Abstract
Identifying objectively measurable seasonal changes in 24-hour activity patterns (rest-activity rhythms or RARs) that occur in seasonal affective disorder (SAD) could help guide research and practice towards new monitoring tools or prevention targets. We quantified rest-activity rhythms (RARs) from actigraphy data using non-parametric and extended cosine based approaches, then compared RARs between people with SAD and healthy controls in the summer (n=70) and winter seasons (n=84). We also characterized the within-person seasonal RAR changes that occurred in the SAD (n=19) and control (n=26) participants who contributed repeated measures. Only controls had significant winter increases in RAR fragmentation (intra-daily variability; in controls mean winter-summer changes (log scale) = 0.05, 0.21 standard deviation, p = 0.03). In SAD participants only, estimated evening settling times (down-mesor) were an average of 30 minutes earlier in the winter compared with the summer (1-hour standard deviation, p=0.045). These RAR characteristics correlated with greater fatigue (Spearman |r|=0.36) but not depression symptom severity. Additional research is needed to ascertain why healthy controls, but not people with SAD, appear to have increased RAR fragmentation in the winter. People with SAD lacked this increase in RAR fragmentation, and instead had earlier evening setting in the winter. Prospective and intervention studies with greater temporal resolution are warranted to ascertain how these seasonal behavioral differences relate to fatigue pathophysiology in SAD. Future research is needed to determine whether extending the winter active period, even in relatively fragmented bouts, could help reduce the fatigue symptoms common in SAD.
Keywords: Rest activity rhythm, seasonal affective disorder, seasonal changes, winter depression
Of people affected by depression, 10–20% experience a seasonal pattern of symptoms known as Seasonal Affective Disorder (SAD; Magnusson (2000)). The predictable relapse and remission pattern observed in people with SAD provides an opportunity to identify linked changes in behaviors and symptoms. One set of behavioral factors that are potentially related to changes in mood state are characteristics of the 24-hour activity pattern (known as the rest-activity rhythm or RAR). For example, people with depression symptoms tend to have less regular RARs (Luik, Zuurbier et al., 2013; Luik, Zuurbier et al., 2015), narrower active periods (Smagula, Boudreau et al., 2015; Smagula, Krafty et al., 2017), and often later activity timing (Robillard, Hermens et al., 2015; Smagula, Boudreau et al., 2015). Less research has examined RARs in SAD. Past research suggests that, compared with healthy controls, children with SAD have lower circadian rhythm amplitude (blunting) and less circadian regularity (Glod, Teicher et al., 1997), and adults with SAD have later RAR timing and lower cross-daily stability (Teicher, Glod et al., 1997).
However, we are unaware of prior evidence investigating the seasonal changes in RARs that could influence mood state in people with SAD. Seasonal RAR changes that relate to the symptoms of SAD would represent logical targets for future research and behaviorally-focused prevention approaches. In general, little is known regarding how RARs change across seasons. One recent general community study found that RAR fragmentation (having less consistent activity levels within days) increased in the winter season compared to the summer (Kume, Makabe et al., 2017). It is unknown whether this winter increase in RAR fragmentation occurs in people with SAD, whether it marks adaptation or pathology, and whether SAD is characterized by additional RAR changes in the winter.
We therefore aimed to characterize the seasonal changes that occur in people with SAD and healthy controls. We also compared RAR characteristics between these groups in the winter and summer seasons. Finally, to begin understanding the psychopathological correlates of the RAR characteristics and seasonal changes associated with SAD, we evaluated their associations with key symptom dimensions (depression, self-rated seasonality, and fatigue symptoms).
Materials and Methods
Sample
Recruitment occurred in Pittsburgh (40.4406° N, 79.9959° W) during the summer and winter months from 2013 to 2017. Individuals with SAD were diagnosed according to Rosenthal’s criteria (Rosenthal, Sack et al., 1984) and the Structured Clinical Interview for DSM-IV and –V Disorders (SCID; (First, Williams et al., 2015). Control participants reported no current or past depressive episodes on the SCID and reported low seasonality on the modified Seasonal Pattern Assessment Questionnaire (global seasonality score < 11; (Rosenthal, Sack et al., 1984)). Participants were excluded if they reported shift-work, a history of bipolar disorder, psychosis, or post-traumatic stress disorder, and current sleep or circadian disorder. Duration of the current SAD episode was at least 2 weeks out of the previous month for study inclusion. The number of previous episodes of SAD was at least two in the past two winters as per DSM-IV and DSM-5 diagnostic criteria. Treatment was not provided in this study, but for ecological validity, we included patients who were taking stable doses of antidepressant medication or bright light therapy if they met current SAD episode criteria as described above. Therefore, regardless of treatment, participants in the SAD group were still meeting diagnostic criteria for a Major Depressive Episode at the winter visit. Whether participants were engaged in treatment was not systematically assessed at every visit.
Procedure
Participants completed questionnaires on intake and then wore an Actiwatch Spectrum (Philips Respironics Inc., Murrysville, PA) for 1–2 weeks. Actigraphy data was collected from 78 participants in the summer and 90 participants in the winter. From this, adequate actigraphy data (defined as at least 3 days of recording) was available from 70 participants in the summer (43 controls, 27 people with SAD), 84 participants in the winter (49 controls, 35 people with SAD); 45 participants (26 controls and 19 people with SAD) had data from both seasons. The average length of recording was 9.6 days (3.3 SD; range 3.7–18.2 days) and did not differ by season (p=0.23).
The Institutional Review Board at the University of Pittsburgh approved all procedures and informed consent was obtained prior to experimental procedures.
Measures
The Modified Seasonal Pattern Assessment Questionnaire was used to assess self-reported seasonality (Rosenthal et al., 1984). The Structured Interview Guide for the Hamilton Depression Rating Scale (Williams, 1988) was administered by trained raters to assess the 17-item Hamilton Depression rating score reflecting severity of depression symptoms over the past week. The 11-item Chalder Fatigue Measure to assess mental and physical fatigue severity rated on a 4 point likert scale (Chalder et al., 1993); higher total scores reflect greater fatigue. For descriptive purposes, we provide Composite Scale of Morningness scores (Smith, Reilly et al., 1989).
RAR Measures
We used both non-parametric (Witting, Kwa et al., 1990) and parametric (extended cosine-based modeling (Marler, Gehrman et al., 2006) approaches.
We implemented a non-parametric approach (Witting, Kwa et al., 1990) using R Software (R Development Core Team, 2013) and the package ‘nparACT’ (Blume, Santhi et al., 2016). We calculated: intradaily variability (IV), interdaily stability (IS), activity during the least active 5 hours (L5), activity during the most active 10 hours (M10), and relative amplitude (RA). Higher IV values reflect more fragmented rhythms within days (e.g., due to frequent daytime napping, bursts of daytime activity, or night-time awakenings). Higher IS values indicate that the typical 24-hour profile accounts for more the overall variability in the recording, reflecting greater stability of the mean 24-hour profile across days. L5 and M10 were defined as the average level of activity during the least active 5 hours and most active 10 hours, respectively. Finally, RA, was operationalized as the difference between the average level of activity during the most active 10 hours of the day and the least active 5 hours of the day and is standardized to overall activity level; higher RA values indicate a greater RAR amplitude.
Parametric measures based on an extended cosinor model, derived using in-house SAS code (by R.T.K.), were: RAR alpha, RAR beta; and three RAR timing measures. RAR alpha is a parameter indicating the relative width of active to rest periods (higher alpha indicates more narrow active relative to resting periods). RAR beta is a parameter indicating the steepness of the RARs (higher values indicate more steep or “square-like” RARs). The three timing parameters were: (1) acrophase, defined as the time when activity peaks in the modeled rhythm; (2) up-mesor, defined as the time when the individual passes through the mesor (middle level of the estimated curve) on the way up, also known as left-half detection point (indicating the time the participants “gets going” in the morning); and (3) down-mesor, defined as the time when the individual passes through the mesor on the way down, also known as right-half detection point (indicating the time the participants “settles down” in the evening).
Illustrations of these metrics have been published previously (e.g., see Luik, Zuurbier et al. (2013); Smagula (2016); Smagula, Krafty et al. (2017)).
Statistical Analysis
Transformations (type) were applied to the following RAR variables that were not initially normally distributed: IV (log+1), RA (square root), alpha (log+1), and beta (log+1). To analyze within-person seasonal changes, we used t-tests to evaluate whether seasonal RAR changes were statistically different from zero (in the SAD and control groups separately). We next compared the RAR characteristics across groups using linear regressions (adjusting for age and sex) for summer and winter separately. Variables were standardized for group analyses to facilitate effect size comparisons across the RAR measures. Finally, in both groups combined, we used Spearman correlations to evaluate whether the identified RAR changes and group differences were associated with winter levels of depression symptom severity (i.e., HRSD), self-reported behavior seasonality (i.e., GSS), and fatigue (i.e., CFM).
Results
Participants showed the expected clinical differences (Table 1).
Table 1.
Descriptive information
| Winter Sample | Summer Sample | ||||||
|---|---|---|---|---|---|---|---|
| Controls (n=49) | SAD (n=35) | p-value | Controls (n=43) | SAD (n=27) | p-value | ||
| Age | 37.5 (13.7) | 39.4 (12.2) | 0.51 | 36.4 (14.0) | 40.1 (12.6) | 0.27 | |
| Female gender, n (%) | 35 (71) | 31 (88) | 0.06 | 26 (60) | 22 (81) | 0.07 | |
| Body mass index | 26.1 (6.0) | 28.3 (10.1) | 0.27 | 26.0 (6.2) | 29.9 (8.4) | 0.04 | |
| Hamilton Depression Rating Scale | 2.5 (3.4) | 16.4 (6.3) | <0.0001 | 2.3 (2.7) | 7.0 (5.3) | <0.0001 | |
| Global Seasonality Scale | 3.0 (2.9) | 14.9 (3.1) | <0.0001 | 3.4 (3.4) | 13.6 (3.3) | <0.0001 | |
| Chalder Fatigue Measure* | 10.6 (4.1) | 16.9 (6.2) | <0.0001 | 10.0 (4.2) | 10.2 (4.7) | 0.90 | |
| Composite Scale for Morningness | 36.7 (7.8) | 34.8 (9.8) | 0.34 | 39.2 (7.6) | 35.3 (8.7) | 0.06 | |
Means and standard deviations shown unless otherwise noted; p-value is from an independent samples t-test or Chi-Square test;
limited data available (n=80/84 in the winter and 43/70 in the summer)
Seasonal changes within individuals (Table 2):
Table 2.
RAR variable change scores by clinical group
| Controls (n=26) | SAD (n=19) | |||
|---|---|---|---|---|
| Change Score | p-value | Change Score | p-value | |
| IS | 0.06 (0.25) | 0.23 | 0.06 (0.18) | 0.16 |
| IV | 0.05 (0.11) | 0.03 | 0.01 (0.09) | 0.50 |
| RA | 0.03 (0.13) | 0.29 | 0.04 (0.09) | 0.07 |
| L5 | −0.25 (0.84) | 0.14 | −0.36 (0.84) | 0.08 |
| M10 | 3.79 (63.6) | 0.76 | 4.80 (39.4) | 0.60 |
| Alpha | 0.05 (0.39) | 0.50 | 0.10 (0.29) | 0.16 |
| Beta | −0.20 (0.77) | 0.19 | −0.04 (0.56) | 0.75 |
| Up-mesor | 0.27 (1.88) | 0.46 | −0.04 (1.00) | 0.85 |
| Acrophase | −0.04 (0.91) | 0.85 | −0.27 (0.74) | 0.13 |
| Down-mesor | −0.34 (2.01) | 0.39 | −0.49 (0.99) | 0.045 |
Means change scores (winter-summer) and standard deviations shown; Acronyms: IS=interdaily stability; IV=intradaily variability; RA=relative amplitude; L5=average activity during the least active five hours; M10=average activity during the most active ten hours
The only significant seasonal changes in RARs were: (1) the control group experienced increases in IV (RAR fragmentation) in the winter compared with summer (IV change score=0.05, standard deviation=0.21, p=0.03; see Figure 1), while the SAD group did not show this change; and (2) the SAD group settled down (down-mesor) about a half hour earlier in the winter (standard deviation=0.99, p=0.045), while the control group did not exhibit this change.
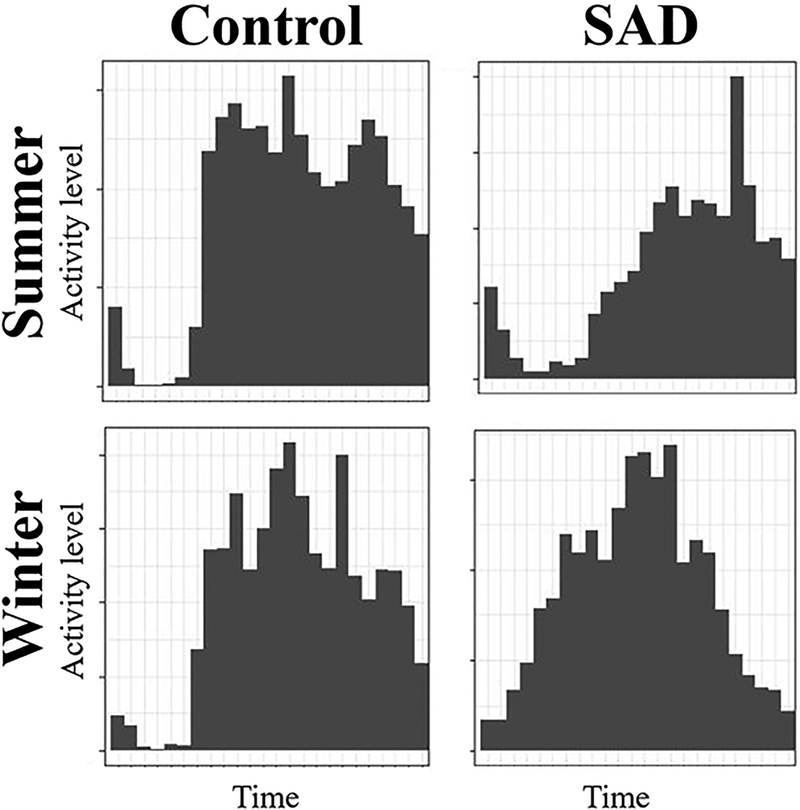
Figure 1. The average daily RAR pattern from one control particpant illustrating winter increases in rest-activity rhythm fragmentation (intra-daily variability) and one SAD participant illustrating earlier evening settling in the winter.
Note that, in the control participant, winter activity is more sporadic with greater and more frequent transitions between activity levels.
Differences between the groups within each season (Table 3):
Table 3.
Standardized effect sizes of clinical group on RAR variables
| Winter | |||
| Controls (n=49) | SAD (n=35) | ||
| β (standard error) | p-value | ||
| IS | Reference | 0.12 (0.22) | 0.59 |
| IV | −0.51 (0.22) | 0.03 | |
| RA | 0.05 (0.20) | 0.81 | |
| L5 | 0.13 (0.20) | 0.54 | |
| M10 | 0.04 (0.22) | 0.86 | |
| Alpha | 0.46 (0.24) | 0.06 | |
| Beta | −0.20 (0.20) | 0.33 | |
| Up-mesor | 0.18 (0.22) | 0.42 | |
| Acrophase | 0.09 (0.20) | 0.66 | |
| Down-mesor | −0.02 (0.21) | 0.91 | |
| Summer | |||
| Controls (n=43) | SAD (n=27) | ||
| β (standard error) | p-value | ||
| IS | Reference | 0.28 (0.24) | 0.23 |
| IV | −0.09 (0.27) | 0.72 | |
| RA | 0.14 (0.25) | 0.58 | |
| L5 | 0.04 (0.26) | 0.87 | |
| M10 | 0.25 (0.26) | 0.34 | |
| Alpha | 0.19 (0.26) | 0.46 | |
| Beta | −0.11 (0.28) | 0.70 | |
| Up-mesor | 0.28 (0.25) | 0.27 | |
| Acrophase | 0.21 (0.22) | 0.36 | |
| Down-mesor | 0.09 (0.21) | 0.69 | |
All models are separate and adjusted for age and sex; Acronyms: IS=interdaily stability; IV=intradaily variability; RA=relative amplitude; L5=average activity during the least active five hours; M10=average activity during the most active ten hours
Compared with the control group, the SAD group had statistically lower IV in the winter season only (standardized β=−0.51, standard error=0.22, p=0.03). There was also a trend where, compared with the control group, the SAD group had higher alpha (indicating relatively narrower active periods) in the winter (standardized β=0.46, standard error=0.24, p=0.06). There were no RAR differences between the groups in the summer.
Correlations between RAR differences and mental health symptoms (Table 4 and Figure 2):
Table 4.
Spearman correlations of the selected RAR variables with clinical outcomes in the winter
| Hamilton Rating Scale for Depression | Global Seasonality Scale | Chalder Fatigue Scale | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | r | p-value | n | r | p-value | n | r | p-value | |
| Change in IV | 45 | −0.23 | 0.12 | 45 | −0.21 | 0.17 | 41 | −0.36 | 0.02 |
| Winter IV | 84 | −0.18 | 0.10 | 84 | −0.18 | 0.09 | 80 | −0.18 | 0.11 |
| Winter down-mesor | 84 | −0.17 | 0.12 | 84 | −0.15 | 0.17 | 80 | −0.36 | 0.001 |
| Change in down-mesor | 45 | −0.06 | 0.68 | 45 | −0.22 | 0.15 | 41 | −0.11 | 0.48 |
| Winter alpha | 84 | 0.16 | 0.16 | 84 | 0.31 | 0.004 | 79 | 0.21 | 0.06 |
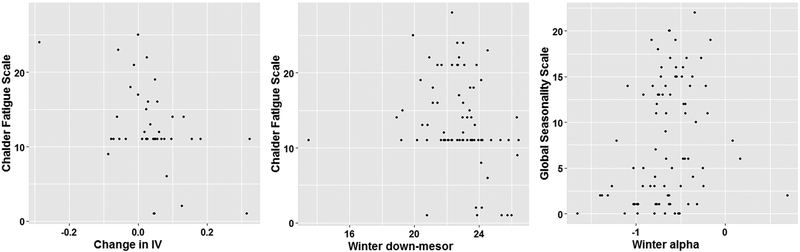
Figure 2. Correlations between RAR and clinical measures.
Notes: IV changes were calculated as winter-summer meaning such that higher values indicate a winter increase; evening settling times are in 24-hour format; for alpha, higher levels indicate relativley narrower active periods.
Greater seasonal increases in IV correlated with less fatigue symptoms (n=41, Spearman r=−0.36, p=0.02), whereas IV in the winter did not. Later down-mesor (evening settling times) in the winter, but not seasonal changes in down-mesor, correlated with less fatigue symptoms (n=80, Spearman r=−0.36, p=0.001). Finally, narrower active periods in the winter (greater alpha levels) correlated with greater self-rated behavior seasonality scores (n=80, Spearman r=0.31, p=0.004). Figure 2 illustrates these findings (note that exclusion of the extreme early down-mesor value did not alter the correlation with fatigue symptoms, i.e., n=79, Spearman r=−0.38, p=0.0005)). No other significantly correlations were identified. In sensitivity analyses stratified by clinical group (Supplemental Tables 1 and 2), the relationship between seasonal IV changes and fatigue was strong and statistically significant in the SAD group only (n=18, Spearman r=−0.60, p=0.009), and the relationship between later winter-down mesor and fatigue only met statistical significance in the control group (n=46, Spearman r=−0.46, p=0.001).
Discussion
We observed different seasonal RAR changes in healthy controls and people with SAD, which correlated with fatigue (a hallmark symptom dimension in SAD). Specifically, we replicated a previous report (Kume, Makabe et al., 2017) that RAR fragmentation (IV) increases in the winter season, and extended this evidence by noting that these winter increases in RAR fragmentation do not occur in participants with SAD. Instead, people with SAD showed a winter change to settle down earlier in the evening (down-mesor). Our interpretation is that, in the winter months, individuals without mood disorders spread their daily activities into distinct bouts (resulting in higher IV, see Figure 1; i.e., less consistent activity levels spread over a relatively wide active period). Given that the L5 measure, marking sleep fragmentation, did not display seasonal changes or group differences, the observed winter increase in IV among controls was not likely due to increased night-time awakenings.
Finding that people with SAD settled earlier in the winter is consistent with prior literature that has demonstrated relatively narrower active periods (Smagula, Boudreau et al., 2015) and earlier evening settling times (Smagula, Krafty et al., 2017) associated with depression symptoms. We extended this literature with our finding that, in SAD, these RAR factors related to fatigue and self-reported behavioral seasonality (and not particularly mood symptoms measured with the HRSD). However, the current design cannot establish how the relationship between fatigue and seasonal RAR changes (earlier evening settling in the winter and a lack of IV increases in the winter) unfold over time. The possibilities include that: (1) fatigue precedes the winter RARs observed, or (2) an earlier evening settling in the winter contributes to feelings of fatigue.
It is interesting to note that our observations are not consistent with past research that found delayed activity timing and unstable rhythms in people with SAD (Glod, Teicher et al., 1997; Teicher, Glod et al., 1997). It is possible that these different characteristics (later timing and RAR instability vs. shortened active periods) reflect distinct sub-groups of patients with SAD. The relatively small sample sizes in these studies (including the present work) may have led to over- or under- sampling of disease sub-groups. Our also appear to be inconsistent with past literature that correlated greater RAR fragmentation (higher IV) with mood symptoms (Luik, Zuurbier et al., 2013; Luik, Zuurbier et al., 2015). However, this past evidence comes from studies of older adults who were past retirement age. In people of working age, higher IV in the winter may have a different meaning, as spreading activity into distinct bouts could be necessary to accomplish both professional and leisure activities. Future research is needed to clarify the differences in the psychosocial meaning of RAR fragmentation across population sub-groups and seasons.
Other limitations of our study include the lack of statistical power in the repeated measures sample to statistically infer group by season interactions. As discussed above, our study design precludes the assessment of temporal relations between RAR changes and psychological symptoms (e.g., fatigue). Therefore, it remains unseen which aspect (RAR changes and fatigue) are upstream, whether modifying the RAR will affect fatigue levels, or whether these RAR factors are simply markers of the underlying mechanisms that must be targeted. We also cannot ascertain whether these effects depend on or differ following the treatment of SAD. Nevertheless, if the lack of fragmentation in the winter for people with SAD was due to treatment effects, we would expect winter fragmentation levels to correlate with depression severity (i.e., the downstream effect of treatment; it did not, see Table 4). Follow-up studies are needed to surpass these limitations.
Specifically, future experimental interventions and longitudinal research with greater temporal resolution are needed to establish temporality in the relationships between RAR changes and changes in the various psychological symptom domains that have their own time-courses in SAD incidence (Young, Watel et al., 1991) and remission (Meyerhoff, Young et al., 2018). If RAR changes do precede symptoms, interventions to monitor and intervene on RARs would be warranted; this would be consistent with one of the guiding principles of cognitive behavioral therapy (which is efficacious for SAD, see Rohan, Meyerhoff et al. (2016)): behavioral modifications can drive changes to thoughts and feelings. Future studies with longer-term tracking can use lagged time-series analysis are needed to clarify which factors (RARs or specific psychological symptoms) should be intervened on early to prevent further mood and energy problems.
Supplementary Material
Declaration of Interest and Acknowledgements:
The authors report no conflicts of interest. Funding for this study was provided by NIH Grant 5 R01 MH 103313–5 (K. Roecklein), NIH Grant K01 MH112683 (S. Smagula), R01 DA033064 (P. Franzen), and R01 GM 113243 (R. Krafty); the NIH had no role in the study design, data analysis and interpretation, report writing, or decision to submit the paper for publication. The authors thank Mary E. Fletcher for her role processing the actigraphy data.
References
- Blume C, Santhi N, Schabus M. (2016). ‘nparACT’ package for R: A free software tool for the non-parametric analysis of actigraphy data. MethodsX. 3:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Williams J, Karg R, Spitzer R. (2015). Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Glod CA, Teicher MH, Polcari A, McGreenery CE, Ito Y. (1997). Circadian rest-activity disturbances in children with seasonal affective disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 36:188–195. [DOI] [PubMed] [Google Scholar]
- Kume Y, Makabe S, Singha-Dong N, Vajamun P, Apikomonkon H, Griffiths J. (2017). Seasonal effects on the sleep-wake cycle, the rest-activity rhythm and quality of life for Japanese and Thai older people. Chronobiology international. 34:1377–1387. [DOI] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Direk N, Hofman A, Van Someren EJ, Tiemeier H. (2015). 24-hour activity rhythm and sleep disturbances in depression and anxiety: a population-based study of middle-aged and older persons. Depression and anxiety. [DOI] [PubMed] [Google Scholar]
- Luik AI, Zuurbier LA, Hofman A, Van Someren EJ, Tiemeier H. (2013). Stability and fragmentation of the activity rhythm across the sleep-wake cycle: the importance of age, lifestyle, and mental health. Chronobiology international. 30:1223–1230. [DOI] [PubMed] [Google Scholar]
- Magnusson A (2000). An overview of epidemiological studies on seasonal affective disorder. Acta Psychiatrica Scandinavica. 101:176–184. [PubMed] [Google Scholar]
- Marler MR, Gehrman P, Martin JL, Ancoli-Israel S. (2006). The sigmoidally transformed cosine curve: a mathematical model for circadian rhythms with symmetric non-sinusoidal shapes. Stat Med. 25:3893–3904. [DOI] [PubMed] [Google Scholar]
- Meyerhoff J, Young MA, Rohan KJ. (2018). Patterns of depressive symptom remission during the treatment of seasonal affective disorder with cognitive-behavioral therapy or light therapy. Depression and anxiety. 35:457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. (2013). R: A language and environment for statistical computing. . In Computing RFfS (Eds). Vienna, Austria. . [Google Scholar]
- Robillard R, Hermens DF, Naismith SL, White D, Rogers NL, Ip TK, Mullin SJ, Alvares GA, Guastella AJ, Smith KL, Rong Y, Whitwell B, Southan J, Glozier N, Scott EM, Hickie IB. (2015). Ambulatory sleep-wake patterns and variability in young people with emerging mental disorders. Journal of psychiatry & neuroscience : JPN. 40:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohan KJ, Meyerhoff J, Ho S-Y, Evans M, Postolache TT, Vacek PM. (2016). Outcomes One and Two Winters Following Cognitive-Behavioral Therapy or Light Therapy for Seasonal Affective Disorder. The American journal of psychiatry. 173:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, Mueller PS, Newsome DA, Wehr TA. (1984). Seasonal affective disorder: a description of the syndrome and preliminary findings with light therapy. Archives of general psychiatry. 41:72–80. [DOI] [PubMed] [Google Scholar]
- Smagula SF. (2016). Opportunities for clinical applications of rest-activity rhythms in detecting and preventing mood disorders. Curr Opin Psychiatry. 29:389–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Boudreau RM, Stone K, Reynolds CF 3rd, Bromberger JT, Ancoli-Israel S, Dam TT, Barrett-Connor E, Cauley JA. (2015). Latent activity rhythm disturbance sub-groups and longitudinal change in depression symptoms among older men. Chronobiology international.1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smagula SF, Krafty RT, Taylor BJ, Martire LM, Schulz R, Hall MH. (2017). Rest-activity rhythm and sleep characteristics associated with depression symptom severity in strained dementia caregivers. Journal of sleep research. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CS, Reilly C, Midkiff K. (1989). Evaluation of three circadian rhythm questionnaires with suggestions for an improved measure of morningness. The Journal of applied psychology. 74:728–738. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Glod CA, Magnus E, Harper D, Benson G, Krueger K, McGreenery CE. (1997). Circadian rest-activity disturbances in seasonal affective disorder. Archives of general psychiatry. 54:124–130. [DOI] [PubMed] [Google Scholar]
- Witting W, Kwa IH, Eikelenboom P, Mirmiran M, Swaab DF. (1990). Alterations in the circadian rest-activity rhythm in aging and Alzheimer’s disease. Biological psychiatry. 27:563–572. [DOI] [PubMed] [Google Scholar]
- Young MA, Watel LG, Lahmeyer HW, Eastman CI. (1991). The temporal onset of individual symptoms in winter depression: differentiating underlying mechanisms. Journal of affective disorders. 22:191–197. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.