The usefulness of underwater techniques of endoscopic submucosal dissection (ESD)1, 2, 3, 4, 5 and EMR6, 7, 8, 9, 10 has been reported in the literature. Most of these studies on underwater ESD (UESD) have used a straight needle-type knife. However, in situations such as vertical position opposite the muscle layer, poor maneuverability of the endoscope, and severe fibrosis, submucosal dissection by use of a straight needle-type knife sometimes becomes difficult because of an increased risk of perforation. Therefore, an approach other than one involving a straight needle-type knife is needed in UESD, as in conventional ESD (CESD).
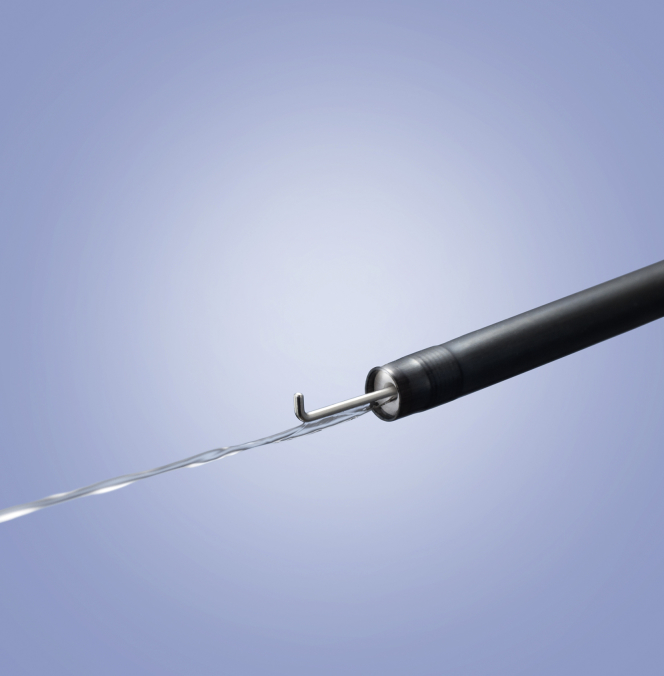
The Hook Knife J (Fig. 1, bent-type, monopolar; Olympus, Tokyo, Japan) is useful and safe because tissue can be dissected away from the muscle layer, thus avoiding perforation. In UESD, we primarily use the Dual Knife J (straight needle-type, tip length 1.5 mm; Olympus)1; in difficult situations, such as those aforementioned, we use the Hook Knife J. While using the Dual Knife J with the VIO300D (Erbe Elektromedizin GmbH, Tübingen, Germany), even in saline solution, it is not necessary to change the electrosurgical unit settings for submucosal dissection (Swift coagulation mode effect 3, 30 W). When the Hook Knife J is used in saline solution, submucosal dissection with the coagulation mode (Swift or Forced coagulation mode) is difficult because of the high electrical conductivity of saline solution. Therefore, unlike the Dual Knife J, the Endocut I mode (effect 3, duration 2, interval 1) is suitable for mucosal incision and submucosal dissection.
Figure 1.
Hook Knife J is a monopolar knife with a bent and rotatable tip along with a water jet function.
Because the coagulation power of the Endocut I is weak, bleeding occurs easily if submucosa with vessels is dissected. In this situation, precoagulation is required by means of a knife-coagulated cut11 with the soft-coagulation mode (effect 3, 40 W) or the use of hemostatic forceps. We used bipolar rotatable hemostatic forceps (Tighturn; Zeon Medical, Tokyo, Japan) with the bipolar soft-coagulation mode (effect 3, 30 W in dry conditions, and effect 5, 50 W in underwater conditions) for precoagulation and hemostasis.
The use of bipolar hemostatic forceps allowed for precoagulation and hemostasis in the saline solution while maintaining an underwater condition. Conversely, precoagulation and hemostasis in saline solution were relatively difficult to achieve using monopolar hemostatic forceps due to the high electrical conductivity of saline solution. Thus, CO2 exposure and a dry condition were assumed to be favorable conditions for precoagulation and hemostasis. However, supplying CO2 for every precoagulation and hemostasis application was complicated. Therefore, bipolar hemostatic forceps were used rather than monopolar hemostatic forceps for UESD.
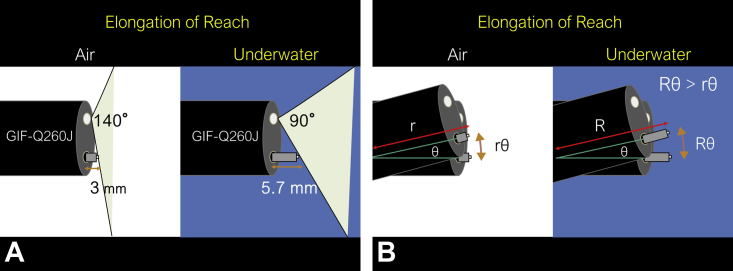
Endoscopists should be aware of the “visible range change” resulting from underwater conditions. Basically, once air is replaced by water, the focal length increases, and far objects may appear closer. In underwater conditions, operators can handle the knife for a longer distance than in air. During use of the GIF-Q260J endoscope (Olympus), an object in air 3 mm from the endoscope will appear the same on screen as an underwater object 5.7 mm from the endoscope because of the zoom effect (Fig. 2A). We coined this effect as “elongation of reach,” which may offer an advantage in specific situations.
Figure 2.
Explanation of “elongation of reach” in underwater condition. A, “Elongation of reach” due to the underwater condition. During use of the GIF-Q260J endoscope (Olympus, Tokyo, Japan), an object in air at a 3-mm distance from the endoscope will appear the same on screen as an underwater object at a 5.7-mm distance from the endoscope because of the zoom effect. B, When the knife is moved with the same sense of distance on the screen and same angle of the endoscope, the moving distance of the knife tip can appear larger in underwater conditions than in air because the arc length is represented by “r (or R) × θ” (r or R, radius; θ, central angle) in the circular measure.
When the knife is moved with the same sense of distance on the screen and same angle of the endoscope, the moving distance of the knife tip can appear larger in underwater conditions than in air because the arc length is represented by “r × θ” (r, radius; θ, central angle) in the circular measure (Fig. 2B). “Elongation of reach” caused by the underwater condition may make the use of the Hook Knife J easier because dissection with this knife is primarily performed by angle operation. However, in an underwater condition, nearby objects cannot be seen because of the “visible range change”; therefore, it is better to prevent excessive nearing of the lesion using a long hood. Generally, for this purpose, we use the ST hood (tip protrusion length, 8.3 mm; Fujifilm Medical, Tokyo, Japan) because of its tapering tip, which is advantageous for maneuvering under the mucosal flap, especially in cases of severe fibrosis.
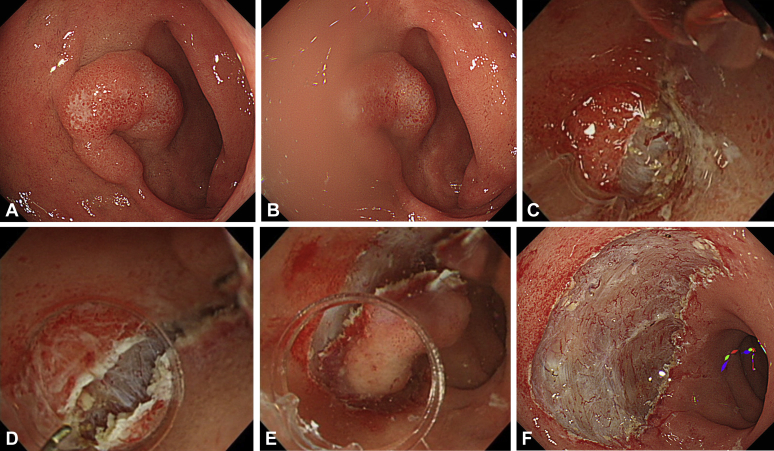
Video 1 (available online at www.VideoGIE.org) shows the usefulness of UESD with the Hook Knife J in a case of duodenal tumor. Duodenal ESD is challenging because of the high rate of perforation and should be limited for use by very experienced endoscopists with expertise in colorectal ESD.12, 13 A flat lesion (25 mm in diameter) was located at the superior duodenal angle (SDA) (Fig. 3A). ESD was arranged instead of EMR to achieve en bloc resection. The SDA was often on the gravitational lower side with the patient in the left lateral decubitus or supine position (Fig. 3B). Water collected around the lesion and the interface between air and water obstructed the visual field. Moreover, approaching the submucosal layer became difficult because the mucosal flap hung down under the influence of gravity.
Figure 3.
Underwater endoscopic submucosal dissection for duodenal tumor. A, The lesion (25 mm in diameter) was located at the superior duodenal angle. B, The lesion was located at the gravitational lower side with the patient in the left lateral decubitus and supine positions. Therefore, water collected around the lesion, and the interface between the air and water obstructed the visual field. C, Owing to severe fibrosis and halation, the boundary between the submucosal and muscle layers was unclear. D, The underwater condition enabled recognition of the boundary between the submucosal and muscle layers. E, Buoyancy aided the opening of the mucosal flap against gravity, making the approach to the submucosal layer easier. F, En bloc resection was achieved without any adverse events. Complete resection was histopathologically proven.
The first injection and mucosal incision were performed under CO2 insufflation without the underwater zoom effect while the whole image was checked. MucoUp (0.4% hyaluronic acid; Boston Scientific, Marlborough, Mass, USA) mixed with 0.4% indigo carmine (Daiichisankyo, Tokyo, Japan) and diluted 200 times was used for submucosal injection.
In underwater conditions, increasing the concentration of indigo carmine increased the cloudiness of the visual field, whereas decreasing the concentration improved the visual field. Saline solution was injected by means of the water jet function of the GIF-Q260J endoscope. The water jet function switch is located in the push button of the endoscope, not in the foot push button. Thus, we could use the water jet function without looking away from the monitor. UESD was initiated in an underwater condition, which was followed by submucosal dissection.
Owing to severe fibrosis and halation, the boundary between the submucosal and muscle layers was unclear (Fig. 3C). The underwater condition facilitated recognition of this boundary as a result of the natural zoom effect and disappearance of halation (Fig. 3D). Buoyancy aided the opening of the mucosal flap against gravity, making the approach to the submucosal layer easier (Fig. 3E). En bloc resection was successfully achieved without any adverse events (Fig. 3F). To prevent delayed perforation, the post-ESD ulcer was completely closed. Histopathologic examination showed tubular adenoma with high-grade dysplasia and complete resection.
Performing UESD or CESD under appropriate situations is crucial. UESD should be selected only when its advantages are more apparent than those of CESD because CESD is more convenient. Situations in which USED can offer more advantages than CESD are those in which (1) the lesion is on the gravitational lower side, (2) severe fibrosis is noted at the submucosa, and (3) the visual field is obstructed by fat tissue. Among these, the first is particularly important. If the lesion is on the gravitational upper side, it is difficult to accumulate saline solution around the lesion. Furthermore, because buoyancy acts on the opposite side of gravity, the buoyancy may obstruct the opening of the mucosal flap, making it difficult to approach the submucosa. In this video case, UESD was indicated by the presence of the first and second situations.
In conclusion, UESD is indicated for lesions with difficult situations, such as those mentioned above. Both the Hook Knife J and the Dual Knife J can be used for UESD. Moreover, the Hook Knife J is mostly useful for operations on regions of fibrosis.
Disclosure
The author disclosed no financial relationships relevant to this publication.
Supplementary data
Underwater endoscopic submucosal dissection with use of the Hook Knife J for a duodenal tumor (25 mm in diameter) at the superior duodenal angle.
References
- 1.Nagata M. Usefulness of underwater endoscopic submucosal dissection in saline solution with a monopolar knife for colorectal tumors (with videos) Gastrointest Endosc. 2018;87:1345–1353. doi: 10.1016/j.gie.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 2.Yahagi N., Nishizawa T., Sasaki M. Water pressure method for duodenal endoscopic submucosal dissection. Endoscopy. 2017;49:E227–E228. doi: 10.1055/s-0043-113556. [DOI] [PubMed] [Google Scholar]
- 3.Akasaka T., Takeuchi Y., Uedo N. “Underwater” endoscopic submucosal dissection for superficial esophageal neoplasms. Gastrointest Endosc. 2017;85:251–252. doi: 10.1016/j.gie.2016.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Akasaka T., Tonai Y., Hamada K. Dive to the underwater world: a water immersion technique for endoscopic submucosal dissection of gastric neoplasms. Am J Gastroenterol. 2017;112:985. doi: 10.1038/ajg.2016.595. [DOI] [PubMed] [Google Scholar]
- 5.Yoshii S., Hayashi Y., Matsui T. “Underwater” endoscopic submucosal dissection: a novel technique for complete resection of a rectal neuroendocrine tumor. Endoscopy. 2016;48:E67–E68. doi: 10.1055/s-0042-101855. [DOI] [PubMed] [Google Scholar]
- 6.Binmoeller K.F., Weilert F., Shah J. “Underwater” EMR without submucosal injection for large sessile colorectal polyps (with video) Gastrointest Endosc. 2012;75:1086–1091. doi: 10.1016/j.gie.2011.12.022. [DOI] [PubMed] [Google Scholar]
- 7.Binmoeller K.F., Shah J.N., Bhat Y.M. “Underwater” EMR of sporadic laterally spreading nonampullary duodenal adenomas (with video) Gastrointest Endosc. 2013;78:496–502. doi: 10.1016/j.gie.2013.03.1330. [DOI] [PubMed] [Google Scholar]
- 8.Chaves D.M., Brito H.P., Chaves L.T. Underwater endoscopic resection: an alternative for difficult colorectal polyps. VideoGIE. 2016;1:82–84. doi: 10.1016/j.vgie.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishaq S., Kuwai T. Rectal polyp reaching the dentate line: underwater EMR without submucosal lift. VideoGIE. 2017;2:53–54. doi: 10.1016/j.vgie.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Girotra M., Friedland S. Underwater endoscopic mucosal resection of anal condyloma. VideoGIE. 2018;3:123–124. doi: 10.1016/j.vgie.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horikawa Y., Toyonaga T., Mizutamari H. Feasibility of knife-coagulated cut in gastric endoscopic submucosal dissection: a case-control study. Digestion. 2016;94:192–198. doi: 10.1159/000450994. [DOI] [PubMed] [Google Scholar]
- 12.Inoue T., Uedo N., Yamashina T. Delayed perforation: a hazardous complication of endoscopic resection for non-ampullary duodenal neoplasm. Dig Endosc. 2014;26:220–227. doi: 10.1111/den.12104. [DOI] [PubMed] [Google Scholar]
- 13.Kakushima N., Kanemoto H., Tanaka M. Treatment for superficial non-ampullary duodenal epithelial tumors. World J Gastroenterol. 2014;20:12501–12508. doi: 10.3748/wjg.v20.i35.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Underwater endoscopic submucosal dissection with use of the Hook Knife J for a duodenal tumor (25 mm in diameter) at the superior duodenal angle.