Abstract
GCH1 encodes the enzyme GTP cyclohydrolase 1, essential for dopamine synthesis in nigrostriatal cells, and rare mutations in GCH1 may lead to Dopa-responsive dystonia (DRD). While GCH1 is implicated in genome-wide association studies in Parkinson disease (PD), only a few studies examined the role of rare GCH1 variants in PD, with conflicting results. In the current study, GCH1 and its 5’ and 3’ untranslated regions were sequenced in 1,113 PD patients and 1,111 controls. To examine the association of rare GCH1 variants with PD, burden analysis was performed. Three rare GCH1 variants, which were previously reported to be pathogenic in DRD, were found in five PD patients and not in controls (SKAT, p=0.024). A common haplotype, tagged by rs841, was associated with a reduced risk for PD (OR= 0.71, 95% CI=0.61–0.83, p= 1.24×10−4), and with increased GCH1 expression in brain regions relevant for PD (www.gtexportal.org). Our results support a role for rare, DRD-related variants, and common GCH1 variants in the pathogenesis of PD.
Keywords: Parkinson disease, Dopa-responsive dystonia (DRD), GTP Cyclohydrolase 1 deficiency, GCH1
1. Introduction
The genetics of Parkinson disease (PD) is a rapidly evolving field, which may help identifying patients with specific variants that will be eligible for future, specific precision medicine. Genetic studies from recent years reported conflicting results on the involvement of rare and common GCH1 variants in PD. GTP-cyclohydrolase 1, encoded by GCH1, controls the first, rate-limiting step of the biosynthesis of tetrahydrobiopterin (BH4), which is an essential cofactor for synthesis of dopamine in nigrostriatal cells (Kurian et al., 2011). Loss-of-function mutations in GCH1 have been shown to cause two rare disorders: autosomal dominant DOPA-responsive dystonia (DRD) and autosomal recessive GCH-deficient hyperphenylalaninemia (HPA) (Furukawa et al., 1998). Co-occurrence of DRD and Parkinsonism has been reported in families with GCH1 mutations (Lewthwaite et al., 2015; Rengmark et al., 2016), and a study on sporadic PD patients demonstrated an increased frequency of pathogenic GCH1 mutations that were previously reported to cause DRD (Mencacci et al., 2014). Three subsequent studies also supported an association between rare GCH1 variants and PD in different populations (Guella et al., 2015; Lewthwaite et al., 2015; Xu et al., 2017). However, other studies did not provide convincing evidence for association of rare GCH1 DRD-causing mutations and PD (Bandres-Ciga et al., 2016; Rengmark et al., 2016; Yan et al., 2018) .
Some conflicting results were also reported on common variants in the GCH1 locus. Large genome wide association studies (GWAS) identified common variants near GCH1, associated with risk for PD (Chang et al., 2017; Nalls et al., 2014). These were replicated is several studies (Chen et al., 2016; Safaralizadeh et al., 2016), but not in others (Newman et al., 2014; Yang et al., 2017; Zou et al., 2018), possibly due to the different sizes and ethnicities of the population studied. Understanding whether rare and common GCH1 variants have a role in PD is of major importance, as GTP-cyclohydrolase 1 can become a target for PD drug development in the upcoming era of precision medicine.
To further examine the potential role of rare and common GCH1 variants in PD, we sequenced its entire coding regions, as well as the 5’ and 3’ untranslated regions and the intronic regions around the exon-intron boundaries in two cohorts of PD patients and controls.
2. Subjects and methods
2.1. Study population
Two cohorts with a total of 1,113 PD patients and 1,111 controls were included: A cohort composed of French and French-Canadian unrelated PD patients (n=538) and controls (n=831), recruited in Quebec (Canada) and in France. Average patient age was 65.7±10.0 years, with 62.9% men. The control population of this cohort included 2 groups, elderly controls (n =201, average age at enrollment of 62.7 ± 8.2 years) and young controls (n = 619, average age at enrollment of 35.4 ± 6.5 years, data on age were not available for 11 controls). There was no significant difference in GCH1 variant frequencies between the 2 groups, which allowed us to combine them for the analysis (average age of 41.9 ± 13.6 years with 51.7% men). The second cohort was recruited in New York (Columbia University) and included 575 PD patients (average age 66.3±10.55 years, 64% men) and 280 unrelated controls (average age 65.0 ±9.7 years, 35.4% men). As detailed below, due to the differences in age and sex, statistical analysis was adjusted. All PD patients were diagnosed by movement disorder specialists according to the UK brain bank criteria (Hughes et al., 1992), without excluding patients with family history of PD. All patients signed informed consent before entering the study, and the institutional review boards approved the study protocols.
2.2. DNA extraction and GCH1 sequencing
DNA was extracted using a standard salting out protocol. The coding sequence and regulatory regions of GCH1 were targeted using molecular inversion probes (MIPs), designed as previously described (O’Roak et al., 2012). MIPs were selected based on their predicted coverage quality and overlap. All MIPs used to sequence GCH1 in the present study are detailed in Supplementary Table 1. Targeted DNA capture and amplification was done as previously described (Ross et al., 2016), and the full protocol is available upon request. The library was sequenced using Illumina HiSeq 2500 platform at the McGill University and Genome Quebec Innovation Centre. Sequence processing was done by Burrows-Wheeler Aligner for alignment (Li and Durbin, 2009), the Genome Analysis Toolkit (GATK, v3.8) for post-alignment cleanup and variant calling (McKenna et al., 2010), and ANNOVAR for annotation (Wang et al., 2010). Data on the frequency of each GCH1 variant were extracted from the public database Exome Aggregation Consortium (ExAC). Only variants with high coverage (>30x) and read quality were included in the analysis. Pathogenicity of variants was examined in ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/), and through specific searches in PubMed. Since exon 1 of GCH1 was not covered well by the targeted sequencing, Sanger sequencing of exon 1 was also done in all samples, using the following primers: forward 5’ – GAGGCAACTCCGGAAACT – 3’, reverse 5’ – GCTCATTCCGCAATAAGTGG – 3’.
2.3. QC steps
During QC filtration using the PLINK software, we excluded SNPs that failed to follow Hardy-Weinberg equilibrium, set at 0.001 threshold, and SNPs with genotyping rate of less than 90%. Same genotyping rate cut-off was used for individual samples. Threshold for missingness between cases and controls was set at 0.05. After the QC, 1,082 patients and 1,110 controls were included in the analysis. The final genotype call rate post QC filtration was greater than 99%.
2.4. Statistical Analysis
The association between common GCH1 variants and PD was analyzed using a logistic regression with the status (patient or control) as a dependent variable. Since there were differences in age and sex between patients and controls, and since the different recruitment sites recruited patients with different ethnical background, age, sex and recruitment site were used as covariates. To analyse all the rare variants (minor allele frequency [MAF] <1%), optimised sequence Kernel association test (SKAT-O, R package) was performed (Lee et al., 2012). Association of presumed pathogenic variants was tested using burden analysis (R package SKAT)(Lee et al., 2012), since the direction of the association was presumed as pathogenic prior to the test. All other statistical analysis was performed using the SPSS software, version 24 (IBM Inc).
3. Results
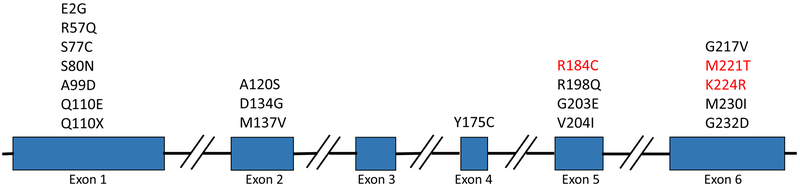
Table 1 details the identified nonsynonymous GCH1 variants, and Figure 1 depicts the location of mutations in GCH1 reported in PD patients in the current and previous studies on PD. A total of 11 rare variants (MAF <1%) and one less-frequent variant P23L (MAF 1–5%) were identified. There were 6 novel variants that were not found in public databases (2 in patients and 4 in controls). Three variants (p.R184C, p.M221T and p.K224R), that were previously reported to be pathogenic in DRD (Chenbhanich et al., 2017; Furukawa et al., 1998; Guella et al., 2015; Leuzzi et al., 2002; Mencacci et al., 2014) were found in five (~0.5%) PD patients, and none in controls (SKAT burden test p=0.024). The other variants are of unknown significance. When analyzing all nonsynonymous variants, regardless of their contribution to DRD, no association was found (SKAT-O, p=0.223). One variant (p.V204I) that is reported in the ClinVar database to have conflicting interpretation of pathogenicity in DRD was found in 3 controls (~0.3%) and in no PD patients.
Table 1.
Summary of all non-synonymous variants detected in the current study
| dbSNP | Position | Substitution | PD N=1082 |
Controls N=1110 |
ExAC MAF in Europeans |
|---|---|---|---|---|---|
| rs41298432 | 14:55369314 | p.P23L | 3 | 8 | 1.502E-02 |
| rs1030068813 | 14:55369276 | p.A36S | 0 | 1 | - |
| - | 14:55369231 | p.D51N | 0 | 1 | - |
| - | 14:55369219 | p.G55S | 0 | 1 | - |
| rs56127440 | 14:55369176 | p.P69L | 2 | 0 | 5.934E-04 |
| - | 14:55312562 | p.R184C* | 1 | 0 | - |
| rs200891969 | 14:55312502 | p.V204I | 0 | 3 | 1.798E-04 |
| - | 14:55310569 | p.N215K | 0 | 1 | - |
| - | 14:55310570 | p.N215I | 0 | 1 | - |
| - | 14:55310841 | p.R216Q | 1 | 0 | - |
| rs104894434 | 14:55310826 | p.M221T* | 1 | 0 | 4.495E-05 |
| rs41298442 | 14:55310817 | p.K224R* | 3 | 0 | 4.195E-04 |
| Total | 11 (1.02%) | 16 (1.44%) |
Variants reported to be pathogenic in DRD.
Figure 1.
Mutations in the guanosine triphosphate cyclohydrolase I (GCH1) gene detected in patients with Parkinson’s Disease. The mutations found in this study are indicated highlighted in red.
When examining common variants, logistic regression showed a common SNP, rs841, to be associated with reduced PD risk in our data (OR= 0.73, 95% CI=0.62–0.86, p= 1.24×10−4). The rs841 SNP was also associated with reduced risk for PD in the PD GWAS portal (www.pdgene.org) (Meta OR=0.91, 95%CI=0.87–0.95, p=1.00×10−6) (Lill, 2016; Nalls et al., 2014). This SNP is in partial linkage disequilibrium (LD) with the GWAS tagging SNP rs11158026 (Chang et al., 2017; Nalls et al., 2014) (D’=0.98, r2=0.5, due to lower frequency of rs841). To examine whether this SNP is associated with GCH1 expression, we accessed the Genotype-Tissue Expression portal (GTEx, www.gtexportal.org). Supplementary Figure 1 demonstrates that this SNP is significantly associated with increased expression of GCH1 in multiple brain tissues, including the substantia nigra and other basal ganglia. The rs841 SNP was not associated with age at onset in 947 patients for which data was available.
Clinical presentation of PD patients with DRD-causing GCH1 mutation
Partial clinical data was available for four of the five patients carrying a DRD-causing GCH1 mutation. Patient 1 (p.K224R) is a male patient that was diagnosed with idiopathic PD at age 58, presenting with right hand tremor, that later progressed to bi-lateral hand tremor. The patient also presented with rigidity in both knees as well as facial freezing and tremors at rest of lower limbs. The patient had good response to L-DOPA. At age 66, he developed mild cognitive impairment. Patient2 (p.K224R) is a male PD patient with age at onset of 64 years old. He is not treated with L-DOPA and information about dystonia is not available. His unified PD rating scale (UPRDS) part III was 15.5 and his Montreal cognitive assessment (MoCA) was 28 in his last examination. Clinical data was not available for the third patient with the p.K224R mutation. Patient 4 (p.R184C) is a male patient with age at onset of 48 years old and family history of PD (father). His initial clinical presentation was mainly left-sided rigidity, yet later tremor became more predominant. He is treated with L-DOPA medication with good response, however, he suffered from dyskinesia and on/off fluctuations. This patient also suffers from anxiety, hallucinations and probable REM sleep behavior disorder, based on a questionnaire. In 2013 the patient went through a successful deep brain stimulation surgery which led to improvement of motor symptoms. Patient 5 (p.M221T) is a female patient with age at onset of 61 years old. She had an excellent response to L-DOPA medication. Her UPDRS and MoCA scores are 11 and 27, respectively.
4. Discussion
The current study provides further support to previous reports suggesting that rare DRD-causing GCH1 variants may also cause PD, and that common GCH1 variants are associated with a small effect on PD risk, perhaps through regulation of GCH1 expression. The negative results previously reported for common and rare GCH1 variants in PD (Cobb et al., 2009; Hertz et al., 2006; Newman et al., 2014; Rengmark et al., 2016; Yang et al., 2017) may be due to small sample sizes, differential sequencing techniques, or population-specific effects (Table 2 details results from previous sequencing and genotyping studies on GCH1 in PD).
Table 2:
Role of GCH1 in PD - Summary of previous reports
| Article | Role in PD |
#controls | #cases | Diagnosis of cases | Ethnicity | GCH1 findings |
|---|---|---|---|---|---|---|
| Hertz et al., 2006 | No | 0 | 87 | EOPD | Danish | No pathogenic GCH1 variants found |
| Cobb et al., 2009 | No | 0 | 53 | familial EOPD, 21 with EOPD+dystonia | North-American Caucasian | No coding changes/CNV |
| Momma et al., 2009 | Yes | 96 | 2 | EOPD | Chinese | 1 rare mutation found in patients |
| Mencacci et al., 2014 | Yes | 5935 | 1318 | PD | North-American of European descent, Estonians | 11 different heterozygous variants at low frequency, 4 of them associated with DRD |
| Nalls et al., 2014 | Yes | 95282 | 13708 | PD | European ancestry | GWAS signal |
| Newman et al., 2014 | No | 862 | 1105 | PD/dystonia | Australian | No association between PD and the analyzed SNPs |
| Weissbach et al., 2014 | Yes | 0 | 15 | PD/Parkinsonism/dystonia | N/A | GCH1 mutation carriers with parkinsonism and idiopathic PD (one had dystonia) |
| Guella et al., 2015 | Yes | 290 | 528 | 361PD/167 atypical Parkinsonism+DLB+MSA+PSP | N/A | Rare heterozygous nonsynonymous substitutions found in patients |
| Lewthwaite et al., 2015 | Yes | 6 | 6 | 2EOPD, 1 Parkinsonism, 3DRD | Caucasian | 1 novel heterozygous substitution found in a very conserved region |
| Bandres-Ciga et al., 2016 | No | 0 | 134 | 97LOPD/28EOPD/9FPD | South Spanish | No mutation carriers for GCH1 |
| Chen et al., 2016 | Yes | 553 | 528 | PD | Taiwanese | rs11158026 increased the risk of developing PD |
| Rengmark et al., 2016 | No | 230 | 509 | LOPD | Norwegian/Swedish | No pathogenic GCH1 variants found |
| Safaralizadeh et al., 2016 | Yes | 1200 | 600 | PD (excluded EOPD,FPD) | Iranian | Replicated the association of rs11158026 with PD |
| Chang et al., 2017 | Yes | 302042 | 6476 | PD | European ancestry | GWAS signal |
| Xu et al., 2017 | Yes | 1565 | 1758 | PD | Chinese | 7 rare heterozygous non-synonymous mutations in patients |
| Yang et al., 2017 | No | 634 | 589 | sporadic PD (FPD excluded) | Han Chinese | No association of rs11158026 with PD |
| Yan et al., 2018 | Yes | 438 | 421 | 170EOPD/251LOPD(FPD excluded) | Han Chinese | 1 LOPD patient (maybe +dystonia) with rare GCH1 mutation(+1 found earlier) |
| Zou et al., 2018 | No | 624 | 579 | sporadic PD (FPD excluded) | East Asians | No association of rs11158026 with PD |
CNV, copy number variation; DLB, dementia with Lewy Bodies; DRD, dopa-responsive dystonia; EOPD, early-onset PD; FPD, familial PD; GWAS, genome wide association study; LOPD, late onset PD; MSA, multiple system atrophy; PSP, progressive supranuclear palsy
A total of 20 rare GCH1 variants have been reported in PD / Parkinsonism (Figure 1). However, it is still not clear if all 20 variants indeed have a pathogenic role in PD, as some of them may be rare benign variants that were randomly found in PD patients. For example, there is conflicting evidence regarding the role of p.V204I; this variant was reported in three PD patients (Mencacci et al., 2014), but was also found in compound heterozygosity with another pathogenic GCH1 variant. If the p. V204I was indeed pathogenic as well, this patient should have had the infant-onset severe phenotype (Weissbach and Klein, 2014). In our study, this variant was found in three controls, and none in patients, further supporting lack of pathogenicity. Furthermore, in ExAC it is found in about 1:500 individuals of South Asian origin (http://exac.broadinstitute.org/variant/14–55312502-C-T), which is a somewhat high frequency for a disease-causing variant. Similarly, we have identified two PD patients with the p. P69L variant, which was previously reported in PD patients with dystonia (Furukawa et al., 2004), but was also reported as a benign variant by others (Mencacci et al., 2014). However, this mutation is slightly less common, found in about 1:1000 Europeans. At this point, the pathogenicity of these variants, or alternatively, their role as risk factors with reduced penetrance (as seen with some GBA variants for example (Hernandez et al., 2016)), cannot be ruled out, and larger genetic studies or functional studies are required to examine their pathogenicity. Other variants such as p.S80N, (Cao et al., 2010; Xu et al., 2017; Yan et al., 2018) p.R184C (Chenbhanich et al., 2017; Dobricic et al., 2017), p.M221T (Furukawa et al., 1998), p.K224R (Guella et al., 2015; Mencacci et al., 2014) are more rare and repeatedly reported in DRD and PD, and thus can be considered as pathogenic for both.
Large GWASs identified a risk locus that includes GCH1 (Chang et al., 2017; Nalls et al., 2014), suggesting that common, possibly regulatory variants in GCH1 may affect the susceptibility for PD. The common SNP rs841, which was identified in our study, seems to be associated with reduced risk of PD. In the GTEx portal (www.gtexportal.org), this SNP is associated with increased GCH1 expression in brain regions important in PD, including the substantia nigra (Supplementary Figure 1). Of note, since this SNP is in LD with the GWAS tagging SNP rs11158026, it is possible that the rs841 SNP is responsible for the association in this locus, however, functional studies are required to determine whether this or other SNPs in this locus drive the association. Considering that rare, probably loss-of-function (LOF) mutations in GCH1 cause DRD or PD, that the biologic function of GTP-cyclohydrolase 1 is in the synthesis of dopamine, together with the protective effect of common variants that are likely to increase expression of GCH1, may suggest that increasing the expression and/or the activity of GCH1/GTP-cyclohydrolase 1 could be an attractive target for drug development for sporadic PD as well.
Clinically, late onset DRD may present as Parkinsonism (Lill, 2016), which may suggest that patients diagnosed with PD who carry a presumed pathogenic GCH1 mutation, actually have a rare phenotype of DRD which presents similarly to PD (Lewthwaite et al., 2015; Mencacci et al., 2014; Rengmark et al., 2016). Alternatively, it is possible that GCH1 mutations may predispose to both, and whether a carrier will develop DRD or PD is dependent on other genetic or environmental factors. For example, in a family with a novel pathogenic GCH1 variant, p.E2G, it seemed to cause both PD and DRD phenotypes in different members of the studied family (Lewthwaite et al., 2015). Further supporting this notion, neuropathological studies of DRD patients with GCH1 mutations demonstrated that in most DRD patients there was an absence of Lewy bodies (LB) pathology, while a subset of patients with late-onset Parkinsonism was positive for LB pathology (Schneider and Alcalay, 2017). Chart reviews of two of our patients from NY confirmed the clinical diagnosis of PD.
Our study has several limitations. First, in the French-Canadian and French controls, some of the controls are significantly younger than the patients. It is important to note that this is a bias towards the null hypothesis, which only means that our results could be more significant had we used an age-matched control group. Furthermore, we accounted for the age and sex differences by demonstrating that there was no difference in frequencies of rare or common variants between the young and elderly controls, and by adjusting the regression model with age and sex as covariates. Lastly, despite having a relatively large cohort, since many of the GCH1 variants are rare, a larger study, or rather a meta-analysis of multiple studies, will be required to determine the role of some of the variants which are still questionable.
Overall, our results support a role for rare GCH1 variants in PD, and for common variants as modifiers of risk for PD. While larger genetic studies, as well as functional studies are still warranted, GCH1 should already be considered as a target for drug development.
Supplementary Material
Acknowledgements:
We thank the patients and control subjects for their participation in this study. This work was financially supported by the Michael J. Fox Foundation, the Canadian Consortium on Neurodegeneration in Aging (CCNA) and the Canadian Glycomics Network (GlycoNet). This research was also undertaken thanks in part to funding from the Canada First Research Excellence Fund (CFREF), awarded to McGill University for the Healthy Brains for Healthy Lives (HBHL) program. The Columbia University cohort is supported by the Parkinson’s Foundation, the National Institutes of Health [K02NS080915, and UL1 TR000040] and the Brookdale Foundation. GAR holds a Canada Research Chair in Genetics of the Nervous System and the Wilder Penfield Chair in Neurosciences. ZGO is supported by the Fonds de recherche du Québec - Santé (FRQS) Chercheurs-boursiers award. The access to part of the participants for this research has been made possible thanks to the Quebec Parkinson’s Network (http://rpq-qpn.ca/en/). We thank Jay Ross, Daniel Rochefort, Helene Catoire, Cathy Mirarchi and Vessela Zaharieva for their assistance. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from the GTEx Portal on 05/15/18.
References:
- Bandres-Ciga S, Mencacci NE, Duran R, Barrero FJ, Escamilla-Sevilla F, Morgan S, Hehir J, Vives F, Hardy J, Pittman AM, 2016. Analysis of the genetic variability in Parkinson’s disease from Southern Spain. Neurobiol. Aging 37, 210 e211–210 e215. [DOI] [PubMed] [Google Scholar]
- Cao L, Zheng L, Tang WG, Xiao Q, Zhang T, Tang HD, He SB, Wang XJ, Ding JQ, Chen SD, 2010. Four novel mutations in the GCH1 gene of Chinese patients with dopa-responsive dystonia. Mov. Disord 25(6), 755–760. [DOI] [PubMed] [Google Scholar]
- Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, International Parkinson’s Disease Genomics, C., andMe Research, T., Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR, 2017. A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat. Genet 49(10), 1511–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CM, Chen YC, Chiang MC, Fung HC, Chang KH, Lee-Chen GJ, Wu YR, 2016. Association of GCH1 and MIR4697, but not SIPA1L2 and VPS13C polymorphisms, with Parkinson’s disease in Taiwan. Neurobiol. Aging 39, 221 e221–225. [DOI] [PubMed] [Google Scholar]
- Chenbhanich J, Sringean J, Bhidayasiri R, 2017. Beyond the Classic Segawa Disease, GCH1-Associated Neurodegenerative Parkinsonism: Practical Considerations for Physicians. J Mov Disord 10(2), 102–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb SA, Wider C, Ross OA, Mata IF, Adler CH, Rajput A, Rajput AH, Wu RM, Hauser R, Josephs KA, Carr J, Gwinn K, Heckman MG, Aasly JO, Lynch T, Uitti RJ, Wszolek ZK, Kapatos G, Farrer MJ, 2009. GCH1 in early-onset Parkinson’s disease. Mov. Disord 24(14), 2070–2075. [DOI] [PubMed] [Google Scholar]
- Dobricic V, Tomic A, Brankovic V, Kresojevic N, Jankovic M, Westenberger A, Rasic VM, Klein C, Novakovic I, Svetel M, Kostic VS, 2017. GCH1 mutations are common in Serbian patients with dystonia-parkinsonism: Challenging previously reported prevalence rates of DOPA-responsive dystonia. Parkinsonism Relat. Disord 45, 81–84. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Filiano JJ, Kish SJ, 2004. Amantadine for levodopa-induced choreic dyskinesia in compound heterozygotes for GCH1 mutations. Mov. Disord 19(10), 1256–1258. [DOI] [PubMed] [Google Scholar]
- Furukawa Y, Kish SJ, Bebin EM, Jacobson RD, Fryburg JS, Wilson WG, Shimadzu M, Hyland K, Trugman JM, 1998. Dystonia with motor delay in compound heterozygotes for GTP-cyclohydrolase I gene mutations. Ann. Neurol 44(1), 10–16. [DOI] [PubMed] [Google Scholar]
- Guella I, Sherman HE, Appel-Cresswell S, Rajput A, Rajput AH, Farrer MJ, 2015. Parkinsonism in GTP cyclohydrolase 1 mutation carriers. Brain 138(Pt 5), e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez DG, Reed X, Singleton AB, 2016. Genetics in Parkinson disease: Mendelian versus non-Mendelian inheritance. J. Neurochem 139 Suppl 1, 59–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz JM, Ostergaard K, Juncker I, Pedersen S, Romstad A, Moller LB, Guttler F, Dupont E, 2006. Low frequency of Parkin, Tyrosine Hydroxylase, and GTP Cyclohydrolase I gene mutations in a Danish population of early-onset Parkinson’s Disease. Eur. J. Neurol 13(4), 385–390. [DOI] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ, 1992. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55(3), 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurian MA, Gissen P, Smith M, Heales S Jr., Clayton PT, 2011. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol 10(8), 721–733. [DOI] [PubMed] [Google Scholar]
- Lee S, Emond MJ, Bamshad MJ, Barnes KC, Rieder MJ, Nickerson DA, Team NGESP-ELP, Christiani DC, Wurfel MM, Lin X, 2012. Optimal unified approach for rare-variant association testing with application to small-sample case-control whole-exome sequencing studies. Am. J. Hum. Genet 91(2), 224–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuzzi V, Carducci C, Carducci C, Cardona F, Artiola C, Antonozzi I, 2002. Autosomal dominant GTP-CH deficiency presenting as a dopa-responsive myoclonus-dystonia syndrome. Neurology 59(8), 1241–1243. [DOI] [PubMed] [Google Scholar]
- Lewthwaite AJ, Lambert TD, Rolfe EB, Olgiati S, Quadri M, Simons EJ, Morrison KE, Bonifati V, Nicholl DJ, 2015. Novel GCH1 variant in Dopa-responsive dystonia and Parkinson’s disease. Parkinsonism Relat. Disord 21(4), 394–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Durbin R, 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25(14), 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill CM, 2016. Genetics of Parkinson’s disease. Mol. Cell. Probes 30(6), 386–396. [DOI] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA, 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20(9), 1297–1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mencacci NE, Isaias IU, Reich MM, Ganos C, Plagnol V, Polke JM, Bras J, Hersheson J, Stamelou M, Pittman AM, Noyce AJ, Mok KY, Opladen T, Kunstmann E, Hodecker S, Munchau A, Volkmann J, Samnick S, Sidle K, Nanji T, Sweeney MG, Houlden H, Batla A, Zecchinelli AL, Pezzoli G, Marotta G, Lees A, Alegria P, Krack P, Cormier-Dequaire F, Lesage S, Brice A, Heutink P, Gasser T, Lubbe SJ, Morris HR, Taba P, Koks S, Majounie E, Raphael Gibbs J, Singleton A, Hardy J, Klebe S, Bhatia KP, Wood NW, International Parkinson’s Disease Genomics, C., consortium, U.C.-e., 2014. Parkinson’s disease in GTP cyclohydrolase 1 mutation carriers. Brain 137(Pt 9), 2480–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalls MA, Pankratz N, Lill CM, Do CB, Hernandez DG, Saad M, DeStefano AL, Kara E, Bras J, Sharma M, Schulte C, Keller MF, Arepalli S, Letson C, Edsall C, Stefansson H, Liu X, Pliner H, Lee JH, Cheng R, International Parkinson’s Disease Genomics, C., Parkinson’s Study Group Parkinson’s Research: The Organized, G.I., andMe, GenePd, NeuroGenetics Research, C., Hussman Institute of Human, G., Ashkenazi Jewish Dataset, I., Cohorts for, H., Aging Research in Genetic, E., North American Brain Expression, C., United Kingdom Brain Expression, C., Greek Parkinson’s Disease, C., Alzheimer Genetic Analysis, G., Ikram MA, Ioannidis JP, Hadjigeorgiou GM, Bis JC, Martinez M, Perlmutter JS, Goate A, Marder K, Fiske B, Sutherland M, Xiromerisiou G, Myers RH, Clark LN, Stefansson K, Hardy JA, Heutink P, Chen H, Wood NW, Houlden H, Payami H, Brice A, Scott WK, Gasser T, Bertram L, Eriksson N, Foroud T, Singleton AB, 2014. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet 46(9), 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JR, Todorovic M, Silburn PA, Sutherland GT, Mellick GD, 2014. Lack of reproducibility in re-evaluating associations between GCH1 polymorphisms and Parkinson’s disease and isolated dystonia in an Australian case--control group. Parkinsonism Relat. Disord 20(6), 668–670. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Fu W, Egertson JD, Stanaway IB, Phelps IG, Carvill G, Kumar A, Lee C, Ankenman K, Munson J, Hiatt JB, Turner EH, Levy R, O’Day DR, Krumm N, Coe BP, Martin BK, Borenstein E, Nickerson DA, Mefford HC, Doherty D, Akey JM, Bernier R, Eichler EE, Shendure J, 2012. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science 338(6114), 1619–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rengmark A, Pihlstrom L, Linder J, Forsgren L, Toft M, 2016. Low frequency of GCH1 and TH mutations in Parkinson’s disease. Parkinsonism Relat. Disord 29, 109–111. [DOI] [PubMed] [Google Scholar]
- Ross JP, Dupre N, Dauvilliers Y, Strong S, Ambalavanan A, Spiegelman D, Dionne-Laporte A, Pourcher E, Langlois M, Boivin M, Leblond CS, Dion PA, Rouleau GA, Gan-Or Z, 2016. Analysis of DNAJC13 mutations in French-Canadian/French cohort of Parkinson’s disease. Neurobiol. Aging 45, 212 e213–212 e217. [DOI] [PubMed] [Google Scholar]
- Safaralizadeh T, Jamshidi J, Esmaili Shandiz E, Movafagh A, Fazeli A, Emamalizadeh B, Manafi N, Taghavi S, Tafakhori A, Darvish H, 2016. SIPA1L2, MIR4697, GCH1 and VPS13C loci and risk of Parkinson’s diseases in Iranian population: A case-control study. J. Neurol. Sci 369, 1–4. [DOI] [PubMed] [Google Scholar]
- Schneider SA, Alcalay RN, 2017. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature. Mov. Disord 32(11), 1504–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li M, Hakonarson H, 2010. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38(16), e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissbach A, Klein C, 2014. Hereditary dystonia and parkinsonism: two sides of the same coin? Brain 137(Pt 9), 2402–2404. [DOI] [PubMed] [Google Scholar]
- Xu Q, Li K, Sun Q, Ding D, Zhao Y, Yang N, Luo Y, Liu Z, Zhang Y, Wang C, Xia K, Yan X, Jiang H, Shen L, Tang B, Guo J, 2017. Rare GCH1 heterozygous variants contributing to Parkinson’s disease. Brain 140(7), e41. [DOI] [PubMed] [Google Scholar]
- Yan YP, Zhang B, Shen T, Si XL, Guo ZY, Tian J, Xu CY, Zhang BR, 2018. Study of GCH1 and TH genes in Chinese patients with Parkinson’s disease. Neurobiol. Aging [DOI] [PubMed] [Google Scholar]
- Yang X, Zheng J, An R, Tian S, Zhao Q, Chen Y, Huang H, Ning PP, Song Y, Xu Y, 2017. Polymorphism in MIR4697 but not VPS13C, GCH1, or SIPA1L2 is associated with risk of Parkinson’s disease in a Han Chinese population. Neurosci. Lett 650, 8–11. [DOI] [PubMed] [Google Scholar]
- Zou M, Li R, Wang JY, Wang K, Wang YN, Li Y, Ji FX, Sun SN, Huang SS, Fan HH, Huang CP, Zhang X, Zhu JH, 2018. Association analyses of variants of SIPA1L2, MIR4697, GCH1, VPS13C, and DDRGK1 with Parkinson’s disease in East Asians. Neurobiol. Aging [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.