Abstract
Psoriasis is a chronic inflammatory skin disease dependent on the IL-23/IL-17 axis, a potent inflammatory pathway involved in pathogen clearance and autoimmunity. Several triggers have been proposed as initiators for psoriasis, including alarmins such as ATP. However, the role of alarmins in psoriasis pathogenesis and cutaneous inflammation has not been well addressed. Herein studies demonstrate that signaling through the P2X7 receptor (P2X7R) pathway underlies the development of psoriasiform inflammation. In this regard, psoriasiform dermatitis induced by IL-23 is dependent on P2X7R signaling. Furthermore, direct activation of the P2X7R is sufficient to induce a well characterized psoriasiform dermatitis. Mechanistic studies determined that P2X7R-induced inflammation is largely dependent on the IL-1β/NLRP3 inflammasome pathway and neutrophils. In conclusion, this work provides basic mechanistic insight into local inflammatory circuits induced following purinergic P2X7R signaling that are likely involved in the pathogenesis of many inflammatory diseases, such as psoriasis.
Keywords: Psoriasis, P2X7 receptor, Purinergic receptors, ATP, Alarmins, neutrophils, IL-23, NLRP3
INTRODUCTION
Psoriasis vulgaris is a chronic inflammatory cutaneous disease that affects approximately 2–3% of the population. Psoriasis is categorized as a psoriasiform dermatitis with histopathological manifestations including epidermal acanthosis, hyperkeratosis, parakeratosis, microabscess formation, vascular expansion, and infiltration of leukocytes into both dermis and epidermis. Several triggers have been proposed as initiator events for psoriasis, including IL-1, IL-6, CAMP/LL-37, TNF-α, and alarmins (Alwan and Nestle, 2015). Alarmins are damage-associated molecular patterns that act as danger-signals, inducing innate and adaptive inflammatory responses. Following trauma, alarmins are released from damaged, stressed, or necrotic cells. In a genetically predisposed environment, it has been suggested that alarmins could lead to the induction of psoriatic lesions by promoting a positive inflammatory feedback loop (Dumitriu et al., 2005, Gallucci et al., 1999). Supporting this is the Koebner phenomenon in which lesions frequently develop at sites of trauma were alarmins are locally released. However, the role of alarmins in psoriasis pathogenesis has not been well addressed. In this context, extracellular ATP is a particularly interesting alarmin that, via purinergic P2X7 receptor (P2X7R) signaling, induces NF-κB activation and the IL-23/IL-17 axis, both of which have been shown to be psoriasis susceptibility pathways (Atarashi et al., 2008, Di Virgilio et al., 2017, Nair et al., 2009). Moreover, the sympathetic nervous system releases ATP during times of stress, thereby linking stress and the exacerbation of psoriasis (Burnstock, 2009, Stohl et al., 2013). Several key studies also support a role for ATP in cutaneous inflammation. For instance, contact hypersensitivity responses were inhibited in P2X7R −/− mice and potentiated in the presence of ATPγS, an ATP analog (Granstein et al., 2005, Weber et al., 2010). We and others have shown that P2X7R is highly upregulated in psoriatic lesions in both humans and mouse models (Geraghty et al., 2017, Killeen et al., 2013, Pastore et al., 2007). Finally, P2X7R signaling in a human ex vivo skin model provokes the expression of innate cutaneous inflammatory cytokines, DC17 differentiation, and Th17 responses (Killeen et al., 2013). Thus, we hypothesize that cutaneous P2X7R signaling is an early trigger of psoriasis pathogenesis.
RESULTS and DISCUSSION
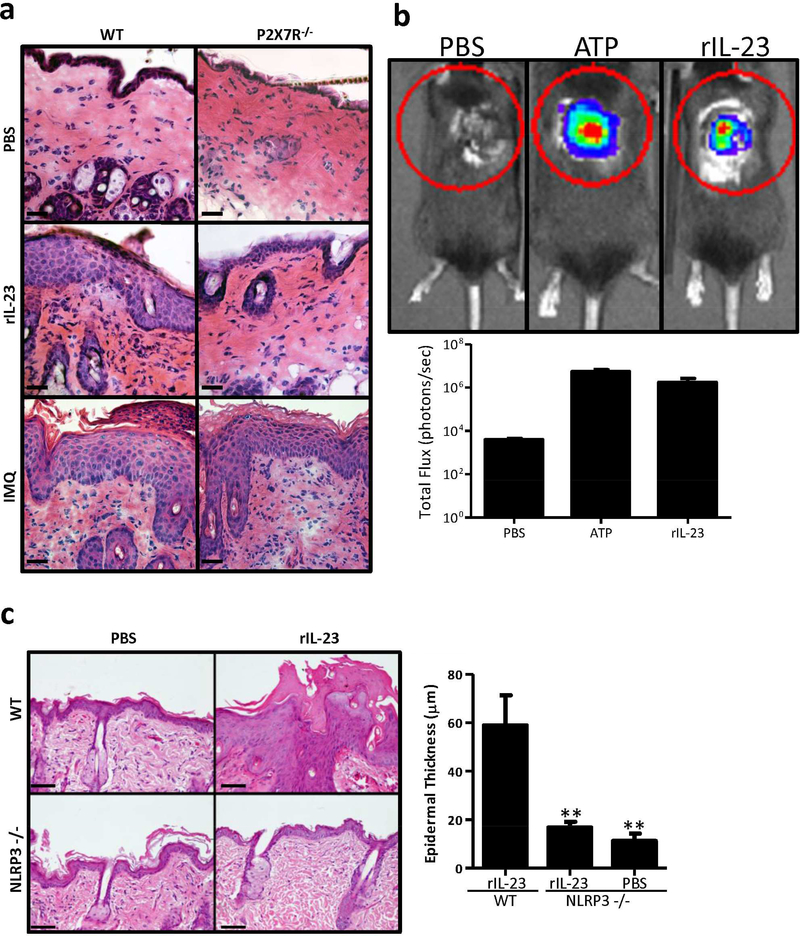
To test our hypothesis, we induced two acute models of psoriasis (rIL-23 and imiquimod; IMQ) in P2X7R −/− mice. Recombinant murine IL-23 was injected intradermally (i.d.) or Imiquimod (IMQ) was topically applied daily to C57BL/6 (wild type; WT) and P2X7R −/− mice. In the acute rIL-23 and IMQ models, psoriasiform dermatitis develops in WT mice, with marked epidermal thickening, increased inflammatory infiltrates, parakeratosis, and microabscess formation (Figure 1a and S1). In line with our hypothesis, rIL-23 did not promote a psoriasis-like phenotype in P2X7R −/− mice (Figure 1a and S1). Conversely, in the IMQ model, P2X7R −/− mice have considerable cutaneous inflammation and epidermal thickening (Figure 1a and S1), consistent with recent findings (Geraghty et al., 2017). Together this data suggests that the P2X7R pathway is necessary in the rIL-23 model to induce a psoriasis-like phenotype but the P2X7R pathway is dispensable for the IMQ model.
Figure 1. P2X7R signaling is necessary for the induction of acute psoriasiform inflammation induced by rIL-23 but not IMQ.
(a) rIL-23 and IMQ were utilized to induce inflammation in P2X7R −/− mice or age-matched C57BL/6 (WT) mice. On day 6 skin samples were collected and stained with H&E. One representative (n=3 mice/group) of two independent experiments. Scale bar = 50 μm. (b) Bioluminescence imaging was utilized to detect extracellular ATP in WT mice treated with rIL-23, PBS (negative control), or ATP (positive control). Bioluminescence was quantified in lower panel. Bars represent the mean ± SEM (n= 3–5 mice/group). One representative of four independent experiments. (c) WT or NLRP3 −/− mice were injected daily for 5 days with rIL-23 or PBS control. On day 6 skin samples were collected and stained with H&E, scale bar = 100 μm. Epidermal thickness was quantitated. Bars represent the mean ± SEM (n=4–5 mice/group), ten independent high powered field (HPF) measurements were averaged from each mouse. One representative of two independent experiments. Asterisks indicate a significant difference compared to WT mice treated with rIL-23. ** = p < 0.01.
We next sought to determine how IL-23 was stimulating the P2X7R pathway. To accomplish this, we cutaneously injected mice with rIL-23 and utilizing in vivo bioluminescence imaging revealed a significant release of ATP in mice injected with rIL-23 compared to vehicle control (Figure 1b). Thus, these data indicate that IL-23 can induce the cutaneous secretion of ATP leading to the activation of purinergic signaling.
ATP/P2X7R interactions initiate activation of the NLRP3 inflammasome and cleavage of pro-IL-1β to its active form (Di Virgilio et al., 2017). To determine if the level of ATP released following rIL-23 injections was biologically significant, we injected rIL-23 into NLRP3 −/− mice. In WT mice, rIL-23 induced a characteristic psoriasiform phenotype. However, in NLRP3 −/− mice injected with rIL-23, the psoriasiform phenotype was lost and there was a significant decrease in epidermal thickness compared to WT mice (Figure 1c). Overall, these data indicate that rIL-23 leads to the secretion of biologically relevant levels of ATP. Moreover, to our knowledge, the contribution of the inflammasome to the development of psoriasis in the rIL-23 model has been previously unreported.
Interestingly, studies have previously demonstrated that inflammation induced by IMQ is independent of the NLRP3 inflammasome (Rabeony et al., 2015, Walter et al., 2013), which is consistent with our finding that IMQ is capable of inducing inflammation in P2X7R −/− mice (Figure 1a). Furthermore, since the IMQ-dependent inflammatory response is independent of the P2X7R/IL-1β/NLRP3 inflammatory circuit the IL-1α or IL-36 pathways are likely involved in the induction of the IL-23/IL-17 axis in the IMQ model of psoriasis (Rabeony et al., 2015, Walter et al., 2013). The mechanisms that lead to psoriasis pathogenesis are poorly understood and likely multiple different pathways lead to the same disease phenotype based on genetic predisposition of the patient. Moreover, mice do not naturally develop psoriasis and due to the major differences between mice and humans, murine models only represent various features of the psoriatic disease. Thus, even though the IMQ model does not signal through the P2X7R pathway, the IMQ model of psoriasis still shares many features of human psoriasis and when utilized appropriately, will continue to provide valuable insight into the pathogenesis of psoriasis (Swindell et al., 2017).
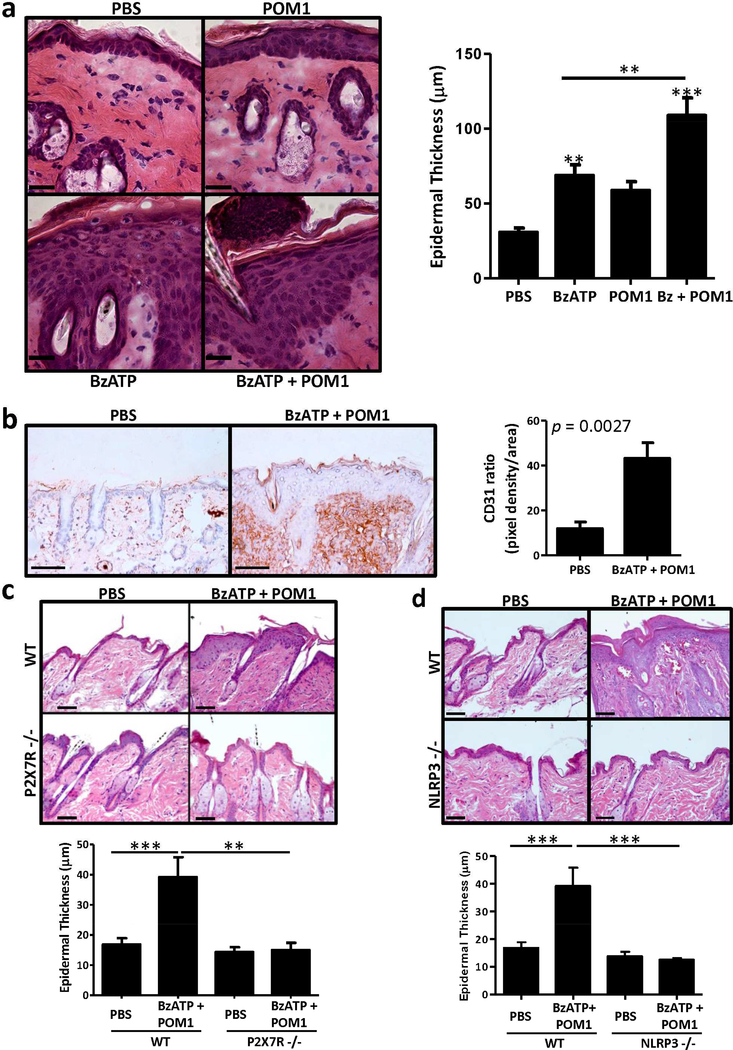
To determine if direct P2X7R signaling is sufficient for the development of psoriasis-like lesions we intradermally injected mice with BzATP, an ATP analog and a potent P2X7R agonist. Titration studies were conducted to determine the appropriate dose of BzATP (Figure S2). Intradermal injections of BzATP induced the development of parakeratosis and a significant increase in epidermal hyperplasia compared to vehicle control (Figure 2a). However, BzATP alone did not induce a full-fledge psoriasiform dermatitis. BzATP, like ATP, is hydrolyzed by ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDase) into the anti-inflammatory adenosine molecule (Kukley et al., 2004). Thus, in conjunction with BzATP, mice were injected with POM1, an inhibitor of E-NTPDases. BzATP + POM1 initiates the development of a full psoriasiform inflammatory response, characterized by prominent parakeratosis, microabscess formation, and a significant increase in epidermal thickness compared to PBS and BzATP or POM1 alone (Figure 2a). Additionally, in the dermis the level of vascularization was significantly increased in mice treated with BzATP + POM1, compared to PBS, as determined by histopathological examination and CD31 expression (Figure 2b). The development of cutaneous inflammation was not different between female and male mice (data not shown). Finally, the psoriasiform dermatitis induced following P2X7R signaling is an acute inflammatory response that begins to resolve after 4 days of treatment (data not shown), likely due to the absence of a genetic predisposition. For instance, the genetic predisposition of psoriasis patients to produce excessive IL-1β is supported, in part, by a gain-of-function mutation in the NLRP3 gene (rs10733113) (Carlström et al., 2012). Thus, P2X7R stimulation of this highly active NLRP3 inflammasome in psoriasis patients may lead to pathological levels of IL-1β that cannot be achieved or sustained naturally in mice.
Figure 2. Signaling via the P2X7R induces a psoriasis-like inflammatory phenotype in mice dependent on NLRP3 inflammasome.
C57BL/6 mice were injected daily for 4 d with BzATP ± POM1, or PBS (vehicle control). (a) On day 5 skin samples were collected and stained with H&E to assess histological phenotype, scale bar = 50 μm. Epidermal thickness was quantitated, bars are the mean ± SEM, ten independent HPF measurements were averaged from each mouse (n=10 combined from multiple independent experiments). (b) On day 5 skin samples were collected and immunohistochemically stained with CD31 (brown) and counter-stained with hematoxylin (blue). The density of CD31 expression was quantitated, the graph represents the ratio of pixel density/area ± SEM (n = 6–7 mice). Scale bar = 100 μm. (c) P2X7R −/− or WT mice were injected daily for 4 d with BzATP + POM1 or PBS. On day 5 skin samples were collected and stained with H&E, scale bar = 100 μm. Epidermal thickness was quantitated, bars are the mean ± SEM, ten independent HPF measurements were averaged from each mouse (n = 4–6 mice). One representative of two independent experiments. (d) NLRP3 −/− or WT mice were injected daily for 4 days with BzATP + POM1 or PBS. On day 5 skin samples were collected and stained with H&E, scale bar = 100 μm. Epidermal thickness was quantitated, bars represent the mean ± SEM, ten independent HPF measurements were averaged from each mouse (n=4–5 mice). One representative of three independent experiments. Asterisks indicate a significant difference compared to PBS control unless otherwise indicated. **= p < 0.01 and *** = p < 0.001. Bz=BzATP.
BzATP is a potent P2X7R agonist, but exhibits partial agonist activity at other purinergic receptors. Thus, to confirm that the observed response occurs through the P2X7R we utilized A438079 (A4), a competitive P2X7R antagonist that is inactive at other P2 receptors (da Silva et al., 2013, Nelson et al., 2006). Treatments with A4 block the cutaneous inflammatory response induced by BzATP + POM1 (Figure S3). Likewise, when P2X7R −/− mice were treated with BzATP + POM1 an inflammatory response did not develop and epidermal thickness was significantly decreased compared to WT mice (Figure 2c). These findings strongly support our hypothesis that signaling directly through the P2X7R can induce the development of a psoriasis-like response.
Studies support a role for an aberrant inflammasome/IL-1β pathway in psoriasis (Carlström et al., 2012). Moreover, the NLRP3 inflammasome/IL-1β pathway is a prototypical downstream pathway of the P2X7R; however, P2X7R signaling can induce both inflammasome-dependent and -independent responses. Therefore, the relationship between the inflammasome/IL-1β pathway and P2X7R signaling was assessed by treating NLRP3 −/− mice with BzATP + POM1. In the NLRP3 −/− mice, inflammation was not induced when mice were treated with BzATP + POM1, compared to WT mice (Figure 2d). Consistent with the histological observations, there was not a significant increase in epidermal thickness in the NLRP3 −/− mice compared to WT mice (Figure 2d). Therefore, this data indicates that the psoriasiform response induced following P2X7R signaling is dependent on the NLRP3 inflammasome pathway. Moreover, these studies are consistent with those in Figure 1, demonstrating that the rIL-23 model of psoriasis was dependent on the NLRP3 inflammasome.
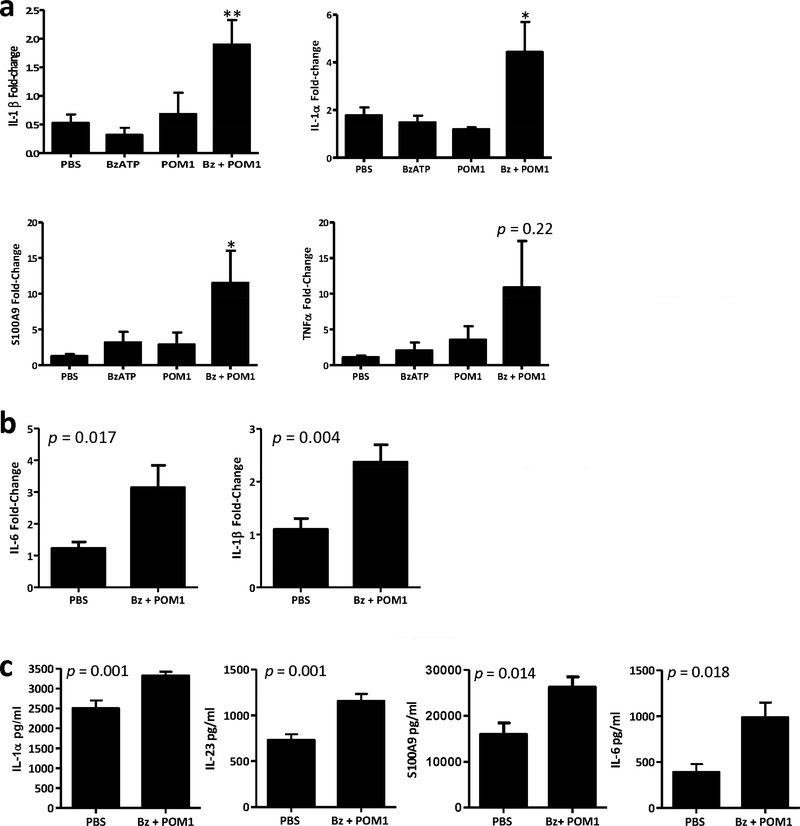
The role of innate cytokines induced following P2X7R signaling was subsequently evaluated in mice treated with BzATP in the presence or absence of POM1. Relevant cutaneous changes were observed in transcript expression at 12 h and 72 h following initial treatment. At the 12 h time point there was a significant increase in IL-1β, IL-1α, and S100A9 in the BzATP + POM1 treatment group compared to controls (Figure 3a). Additionally, there was a trend for increased TNF-α which did not reach statistical significance. By 72 h there was a significant increase in IL-6 in the BzATP + POM1 treatment group compared to controls (Figure 3b). The significant increase in IL-1β was also sustained in the skin for 72 h (Figure 3b). We next expanded our studies to assess protein expression. At 72 h and 120 h significant changes in protein expression were detected. Compared to controls, IL-1α, IL-23, and S100A9 were significantly increased at 72 h and IL-6 was significantly increased at 120 h in the BzATP + POM1 treatment group (Figure 3c). These data are consistent with our ex vivo studies demonstrating the capacity of P2X7R signaling to induce the expression of characteristic psoriasiform cytokines (Killeen et al., 2013).
Figure 3. Innate cytokines are induced following cutaneous signaling through the P2X7R.
(a and b) Bar graphs demonstrate the relative fold change in mRNA expression of IL-1β, IL-1α, S100A9, TNF-α, and IL-6 (a) 12h (IL-1β, IL-1α, S100A9 and TNFα) and (b) 72 h (IL-1β and IL-6) following cutaneous injections with BzATP ± POM1 normalized to PBS injected controls. (c) Bar graphs represent the protein concentration of IL-1α and IL-23 at 72 h and S100A9 and IL-6 at 120 h. (a-c) Data are expressed as mean ± SEM (n=4–10 mice) combined from two independent experiments, each sample was ran in triplicate for RT-PCR and duplicate for Luminex protein samples. Asterisk indicates a significant difference compared to PBS from multiple comparison groups, * = p < 0.05 and ** = p < 0.01. Bz=BzATP.
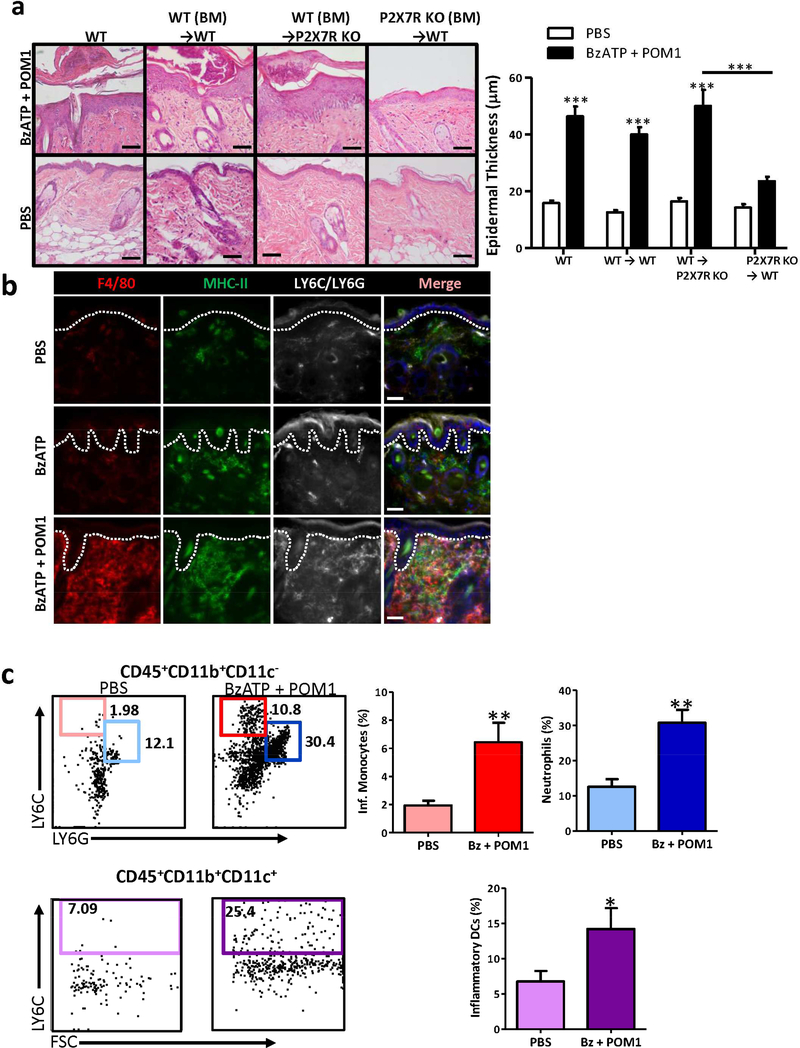
Psoriasis is dependent on environmental and genetic influences that render the skin susceptible to an imbalance in pro-inflammatory cytokines, chemokines, and growth factors stimulating aberrant immunity and keratinocyte functions. Whether keratinocytes have a direct role in inducing lesions through the stimulation of an aberrant immune response or if they are targets of an imbalanced immune response is not completely understood. To determine whether P2X7R expression and stimulation is necessary on keratinocytes and/or hematopoietic cells we generated chimeric mice in which WT bone marrow (BM) was transferred into lethally irradiated P2X7R deficient recipient mice. We determined that there was not a significant difference in inflammation and epidermal thickness when WT BM was transferred into P2X7R −/− mice compared to non-irradiated WT mice or when WT BM was transferred into WT recipient mice and stimulated with BzATP + POM1 (Figure 4a). Conversely, when P2X7R −/− BM was transferred into WT recipient mice there was a significant reduction in the development of inflammation and epidermal thickness, compared to WT controls (Figure 4a). Overall, these data reveal that hematopoietic cells expressing P2X7R are essential for the development of psoriasiform dermatitis in mice following stimulation with exogenous P2X7R agonists.
Figure 4. P2X7R-dependent cutaneous inflammation is dependent on hematopoietic cells.
(a) Lethally irradiated WT or P2X7R −/− mice were reconstituted with total BM from WT or P2X7R −/− mice. Congenic mice were treated with BzATP + POM1 or PBS. On day 5 skin samples were collected and stained with H&E, scale bar = 100 μm. Bar graph represents the mean increase ± SEM in epidermal thickness, ten independent HPF measurements were averaged from each mouse (n=6–16 mice combined from four independent experiments). (b) WT mice were injected daily for 4 d with BzATP ± POM1 or PBS. On day 5 skin sections were collected and immunofluorescently labeled with F4/80 (red), MHC-II (green), and GR-1 (LY6C/LY6G) (white) specific Abs. Merged panels include all three stains and DAPI nuclear counter-stain. Dashed line indicates epidermal-dermal junction. Scale bar = 50 μm (c) Phenotypic analysis of cutaneous infiltration was performed on day 5 by flow cytometry. Single-cell suspensions were assessed for CD45+CD11b+CD11c-LY6C+ inflammatory monocytes, CD45+CD11b+CD11c-LY6G+ neutrophils (top panels) and CD45+CD11b+CD11c+LY6C+ inflammatory DCs (bottom panels). In the right panels the bars represent the mean ± SEM of the percentage of each population indicated (n=6 mice). One representative of three independent experiments. Asterisks indicate a significant difference between indicated groups.*= p < 0.05, **= p < 0.01, and *** = p < 0.001. Bz=BzATP.
To determine which hematopoietic cells are important for the development of cutaneous inflammation the inflammatory infiltrate induced following P2X7R signaling was assessed. By day 5, compared to the PBS treatment group, in the BzATP group infiltrates were highly enriched for cells expressing MHC-class II (Figure 4b). Compared to both the PBS and BzATP treatment groups, BzATP + POM1 induced a marked increase into the papillary dermis and the superficial perivascular areas of F4/80+ (macrophages), MHC class II+ (antigen presenting cells), and LY6C/LY6G+ (granulocytes; Figure 4b). Cutaneous single-cell suspensions of the infiltrate were prepared and further characterized on day 5 by flow cytometry. Initially, we examined general cellular markers typically present in psoriatic lesions. We did not observe an increase in total CD3+ T cells, αβ+ T cells, γδ+ T cells, nor CD127+ ILCs (Figure S4). We detected a decrease in CD11c+ dendritic cells (DCs) (Figure S4), likely due to DCs migrating from the skin to the dLNs. Finally, there was an increase in LY6C+, CD11b+, and LY6G+ cells (Figure S4). To further delineate and quantitate the cutaneous cells increased in the skin we utilized a gating strategy previously described (Pommier et al., 2013). It was determined that LY6ChighCD11b+CD11c- inflammatory monocytes, LY6GhighCD11b+CD11c- neutrophils, and LY6ChighCD11b+CD11c+ inflammatory DCs were significantly increased following BzATP + POM1 treatments, compared to controls (Figure 4c).
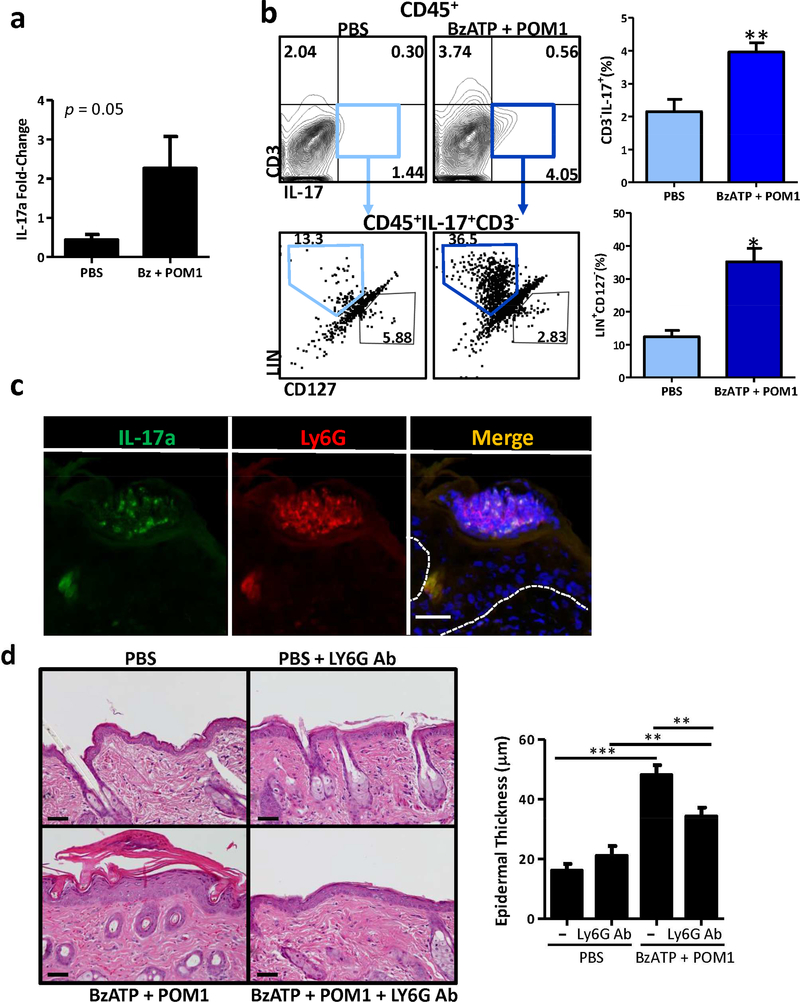
IL-17 is a main effector cytokine in psoriasis that indirectly and directly induces keratinocyte proliferation and hyperplasia along with the secretion of inflammatory cytokines and chemokines forming a self-amplifying feed forward response (Hawkes et al., 2017). To ascertain if IL-17A is increased in the skin following P2X7R stimulation we first determined that the IL-17A mRNA transcript levels are increased following treatment with BzATP + POM1, compared to controls (Figure 5a). Furthermore, the production of IL-17A was confirmed by flow cytometry; mice treated with BzATP + POM1 had a significant increase in cutaneous IL-17A-secreting cells, compared to PBS alone (Figure 5b, top panels). Studies in murine models of psoriasis have demonstrated that γδ T cells, neutrophils, and ILCs are the cells secreting the majority of IL-17A compared to adaptive Th17 cells (Cai et al., 2011, Gladiator and LeibundGut-Landmann, 2013, Mabuchi et al., 2011, Pantelyushin et al., 2012, Sutton et al., 2012). However, in our model we determined that the cells expressing IL-17A were CD45+CD3-CD127-Lineage+ (Figure 5b, bottom panels). In this regard, there was a significant increase in CD45+CD3-CD127-Lineage+IL-17+ cells following BzATP + POM1 treatment compared to control (Figure 5b, bottom panels). The markers utilized eliminated Th17 cells (CD3+), γδ T cells (CD3+), and ILCs (CD127+) as producers of IL-17A following P2X7R signaling. Within the lineage population are neutrophils, which have been demonstrated to express IL-17A (Lin et al., 2011, Reich et al., 2015) and are significantly increased in the skin following BzATP + POM1 injections (Figure 4b and c). To further confirm that neutrophils were expressing IL-17A following BzATP + POM1 treatments we utilized an IL-17A fate-tracking reporter mouse (IL-17eYFP) (Hirota et al., 2011, Huppler et al., 2015). For this IL-17eYFP mice were treated with BzATP + POM1 and cutaneous cross-sections were stained with LY6G-specific antibodies. We demonstrate that neutrophils in the epidermal microabscess were capable of expressing IL-17A in our model of psoriasiform dermatitis (Figure 5c). Thus, this data indicates that neutrophils are an early infiltrate expressing IL-17A in the skin following P2X7R stimulation, which is consistent with the finding that neutrophils are a predominant cell type expressing IL-17A in psoriasis (Lin et al., 2011, Reich et al., 2015).
Figure 5. Acute inflammation induced following P2X7R-stimulation is dependent on neutrophils.
(a) Bar graph demonstrates the relative fold change in mRNA expression of IL-17 72 h following cutaneous injections with BzATP + POM1. Data are expressed as mean ± SEM (n=17–21 mice). The p value is indicated. (b) C57BL/6 mice were injected with BzATP + POM1 or PBS. Skin was collected on day 5 and single-cell suspensions were stained with CD45-, IL-17- CD3-, and Lineage (LIN)-specific Abs, and viability dye then analyzed by flow cytometry. The top left panels were gated on live CD45+ cells and the top right graph represents the mean ± SEM (n=3–5 mice) of the percentage of CD3-IL-17+ cells. The bottom left panels were further gated on the CD3-IL-17+ cells (light blue and dark blue boxes). The bottom right graph represents the mean ± SEM (n=3–5 mice) of the percentage of the LIN+CD127- cells. One representative of two independent experiments. (c) IL-17eYFP (green) mice were injected with BzATP + POM1 and on day five cutaneous sections were collected and immunofluorescently labeled with LY6G (red). Dashed line indicates epidermal/dermal junction. Scale bar = 50 μm (d) BzATP + POM1 or PBS was injected in the presence or absence of LY6Gspecific antibodies. On day 5 skin samples were collected and stained with H&E, scale bar = 100 μm. Epidermal thickness was quantitated. Bars represent mean ± SEM (n=5 mice), ten independent HPF measurements were averaged from each mouse. One representative of two independent experiments. Asterisks indicate a significant difference compared to PBS treatment unless otherwise indicated. *= p < 0.05, **= p < 0.01, and *** = p < 0.001.
Finally we sought to directly determine if neutrophils are required for the development of P2X7R-dependent inflammation. To accomplish this, mice were treated with a LY6G Ab to deplete neutrophils. Consistent with previous data, BzATP + POM1 induced a psoriasis-like phenotype with a significant increase in epidermal thickness (Figure 5d). Mice treated with BzATP + POM1 in the presence of a LY6G Ab did not develop a prominent psoriatic phenotype, compared to PBS controls (Figure 5d). Thus, this data indicates that neutrophils are necessary for the development of the psoriasiform dermatitis induced following P2X7R signaling. Likewise, neutrophils have been associated with the pathophysiology of psoriasis and studies have demonstrated that an increase in neutrophil extracellular traps and NETosis are positively correlated with disease severity (Hu et al., 2016).
It should be noted that while we only detected IL-17A expression in neutrophils we are not ruling out the possibility that other cell populations, such as T cells, express IL-17A in psoriatic disease. Our results indicate that innate inflammation induced following P2X7R stimulation is capable of initiating a psoriasiform dermatitis in the absence of T cell activation. In this regard, psoriasis has recently been described as a bimodal immune response with the initiation of lesions being developed by an innate autoinflammatory response that later develops into an adaptive autoimmune response (Christophers et al., 2014). Importantly, the response observed following the cutaneous injections of BzATP + POM1 reflect the innate autoinflammatory phase of psoriasis. Recent studies suggest that IL-17+ resident memory T cells (Trm) are important in the adaptive phase of human psoriasis but Trm are virtually absent in naïve specific pathogen free laboratory mice, which may explain why P2X7R stimulation does not lead to the chronic adaptive phase observed in human disease (Beura et al., 2016, Clark, 2015).
Conclusions
The present study demonstrates that ATP signaling through the P2X7R is necessary to develop a psoriasis-like inflammatory response induced by IL-23. A finding that has emerged from these studies is that IL-23 leads to the active secretion of biologically relevant levels of ATP necessary for P2X7R activation. Moreover, directly signaling through the cutaneous P2X7R leads to the development of a psoriasiform inflammatory response that mirrors the pathophysiologic immune response and histopathologic phenotype of psoriasis in humans. Importantly, these data also suggest that ATP released following trauma and/or stress can result in the development of a psoriatic Koebner response in a susceptible microenvironment. Finally, this study supports a basic immunology model in which IL-23 and P2X7R signaling form a positive feedback loop that facilitates the development of psoriatic dermatitis (Figure S5).
MATERIALS and METHODS
Mice
C57BL/6 and P2X7R −/− mice were purchased from Jackson Laboratories (Bar Harbor, Maine). NLRP3 −/− mice were obtained from Lexicon Genetics Incorporated (The Woodlands, TX), IL-23 −/− mice were obtained from Genentech (San Francisco, CA), and Il17ACreRosa26ReYFP (IL-17eYFP) mice were a kind gift from Sarah Gaffen and were all bred and housed in the University of Pittsburgh animal facility. Male and female mice were used between the ages of 6 and 12 weeks. Mice were housed under specific-pathogen-free conditions. All mice were treated according to the NIH guide for the care and use of laboratory animals and experiments and protocols were approved by the University of Pittsburgh’s IACUC.
Experimental design
Mice were injected i.d. with 100 μL of either BzATP [(350 μM, unless otherwise indicated) Sigma-Aldrich; St. Louis, MO)], or BzATP in combination with sodium polyoxotungstate [POM1 (3.2 mg/kg; Tocris Bioscience; Bristol, United Kingdom)], ± A438079 (A4; 80 μmol/kg; Tocris, Minneapolis, MN) in PBS (vehicle control), at two sites daily on the shaved backs for 4 consecutive days. In some experiments, mice were also intraperitoneally injected every other day with 0.1 mg rat anti-mouse LY6G Ab (clone 1A8; BioXCell; Lebanon, NH) dissolved in 200 μl PBS. Injections of LY6G Ab were initiated on day −1 of BzATP injections. In separate experiments lesional development was induced in mice by daily i.d. injections of murine rIL-23 (500 ng; eBiosciences; San Diego, CA or Miltenyi Biotec; Auburn, CA) in 100 μl of PBS on the shaved backs for 5 days or 62.5 mg of IMQ (5% Aldara; 3M Pharmaceuticals; St. Paul, MN) was topically administered daily for 5 days.
In vivo detection of ATP
In vivo detection of ATP was performed utilizing bioluminescence imaging as previously described (Di Virgilio et al., 2016, Weber et al., 2010). In brief, mice were injected i.d. daily with rIL-23 or PBS (negative control) on days 1–4. On day 4, 2 ˣ 106 HEK293-pmeLuc cells, a kind gift from Francesco Di Virgilio (Pellegatti et al., 2008) were injected i.d. in 100 μl of DMEM at the same injection site as rIL-23. 24 h following cellular injections mice were treated with a final dose of rIL-23 or PBS. 4 h following rIL-23 injections, some mice were treated i.d. with ATP in 100 μl (positive control; 1 mM) followed by intraperitoneal injections of D-luciferin (3mg/mouse) in PBS. 20 min later mice were imaged with the IVIS 200 Luminometer (Perkin Elmer, Waltham, MA). The acquisition time was 3 min/acquisition and binning was 4.
Generation of bone marrow-chimeric mice
Mice were gamma-irradiated with two separate exposures to 550 rads of whole body irradiation 6 h apart for a total of 1100 rads. Within 24 h mice were intravenously reconstituted with 1 ˣ 107 undepleted bone marrow cells. Mice were fed Uniprim chow (Envigo, Madison WI) for one week post-irradiation and used in experiments 6–8 weeks following bone marrow reconstitution.
Histology and Immunohistochemistry
Cutaneous tissue samples were collected and processed for H&E staining and immunohistochemistry. For this, tissues were blocked with 3% H2O2 and then with 0.5% BSA. Samples were incubated with rat anti-CD31 antibody (ThermoFisher, Grand Island, NY) followed by biotinylated donkey anti-rat IgG secondary Ab (Jackson ImmunoResearch Laboratories; West Grove, PA). Immunoreactivity was detected by incubation with a 3,3’-diaminobenzidine peroxidase substrate kit according to manufacturer’s instructions (Vector Laboratories; Burlingame, CA). Sections were then counter-stained with hematoxylin. Quantification of vascularity was based on CD31 staining utilizing ImageJ software (NIH; Bethesda, MD).
Immunofluorescence
Cross-sections of mouse back skin were prepared and stained as previously described (Mathers et al., 2009). Sections were immunofluorescently labeled with MHC class II: Alexa488 (BD Biosciences; San Jose, CA), GR-1(LY6C/LY6G): Alexa 647 (Biolegend; San Diego, CA), and F4/80: Biotin (Biolegend) followed by SA-Cy3 (Jackson ImmunoResearch). Nuclei were counter-stained with DAPI (Molecular Probes; Eugene, OR).
Tissue Cytokines
At indicated time points, skin samples (4 mm) were collected, minced, and placed into Cell Lysate Buffer (RayBiotech; Norcross, GA) supplemented with protease inhibitors. Lysates were diluted 1:2 and cytokine concentrations were measured in duplicate utilizing Luminex technology with the Fluorokine® Multianalyte Profiling kit according to manufacturer’s instructions (R&D systems; Minneapolis, MN). Samples were read on a Bio-Plex 200 system (BioRad; Hercules, CA) using the Bioplex 6.1 software.
Quantitative RT-PCR (qRT-PCR)
Real-time qRT-PCR experiments were conducted using total RNA, which was isolated using TRIzol reagent (Invitrogen). For each RT assay, 2 μg RNA was converted to cDNA utilizing RNA to cDNA High Capacity Master Mix (Applied Biosystems, Carlsbad, CA). Gene expression was determined with the following TaqMan assays: IL-6, IL-1α, IL-1β, TNFα, IL-17A, and S100A9 (Applied Biosystems). Endogenous control was GusB. All cDNA samples were ran in triplicate, amplified with the Veriquest PCR Master Mix (Affymetrix; Santa Clara, CA), and analyzed using the real-time StepOnePlus sequence detection system (Applied Biosystems). Relative fold changes were calculated based on the 2-ΔΔCt method.
Flow cytometry
Skin biopsies (10 mm) were collected, minced, and then enzymatically digested in 1 mg/ml Collagenase D (Roche; Indianapolis, IN), 1 mg/ml DNAse (Roche), 10 mg/ml hyaluronidase (Sigma-Aldrich), and 0.1% BSA in IMDM (ThermoFisher) for 45 min at 37 °C. 10 mM EDTA was added for an additional 5 min at room temperature. To make a single-celled suspension samples were passed over a cell-strainer (Corning). Cells were blocked with Fc block (CD16/CD32; BD Biosciences) and 10% donkey serum then stained with antibodies listed in Supplemental Methods. The lineage stain includes CD3/GR-1/CD11b /B220/Ter-119 antibodies. Populations were initially gated on live cells in the forward versus side scatter and by CD45 vs eFluor 780 viability dye (eBioscience). Gates were then set based on negative controls, single-staining, and fluorescence minus one controls.
Statistics
Multiple groups were compared using a one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison post-hoc test or a two-way AVOVA was utilized followed by a Bonferroni post-hoc test if two affecting factors were present. Comparison of two means was performed by a 2-tailed Student’s T-test. A p value < 0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Tina Sumpter and Daniel Kaplan for critical reading of the manuscript. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the NIH under Award Number R01AR067746 (to A.R.M.). Tissue sample imaging was performed in the Center for Biological Imaging, which is supported by NIH Grant U54 RR022241. This project used the UPCI Cancer Biomarkers Facility: Luminex Core Laboratory that is supported in part by award P30CA047904 and the Hillman In vivo Imaging Facility that is supported in part by award P30CA047904. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS:
- BzATP
2’(3’)-O-(4-Benzoylbenzoyl) adenosine 5’-triphosphate
- P2X7R
P2X7 receptor
- qRT-PCR
quantitative RT-PCR
- IMQ
Imiquimod
- E-NTDPase
ecto-nucleoside triphosphate diphosphohydrolases
Footnotes
CONFLICTS of INTEREST
LDF has financial interest in SkinJect and Brainstage. The other authors have declared that no conflict of interest exists.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clin Exp Rheumatol 2015;33(5 Suppl 93):S2–6. [PubMed] [Google Scholar]
- Atarashi K, Nishimura J, Shima T, Umesaki Y, Yamamoto M, Onoue M, et al. ATP drives lamina propria TH17 cell differentiation. Nature 2008;455:808–12 [DOI] [PubMed] [Google Scholar]
- Beura LK, Hamilton SE, Bi K, Schenkel JM, Odumade OA, Casey KA, et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 2016;532:512–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G Purinergic cotransmission. Exp Physiol 2009;94(1):20–24. [DOI] [PubMed] [Google Scholar]
- Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity 2011;35(4):596–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlström M, Ekman A-K, Petersson S, Söderkvist P, Enerbäck C. Genetic support for the role of the NLRP3 inflammasome in psoriasis susceptibility. Exp Dermatol 2012;21(12):932–37. [DOI] [PubMed] [Google Scholar]
- Christophers E, Metzler G, Rocken M. Bimodal immune activation in psoriasis. Br J Dermatol 2014;170(1):59–65. [DOI] [PubMed] [Google Scholar]
- Clark RA. Resident memory T cells in human health and disease. Science Translational Medicine 2015;7(269):269rv1–69rv1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva GL, Sperotto NDM, Borges TJ, Bonorino C, Takyia CM, Coutinho-Silva R, et al. P2X7 receptor is required for neutrophil accumulation in a mouse model of irritant contact dermatitis. Exp Dermatol 2013;22(3):184–88. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017;47(1):15–31. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F, Pinton P, Falzoni S. Assessing Extracellular ATP as Danger Signal In Vivo: The pmeLuc System. Methods Mol Biol 2016;1417:115–29. [DOI] [PubMed] [Google Scholar]
- Dumitriu IE, Baruah P, Manfredi AA, Bianchi ME, Rovere-Querini P. HMGB1: guiding immunity from within. Trends Immunol 2005;26(7):381–87. [DOI] [PubMed] [Google Scholar]
- Gallucci S, Lolkema M, Matzinger P. Natural adjuvants: Endogenous activators of dendritic cells. Nat Med 1999;5(11):1249–55. [DOI] [PubMed] [Google Scholar]
- Geraghty NJ, Mansfield KJ, Fuller SJ, Watson D, Sluyter R. The P2X7 receptor is not essential for development of imiquimod-induced psoriasis-like inflammation in mice. Purinergic Signal 2017;13(4):405–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladiator A, LeibundGut-Landmann S. Innate lymphoid cells: new players in IL-17-mediated antifungal immunity. PLoS Pathog 2013;9(12):e1003763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granstein RD, Ding W, Huang J, Holzer A, Gallo RL, Di Nardo A, et al. Augmentation of Cutaneous Immune Responses by ATPγS: Purinergic Agonists Define a Novel Class of Immunologic Adjuvants. J Immunol 2005;174(12):7725–31. [DOI] [PubMed] [Google Scholar]
- Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol 2017;140(3):645–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, et al. Fate mapping of interleukin 17-producing T cells in inflammatory responses. Nat Immunol 2011;12(3):255–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SC-S, Yu H-S, Yen F-L, Lin C-L, Chen G-S, Lan C-CE. Neutrophil extracellular trap formation is increased in psoriasis and induces human β-defensin-2 production in epidermal keratinocytes. Sci Rep 2016;6:31119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppler AR, Verma AH, Conti HR, Gaffen SL. Neutrophils Do Not Express IL-17A in the Context of Acute Oropharyngeal Candidiasis. Pathogens 2015;4(3):559–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killeen ME, Ferris L, Kupetsky EA, Falo L Jr., Mathers AR. Signaling through purinergic receptors for ATP induces human cutaneous innate and adaptive Th17 responses: implications in the pathogenesis of psoriasis. J Immunol 2013;190(8):4324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukley M, Stausberg P, Adelmann G, Chessell IP, Dietrich D. Ecto-Nucleotidases and Nucleoside Transporters Mediate Activation of Adenosine Receptors on Hippocampal Mossy Fibers by P2X7 Receptor Agonist 2’−3’-O-(4-Benzoylbenzoyl)-ATP. J Neurosci 2004;24(32):7128–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S, et al. Mast Cells and Neutrophils Release IL-17 through Extracellular Trap Formation in Psoriasis. J Immunol 2011;187(1):490–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mabuchi T, Takekoshi T, Hwang ST. Epidermal CCR6+ gammadelta T cells are major producers of IL-22 and IL-17 in a murine model of psoriasiform dermatitis. J Immunol 2011;187(10):5026–31. [DOI] [PubMed] [Google Scholar]
- Mathers AR, Janelsins BM, Rubin JP, Tkacheva OA, Shufesky WJ, Watkins SC, et al. Differential capability of human cutaneous dendritic cell subsets to initiate Th17 responses. J Immunol 2009;182(2):921–33. [DOI] [PubMed] [Google Scholar]
- Nair RP, Duffin KC, Helms C, Ding J, Stuart PE, Goldgar D, et al. Genome-wide scan reveals association of psoriasis with IL-23 and NF-κB pathways. Nat Genet 2009;41(2):199–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DW, Gregg RJ, Kort ME, Perez-Medrano A, Voight EA, Wang Y, et al. Structure−Activity Relationship Studies on a Series of Novel, Substituted 1-Benzyl-5-phenyltetrazole P2X7 Antagonists. J Med Chem 2006;49(12):3659–66. [DOI] [PubMed] [Google Scholar]
- Pantelyushin S, Haak S, Ingold B, Kulig P, Heppner FL, Navarini AA, et al. Rorγt+ innate lymphocytes and γδ T cells initiate psoriasiform plaque formation in mice. J Clin Invest 2012;122(6):2252–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastore S, Mascia F, Gulinelli S, Forchap S, Dattilo C, Adinolfi E, et al. Stimulation of purinergic receptors modulates chemokine expression in human keratinocytes. J Invest Dermatol 2007;127(3):660–67. [DOI] [PubMed] [Google Scholar]
- Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 2008;3(7):e2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pommier A, Audemard A, Durand A, Lengagne R, Delpoux A, Martin B, et al. Inflammatory monocytes are potent antitumor effectors controlled by regulatory CD4+ T cells. P Natl Acad Sci USA 2013;110(32):13085–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabeony H, Pohin M, Vasseur P, Petit-Paris I, Jégou J-F, Favot L, et al. IMQ-induced skin inflammation in mice is dependent on IL-1R1 and MyD88 signaling but independent of the NLRP3 inflammasome. Eur J Immunol 2015;45(10):2847–57. [DOI] [PubMed] [Google Scholar]
- Reich K, Papp KA, Matheson RT, Tu JH, Bissonnette R, Bourcier M, et al. Evidence that a neutrophil-keratinocyte crosstalk is an early target of IL-17A inhibition in psoriasis. Exp Dermatol 2015;24(7):529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl LL, Zang JB, Ding W, Manni M, Zhou XK, Granstein RD. Norepinephrine and adenosine-5’-triphosphate synergize in inducing IL-6 production by human dermal microvascular endothelial cells. Cytokine 2013;64(2):605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Mielke LA, Mills KHG. IL-17-producing γδ T cells and innate lymphoid cells. Eur J Immunol 2012;42(9):2221–31. [DOI] [PubMed] [Google Scholar]
- Swindell WR, Michaels KA, Sutter AJ, Diaconu D, Fritz Y, Xing X, et al. Imiquimod has strain-dependent effects in mice and does not uniquely model human psoriasis. Genome Med 2017;9(1):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter A, Schafer M, Cecconi V, Matter C, Urosevic-Maiwald M, Belloni B, et al. Aldara activates TLR7-independent immune defence. Nat Comm 2013;4:1560. [DOI] [PubMed] [Google Scholar]
- Weber FC, Esser PR, Muller T, Ganesan J, Pellegatti P, Simon MM, et al. Lack of the purinergic receptor P2X(7) results in resistance to contact hypersensitivity. J Exp Med 2010;207(12):2609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.