Abstract
Leukocyte activation during cardiopulmonary bypass (CPB) promotes a systemic inflammatory response that contributes to organ injury and post-operative organ dysfunction. A leukocyte modulatory device (L-MOD) for use during (and after) CPB to limit leukocyte mediated organ injury was tested in a pre-clinical model. Twenty-two pigs underwent 180 minutes of CPB and 5 hours post-operative observation. Pigs received no intervention (Group 1, n= 9), 3 hours of therapy by incorporation of L-MOD into the CPB circuit (Group 2, n=6) or 8 hours of therapy using a femoral venovenous L-MOD circuit during and after CPB (Group 3, n=7). Leukocyte activation was increased at end of CPB and leukocyte counts, namely neutrophils, increased post-operatively in most animals. These indices trended much lower in Group 3. Systemic vascular resistance was not as reduced post CPB for the L-MOD treated pigs and urine output was significantly greater for Group 3 (p<0.01). At 5 hours post CPB, Group 3 had a lower troponin-I (1.59±0.68 ng/ml) than Group 1 or Group 2 (3.97±2.63 and 3.55±2.04 ng/ml, respectively, P<0.05) and a lower urine NGAL (7.57±3.59ng/ml) than the average of the other groups (50.71±49.17, p<0.05). These results demonstrate the therapeutic potential of L-MOD therapy to mitigate the inflammatory response to CPB. Eight hours of venovenous L-MOD resulted in less organ injury and post-op organ dysfunction in this model.
Keywords: systemic inflammatory response, immunomodulation, multi-organ dysfunction, cardiopulmonary bypass, cytopheresis
INTRODUCTION
Cardiopulmonary bypass (CPB) is used for intraoperative patient support during many cardiothoracic procedures. However, an inflammatory response occurs in association with CPB which can intensify to an excessive systemic inflammatory state with associated morbidity and even death 1. High levels of inflammatory markers have been correlated with organ dysfunction following cardiac surgery with CPB 2 and varying degrees of cardiovascular depression, vasomotor, renal, and pulmonary dysfunction and altered mentation following CPB are not uncommon. The priming and activation of leukocytes is recognized as a major contributor to the pathogenesis and progression of the inflammatory response to CPB. Activation of both neutrophils (NE) and monocytes (MO) during CPB incites downstream cellular events including production of inflammatory mediators, increased NE-endothelial interactions, and NE degranulation which in turn promote endothelial dysfunction, edema formation and extravasation of inflammatory cells that contribute to organ dysfunction and additional tissue injury 3.
Accordingly, leukocyte depleting filters for use during CPB have been extensively evaluated in preclinical and clinical trials. Organ protection has been demonstrated with leukocyte depletion 4, 5, however, inconsistent reduction of circulating leukocyte counts, filter saturation and evidence of activation of filter trapped leukocytes has hampered universal clinical support for leukocyte filtration during CPB 6.
Our group has identified a novel biomimetic membrane formulation that instead of depleting cells, sequesters activated leukocytes within a selective cytopheretic device (SCD) and inhibits the inflammatory activities of these cells. Previously, we showed that addition of an SCD to a CPB circuit could reduce circulating leukocyte counts both in vitro and in vivo 7. Use of an SCD with regional citrate anticoagulation (RCA) in these studies prevented the appearance of immature NEs in the circulation, suggesting alteration of the systemic inflammatory response to CPB rather than simple leukoreduction. Amelioration of the multi-organ dysfunction effects of systemic inflammation and reduced mortality rate have been clinically demonstrated using this device formulation in ICU patients with a systemic inflammatory response syndrome (SIRS) and multi-organ failure receiving continuous renal replacement therapy 8–10. Therapy using an SCD with RCA for the cardiothoracic surgery patient has been termed Leukocyte Modulator (L-MOD) therapy, and experiments to investigate the potential clinical benefits for this indication were undertaken using a porcine model of a simulated cardiothoracic procedure with CPB.
MATERIALS AND METHODS
(Details are found in Appendix 1)
Animal Model
Animal use adhered to the Guide for Care and Use of Laboratory Animals 11 and was approved by University of Michigan (USA) institutional committee for care and use of animals. Twenty-two domestic swine (weight 50 ± 5 kg) visibly free of disease were instrumented for right atrial to aortic CPB and continuous hemodynamic monitoring. Pigs were heparinized to achieve an activated clotting time of ≥600 seconds for CPB which was performed using a non-heparin coated circuit with a multi-flow roller pump. During CPB, perfusion was maintained at 2.4-3.0 L/min/m2 body surface area with moderate hypothermia (33 ± 1 ○C) and a mean arterial pressure (MAP) of 50 ± 5 mmHg. Sixty minutes into bypass, the ascending aorta was cross clamped and 3:1 cold blood cardioplegia administered every 15 minutes. The clamp was removed after 45 min, pigs warmed to 37○C and electrocardioversion performed, if needed. CPB was maintained for a total of 180 min. Fluid administration at 5 mL/kg/h with boluses of 0.9% NaCl and 6% hydroxyethylstarch was provided to maintain baseline cardiac filling pressures. If required, phenylephrine (0-200 μg/min) and/or norepinephrine (0-4 μg/min) infusions were administered targeting a minimum MAP of 60 mmHg and cardiac index of 2.0 L/min/m2 post CPB. Glucose and electrolytes were supplemented as needed. Pigs were monitored for 5 hours post CPB, then euthanized by overdose of sodium pentobarbital.
L-MOD therapy was provided by an SCD with a fiber surface area of 1.4 m2 within a 300mL extracorporeal circuit at blood flow rates of 100-200 mL/min. Essential to the leukocyte inhibitory action of the therapy, a low ionized calcium (iCa) environment of <0.40 mM, was achieved within the L-MOD using RCA with anticoagulant-citrate-dextrose formula a solution (ACD-A, Fenwal). Systemic iCa levels were measured bedside using an i-STAT handheld blood analyzer (Abbot Point of Care) and maintained in the physiologic range (0.9-1.1 mmol/L) by adjusting calcium infusion into the return line according to RCA protocol. No ultrafiltration was performed.
Three groups were investigated: Group 1) standard CPB (no intervention), Group 2) 3 hours of L-MOD therapy during CPB by incorporation of L-MOD into the arterial limb of the CPB circuit and Group 3) 8 hours of L-MOD therapy (during CPB + 5 hour post-op). In order to facilitate continuation of therapy into the post-operative period for Group 3, a double lumen hemodialysis catheter was placed in the femoral vein for administration of L-MOD therapy via its own extracorporeal circuit during and post CPB. Schematics of the circuits for each group are shown in Figure 1.
Figure 1.
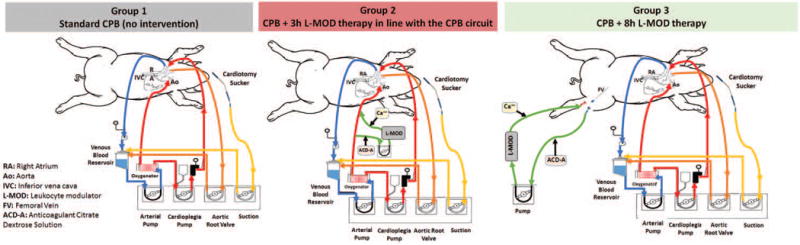
Schematic representations of the extracorporeal circuits. Group 1: Standard right atrial to aortic CPB was performed using a clinical circuit. Group 2: The L-MOD was incorporated into the CPB circuit by creation of a parallel blood flow path post oxygenator. Regional citrate administration, which is essential to the therapeutic action, was performed and modulated blood was delivered into the arterial limb of the CPB circuit prior to blood returning to the animal. Group 3: L-MOD therapy was administered via regional citrate anticoagulation of an independent extracorporeal circuit with femoral venous access which allowed for continuation of therapy during the post-operative period.
Blood Sampling and Leukocyte Analysis
Blood was sampled hourly to determine activated clotting time, blood gas tensions and leukocyte counts. Timepoints for additional sampling were immediately prior to initiating CPB (BL), 5 minutes after cessation of CPB (END CPB) and 5 hours after cessation of CPB (5h). Whole blood was analyzed by flow cytometry for NE and MO activation based on expression of the CD11R3 integrin. After each experiment, L-MOD adherent cells were eluted from the device and quantified as previously described 12. Eluted NE were evaluated for CD11R3 expression and for apoptosis using Annexin V staining.
Markers of Systemic Inflammation and End-Organ Injury
Plasma levels of representative pro (TNFα) and anti (IL-10) inflammatory cytokines, and troponin-I, were measured using porcine specific ELISA kits (R&D Systems; TNFα, PTA00; and IL-10, P1000 and Life Diagnostics CTNI-9-US). Samples obtained from a 60-minute urine collection were centrifuged and frozen until assay. Urine NE gelatinase-associated lipocalin (NGAL), a marker of acute kidney injury, was measured by ELISA (Alpco BioPorto 54-044).
Hemodynamic parameters, medications and fluid requirements were recorded every 15 minutes. As described by Cruz et al 13, a vasopressor dependency index was calculated using the dose of vasoactive medications and MAP; the higher the score, the greater the vasopressor requirement. The alveolar to arterial (A:a) gradient of oxygen was calculated as an index of pulmonary function. Lung edema (based on water content) and immunohistochemical assessment of the accumulation of activated leukocytes in pulmonary tissue at post mortem served as indices of pulmonary injury.
Statistical Analysis
Results are reported as mean ± S.D. and were compared using Analysis of Variance for Repeated Measures (ANOVA) with Tukey's correction for multiple comparisons. Significant findings were then analyzed using one (increase in leukocyte activation) or two tailed student’s t tests (all other comparisons). Analyses were made with the SPSS package (version 7.0; SPSS, Inc, Chicago, Ill) with a p value <0.05 deemed significant.
RESULTS
A complex cardiac surgical case prone to develop a potentially injurious inflammatory response was simulated using 180 min of CPB. A cross clamp time of 45 min provided survivable cardiac ischemia with reperfusion in this model as longer periods in pilot studies had limited success in reproducible restoration of viable cardiac activity. All 22 animals (Group1 n=9, Group 2 n=6, Group 3 n=7) regained sinus rhythm and were weaned off CPB. An aortic tear caused loss of one pig immediately post CPB in Group 1 and a pig in Group 2 died with hyperkalemia at 4h post CPB. Data available at each time point was used in analysis. Hemodynamic parameters, blood gas values and management data are presented in Supplemental Table 1. Prudent administration of fluids, ionotropic and vasopressor medications was used to ensure survival of pigs through the observation period, but this obscured the innate hemodynamic responses of the animals and most of these indices were similar across groups at each time point. Notably, systemic vascular resistance (SVR) was different between groups post CPB with higher values observed in the L-MOD treated groups. Initially maintained near the baseline value in Group 2, by 3 hours after cessation of L-MOD therapy, the trend in SVR followed that of Group 1. In Group 3, SVR was maintained, being significantly greater than Group 1 at hours 3-5 (Figure 2). There was no difference in total fluids or vasoactive medications administered yet the pressor dependency index scores were lowest for Group 3.
Figure 2.
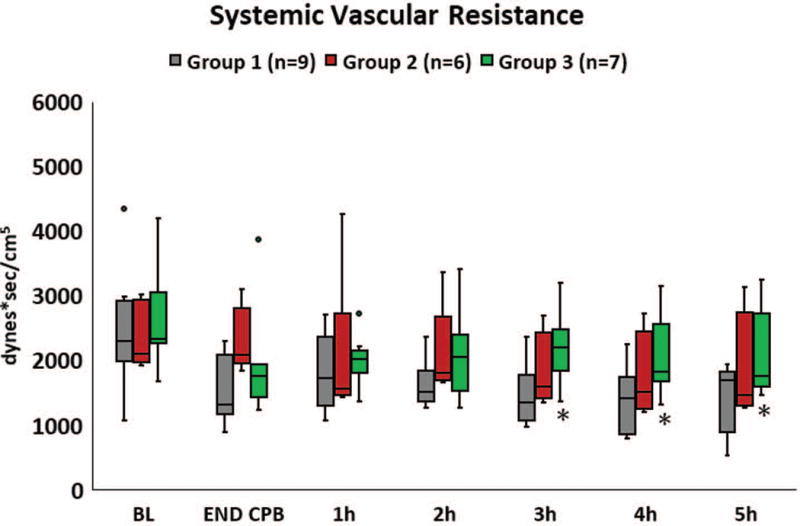
Systemic vascular resistance was reduced during the early postoperative period. The reduction in SVR was not as pronounced in pigs that received LMOD therapy with a significantly greater SVR observed in pigs receiving 8 hours of L-MOD therapy (Group 3) compared to Group 1 for hours 3-5. * p < 0.05.
Biomarkers of cardiac and renal injury were increased in many pigs at END CPB (troponin-I) and at 5h post (NGAL, troponin-I). Troponin-I was significantly lower for Group 3 at 5h (1.59 ± 0.68 ng/mL) compared to Group 1 (3.97 ± 2.63 ng/mL) and to Group 2 (3.55 ± 2.04 ng/mL, P < 0.05 for each), Figure 3A. Renal tubule injury occurred in at least 18% of the pigs, evidenced by urine NGAL concentration that was 10 fold higher at 5h for 2 pigs each in Group 1 and Group 2 compared to the average concentration at END CPB, Figure 3B. No pig in Group 3 had a post CPB urine NGAL concentration that was greater than BL and the 5h NGAL was significantly lower for Group 3 (7.57 ± 3.6 ng/ml) compared to the average of all other pigs (50.71 ± 49.17 ng/ml, P < 0.05). Post- operative urine output was significantly greater for Group 3 (Supplemental Table 1).
Figure 3.
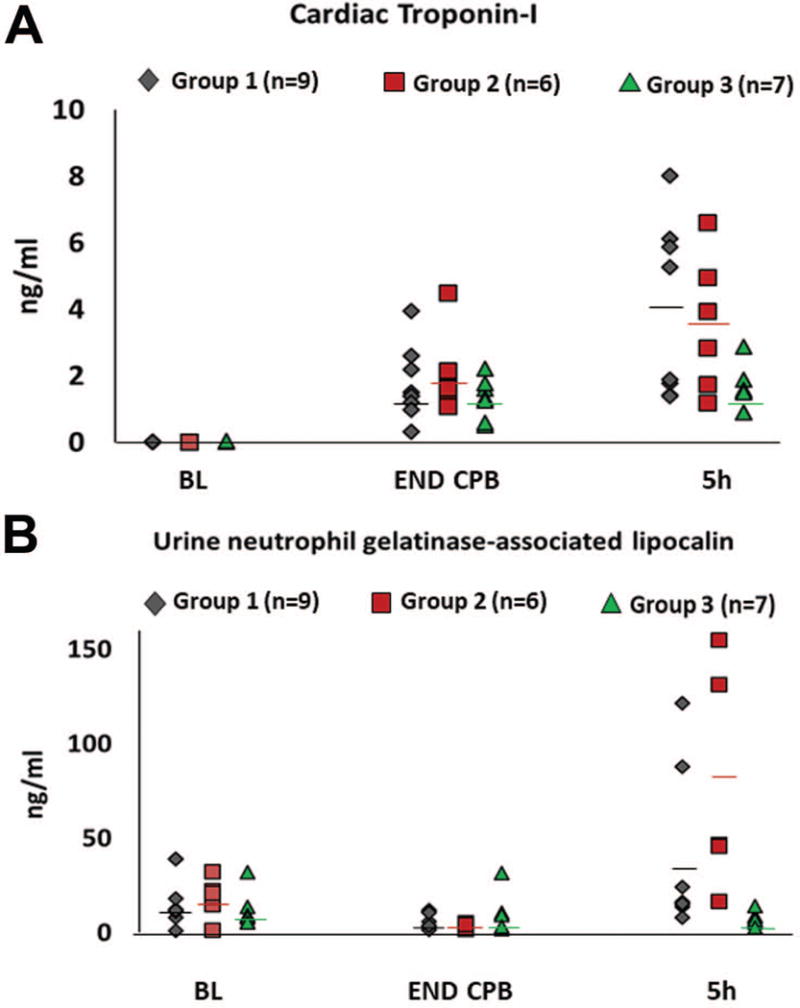
Biomarkers of organ injury were significantly lower in pigs that received 8 hours of L-MOD therapy. Panel A) Serum concentration of cardiac Troponin -I was increased at the end of CPB (END CPB) and at 5 hours post CPB (5h). A cardioprotective effect of L-MOD was observed in Group 3 with the average value for this myocardial injury marker being significantly lower at 5 hours post CPB compared to both Group 1 and to Group 2 (p <0.05). Panel B) At 5 hours post CPB, urine levels neutrophil gelatinase associated lipocalin (NGAL), a marker of acute kidney injury, were increased several-fold in multiple animals from Group 1 and Group 2. No pig in Group 3 had a urine NGAL concentration that exceeded the average baseline value and the mean NGAL concentration at 5 hours post CPB was significantly lower for Group 3 (7.57 ± 3.6 ng/ml) compared to the mean of all other pigs (50.71 ± 49.17 ng/ml, p <0.05). Horizontal lines, mean value.
Moderate hypothermia was maintained during CPB until cross clamp removal then pigs were warmed to 37○C by the heat exchanger. Postoperatively, a progressive increase in rectal temperature was observed in Group 1 and to a lesser extent in Group 2, averaging 40.8 ± 0.4○C and 39.4 ± 0.7○C, respectively at 5h. In contrast, a peak temperature of 38.6○C at END CPB was seen in Group 3, followed by a subtle decrease that plateaued at 37.5 ± 0.4○C for hours 4 and 5 post CPB (P< 0.05)
Lung function was impaired post CPB with the A:a gradient of O2 increased similarly across groups (see Supplemental Table 1). Lung water content at post mortem was not different between groups.
Effect of L-MOD Therapy on Leukocytes
WBC decreased in all pigs initially during CPB due to hemodilution, which, based on changes in hemoglobin concentration, was similar even with the additional extracorporeal circuit volume for the L-MOD treated groups. The general trend was then a progressive increase in WBC, which was primarily due to increases in circulating NE, namely immature NE. Leukocyte activation, as evaluated by measurement of CD11R3 (the porcine analog of human CD11b) on NE and MO was significantly increased by surgery and CPB. The magnitude of these inflammatory responses, depicted in Figures 4 and 5, varied widely among pigs in Groups 1 and 2 but were consistently low in Group 3. An END CPB average WBC of 11,290 ± 5,210 cells/μL with MFI of CD11R3 at 13,313 ± 13,482 was observed for Group 1. This was similar for Group 2 with WBC of 11,560 ± 2,630 cells/μL and MFI of CD11R3 at 16,022 ± 11,376. These LE indices trended much lower in Group 3, with WBC of 8,770 ± 4,260 cells/μL with an MFI of CD11R3 at 6,232 ± 2,564 at END CPB. Despite screening animals to utilize only those with a total WBC within the normal range for conventional pigs 14, ad hoc evaluation revealed that pigs with NE counts <2000 cells/uL at BL experienced minimal stimulation of the innate immune system by the CPB procedure, with only modest increases in NE count or CD11R3 expression. In order to more clearly determine the impact of L-MOD therapy to limit excessive inflammatory responses, leukocyte parameters were also evaluated without these seemingly non-reactive animals which consisted of one pig each in Groups 2 and 3 and four animals from Group 1. This analysis revealed that MFI of CD11R3 for Group 3 at END CPB (6,190 ± 1146) was not significantly increased from BL (4,043 ± 1,789). Furthermore, WBC for Group 3 was significantly lower at every post operative time point (p < 0.05 for hours 1-2 and p < 0.01 for hours 3-5). Total NE count was significantly lower hours 3-5 (p < 0.05) and a significant difference in immature NE count emerged at 5h post CPB (Group 3 v. Group 1, 3.27 ± 1.90 v. 8.30 ± 3.89, p < 0.05).
Figure 4.
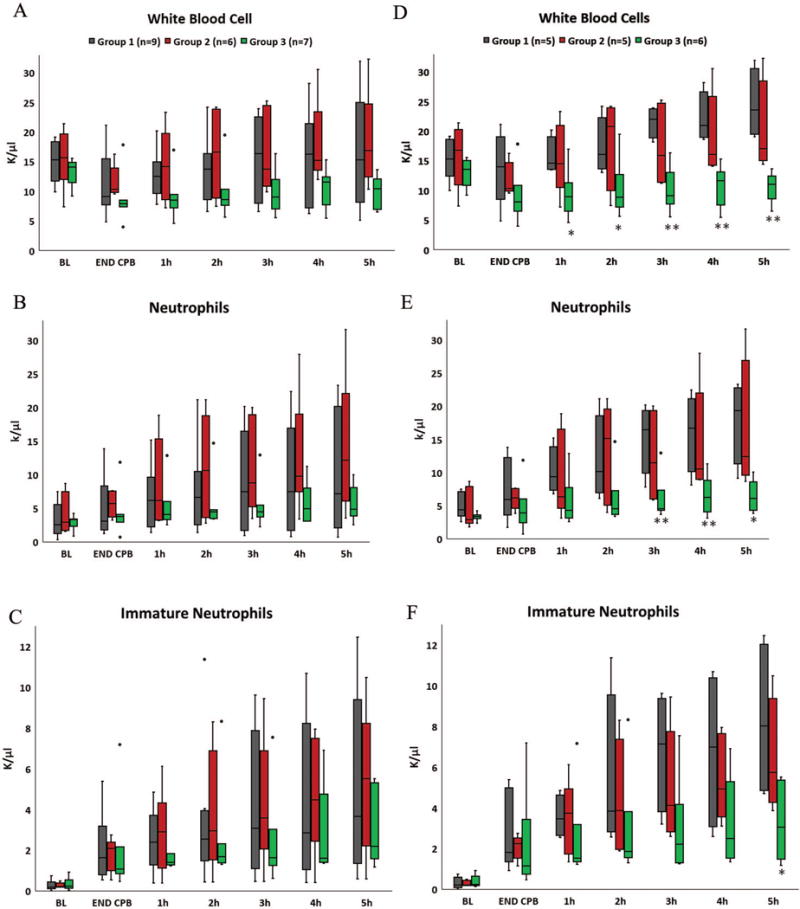
L-MOD therapy is intended to prevent the excessive inflammatory response to CPB. Because pigs with NE counts <2000 cells/μL at baseline (BL) did not demonstrate a measurable inflammatory response to CPB, analysis was performed both with (LEFT panels A-C) and without (RIGHT panels D-F) those animals. When considering all pigs, a trend for lower white blood cell (WBC) count during CPB was observed with L-MOD therapy (Panel A). WBC rebounded when L-MOD therapy was discontinued at END CPB (Group 2) but remained below the baseline (BL) average with continuation therapy (Group 3). Post-operative increases in WBC were due to a progressive increase in circulating NE, which was less for pigs in Group 3 (Panel B). However, the neutrophil responses varied widely. Among the pigs that displayed a robust inflammatory reaction to CPB, the neutrophilic response was clearly modulated during the 8 hours of venovenous LMOD therapy as significantly lower post-operative WBC were observed in Group 3 compared to Group 1 (Panel D). Total neutrophil counts were significantly lower hours 3-5 (Panel E) and a significant difference in immature neutrophil count emerged at 5h post CPB (Panel F). * p <0.05, ** p<0.01. Endpoint of upper whisker, maximum; upper edge of box, third quartile; horizontal line inside box, median; lower edge of box, first quartile; endpoint of lower whisker, minimum; Colored circles represent outlier values.
Figure 5.
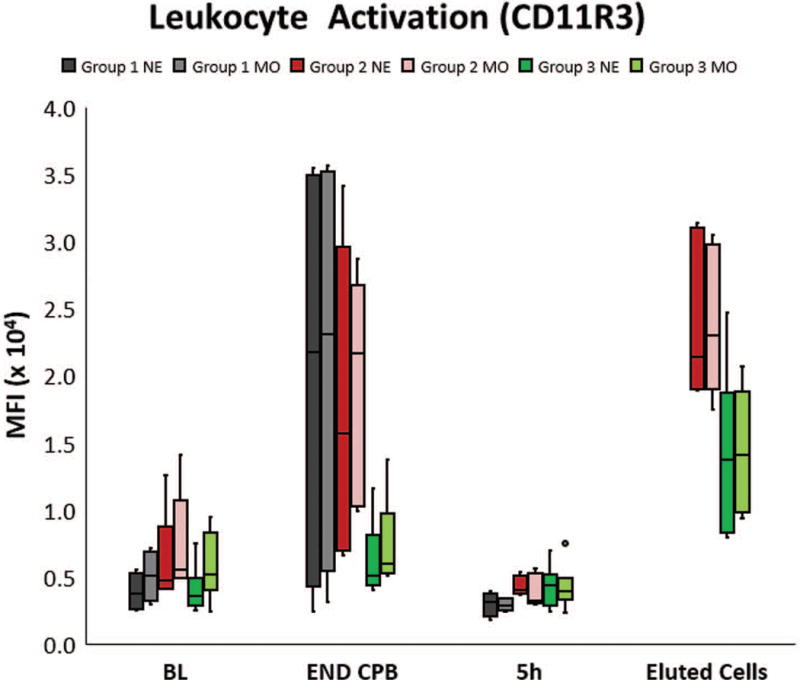
Leukocyte activation was quantified using flow cytometry by measuring the mean fluorescent intensity (MFI) of surface expression of CD11R3, the porcine analog to human CD11b, on neutrophils (NE) and monocytes (MO) in whole blood. Most pigs in Group 1 and Group 2 had a significant increase in CD11R3 expression at the end of CPB (END CPB) compared to baseline (BL), although individual animal responses varied. The magnitude of this change trended much lower in Group 3. Cells eluted from the L-MOD cartridges post therapy in each of the treated groups had a very high expression of CD11R3, demonstrating that activated leukocytes were sequestered within the device. By 5h post CPB, MFI of circulating cells had returned to the baseline value in all groups, presumably due to extravasation of the activated cells Endpoint of upper whisker, maximum; upper edge of box, third quartile; horizontal line inside box, median; lower edge of box, first quartile; endpoint of lower whisker, minimum; colored dots represent outlier values.
The number of cells eluted from the L-MOD upon discontinuation of therapy was similar for devices used in Group 2 (range 4.28×107 to 1.86×1010 cells) and Group 3 (range 4.58×107 to 1.04×1010 cells) and demonstrated preferential sequestration of NE and MO. The leukocyte differential in systemic blood at BL was approximately 60% lymphocytes, 35-45% NE, and only 3-5% MO. L-MOD eluted from Group 2 contained 70 ± 5 % NE, 25 ± 7 % MO and < 5 % other cells and L-MOD used in Group 3 contained 58 ± 6 % NE, 39 ± 6% MO, and 4 % other cells. MFI of CD11R3 on NE associated with the L-MOD membranes were higher than that seen in the systemic circulation at the time of device removal and exceeded the peak MFI observed on circulating cells (Figure 5). NE activation results in prolongation of cell life span by the delay of apoptosis. Per Annexin V staining, the NE population recovered from L-MOD in Group 2 consisted of 38 ± 14 % cells that were resistant to apoptosis (activated), while 70 ± 7 % of the NE recovered from the L-MOD in Group 3 were activated.
The presence of activated leukocytes within pulmonary tissue at 5h post CPB was slightly lower, but not statistically significant, for the L-MOD treated Groups at 15 ± 5 % for Group 2 and 16 ± 4 % for Group 3 versus 19 ± 7 % for Group 1.
Systemic levels of TNFα and IL-10 were highly variable with no differences detected in the mean levels of these cytokines over time or between groups (data not shown).
DISCUSSION
CPB promotes an excessive systemic inflammatory state with activation of leukocytes implicated as a key factor in this inflammatory response and subsequent organ dysfunction. Leukodepletion during CPB has been associated with protective effects on pulmonary, renal and neurologic function 6, however, leukodepletion filters have been shown to become saturated during CPB, rendering them ineffective in capturing leukocytes 15 and to cause activation of leukocytes, resulting in the release of inflammatory mediators 15. Accordingly, results have been inconsistent in demonstrating clinical improvement with the use of these filters during CPB.
While similar in size and materials to a hemofilter, the L-MOD is not a leukocyte filter, but rather, a cell processing device. L-MOD therapy utilizes a biomimetic membrane composed of polysulfone-based fibers with specialized surface characteristics and a unique low shear blood flow path which permits sequestration of cells. A low iCa environment within the device, safely afforded by infusion of citrate, is an integral part of the therapy providing an inhibitory effect on sequestered leukocytes and promoting release of cells from the membrane back to the systemic circulation 12. This combination of activated leukocyte sequestration, inhibition and subsequent release promotes a lower systemic leukocyte activation profile during and after CPB as demonstrated in our prior work and this study.
A porcine model was used to investigate the potential impact of L-MOD therapy on the systemic inflammatory response to CPB and postoperative organ dysfunction. Eight hours of L-MOD therapy in Group 3 demonstrated the capability for amelioration of the inflammatory leukocyte response to CPB and resulted in significantly lower biomarkers of organ injury, improved SVR and increased urine output post-CPB. The inter-animal variation in this study dampened the measured impact of L-MOD on leukocyte parameters but immune modulation was clearly demonstrated when comparing pigs capable of robust inflammatory responses. The trend for lower leukocyte activation and altered kinetics of circulating NE post-operatively observed in Group 3 suggests modulation of the inflammatory response to CPB. The fact that a lower number of circulating NE at the time of surgery was associated with a minimal immune response in some pigs supports the premise that these cardinal effector cells of the innate immune system play a critical role in initiation of the inflammatory response to CPB. With lower NE counts and activation observed at END CPB in Group 3, L-MOD created a similar less inflammatory immune profile, demonstrating that a therapy that alters the activity of these cells can modify the innate immune response and thus has potential to ameliorate inflammatory complications of CPB.
Multiple therapeutic benefits associated with immunomodulation of the circulating leukocyte pool were seen in this porcine model. Cardiovascular effects of L-MOD therapy demonstrated improved SVR post CPB, potentially due to improved vascular tone and/or diminished myocardial injury. Consistent with a myocardial protective effect, a significantly reduced rise in troponin-I was observed in Group 3. A renoprotective effect was also observed with 8 hours of L-MOD therapy, with no rise in the well accepted AKI marker, NGAL, and greater urine output in Group 3. Administration of venovenous L-MOD therapy was also associated with a plateau in body temperature compared to the elevation observed in the other groups. This significantly different temperature pattern is likely due in part to continued extracorporeal circulation but may also be a systemic consequence of amelioration the CPB promoted inflammatory reaction and may well have clinical relevance as post-operative hyperthermia has been linked to greater cognitive dysfunction after cardiac surgery 16. In this study, we were unable to detect any measurable influence of L-MOD therapy on respiratory parameters or lung injury. CPB results in a reperfusion injury to the lung with LE accumulation along damaged endothelium, contributing to postoperative pulmonary dysfunction 3. As improved pulmonary parameters have been observed with 12 hours or more of SCD therapy in both pre-clinical and clinical trials, 8–10, 12 it is possible that any beneficial effects of immunomodulation on the severity of lung injury may not have had sufficient time to manifest in these 8 hour experiments.
The L-MOD is designed to sequester activated LE using low shear flow and the surface characteristics of the membranes which encourage binding of these cells. Analysis of the cells eluted from the L-MOD confirmed preferential sequestration of NE and MO. These cells were found to be highly activated based on MFI of CD11R3 and a high percentage of the bound NE being resistant to apoptosis. Unlike standard leukodepletion filters, activation of cells while they are sequestered within the L-MOD is unlikely due to the citrate induced low iCa environment, which we have previously shown to reduce the release of inflammatory proteins from exocytic vesicles of NE as well as reduce cytokine production and CD11b expression in whole blood12. The number of cells eluted from each L-MOD ranged over several orders of magnitude, yet the numbers were similar after 3 hours or 8 hours of L-MOD treatment. This finding is aligned with the “catch and release” mechanism of action of the device which has been demonstrated in vitro12, although we cannot be sure that the highest quantities of cells found associated with the membrane in these studies were not indicative of membrane saturation. The percentage of MO and apoptosis resistant (i.e. activated) NE among the bound cells increased with duration of therapy, a trend we have observed in other models of SCD therapy (unpublished data). This phenomenon may reflect differing binding kinetics or turnover for various cell types or activation levels, with prolonged sequestration of the most immunologically active cells potentially offering increased therapeutic benefit as was observed with 8 hours of therapy in Group 3.
From these experiments, it is not clear if the duration of treatment or if location of the L-MOD device is most important for its measured effects to reduce organ injury and dysfunction. Nominal and/or transitory immunomodulation was observed with administration of L-MOD therapy only during CPB in Group 2 which prompted the creation of Group 3 to investigate durability of any therapeutic effects by extending L-MOD therapy post CPB. An independent venovenous L-MOD circuit was elected for extended therapy to permit seamless transition into the post-operative period, as, similar to use of the SCD in patients with AKI and MOF, this would be the anticipated method of administration in the ICU. Unexpectedly, placement of the device in a separate extracorporeal circuit from the CPB perfusion circuit was found to more effectively modulate systemic leukocyte activation during the CPB procedure, as seen in the MFI of CD11R3 expression on circulating leukocytes at END CPB. Expression of the CD11b epitope in humans is known to be calcium dependent and chelation of calcium prevents expression and mobilization of intracellular stores of this surface integrin15. The venovenous circuit set-up for Group 3 allowed for modulation of leukocytes within the low iCa environment of the L-MOD preceding their exposure to the activating stimuli of the CPB circuit and this may have prevented the upregulation of CD11R3 on these cells, a phenomenon not observed when the L-MOD was incorporated within the arterial limb of the CPB circuit. Perhaps incorporation of L-MOD into the venous limb of the CPB circuit may have proven as effective in limiting LE activation as venovenous delivery and deserves further investigation. Cardiac and renal protection, in addition to maintained suppression of WBC were observed with 8 hours of venovenous L-MOD therapy in comparison to terminating L-MOD therapy with the CPB circuit in Group 2. However, with the different manner of delivery of L-MOD therapy for the groups, it is unclear if these therapeutic benefits resulted from the increased efficacy of immunomodulation with placement of the device in a dedicated venous circuit or from prolongation of therapy, or both. Varied configurations for incorporation of the L-MOD into the CPB circuit as well as cohorts receiving venovenous L-MOD therapy only during CPB should provide additional insight towards the optimal application of this therapy and are planned for subsequent studies.
Limitations
The inflammatory response and its consequences undoubtedly exceed the 5-hour post CPB timeframe as well as the scope of parameters that were measured in this animal model. The heterogeneous immunologic responses of pigs to CPB likely mirrors the clinical scenario, but this limited the power of our study to detect differences in LE activation and LE counts unless only considering subjects with a robust inflammatory response. As patients prone to developing a severe or sustained inflammatory response represent the target population for L-MOD therapy, we feel these results do provide support to proceed toward clinical evaluation, since we were able to clearly demonstrate clinically relevant organ protection with the use of venovenous L-MOD. A dose escalation study based on duration of L-MOD therapy post CPB along with a more comprehensive evaluation of systemic markers of inflammation and the organ systems affected by CPB related complications, including those not evaluated in this study, such as the neurologic and hemostatic systems, are needed to further ascertain the impact of L-MOD therapy on systemic inflammation and organ dysfunction following CPB.
Conclusions
These studies suggest that L-MOD therapy has the potential to ameliorate the natural progression of excessive inflammation by blunting the systemic leukocyte response and limiting cardiac and renal injury leading to improved cardiovascular stability and renal function post CPB. The benefits of this leukocyte modulation may also extend to the pulmonary and neurologic systems and further study is warranted to elucidate the impact of L-MOD therapy on thermoregulation as well as determine if L-MOD is able to mediate pulmonary injury following reperfusion. A preventive therapeutic approach to reduce CPB promoted inflammatory leukocyte responses and thereby lessen organ injury and progression to organ dysfunction could drastically reduce the morbidity associated with CPB. Riding on an excellent safety profile and compelling clinical results for SCD therapy in SIRS associated multi-organ failure, translation to the cardiothoracic surgery patient is underway for evaluation of L-MOD therapy in a pilot safety and efficacy clinical trial.
Supplementary Material
Acknowledgments
We recognize Christopher Pino, Liandi Lou, Linda Charles, Marie Cornell and Kristopher Deatrick (Barry) for their technical expertise and thank The Undergraduate Research Opportunity Program at the University of Michigan for its student support.
Source of Funding
Support for the studies described in this manuscript was provided by the NIH SBIR award number: 1R43HL127830-01.
Footnotes
Conflicts of Interest
H. David Humes, M.D. is a shareholder of Innovative BioTherapies and Cyto Pherx, Inc. biotechnology spin our companies of the University of Michigan. Kimberly A. Johnston, Angela J. Westover, Deborah A. Buffington, are employees of Innovative BioTherapies, Inc.
References
- 1.Holmes JHt, Connolly NC, Paull DL, et al. Magnitude of the inflammatory response to cardiopulmonary bypass and its relation to adverse clinical outcomes. Inflamm Res. 2002;51:579–586. doi: 10.1007/pl00012432. [DOI] [PubMed] [Google Scholar]
- 2.de Mendonca-Filho HT, Pereira KC, Fontes M, et al. Circulating inflammatory mediators and organ dysfunction after cardiovascular surgery with cardiopulmonary bypass: A prospective observational study. Crit Care. 2006;10:R46. doi: 10.1186/cc4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Warren OJ, Smith AJ, Alexiou C, et al. The inflammatory response to cardiopulmonary bypass: Part 1–mechanisms of pathogenesis. J Cardiothorac Vasc Anesth. 2009;23:223–231. doi: 10.1053/j.jvca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Rubino AS, Serraino GF, Mariscalco G, Marsico R, Sala A, Renzulli A. Leukocyte depletion during extracorporeal circulation allows better organ protection but does not change hospital outcomes. Ann Thorac Surg. 2011;91:534–540. doi: 10.1016/j.athoracsur.2010.09.077. [DOI] [PubMed] [Google Scholar]
- 5.Tao K, An Q, Lin K, et al. Which is better to preserve pulmonary function: Short-term or prolonged leukocyte depletion during cardiopulmonary bypass? J Thorac Cardiovasc Surg. 2009;138:1385–1391. doi: 10.1016/j.jtcvs.2009.07.059. [DOI] [PubMed] [Google Scholar]
- 6.Boodram S, Evans E. Use of leukocyte-depleting filters during cardiac surgery with cardiopulmonary bypass: A review. J Extra Corpor Technol. 2008;40:27–42. [PMC free article] [PubMed] [Google Scholar]
- 7.Pino CJ, Lou L, Smith PL, et al. A selective cytopheretic inhibitory device for use during cardiopulmonary bypass surgery. Perfusion. 2012;27:311–319. doi: 10.1177/0267659112444944. [DOI] [PubMed] [Google Scholar]
- 8.Ding F, Yevzlin AS, Xu ZY, et al. The effects of a novel therapeutic device on acute kidney injury outcomes in the intensive care unit: A pilot study. ASAIO J. 2011;57:426–432. doi: 10.1097/MAT.0b013e31820a1494. [DOI] [PubMed] [Google Scholar]
- 9.Tumlin JA, Chawla L, Tolwani AJ, et al. The effect of the selective cytopheretic device on acute kidney injury outcomes in the intensive care unit: A multicenter pilot study. Semin Dial. 2013;26:616–623. doi: 10.1111/sdi.12032. [DOI] [PubMed] [Google Scholar]
- 10.Tumlin JA, Galphin CM, Tolwani AJ, et al. A multi-center, randomized, controlled, pivotal study to assess the safety and efficacy of a selective cytopheretic device in patients with acute kidney injury. PLoS One. 2015;10:e0132482. doi: 10.1371/journal.pone.0132482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Research Council. Guide for the care and use of laboratory animals. Eighth. Washington (DC): 2011. [Google Scholar]
- 12.Ding F, Song JH, Jung JY, et al. A biomimetic membrane device that modulates the excessive inflammatory response to sepsis. PLoS One. 2011;6:e18584. doi: 10.1371/journal.pone.0018584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cruz DN, Antonelli M, Fumagalli R, et al. Early use of polymyxin b hemoperfusion in abdominal septic shock: The euphas randomized controlled trial. JAMA. 2009;301:2445–2452. doi: 10.1001/jama.2009.856. [DOI] [PubMed] [Google Scholar]
- 14.Jackson PG, Cockcroft PD. Handbook of pig medicine. Saunders, LTD; 2007. [Google Scholar]
- 15.Thurlow PJ, Doolan L, Sharp R, Sullivan M, Smith B, Andersen LW. Studies of the effect of pall leucocyte filters lg6 and av6 in an in vitro simulated extracorporeal circulatory system. Perfusion. 1995;10:291–300. doi: 10.1177/026765919501000503. [DOI] [PubMed] [Google Scholar]
- 16.Grocott HP, Mackensen GB, Grigore AM, et al. Postoperative hyperthermia is associated with cognitive dysfunction after coronary artery bypass graft surgery. Stroke. 2002;33:537–541. doi: 10.1161/hs0202.102600. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


