Abstract
Mammalian Pumilio proteins, PUM1 and PUM2, are members of the PUF family of sequence specific RNA-binding proteins. This review explores their mechanisms, regulatory networks, biological functions, and relevance to diseases. Pumilio proteins bind an extensive network of mRNAs and repress protein expression by inhibiting translation and promoting mRNA decay. Opposingly, in certain contexts they can activate protein expression. Pumilio proteins also regulate non-coding RNAs. The non-coding RNA, NORAD, can in turn modulate Pumilio activity. Genetic analysis provides new insights into Pumilio protein function. They are essential for growth and development. They control diverse processes including stem cell fate and neurological functions such as behavior and memory formation. Novel findings show that their dysfunction contributes to neurodegeneration, epilepsy, movement disorders, intellectual disability, infertility, and cancer.
Keywords: Pumilio, PUM1, PUM2, RNA-binding protein, Post-transcriptional gene regulation
RNA decay
Pumilio proteins are conserved regulators of mRNA fate
Post-transcriptional mechanisms dynamically control the magnitude and spatio-temporal pattern of protein expression, orchestrated by cis-acting RNA features and trans-acting RNA-binding factors [1, 2]. This review focuses on mammalian Pumilio proteins, which belong to the eukaryotic PUF family of sequence-specific, RNA-binding proteins that control mRNA fate [3]. Pumilio was discovered in Drosophila by virtue of its essential role in embryonic development [4–6]. Thereafter, Pumilio proteins have emerged as archetypal post-transcriptional regulators, and much is known about invertebrate orthologs [3, 4, 7, 8]. This review describes progress in understanding the regulatory roles of mammalian Pumilio proteins, including new insights into their functions in stem cells, fertility, development, and the nervous system and how their dysfunction contributes to neurological diseases and cancer.
Defining characteristics of Pumilio proteins
Mammals possess two cytoplasmic ‘classical’ Pumilio proteins, PUM1 and PUM2, which are closely related to each other (76% identical) and to Drosophila Pumilio (30% identical) (Figure 1). The classical Pumilio proteins are characterized by exquisite sequence specificity dictated by a unique, highly conserved RNA-binding domain, the Pumilio Homology Domain (Pum-HD) (80% identical between human and Drosophila). The Pum-HD comprises eight structural repeats of a ~36 amino acid motif (Figure 1A). These motifs were recognized in founding members Drosophila Pumilio (Figure 1B) and Caenorhabditis elegans fem-3 binding factor (FBF) and are referred to as Pum/PUF repeats [9–12].
Figure 1. Features of classical cytoplasmic PUMs and divergent nucleolar PUMs.
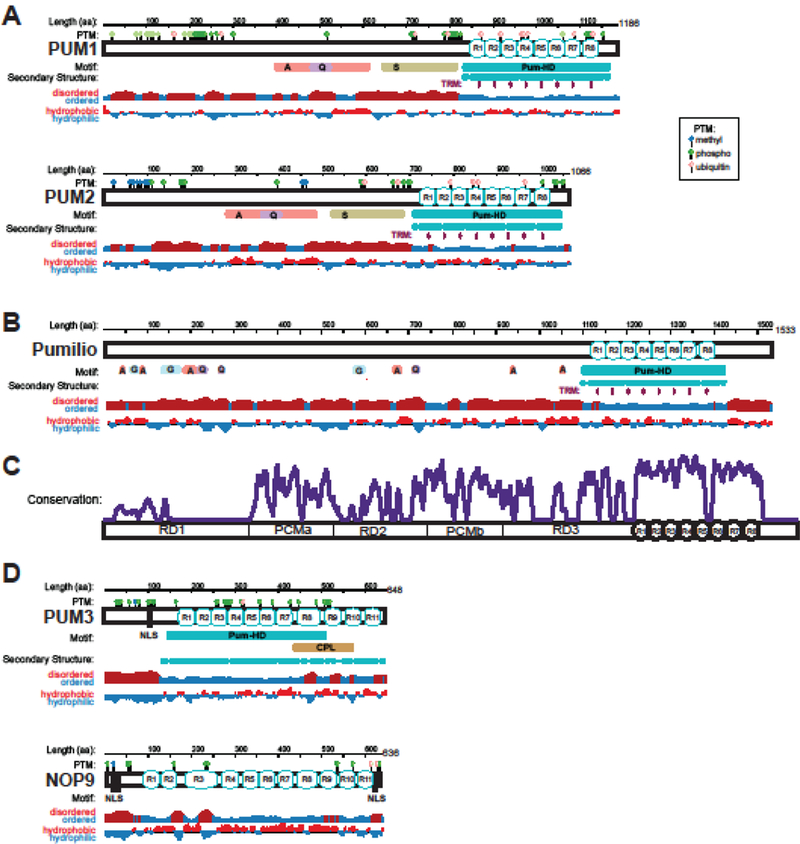
(A) Diagrams of human PUM1 and PUM2 proteins showing length in amino acid (aa) residues, sequence motifs, secondary structure, disordered versus ordered regions (computed by JRONN) [119] and hydrophobic versus hydrophilic amino acid content (adapted from Protein Data Bank: https://www.rcsb.org). Post-translational modifications (PTM) including methylation, phosphorylation, and ubiquitylation from Uniprot (https://www.uniprot.org) and Phosphosite (https://www.phosphosite.org) are shown at the top. Motifs designated by a single letter represent low complexity regions enriched for that amino acid residue (A = alanine rich, Q = glutamine rich, S = serine rich, G = glycine rich). The TRMs within each Pum repeat (R1-R8) are also shown. (B) Diagram for founding member, Drosophila Pumilio, is shown for comparison. (C) Plot of relative sequence conservation versus amino acid residue position of 82 Pumilio protein orthologs including insects, fish, reptiles, birds, marsupials, mammals, primates and humans, generated using Clustal Omega [120], Consurf server [121], and Emboss Plotcon (http://www.bioinformatics.nl/cgi-bin/emboss/plotcon). For reference, conservation is plotted relative to functional domains defined for Drosophila Pumilio including three repression domains (RD1–3), Pumilio Conserved Motifs (PCMa and PCMb), and the Pum repeats (R1-R8) of the Pum- HD. Troughs represent sites of insertion. Peak height is proportional to conservation of sequence identity. (D) Diagrams of the divergent Pumilio orthologs PUM3 and NOP9. Motifs include predicted nuclear localization signals (NLS) and C-terminal Penguin Like (CPL) motif (PFam PF08144).
Classical PUMs ranging from insects to humans also share a unique N-terminal region that lacks homology to other proteins and is predicted to be intrinsically disordered overall (Figure 1A and B)[13]. The N-terminal regions of PUMs show higher rates of evolution, including variable low complexity regions, insertions, and deletions, with the exception of two segments designated Pumilio Conserved Motifs (PCMa and PCMb)(~37% and ~62% identity between Drosophila and human PUMs, respectively)(Figure 1C)[13]. It is noteworthy that multiple isoforms of PUM1 and PUM2 encoding mRNAs have been cataloged (for example, see NCBI Gene ID: 9698 for human PUM1 and Gene ID: 23369 for human PUM2), including alternative transcription initiation sites, 5´ exons, internal exons, and 3´ processing events. These mRNA isoforms have the potential to impact the function and expression of the PUMs, an area of inquiry that remains unexplored.
Mammals also have two ‘divergent’ PUMs, PUM3/Puf-A and NOP9 (Figure 1D) that are conserved in eukaryotes [14]. They have Pum-HD regions; however, their RNA-binding domains are extended with 11 Pum repeats [15, 16]. In contrast to classical PUMs, divergent PUMs have predicted nuclear localization signals (NLS), are predominantly nucleolar, and function in ribosome biogenesis [17, 18]. The PUM3 family appears to bind to structured RNA or DNA and lacks sequence specificity, making these proteins quite distinct from classical PUMs [15]. NOP9 proteins are a hybrid of classical PUM and PUM3 traits; they bind to both specific sequences or structured elements in pre-ribosomal RNA [16, 19–21]. As less is known about their functions in mammals, this review does not address divergent PUMs further.
Structure and specificity of Pumilio proteins
Epiphanies in Pumilio function came when Drosophila Pumilio was shown to bind short RNA motifs in the 3´UTR of the hunchback (hb) mRNA to control embryogenesis [11, 22, 23] and C. elegans FBF was shown to bind a related motif in the fem-3 mRNA to control gametogenesis [12]. In both instances, the Pum-HD regions conferred specific RNA interaction and resulted in repression of their respective target mRNA. Although the functions of mammalian PUM1/2 were not known at that time, their Pum-HDs bound to the same RNA motifs in the hb mRNA [11, 24]. The binding specificities for Pumilio proteins from a variety of species, including Drosophila to humans, are now well-characterized [11, 22, 24–35]. The consensus binding site for proteins closely related to Pumilio, 5´UGUAHAUA, herein referred to as the Pumilio Recognition/Response Element (PRE), is well conserved.
Structural studies illuminated how Pumilio proteins specifically recognize RNA sequences. Crystal structures of the Pum-HDs of Drosophila Pumilio and human PUM1 revealed α-helical Pum repeats that formed a curved structure (Figure 2A)[36, 37]. The most highly conserved amino acid residues line the inner concave surface of the Pum-HD. Crystal structures of PUM1 in complex with the PRE from hb mRNA illuminated the elegant mechanism of RNA sequence recognition by Pum repeats [31]. Each repeat employs three side chains, referred to as a tripartite recognition motif (TRM)[38], to recognize an RNA base. Two side chains form hydrogen bond or van der Waals contacts with an edge of the base and a third side chain forms a stacking interaction. Remarkably, the particular sets of side chains specify the RNA base recognized [39]. For example, the TRM motif NYxxQ (x is a hydrophobic residue) uses edge-interacting Asn and Gln and base-stacking Tyr to specify uracil (Figure 2B). This TRM can be abbreviated NQ/Y. The PUM1-RNA structure also revealed TRMs that specify adenine (CQ/R or SQ/R) and guanine (SE/N). Selection experiments later identified TRMs that specify cytosine (SR/Y)[40, 41].
Figure 2. Structure and RNA recognition by PUM1 and PUM2.

(A) High conservation of the RNA-binding surface of PUM proteins. Ribbon diagram of a crystal structure of human PUM1 in complex with hb NRE RNA is shown colored according to the degree of amino acid sequence conservation calculated using the Consurf server [117]. The most highly conserved positions are colored maroon, and the least conserved are colored cyan. The specific PUM1 amino acid residues that are mutated in PADDAS (R1139W and R1147W) and the PRCA (T1035S) are indicated by space-filling spheres. (B) PUM1 RNA recognition code. Specific interaction of Pum repeats with U, A, G, and C nucleotides is shown. (C) Ribbon diagram of a crystal structure of human PUM2 in complex with erk2 PRE RNA. The RNA-binding helices are colored maroon. PUM2 binds to the erk2 PRE RNA using the alternative ‘base-omission mode,’ where bases A4 and C5 (green) are directly stacked and R888 in repeat 5 (green) does not stack between bases A4 and C5.
Once mRNA targets of mammalian PUMs were identified, crystal structures of human PUM1 and PUM2 in complex with their PREs showed that PUM1 and PUM2 bind similarly to these sequences as to hb RNA (Figure 2C)[32]. These structures also showed that sequence variations at the 5th nucleotide position result in different modes of RNA recognition in that region. The Hoogsteen edge of an A5 base may be recognized instead of the Watson-Crick edge, and a C5 base may stack directly with the A4 base and not contact the protein (highlighted in Figure 2C). The fact that Drosophila, mouse, and human PUMs have identical TRMs embedded within highly similar Pum-HDs of nearly identical structure (1.2–1.6 Å rmsd between Drosophila and human, 1.4 Å rmsd between human PUM1 and PUM2) can explain their conserved PRE specificity ([25, 36 37, 42]. The modular recognition of RNA sequence has enabled prediction of PUM binding sites in target RNAs and rational design of Pumilio proteins with engineered RNA specificity and novel functions [31, 38, 43–49].
Mechanisms of PUM-mediated repression (PMR)
Discovery of the mechanisms of mRNA regulation by Pumilio proteins is crucial for understanding their functions. Analyses of invertebrate orthologs established that they repress protein and mRNA levels of target mRNAs to which they bind [3, 4, 7, 8]. This inhibitory function carries over to mammals; PUM1 and PUM2 repress PRE-containing target mRNAs, manifested by reduced levels of that mRNA and the encoded protein [50], hereon referred to as PUM-mediated repression (PMR).
Stability and translation of mRNAs are intermingled. Translation is promoted by two key mRNA modifications, the 5´ 7-methyl guanosine cap (5´ cap) and the 3´ poly-adenosine tail, which are bound by eIF4E and poly(A) binding proteins (PABP), respectively [51]. The cap and poly(A) tail also protect the RNA from spurious degradation, and their removal typically initiates mRNA decay [52]. Given their pivotal roles in translation and stability, the 5´ cap and 3´ poly(A) tail are nodes for regulatory processes. Indeed, invertebrate Pumilio proteins repress by promoting deadenylation and, in several instances, decapping [53–56].
In mammals, PMR results in degradation of target mRNAs [29, 50, 57]. Biochemical analysis showed that components of the major deadenylation machine, the Ccr4-Not complex (CNOT), copurify with human PUM1 and PUM2 [50, 54, 58]. Recruitment of the CNOT complex is a conserved mechanism of PMR, as the Pum-HD of Pumilio orthologs from yeast to humans directly interact with the Pop2/Caf1/CNOT7/CNOT8 deadenylase enzyme subunit (Figure 3)[54, 59–62]. This conclusion is supported by functional analyses, which showed that blocking CNOT activity by deletion, RNAi depletion, or dominant negative approaches reduced PMR in multiple species including humans [50, 54, 55, 60–63].
Figure 3. Model of PUM1/2 repression mechanisms.

PUM proteins bind the PRE, typically located in the 3´UTR of target mRNAs. Repression of the target mRNA is mediated in at least three ways: (A) The Pum-HDs of PUMs antagonize the translational activity of poly(A) binding protein (PABPC1). (B) PUMs promote translational repression and mRNA decay by recruiting the CCR4-NOT deadenylase complex (CNOT). (C) The N-terminal regions of PUM orthologs confer repression, but the mechanism remains to be determined.
Analysis of Drosophila Pumilio identified three additional repression domains (RDs) in the N terminus (Figure 1C) that reduce protein and mRNA expression levels [13]. The PUM RDs can function autonomously when artificially directed to an mRNA. These novel RDs do not share sequence homology with each other or known domains. While the RDs are among the least conserved parts of PUMs (Figure 1C), experimental evidence indicates that analogous regions of human PUM1/2 possess repression activity (Figure 3)[13]. An important future goal will be to determine the regulatory mechanisms of the PUM N-terminal RDs.
The generality of PUM-mediated RNA decay has been borne out by transcriptome-wide analyses which found that the PRE is among the most strongly correlated features with mRNA instability [64–66]. Massive parallel reporter assays showed that PREs destabilize mRNAs during vertebrate embryogenesis [67]. Analysis with reporter mRNAs indicated that the major effect of PREs is to reduce mRNA levels [33, 50, 68]. Moreover, depletion of PUM1/2 in human cells stabilized hundreds of PRE-containing transcripts, whereas over-expression of PUM1/2 destabilized their target mRNAs [33, 69].
Inhibition of translation is also involved in PMR. Compelling evidence supports that antagonism of the translational activity of poly(A) and PABP is a second conserved repression mechanism (Figure 3) [50, 61]. Biochemical and cell-based data from invertebrates and humans indicate that PABP and poly(A) are required for efficient repression by the Pum-HD of multiple orthologs [61, 70]. Mechanistically, the Pum-HD is thought to associate with PABP and to disrupt its ability to promote translation, apparently without dislodging it from the mRNA [61, 70]. At this time it is unclear if mammalian PUMs interact with PABP and how this mechanism interfaces with deadenylation.
Additional mechanisms of PUM-mediated translational inhibition have been proposed. First, Xenopus PUM2 was shown to bind to the 5´ cap of mRNAs, and mutation of the cap-binding motif alleviated translational repression in oocytes [71]. This cap-binding motif is in PCMb, and does not coincide with the Pumilio N-terminal RDs (Figure 1C). The potential for this mechanism to contribute to PMR by mammalian PUMs has not been addressed. Second, PUM orthologs were reported to inhibit translation elongation in vitro by inhibiting translation elongation factor eEF1a through an Argonaute-mediated mechanism [72]; however, disrupting this potential mechanism did not affect PMR in cells [61].
Do PUMs act on mRNAs before or after they engage the translation apparatus? Conflicting observations have been reported; in one case PUM2 did not associate with poly-ribosome bound mRNAs [73], whereas another study observed that a minor population of PUM1/2 cofractionates with poly-ribosomes [34]. A recent study showed that PMR resulted in diminished poly-ribosome association of mRNAs with two or more PREs, but the magnitude of the effect was modest relative to the effect on mRNA abundance [68]. The effect of PUMs on translation may be dependent on the context of specific mRNA or biological conditions. For instance, protein expression of several mRNAs is affected by PMR to a larger extent than changes in the level of that mRNA [33, 34]. Future analyses of the transcriptome-wide impact of PUMs on translation may help address these issues.
The PUM-mediated activation (PMA) paradox
New data reveal that mammalian PUM1/2 can activate certain mRNAs. For example, PUM1/2 bind PREs in the 3´UTR of the mRNA encoding the transcription factor FOXP1 and enhance its expression [74]. Another study identified more than 100 PUM-activated mRNAs that contain a PRE, half of which are known to be bound by PUMs [33]. While seemingly paradoxical, such bifunctional control of mRNAs is not without precedent; for example, the Iron Response Element Protein has positive or negative effects, depending on the context of its binding site [75]. Also, the cytoplasmic poly-adenylation element binding protein (CPEB) can switch from repressor to activator in response to developmental cues [76]. In fact, activation of mRNAs has been observed for PUM orthologs in multiple species [60, 77–80]. While the mechanism of PMA in mammals is unknown, the phenomenon is transcript-specific, suggesting that mRNA features may be key determinants. Evidence in non-mammalian model organisms indicates that activation by PUM orthologs involves mRNA stabilization, cytoplasmic poly-adenylation, and enhancement of translation [60, 78–80]. Perhaps PUM1/2 collaborates with other regulatory factors, such as poly- adenylation factors, to promote mRNA stability and/or translation or, alternatively, displaces a dominant regulator from the target mRNA.
Overlapping functions of PUMs
The presence of two classical mammalian PUMs raises the question: does each PUM have a specialized, unique function or are they redundant? Their Pum-HDs are 90% identical, have identical TRMs that confer indistinguishable RNA specificities, and associate with overlapping sets of mRNAs [29, 30, 32, 34, 35]. Both PUMs repress target mRNAs with comparable magnitude [50]. Moreover, the two PUMs are functionally redundant in cells; both must be simultaneously depleted to fully alleviate PRE-mediated repression [33, 50]. In the case of PUM-activated mRNAs, again both PUMs appear to participate [33, 74]. Based on these observations, it is likely that PUMs are redundant with overlapping functions, consequently both PUMs should be investigated when analyzing mRNA regulation.
PUM1 and PUM2 proteins and mRNAs are coincidently expressed in a wide range of tissues and cell types throughout development (Figure 4)[27, 81, 82](see also Illumina Body Map 2 Project and the Human Protein Atlas [83]). Based on these observations, it is reasonable to predict that their regulatory functions broadly overlap. In certain tissues, enrichment of either PUM1 or PUM2 mRNAs are observed (Figure 4). For instance, PUM2 mRNA is more abundant in blood and the cerebellum of the brain than PUM1, whereas PUM1 is more prevalent in skeletal muscle. Still, our knowledge of differential expression of the two PUM proteins in diverse cell types and stages of development remains incomplete, and deeper understanding could reveal unique, context- specific roles.
Figure 4. Gene expression of human PUM1 and PUM2 mRNAs across 53 tissue types.
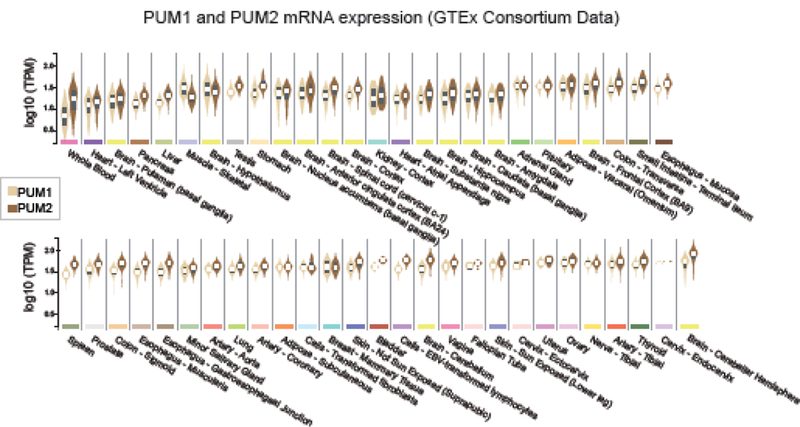
Data were obtained from the GTEx consortium database. Violin plots showing median, interquartile range, and density for expression values of mRNAs for each PUM, in normalized, logio Transcripts per Million units (TPM), were generated for the indicated tissues using the GTEx portal website (http://www.gtexportal.org/) on 9/5/2018 using data from 10,294 samples.
Insights into the PUM regulatory networks
To understand the impact of PUMs on gene expression, it is necessary to define the repertoire of transcripts that they regulate. Based on current knowledge, three criteria should be applied to define PUM target RNAs, including (1) the presence of a PRE site that is (2) bound by PUM1/2 resulting in (3) a functional consequence on the fate of that target RNA, including PMR or PMA. Numerous studies support these conclusions for individual genes (cataloged in Table S1, Bohn et al, 2018)[33]. Here, we summarize transcriptome-wide analyses of PUM1/2 regulatory networks based on one or more of these criteria.
High resolution definition of the PRE [11, 28–32, 35, 84] enabled prediction of thousands of potential PUM targets across the transcriptome. In humans, mRNAs and non-coding RNAs from 7822 genes contain PREs, ranging from 1–19 sites (cataloged in Table S6 of Bohn et al, 2018)[33]. Interestingly, several ncRNAs contain the highest density of PREs [33, 69, 85]. These observations suggest that PUMs may broadly impact gene expression.
An extensive collection of PUM1/2-bound RNAs from more than 4000 unique genes have been identified from human or mouse cells and several tissues [27, 29, 30, 34, 35, 85]. PRE sequences are enriched in more than half of these mRNAs, consistent with direct PUM targeting[33]. The remainder may be indirectly bound (i.e. PUM1/2 bind another RBP that is in direct contact with that RNA), could be recognized via combinatorial mechanisms that alter PUM specificity, or in some instances might be false positives.
PUM-mediated regulation of the transcriptome has been investigated by either deleting, depleting, or over-expressing PUM1 and/or PUM2, thereby identifying many hundreds of affected RNAs [33, 34, 69, 85]. Of those, hundreds of RNAs are direct targets, based on their inclusion in PREcontaining and PUM-bound datasets [33]. These analyses were restricted to a few cell/tissue types; thus, to capture the full impact of PUMs on gene expression, future analyses should pursue PUM targets in multiple contexts. Moreover, previous global analyses focused on RNA levels. To fully measure the influence of PUMs, ribosome profiling and quantitative proteomic approaches should be applied to measure changes in the translated proteome.
What can we learn from these PUM regulatory networks? First, the location of PREs is a determinant of regulation. PREs are most prevalent in 3´UTRs in the predicted, bound, and regulated target mRNAs [27, 29, 30, 33] and are less prevalent in 5´UTR and coding sequences. PREs in 3´UTRs correlate most strongly with PMR, as do PREs in ncRNAs, whereas PREs in coding regions show a weaker correlation [33]. In contrast, 5´UTR PREs do not contribute to PUM-induced changes in RNA levels. A single PRE is necessary and sufficient for regulation, and more PREs correlates with increased regulation, an effect that saturates at 3–4 PREs [29, 33, 68]. Still, computational modeling indicates that we have more to learn, because parameters of PRE number, location, and PUM occupancy do not fully account for the observed regulation. Given that each PUM target RNA likely has a unique constellation of cis-elements and trans-acting RNA- binding factors that control its fate, it is necessary to determine how PUM1/2-mediated regulation integrates with those factors in the context of individual transcripts.
Characterization of PUM1/2 regulatory networks provides insights into the diverse cellular processes and molecular functions that they can affect. Here we summarize the significantly enriched gene ontology categories among the predicted, bound, and regulated PUM targets (Table 1) [27, 29, 30, 33–35, 85–90]. Future research should focus on investigating the direct impact of PUMs on these pathways and processes in normal and disease states. Non-coding RNAs were also present in the PUM regulatory network, but as few have documented functions, interpreting these connections awaits characterization of the ncRNAs themselves.
Table 1:
Gene ontology categories enriched in PUM regulatory networks.
| Gene Ontology Category | Examples | References |
|---|---|---|
| Signaling pathways | RAS, GTPase, WNT, Hedgehog, Notch, PDGF, EGF receptor, MAP kinase, serine/threonine phosphatase, TGF beta, and insulin receptor binding | [27, 29, 30, 33, 34, 89, 122] |
| Control of cell death | Negative regulation of apoptosis | [27, 30, 33, 34] |
| Cell migration and adhesion | Extracellular matrix glycoproteins, intracellular adhesion molecules, integrins, and cadherins | [29, 33, 34, 89] |
| Core cellular functions | Genome replication and stability, DNA repair, mitosis, spindle assembly and chromosome segregation | [29, 30, 33, 85] |
| Cell cycle and proliferation regulators | CDK/Cyclins, CDK inhibitors, and G1/S transition factors | [27, 29, 30, 33, 34, 86, 89] |
| Metabolic pathways | Oxidative phosphorylation, ubiquinone metabolism, nucleotide metabolism, fatty acid metabolism, pantothenate kinase activity | [27, 29, 30, 33, 34] |
| Transcription regulators | DNA-binding transcription factors including those involved in cancer and stem cell fate | [29, 30, 33, 88, 89] |
| Neurological functions | Nervous system development, differentiation, morphology, axon guidance, nerve signaling and impulse transmission, neurotransmitter transport, secretion, binding, and metabolism, and voltage-gated ion channels | [30, 33, 34, 57, 89, 90] |
| Neurodegenerative disease genes | Parkinson’s, cerebellar ataxia, fragile X syndrome, and Alzheimer’s disease | [30, 33, 34, 57, 105] |
| Cancer genes | 330 cancer genes have PREs, 209 were bound by PUM1/2, and at least 45 have been shown to be regulated by PUMs, including tumor suppressors and oncogenes | [29, 30, 33, 86, 87] |
Localization of PUM1/2 to intracellular granules
Specific RNAs and proteins can condense into membrane-less ribonucleoprotein (RNP) granules. Classical PUMs operate in the cytoplasm and several studies report that PUM1/2 can be enriched in three types of cytoplasmic RNP granules: stress granules, processing bodies (P-bodies), and neuronal transport granules [91–94]. Stress granules form in response to environmental stresses and are defined by their content of translationally inactive RNPs containing certain translation factors, RNA-binding proteins, and mRNAs [95]. Both PUM1 and PUM2 were observed in stress granules induced by oxidative stress and viral infection [29, 93, 96]. Moreover, over-expression of PUM2 can induce stress granule formation in lieu of stress [91]. PUM1/2 are enriched in purified P-bodies [94], which are RNP granules that are enriched with mRNA decay factors and translationally inactive mRNAs. In neurons, RNP granules are actively transported along dendrites to deliver mRNAs to synapses, where they can be locally translated in response to synaptic activity. PUM2 is present in RNP granules that traffic in dendrites, in addition to being present throughout the neuronal cell body [73, 91].
The presence of PUM1/2 in RNP granules raises important questions. Foremost, does localization have a functional consequence? Could RNP granules act to sequester or store PUMs and their target mRNAs for subsequent re-animation? Alternatively, does granule localization contribute to PUM-mediated repression? Because PUM1/2 repress mRNAs under conditions where RNP granules are not present, localization to granules is unlikely to be obligatory. In the case of neuronal RNP granules, PUM2 may bind and translationally repress specific transcripts whilst they are being localized to the synapse; those mRNAs could then be de-repressed in response to synaptic activation. The determinants of PUM1/2 incorporation into granules remains unknown. Low complexity, intrinsically disordered regions of proteins facilitate RNP granule formation [95] and properties of RNAs can also drive formation [93, 94, 97]. In the case of PUMs, both concepts may be relevant; PRE-containing mRNAs and ncRNAs are enriched in stress granules [93], and the Pum-HD and disordered N terminus are necessary for PUM2 association with stress granules [91].
Insights into regulation of PUM1/2 activity
The activities of PUMs must be modulated to avoid deleterious effects on gene expression. One homeostatic mechanism is auto- and cross-regulation through negative feedback. PUM1 and PUM2 mRNAs have 8 and 13 PREs, respectively [33], and multiple studies reported PUM1/2 binding to their own and each other’s mRNAs in cultured cells, stem cells, and brain [29, 30, 34, 88], and experimental evidence supports that depletion of one PUM can lead to increased expression of the other PUM [34, 86].
The activities of PUM1 and PUM2 may be modulated by post-translational modifications. Multiple sites of phosphorylation, ubiquitylation, and methylation have been mapped on each PUM (Figure 1), though the modifying enzymes and consequences of these modifications remain unknown. Thus far, only one site has been shown to affect PUM activity; phosphorylation of S714 of PUM1 is stimulated by growth factors and promotes binding and repression of the CDKN1B/p27 mRNA [86]. Surprisingly, S714 is outside of the RNA-binding domain and it is unclear how this modification affects the PUM-RNA interaction.
PUM activity can be modulated by a cytoplasmic, long ncRNA NORAD (NcRNA Activated by DNA damage)[69, 85]. NORAD contains 17 PREs, is avidly bound by PUMs, and therefore was proposed to act as a competitive inhibitor. Indeed, depletion of NORAD enhanced PMR of PUM- bound, PRE-containing mRNAs, whereas its over-expression reduced PMR. NORAD is induced by DNA damage, and depletion of NORAD leads to genome instability [85]. Likewise, PUMs affect genome stability, and this effect partly depends on NORAD. Transcriptome-wide analysis indicates that PUM1/2 and NORAD have opposing effects on an overlapping set of 193 genes, including replication, repair, and mitotic factors [85].
Even with its high PRE content and abundant expression, NORAD contains a minor fraction of all PREs in expressed transcripts [33, 98]. How can NORAD competitively inhibit PUM? A new study reports that NORAD binding to PUM1/2 may be facilitated by the RNA-binding protein SAM68 [98]. Like NORAD, SAM68 counteracts PUM2 repression and is necessary for NORAD to inhibit PMR. Mechanistically, PUM2 and SAM68 interact in an RNase-resistant manner. Based on these observations, the NORAD-SAM68 RNP is proposed to inhibit PUMs by binding them more avidly than other PRE-containing target RNAs. Interestingly, NORAD is itself subject to PMR, indicative of a PUM-NORAD feedback loop [33]. Thus PUM and NORAD functions are intertwined, but much remains to be learned with regard to when, where, and how the PUM-NORAD regulatory mechanism controls gene expression.
Combinatorial control can alter the regulatory activity and target specificity of invertebrate Pumilio orthologs [4, 25, 79, 99]. In mammals, a collection of RNA-binding proteins have been reported to associate with PUMs, including orthologs of Nanos (i.e. NANOS1, 2, 3)[100–103], Brain T umor (i.e. TRIM71)[104], and CPEB [99]. These observations suggest combinatorial control of PUM1/2 RNA-binding and regulatory activities; however, this remains to be proven. The FMRP protein is an intriguing example; it associates with PUM1 and PUM2, and they bind partially overlapping sets of nearly 200 mRNAs, including each other’s mRNAs [29, 30, 34]. While PUMs and FMRP share some neurodevelopmental phenotypes [34, 57, 105, 106], their functional relationship has not been investigated. Additionally, members of the DAZ/Boule family of RNA-binding proteins, which have important roles in the germline, were reported to associate with human PUM2, though their influence on its activity remains unknown [107].
PUM1/2 may interface with microRNA (miRNA)-mediated regulation. Binding sites of miRNAs are enriched near PREs in a subset of PUM target mRNAs [30, 33]. Further, PUM1/2 associate with Argonaute proteins, the core component of the miRNA-induced silencing complex (RISC) [61, 72]. While Argonautes are not required for PMR per se [57, 61], they could act in a combinatorial manner. Two studies reported functional interactions between PUMs and miRNAs. In the first case, miR-221 repression of the CDKN1B/p27 mRNA was reported to be PUM dependent [86]. In this mRNA, the miR-221 binding sites can base pair with the PREs, and PUM was proposed to disrupt that interaction, thereby enabling repression by miR-221. But CDKN1B mRNA is likely the exception, because complementarity of a PRE with a miRNA site is rare. In the second example, PUMs and PREs in the E2F3 mRNA were shown to potentiate repression by miRNAs that target E2F3, though the mechanism remains unknown [87]. A germane study systematically assessed the functional interplay of PRE and let-7 miRNA sites and found that they operate independently, with additive effects on mRNA regulation [68].
Further research is necessary to determine the ability of PUM1/2 partners to modulate RNA binding, PMR, or PMA. We suggest the following criteria for interrogating combinatorial control: Both RBPs should bind to the same mRNA; they may do so cooperatively and/or with altered specificity. Both RBPs should make significant contributions to controlling the fate of that mRNA. Their combined activities may be additive, synergistic or, alternatively, their partnership may cause a new regulatory outcome.
Biological functions of mammalian Pumilio proteins
New studies illuminate the biological functions of mammalian PUM1/2 and the disease phenotypes resulting from their dysfunction, as summarized in Table 2. Here we explore their functions in stem cell biology, growth and development, in the germline and nervous system. We also discuss discoveries that link PUMs to cancer, infertility, and neurological disorders.
Table 2:
Major functions of PUM1 and PUM2 and consequences of their dysfunction.
| PUM Function | PUM Dysfunction | Approaches | References |
|---|---|---|---|
| Growth | Reduced body size | Mouse knockout, naturally occurring human mutations | [27, 34, 105, 108–110] |
| Development | Embryonic lethality, developmental disability | Mouse knockout, naturally occurring human mutations | [34, 105] |
| Stem cell fate | Defects in embryonic, germline, hematopoietic, and neural stem cell proliferation and differentiation | Mouse knockout, RNAi in human and mouse cells | [27, 34, 74, 82, 88] |
| Gametogenesis | Reduced fertility | Mouse knockout | [27, 109, 110] |
| Hematopoiesis | Promote acute myeloid leukemia | RNAi in human and mouse cells, analysis of mouse | [74, 82] |
| Neurogenesis | Neurodegeneration | Mouse knockout, RNAi in mouse brain | [34, 57, 89] |
| Neural electrophysiology | Epilepsy | Mouse knockout, RNAi in rat neurons, naturally occurring human mutations | [90, 105, 108, 114] |
| Behavior | Hyperactivity, deficient nesting behavior | Mouse knockout | [57, 90, 108] |
| Learning and memory formation | Diminished spatial memory, intellectual disability | Mouse knockout, naturally occurring human mutations | [34, 57, 105] |
| Motor function | Cerebellar ataxia | Mouse knockout, naturally occurring human mutations | [57, 105] |
Control of stem cell fate
A prescient review suggested that the “primordial function of PUF proteins is to sustain mitotic proliferation of stem cells” [3]. Since then, accumulating data reveal that mammalian PUMs regulate stem cell fate in diverse contexts. In cultured embryonic stem cells (ESCs), PUM1 is required to exit self renewal, wherein it promotes differentiation by repressing pluripotency transcription factors [88]. In effect, PUM1 rewires the gene expression circuitry from ESC towards differentiation. While depletion of PUM1 reduced differentiation of ESCs, depletion of PUM2 had no effect, distinguishing its function from PUM1. As discussed below, PUMs also regulate the fate of neuronal, germline, and hematopoietic stem cells.
Regulation of growth and development
Genetic analysis in mice revealed that PUMs are necessary for viability, growth and development. Combined knockout of PUM1 and PUM2 (PUM1&2) resulted in lethality during embryogenesis [34], whereas individual PUM knockouts cause diminished growth and size throughout lifespan [27, 34, 108–110]. This function appears to be relevant to humans, as PUM1 mutations are linked to reduced growth and development [105]. Interestingly, neural-specific PUM1&2 knockout also resulted in reduced growth and body weight, indicating an important role of brain function in this phenotype [34].
Regulation of gametogenesis
Pumilio orthologs in Drosophila and C. elegans have roles in germline stem cells wherein they regulate gametogenesis, and their disruption negatively impacts fertility [12, 111–113]. This role is conserved in mammals, as knockout of PUM1 or PUM2 reduces germline function. PUM1 is important for male fertility, wherein it suppresses activation of the p53 pathway within germ cells [27]. In the absence of PUM1, the testes are smaller and the germ cells undergo premature apoptosis as spermatocytes. Male mice with a PUM2 gene trap mutation have smaller testes and degenerated seminiferous tubules [109]. While mice lacking either PUM gene exhibit reduced gametogenesis, they can still reproduce, perhaps sustained by the remaining PUM.
In the female germline, PUM1 is important for fertility and production of viable oocytes [110]. PUM1 knockout mice are subfertile, produce smaller litters, and exhibit reduced ovarian follicle formation and oocyte development. Meiosis is delayed with defective chromosome synapsis. The target mRNAs underlying these phenotypes remain unknown. PUM1 does affect expression of the synaptonemal complex component, Sycp1, though this mRNA does not possess a PRE, suggesting that PUM1 may regulate synaptonemal complex disassembly or stability, rather than Sycp1 mRNA directly. In contrast, females with PUM2 knockout have normal fertility [109, 110], providing an example of PUM1 specific function. While both PUMs are present in ovaries, PUM1 protein is enriched in oocytes relative to PUM2; thus, PUM2 levels in the female germline may be insufficient to compensate.
Roles in neurogenesis, neuronal function, behavior, learning and memory formation
Drosophila and C. elegans PUM orthologs function in the nervous system to control memory formation and neural morphology and electrophysiology [4, 77]. Astonishingly, mammalian PUMs also regulate these and other neurological processes. PUM1 and PUM2 are expressed in neural stem cells, progenitors, and mature neurons throughout the developing brain [34, 57, 89, 91, 108]. PUM2 was shown to control morphology and electrophysiology of cultured hippocampal neurons wherein it directly regulates mRNAs encoding voltage-gated sodium channel Nav1.1 (SCN1A) and Nav1.6 (SCN8A gene)[73, 114]. Transcripts from ten voltage-gated sodium ion channels have PREs and are potential PUM targets [33], suggesting a general role for PUMs in control of electrophysiology. Inactivation of PUM2 by gene trap caused hyperactivity and behavioral deficiencies in spatial and memory tests and nest building [90, 108]. This latter phenotype is shared by PUM1 knockout mice [57].
Loss of PUM function contributes to seizures. In humans, PUM1 mutations are linked to epilepsy [105]. The relationship of PUM1 to seizures has not been reported in mouse models; however, inactivation of PUM2 increased the propensity for seizures [90, 108]. PUM2 inactivation did not affect overall hippocampal neuronal activity, but decreased paired-pulse inhibition, which can contribute to spontaneous seizures. Hundreds of mRNAs were upregulated in response to PUM2 inactivation in the brain, including those involved in synaptic transmission and structure [90]. Moreover, PUM2 dynamically affected expression of Nav1.1 (SCN1A), Nav1.2 (SCN2A), and Nav1.6 (SCN8A) encoding mRNAs, which are involved in epilepsy [114, 115]. Perturbation of gamma-aminobutyric acid (GABA) activity, an inhibitory neurotransmitter, may also contribute to propensity of seizures [116]. PUM1/2 repress the ABAT mRNA, which encodes the enzyme that catabolizes GABA [33]; and therefore may stabilize GABA. PUM2 also represses GABRA2, a subunit of the GABA type A ligand-gated chloride channel, which is important for GABA mediated inhibitory activity [90]. Thus, deficiency of PUM1/2 activity may dysregulate GABA and its receptor, which theoretically could contribute to increased susceptibility to seizures.
New studies establish key roles of mammalian PUMs in brain development, neurogenesis and neurological functions [34, 57, 73, 89]. Simultaneous neural-specific PUM1&2 knockout caused deficiency in spatial learning and memory [34]. The brains of these PUM1&2 knockout mice exhibited reduction of the dentate gyrus region of the hippocampus, which is important for learning and memory [117]. The dentate gyrus contains the neural stem cells that produce progenitors which subsequently differentiate into hippocampal neurons. The neural stem cell population in the brains of the PUM1&2 knockout mice did not appear to change, but the population of neural progenitors increased whereas the number of immature neurons decreased, indicating that PUMs promote proliferation and differentiation [34]. PUMs are necessary for survival of immature neurons, as apoptosis increased in the dentate gyrus of the double knockout mice. These observations were corroborated by neurosphere assays, wherein neural stem cells from PUM1&2 knockout showed reduced proliferation, self-renewal and differentiation, along with increased apoptosis. Another study analyzed the role of PUM2 in brain development and provides evidence for its importance in cortical neurogenesis and differentiation [89].
An extensive set of mRNAs are likely controlled by PUMs in the brain. Hundreds of PREcontaining mRNAs bound by PUM1 and PUM2 in mouse brain were identified and are enriched in neuro-developmental functions [34, 89]. Widespread changes in gene expression were observed in the PUM1&2 knockout brains, with more than 7000 differentially expressed genes, whereas single PUM knockouts had a small number of affected mRNAs [34]. More than 300 of the differentially expressed mRNAs were bound by PUMs, with evidence of both PMR and PMA.
In several cases, the levels of PUM-bound target mRNAs did not change in the PUM1&2 knockout brain, but the level of encoded protein was altered, indicative of translational control [34]. A new study suggests a mechanism where PUM2 may collaborate with eIF4E-Transporter (4E-T) to inhibit expression of proteins during neuronal differentiation [89]. 4E-T inhibits translation by binding to the translation initiation factor eIF4E [118]. In the brain, PUM2 and 4E-T target an overlapping set of nearly 300 mRNAs, including those of neuronal specification transcription factors Brn1 and Tle4 [89]. During embryonic development, Brn1 and Tle4 mRNAs are coexpressed in progenitor cells whereas in postnatal stages they are expressed in mutually exclusive sets of neurons. Depletion of PUM2 or 4E-T caused aberrant co-expression of Tle4 and Brn1 in neuronal precursors and differentiating neurons. PUM2 and 4E-T co-immunoprecipitate, but whether this results from direct protein contacts or co-occupancy of target mRNAs remains unknown. Overall, this work suggests that PUM2 and 4E-T collaborate to repress expression of specification proteins during neurogenesis [89]. It is noteworthy that PUM2 and 4E-T also independently target many mRNAs, indicating that their regulatory function is not likely fully interdependent.
Roles in neurodegeneration
Recent evidence links PUM1 to a neurodegenerative disease [57]. In mice, PUM1 knockout or haploinsufficiency caused neurodegeneration. The mechanism involves PUM1 repression of Ataxin1 mRNA (ATXN1). In humans, poly-glutamine expansion in Ataxin1 leads to spinocerebellar ataxia type 1 (SCA1), a neurodegenerative disease characterized by loss of coordination and balance, muscle stiffness, weakness, cognitive and sensory impairment, and death. In mice, over-expression of Ataxin1 causes SCA1-related phenotypes. Knockout or depletion of PUM1 results in increased Ataxin1 expression, motor dysfunction, incoordination and loss of Purkinje neurons and reduced neuronal arborization in the cerebellum, resembling SCA1 [57]. Consistent with importance of PUM1 repression of Ataxin1 protein expression, haploinsufficiency of Ataxin1 alleviated the effect. Moreover, over-expression of PUM1 suppressed neurodegeneration caused by Ataxin1 with poly-Q expansion, whereas PUM1 haploinsufficiency exacerbated the disease phenotype.
Genomics provided the first evidence linking human PUM1 to neurological disorders and emphasizes its neurodevelopmental roles [105]. Two classes of PUM1-associated neurological disorders were characterized by developmental delay, intellectual disability, seizures, and ataxia. PADDAS is an infantile onset PUM1-associated disorder with developmental disability, ataxia, and seizures. PRCA is an adult onset PUM1-related cerebellar ataxia disorder. These syndromes were discovered in a cohort of 15 patients, are likely quite rare, and it remains possible that other loci may contribute. Most PADDAS patients had heterozygous deletions encompassing the PUM1 gene. Two PADDAS patients had either of two PUM1 missense mutations. A patient with PUM1 R1139W had ataxia, incoordination, chorea, spasticity, and reduced growth. Another patient with PUM1 R1147W had epilepsy, ataxia, hypotonicity, developmental delay, visual impairment, scoliosis, facial dysmorphia, low bone density, and brain morphology abnormality. In the case of PRCA, an afflicted family was identified with a PUM1 missense mutation, T1035S, which is associated with a partially penetrant autosomal dominant syndrome with cerebellar degeneration, ataxia, incoordination, and speech disorder. It remains unclear how these mutations affect PUM1 protein function. The PADDAS mutations R1139W and R1147W reside in the eighth repeat of the Pum-HD and are surface-exposed, whereas the PRCA PUM1 T1035S mutation is within the sixth repeat of the Pum-HD and is partially exposed (Figure 2A). None of these changes are on the RNA-binding surface. All three mutants exhibited reduced PUM1 protein levels, which may manifest in the disease state. In cultured cells from PADDAS and PRCA patients, expression of several PUM targets was affected, including ATXN1, supporting a loss of function. Further investigation by over-expression of PUM1 mutants showed that R1147W repressed several target mRNAs, whereas R1139W or T1035S did not. In hippocampal neurons, wild type PUM1 and T1035S reduced arborization when over-expressed, whereas R1139W and R1147W had less of an effect. Additional research is necessary to determine the impact of PADDAS and PRCA mutations on PUM1 protein turnover, RNA binding, repression, protein interactions with corepressors, and intracellular localization.
Roles in hematopoiesis and leukemia
Mammalian PUM1/2 have been implicated in hematopoiesis and leukemia. Normally, hematopoietic stem cells (HSCs) in bone marrow differentiate to produce the different types of blood cells. Leukemia occurs when cancer-driving mutations give rise to leukemia stem cells (LSCs) that proliferate and disrupt blood and tissue function. New evidence indicates that PUM1/2 play a critical regulatory role in the maintenance and proliferation of normal HSCs and impact acute myeloid leukemia (AML)[74]. PUM1/2 are highly expressed in human and mouse HSCs and are over-expressed in primary AML samples and cell lines [74, 82]. Moreover, upon individual depletion of PUM1/2, the cell cycle was disrupted, proliferation decreased and apoptosis increased in both normal human and mouse HSCs as well as AML cells [74]. FOXP1 mRNA, encoding a transcription factor involved in cell proliferation and differentiation, was identified as a direct target of PUM1/2. As noted earlier, PUM1/2 directly bind and activate the FOXP1 mRNA, which in turn represses expression of cell cycle inhibitors such as CDKN1B/p27, thereby promoting proliferation. Whether PMA is widespread in hematopoiesis or leukemogenesis remains unknown. Notably, PUM1 can also directly repress expression of CDKN1B/p27 tumor suppressor [33, 86], potentially reinforcing the FOXP1 pathway in HSCs and AML cells. Numerous other mRNAs are likely also affected by PUM1/2 in HSCs and LSCs and remain to be discovered.
Concluding Remarks
Tremendous progress has been made in elucidating the regulatory roles and biological functions of mammalian Pumilio proteins. Still, many important questions remain regarding how they regulate target mRNAs in diverse biological contexts. PUMs are broadly expressed in the human body and are predicted to bind a wide array of the transcriptome, thus they are likely to affect many additional biological processes. It is our hope that this review will serve to focus and accelerate new discoveries. This important endeavor will promote our understanding of diseases that result from dysfunction of Pumilio proteins and will enable therapeutic strategies to correct those defects.
Highlights:
Mammalian Pumilio proteins recognize specific RNA sequences via a highly conserved Pum-HD domain.
Pumilio proteins bind and regulate a large number of RNAs.
Pumilio proteins repress target mRNAs by antagonizing translation and promoting RNA degradation.
In certain contexts, Pumilio proteins may activate gene expression.
Pumilio proteins regulate stem cell fate, development, and neurological functions.
Dysfunction of Pumilio proteins contributes to neurodegeneration, epilepsy, ataxia, infertility, and cancer.
Outstanding Questions:
What are the biological roles of PUMs?
What diseases are caused by dysfunction of PUMs?
Which mRNAs are regulated by PUMs in relevant cell, tissue types, and developmental contexts?
What are the precise mechanisms of PUM-mediated regulation?
How is PUM activity regulated?
Acknowledgments
We thank Dr. Peter Freddolino, Univ. of Michigan, for discussions and input on this work. This work was supported by grant R01GM105707 from the National Institute of General Medical Sciences, National Institutes of Health (ACG) and the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences (TMTH). We sincerely apologize to colleagues whose work we were unable to include due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore MJ (2005) From birth to death: the complex lives of eukaryotic mRNAs. Science 309 (5740), 1514–8. [DOI] [PubMed] [Google Scholar]
- 2.Gerstberger S et al. (2014) A census of human RNA-binding proteins. Nat Rev Genet 15 (12), 829–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wickens M et al. (2002) A PUF family portrait: 3’UTR regulation as a way of life. Trends Genet 18 (3), 150–7. [DOI] [PubMed] [Google Scholar]
- 4.Arvola RM et al. (2017) Combinatorial control of messenger RNAs by Pumilio, Nanos and Brain Tumor Proteins. RNA Biol 14 (11), 1445–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lehmann R and Nusslein-Volhard C (1987) Involvement of the pumilio gene in the transport of an abdominal signal in the Drosophila embyro. Nature 329, 167–170. [Google Scholar]
- 6.Nusslein-Volhard C et al. (1987) Determination of anteroposterior polarity in Drosophila. Science 238 (4834), 1675–81. [DOI] [PubMed] [Google Scholar]
- 7.Miller MA and Olivas WM (2011) Roles of Puf proteins in mRNA degradation and translation. Wiley Interdiscip Rev RNA 2 (4), 471–92. [DOI] [PubMed] [Google Scholar]
- 8.Quenault T et al. (2011) PUF proteins: repression, activation and mRNA localization. Trends Cell Biol 21 (2), 104–12. [DOI] [PubMed] [Google Scholar]
- 9.Barker DD et al. (1992) Pumilio is essential for function but not for distribution of the Drosophila abdominal determinant Nanos. Genes Dev 6 (12A), 2312–26. [DOI] [PubMed] [Google Scholar]
- 10.Macdonald PM (1992) The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development 114 (1), 221–32. [DOI] [PubMed] [Google Scholar]
- 11.Zamore PD et al. (1997) The Pumilio protein binds RNA through a conserved domain that defines a new class of RNA-binding proteins. Rna 3 (12), 1421–33. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang B et al. (1997) A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390 (6659), 477–84. [DOI] [PubMed] [Google Scholar]
- 13.Weidmann CA and Goldstrohm AC (2012) Drosophila Pumilio protein contains multiple autonomous repression domains that regulate mRNAs independently of Nanos and brain tumor. Mol Cell Biol 32 (2), 527–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang CD et al. (2012) Characteristics and evolution of the PUF gene family in Bombyx mori and 27 other species. Mol Biol Rep 39 (1), 675–83. [DOI] [PubMed] [Google Scholar]
- 15.Qiu C et al. (2014) A divergent Pumilio repeat protein family for pre-rRNA processing and mRNA localization. Proc Natl Acad Sci U S A 111 (52), 18554–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J et al. (2016) Nop9 is a PUF-like protein that prevents premature cleavage to correctly process pre-18S rRNA. Nat Commun 7, 13085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson E et al. (2007) Nop9 is an RNA binding protein present in pre-40S ribosomes and required for 18S rRNA synthesis in yeast. RNA 13 (12), 2165–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z et al. (2009) Rational extension of the ribosome biogenesis pathway using network- guided genetics. PLoS Biol 7 (10), e1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang C and Muench DG (2015) A Nucleolar PUF RNA-binding Protein with Specificity for a Unique RNA Sequence. J Biol Chem 290 (50), 30108–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang B and Ye K (2017) Nop9 binds the central pseudoknot region of 18S rRNA. Nucleic Acids Res 45 (6), 3559–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bao H et al. (2017) Structural basis for the specific recognition of 18S rRNA by APUM23. Nucleic Acids Res 45 (20), 12005–12014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata Y and Wharton RP (1995) Binding of pumilio to maternal hunchback mRNA is required for posterior patterning in Drosophila embryos. Cell 80 (5), 747–56. [DOI] [PubMed] [Google Scholar]
- 23.Wharton RP et al. (1998) The Pumilio RNA-binding domain is also a translational regulator. Mol Cell 1 (6), 863–72. [DOI] [PubMed] [Google Scholar]
- 24.Zamore PD et al. (1999) The PUMILIO-RNA interaction: a single RNA-binding domain monomer recognizes a bipartite target sequence. Biochemistry 38 (2), 596–604. [DOI] [PubMed] [Google Scholar]
- 25.Weidmann CA et al. (2016) Drosophila Nanos acts as a molecular clamp that modulates the RNA-binding and repression activities of Pumilio. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laver JD et al. (2015) Brain tumor is a sequence-specific RNA-binding protein that directs maternal mRNA clearance during the Drosophila maternal-to-zygotic transition. Genome Biol 16, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen D et al. (2012) Pumilio 1 Suppresses Multiple Activators of p53 to Safeguard Spermatogenesis. Curr Biol 22 (5), 420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White EK et al. (2001) PUM2, a novel murine puf protein, and its consensus RNA-binding site. Rna 7 (12), 1855–66. [PMC free article] [PubMed] [Google Scholar]
- 29.Morris AR et al. (2008) Ribonomic analysis of human Pum1 reveals cis-trans conservation across species despite evolution of diverse mRNA target sets. Mol Cell Biol 28 (12), 4093–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Galgano A et al. (2008) Comparative analysis of mRNA targets for human PUF-family proteins suggests extensive interaction with the miRNA regulatory system. PLoS One 3 (9), e3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X et al. (2002) Modular recognition of RNA by a human pumilio-homology domain. Cell 110 (4), 501–12. [DOI] [PubMed] [Google Scholar]
- 32.Lu G and Hall TM (2011) Alternate modes of cognate RNA recognition by human PUMILIO proteins. Structure 19 (3), 361–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bohn JA et al. (2018) Identification of diverse target RNAs that are functionally regulated by human Pumilio proteins. Nucleic Acids Res 46 (1), 362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang M et al. (2017) Post-transcriptional regulation of mouse neurogenesis by Pumilio proteins. Genes Dev 31 (13), 1354–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hafner M et al. (2010) Transcriptome-wide identification of RNA-binding protein and microRNA target sites by PAR-CLIP. Cell 141 (1), 129–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edwards TA et al. (2001) Structure of Pumilio reveals similarity between RNA and peptide binding motifs. Cell 105 (2), 281–9. [DOI] [PubMed] [Google Scholar]
- 37.Wang X et al. (2001) Crystal structure of a Pumilio homology domain. Mol Cell 7 (4), 855–65. [DOI] [PubMed] [Google Scholar]
- 38.Campbell ZT et al. (2014) A protein-RNA specificity code enables targeted activation of an endogenous human transcript. Nat Struct Mol Biol 21 (8), 732–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheong CG and Hall TM (2006) Engineering RNA sequence specificity of Pumilio repeats. Proc Natl Acad Sci U S A 103 (37), 13635–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong S et al. (2011) Specific and modular binding code for cytosine recognition in Pumilio/FBF (PUF) RNA-binding domains. J Biol Chem 286 (30) 26732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Filipovska A et al. (2011) A universal code for RNA recognition by PUF proteins. Nat Chem Biol 7 (7) 425–7. [DOI] [PubMed] [Google Scholar]
- 42.Jenkins HT et al. (2009) Structure and RNA binding of the mouse Pumilio-2 Puf domain. J Struct Biol 167 (3), 271–6. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y et al. (2009) Engineering splicing factors with designed specificities. Nat Methods 6(11), 825–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Opperman L et al. (2005) A single spacer nucleotide determines the specificities of two mRNA regulatory proteins. Nat Struct Mol Biol 12 (11), 945–51. [DOI] [PubMed] [Google Scholar]
- 45.Ozawa T et al. (2007) Imaging dynamics of endogenous mitochondrial RNA in single living cells. Nat Methods 4 (5), 413–9. [DOI] [PubMed] [Google Scholar]
- 46.Cooke A et al. (2011) Targeted translational regulation using the PUF protein family scaffold. Proc Natl Acad Sci U S A 108 (38), 15870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choudhury R et al. (2012) Engineering RNA endonucleases with customized sequence specificities. Nat Commun 3, 1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Adamala KP et al. (2016) Programmable RNA-binding protein composed of repeats of a single modular unit. Proc Natl Acad Sci U S A 113 (19), E2579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao YY et al. (2018) Expanding RNA binding specificity and affinity of engineered PUF domains. Nucleic Acids Res 46 (9), 4771–4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Etten J et al. (2012) Human Pumilio proteins recruit multiple deadenylases to efficiently repress messenger RNAs. J Biol Chem 287 (43), 36370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jackson RJ et al. (2010) The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol 11 (2), 113–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garneau NL et al. (2007) The highways and byways of mRNA decay. Nat Rev Mol Cell Biol 8 (2), 113–26. [DOI] [PubMed] [Google Scholar]
- 53.Olivas W and Parker R (2000) The Puf3 protein is a transcript-specific regulator of mRNA degradation in yeast. Embo J 19 (23), 6602–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goldstrohm AC et al. (2006) PUF proteins bind Pop2p to regulate messenger RNAs. Nat Struct Mol Biol 13 (6), 533–9. [DOI] [PubMed] [Google Scholar]
- 55.Goldstrohm AC et al. (2007) PUF protein-mediated deadenylation is catalyzed by Ccr4p. J Biol Chem 282 (1), 109–14. [DOI] [PubMed] [Google Scholar]
- 56.Blewett NH and Goldstrohm AC (2012) A eukaryotic translation initiation factor 4E-binding protein promotes mRNA decapping and is required for PUF repression. Mol Cell Biol 32 (20), 4181–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gennarino VA et al. (2015) Pumilio1 haploinsufficiency leads to SCA1-like neurodegeneration by increasing wild-type Ataxin1 levels. Cell 160 (6), 1087–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lau NC et al. (2009) Human Ccr4-Not complexes contain variable deadenylase subunits. Biochem J 422 (3), 443–53. [DOI] [PubMed] [Google Scholar]
- 59.Kadyrova LY et al. (2007) Translational control of maternal Cyclin B mRNA by Nanos in the Drosophila germline. Development 134 (8), 1519–27. [DOI] [PubMed] [Google Scholar]
- 60.Suh N et al. (2009) FBF and its dual control of gld-1 expression in the Caenorhabditis elegans germline. Genetics 181 (4), 1249–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weidmann CA et al. (2014) The RNA binding domain of Pumilio antagonizes poly-adenosine binding protein and accelerates deadenylation. RNA 20 (8), 1298–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joly W et al. (2013) The CCR4 deadenylase acts with Nanos and Pumilio in the fine-tuning of Mei-P26 expression to promote germline stem cell self-renewal. Stem Cell Reports 1 (5), 411–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brocard M et al. (2018) Pumilio directs deadenylation-associated translational repression of the cyclin-dependent kinase 1 activator RGC-32. Nucleic Acids Res 46 (7), 3707–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang E et al. (2003) Decay rates of human mRNAs: correlation with functional characteristics and sequence attributes. Genome Res 13 (8), 1863–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schwanhausser B et al. (2011) Global quantification of mammalian gene expression control. Nature 473 (7347), 337–42. [DOI] [PubMed] [Google Scholar]
- 66.Sharova LV et al. (2009) Database for mRNA half-life of 19 977 genes obtained by DNA microarray analysis of pluripotent and differentiating mouse embryonic stem cells. DNA Res 16 (1), 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rabani M et al. (2017) A Massively Parallel Reporter Assay of 3´ UTR Sequences Identifies In Vivo Rules for mRNA Degradation. Mol Cell 68 (6), 1083–1094 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cottrell KA et al. (2018) PTRE-seq reveals mechanism and interactions of RNA binding proteins and miRNAs. Nat Commun 9 (1), 301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tichon A et al. (2016) A conserved abundant cytoplasmic long noncoding RNA modulates repression by Pumilio proteins in human cells. Nat Commun 7, 12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chritton JJ and Wickens M (2011) A role for the poly(A)-binding protein Pab1p in PUF protein-mediated repression. J Biol Chem 286 (38), 33268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao Q et al. (2010) Pumilio 2 controls translation by competing with eIF4E for 7-methyl guanosine cap recognition. Rna 16 (1), 221–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Friend K et al. (2012) A conserved PUF-Ago-eEF1A complex attenuates translation elongation. Nat Struct Mol Biol 19 (2), 176–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vessey JP et al. (2010) Mammalian Pumilio 2 regulates dendrite morphogenesis and synaptic function. Proc Natl Acad Sci U S A 107 (7), 3222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Naudin C et al. (2017) PUMILIO/FOXP1 signaling drives expansion of hematopoietic stem/progenitor and leukemia cells. Blood 129 (18), 2493–2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hentze MW et al. (2004) Balancing acts: molecular control of mammalian iron metabolism. Cell 117 (3), 285–97. [DOI] [PubMed] [Google Scholar]
- 76.Ivshina M et al. (2014) Cytoplasmic polyadenylation element binding proteins in development, health, and disease. Annu Rev Cell Dev Biol 30, 393–415. [DOI] [PubMed] [Google Scholar]
- 77.Kaye JA et al. (2009) A 3´UTR pumilio-binding element directs translational activation in olfactory sensory neurons. Neuron 61 (1), 57–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee CD and Tu BP (2015) Glucose-Regulated Phosphorylation of the PUF Protein Puf3 Regulates the Translational Fate of Its Bound mRNAs and Association with RNA Granules. Cell Rep 11 (10), 1638–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pique M et al. (2008) A combinatorial code for CPE-mediated translational control. Cell 132(3), 434–48. [DOI] [PubMed] [Google Scholar]
- 80.Archer SK et al. (2009) Trypanosoma brucei PUF9 regulates mRNAs for proteins involved in replicative processes over the cell cycle. PLoS Pathog 5 (8), e1000565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Consortium GT (2013) The Genotype-Tissue Expression (GTEx) project. Nat Genet 45 (6), 580–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Spassov DS and Jurecic R (2003) Mouse Pum1 and Pum2 genes, members of the Pumilio family of RNA-binding proteins, show differential expression in fetal and adult hematopoietic stem cells and progenitors. Blood Cells Mol Dis 30 (1), 55–69. [DOI] [PubMed] [Google Scholar]
- 83.Uhlen M et al. (2015) Proteomics. Tissue-based map of the human proteome. Science 347 (6220), 1260419. [DOI] [PubMed] [Google Scholar]
- 84.Ray D et al. (2013) A compendium of RNA-binding motifs for decoding gene regulation. Nature 499 (7457), 172–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee S et al. (2016) Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 164 (1–2), 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kedde M et al. (2010) A Pumilio-induced RNA structure switch in p27–3’ UTR controls miR- 221 and miR-222 accessibility. Nat Cell Biol 12 (10), 1014–20. [DOI] [PubMed] [Google Scholar]
- 87.Miles WO et al. (2012) Pumilio facilitates miRNA regulation of the E2F3 oncogene. Genes Dev 26 (4), 356–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Leeb M et al. (2014) Genetic exploration of the exit from self-renewal using haploid embryonic stem cells. Cell Stem Cell 14 (3), 385–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zahr SK et al. (2018) A Translational Repression Complex in Developing Mammalian Neural Stem Cells that Regulates Neuronal Specification. Neuron 97 (3), 520–537 e6. [DOI] [PubMed] [Google Scholar]
- 90.Follwaczny P et al. (2017) Pumilio2-deficient mice show a predisposition for epilepsy. Dis Model Mech 10 (11), 1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Vessey JP et al. (2006) Dendritic localization of the translational repressor Pumilio 2 and its contribution to dendritic stress granules. J Neurosci 26 (24), 6496–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Youn JY et al. (2018) High-Density Proximity Mapping Reveals the Subcellular Organization of mRNA-Associated Granules and Bodies. Mol Cell 69 (3), 517–532 e11. [DOI] [PubMed] [Google Scholar]
- 93.Khong A et al. (2017) The Stress Granule Transcriptome Reveals Principles of mRNA Accumulation in Stress Granules. Mol Cell 68 (4), 808–820 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hubstenberger A et al. (2017) P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol Cell 68 (1), 144–157 e5. [DOI] [PubMed] [Google Scholar]
- 95.Protter DS and Parker R (2016) Principles and Properties of Stress Granules. Trends Cell Biol 26 (9), 668–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Narita R et al. (2014) A novel function of human Pumilio proteins in cytoplasmic sensing of viral infection. PLoS Pathog 10 (10), e1004417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith J et al. (2016) Spatial patterning of P granules by RNA-induced phase separation of the intrinsically-disordered protein MEG-3. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tichon A et al. (2018) SAM68 is required for regulation of Pumilio by the NORAD long noncoding RNA. Genes Dev 32 (1), 70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Campbell ZT et al. (2012) Identification of a conserved interface between PUF and CPEB proteins. J Biol Chem 287 (22), 18854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jaruzelska J et al. (2003) Conservation of a Pumilio-Nanos complex from Drosophila germ plasm to human germ cells. Dev Genes Evol 213 (3), 120–6. [DOI] [PubMed] [Google Scholar]
- 101.Lolicato F et al. (2008) Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol 313 (2), 725–38. [DOI] [PubMed] [Google Scholar]
- 102.Barrios F et al. (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123 (Pt 6), 871–80. [DOI] [PubMed] [Google Scholar]
- 103.Suzuki A et al. (2016) Dead end1 is an essential partner of NANOS2 for selective binding of target RNAs in male germ cell development. EMBO Rep 17 (1), 37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Loedige I et al. (2013) The mammalian TRIM-NHL protein TRIM71/LIN-41 is a repressor of mRNA function. Nucleic Acids Res 41 (1), 518–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gennarino VA et al. (2018) A Mild PUM1 Mutation Is Associated with Adult-Onset Ataxia, whereas Haploinsufficiency Causes Developmental Delay and Seizures. Cell 172 (5), 924–936 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hagerman RJ et al. (2017) Fragile X syndrome. Nat Rev Dis Primers 3, 17065. [DOI] [PubMed] [Google Scholar]
- 107.Moore FL et al. (2003) Human Pumilio-2 is expressed in embryonic stem cells and germ cells and interacts with DAZ (Deleted in AZoospermia) and DAZ-like proteins. Proc Natl Acad Sci U S A 100 (2), 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Siemen H et al. (2011) Pumilio-2 function in the mouse nervous system. PLoS One 6 (10), e25932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu EY et al. (2007) A gene trap mutation of a murine homolog of the Drosophila stem cell factor Pumilio results in smaller testes but does not affect litter size or fertility. Mol Reprod Dev 74 (7), 912–21. [DOI] [PubMed] [Google Scholar]
- 110.Mak W et al. (2016) An Important Role of Pumilio 1 in Regulating the Development of the Mammalian Female Germline. Biol Reprod 94 (6), 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Forbes A and Lehmann R (1998) Nanos and Pumilio have critical roles in the development and function of Drosophila germline stem cells. Development 125 (4), 679–90. [DOI] [PubMed] [Google Scholar]
- 112.Lin H and Spradling AC (1997) A novel group of pumilio mutations affects the asymmetric division of germline stem cells in the Drosophila ovary. Development 124 (12), 2463–76. [DOI] [PubMed] [Google Scholar]
- 113.Crittenden SL et al. (2002) A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature 417 (6889), 660–3. [DOI] [PubMed] [Google Scholar]
- 114.Driscoll HE et al. (2013) Pumilio-2 regulates translation of Nav1.6 to mediate homeostasis of membrane excitability. J Neurosci 33 (23), 9644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaplan DI et al. (2016) Role of Sodium Channels in Epilepsy. Cold Spring Harb Perspect Med 6 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Treiman DM (2001) GABAergic mechanisms in epilepsy. Epilepsia 42 Suppl 3, 8–12. [DOI] [PubMed] [Google Scholar]
- 117.Goncalves JT et al. (2016) Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 167 (4), 897–914. [DOI] [PubMed] [Google Scholar]
- 118.Kamenska A et al. (2014) eIF4E-binding proteins: new factors, new locations, new roles. Biochem Soc Trans 42 (4), 1238–45. [DOI] [PubMed] [Google Scholar]
- 119.Troshin PV et al. (2018) JABAWS 2.2 distributed web services for Bioinformatics: protein disorder, conservation and RNA secondary structure. Bioinformatics 34 (11), 1939–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McWilliam H et al. (2013) Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res 41 (Web Server issue), W597–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ashkenazy H et al. (2016) ConSurf 2016: an improved methodology to estimate and visualize evolutionary conservation in macromolecules. Nucleic Acids Res 44 (W1), W344–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lee MH et al. (2007) Conserved regulation of MAP kinase expression by PUF RNA-binding proteins. PLoS Genet 3 (12), e233. [DOI] [PMC free article] [PubMed] [Google Scholar]


