Abstract
Although attention deficit hyperactivity disorder is associated with deficits in docosahexaenoic acid (DHA), an omega-3 fatty acid implicated in dopamine and glutamate synaptic plasticity, its role in neuroplastic brain changes that occur following repeated amphetamine (AMPH) treatment are not known. This study used pharmacological magnetic resonance imaging to investigate the impact of repeated AMPH exposure and alterations in brain DHA levels on AMPH-induced brain activation patterns. Male rats were fed a diet with no n-3 fatty acids (Deficient, DEF, n = 20), a diet fortified with preformed DHA (fish oil, FO, n = 20), or a control diet fortified with alpha-linolenic acid (n = 20) from P21 to P90. During adolescence (P40–60), one- half of each diet group received daily AMPH injections escalated weekly (0.5, 1.0, 2.5, 5.0 mg/kg/d) or drug vehicle. Following a 30-d abstinence period blood oxygen level dependent (BOLD) responses were determined in a 7 T Bruker Biospec system following an AMPH challenge (7.5 mg/kg, i.v). Postmortem erythrocyte and forebrain DHA composition were determined by gas chromatography. Compared with control rats, forebrain and erythrocyte DHA levels were significantly lower in DEF rats and significantly higher in FO rats. Across AMPH doses DEF rats exhibited greater locomotor activity compared to control and FO rats. In AMPH-naive rats, the AMPH challenge increased BOLD activity in the substantia nigra and basal forebrain and no diet group differences were observed. In AMPH-pretreated control and FO rats, the AMPH challenge similarly increased BOLD activation in the bilateral caudate putamen, thalamus, and motor and cingulate cortices. In contrast, BOLD activation in AMPH-pretreated DEF rats was similar to AMPH-naive DEF animals, and AMPH-pretreated DEF rats exhibited attenuated frontostriatal BOLD activation compared with AMPH-pretreated control and FO rats. These findings demonstrate that chronic escalating AMPH treatment induces enduring frontostriatal recruitment and that peri-adolescent deficits in brain DHA accrual impair this response.
Keywords: Omega-3 fatty acids, Docosahexaenoic acid (DHA), Sensitization, Magnetic resonance imaging, Amphetamine, Rat
Introduction
Because psychostimulant drugs including amphetamine (AMPH) are an effective treatment for youth with attention deficit hyperactivity disorder (ADHD), understanding their long-term effects on brain structure and function may provide insight into the pathophysiology of ADHD. Prior rodent studies have found that repeated treatment with AMPH leads to enduring synaptic reorganization within pre- frontal and basal forebrain circuits. Specifically, chronic treatment with, or self-administration of, an escalating dose of AMPH increases dendritic spine density in the medial prefrontal cortex, nucleus accum- bens, and hippocampus (CA1), and reduces dendritic spine density in parietal and orbitofrontal cortices.1–3 Repeated AMPH exposure is also associated with a long-standing modification in mesolimbic dopamine release and behavioral sensitization,4,5 and regional alterations in glutamate neurotransmission6–8 and neuronal excitability.9,10 Despite this body of evidence for regional neuroadaptations following repeated AMPH exposure, their effects on global and regional brain activation patterns remain poorly understood.
Meta-analyses indicate that adults and children with ADHD exhibit significant blood omega-3 polyunsaturated fatty acid (n-3 PUFA) deficits,11 and that adjunctive n-3 PUFA supplementation may have therapeutic benefits in ADHD patients.12 Docosahexaenoic acid (DHA) is the most abundant n-3 PUFA in the mammalian brain, and animal studies indicate that DHA has neurotrophic, anti-inflammatory, and neuroprotective properties.13 In rodents, deficits in brain DHA accrual during development induce enduring changes in mesolimbic and mesocortical dopamine systems,14–17 and abnormalities in dopamine-mediated behavior.18,19 Brain DHA deficits are also associated with impaired glutamate synaptogenesis, synaptic plasticity, and homeostasis,20–22 as well as deficits in regional functional connectivity.23 In contrast, dietary DHA supplementation increases dendritic spine density24 and frontal cortex dopamine levels.25 While these findings suggest that DHA modulates dopamine and glutamate circuit plasticity, its role in the progressive neuroplastic brain changes that occur in response to repeated AMPH treatment are not known.
The present study investigated the impact of repeated AMPH exposure on AMPH-induced regional brain activation patterns in vivo using pharmacological magnetic resonance imaging (phMRI). MRI measures regional brain changes in blood oxygen level dependent (BOLD) signal which are attributable to a rise in oxygen-dependent synaptic activity and associated increases in local blood volume.26 Prior rat phMRI studies have demonstrated that an acute AMPH challenge is associated with increased BOLD signal in dopamine terminal regions, and that this response is correlated with increases in extracellular dopamine levels and mediated by dopamine receptors.27–29 To investigate the role of brain DHA levels on AMPH-induced brain activation patterns, a peri-adolescent feeding model was used to generate three groups of rats with graded brain DHA levels.21 Based on the evidence reviewed above it was hypothesized that repeated prior AMPH exposure would be associated with enduring changes in regional BOLD activation, and that these changes will be augmented in rats with high DHA levels and blunted in rats with low DHA levels.
Materials and methods
Animals and diets
Post-weaning (P20) male Long-Evans hooded rats were purchased (Harlan Farms, Indianapolis, IN, USA) and randomized (n = 20/diet group) to one of three diets (Harlan-TEKLAD, Madison, WI, USA) upon arrival (P21) until the end of the experiment (P90). Control rats were maintained on an a-linolenic acid (ALA, 18:3n-3)-fortified diet (TD.04285), and deficient (DEF) rats were maintained on an ALA- free diet (TD.04286). n-3 PUFA-enriched rats were maintained on diet containing 1.1% fish oil in place of ALA (FO, TD.110837). Diets were closely matched for all non-fat nutrients and fatty acid composition with the exception of ALA, which was absent from the DEF and FO diets, and preformed DHA and eicosapentaenoic acid (EPA, 20:5n-3) which were present in the FO diet but not the control or DEF diets (Supplemental Table 1). Rats were pair-housed under standard vivarium conditions on a 12:12 hour light:dark cycle with free access to food and water. All experimental procedures were approved on March 12, 2012-present by the University of Cincinnati and Children’s Hospital Institutional Animal Care and Use Committees, and adhere to the guidelines set by the National Institutes of Health.
AMPH pretreatment
From P40 to P60 rats received daily subcutaneous injections with D-AMPH sulfate (Sigma-Aldrich Co. LLC, St. Louis, MO, USA)(n = 10/diet group) or an equivalent volume of drug vehicle (0.9% saline, ml/kg, n = 10/diet group). Each dose (0.5, 1.0, 2.5, 5.0 mg/ml/kg) was administered for 5 consecutive days, followed by 2 drug-free days, and the AMPH dose was escalated weekly over 4 consecutive weeks. An escalating AMPH dosing regimen was selected based on prior evidence that it produces robust behavioral sensitization as well as frontostriatal synaptic reorganization.1–3 Injections were administered in automated activity chambers (46 × 24 × 20 cm) equipped with an external 8 × 4 photo beam array positioned 6 cm above the chamber floor (San Diego Instruments, San Diego, CA, USA). Horizontal locomotor activity (indexed by number of beam breaks) was collected for 1 hour post-injection on the first and fifth injection day of each dose week. Activity chambers were located in a separate room and rats were returned to their home cage following each session. Following the completion of this treatment protocol, rats resided in their home cage for a 30 d abstinence period prior to scanning on P90.
phMRI
Image acquisition
On P90, rats from each treatment group were anesthetized with 1.5–2.5% isoflurane in air, positioned supine with their teeth in a bite bar, and the head centered inside a 38 mm Litz coil (Doty Scientific, Inc., Columbia, SC, USA). Respiration was monitored and body temperature was maintained at 36–38°C using an animal monitoring system (SAI Inc., Stony Brook, NY, USA). The coil and animal were positioned in a 7 T Bruker Biospec system (Bruker BioSpin, Ettlingen, Germany), and a set of localizers from each orthogonal plane were collected. Following localizer acquisition, RARE images (Axial images: effective TE 45 ms, TR 3500 ms, RARE factor 16, matrix 256 × 256, FOV 36 mm, 11 slices in the axial direction; and sagittal images: effective TE 45 ms, TR 2000ms, RARE factor 16, matrix 256 × 192, FOV 51.2 × 38.4 mm 11 slices in the sagittal direction) were collected. A 3D data set was also acquired for registration of the MRI data (Sagittal orientation, effective TE 60.8 ms, TR 1000 ms, RARE factor 16, matrix 256 × 160 × 180, FOV 51.2 × 32 × 36 mm3). MRI data were then acquired using a RARE acquisition (repetition time 2000 ms, effective echo time 20 ms, RARE factor 8, 80 repetitions, 38.4 mm x 38.4 mm field-of-view, matrix 128 × 128, slice thickness 1mm, 11 slices). The AMPH challenge (7.5 mg/kg) was administered via an indwelling intravenous catheter following the 10th repetition and scanning continued for the remaining repetitions for approximately 30 minutes post-injection. Pilot studies found that this challenge dose produced consistent forebrain activation under the current experimental parameters.
Image processing
MRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). To ensure compatibility with the software, image data were cropped to contain primarily brain and the resolution was scaled by a factor of 10. Registration to a standard space based on the Paxinos and Watson atlas30 was carried out using FLIRT.31,32 The following pre-statistics processing were applied; motion correction using MCFLIRT,32 spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma =1120.0s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction.33 Z (Gaussianised T/F) statistic images were thre- sholded using clusters determined by Z > 2.3 and a (corrected) cluster significance threshold of P ≤ 0.05.34
Tissue collection
Isoflurane-anesthetized rats were sacrificed by decapitation immediately following scanning. Whole venous trunk blood was collected into EDTA-coated tubes, and centrifuged for 20 minutes (1500×g). Plasma and buffy coat were then removed and erythrocytes washed three times with 0.9% NaCl and stored at −80°C. The brain was dissected on ice to isolate the forebrain which was stored at −80°C.
Gas chromatography
Erythrocyte and forebrain total fatty acid composition were determined by gas chromatography with a Shimadzu GC-2014 Shimadzu Scientific Instruments Inc., Columbia, MD, USA), as described in detail previously.35 The column was a DB-23 (123–2332): 30 m (length), I.D. 0.32 mm wide bore, film thickness of 0.25 μM (J&W Scientific, Folsom, CA, USA). Fatty acid identification was determined using retention times of authenticated fatty acid methyl ester standards (Matreya LLC Inc., Pleasant Gap, PA, USA). Analysis of fatty acid methyl esters is based on areas calculated with EZstart 7.4 software. Fatty acid composition is expressed as weight percent of total fatty acids (mg fatty acid/100 mg fatty acids). All samples were processed by a technician blinded to treatment. The primary measures of interest were DHA, arachi- donic acid (AA, 20:4n-6), and the AA/DHA ratio.
Statistical analyses
Group differences in fatty acid measures were determined with a two-way ANOVA, with Diet (control, FO, DEF) and Treatment (SAL, AMPH) as the independent variables. Post hoc comparisons were made with Fisher’s LSD tests. Locomotor activity data (total number of beam breaks 30 minutes post-injection) were analyzed with a four-way ANOVA with repeated measures, with Diet (control, FO, DEF) and Treatment (SAL, AMPH) as the between subjects variables, and with Dose (0.5, 1.0, 2.5, 5.0 mg/kg) and injection Day (1st, 5th) as the repeated measures. Additionally, three-way ANOVAs (Diet × Drug × Day) with Day as a repeated measure were performed for each dose. Post hoc comparisons were made using Fisher’s LSD tests. Statistical analyses were completed using the Statistical Package for the Social Sciences (SPSS, IBM Corp., IL, USA).
Results
Fatty acid composition
Significant main effects of Diet were observed for erythrocyte DHA, F(2,57) = 284.7, P ≤ 0.0001, AA, F (2,57) = 394.9, P ≤ 0.0001, and the AA/DHA ratio, F(2,57) = 455.2, P ≤ 0.0001, and the main effects of Treatment and the Diet × Treatment interaction were not significant. (Fig. 1A-C). Significant main effects of Diet were observed for forebrain DHA, F(2,57) = 556.3, P ≤ 0.0001, AA, F(2,57) = 9.7, P ≤0.0001, and the AA/DHA ratio, F(2,57) = 3.1, P ≤ 0.0001. The main effect of Treatment and the Diet × Treatment interaction were not significant (Fig. 1D- F). After collapsing across treatment groups, forebrain DHA composition was significantly lower in DEF rats (−35%, P ≤ 0.0001) and significantly higher in FO rats (+6%, P ≤ 0.0001) compared with control rats (Fig. 1D). Compared with control rats, AA composition was significantly higher in DEF rats (+4%, P = 0.002) and significantly lower in FO rats (−5%, P ≤ 0.0001) (Fig. 1E). Compared with control rats, the AA/DHA ratio composition was significantly higher in DEF rats (+37%, P ≤ 0.0001) and significantly lower in FO rats (−10%, P ≤ 0.0001) (Fig. 1F). Across all treatment groups (n = 60), forebrain and erythrocyte DHA (r =+0.95, P ≤ 0.0001), AA (r =+0.66, P ≤ 0.0001), and AA/DHA ratio (r = +0.89, P ≤ 0.0001) were positively correlated.
Figure 1.
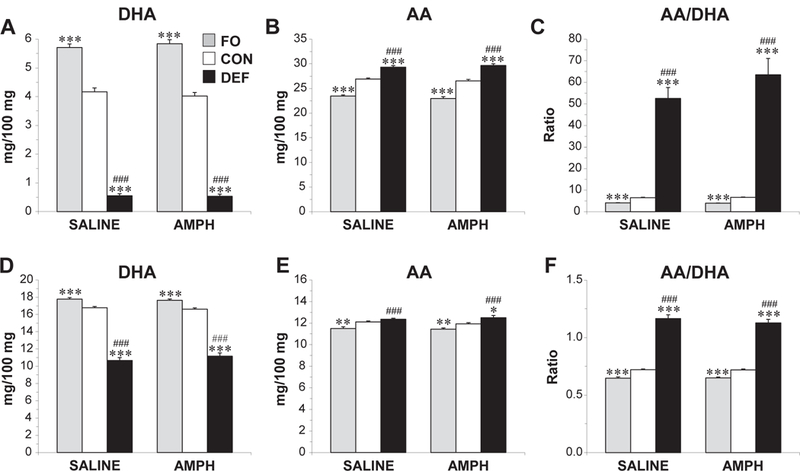
DHA (A,D) and arachidonic acid (AA) (B,E) composition, and the AA/DHA ratio (C,F) in erythrocytes (A–C) and forebrain, (D–F) of adult rats maintained on the control diet (CON), n-3-free diet (DEF), and fish oil-fortified diet (FO) during peri-adolescent, development. Diet groups are separated into those that received prior saline or AMPH dosing. Values are group mean ± S.E.M., *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.0001 vs. CON, ###P ≤ 0.0001 vs. FO
Locomotor activity
There was a significant overall main effect of drug, F(1,41)= 432.3, P < 0.0001, with AMPH-treated rats exhibiting greater locomotor activity across all doses compared with saline-treated rats (Fig. 2A). The overall main effect of diet was also significant, F(2,41)= 5.6, P = 0.007, with DEF rats being significantly more active than control (P = 0.029) and FO (P = 0.002) rats across doses. Locomotor responses on the first day of each dose are illustrated in Fig. 2B. At the 0.5 mg/kg dose, the main effect of diet was not significant, F(2,42) = 1.0, P = 0.36, and the main effect of day, F(1,42) = 5.9, P = 0.019, and the Day × Drug Interaction, F(1,42) = 11.1, P = 0.002, were significant. Locomotor activity decreased from day 1 to day 5 in rats receiving saline, whereas locomotor activity increased from day 1 to day 5 in rats receiving AMPH. At 0.5 mg/kg AMPH-treated DEF rats were more active relative to AMPH-treated control rats (P = 0.046). The main effect of diet was significant for 1.0 mg/kg, F(2,41) = 5.481, P = 0.008, with DEF rats exhibiting greater locomotor activity compared with control (P = 0.007) or FO (P = P = 0.001) rats. The main effect of diet was not significant at 2.5 mg/kg, F(2,42) = 1.5, P = 0.2, or 5.0 mg/kg, F= 2.9, P = 0.07, nor were the main effects of day or interaction terms.
Figure 2.
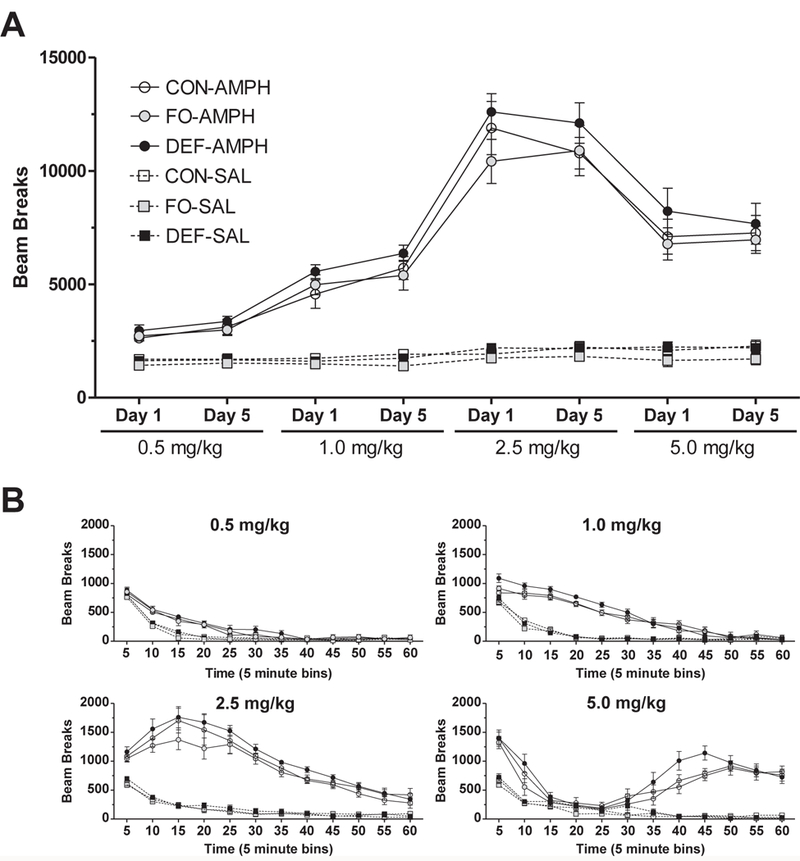
(A) Horizontal locomotor activity (beam breaks) for the first 30 minutes following administration of saline (SAL, 1.0 ml/kg) or escalating AMPH doses (0.5, 1.0, 2.5, 5.0 mg/kg) on the first (Day 1) and fifth (Day 5) injection day of each dose week in adolescent rats maintained on the control diet (CON), n-3-free diet (DEF), and fish oil-fortified diet (FO). (B) Horizontal locomotor activity following administration of SAL or escalating AMPH doses on the first day of each dose in rats maintained on CON, FO, or DEF diets. Values are group mean beam breaks ± S.E.M.
phMRI
No significant negative BOLD responses were observed in any analysis. In SAL-pretreated (AMPH- naive) control rats, the AMPH challenge induced bilateral increases in BOLD activity in the substantia nigra and basal forebrain dopamine structures including the islands of Calleja, ventral pallidum, anterior amygdala, lateral hypothalamus, as well as the subiculum, and a similar response pattern was observed in SAL- pretreated FO and DEF rats (Fig. 3A). There were no significant diet group differences among SAL-pretreated rats (P ≤ 0.05 corrected). In AMPH-pretreated control rats, the AMPH challenge induced widespread bilateral BOLD activation in multiple regions, including substantia nigra, ventral tegmentum, caudate putamen, amygdala, hippocampus, thalamus, and cin- gulate and motor cortices (Fig. 3B). A similar, albeitless robust, pattern was observed in AMPH-pretreated FO rats, whereas AMPH-pretreated DEF rats did not exhibit widespread bilateral BOLD activation (Fig. 3B). Compared with SAL-pretreated control rats, AMPH-pretreated control rats revealed significantly greater BOLD activation in multiple cortical and subcortical regions, including the bilateral caudate putamen, thalamus, frontoparietal and motor cortices, and a similar pattern was observed between SAL- and AMPH-pretreated FO rats (Fig. 3C). Contrasts of SAL- and AMPH-pretreated DEF rats did not reveal any significant differences in BOLD activation. Compared with AMPH-pretreated control and FO rats, AMPH-pretreated DEF rats exhibited significantly lower BOLD activity in bilateral caudate putamen, thalamus, frontoparietal and motor cortices, and there were no differences between AMPH-pre- treated control and FO rats (Fig. 4).
Figure 3.
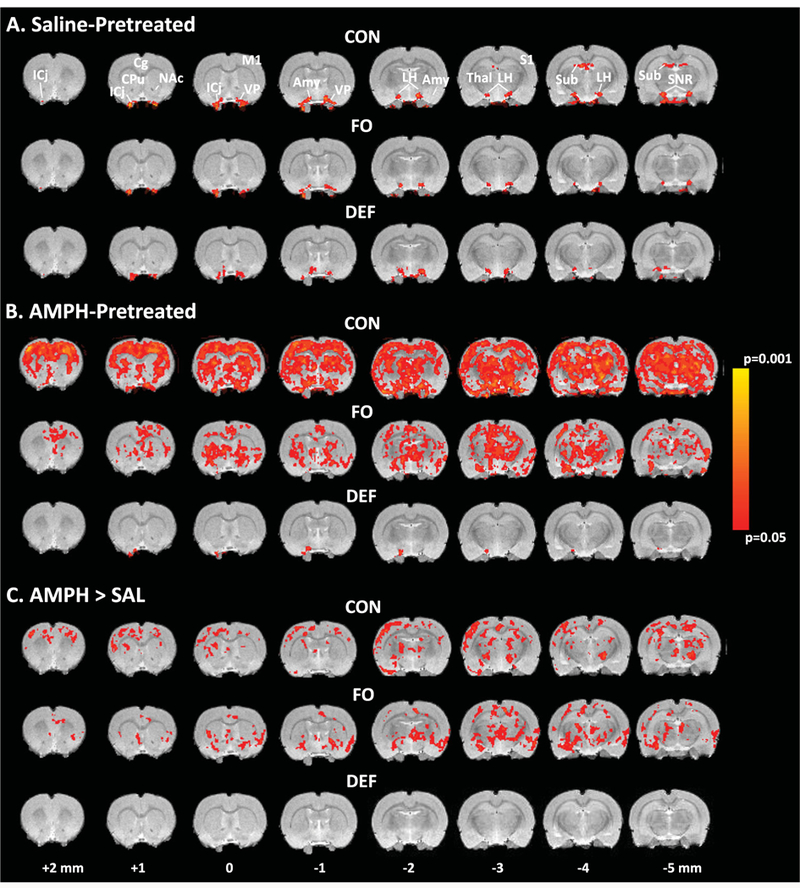
Voxel-based analysis illustrating AMPH-induced BOLD activity in saline-pretreated (AMPH-naïve) (A) and AMPHpretreated, (B) adult (P90) rats maintained on the control diet (CON), fish oil-fortified diet (FO), and n-3-free diet (DEF) during periadolescent, development (P21–P90). (C) Contrasts illustrating differential AMPH-induced BOLD activity in AMPH-pretreated versus saline-pretreated rats maintained on CON, FO, or DEF diets. All contrasts used cluster significance threshold of P ≤ 0.05 (corrected). Bregma coordinates of each 1 mmslice are indicated. Abbreviations: ICj, islands of Calleja; Cg, cingulate cortex; M1, motor cortex; CPu, caudate putamen; NAc, nucleus accumbens; VP, ventral pallidum; Amy, amydgala; LH, lateral hypothalamus;Thal, thalamus; S1, somatosensory cortex; Sub, subiculum; SNR, substantia nigra.
Figure 4.
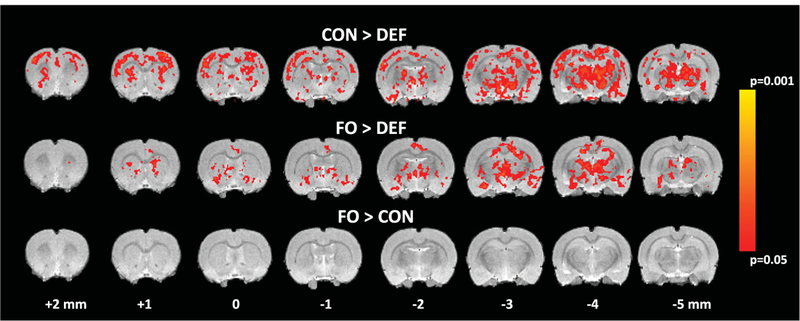
Voxel-based analysis illustrating differential AMPH-induced BOLD activity in AMPH-pretreated adult rats maintained on the CON diet versus DEF diet (CON > DEF), FO diet versus DEF diet (FO > DEF), and FO diet versus CON diet (FO > CON). All contrasts used cluster significance threshold of P ≤ 0.05 (corrected). Bregma coordinates of each 1 mm slice are indicated
Discussion
This phMRI study investigated the hypothesis that repeated prior AMPH exposure would be associated with enduring changes in regional BOLD activation, and that these changes would be augmented in rats with high DHA levels and blunted in rats with low DHA levels. Compared with control rats, forebrain and erythrocyte DHA levels were significantly lower in DEF rats and significantly higher in FO rats. Across AMPH pretreatment doses DEF rats exhibited greater locomotor activity compared with control and FO rats. In AMPH-naive rats, the AMPH challenge increased activation (BOLD signal) primarily in basal forebrain dopamine terminal regions, including the islands of Calleja, ventral pallidum, and lateral hypothalamus, and no diet group differences were observed. In support of our hypothesis, prior chronic escalating AMPH treatment was associated with an enduring (30 day) increase in AMPH-induced activation in multiple regions beyond the basal forebrain, including the bilateral caudate putamen, thalamus, and cingulate and motor cortices (hereafter referred to as ‘frontostriatal’ recruitment), and this response was blunted in DEF rats. In contrast to our hypothesis, frontostriatal recruitment was not augmented in FO rats, though the increase in forebrain DHA levels observed in FO rats relative to control rats (+6%) may not have been sufficiently robust to further increase activation. Together, these results demonstrate that chronic escalating AMPH treatment is associated with enduring frontostriatal recruitment and that this response is markedly blunted in DHA- deficient rats.
In response to the AMPH challenge AMPH-naive control rats exhibited increased activation in the substantia nigra and basal forebrain dopamine terminal regions including the islands of Calleja, ventral pallidum, and lateral hypothalamus. It is notable that this relatively discrete activation pattern differs from the widespread activation observed in response to acute AMPH challenge in prior phMRI studies.27,28 The reason for this discrepancy may be due in part to methodological differences including anesthetic (isoflurane vs. halothane), magnet strength (7 T vs. 2.3–4.7 T), AMPH challenge dose (7.5 mg/kg vs. 3.0 mg/kg), and rat strain (LEH vs. Sprague-Dawley). However, the BOLD pattern observed in AMPH-naive control rats is very similar to the pattern observed following a methylphenidate challenge in a recent phMRI study that also used isoflurane anesthetic.36 In the present study, we additionally demonstrate that this activation pattern was not significantly altered by either increases or decreases in brain DHA levels. Prior in vivo microdialysis studies indicate that larger brain DHA deficits (−70%) resulting from perinatal n-3 fatty acid deficiency produce long-standing deficits in AMPH- or tyramine-stimulated increases in extracellular dopamine levels in the basal forebrain and pre- frontal cortex.16,17 Although the effects of moderate reductions in brain DHA levels observed in the present study on dopamine release dynamics are not known, the present phMRI evidence would suggest that they are not robustly impacted by peri-adolescent n-3 fatty acid deficiency.
A central finding of the present study is that prior exposure to chronic escalating AMPH dosing is associated with an enduring expansion of activation in multiple regions outside of the basal forebrain, including the bilateral caudate putamen, thalamus, and cingulate and motor cortices. While the neuroplastic mechanisms mediating this ‘recruitment’ of frontos- triatal structures following prior AMPH exposure are poorly understood, chronic escalating AMPH dosing was previously found to induce enduring synaptic reorganization in frontostriatal regions which may mediate increased regional functional connectivity.2,3 However, other mechanisms including the sensitization of mesocortical and mesostriatal dopamine neurotransmission also likely play a role and additional studies are warranted to delineate mechanisms. It is notable that a similar pattern of frontostriatal recruitment was observed in a recently published study that used an AMPH dosing regimen found to have dopamine terminal neurotoxic effects.36 Moreover, frontostriatal recruitment was not observed in rats challenged with AMPH following 3-week treatment with methylphenidate, which does not have dopamine terminal neurotoxic effects.37 Although we did not evaluate indices of dopamine neurotoxicity, the AMPH dosing regimen used in the present study has previously been shown to not be associated with dopamine terminal degeneration.38 Nevertheless, additional studies will be required to better understand the mechanisms mediating neuroplastic changes within frontostriatal circuitry following repeated AMPH exposure.
A second important finding is that the frontostriatal recruitment observed in control and FO rats following chronic AMPH exposure was not observed in DEF rats. Indeed, the regional activation pattern following the AMPH challenge in AMPH-pretreated and AMPH-naive DEF rats did not differ significantly. While the reason for this blunted response is not known, prior evidence indicates that DHA deficiency is associated with impaired glutamatergic synaptic plasticity (i.e. long-term potentiation)20 whereas increasing DHA intake increases dendritic spine density.24 Therefore, DHA deficiency may have impaired regional alterations in dendritic spine density and synaptic remodeling observed following chronic AMPH exposure.1–3 Additionally or alternatively, DHA has been found to be protective against neurotoxic dopamine lesions which may be mediated in part by elevated pro-inflammatory signaling,39–41 and larger brain DHA deficits are associated with constitutive increases in pro-inflammatory cytokine levels42 and dopamine cell loss.14 However, a neurotoxic mechanism is not supported by the observation that DEF rats exhibited greater locomotor activity during AMPH pretreatment, and frontostriatal recruitment is also observed following a neurotoxic AMPH dosing regimen.36 Nevertheless, additional studies are warranted to determine whether AMPH- induced dopamine neurotoxicity and/or synaptic remodeling are altered in DEF rats.
The present findings may take on additional significance in view of evidence that medication-naive ADHD patients exhibit blunted frontostriatal activation and functional connectivity during performance of cognitive tasks, and that blunted frontostriatal activation is normalized following psychostimulant treatment.43–47 The present results additionally suggest that DHA deficits commonly observed in ADHD patients11 may hinder AMPH- induced neuroplastic adaptations in frontostriatal circuits, and that the therapeutic benefits associated with n-3 PUFA supplementation in ADHD patients may be mediated in part by promoting frontostriatal activation.12 It is also notable that DEF rats exhibited elevated locomotor activity during AMPH pretreatment across doses, suggesting that impaired frontostriatal recruitment is associated with behavioral hyperactivity rather than hypoactivity. The latter, apparently paradoxical, effect may be due in part to a failure to recruit medial prefrontal-mediated inhibitory feedback on ventral striatum dopamine systems (i.e. nucleus accumbens)48 as is observed following medial prefrontal cortex lesions.49 These and other findings support the translational hypothesis that optimal n-3 PUFA levels are required for AMPH- induced frontostriatal recruitment, and prospective fMRI studies are warranted to evaluate this mechanism in medication-naive ADHD patients.
This study has a number of limitations. First, rats were anesthetized with isoflurane during MRI acquisition which may have artificially altered BOLD signal. However, all groups were anesthetized with similar levels of isoflurane and reliable AMPH- induced changes in BOLD activity were observed. Second, we did not obtain locomotor activity data at P90 to confirm that the AMPH pretreatment regimen resulted in behavioral sensitization. Third, we did not determine indices of dopamine neurotoxicity or synaptic remodeling (i.e. dendritic spine density) to evaluate their potential contribution to the present findings. Strengths of this study include high magnetic field strength (7 Tesla), voxel-based determination of regional brain activation patterns in vivo, measurement of behavioral responses to AMPH treatment, and selective manipulation of diets and postmortem evaluation of fatty acid levels.
In conclusion, the present findings provide preclinical evidence that chronic escalating AMPH treatment leads to an enduring recruitment of frontostriatal regions, and that this response in impaired in rats subjected to peri-adolescent deficits in brain DHA accrual. These findings compliment prior evidence for enduring synaptic reorganization in frontostriatal regions following similar chronic escalating AMPH dosing, and support additional research to determine whether enduring synaptic reorganization within frontostriatal structures contributes to enduring increases in activation in response to AMPH. Together these findings may have implications for understanding the neurobiological substrates mediating the therapeutic actions of psychostimulant medications in ADHD, and further suggest that these neuroplastic processes are impaired by low brain DHA levels.
Supplementary Material
Acknowledgments
Acknowledgements
The NIH did not have any role in the design, implementation, analysis or interpretation of the research.
Funding R.K.M. has received research support from NARSAD, Martek Biosciences Corporation, Ortho- McNeil Janssen, AstraZeneca, Eli Lilly and Company, Kyowa Hakko Bio Co., LTD, Royal DSM Nutritional Products, LLC, and the Inflammation Research Foundation (IRF). This work was supported in part by National Institute of Health grants MH107378, DK097599, MH097818, and NCRR UL1 RR026314 to R.K.M.
Footnotes
Disclaimer statements
Conflicts of interest R.K.M. was a member of the IRF scientific advisory board, and served as a paid consultant for VAYA Pharma Inc., and Vifor Pharma Inc.
Ethics approval All experimental procedures were approved on 2012-present by the University of Cincinnati and Children’s Hospital Institutional Animal Care and Use Committees, and adhere to the guidelines set by the National Institutes of Health.
Supplemental data for this article can be accessed at doi:10.1080/1028415X.2017.1419550
References
- 1.Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex 2005;15:341–8. [DOI] [PubMed] [Google Scholar]
- 2.Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefron- tal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci 1999;11:1598–604. [DOI] [PubMed] [Google Scholar]
- 3.Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997;17: 8491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Imperato A, Obinu MC, Carta G, Mascia MS, Casu MA, Gessa GL. Reduction of dopamine release and synthesis by repeated amphetamine treatment: role in behavioral sensitization. Eur J Pharmacol 1996;317:231–7. [DOI] [PubMed] [Google Scholar]
- 5.Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+—)-amphetamine: a microdialysis study in freely moving rats. Brain Res 1988;462:211–22. [DOI] [PubMed] [Google Scholar]
- 6.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, et al. The nucleus accumbens: mechanisms of addiction across drug classes reflect the importance of glutamate homeostasis. Pharmacol Rev 2016;68:816–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu W, Wolf ME. Repeated amphetamine administration alters AMPA receptor subunit expression in rat nucleus accumbens and medial prefrontal cortex. Synapse 1999;32:119–31. [DOI] [PubMed] [Google Scholar]
- 8.White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther 1995;273:445–54. [PubMed] [Google Scholar]
- 9.Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci 2006;26:8025–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gulley JM, Stanis JJ. Adaptations in medial prefrontal cortex function associated with amphetamine-induced behavioral sensitization. Neuroscience 2010;166:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang JC, Su KP, Mondelli V, Pariante CM. Omega-3 polyunsaturated fatty acids in youths with attention deficit hyperactivity disorder (ADHD): a systematic review and meta-analysis of clinical trials and biological studies. Neuropsychopharmacology 2017. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bloch MH, Qawasmi A. Omega-3 fatty acid supplementation for the treatment of children with attention-deficit/hyperactivity disorder symptomatology: systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2011;50:991–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara RK, Valentine CJ. Role of long-chain omega-3 fatty acids in cognitive and emotional development In Dye L, Campoy C (eds.) Nutrition for brain health and cognitive performance. London: Taylor & Francis Group; 2015, p. 151–88. [Google Scholar]
- 14.Ahmad SO, Park JH, Radel JD, Levant B. Reduced numbers of dopamine neurons in the substantia nigra pars compacta and ventral tegmental area of rats fed an n-3 polyunsaturated fatty acid-deficient diet: a stereological study. Neurosci Lett 2008;438:303–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bondi CO, Taha AY, Tock JL, Totah NK, Cheon Y, Torres GE, et al. Adolescent behavior and dopamine availability are uniquely sensitive to dietary omega-3 fatty acid deficiency. Biol Psychiatry 2014;75:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kodas E, Vancassel S, Lejeune B, Guilloteau D, Chalon S. Reversibility of n-3 fatty acid deficiency-induced changes in dopaminergic neurotransmission in rats: critical role of developmental stage. J Lipid Res 2002;43:1209–19. [PubMed] [Google Scholar]
- 17.Zimmer L, Vancassel S, Cantagrel S, Breton P, Delamanche S, Guilloteau D, et al. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am J Clin Nutr 2002;75:662–7. [DOI] [PubMed] [Google Scholar]
- 18.Levant B, Radel JD, Carlson SE. Decreased brain docosahexaenoic acid during development alters dopamine-related behaviors in adult rats that are differentially affected by dietary remediation. Behav Brain Res 2004;152:49–57. [DOI] [PubMed] [Google Scholar]
- 19.McNamara RK, Sullivan J, Richtand NM, Jandacek R, Rider T, Tso P, et al. Omega-3 fatty acid deficiency augments amphetamine-induced behavioral sensitization in adult DBA/2J mice: relationship with ventral striatum dopamine concentrations. Synapse 2008;62:725–35. [DOI] [PubMed] [Google Scholar]
- 20.Cao D, Kevala K, Kim J, Moon HS, Jun SB, Lovinger D, et al. Docosahexaenoic acid promotes hippocampal neuronal development and synaptic function. J Neurochem 2009;111:510–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNamara RK, Asch RH, Schurdak JD, Lindquist DM. Glutamate homeostasis in the adult rat prefrontal cortex is altered by cortical docosahexaenoic acid accrual during adolescence: An in vivo 1H MRS study. Psychiatry Res 2017;270: 39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida S, Yasuda A, Kawazato H, Sakai K, Shimada T, Takeshita M, et al. Synaptic vesicle ultrastructural changes in the rat hippocampus induced by a combination of alpha-linole-nate deficiency and a learning task. J Neurochem 1997;68: 1261–8. [DOI] [PubMed] [Google Scholar]
- 23.Grayson DS, Kroenke CD, Neuringer M, Fair DA. Dietary omega-3 fatty acids modulate large-scale systems organization in the rhesus macaque brain. J Neurosci 2014;34:2065–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sakamoto T, Cansev M, Wurtman RJ. Oral supplementation with docosahexaenoic acid and uridine-5’-monophosphate increases dendritic spine density in adult gerbil hippocampus. Brain Res 2007;1182:50–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chalon S, Delion-Vancassel S, Belzung C, Guilloteau D, Leguisquet AM, Besnard JC, et al. Dietary fish oil affects mono- aminergic neurotransmission and behavior in rats. J Nutr 1998;128:2512–9. [DOI] [PubMed] [Google Scholar]
- 26.Hess A, Stiller D, Kaulisch T, Heil P, Scheich H. New insights into the hemodynamic blood oxygenation level-dependent response through combination of functional magnetic resonance imaging and optical recording in gerbil barrel cortex. J Neurosci 2000;20:3328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen YC, Galpern WR, Brownell AL, Matthews RT, Bogdanov M, Isacson O, et al. Detection of dopaminergic neurotransmitter activity using pharmacologic MRI: correlation with PET, microdialysis, and behavioral data. Magn Reson Med 1997;38:389–98. [DOI] [PubMed] [Google Scholar]
- 28.Dixon AL, Prior M, Morris PM, Shah YB, Joseph MH, Young AM. Dopamine antagonist modulation of amphetamine response as detected using pharmacological MRI. Neuropharmacology 2005;48:236–45. [DOI] [PubMed] [Google Scholar]
- 29.Schwarz A, Gozzi A, Reese T, Bertani S, Crestan V, Hagan J, et al. Selective dopamine D(3) receptor antagonist SB-277011- A potentiates phMRI response to acute amphetamine challenge in the rat brain. Synapse 2004;54:1–10. [DOI] [PubMed] [Google Scholar]
- 30.Schweinhardt P, Fransson P, Olson L, Spenger C, Andersson JL. A template for spatial normalisation of MR images of the rat brain. J Neurosci Methods 2003;129:105–13. [DOI] [PubMed] [Google Scholar]
- 31.Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Anal 2001;5:143–56. [DOI] [PubMed] [Google Scholar]
- 32.Jenkinson M, Bannister P, Brady M, Smith S. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. Neuroimage 2002;17:825–41. [DOI] [PubMed] [Google Scholar]
- 33.Woolrich MW, Ripley BD, Brady JM, Smith SM. Temporal autocorrelation in univariate linear modelling of fMRI data. Neuroimage 2001;14:1370–86. [DOI] [PubMed] [Google Scholar]
- 34.Worsley KJ. Statistical analysis of activation images Ch 14. In Jezzard P, Matthews PM, Smith SM (eds.) Functional MRI: an introduction to methods. Oxford: : Oxford University Press; 2001. [Google Scholar]
- 35.McNamara RK, Able J, Jandacek R, Rider T, Tso P. Inbred C57BL/6J and DBA/2J mouse strains exhibit constitutive differences in regional brain fatty acid composition. Lipids 2009;44:1–8. [DOI] [PubMed] [Google Scholar]
- 36.Schrantee A, Tremoleda JL, Wylezinska-Arridge M, Bouet V, Hesseling P, Meerhoff GF, et al. Repeated dexamphetamine treatment alters the dopaminergic system and increases the phMRI response to methylphenidate. PLoS One 2017;12: e0172776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Marel K, Klomp A, Meerhoff GF, Schipper P, Lucassen PJ, Homberg JR, et al. Long-term oral methylphenidate treatment in adolescent and adult rats: differential effects on brain morphology and function. Neuropsychopharmacology 2014;39: 263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Advokat C Update on amphetamine neurotoxicity and its relevance to the treatment of ADHD. J Atten Disord 2007;11: 8–16. [DOI] [PubMed] [Google Scholar]
- 39.Bousquet M, Gue K, Emond V, Julien P, Kang JX, Cicchetti F, et al. Transgenic conversion of omega-6 into omega-3 fatty acids in a mouse model of Parkinson’s disease. J Lipid Res 2011;52: 263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ozsoy O, Seval-Celik Y, Hacioglu G, Yargicoglu P, Demir R, Agar A, Aslan M. The influence and the mechanism of docosa- hexaenoic acid on a mouse model of Parkinson’s disease. Neurochem Int 2011;59:664–70. [DOI] [PubMed] [Google Scholar]
- 41.Tanriover G, Seval-Celik Y, Ozsoy O, Akkoyunlu G, Savcioglu F, Hacioglu G, et al. The effects of docosahexaenoic acid on glial derived neurotrophic factor and neurturin in bilateral rat model of Parkinson’s disease. Folia Histochem Cytobiol 2010;48:434–41. [DOI] [PubMed] [Google Scholar]
- 42.McNamara RK, Rider T, Jandacek RJ, Tso P, Cole-Strauss A, Lipton JW. Omega-3 fatty acid deficiency increases constitutive pro-inflammatory cytokine production in rats: relationship with central serotonin turnover. Prostaglandins Leukot Essent Fatty Acids 2010;83:185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K. Fronto- striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/ hyperactivity disorder and persistent symptoms. Psychiatry Res 2011;193:17–27. [DOI] [PubMed] [Google Scholar]
- 44.Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Metaanalysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry 2013;70:185–98. [DOI] [PubMed] [Google Scholar]
- 45.Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology 2009;57:640–52. [DOI] [PubMed] [Google Scholar]
- 46.Rubia K, Halari R, Mohammad AM, Taylor E, Brammer M. Methylphenidate normalizes frontocingulate underactivation during error processing in attention-deficit/hyperactivity disorder. Biol Psychiatry 2011;70:255–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaidya CJ, Austin G, Kirkorian G, Ridlehuber HW, Desmond JE, Glover GH, et al. Selective effects of methylphenidate in attention deficit hyperactivity disorder: a functional magnetic resonance study. Proc Natl Acad Sci USA 1998;95:14494–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson TL, Moss RL. In vivo stimulated dopamine release in the nucleus accumbens: modulation by the prefrontal cortex. Brain Res 1995;686:93–8. [DOI] [PubMed] [Google Scholar]
- 49.Jaskiw GE, Karoum F, Freed WJ, Phillips I, Kleinman JE, Weinberger DR. Effect of ibotenic acid lesions of the medial pre- frontal cortex on amphetamine-induced locomotion and regional brain catecholamine concentrations in the rat. Brain Res 1990;534:263–72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


