Macaque. The FASEB Journal. 2016;30(1 Supplement):689.2, 689.2. [Google Scholar]
- 40.Muriach M, Bosch-Morell F, Alexander G, Blomhoff R, Barcia J, Arnal E, et al. Lutein effect on retina and hippocampus of diabetic mice. Free Radical Biology and Medicine. 2006;41(6):979–84. [DOI] [PubMed] [Google Scholar]
- 41.Lieblein-Boff JC, Johnson EJ, Kennedy AD, Lai C, Kuchan MJ. Exploratory metabolomic analyses reveal compounds correlated with lutein concentration in frontal cortex, hippocampus, and occipital cortex of human infant brain. PloS one. 2015;10(8):e0136904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahl W, Nicolai S, Briviba K, Hanusch M, Broszeit G, Peters M, et al. Biological activities of natural and synthetic carotenoids: induction of gap junctional communication and singlet oxygen quenching. Carcinogenesis. 1997. January;18(1):89–92. [DOI] [PubMed] [Google Scholar]
- 43.Hammond BR, Jr, Fuld K, Curran-Celentano J. Macular pigment density in monozygotic twins. Invest Ophthalmol Vis Sci. 1995. November;36(12):2531–41. [PubMed] [Google Scholar]
- 44.Meyers KJ, Johnson EJ, Bernstein PS, Iyengar SK, Engelman CD, Karki CK, et al. Genetic Determinants of Macular Pigments in Women of the Carotenoids in Age-Related Eye Disease StudyGenetic Predictors of MPOD. Invest Ophthalmol Vis Sci. 2013;54(3):2333–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams KM, Bentham GCG, Young IS, McGinty A, McKay GJ, Hogg R, Hammond CJ, Chakravarthy U, Rahu M, Seland J, Soubrane G, Tomazzoli L, Topouzis F, Fletcher AE. Association Between Myopia, Ultraviolet B Radiation Exposure, Serum Vitamin D Concentrations, and Genetic Polymorphisms in Vitamin D Metabolic Pathways in a Multicountry European Study. JAMA Ophthalmol. 2017; 135(1): 47–53. [DOI] [PubMed] [Google Scholar]
- 46.Kamijo K, Khan NA, Pontifex MB, Scudder MR, Drollette ES, Raine LB, et al. The relation of adiposity to cognitive control and scholastic achievement in preadolescent children. Obesity. 2012;20(12):2406–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamijo K, Pontifex MB, Khan NA, Raine LB, Scudder MR, Drollette ES, et al. The Negative Association of Childhood Obesity to Cognitive Control of Action Monitoring. Cerebral Cortex. 2014. March 01;24(3):654–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hillman CH, Buck SM, Themanson JR, Pontifex MB, Castelli DM. Aerobic fitness and cognitive development: Event-related brain potential and task performance indices of executive control in preadolescent children. Dev Psychol. 2009;45(1):114. [DOI] [PubMed] [Google Scholar]
- 49.Rao AV, Rao LG. Carotenoids and human health. Pharmacological research. 2007;55(3):207–16. [DOI] [PubMed] [Google Scholar]