Abstract
HIV infection of the CNS causes neuroinflammation and damage that contributes to the development of HIV associated neurocognitive disorders (HAND) in greater than 50% of HIV infected individuals, despite antiretroviral therapy (ART). Opioid abuse is a major risk factor for HIV infection. It has been shown that opioids can contribute to increased HIV CNS pathogenesis, in part, by modulating the function of immune cells. HIV enters the CNS within two weeks after peripheral infection by transmigration of infected monocytes across the blood brain barrier (BBB). CD14+CD16+ monocytes are a mature subpopulation that is increased in number in the peripheral blood of HIV infected people. Mature monocytes can be productively infected with HIV, and they transmigrate preferentially across the BBB in response to CCL2, a chemokine elevated in the CNS and CSF of HIV infected people even with ART. Buprenorphine, an opioid derivate, is an opioid replacement therapy for heroin addiction. It is a partial agonist of MOR and full antagonist of KOR. The effects of buprenorphine on CCL2-mediated CD14+CD16+ monocytes transmigration across the BBB, a critical mechanism that promotes neuroinflammation and HAND, have not been characterized. We showed for the first time that buprenorphine decreases several steps of CCL2-mediated human mature monocyte transmigration. We propose that buprenorphine treatment in the context of HIV infection could serve a dual purpose, to treat opioid addiction and also to reduce neuroinflammation. Additionally, buprenorphine may be used as a treatment for HAND not only in the context of opioid abuse.
Keywords: Opioids, neuroAIDS, chemotaxis, adhesion, FROUNT, human
Graphical Abstract
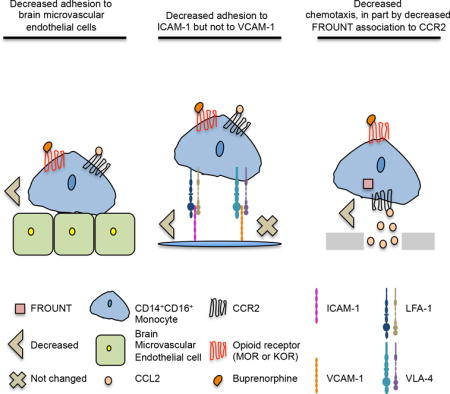
Introduction
HIV enters the CNS within two weeks after peripheral infection [1–3], ultimately resulting in neuroinflammation and neuronal damage that leads to the development of HAND in approximately 50% of HIV infected people, despite ART [4, 5]. One mechanism by which HIV enters the CNS is by transmigration of infected monocytes across the BBB in response to chemokines such as CCL2 [6–10]. CCL2 is a potent monocyte chemoattractant, and its levels are increased in the CNS of HIV infected people despite successful ART [11, 12]. Monocyte transmigration is a tightly regulated multi-step process [13, 14]. CCL2, presented on the apical surface of brain microvascular endothelial cells (BMVEC) of the BBB, binds to its receptor, CCR2, on the surface of monocytes triggering signaling pathways that lead to the activation of the monocyte integrins lymphocyte function associated antigen-1 (LFA-1) and very late antigen-4 (VLA-4) [15–17]. Activated LFA-1 and VLA-4 bind to their targets, ICAM-1 and VCAM-1, respectively, on BMVEC resulting in firm adhesion and subsequent monocyte transmigration into the CNS [18, 19]. FROUNT is a protein that associates with CCR2 within 30(s) after CCL2’s binding. It is important for CCR2 signaling that promote monocyte migration [20].
Peripheral blood monocytes are a heterogeneous population that can be classified by surface CD14, the LPS receptor, and CD16, the FcγIII receptor, with some expressing CD14 only and others expressing both [21]. Monocytes that express both CD14 and CD16 are mature cells that are key to HIV neuropathogenesis [8, 22–25]. Mature monocytes are increased in number in the peripheral blood of HIV infected people [26, 27]. Additionally, they can be productively infected with HIV, and they transmigrate preferentially across the BBB in response to CCL2 [9, 28, 29].
Injection drug use is a major risk factor for HIV infection [30, 31]. Opioids, in particular heroin, are commonly abused intravenously [32]. Currently in the United States, opiate abuse, including heroin and prescription opioids, is a major epidemic [33, 34]. Deaths associated with the overdose of both types of opioids continue to increase [34]. Additionally, the number of people seeking treatment for prescription opioid abuse has increased significantly [35]. Opioid abuse is important in the context of HIV infection and neuropathogenesis since some studies have shown that HIV infected people who use opioids have increased CNS disease compared to infected non-opioid abusers [36–39]. The mechanisms by which opioids contribute to HIV neuropathogenesis are not fully understood, but regulation of immune cell functions is believed to be important [40–42].
Opioid substitution therapies are used for opioid addiction [43]. One such therapeutic is buprenorphine, an opioid derivate, and a partial agonist of the mu opioid receptor (MOR), and full antagonist of the kappa opioid receptor (KOR) [44, 45]. However, the effects of buprenorphine on HIV neuropathogenesis and specifically on CCL2 regulated mature monocyte migration across the BBB are not well understood. We demonstrated for the first time that buprenorphine treatment could decrease mature monocyte mediated neuroinflammation in the context of HIV infection by reducing their CCL2-mediated adhesion and chemotaxis, important steps for monocyte transmigration across the BBB. We propose that buprenorphine, despite being an opioid, may decrease chronic CNS inflammation and its associated damage in HIV infected people, and thus will have an important additional therapeutic impact by reducing HAND.
Material and Methods
Materials
2-Mercaptoethanol (BME), CaCl2, MgCl2, buprenorphine, sodium bicarbonate, ascorbic acid, heparin sodium salt, and endothelial cell growth factor supplement were from Sigma-Aldrich (St. Louis, MO, USA). Ficoll-Paque PLUS was from GE Healthcare (Uppsala, Sweden). Penicillin/Streptomycin (P/S), PBS, RPMI, and human serum-type AB (HS) were from Corning (Corning, NY, USA). CCL2 was from PreproTech (Rocky Hill, NJ, USA) and R&D Systems (Minneapolis, MN, USA). Macrophage colony stimulating factor (MCSF-1) was from PreproTech, digitonin from Invitrogen (Carlsbad, CA, USA), EDTA from Promega (Madison, WI, USA), and Bradford protein assay was from Bio-Rad (Hercules, CA, USA). Protein A/G and protein-A agarose beads were from Santa Cruz (Dallas, TX, USA). HEPES was from Technova (Hollister, CA, USA), bovine serum albumin from Life Sciences US biological (Salem, MA, USA), paraformaldehyde (PFA) from Electron Microscopy Sciences (Hatfield, PA, USA) and nonfat dry milk was from LabScientific (Highlands, NJ, USA). Fetal bovine serum (FBS), M199, HBSS, TRIS-HCL, NaCl, calcein-AM, glycerol, Medium 199 (M199), L-glutamine, and new born calf serum were from Thermo Fischer Scientific (Waltham, MA, USA). Bovine brain extract was from Clonetics/Lonza (Walkersville, MD, USA).
Antibodies
CD14-FITC (clone M5E2), IgG2a,k-FITC (clone G155-178), CD16-PECy7 (clone 3G8), IgG1,k-PECy7 (clone MOPC-21) and IgG2b,k-APC (clone 27-35) were from Becton Dickinson and Company (BD) (Franklin Lakes, NJ, USA). CCR2-APC (clone 48607) was from R&D Systems. MOR-1 (N-20), KOR-1 (N-19) and normal goat IgG were from Santa Cruz. Anti-Goat IgG-PE (whole molecule) was from Sigma-Aldrich. Anti-FROUNT (NUP85) was from Bethyl Laboratories (Montgomery, TX, USA). Anti-CCR2 (clone D14H7) and Rabbit IgG (clone DA1E) for IP and WB were from Cell signaling (Beverly, MA, USA). Goat anti-Rabbit IRDye 800 CW and IRDye 680RD were from LI-COR (Lincoln, NE, USA).
Cell isolation
Concentrated blood was obtained from leukopaks from the New York Blood Center according to established protocols at the Albert Einstein College of Medicine. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Paque PLUS density gradient centrifugation. CD14+ monocytes were isolated from PBMC by positive selection using the CD14 EasySep separation kit (Stem Cell Technologies, Vancouver, Canada). To mature CD14+ monocytes we used a previously established in vitro culture system [46]. Briefly, freshly isolated CD14+ monocytes were cultured nonadherently for 3 days in Teflon coated flasks (BD) at a density of 2 × 106 cells/ml in RPMI, 5% FBS, 10% HS, 1% HEPES, and 1% P/S (monocyte media) supplemented with 10 ng/ml MCSF-1. After 3 days in culture more than 80% of monocytes were CD14+CD16+, as demonstrated by flow cytometry.
THP-1 cell culture
The human monocytic THP-1 cells line was a gift from Dr. Evelyn Aranda Jaque (Albert Einstein College of Medicine, Bronx, New York, USA). Cells were cultured at a density of 2.5×105 cells/mL in RPMI supplemented with 10% FBS, 1% HEPES, 1% P/S and 2% BME at 37°C 5% CO2. Cells were subcultured with fresh media until 1×106 cells/mL density was reached.
Flow cytometry
To analyze surface expression of MOR and KOR on CD14+CD16+ monocytes in PBMC or from culture, 4×105 or 2×105 cells per tube respectively were stained with CD14-FITC (1.2 μg/reaction) and CD16-PECy7 (0.4 μg/reaction). Antibodies were previously tittered and optimized to obtain optimal signal. After staining, cells were washed, and then stained with either anti-MOR-1 (2 μg/reaction) or anti-KOR-1 (2 μg/reaction). After staining, cells were washed twice and stained with anti-Goat IgG-PE (1:10 dilution). Finally cells were washed twice and resuspended in PBS with 2% PFA until acquired and all staining was performed in a final volume of 50-100 μL of 1X PBS with 1% BSA on ice for 30 minutes. Irrelevant isotype matched antibodies were used as controls for nonspecific signals. Additionally, compensation controls were stained for each individual donor analyzed. For THP-1, 2×105 cells per condition were immediately stained for MOR, KOR, or CCR2-APC using the same conditions described above. Stained cells were acquired in a BD FACSCantoII flow cytometer, and data were analyzed using FlowJo software (v. 10.0.6, TreeStar, Ashland, OR, USA). Singlets were gated using a FSC-H/FSC-A plot. Monocytes and THP-1 cells were gated by their size using a SSC-A/FSC-A gate. Monocyte gating was confirmed by CD14 expression and populations were identified according to their expression of CD14 and CD16, when appropriate. Receptor expression on the surface of monocytes was represented as the geometric mean intensity for the respective receptor minus the geometric mean intensity of the irrelevant isotype matched antibody.
Monocyte adhesion assays
For BMVEC (Applied Cell Biology Research Institute, Kirkland, WA, USA) adhesion assays, cells were cultured 2 days prior to the assay on tissue culture treated, 96 well black, clear bottom plates (Corning) at a density of 25×103 to 50×103 cells per well in M199 supplemented with 0.05 M sodium bicarbonate, 0.03 μM HEPES, 0.8% L-glutamine, 1% P/S, 50 mg/mL ascorbic acid, 25 mg/mL heparin sodium salt, 3 mg/mL endothelial cell growth supplement, 9 mg/mL bovine brain extract, 5% HS, and 20% newborn calf serum. On the day of the assay, cells were washed once with 1X HBSS, and low serum M199 (10% new born calf serum and no HS) was added prior adding the treated cells. For recombinant human protein adhesion assays, 96 well black, clear bottom high binding plates (Corning) were coated with Fc-ICAM-1 (R&D) or Fc-VCAM-1 (R&D) at 10 μg/mL each in a 50 μL volume per well for 2h at 37°C, 5% CO2. After coating, wells were washed twice with warm 1X PBS and blocked with 5% non-fat milk in PBS for 1h at 37°C, 5% CO2. After blocking the plate was washed twice with PBS. Mature monocytes were labeled with 5 μM of calcein-AM, for 30 minutes at 37°C at a density of 1×106 cells per mL in RPMI with 10% FBS. After labeling, monocytes were washed once with PBS to remove free calcein-AM. Monocytes were then counted and resuspended in low serum M199 media for BMVEC assays or HBSS with 1mM CaCl2, 1mM MgCl2, and 2% FBS for recombinant protein assay at a density of 10×103 cells per 100 μL. Monocytes were treated with CCL2 (200 ng/mL), CCL2 plus buprenorphine (40 nM), or left untreated. After treatment, monocytes were immediately added to the confluent monolayer of BMVEC, for 5 minutes at 37°C, or to Fc-ICAM-1 and Fc-VCAM-1 coated wells for 10 minutes at 37°C. After incubation, wells were washed twice with HBSS and fluorescence in each wells was measured at the bottom of the well on a Spectra Max 5e fluorimeter (Molecular Devices, Sunnyvale, CA, USA), at 37°C using 485 nm excitation and 538 nm emission with a 530 nm cut off. Data were represented as fold change adhesion over media treated cells (base line adhesion).
Chemotaxis
Chemotaxis was assayed using a 48-well microchemotaxis chamber (Neuro Probe, Gaithersburg, MD, USA). Media (base line migration) or CCL2 (200 ng/mL) was added to the wells of the bottom portion of the chamber. Mature monocytes were treated with buprenorphine 40 (nM) or left untreated and added immediately to the wells of the top portion of the chamber. After 1h at 37°C, chemotaxis was assayed by staining the migrated cells adhered to the underside of a filter that separates the top and bottom of the chamber using Diff-Quick™ Stain Set (Siemens Healthcare Diagnostics, Malvern, PA, USA). Migration was quantified by densitometry with UN-SCAN-IT (Silk Scientific, Orem, UT, USA) software. Data were expressed as fold change over baseline migration.
FROUNT immunoprecipitation from THP-1 cells
FROUNT immunoprecipitation (IP) was based on a previously published protocol [20]. Briefly, 5×106 cells per condition were left untreated, treated with CCL2 (200 ng/mL), or CCL2 plus buprenorphine (40 nM), for 30 seconds at 37°C. After treatment, cells were incubated on ice in cold 1X PBS for 30 seconds and spun immediately. Cell pellets were resuspended with 200 μL of digitonin lysis buffer (20 mM TRIS pH: 8, 300 mM NaCl, 2 mM EDTA, 20% Glycerol, and 1% digitonin). Lysates were sonicated, and debris removed by centrifugation. Supernatants were stored at -80°C until use. IP was performed using 500 μg of protein per condition, as measured by a Bradford protein assay. Lysates were incubated with protein A/G agarose beads and an irrelevant isotype matched antibody to preclear any nonspecific interactions. Beads were removed by centrifugation and FROUNT antibody added (10-15 μg/per condition), and incubated at 4°C overnight with agitation. Protein A-agarose beads were added and incubated overnight at 4°C. Beads were washed three times and resuspended in 1X Laemmli loading buffer (Bio-Rad). As a control, an IP with an isotype matched irrelevant antibody was included. IP proteins were resolved by SDS-PAGE electrophoresis and transferred to nitrocellulose membranes. Immunoblot assays were performed to detect CCR2 using anti-CCR2 antibody (1:1000 dilution), and LI-COR secondary antibodies (1:40.000 dilution, 680RD). For normalization the membranes were stripped and probed for FROUNT using the same antibody for IP (1:4000 dilution) and detected with LI-COR secondary antibody (1:40.000, 800CW). Fluorescence signal was detected at 700 nm (CCR2) and 800 nm (FROUNT) for two minutes using Odyssey Fc imaging system (LI-COR, model 2800) and data analyzed with Image Studio lite (LI-COR, version 5.2). Data were expressed as fold change over base line IP. CCR2 signal was normalized to the signal of FROUNT for each condition.
Statistical analysis
Statistical analyses were performed using Prism 7.0c (GraphPad Software, Inc., San Diego, CA, USA). A Wilcoxon test was used for paired non-parametric measures, and student two tailed paired t test for parametric measures. p<0.05 was considered to be statistically significant.
Results
Opioid receptor expression is higher on CD14+CD16+ monocytes than on CD14+CD16− monocytes
We examined surface expression of MOR and KOR on CD14+CD16− and mature CD14+CD16+ monocytes in PBMC from many uninfected human donors by flow cytometry. We found that CD14+CD16+ monocytes express significantly more MOR (MFI: 686.8 ± 367.7; p<0.005; n= 15; Fig. 1A), as well as KOR (MFI: 378 ± 243.7; p<0.0001; n=15; Fig. 1B), on their surface as compared to CD14+CD16− monocytes (MOR MFI: 517.4 ± 299.8; n=15; Fig. 1A and KOR MFI: 147.5 ± 74.7; n=15; Fig. 1B). These results indicate that both populations of monocytes express surface receptors that bind buprenorphine, and that buprenorphine may therefore mediate functions of these cells, especially of CD14+CD16+ monocytes.
Figure 1.
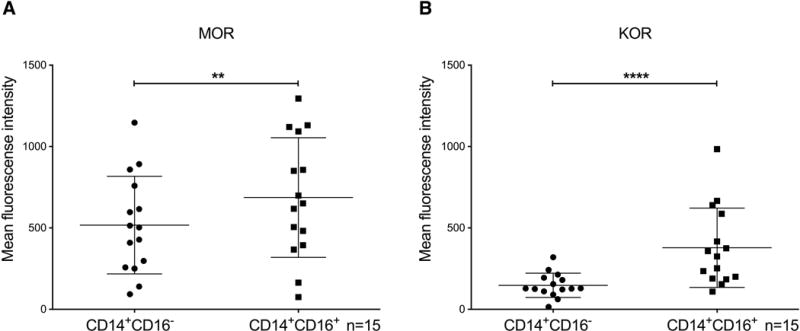
CD14+CD16+ monocytes have higher expression of MOR and KOR as compared to CD14+CD16− monocytes in PBMC from uninfected people. PBMC were isolated from peripheral blood by Ficoll gradient centrifugation. Cells were analyzed by flow cytometry for surface CD14, CD16, MOR, and KOR. Expression of (A) MOR and (B) KOR on CD14+CD16− and CD14+CD16+ monocytes from uninfected individuals. Data points represent the geometric mean fluorescence intensity that was obtained after subtracting the MFI of an isotype matched irrelevant antibody. Data are represented as the mean ± SD. n = 15 independent donors. **p<0.005, ****p<0.0001.
Opioid receptor expression on MCSF-1 matured CD14+CD16+ monocytes
In healthy individuals, most peripheral blood monocytes are CD14+CD16−, and only a small fraction (2-10%) are CD14+CD16+ [47]. To obtain sufficient numbers of mature CD14+CD16+ monocytes to examine the effects of buprenorphine on CCL2-mediated migration, we used a monocyte maturation system that we previously developed [46]. Freshly isolated human CD14+ monocytes were cultured non-adherently for 3 days with MCSF-1, a growth factor that stimulates monocyte maturation [48]. After 3 days of culture, on average more than 70% of the monocytes are CD14+CD16+ [29]. We analyzed surface MOR and KOR on these mature monocytes by flow cytometry and found that they express both opioid receptors (MOR MFI: 3000.7 ± 1148; n=15; Fig. 2A and KOR MFI: 617.6 ± 293.1; n=15; Fig. 2B), indicating that these cells can be used in our studies to examine the effects of buprenorphine.
Figure 2.
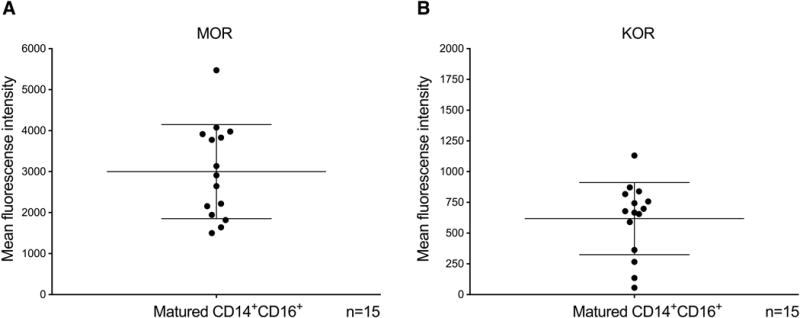
Mature monocytes express surface MOR and KOR. CD14+ monocytes were isolated from PBMC using magnetic beads and cultured non-adherently for three days with MCSF-1. After culture, more than 70% of the monocytes were CD14+CD16+. Surface MOR and KOR on mature monocytes was quantified by flow cytometry. Mature monocytes express high amounts of (A) MOR and (B) KOR. Data are expressed as the geometric mean fluorescent intensity ± SD, normalized by subtracting the MFI of an isotype matched irrelevant antibody. n = 15 independent donors.
Buprenorphine decreases CCL2-mediated adhesion of mature monocyte to BMVEC
We studied the effect of buprenorphine on CCL2-mediated mature monocyte adhesion to BMVEC using an in vitro binding assay. Briefly, we cultured BMVEC in 96 well plates until confluent. Calcein-AM labeled mature monocytes were added to the monolayers with media alone (untreated), CCL2, or CCL2 plus buprenorphine (C+B). After 5 minutes of incubation, the wells were washed and fluorescence measured. CCL2 significantly induced the adhesion of mature monocytes to BMVEC compared to baseline adhesion (untreated cells) (CCL2: 1.56 ± 0.35 fold; p<0.0001; n=15; Fig. 3), and co-treatment with buprenorphine significantly reduced this binding (C+B: 1.30 ± 0.42 fold; p<0.005; n=15; Fig. 3). This indicates that buprenorphine may decrease an early step of monocyte transmigration across the BBB [49].
Figure 3.
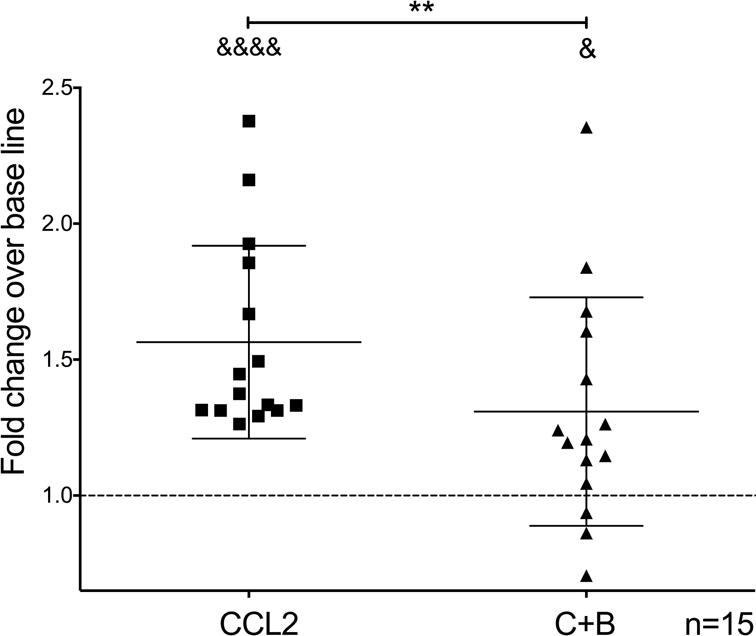
Buprenorphine decreases CCL2-mediated adhesion of mature monocytes to BMVEC. Mature monocytes labeled with calcein-AM were untreated or treated with CCL2 (200 ng/mL) or CCL2 plus buprenorphine (40 nM) (C+B), and added immediately to confluent monolayers of BMVEC. After 5 minutes of incubation at 37°C, monocytes were washed and fluorescence was measured using a fluorimeter. Data are shown as the fold change in adhesion of monocytes with respect to baseline adhesion (untreated cells), which was set to one and depicted as a dotted line. Data are represented as the mean ± SD. n = 15 independent donors. &&&&p<0.0001 and &p<0.05 compared to base line, **p<0.005, C+B compared to CCL2.
Buprenorphine decreases CCL2-mediated adhesion of mature monocytes to ICAM-1 but not to VCAM-1
BMVEC express surface adhesion molecules ICAM-1 and VCAM-1 that enable firm monocyte binding [50, 51]. We examined the effects of buprenorphine on CCL2-mediated mature monocyte adhesion to ICAM-1 and VCAM-1 using a similar in vitro adhesion assay as the one described above, but with Fc-ICAM-1 and Fc-VCAM-1 coated wells. We found that CCL2 increases mature monocyte binding to ICAM-1 (CCL2: 2.12 ± 0.68 fold; p<0.005; n=11; Fig. 4A) and VCAM-1 over base line (CCL2: 1.62 ± 0.24 fold; p<0.005; n=11; Fig. 4B). Concomitant treatment with buprenorphine decreases the binding of mature monocytes to ICAM-1 (C+B: 1.69 ± 0.48 fold; p<0.005; n=11; Fig. 4A), but not to VCAM-1 (C+B: 1.56 ± 0.21 fold; p=0.81; n=11; Fig. 4B). Thus, buprenorphine decrease CCL2-mediated matured monocyte adhesion to BMVEC by a mechanism that specifically involves ICAM-1 (Fig. 4).
Figure 4.
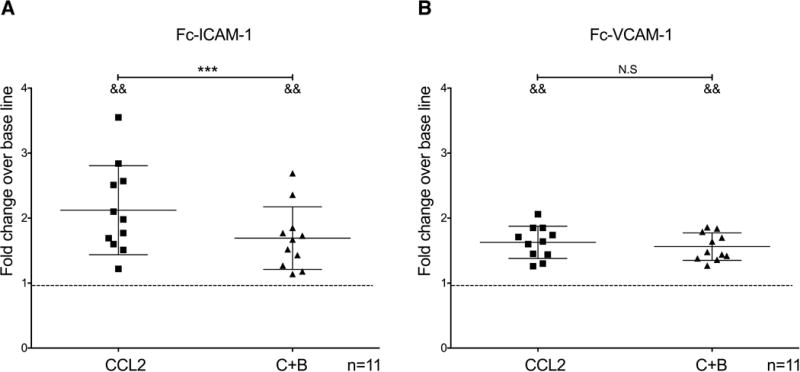
Buprenorphine decreases CCL2-mediated binding of mature monocytes to ICAM-1 but not to VCAM-1. Calcein-AM labeled mature monocytes were untreated or treated with CCL2 (200 ng/mL) or with CCL2 plus buprenorphine (40 nM) (C+B), and added immediately to wells of a 96 well plate coated with Fc-ICAM-1 (10 μg/mL) or Fc-VCAM-1 (10 μg/mL). After 10 minutes of incubation at 37°C, wells were washed and fluorescence measured using a fluorimeter. (A) Fc-ICAM-1 and (B) Fc-VCAM-1 adhesion assays. Data are expressed as mean fold change over base line adhesion (untreated cells), which was set to one and depicted as a dotted line, ± SD. n = 11 independent donors. &&p<0.005 compared to base line, **p<0.005, C+B compared to CCL2, N.S no statistical significance.
Buprenorphine decreases CCL2-mediated mature monocyte chemotaxis
We characterized the effects of buprenorphine on CCL2-mediated mature monocyte chemotaxis, another step in the transmigration process [52]. We used a modified Boyden chamber in which monocytes were added to the top of the chamber, with (Bup) or without (Untx) buprenorphine, and chemotaxis to CCL2 or to media as a control for baseline movement, located in the bottom of the chamber, was assayed. After incubation, a filter separating the top and bottom chambers that retains migrated cells on its underside was stained, and the migrated cells were quantified by densitometry. We found that CCL2 increases chemotaxis to CCL2 as compared to media (Top: Untx - Bottom: CCL2; 1.36 ± 0.12 fold; p<0.05; n=7; Fig. 5), and that buprenorphine decreases this CCL2-mediated monocyte chemotaxis (Top: Bup – Bottom: CCL2; 1.18 ± 0.14 fold; p<0.05; n=7; Fig. 5). Taken together with the adhesion assay data, the results indicate that buprenorphine decreases the ability of monocytes to migrate in response to CCL2 at two different steps in this process.
Figure 5.
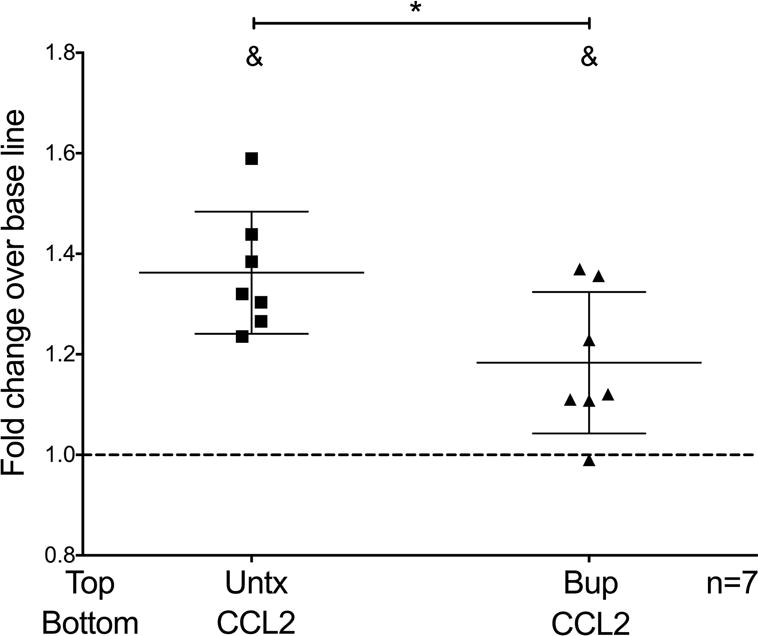
Buprenorphine decreases CCL2-mediated chemotaxis of mature monocytes. Monocytes were untreated (Untx) or treated with buprenorphine (40 nM) (Bup) and immediately added to the top wells of a modified Boyden chamber. Chemotaxis was assayed to media or CCL2 (200 ng/mL) located at the bottom of the chamber. After 1h incubation at 37°C, cell migration was quantified by staining the cells that attached to the underside of the filter separating the top and bottom of the chamber. Data are expressed as mean fold change over base line chemotaxis, which was set to one as depicted by the dotted line, ± SD. n = 7 independent donors. &p<0.05 compared to baseline, *p<0.05 Top: Bup - Bottom: CCL2 compared to Top: untx - Bottom: CCL2.
Buprenorphine decreases CCL2-mediated FROUNT association with CCR2
FROUNT is a protein that binds to the proximal C-terminal domain of CCR2 within 30 seconds after CCL2 treatment and activates signaling pathways that lead to monocyte chemotaxis [20]. We examined the effects of buprenorphine on CCL2-mediated FROUNT association to CCR2 in THP1-cells to characterize a possible mechanism by which buprenorphine decreases CCL2-mediated monocyte chemotaxis. We chose THP-1 monocytic cells to minimize the variability among experiments inherent in using primary human monocytes from different donors. Additionally, we showed that THP-1 cells express similar levels of MOR, KOR, and CCR2 as do mature monocytes from our culture system (Fig. 6A). THP-1 cells were treated with media alone, CCL2, or CCL2 plus buprenorphine for 30 seconds. After treatment cells were lysed, FROUNT was immunoprecipitated, and CCR2 association with FROUNT was analyzed by Western blot. CCR2 signal was normalized by the amount of immunprecipitated FROUNT in each condition (compare bands in the rectangular black box in lanes Untx, C, and C+B on the WB: Anti-FROUNT; Fig. 6B right panel). We found that with CCL2 treatment, FROUNT association with CCR2 is increased, as shown by an increase in CCR2 in the immunoprecipitated fraction over base line (compare bands in the rectangular box in the lanes Untx compared to C on the WB: Anti-CCR2; Fig. 6B left panel) (CCL2: 1.64 ± 0.52 fold; p<0.05; n=6; Fig. 6B right panel). Concomitant treatment with buprenorphine significantly decreased this association (compare bands in the rectangular box in the lanes C compared to C+B on the WB: Anti-CCR2; Fig. 6B left panel) (C+B: 1.06 ± 0.1784 fold; p<0.05; n=6; Fig. 6B right panel). Thus, buprenorphine decreases monocyte chemotaxis in part, by decreasing FROUNT association to CCR2, an early signaling event of CCR2 after binding of CCL2 [20].
Figure 6.
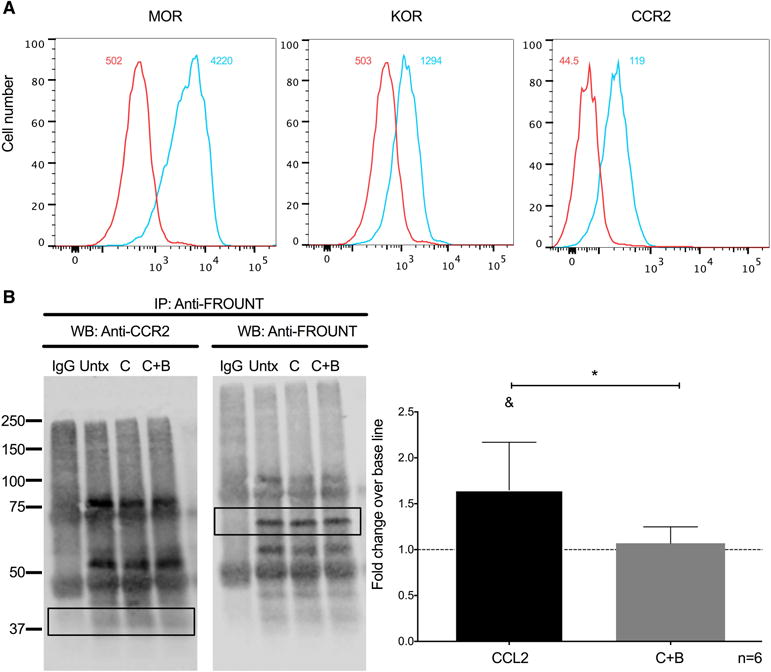
Buprenorphine decreases CCL2-mediated association of FROUNT with CCR2 on THP-1 cells. (A) Expression of MOR, KOR, and CCL2 on THP-1 cells analyzed by flow cytometry. The number depicted in the upper right of each panel is the mean fluorescence intensity for that receptor, and the value in the upper left is the mean fluorescence intensity for the irrelevant isotype matched antibody. (B) THP-1 cells were treated with CCL2 (200 ng/mL) (C), CCL2 plus buprenorphine (40 nM) (C+B), or left untreated (Untx). After 30 seconds of treatment, cells were washed and lysed using digitonin lysis buffer. Immunoprecipitation of FROUNT was performed with 10-15 μg of antibody. Immunoprecipitated proteins were resolved with SDS-PAGE, and analyzed by Western blotting for CCR2 (B; WB: Anti-CCR2; Left panel). As a normalization control, the same membrane was stripped and probed for immunoprecipitated FROUNT (B; WB: Anti-FROUNT; Left panel). Densitrometry was performed on the bands enclosed by the rectangular box in each image. The CCR2 signal was normalized to the FROUNT signal for each condition and then compared to base line. (B, right panel) Results are expressed as mean fold change over base line association of FROUNT to CCR2 (Untx), which was set to one and depicted as a dotted line, ± SD. n = 6 independent experiments. &p<0.05 compared to baseline, *p<0.05 C+B compared to CCL2.
Discussion
NeuroAIDS persists despite successful ART [5, 53]. As people live longer, viral reservoirs within the CNS, and chronic low-level neuroinflammation promote the development of HAND in approximately 50% of infected people [4]. Currently there are no therapies for HAND. A key mediator of chronic CNS inflammation and damage is the mature subpopulation of monocytes that expresses CD14+CD16+ [8, 23, 54]. These cells preferentially transmigrate across the BBB to CCL2, a chemokine increased in the CNS of HIV infected individuals, even on successful ART [11, 12, 46]. Targeting CCL2-mediated mature monocyte migration into the CNS could provide a therapeutic strategy to reduce and even ultimately eradicate HAND.
Intravenous drug abuse, in particular heroin, is a major risk factor for HIV infection [55]. Additionally, in the last decade prescription opioid abuse has become a highly significant public health problem contributing to both opioid abuse and HIV epidemics in the US [56–58]. Opioids have been shown in some studies to augment HIV neuropathogensis [37–39] and their effects are still present in the current ART era [36]. One mechanism by which opioids may increase HIV neuropathogenesis is by modulating the functions of immune cells [40–42]. This is supported by the fact that these cells, including monocytes, express opioid receptors [59–63]. We showed that mature monocytes express surface opioid receptors, making them targets for these drugs (Fig. 1 and Fig. 2). Opioid substitution therapy is used to treat opioid dependency [43]. Buprenorphine, an opioid derivate, is one such therapeutic, and is a partial agonist of MOR and full antagonist of KOR [44, 45]. The effects of buprenorphine on CCL2-mediated mature monocyte migration, important for development of neuroinflammation and subsequent HAND, were not characterized.
We demonstrated that buprenorphine decreases CCL2-mediated mature monocyte adhesion to BMVEC, a key step in monocyte transmigration across the BBB (Fig. 3). CCL2-mediated monocyte adhesion is a highly regulated process that depends on the activation of integrins on their surface and their binding to adhesion molecules expressed on the surface of endothelial cells [64, 65]. LFA-1 and VLA-4 are members of the β2 and β1 family respectively and are expressed by mature monocytes [66]. LFA-1 binds to ICAM-1 and VLA-4 binds to VCAM-1 on the surface of endothelial cells [14, 67]. ICAM-1 is constitutively expressed at low levels on the surface of BMVEC [68, 69]. ICAM-1 and VCAM-1 are both upregulated by inflammatory signals [68, 70].
Activation of integrins on the surface of cells is a complex process that requires multiple steps [71]. Integrins are dimers composed by one β and one α chain that localize on the surface of cells in a closed conformation [71]. Chemokines activate signaling pathways through their receptors that promote conformational changes of the integrin dimer leading to an open conformation that is able to bind to its ligand [72]. This early initial activation can be monitored by the exposure of epitopes that were previously masked in the closed conformation [73]. We showed by flow cytometry that CCL2 is able to induce exposure of the m24 activation epitope of LFA-1 α chain on the surface of mature monocytes in the absence of ICAM-1 (data not shown), albeit at low levels [73]. This result is consistent with the observation that full activation of integrins requires binding to their immobilized ligands, that mediates additional mechanisms that promote their full activation [74].
We studied the effects of buprenorphine on CCL2-mediated mature monocyte adhesion in the presence of recombinant human ICAM-1 and VCAM-1 (Fig. 4). This enabled us to isolate the effects of buprenorphine on monocytes because no endothelial cells were present, as well as to study the effects of buprenorphine on mature monocyte adhesion with full integrin conformation changes that enable binding to ICAM-1 and VCAM-1. We found that buprenorphine decreases CCL2- mediated mature monocyte adhesion to ICAM-1, but not to VCAM-1 (Fig. 4). Previously it was shown that a specific isoform of the catalytic subunit of the PI(3)K, P110δ was necessary for CCL2-mediated monocyte binding to ICAM-1 but not VCAM-1 [75]. We propose that buprenorphine may be inhibiting CCL2 activation of P110δ, in mature monocytes, thus decreasing CCL2-mediated adhesion specifically to ICAM-1. Is not known at which step of the integrin activation pathway buprenorphine is acting, early after the initial activation of the integrin dimer, or later when the pre-activated integrin binds to its target. We are currently pursuing these studies.
We showed that buprenorphine decreased mature monocyte chemotaxis to CCL2, thus limiting an additional step in the transmigration process (Fig. 5). Blocking of monocyte entry into the CNS could reduce the ongoing low-level inflammation that leads to neuronal injury. It was shown in a model of SIV infection that treatment with Natalizumab decreased neuronal injury and SIV infection of the CNS when given at the time or late after infection [10]. Natalizumab is an antibody that binds the α4 chain of the α4β1 and the α4β7 integrins expressed by leukocytes and decreases their migration into the CNS [76–78]. Natalizumab treatment was found to reduce the migration of leukocytes, including monocyte/macrophages, into the CNS and this decrease was associated with improved CNS disease [10]. However, excessive blocking of leukocyte trafficking into the CNS may lead to improper immune responses [79]. One example is reactivation of JC virus in Natalizumab treated MS patients [80]. Treatment with this antibody led to reactivation of the virus, which causes progressive multifocal leukoencephalopathy [80, 81]. It is important to note that buprenorphine decreased CCL2-mediated adhesion and chemotaxis of mature monocytes, but not to base line, suggesting that some mature monocytes still will be able to transmigrate in response to this chemokine and patrol the CNS to maintain proper immune surveillance. The effects of buprenorphine on migration of other leukocytes, including T cells, are not known and will be addressed in future studies.
CCL2’s binding to CCR2 activates signaling molecules that trigger monocyte adhesion and chemotaxis [82–84]. FROUNT is an adaptor protein that binds to the proximal C-terminal domain of CCR2 within 30 seconds of treatment with CCL2, and promotes the activation of PI(3)K and Rac, that leads to monocyte migration [20]. As a potential mechanism by which buprenorphine may regulate activation of the CCL2/CCR2 signaling pathway we studied the effects of buprenorphine on FROUNT association to CCR2 [20]. We showed that buprenorphine decreased this association, demonstrating that buprenorphine is able to inhibit early signaling of CCL2/CCR2 important for monocyte migration (Fig. 6). This result complements our previous findings in which we showed that buprenorphine decreased CCL2-mediated phosphorylation of signaling molecules and delayed CCR2 recycling to the surface in the total population of human monocytes [85]. Thus, we propose that buprenorphine is inhibiting several steps of the CCL2/CCR2 signaling pathway in monocytes, including early events.
CCL2-mediated monocyte migration is a key component of the inflammatory response and is involved in other CNS diseases such as multiple sclerosis (MS) [86–88]. It was shown that mature monocytes are increased in the peripheral blood of people with MS and that they display a more inflammatory phenotype compared to healthy individuals, including increased surface CCR2 [89]. Additionally, mature monocytes were shown to be present in the perivascular space of active MS lesions [90]. Mature monocytes and macrophages that derive from these infiltrated cells may contribute to these lesions by producing cytokines that mediate demyelination, and by facilitating the migration of other immune cells, including T cells [90, 91]. Mature monocyte infiltration plays a role as well in peripheral inflammatory diseases such as atherosclerosis, rheumatoid arthritis, and Crohn’s disease [92, 93]. Decreasing CCL2-mediated mature monocyte infiltration by buprenorphine in the context of these inflammatory central nervous system and peripheral pathologies may also contribute to reduced chronic inflammation, suggesting a broader use of this therapeutic.
We showed that buprenorphine decreases several steps of CCL2-mediated mature monocyte migration. Our results suggest that, despite being an opioid, buprenorphine may be beneficial, given that it may contribute to decrease mature monocyte migration, chronic neuroinflammation and neuronal damage, and thus reduce or even eventually eradicate HAND. We propose that buprenorphine could also be used in HIV infected non-opioid abusers [44, 94]. Additionally, understanding the effects of buprenorphine on CCL2/CCR2 signaling in monocytes could provide new understanding of how activation of opioid receptors impacts these cells, and further broaden its use to treat other chronic inflammatory diseases.
Summary sentence.
Buprenorphine may decrease neuroinflammation, important in the context of HIV associated neurocognitive disorders, by limiting CCL2-mediated CD14+CD16+ monocytes migration.
Acknowledgments
This work was supported by National Institutes of Health grant T32 AI007501 (to MJB) - ‘Training in HIV/AIDS Pathogenesis; Basic and Translational Research’, and by National Institutes of Health grant 5R01DA041931-02 (MJB, LL, and JWB).
Abbreviations
- ART
Antiretroviral therapy
- BBB
Blood brain barrier
- BME
2-mercaptoethanol
- BMVEC
Brain microvascular endothelial cells
- CNS
Central nervous system
- CSF
Cerebrospinal fluid
- Fc
Fragment crystallizable region
- FSC-A
Forward scatter area
- HAND
HIV associated neurocognitive disorders
- HBSS
Hank’s balanced salt solution
- HEPES
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
- HS
Human serum-type AB
- ICAM-1
Intracellular adhesion molecule-1
- IP
Immunoprecipitation
- JC
John Cunningham virus
- KOR
κ-opioid receptor
- LFA-1
Lymphocyte function-associated antigen-1
- MCSF-1
Macrophage colony stimulating factor-1
- MOR
μ-opioid receptor
- MS
Multiple sclerosis
- PBMC
Peripheral blood mononuclear cells
- PFA
Paraformaldehyde
- PI(3)K
Phosphatidyl-inositol-3-kinase
- P/S
Penicillin-streptomycin
- RPMI
Roswell Park Memorial Institute medium
- SDS-PAGE
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SIV
Simian immunodeficiency virus
- SSC-A
Side scatter area
- VCAM-1
Vascular cell adhesion molecule-1
- VLA-4
Very late antigen-4
- WB
Western blot
Footnotes
Authorship
MJB performed the majority of the experiments, as well as contributed to the experimental design and manuscript writing. LL performed the immunoprecipitation experiments. JWB contributed to the study’s conception, design, data analysis and interpretation, and manuscript writing.
Conflict of interest disclosure
The authors declare no conflict of interest.
References
- 1.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, Suwanwela NC, Jagodzinski L, Michael N, Spudich S, van Griensven F, de Souza M, Kim J, Ananworanich J, Group, R.S.S Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis. 2012;206:275–82. doi: 10.1093/infdis/jis326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis LE, Hjelle BL, Miller VE, Palmer DL, Llewellyn AL, Merlin TL, Young SA, Mills RG, Wachsman W, Wiley CA. Early viral brain invasion in iatrogenic human immunodeficiency virus infection. Neurology. 1992;42:1736–9. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 3.Witwer KW, Gama L, Li M, Bartizal CM, Queen SE, Varrone JJ, Brice AK, Graham DR, Tarwater PM, Mankowski JL, Zink MC, Clements JE. Coordinated regulation of SIV replication and immune responses in the CNS. PLoS One. 2009;4:e8129. doi: 10.1371/journal.pone.0008129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saylor D, Dickens AM, Sacktor N, Haughey N, Slusher B, Pletnikov M, Mankowski JL, Brown A, Volsky DJ, McArthur JC. HIV-associated neurocognitive disorder - pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12:309. doi: 10.1038/nrneurol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Mindt MR, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, Group, C., Group, H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96. doi: 10.2174/1570162x12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eugenin EA, Osiecki K, Lopez L, Goldstein H, Calderon TM, Berman JW. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: a potential mechanism of HIV-CNS invasion and NeuroAIDS. J Neurosci. 2006;26:1098–106. doi: 10.1523/JNEUROSCI.3863-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer-Smith T, Croul S, Sverstiuk AE, Capini C, L’Heureux D, Regulier EG, Richardson MW, Amini S, Morgello S, Khalili K, Rappaport J. CNS invasion by CD14+/CD16+ peripheral blood-derived monocytes in HIV dementia: perivascular accumulation and reservoir of HIV infection. J Neurovirol. 2001;7:528–41. doi: 10.1080/135502801753248114. [DOI] [PubMed] [Google Scholar]
- 9.Williams DW, Calderon TM, Lopez L, Carvallo-Torres L, Gaskill PJ, Eugenin EA, Morgello S, Berman JW. Mechanisms of HIV entry into the CNS: increased sensitivity of HIV infected CD14+CD16+ monocytes to CCL2 and key roles of CCR2, JAM-A, and ALCAM in diapedesis. PLoS One. 2013;8:e69270. doi: 10.1371/journal.pone.0069270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell JH, Ratai EM, Autissier P, Nolan DJ, Tse S, Miller AD, Gonzalez RG, Salemi M, Burdo TH, Williams KC. Anti-alpha4 antibody treatment blocks virus traffic to the brain and gut early, and stabilizes CNS injury late in infection. PLoS Pathog. 2014;10:e1004533. doi: 10.1371/journal.ppat.1004533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–43. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan L, Qiao L, Wei F, Yin J, Liu L, Ji Y, Smith D, Li N, Chen D. Cytokines in CSF correlate with HIV-associated neurocognitive disorders in the post-HAART era in China. J Neurovirol. 2013;19:144–9. doi: 10.1007/s13365-013-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamei M, Carman CV. New observations on the trafficking and diapedesis of monocytes. Curr Opin Hematol. 2010;17:43–52. doi: 10.1097/MOH.0b013e3283333949. [DOI] [PubMed] [Google Scholar]
- 14.Imhof BA, Aurrand-Lions M. Adhesion mechanisms regulating the migration of monocytes. Nat Rev Immunol. 2004;4:432–44. doi: 10.1038/nri1375. [DOI] [PubMed] [Google Scholar]
- 15.Weber KS, Klickstein LB, Weber C. Specific activation of leukocyte beta2 integrins lymphocyte function-associated antigen-1 and Mac-1 by chemokines mediated by distinct pathways via the alpha subunit cytoplasmic domains. Mol Biol Cell. 1999;10:861–73. doi: 10.1091/mbc.10.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weber C, Alon R, Moser B, Springer TA. Sequential regulation of alpha 4 beta 1 and alpha 5 beta 1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–73. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge S, Song L, Serwanski DR, Kuziel WA, Pachter JS. Transcellular transport of CCL2 across brain microvascular endothelial cells. J Neurochem. 2008;104:1219–32. doi: 10.1111/j.1471-4159.2007.05056.x. [DOI] [PubMed] [Google Scholar]
- 18.Seguin R, Biernacki K, Rotondo RL, Prat A, Antel JP. Regulation and functional effects of monocyte migration across human brain-derived endothelial cells. J Neuropathol Exp Neurol. 2003;62:412–9. doi: 10.1093/jnen/62.4.412. [DOI] [PubMed] [Google Scholar]
- 19.Nottet HS, Persidsky Y, Sasseville VG, Nukuna AN, Bock P, Zhai QH, Sharer LR, McComb RD, Swindells S, Soderland C, Gendelman HE. Mechanisms for the transendothelial migration of HIV-1-infected monocytes into brain. J Immunol. 1996;156:1284–95. [PubMed] [Google Scholar]
- 20.Terashima Y, Onai N, Murai M, Enomoto M, Poonpiriya V, Hamada T, Motomura K, Suwa M, Ezaki T, Haga T, Kanegasaki S, Matsushima K. Pivotal function for cytoplasmic protein FROUNT in CCR2-mediated monocyte chemotaxis. Nat Immunol. 2005;6:827–35. doi: 10.1038/ni1222. [DOI] [PubMed] [Google Scholar]
- 21.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, Leenen PJ, Liu YJ, MacPherson G, Randolph GJ, Scherberich J, Schmitz J, Shortman K, Sozzani S, Strobl H, Zembala M, Austyn JM, Lutz MB. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 22.Williams DW, Byrd D, Rubin LH, Anastos K, Morgello S, Berman JW. CCR2 on CD14(+)CD16(+) monocytes is a biomarker of HIV-associated neurocognitive disorders. Neurol Neuroimmunol Neuroinflamm. 2014;1:e36. doi: 10.1212/NXI.0000000000000036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DW, Anastos K, Morgello S, Berman JW. JAM-A and ALCAM are therapeutic targets to inhibit diapedesis across the BBB of CD14+CD16+ monocytes in HIV-infected individuals. J Leukoc Biol. 2015;97:401–12. doi: 10.1189/jlb.5A0714-347R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulliam L, Gascon R, Stubblebine M, McGuire D, McGrath MS. Unique monocyte subset in patients with AIDS dementia. Lancet. 1997;349:692–5. doi: 10.1016/S0140-6736(96)10178-1. [DOI] [PubMed] [Google Scholar]
- 25.Kusao I, Shiramizu B, Liang CY, Grove J, Agsalda M, Troelstrup D, Velasco VN, Marshall A, Whitenack N, Shikuma C, Valcour V. Cognitive performance related to HIV-1-infected monocytes. J Neuropsychiatry Clin Neurosci. 2012;24:71–80. doi: 10.1176/appi.neuropsych.11050109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allen JB, Wong HL, Guyre PM, Simon GL, Wahl SM. Association of circulating receptor Fc gamma RIII-positive monocytes in AIDS patients with elevated levels of transforming growth factor-beta. J Clin Invest. 1991;87:1773–9. doi: 10.1172/JCI115196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thieblemont N, Weiss L, Sadeghi HM, Estcourt C, Haeffner-Cavaillon N. CD14lowCD16high: a cytokine-producing monocyte subset which expands during human immunodeficiency virus infection. Eur J Immunol. 1995;25:3418–24. doi: 10.1002/eji.1830251232. [DOI] [PubMed] [Google Scholar]
- 28.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, Lewin SR, Gorry PR, Jaworowski A, Greene WC, Sonza S, Crowe SM. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–9. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 29.Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS Viral Seeding by HIV(+) CD14(+) CD16(+) Monocytes: Establishment and Reseeding of Viral Reservoirs Contributing to HIV-Associated Neurocognitive Disorders. MBio. 2017;8:e01280–17. doi: 10.1128/mBio.01280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Escudero DJ, Lurie MN, Mayer KH, King M, Galea S, Friedman SR, Marshall BDL. The risk of HIV transmission at each step of the HIV care continuum among people who inject drugs: a modeling study. BMC Public Health. 2017;17:614. doi: 10.1186/s12889-017-4528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dyer TV, Khan MR, Sandoval M, Acheampong A, Regan R, Bolyard M, Mateu-Gelabert P, Friedman SR. Drug Use and Sexual HIV Transmission Risk Among Men Who have Sex with Men and Women (MSMW), Men Who have Sex with Men only (MSMO), and Men Who have Sex with Women Only (MSWO) and the Female Partners of MSMW and MSWO: A Network Perspective. AIDS Behav. 2017;21:3590–3598. doi: 10.1007/s10461-017-1736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz T, Chu SY, Byers RH, Jr, Hersh BS, Conti L, Rietmeijer CA, Mokotoff E, Fann SA, Boyd D, Iglesias L, et al. The types of drugs used by HIV-infected injection drug users in a multistate surveillance project: implications for intervention. Am J Public Health. 1994;84:1971–5. doi: 10.2105/ajph.84.12.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martins SS, Sarvet A, Santaella-Tenorio J, Saha T, Grant BF, Hasin DS. Changes in US Lifetime Heroin Use and Heroin Use Disorder: Prevalence From the 2001-2002 to 2012-2013 National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry. 2017;74:445–455. doi: 10.1001/jamapsychiatry.2017.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Compton WM, Jones CM, Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374:154–63. doi: 10.1056/NEJMra1508490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, Alexander GC. The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559–74. doi: 10.1146/annurev-publhealth-031914-122957. [DOI] [PubMed] [Google Scholar]
- 36.Smith DB, Simmonds P, Bell JE. Brain viral burden, neuroinflammation and neurodegeneration in HAART-treated HIV positive injecting drug users. J Neurovirol. 2014;20:28–38. doi: 10.1007/s13365-013-0225-3. [DOI] [PubMed] [Google Scholar]
- 37.Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–91. doi: 10.1007/s11481-006-9018-2. [DOI] [PubMed] [Google Scholar]
- 38.Bell JE, Arango JC, Robertson R, Brettle RP, Leen C, Simmonds P. HIV and drug misuse in the Edinburgh cohort. J Acquir Immune Defic Syndr. 2002;31(Suppl 2):S35–42. doi: 10.1097/00126334-200210012-00003. [DOI] [PubMed] [Google Scholar]
- 39.Bokhari SM, Hegde R, Callen S, Yao H, Adany I, Li Q, Li Z, Pinson D, Yeh HW, Cheney PD, Buch S. Morphine potentiates neuropathogenesis of SIV infection in rhesus macaques. J Neuroimmune Pharmacol. 2011;6:626–39. doi: 10.1007/s11481-011-9272-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jaureguiberry-Bravo M, Wilson R, Carvallo L, Berman JW. Opioids and Opioid Maintenance Therapies: Their Impact on Monocyte-Mediated HIV Neuropathogenesis. Curr HIV Res. 2016;14:417–430. doi: 10.2174/1570162x14666160324124132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dutta R, Roy S. Mechanism(s) involved in opioid drug abuse modulation of HAND. Curr HIV Res. 2012;10:469–77. doi: 10.2174/157016212802138805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Banerjee A, Strazza M, Wigdahl B, Pirrone V, Meucci O, Nonnemacher MR. Role of mu-opioids as cofactors in human immunodeficiency virus type 1 disease progression and neuropathogenesis. J Neurovirol. 2011;17:291–302. doi: 10.1007/s13365-011-0037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farrell M, Wodak A, Gowing L. Maintenance drugs to treat opioid dependence. BMJ. 2012;344:e2823. doi: 10.1136/bmj.e2823. [DOI] [PubMed] [Google Scholar]
- 44.Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE. Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–80. doi: 10.1038/clpt.1994.71. [DOI] [PubMed] [Google Scholar]
- 45.Leander JD. Buprenorphine has potent kappa opioid receptor antagonist activity. Neuropharmacology. 1987;26:1445–7. doi: 10.1016/0028-3908(87)90112-2. [DOI] [PubMed] [Google Scholar]
- 46.Buckner CM, Calderon TM, Willams DW, Belbin TJ, Berman JW. Characterization of monocyte maturation/differentiation that facilitates their transmigration across the blood-brain barrier and infection by HIV: implications for NeuroAIDS. Cell Immunol. 2011;267:109–23. doi: 10.1016/j.cellimm.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Zhang L, Yu C, Yang XF, Wang H. Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res. 2014;2:1. doi: 10.1186/2050-7771-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. 2014;6 doi: 10.1101/cshperspect.a021857. pii: a021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeshita Y, Ransohoff RM. Inflammatory cell trafficking across the blood-brain barrier: chemokine regulation and in vitro models. Immunol Rev. 2012;248:228–39. doi: 10.1111/j.1600-065X.2012.01127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong D, Dorovini-Zis K. Expression of vascular cell adhesion molecule-1 (VCAM-1) by human brain microvessel endothelial cells in primary culture. Microvasc Res. 1995;49:325–39. doi: 10.1006/mvre.1995.1028. [DOI] [PubMed] [Google Scholar]
- 51.Sobel RA, Mitchell ME, Fondren G. Intercellular adhesion molecule-1 (ICAM-1) in cellular immune reactions in the human central nervous system. Am J Pathol. 1990;136:1309–16. [PMC free article] [PubMed] [Google Scholar]
- 52.Gras G, Kaul M. Molecular mechanisms of neuroinvasion by monocytes-macrophages in HIV-1 infection. Retrovirology. 2010;7:30. doi: 10.1186/1742-4690-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 54.Ndhlovu LC, Umaki T, Chew GM, Chow DC, Agsalda M, Kallianpur KJ, Paul R, Zhang G, Ho E, Hanks N, Nakamoto B, Shiramizu BT, Shikuma CM. Treatment intensification with maraviroc (CCR5 antagonist) leads to declines in CD16-expressing monocytes in cART-suppressed chronic HIV-infected subjects and is associated with improvements in neurocognitive test performance: implications for HIV-associated neurocognitive disease (HAND) J Neurovirol. 2014;20:571–82. doi: 10.1007/s13365-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vlahov D, Robertson AM, Strathdee SA. Prevention of HIV infection among injection drug users in resource-limited settings. Clin Infect Dis. 2010;50(Suppl 3):S114–21. doi: 10.1086/651482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones CM. Trends and key correlates of prescription opioid injection misuse in the United States. Addict Behav. 2017;78:145–152. doi: 10.1016/j.addbeh.2017.10.018. [DOI] [PubMed] [Google Scholar]
- 57.Peters PJ, Pontones P, Hoover KW, Patel MR, Galang RR, Shields J, Blosser SJ, Spiller MW, Combs B, Switzer WM, Conrad C, Gentry J, Khudyakov Y, Waterhouse D, Owen SM, Chapman E, Roseberry JC, McCants V, Weidle PJ, Broz D, Samandari T, Mermin J, Walthall J, Brooks JT, Duwve JM, Indiana HIVOIT. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014-2015. N Engl J Med. 2016;375:229–39. doi: 10.1056/NEJMoa1515195. [DOI] [PubMed] [Google Scholar]
- 58.Campbell EM, Jia H, Shankar A, Hanson D, Luo W, Masciotra S, Owen SM, Oster AM, Galang RR, Spiller MW, Blosser SJ, Chapman E, Roseberry JC, Gentry J, Pontones P, Duwve J, Peyrani P, Kagan RM, Whitcomb JM, Peters PJ, Heneine W, Brooks JT, Switzer WM. Detailed Transmission Network Analysis of a Large Opiate-Driven Outbreak of HIV Infection in the United States. J Infect Dis. 2017;216:1053–1062. doi: 10.1093/infdis/jix307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chao CC, Hu S, Shark KB, Sheng WS, Gekker G, Peterson PK. Activation of mu opioid receptors inhibits microglial cell chemotaxis. J Pharmacol Exp Ther. 1997;281:998–1004. [PubMed] [Google Scholar]
- 60.Bidlack JM. Detection and function of opioid receptors on cells from the immune system. Clin Diagn Lab Immunol. 2000;7:719–23. doi: 10.1128/cdli.7.5.719-723.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chuang TK, Killam KF, Jr, Chuang LF, Kung HF, Sheng WS, Chao CC, Yu L, Chuang RY. Mu opioid receptor gene expression in immune cells. Biochem Biophys Res Commun. 1995;216:922–30. doi: 10.1006/bbrc.1995.2709. [DOI] [PubMed] [Google Scholar]
- 62.Lopker A, Abood LG, Hoss W, Lionetti FJ. Stereoselective muscarinic acetylcholine and opiate receptiors in human phagocytic leukocytes. Biochem Pharmacol. 1980;29:1361–5. doi: 10.1016/0006-2952(80)90431-1. [DOI] [PubMed] [Google Scholar]
- 63.Gaveriaux C, Peluso J, Simonin F, Laforet J, Kieffer B. Identification of kappa- and delta-opioid receptor transcripts in immune cells. FEBS Lett. 1995;369:272–6. doi: 10.1016/0014-5793(95)00766-3. [DOI] [PubMed] [Google Scholar]
- 64.Luscinskas FW, Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Rosenzweig A. C-C and C-X-C chemokines trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Ann N Y Acad Sci. 2000;902:288–93. doi: 10.1111/j.1749-6632.2000.tb06324.x. [DOI] [PubMed] [Google Scholar]
- 65.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398:718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 66.Kulkarni M, Bowman E, Gabriel J, Amburgy T, Mayne E, Zidar DA, Maierhofer C, Turner AN, Bazan JA, Koletar SL, Lederman MM, Sieg SF, Funderburg NT. Altered Monocyte and Endothelial Cell Adhesion Molecule Expression Is Linked to Vascular Inflammation in Human Immunodeficiency Virus Infection. Open Forum Infect Dis. 2016;3:ofw224. doi: 10.1093/ofid/ofw224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hyduk SJ, Cybulsky MI. Role of alpha4beta1 integrins in chemokine-induced monocyte arrest under conditions of shear stress. Microcirculation. 2009;16:17–30. doi: 10.1080/10739680802425195. [DOI] [PubMed] [Google Scholar]
- 68.Hess DC, Bhutwala T, Sheppard JC, Zhao W, Smith J. ICAM-1 expression on human brain microvascular endothelial cells. Neurosci Lett. 1994;168:201–4. doi: 10.1016/0304-3940(94)90450-2. [DOI] [PubMed] [Google Scholar]
- 69.Navratil E, Couvelard A, Rey A, Henin D, Scoazec JY. Expression of cell adhesion molecules by microvascular endothelial cells in the cortical and subcortical regions of the normal human brain: an immunohistochemical analysis. Neuropathol Appl Neurobiol. 1997;23:68–80. [PubMed] [Google Scholar]
- 70.Stins MF, Gilles F, Kim KS. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–90. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 71.Campbell ID, Humphries MJ. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a004994. pii: a004994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laudanna C, Kim JY, Constantin G, Butcher E. Rapid leukocyte integrin activation by chemokines. Immunol Rev. 2002;186:37–46. doi: 10.1034/j.1600-065x.2002.18604.x. [DOI] [PubMed] [Google Scholar]
- 73.Chan JR, Hyduk SJ, Cybulsky MI. Detecting rapid and transient upregulation of leukocyte integrin affinity induced by chemokines and chemoattractants. J Immunol Methods. 2003;273:43–52. doi: 10.1016/s0022-1759(02)00417-9. [DOI] [PubMed] [Google Scholar]
- 74.Schurpf T, Springer TA. Regulation of integrin affinity on cell surfaces. EMBO J. 2011;30:4712–27. doi: 10.1038/emboj.2011.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ferreira AM, Isaacs H, Hayflick JS, Rogers KA, Sandig M. The p110delta isoform of PI3K differentially regulates beta1 and beta2 integrin-mediated monocyte adhesion and spreading and modulates diapedesis. Microcirculation. 2006;13:439–56. doi: 10.1080/10739680600776062. [DOI] [PubMed] [Google Scholar]
- 76.Yu Y, Schurpf T, Springer TA. How natalizumab binds and antagonizes alpha4 integrins. J Biol Chem. 2013;288:32314–25. doi: 10.1074/jbc.M113.501668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stuve O, Marra CM, Jerome KR, Cook L, Cravens PD, Cepok S, Frohman EM, Phillips JT, Arendt G, Hemmer B, Monson NL, Racke MK. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol. 2006;59:743–7. doi: 10.1002/ana.20858. [DOI] [PubMed] [Google Scholar]
- 78.Mishra MK, Yong VW. Myeloid cells - targets of medication in multiple sclerosis. Nat Rev Neurol. 2016;12:539–51. doi: 10.1038/nrneurol.2016.110. [DOI] [PubMed] [Google Scholar]
- 79.Prinz M, Priller J. The role of peripheral immune cells in the CNS in steady state and disease. Nat Neurosci. 2017;20:136–144. doi: 10.1038/nn.4475. [DOI] [PubMed] [Google Scholar]
- 80.Warnke C, Menge T, Hartung HP, Racke MK, Cravens PD, Bennett JL, Frohman EM, Greenberg BM, Zamvil SS, Gold R, Hemmer B, Kieseier BC, Stuve O. Natalizumab and progressive multifocal leukoencephalopathy: what are the causal factors and can it be avoided? Arch Neurol. 2010;67:923–30. doi: 10.1001/archneurol.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chalkias S, Dang X, Bord E, Stein MC, Kinkel RP, Sloane JA, Donnelly M, Ionete C, Houtchens MK, Buckle GJ, Batson S, Koralnik IJ. JC virus reactivation during prolonged natalizumab monotherapy for multiple sclerosis. Ann Neurol. 2014;75:925–34. doi: 10.1002/ana.24148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ashida N, Arai H, Yamasaki M, Kita T. Distinct signaling pathways for MCP-1-dependent integrin activation and chemotaxis. J Biol Chem. 2001;276:16555–60. doi: 10.1074/jbc.M009068200. [DOI] [PubMed] [Google Scholar]
- 83.Maus U, Henning S, Wenschuh H, Mayer K, Seeger W, Lohmeyer J. Role of endothelial MCP-1 in monocyte adhesion to inflamed human endothelium under physiological flow. Am J Physiol Heart Circ Physiol. 2002;283:H2584–91. doi: 10.1152/ajpheart.00349.2002. [DOI] [PubMed] [Google Scholar]
- 84.Yen H, Zhang Y, Penfold S, Rollins BJ. MCP-1-mediated chemotaxis requires activation of non-overlapping signal transduction pathways. J Leukoc Biol. 1997;61:529–32. doi: 10.1002/jlb.61.4.529. [DOI] [PubMed] [Google Scholar]
- 85.Carvallo L, Lopez L, Che FY, Lim J, Eugenin EA, Williams DW, Nieves E, Calderon TM, Madrid-Aliste C, Fiser A, Weiss L, Angeletti RH, Berman JW. Buprenorphine decreases the CCL2-mediated chemotactic response of monocytes. J Immunol. 2015;194:3246–58. doi: 10.4049/jimmunol.1302647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mahad D, Callahan MK, Williams KA, Ubogu EE, Kivisakk P, Tucky B, Kidd G, Kingsbury GA, Chang A, Fox RJ, Mack M, Sniderman MB, Ravid R, Staugaitis SM, Stins MF, Ransohoff RM. Modulating CCR2 and CCL2 at the blood-brain barrier: relevance for multiple sclerosis pathogenesis. Brain. 2006;129:212–23. doi: 10.1093/brain/awh655. [DOI] [PubMed] [Google Scholar]
- 87.Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 88.Mahad DJ, Ransohoff RM. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 89.Chuluundorj D, Harding SA, Abernethy D, La Flamme AC. Expansion and preferential activation of the CD14(+)CD16(+) monocyte subset during multiple sclerosis. Immunol Cell Biol. 2014;92:509–17. doi: 10.1038/icb.2014.15. [DOI] [PubMed] [Google Scholar]
- 90.Waschbisch A, Schroder S, Schraudner D, Sammet L, Weksler B, Melms A, Pfeifenbring S, Stadelmann C, Schwab S, Linker RA. Pivotal Role for CD16+ Monocytes in Immune Surveillance of the Central Nervous System. J Immunol. 2016;196:1558–67. doi: 10.4049/jimmunol.1501960. [DOI] [PubMed] [Google Scholar]
- 91.Yamasaki R, Lu H, Butovsky O, Ohno N, Rietsch AM, Cialic R, Wu PM, Doykan CE, Lin J, Cotleur AC, Kidd G, Zorlu MM, Sun N, Hu W, Liu L, Lee JC, Taylor SE, Uehlein L, Dixon D, Gu J, Floruta CM, Zhu M, Charo IF, Weiner HL, Ransohoff RM. Differential roles of microglia and monocytes in the inflamed central nervous system. J Exp Med. 2014;211:1533–49. doi: 10.1084/jem.20132477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stansfield BK, Ingram DA. Clinical significance of monocyte heterogeneity. Clin Transl Med. 2015;4:5. doi: 10.1186/s40169-014-0040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mestas J, Ley K. Monocyte-endothelial cell interactions in the development of atherosclerosis. Trends Cardiovasc Med. 2008;18:228–32. doi: 10.1016/j.tcm.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bridge TP, Fudala PJ, Herbert S, Leiderman DB. Safety and health policy considerations related to the use of buprenorphine/naloxone as an office-based treatment for opiate dependence. Drug Alcohol Depend. 2003;70:S79–85. doi: 10.1016/s0376-8716(03)00061-9. [DOI] [PubMed] [Google Scholar]


