Abstract
There is growing evidence for dysfunctional glutamatergic excitation and/or gamma-aminobutyric acid (GABA)ergic inhibition in patients with multiple sclerosis (MS). Cognitive impairment may occur during the early stages of MS and hippocampal abnormalities have been suggested as biomarkers. However, researchers have not clearly determined whether changes in hippocampal GABA and glutamate (Glu) levels are associated with cognitive impairment and aberrant neural activity in patients with MS. We used magnetic resonance spectroscopy to measure GABA+ and Glu levels in the left hippocampal region of 29 patients with relapsing-remitting MS and 29 healthy controls (HCs). Resting-state functional connectivity (FC) with the hippocampus was also examined. Compared to HCs, patients exhibited significantly lower GABA+ and Glu levels, which were associated with verbal and visuospatial memory deficits, respectively. Patients also showed decreased FC strengths between the hippocampus and several cortical regions, which are located within the default mode network. Moreover, hippocampal GABA+ levels and Glu/GABA+ ratios correlated with the FC strengths in HCs but not in patients with MS. This study describes a novel method for investigating the complex relationships among excitatory/inhibitory neurotransmitters, brain connectivity and cognition in health and disease. Strategies that modulate Glu and GABA neurotransmission may represent new therapeutic treatments for patients with MS.
Keywords: multiple sclerosis, gamma-aminobutyric acid, glutamate, hippocampus, functional connectivity
Introduction
Multiple sclerosis (MS) is the most common chronic inflammatory demyelinating disease in the central nervous system of young adults. Although demyelinated plaques in the white matter (WM) are the hallmark of multiple sclerosis pathology, recent autopsy findings have indicated that demyelination occurs in the normal-appearing WM and the cortex in patients with early-stage MS (Barnett et al., 2009; Lucchinetti et al., 2011). MS can result in a broad range of symptoms, of which cognitive disability is of particular interest because it occurs in 40% to 70% of patients at both the earlier and later stages (Chiaravalloti and DeLuca, 2008). Based on recent convergent research, the pathogenic effects of hippocampal abnormalities are implicated in MS-related cognitive impairment (Preziosa et al., 2016; Sacco et al., 2015; Sumowski et al., 2016; van Geest et al., 2016). The hippocampus is a key region involved in memory (Eichenbaum, 2000) and is vulnerable in patients with relapsing-remitting MS (RRMS) (Chiaravalloti and DeLuca, 2008; MacKenzie-Graham et al., 2016). Decreased fractional anisotropy (Planche et al., 2016), reduced functional activity (Hulst et al., 2015; Sweet et al., 2004), microglial activation and extensive demyelination (Geurts et al., 2007) in the hippocampus have been reported in previous imaging and postmortem studies.
Gamma-aminobutyric acid (GABA) and glutamate (Glu) are the main inhibitory and excitatory neurotransmitters in the human brain, respectively, which could regulate the spatial and temporal extent of neural activity throughout the brain (Akerman and Cline, 2007; Tessier and Broadie, 2009). In vivo proton magnetic resonance spectroscopy (1H-MRS) provides a unique opportunity to non-invasively measure Glu levels in the human brain. Recent technical advances have been developed to quantify GABA levels using spectral editing techniques, such as the Mescher-Garwood Point Resolved Spectroscopy sequence (MEGA-PRESS) (Mescher et al., 1998). The MEGA-PRESS method allows GABA signals to be separated from other metabolites by taking advantage of known couplings within the GABA molecule (Harris et al., 2017; Mullins et al., 2014). It has successfully been applied to measure GABA levels in patients with neuropsychiatric disorders (Bhattacharyya et al., 2013; Gao et al., 2015; Robertson et al., 2016) and in healthy subjects (Balz et al., 2016; Gao et al., 2013).
Recently, several clinical studies have investigated GABA levels in patients with MS using MEGA-PRESS, with diverse results. For instance, in sensorimotor regions, higher GABA levels have been reported in RRMS patients compared to healthy controls (HCs) (Nantes et al., 2017) but lower GABA levels have also been reported in secondary progressive MS patients (Cawley et al., 2015). Additionally, lower hippocampal GABA levels were also reported in secondary progressive MS (Cawley et al., 2015). However, it remains to be determined whether GABA levels change in the hippocampus at the RRMS stage. Moreover, the glutamatergic and GABAergic signaling are tightly linked (Akerman and Cline, 2007), and the resulting balance of excitation and inhibition in the brain regions influences individual differences in cognitive ability (de la Vega et al., 2014). GABA enhancer (Sodium valproate) can relieve clinical symptoms in an animal model of MS, which is thought to be mediated by the inhibition of enhanced Glu excitotoxicity (Mandolesi et al., 2015). In contrast, another MS study observed reduced Glu levels, but not significant, in hippocampal region, which correlated with worse memory function (Muhlert et al., 2014). These inconsistent conclusions suggest that the changes in neurotransmitter levels in MS may be complex and specific for different brain circuits, which raises a fundamental question to be investigated.
GABA and Glu regulate the spatial and temporal extent of neural activity throughout the brain (Akerman and Cline, 2007; Tessier and Broadie, 2009), which is essential for the control of information processing and transfer between brain regions (Farrant and Nusser, 2005). Spontaneous neuronal activities, identified by slow fluctuations in the blood oxygen level-dependent (BOLD) signals, are present in the brain at rest and well organized into specific functional networks, which represent spatial maps of correlations of these BOLD signal fluctuations within anatomically separate brain regions (Fox and Raichle, 2007). Resting-state functional magnetic resonance imaging (fMRI) studies have shown that the cognitive deficits in patients with MS are associated with aberrant hippocampal functional connectivity (FC) with temporal regions (Cruz-Gomez et al., 2016), posterior cingulate cortex (PCC) (Hulst et al., 2015), and other regions (Tona et al., 2014) in patients with RRMS.
We hypothesized that patients with RRMS would present aberrant GABA and Glu levels, which might underlie the cognitive disability and disturbed functional integrations between hippocampus and other brain regions. 1H-MRS with MEGA-PRESS and PRESS were used to investigate GABA and Glu levels in the hippocampus in patients with RRMS and HCs. Additionally, the intrinsic FC of the hippocampus was investigated using a resting-state seed-based FC analysis. The relationships between cognitive performance, MRS measurements and FC strengths were analyzed to test our hypothesis.
Materials and Methods
Subjects
Twenty-nine patients with RRMS, defined according to the revised McDonald criteria (Polman et al., 2011), from the Department of Neurology, Shandong Provincial Hospital were recruited in this study. Exclusion criteria included a diagnosis of other neurological or psychiatric disorders and head trauma; relapse and steroid treatment within the preceding 3 months; hippocampal lesions that were visible on the MR images; intake of GABAergic agents (e.g., baclofen) before enrollment; severe depression (Beck Depression Inventory > 27) or use of antidepressant treatments; and a limitation of full activity with as Expanded Disability Status Scale (EDSS) score > 4. Twenty-nine age- and sex-matched HCs without neurological or psychiatric diseases were recruited from the local community. The demographic information was summarized in Table 1.
Table 1.
Participants’ demographic and cognitive data
| Characteristics | Patients (n = 29) |
HCs (n = 29) |
p value |
|---|---|---|---|
| Gender (male/female) | 8/21 | 10/19 | 0.57 |
| Age (years) | 36.38 ± 9.86 (range: 18–51) | 37.38 ± 10.46 (range: 22–53) | 0.71 |
| Education (years) | 11.86 ± 2.82 | 12.69 ± 4.56 | 0.41 |
| Disease duration (years) | 5.38 ± 3.87 | -- | -- |
| EDSS | 2.09 ± 1.27 (range: 0–4) | -- | -- |
| AVLT | 48.28 ± 11.36 | 61.76 ± 10.73 | < 0.001* |
| ROCF-IR | 23.86 ± 7.44 | 27.48 ± 5.65 | 0.03* |
| ROCF-DR | 22.83 ± 8.68 | 25.41 ± 6.56 | 0.17 |
| SDMT | 45.21 ± 12.59 | 55.45 ± 14.41 | 0.009* |
| TMT (B-A) | 74.97 ± 21.01 | 66.03 ± 21.03 | 0.31 |
The data are presented as means ± standard deviations.
indicates p < 0.05, corrected for multiple comparisons using the Holm-Sidak approach.
AVLT, Auditory Verbal Learning Test; EDSS, Expanded Disability Status Scale; HCs, healthy controls; ROCF-IR (or DR), Rey–Osterrieth Complex Figure Test-Immediate Recall (or Delayed Recall); SDMT, Symbol Digit Modalities Test; TMT, Trail-Making Test.
All subjects were right-handed, as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). None of the subjects had a history of substance abuse. Smoking, alcohol and caffeine were prohibited for 12 hours prior to the MR scanning. The study was performed in accordance with the Declaration of Helsinki and was approved by the institutional review board of Shandong Medical Imaging Research Institute, Shandong University. Written informed consent was obtained from all subjects.
Cognitive Tests
The subjects’ neuropsychological statuses were tested using the Auditory Verbal Learning Test (AVLT, Chinese version) for verbal learning and memory (Zhao et al., 2012), the Rey-Osterrieth Complex Figure Test (ROCF) for visuospatial memory (Shin et al., 2006), the Symbol Digit Modalities Test (SDMT) for psychomotor speed (Van Schependom et al., 2014), and the Trail-Making Test (TMT B-A) for executive control (Sanchez-Cubillo et al., 2009). The neuropsychological tests were specifically designed to assess memory and executive domains, which were vulnerable in patients with RRMS (Genova et al., 2013; Hulst et al., 2015). Each participant took approximately 60 min to complete all tests in a fixed order.
MRI Data Acquisition
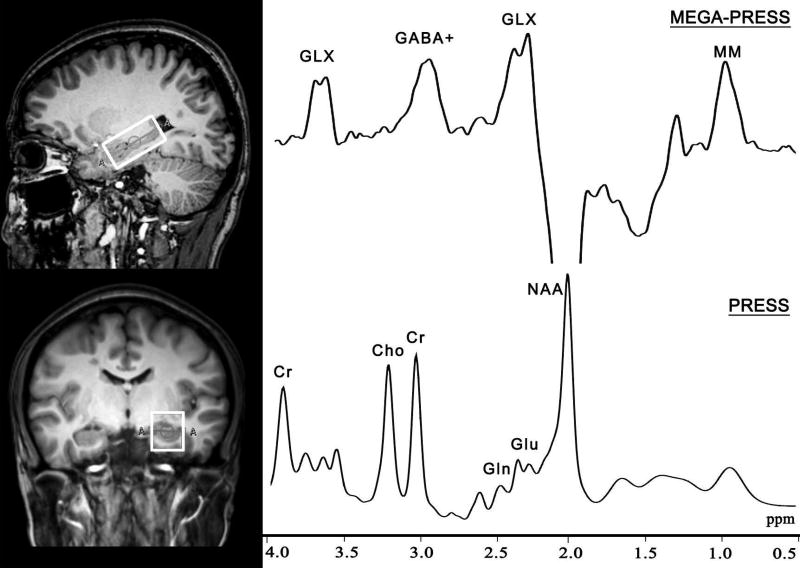
All subjects were scanned with a 3.0 T scanner (Philips ‘Achieva’ TX, Best, The Netherlands) using an eight-channel phased-array head coil. The T1-weighted 3D turbo field echo sequence was used as a localizer and was acquired using the following parameters: repetition time (TR) = 8.1 ms; TE = 3.7 ms; slice thickness = 1 mm; field of view = 24 × 24 cm2; and voxel size = 1 × 1 × 1 mm3. The volume of interest (VOI) with a size of 4 × 2 × 2 cm3 was centered on the left hippocampus and positioned parallel to the long axis of the hippocampal body in a parasagittal section (Fig. 1). The 3 ppm resonance of GABA was measured using the MEGA-PRESS sequence (Mescher et al., 1998) with the following parameters: TR = 2000 ms; TE = 68 ms; 256 averages; and acquisition bandwidth = 2000 Hz. The Glu level was obtained from the same VOI using a PRESS sequence (TR = 2000 ms; TE = 35 ms; 32 averages; and acquisition bandwidth = 2000 Hz). For quantification, a shorter measurement (4 averages) of the unsuppressed water signal was obtained. The resting-state fMRI data were acquired using an echo-planar gradient-echo pulse sequence: TR = 2000 ms; TE = 35 ms; field of view = 24 × 24 cm2; and slice thickness = 4 mm. Fluid-attenuated inversion recovery (FLAIR) images (TR = 11000 ms; TE = 125 ms; slice thickness = 3 mm; field of view = 24 × 20 cm2; and voxel size = 1 × 1 × 3 mm3) were acquired to evaluate WM lesions. Details of the MRI data acquisition procedures are provided in the Supplemental Methods.
Fig. 1.
Representative proton magnetic resonance spectra obtained from the left hippocampus using the MEGA-PRESS and PRESS techniques. Cho, choline; Cr, creatine; GABA, gamma-aminobutyric acid; Gln, glutamine; Glu, glutamate; GLX, glutamine-glutamate complex; MEGA-PRESS, Mescher-Garwood Point Resolved Spectroscopy sequence; MM, macromolecular; NAA, N-acetylaspartate.
Volumetric Data Processing
The absolute volumes of the left and right hippocampi were quantified from 3D T1-weighted images using FMRIB’s Integrated Registration and Segmentation Tool (FSL FIRST) according to the standard procedure (Patenaude et al., 2011). Normalized brain volume (NBV) was estimated with SIENAX (Smith et al., 2002) based on tissue-type segmentation with partial volume estimation. WM lesions in the FLAIR images were segmented with a lesion prediction algorithm (Schmidt et al., 2012) as implemented in the Lesion Segmentation Tool (see Supplemental Methods for details).
Spectral Quantification
The MEGA-PRESS data were analyzed using ‘Gannet’ (GABA-MRS Analysis Tool) in Matlab 2010b (Mathworks) with Gaussian curve fitting of the GABA+ peaks (Edden et al., 2014). A 3 Hz exponential line broadening was applied. Because the signal detected at 3.02 ppm using these experimental parameters is also expected to contain contributions from both macromolecules (MM) and homocarnosine (Rothman et al., 1997), in the rest of this manuscript this signal is labeled GABA+ rather than GABA, to indicate the presence of these other compounds. The PRESS data were quantified using LCModel (version 6.3–0D) (Provencher, 1993). The ratios of the integrals of neurotransmitter (GABA+ or Glu) and water signals, making corrections for T1 and T2 relaxation times and tissue composition, were used to calculate water-scaled GABA+ or Glu levels in institutional units (iu) (Gasparovic et al., 2006; Harris et al., 2015; Mullins et al., 2014) (see Supplemental Methods for details).
Gannet provides normalized residual fitting errors for GABA+ levels, which can be interpreted quantitatively to assess measurement quality. Only spectra with a GABA+ fitting error of less than 15% were included in the final analysis. The reliability of the Glu measurement was assessed using the Cramer-Rao lower bounds (CRLB) as represented in LCModel and a commonly accepted CRLB criterion of 20% was chosen to reject low-quality spectra.
Each pixel in the 3D T1-weighted brain images was segmented as gray matter (GM), WM, or cerebrospinal fluid (CSF) using an automatic brain segmentation program, FAST (FMRIB’s automated segmentation tool) in the FSL package (Oxford University, Oxford, UK) (Zhang et al., 2001). VOIs were co-registered to the anatomical images using the “Re-creation of VOI” Matlab tool (Montelius et al., 2009). Tissue GM fractions were obtained by calculating the ratio of GM volume to the GM+WM volumes in the VOIs.
Hippocampal FC Analysis
Preprocessing of the fMRI data was carried out using the toolbox for Data Processing & Analysis of Brain Imaging (DPABI, version 2.3) (Fan et al., 2016), which is based on Statistical Parametric Mapping software (SPM, version 12). Structural images were coregistered to the functional images and then segmented and registered to standard space using the nonlinear registration algorithm (Ashburner, 2007). Functional images were motion-corrected, and nuisance regressors were removed (see Supplemental Methods for details). The left hippocampus from the Brainnetome Atlas (Fan et al., 2016), which contains information on both anatomical and functional connections, was chosen as the seed for the FC analysis. A Pearson-Moment correlation analysis was performed between the BOLD time course within the hippocampus (averaged across all voxels) and each voxel in the brain. This process resulted in an FC coefficient map for each subject, which were converted into z-maps using Fisher’s r-to-z transformation for the subsequent statistical analyses.
Calculation of FC Strengths Between Clusters
The FC strengths for each participant were then extracted using the clusters showing significant between-group differences in the hippocampal FC analysis. Briefly, the mean time course within each seed was extracted by averaging the time courses of all the voxels belonging to the seed. Subsequently, the mean time course was further used to compute correlation coefficients with the time courses of the left hippocampus. The resulting correlation coefficients were then converted to z-scores using Fisher’s r-to-z transformation to improve the normality.
Statistical Analysis
All variables were assessed for normality using the Kolmogorov-Smirnov testing and histogram inspection. We used multivariate general linear model analyses to assess group differences and all analyses were corrected for age and gender. For the cognitive tests, education levels and NBV were also added as additional covariates. Gender-specific group differences were analyzed using the chi-square test.
Group differences in the FC z-maps were assessed using 2-sample t tests when age and gender were included as covariates (Colasanti et al., 2016). Analyses were limited to include only regions that were recruited by at least one of the groups. These masks were computed by thresholding, binarizing and then combining each within-group analysis (see Supplemental Methods). The analysis was corrected for multiple comparisons using the Gaussian Random Field theory (Z value for voxel > 2.3, cluster-wise p < 0.05).
Partial correlation analyses between the hippocampal neurotransmitter levels and FC strengths, were performed separately for patients with MS and HCs, while controlling for age and gender. Age was considered as an important confounding factor, as we have previously observed an age-related decline in GABA+ levels (Gao et al., 2013). Gender-specific differences in hippocampal FC (Lopez-Larson et al., 2011) have also been reported. The Holm-Sidak approach (Aickin and Gensler, 1996) was applied to correct for multiple comparisons, and a 2-tailed p value of < 0.05 was considered significant. Multiple stepwise regression analyses were then performed on the MS group, with cognitive scores or EDSS as the dependent variable and demographic and imaging (volumetric, spectroscopic and FC) measurements as the predictive variables. All statistical analyses were conducted using PASW software (version 17.0, Chicago, IL, USA).
Results
Demographic and Cognitive Characteristics
The demographic and cognitive characteristics are listed in Table 1. The two groups did not exhibit significant differences in age, gender or education level. All scores of the trials in the AVLT were significantly lower in the patients with MS (all p values < 0.005, Supplementary Table S1). Therefore, the total score for the AVLT was presented here. Compared to the HCs, patients with MS performed worse on the AVLT, immediate recall of the ROCF and SDMT (p < 0.05).
Volumetric Differences
The patients showed reduced NBV compared with the HCs (p < 0.05, Table 2). There were no significant differences in the WM lesions volume, left or right hippocampal volume between the two groups.
Table 2.
Between-groups differences in the volumetric measurements
| Characteristics | Patients (n = 29) |
HCs (n = 29) |
p value |
|---|---|---|---|
| NBV (dm3) | 1.49 ± 0.07 | 1.52 ± 0.05 | 0.03 |
| Left HV (mm3) | 3657.19 ± 408.42 | 3604.55 ± 417.24 | 0.51a |
| Right HV(mm3) | 3831.33 ± 358.58 | 3866.56 ± 417.67 | 0.95a |
| Lesions volume (mm3) | 11069.69 ± 13782.86 | -- | -- |
The data are presented as means ± standard deviations.
indicates analyses that were corrected for inter-individual differences in age, gender and normalized brain volume (NBV).
HCs, healthy controls; HV, hippocampal volume.
MRS
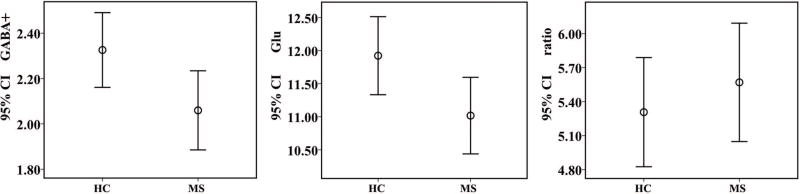
The fitting error of GABA+ and CRLB of Glu in all subjects was less than 15% and 20%, respectively. No difference was observed in the spectral quality between HC and MS groups (fitting error of GABA+: 7.93 ± 1.41 % vs. 7.99 ± 2.01%, p = 0.90; CRLB of Glu: 7.34 ± 1.08 % vs. 7.62 ± 1.15 %, p = 0.35). No difference was observed in the GM fractions within the spectroscopic VOI between groups (HCs vs. MS: 53.08 ± 3.82 % vs. 54.11 ± 3.57 %, p = 0.34). Neither hippocampal volume nor GM fractions within the spectroscopic VOI did correlate with neurotransmitter measurements in the HC and MS groups. Compared with the HCs, patients with MS showed significantly lower GABA+ levels in the left hippocampus, which were reduced by 11.59% (2.33 ± 0.43 iu vs. 2.06 ± 0.46 iu, p = 0.03, Fig. 2). In addition, the difference in Glu levels also reached significance (11.92 ± 1.55 iu vs. 11.02 ± 1.52 iu, p = 0.03, Fig. 2). No significant difference in the Glu/GABA+ ratio (5.31 ± 1.27 vs. 5.57 ± 1.37, p = 0.50, Fig. 2) was observed between groups. The coefficient of variance of GABA+ and Glu levels in the HC group was at 18% and 13%, respectively.
Fig. 2.
Differences in the levels of neurotransmitter between the healthy control (HC) group and patients with multiple sclerosis (MS). CI, confidence interval; Ratio = Glu/GABA+.
Hippocampal FC
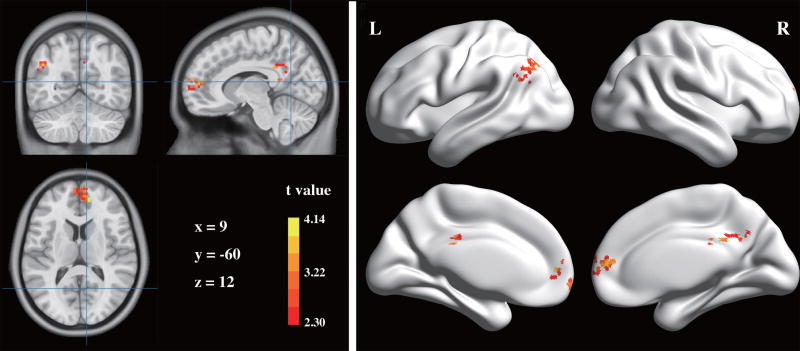
Compared with the HCs, MS patients exhibited lower connectivity with the left hippocampus in three clusters, including the bilateral medial prefrontal cortices (MPFC), left angular gyrus (AG) and bilateral PCC (Fig. 3 and Table 3). No areas showed higher hippocampal connectivity in the MS group. The seed-based FC analysis confirmed that the mean FC strengths between the hippocampus and the three clusters were all significantly decreased in patients with MS compared with those in the HCs (all p values < 0.01, Table 3).
Fig. 3.
Brain regions showing lower FC with the left hippocampus of patients with MS. Left panel: The FC map is overlaid on the MNI standard brain image (x, y and z indicate the MNI coordinates of the crosses). Right panel: The FC map was projected onto the hemispheric surfaces using BrainNet Viewer (http://www.nitrc.org/projects/bnv/) for visualization. L, left; R, right.
Table 3.
Between-group differences in hippocampal FC
| Brain regions | BA | FC strengths | MNI coordinates | Voxels | Max t value |
|||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Patients | HCs | x | y | z | ||||
| Bilateral MPFC | 10 | 0.36 ± 0.17 | 0.54 ± 0.22 | 12 | 51 | 12 | 107 | 4.14 |
| Left AG | 39 | 0.32 ± 0.19 | 0.46 ± 0.20 | −45 | −60 | 33 | 59 | 3.51 |
| Bilateral PCC | 31, 23 | 0.34 ± 0.16 | 0.48 ± 0.19 | 9 | −42 | 30 | 58 | 3.29 |
AG, angular gyrus; BA, Brodmann area; FC, functional connectivity; MNI, Montreal Neurological Institute; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex.
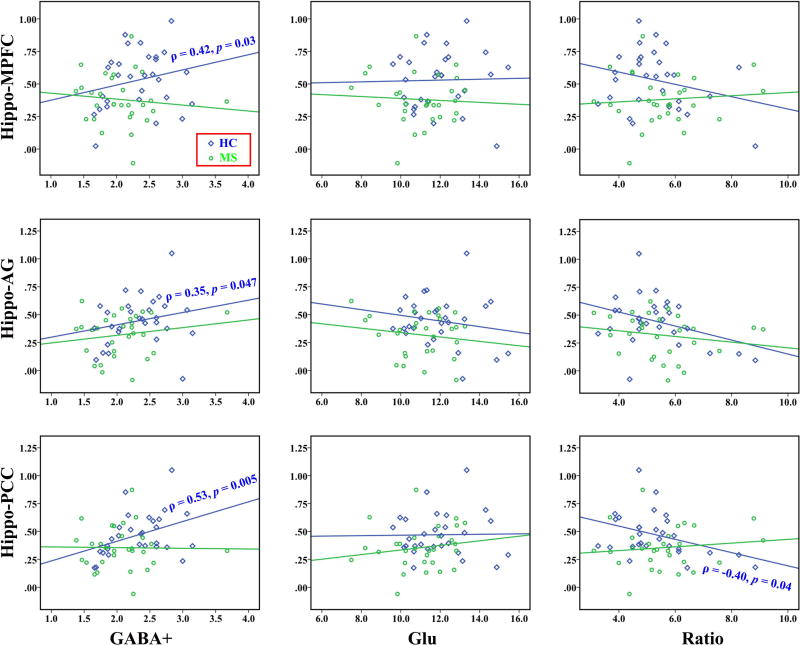
Relationships between Hippocampal GABA+/Glu Levels and FC Strengths
In the HC group (Fig. 4), partial correlation analyses revealed that the GABA+ in the left hippocampus was positively correlated with the hippocampal FC with the AG (ρ = 0.35, p = 0.047) and the PCC (ρ = 0.53, p = 0.005), whereas the Glu/GABA+ ratio was negatively correlated with the hippocampus-PCC connectivity (ρ = −0.40, p = 0.04). GABA+ levels also tended to be associated with the hippocampus-MPFC connectivity (ρ = 0.43, p = 0.09). In addition, better performance on the AVLT was moderately associated with stronger connectivity between the hippocampus and AG (ρ = 0.58, p = 0.01). No other significant correlations were found between clinical performance and imaging parameters in the HC group. In the MS group, correlations were not observed between neurotransmitter levels and FC strengths (p > 0.05, uncorrected; Fig. 4).
Fig. 4.
Correlations between hippocampal neurotransmitter measurements and FC strengths, as well as their relationships with the cognitive performance within each group. The blue and red lines represent positive and negative correlations, respectively. The numbers below the lines represent the partial correlation coefficients. * and ** indicate Holm-Sidak corrected p < 0.05 and 0.01, respectively. The analyses that achieved uncorrected p < 0.05 are also indicated with dashed lines. AG, angular gyrus; Hipp, hippocampus; MPFC, medial prefrontal cortex; PCC, posterior cingulate cortex.
Relationships between clinical performance and imaging measurements
In the MS group, the regression analyses found that the best predictors for AVLT scores (R2 = 0.36, p = 0.003) were the GABA+ levels (β = 0.53, p = 0.002) and age (β = −0.33, p = 0.049), whereas age (β = −0.54, p = 0.001) and Glu levels (β = 0.38, p = 0.02) were used to explain the total variability in ROCF scores (R2 = 0.41, p = 0.001). No significant model was identified for the EDSS score. Neither hippocampal volume nor GM fractions within the spectroscopic VOI did correlate with clinical performance in the HC and MS groups.
Discussion
In the present study, we explored the changes of excitatory and inhibitory neurotransmitter levels in the hippocampus and their relationships with aberrant FC patterns and cognitive impairments in patients with RRMS. The findings support that: (I) the levels of GABA+ and Glu in the hippocampus were lower in patients with MS than in HCs; (II) these decreased levels were separately associated with distinct memory deficits; (III) patients with MS exhibited decreased FC between the hippocampus and certain regions, including the MPFC, AG and PCC; and (IV) the GABA+ levels and Glu/GABA+ ratios were coupled with the FC strengths between the hippocampus and the abovementioned regions in the HCs but not in the MS group.
Several pioneering MRS studies have revealed significantly lower GABA+ levels in the hippocampus of patients with secondary progressive MS (Cawley et al., 2015), and lower Glu levels in the sensorimotor and parietal regions in patients with RRMS (Nantes et al., 2017). This study is the first to show that patients with RRMS exhibit both lower GABA+ and Glu levels in the hippocampus, which may reflect dysfunctional glutamatergic and GABAergic systems. This finding is consistent with a number of previous animal studies: decreased levels of Glu, numbers of GABAergic interneurons and expression of glutamic acid decarboxylase (GAD), the enzyme responsible for synthesizing GABA, have been found in the cortex of mouse model of MS (Falco et al., 2014; Massella et al., 2012; Orije et al., 2015). The abnormalities in the excitatory and inhibitory neurotransmitter systems may be attributed to neurodegeneration and demyelination, which are prominent features observed in the hippocampus of patients with MS (Papadopoulos et al., 2009). Moreover, the MS-induced demyelination would result in significant decreases in synaptic density and the levels of neuronal proteins required for glutamatergic and GABAergic neurotransmission (Dutta et al., 2011).
Consistent with previous results (Schoonheim et al., 2012; Van Schependom et al., 2014), cognitive dysfunction in patients with RRMS was most prominent in the domains of episodic memory and information processing speed. The reduced verbal memory and visuospatial memory observed in patients with MS correlated with decreased levels of GABA+ and of Glu in the hippocampus, respectively, but did not correlate with hippocampal FC strength. The association between memory and GABA+/Glu levels may not depend on macroscopic changes in the hippocampus, as both the hippocampal volume and GM fractions within the spectroscopic VOI did not correlate with memory or neurotransmitter measurements. In line with the current findings, a significant positive correlation was observed between Glu levels in the hippocampus and visuospatial memory in patients with RRMS, but not in healthy controls (Muhlert et al., 2014). One possible explanation for this finding is that learning and memory function in the hippocampus is largely dependent on the glutamate-dependent synaptic pathways from the entorhinal cortex to the dentate gyrus (Tamminga et al., 2012), whose volume has been shown to be related to visuospatial memory (Travis et al., 2014). Brain microdialysis in a rat model of amnesia also revealed that hippocampal glutamate transmission closely correlated with the extent of the spatial memory impairment (Shimizu et al., 1998). On the other hand, the impairments in language ability and word learning in patients with MS are associated with changes in the CA1 subfield (Rocca et al., 2016; Sicotte et al., 2008), which contains GABAergic neurons (Pettit and Augustine, 2000).
Resting-state FC between the hippocampus and its anatomic input or target areas, including the MPFC, PCC and AG, was significantly decreased in patients with MS. Additionally, better AVLT performance was positively associated with hippocampal FC to the AG in the HCs, but not patients. The hippocampus monosynaptically connects with the MPFC and PCC (Small et al., 2011), and decreased resting-state FC between the hippocampus and these two target areas has been reported in patients with RRMS (Rocca et al., 2015; Roosendaal et al., 2010). The AG is considered part of the ventral frontoparietal attention system (Chambers et al., 2004), and the posterior part shows strong structural connectivity with the hippocampus via the inferior longitudinal fasciculus (Uddin et al., 2010). The decrease in FC between the hippocampus and AG was consistent with one previous MS study (Rocca et al., 2015). Moreover, one meta-analysis of 120 functional neuroimaging studies also identified the left AG as a core region for verbal memory tasks (Binder et al., 2009), which may explain the observed relationship between ALVT and hippocampal FC to the AG in the HCs.
Despite the widely accepted concept that FC is disturbed in patients with MS, the precise mechanisms are often poorly specified, mainly due to the limited techniques available to delineate the nature of brain FC in vivo. Our analysis in the HC group closely links the hippocampal FC strengths with the GABA+ levels and Glu/GABA+ ratios, providing a more precise delineation of the biochemical underpinnings of macro-scale brain connectivity. Intrinsic neural activity is generally regulated by the excitatory/inhibitory balance, which is closely related to the levels of Glu and GABA (Duncan et al., 2014). In line with the current findings, a significant positive correlation was observed between the right amygdala–mPFC connectivity and GABA+ levels within the mPFC (Delli Pizzi et al., 2017). In fact, the MPFC, PCC and medial temporal lobes, including the hippocampus (Greicius et al., 2009), and the AG (Passow et al., 2015; Uddin et al., 2009), are the major hubs of the default mode network (DMN), which is coherently active during the resting state and may promote mnemonic processing (Salami et al., 2014). Both significant (Hu et al., 2013; Kapogiannis et al., 2013) and non-significant (Passow et al., 2015) correlations between excitatory (or inhibitory) neurotransmitters and entire connectivity of the DMN have been reported, depending on the choice of regions of interest.
In contrast, the hippocampal FC strengths were uncoupled with the GABA+ levels or Glu/GABA+ ratios in patients with MS. This finding indicated altered neurotransmitter control of synchronized BOLD signal fluctuations between the hippocampus and its input or target areas. Future work will aim to examine whether patients with MS also show changes in GABA and/or glutamate levels in other regions connected to the hippocampus, and whether these changes help to explain the cognitive impairment and decrease in connectivity strengths. In fact, WM lesions also impair FC between the hippocampus and other brain regions. This hypothesis was validated by Rocca and colleagues (Rocca et al., 2015), who reported a strong correlation between a high brain T2 lesion volume and reduced hippocampal resting-state FC. However, similar results were not observed in the current study and other FC studies in patients with MS (Colasanti et al., 2016; Hulst et al., 2015; Rocca et al., 2014). These inconsistencies might be attributed to the diverse locations of the lesions in the frontal, temporal, parietal and cingulate regions, and therefore whole-brain lesion volume might not be a stable and precise index to explain the abnormalities of hippocampal circuits in patients with MS.
The determination of cognitive ability by excitatory and inhibitory neurotransmitters levels rather than FC in patients with MS strengthens the use of MRS as a useful tool for the objective assessment of MS-induced cognitive impairments. Furthermore, the fact that cognitive impairments are related to the levels of excitatory and inhibitory neurotransmitters in the hippocampus of patients in the remitting stage rather than in the progressive stage (Cawley et al., 2015) helps to address a problem regarding the window of opportunity for clinical intervention. A previous animal study also supports the potential of using positive GABA receptor modulators to manage MS (Gilani et al., 2014). Therefore, pharmacological therapies targeting these neurotransmitter systems might be valuable in the future.
There is currently no consensus in terms of the relative involvement of the left and right hippocampi in MS. The majority of structural MRI studies found that they were affected equally (Hulst et al., 2015; Sicotte et al., 2008). Previous functional MRI study also revealed similar patterns of FC abnormalities for the left and right hippocampi (Rocca et al., 2015). Moreover, the reduced GABA concentration in the right hippocampus had been reported in patients with progressive MS (Cawley et al., 2015). Therefore, although the current study only investigated the MRS and FC changes for the left hippocampus, the symmetric findings are to be expected for the contralateral one.
The primary limitation of this study is that the relatively low amplitude of the GABA signal required the use of a relatively large VOI, a volume that contains, but is by no means restricted to, left hippocampus. The VOI also included some WM from surrounding tissue, and small parts of neighbouring medial temporal lobe structures. However, patients and controls showed no differences in the GM fractions. Moreover, to account for differences in metabolite levels between GM and WM, GABA+ and Glu levels were corrected to a voxel of pure GM using the method proposed by Harris et al. (Harris et al., 2015). Additionally, it has been estimated that GABA is ~7× greater in GM than in WM, which suggests that the majority of the GABA level quantified with MRS derives from the GM (Ganji et al., 2014). Second, the edited GABA signal that was detected in this study contains a significant contribution from co-edited macromolecules. New methods for macromolecule suppression are being developed (Edden et al., 2016). However, it is currently thought that the GABA+ measurement is the most robust one available, despite its contaminations (Mullins et al., 2014). Third, the temporal resolution of fMRI precludes determining whether FC between two regions reflects a direct (potentially monosynaptic) connection or is conducted across multiple synapses via intermediate nodes (Greicius et al., 2009). Fourth, although we excluded patients with macroscopic lesions in the hippocampi, the imaging measurements may be contaminated by smaller foci that only could be detected by MRI with special sequences (Roosendaal et al., 2008) or higher fields (Kilsdonk et al., 2016). Fifth, The majority of GABA is located in two pools within neurons—the cytoplasm and the presynaptic vesicles (Kwon et al., 2014). However, MRS is only capable of detecting total GABA within the prescribed localized region, and it cannot distinguish between these separate functional pools of GABA (Stagg et al., 2011). Therefore, the scope of our study to introduce GABAergic neurotransmission as a treatment target for MS may be limited.
Conclusion
By combining J-difference-edited MRS and resting-state fMRI, we provided evidence that hippocampal GABA+ and Glu levels, as well as strengths of FC within brain regions belonging to the DMN, were abnormal in patients with MS. In addition, the reduced verbal memory and visuospatial memory observed in patients with MS correlated with decreased levels of GABA+ and of Glu, respectively. Finally, GABA+ levels and Glu/GABA+ ratios were associated with FC strengths in HCs but not in patients with MS. This study offers a novel combination of methods investigating the complex relationships among excitatory/inhibitory neurotransmitters, brain connectivity and cognitive function in health and disease states. Modulation of Glu and GABA neurotransmission may enable the development of new therapeutic strategies for the early stages of MS.
Supplementary Material
Acknowledgments
This work was supported by the National Natural Science Foundation of China for Young Scholars (no. 81601479); Chongqing Leading Edge and Applied Basic Research Project (no. cstc2015jcyjA10121); Shandong Provincial Key Research and Development Plan of China (no. 2016ZDJS07A16); Shandong Provincial Natural Science Foundation of China (no. BS2015YY003); Shandong Provincial Key Research and Development Plan of China (no. 2016GSF201090); China Postdoctoral Science Foundation funded project (no. 2017M621089); Shandong Provincial Medical and Healthy Technology Development Program of China (no. 2015WS0176); and National Key Research and Development Plan of China (no. 2016YFC0107112). This work applies methods developed under the National Institutes of Health (nos. R01 EB016089, P41 EB015909).
Footnotes
Confict of Interest The authors declare that they have no conflict of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version.
References
- Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. American Journal of Public Health. 1996;86(5):726–728. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30(8):382–9. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38(1):95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Balz J, Keil J, Roa Romero Y, Mekle R, Schubert F, Aydin S, Ittermann B, Gallinat J, Senkowski D. GABA concentration in superior temporal sulcus predicts gamma power and perception in the sound-induced flash illusion. Neuroimage. 2016;125:724–30. doi: 10.1016/j.neuroimage.2015.10.087. [DOI] [PubMed] [Google Scholar]
- Barnett MH, Parratt JD, Cho ES, Prineas JW. Immunoglobulins and complement in postmortem multiple sclerosis tissue. Ann Neurol. 2009;65(1):32–46. doi: 10.1002/ana.21524. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya PK, Phillips MD, Stone LA, Bermel RA, Lowe MJ. Sensorimotor cortex gamma-aminobutyric acid concentration correlates with impaired performance in patients with MS. AJNR Am J Neuroradiol. 2013;34(9):1733–9. doi: 10.3174/ajnr.A3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19(12):2767–96. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawley N, Solanky BS, Muhlert N, Tur C, Edden RA, Wheeler-Kingshott CA, Miller DH, Thompson AJ, Ciccarelli O. Reduced gamma-aminobutyric acid concentration is associated with physical disability in progressive multiple sclerosis. Brain. 2015;138(Pt 9):2584–95. doi: 10.1093/brain/awv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers CD, Payne JM, Stokes MG, Mattingley JB. Fast and slow parietal pathways mediate spatial attention. Nature Neuroscience. 2004;7(3):217–8. doi: 10.1038/nn1203. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurol. 2008;7(12):1139–51. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Colasanti A, Guo Q, Giannetti P, Wall MB, Newbould RD, Bishop C, Onega M, Nicholas R, Ciccarelli O, Muraro PA, et al. Hippocampal Neuroinflammation, Functional Connectivity, and Depressive Symptoms in Multiple Sclerosis. Biol Psychiatry. 2016;80(1):62–72. doi: 10.1016/j.biopsych.2015.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Gomez AJ, Belenguer-Benavides A, Martinez-Bronchal B, Fittipaldi-Marquez MS, Forn C. Structural and functional changes of the hippocampus in patients with multiple sclerosis and their relationship with memory processes. Rev Neurol. 2016;62(1):6–12. [PubMed] [Google Scholar]
- de la Vega A, Brown MS, Snyder HR, Singel D, Munakata Y, Banich MT. Individual differences in the balance of GABA to glutamate in pFC predict the ability to select among competing options. J Cogn Neurosci. 2014;26(11):2490–502. doi: 10.1162/jocn_a_00655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Pizzi S, Chiacchiaretta P, Mantini D, Bubbico G, Ferretti A, Edden RA, Di Giulio C, Onofrj M, Bonanni L. Functional and neurochemical interactions within the amygdala-medial prefrontal cortex circuit and their relevance to emotional processing. Brain Struct Funct. 2017;222(3):1267–1279. doi: 10.1007/s00429-016-1276-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan NW, Wiebking C, Northoff G. Associations of regional GABA and glutamate with intrinsic and extrinsic neural activity in humans-a review of multimodal imaging studies. Neurosci Biobehav Rev. 2014;47:36–52. doi: 10.1016/j.neubiorev.2014.07.016. [DOI] [PubMed] [Google Scholar]
- Dutta R, Chang AS, Doud MK, Kidd GJ, Ribaudo MV, Young EA, Fox RJ, Staugaitis SM, Trapp BD. Demyelination Causes Synaptic Alterations in Hippocampi from Multiple Sclerosis Patients. Annals of Neurology. 2011;69(3):445–454. doi: 10.1002/ana.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Oeltzschner G, Harris AD, Puts NA, Chan KL, Boer VO, Schar M, Barker PB. Prospective frequency correction for macromolecule-suppressed GABA editing at 3T. J Magn Reson Imaging. 2016;44(6):1474–1482. doi: 10.1002/jmri.25304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edden RA, Puts NA, Harris AD, Barker PB, Evans CJ. Gannet: A batch-processing tool for the quantitative analysis of gamma-aminobutyric acid-edited MR spectroscopy spectra. J Magn Reson Imaging. 2014;40(6):1445–52. doi: 10.1002/jmri.24478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1(1):41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Falco A, Pennucci R, Brambilla E, de Curtis I. Reduction in parvalbumin-positive interneurons and inhibitory input in the cortex of mice with experimental autoimmune encephalomyelitis. Exp Brain Res. 2014;232(7):2439–49. doi: 10.1007/s00221-014-3944-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan L, Li H, Zhuo J, Zhang Y, Wang J, Chen L, Yang Z, Chu C, Xie S, Laird AR, et al. The Human Brainnetome Atlas: A New Brain Atlas Based on Connectional Architecture. Cereb Cortex. 2016;26(8):3508–26. doi: 10.1093/cercor/bhw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6(3):215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–11. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Ganji SK, An Z, Banerjee A, Madan A, Hulsey KM, Choi C. Measurement of regional variation of GABA in the human brain by optimized point-resolved spectroscopy at 7 T in vivo. NMR Biomed. 2014;27(10):1167–75. doi: 10.1002/nbm.3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Edden RA, Li M, Puts NA, Wang G, Liu C, Zhao B, Wang H, Bai X, Zhao C, et al. Edited magnetic resonance spectroscopy detects an age-related decline in brain GABA levels. Neuroimage. 2013;78:75–82. doi: 10.1016/j.neuroimage.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Wang G, Ma W, Ren F, Li M, Dong Y, Liu C, Liu B, Bai X, Zhao B, et al. Decreased auditory GABA+ concentrations in presbycusis demonstrated by edited magnetic resonance spectroscopy. Neuroimage. 2015;106:311–6. doi: 10.1016/j.neuroimage.2014.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparovic C, Song T, Devier D, Bockholt HJ, Caprihan A, Mullins PG, Posse S, Jung RE, Morrison LA. Use of tissue water as a concentration reference for proton spectroscopic imaging. Magn Reson Med. 2006;55(6):1219–26. doi: 10.1002/mrm.20901. [DOI] [PubMed] [Google Scholar]
- Genova HM, DeLuca J, Chiaravalloti N, Wylie G. The relationship between executive functioning, processing speed, and white matter integrity in multiple sclerosis. J Clin Exp Neuropsychol. 2013;35(6):631–41. doi: 10.1080/13803395.2013.806649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts JJG, Bo L, Roosendaal SD, Hazes T, Daniels R, Barkhof F, Witter MP, Huitinga I, van der Valk P. Extensive hippocampal demyelination in multiple sclerosis. Journal of Neuropathology and Experimental Neurology. 2007;66(9):819–827. doi: 10.1097/nen.0b013e3181461f54. [DOI] [PubMed] [Google Scholar]
- Gilani AA, Dash RP, Jivrajani MN, Thakur SK, Nivsarkar M. Evaluation of GABAergic Transmission Modulation as a Novel Functional Target for Management of Multiple Sclerosis: Exploring Inhibitory Effect of GABA on Glutamate-Mediated Excitotoxicity. Adv Pharmacol Sci. 2014;2014:632376. doi: 10.1155/2014/632376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Puts NA, Edden RA. Tissue correction for GABA-edited MRS: Considerations of voxel composition, tissue segmentation, and tissue relaxations. J Magn Reson Imaging. 2015;42(5):1431–40. doi: 10.1002/jmri.24903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AD, Saleh MG, Edden RA. Edited 1 H magnetic resonance spectroscopy in vivo: Methods and metabolites. Magn Reson Med. 2017;77(4):1377–1389. doi: 10.1002/mrm.26619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Chen X, Gu H, Yang Y. Resting-state glutamate and GABA concentrations predict task-induced deactivation in the default mode network. J Neurosci. 2013;33(47):18566–73. doi: 10.1523/JNEUROSCI.1973-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst HE, Schoonheim MM, Van Geest Q, Uitdehaag BM, Barkhof F, Geurts JJ. Memory impairment in multiple sclerosis: Relevance of hippocampal activation and hippocampal connectivity. Mult Scler. 2015;21(13):1705–12. doi: 10.1177/1352458514567727. [DOI] [PubMed] [Google Scholar]
- Kapogiannis D, Reiter DA, Willette AA, Mattson MP. Posteromedial cortex glutamate and GABA predict intrinsic functional connectivity of the default mode network. Neuroimage. 2013;64:112–9. doi: 10.1016/j.neuroimage.2012.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilsdonk ID, Jonkman LE, Klaver R, van Veluw SJ, Zwanenburg JJM, Kuijer JPA, Pouwels PJW, Twisk JWR, Wattjes MP, Luijten PR, et al. Increased cortical grey matter lesion detection in multiple sclerosis with 7 T MRI: a post-mortem verification study. Brain. 2016;139:1472–1481. doi: 10.1093/brain/aww037. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Scheinost D, Lacadie C, Benjamin J, Myers EH, Qiu M, Schneider KC, Rothman DL, Constable RT, Ment LR. GABA, resting-state connectivity and the developing brain. Neonatology. 2014;106(2):149–55. doi: 10.1159/000362433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Larson MP, Anderson JS, Ferguson MA, Yurgelun-Todd D. Local brain connectivity and associations with gender and age. Dev Cogn Neurosci. 2011;1(2):187–97. doi: 10.1016/j.dcn.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti CF, Popescu BFG, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, et al. Inflammatory Cortical Demyelination in Early Multiple Sclerosis. New England Journal of Medicine. 2011;365(23):2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKenzie-Graham A, Kurth F, Itoh Y, Wang HJ, Montag MJ, Elashoff R, Voskuhl RR. Disability-Specific Atlases of Gray Matter Loss in Relapsing-Remitting Multiple Sclerosis. JAMA Neurol. 2016;73(8):944–53. doi: 10.1001/jamaneurol.2016.0966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandolesi G, Gentile A, Musella A, Centonze D. IL-1beta dependent cerebellar synaptopathy in a mouse mode of multiple sclerosis. Cerebellum. 2015;14(1):19–22. doi: 10.1007/s12311-014-0613-0. [DOI] [PubMed] [Google Scholar]
- Massella A, D'Intino G, Fernandez M, Sivilia S, Lorenzini L, Giatti S, Melcangi RC, Calza L, Giardino L. Gender effect on neurodegeneration and myelin markers in an animal model for multiple sclerosis. BMC Neurosci. 2012;13:12. doi: 10.1186/1471-2202-13-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. Nmr in Biomedicine. 1998;11(6):266–72. doi: 10.1002/(sici)1099-1492(199810)11:6<266::aid-nbm530>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Montelius M, Ljungbreg A, Carlsson G, Starck E, ForessellAronsson Matlab tool for segmentation and re-creation of MRS volumes of interst in MRI image stacks. Proc Eur Soc Mag Resonan Med Biol 2009 Congr. 2009:320–321. [Google Scholar]
- Muhlert N, Atzori M, De Vita E, Thomas DL, Samson RS, Wheeler-Kingshott CA, Geurts JJ, Miller DH, Thompson AJ, Ciccarelli O. Memory in multiple sclerosis is linked to glutamate concentration in grey matter regions. J Neurol Neurosurg Psychiatry. 2014;85(8):833–9. doi: 10.1136/jnnp-2013-306662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins PG, McGonigle DJ, O'Gorman RL, Puts NA, Vidyasagar R, Evans CJ, Cardiff Symposium on MRSoG. Edden RA. Current practice in the use of MEGA-PRESS spectroscopy for the detection of GABA. Neuroimage. 2014;86:43–52. doi: 10.1016/j.neuroimage.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantes JC, Proulx S, Zhong J, Holmes SA, Narayanan S, Brown RA, Hoge RD, Koski L. GABA and glutamate levels correlate with MTR and clinical disability: Insights from multiple sclerosis. Neuroimage. 2017 doi: 10.1016/j.neuroimage.2017.01.033. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Orije J, Kara F, Guglielmetti C, Praet J, Van der Linden A, Ponsaerts P, Verhoye M. Longitudinal monitoring of metabolic alterations in cuprizone mouse model of multiple sclerosis using 1H-magnetic resonance spectroscopy. Neuroimage. 2015;114:128–35. doi: 10.1016/j.neuroimage.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19(2):238–53. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passow S, Specht K, Adamsen TC, Biermann M, Brekke N, Craven AR, Ersland L, Gruner R, Kleven-Madsen N, Kvernenes OH, et al. Default-mode network functional connectivity is closely related to metabolic activity. Hum Brain Mapp. 2015;36(6):2027–38. doi: 10.1002/hbm.22753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56(3):907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit DL, Augustine GJ. Distribution of functional glutamate and GABA receptors on hippocampal pyramidal cells and interneurons. J Neurophysiol. 2000;84(1):28–38. doi: 10.1152/jn.2000.84.1.28. [DOI] [PubMed] [Google Scholar]
- Planche V, Ruet A, Coupe P, Lamargue-Hamel D, Deloire M, Pereira B, Manjon JV, Munsch F, Moscufo N, Meier DS, et al. Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Mult Scler. 2016 doi: 10.1177/1352458516675750. [DOI] [PubMed] [Google Scholar]
- Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosa P, Rocca MA, Pagani E, Stromillo ML, Enzinger C, Gallo A, Hulst HE, Atzori M, Pareto D, Riccitelli GC, et al. Structural MRI correlates of cognitive impairment in patients with multiple sclerosis: A Multicenter Study. Hum Brain Mapp. 2016;37(4):1627–44. doi: 10.1002/hbm.23125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–9. doi: 10.1002/mrm.1910300604. [DOI] [PubMed] [Google Scholar]
- Robertson CE, Ratai EM, Kanwisher N. Reduced GABAergic Action in the Autistic Brain. Curr Biol. 2016;26(1):80–5. doi: 10.1016/j.cub.2015.11.019. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Absinta M, Amato MP, Moiola L, Ghezzi A, Veggiotti P, Capra R, Portaccio E, Fiorino A, Pippolo L, et al. Posterior brain damage and cognitive impairment in pediatric multiple sclerosis. Neurology. 2014;82(15):1314–21. doi: 10.1212/WNL.0000000000000309. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Morelli ME, Amato MP, Moiola L, Ghezzi A, Veggiotti P, Capra R, Pagani E, Portaccio E, Fiorino A, et al. Regional hippocampal involvement and cognitive impairment in pediatric multiple sclerosis. Mult Scler. 2016;22(5):628–40. doi: 10.1177/1352458515598569. [DOI] [PubMed] [Google Scholar]
- Rocca MA, Pravata E, Valsasina P, Radaelli M, Colombo B, Vacchi L, Gobbi C, Comi G, Falini A, Filippi M. Hippocampal-DMN disconnectivity in MS is related to WM lesions and depression. Hum Brain Mapp. 2015;36(12):5051–63. doi: 10.1002/hbm.22992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology. 2010;255(2):595–604. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Moraal B, Vrenken H, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ. In vivo MR imaging of hippocampal lesions in multiple sclerosis. J Magn Reson Imaging. 2008;27(4):726–31. doi: 10.1002/jmri.21294. [DOI] [PubMed] [Google Scholar]
- Rothman DL, Behar KL, Prichard JW, Petroff OA. Homocarnosine and the measurement of neuronal pH in patients with epilepsy. Magn Reson Med. 1997;38(6):924–9. doi: 10.1002/mrm.1910380611. [DOI] [PubMed] [Google Scholar]
- Sacco R, Bisecco A, Corbo D, Della Corte M, d'Ambrosio A, Docimo R, Gallo A, Esposito F, Esposito S, Cirillo M, et al. Cognitive impairment and memory disorders in relapsing-remitting multiple sclerosis: the role of white matter, gray matter and hippocampus. J Neurol. 2015;262(7):1691–7. doi: 10.1007/s00415-015-7763-y. [DOI] [PubMed] [Google Scholar]
- Salami A, Pudas S, Nyberg L. Elevated hippocampal resting-state connectivity underlies deficient neurocognitive function in aging. Proc Natl Acad Sci U S A. 2014;111(49):17654–9. doi: 10.1073/pnas.1410233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, Tirapu J, Barcelo F. Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc. 2009;15(3):438–50. doi: 10.1017/S1355617709090626. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Forschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, et al. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage. 2012;59(4):3774–83. doi: 10.1016/j.neuroimage.2011.11.032. [DOI] [PubMed] [Google Scholar]
- Schoonheim MM, Popescu V, Rueda Lopes FC, Wiebenga OT, Vrenken H, Douw L, Polman CH, Geurts JJ, Barkhof F. Subcortical atrophy and cognition: sex effects in multiple sclerosis. Neurology. 2012;79(17):1754–61. doi: 10.1212/WNL.0b013e3182703f46. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Matsubara K, Uezono T, Kimura K, Shiono H. Reduced dorsal hippocampal glutamate release significantly correlates with the spatial memory deficits produced by benzodiazepines and ethanol. Neuroscience. 1998;83(3):701–6. doi: 10.1016/s0306-4522(97)00339-4. [DOI] [PubMed] [Google Scholar]
- Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey-Osterrieth Complex Figure Test. Nature Protocols. 2006;1(2):892–9. doi: 10.1038/nprot.2006.115. [DOI] [PubMed] [Google Scholar]
- Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131(Pt 4):1134–41. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- Small SA, Schobel SA, Buxton RB, Witter MP, Barnes CA. A pathophysiological framework of hippocampal dysfunction in ageing and disease. Nat Rev Neurosci. 2011;12(10):585–601. doi: 10.1038/nrn3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17(1):479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. What are we measuring with GABA magnetic resonance spectroscopy? Commun Integr Biol. 2011;4(5):573–5. doi: 10.4161/cib.4.5.16213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumowski JF, Rocca MA, Leavitt VM, Riccitelli G, Sandry J, DeLuca J, Comi G, Filippi M. Searching for the neural basis of reserve against memory decline: intellectual enrichment linked to larger hippocampal volume in multiple sclerosis. Eur J Neurol. 2016;23(1):39–44. doi: 10.1111/ene.12662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet LH, Rao SM, Primeau M, Mayer AR, Cohen RA. Functional magnetic resonance imaging of working memory among multiple sclerosis patients. J Neuroimaging. 2004;14(2):150–7. [PubMed] [Google Scholar]
- Tamminga CA, Southcott S, Sacco C, Wagner AD, Ghose S. Glutamate dysfunction in hippocampus: relevance of dentate gyrus and CA3 signaling. Schizophr Bull. 2012;38(5):927–35. doi: 10.1093/schbul/sbs062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier CR, Broadie K. Activity-dependent modulation of neural circuit synaptic connectivity. Front Mol Neurosci. 2009;2:8. doi: 10.3389/neuro.02.008.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tona F, Petsas N, Sbardella E, Prosperini L, Carmellini M, Pozzilli C, Pantano P. Multiple sclerosis: altered thalamic resting-state functional connectivity and its effect on cognitive function. Radiology. 2014;271(3):814–21. doi: 10.1148/radiol.14131688. [DOI] [PubMed] [Google Scholar]
- Travis SG, Huang Y, Fujiwara E, Radomski A, Olsen F, Carter R, Seres P, Malykhin NV. High field structural MRI reveals specific episodic memory correlates in the subfields of the hippocampus. Neuropsychologia. 2014;53:233–45. doi: 10.1016/j.neuropsychologia.2013.11.016. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–37. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ, Supekar K, Amin H, Rykhlevskaia E, Nguyen DA, Greicius MD, Menon V. Dissociable Connectivity within Human Angular Gyrus and Intraparietal Sulcus: Evidence from Functional and Structural Connectivity. Cerebral Cortex. 2010;20(11):2636–2646. doi: 10.1093/cercor/bhq011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geest Q, Westerik B, van der Werf YD, Geurts JJ, Hulst HE. The role of sleep on cognition and functional connectivity in patients with multiple sclerosis. J Neurol. 2016 doi: 10.1007/s00415-016-8318-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schependom J, D'Hooghe MB, Cleynhens K, D'Hooge M, Haelewyck MC, De Keyser J, Nagels G. The Symbol Digit Modalities Test as sentinel test for cognitive impairment in multiple sclerosis. Eur J Neurol. 2014;21(9):1219–25. e71–2. doi: 10.1111/ene.12463. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Lv Y, Zhou Y, Hong Z, Guo Q. Short-term delayed recall of auditory verbal learning test is equivalent to long-term delayed recall for identifying amnestic mild cognitive impairment. PLoS One. 2012;7(12):e51157. doi: 10.1371/journal.pone.0051157. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.