Abstract
Mitochondrial targeting is a promising approach for solving current issues in clinical application of chemotherapy and diagnosis of several disorders. Here, we discuss direct conjugation of mitochondrial-targeting moieties to anticancer drugs, antioxidants and sensor molecules. Among them, the most widely applied mitochondrial targeting moiety is triphenylphosphonium (TPP), which is a delocalized cationic lipid that readily accumulates and penetrates through the mitochondrial membrane due to the highly negative mitochondrial membrane potential. Other moieties, including short peptides, dequalinium, guanidine, rhodamine, and F16, are also known to be promising mitochondrial targeting agents. Direct conjugation of mitochondrial targeting moieties to anticancer drugs, antioxidants and sensors results in increased cytotoxicity, anti-oxidizing activity and sensing activity, respectively, compared with their non-targeting counterparts, especially in drug-resistant cells. Although many mitochondria-targeted anticancer drug conjugates have been investigated in vitro and in vivo, further clinical studies are still needed. On the other hand, several mitochondria-targeting antioxidants have been analyzed in clinical phases I, II and III trials, and one conjugate has been approved for treating eye disease in Russia. There are numerous ongoing studies of mitochondria-targeted sensors.
Abbreviations: 4-AT, 4-amino-TEMPO; Aβ, beta amyloid; AD, Alzheimer׳s disease; AIE, aggregation-induced emission; Arg, arginine; ATP, adenosine triphosphate; BODIPY, boron-dipyrromethene; CAT, catalase; C-dots, carbon dots; CoA, coenzyme A; COX, cytochrome c oxidase; CZBI, carbazole and benzo[e]indolium; DDS, drug delivery system; DEPMPO, 5-(diethylphosphono)-5-methyl-1-pyrroline N-oxide; DIPPMPO, 5-(diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide; Dmt, dimethyltyrosine; DQA, dequalinium; EPR, enhanced permeability and retention; F16, (E)-4-(1H-indol-3-ylvinyl)-N-methylpyridinium iodide; 5-FU, 5-Fluorouracil; (Fx, r)3, (l-cyclohexyl alanine-d-arginine)3; GPX, glutathione peroxidase; GS, gramicidin S; HTPP, 5-(4-hydroxy-phenyl)-10,15,20-triphenylporphyrin; IMM, inner mitochondrial membrane; IMS, intermembrane space; IOA, imidazole-substituted oleic acid; LA, lipoic acid; LAH2, dihydrolipoic acid; Lys, lysine; MET, mesenchymal-epithelial transition; MitoChlor, TPP-chlorambucil; MitoE, TPP-vitamin E; MitoLA, TPP-lipoic acid; MitoQ, TPP-ubiquinone; mitoTEMPO, (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl)triphenylphosphonium); MitoVES, TPP-vitamin E succinate; MLS, mitochondria localization sequences; MPO, myeloperoxidase; MPP, mitochondria-penetrating peptides; mtCbl, (Fx,r)3-chlorambucil; mtDNA, mitochondrial DNA; mtPt, mitochondria-targeting (Fx,r)3-platinum(II); nDNA, nuclear DNA; Nit, nitrooxy; NitDOX, nitrooxy-DOX; OMM, outer mitochondrial membrane; OXPHOS, oxidative phosphorylation; PD, Parkinson׳s disease; PDT, photodynamic therapy; PET, photoinduced electron transfer; Phe, phenylalanine; PS, photosensitizer; PTPC, permeability transition pore complex; RNS, reactive nitrogen species; ROS, reactive oxygen species; SkQ1, Skulachev ion-quinone; SOD, superoxide dismutase; SS peptide, Szeto-Schiller peptides; TEMPOL, 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl; TPEY-TEMPO, [2-(1-oxyl-2,2,6,6-tetramethylpiperidin-4-ylimino)-ethyl]-triphenyl-phosphonium; TPP, triphenylphosphonium; Tyr, tyrosine; VDAC/ANT, voltage-dependent anion channel/adenine nucleotide translocase; VES, vitamin E succinate; XO, xanthine oxidase; αTOS, alpha-tocopheryl succinate.
KEY WORDS: Anticancer agents, Antioxidants, Direct conjugation, Mitochondria-targeting, Sensing agents
Graphical abstract
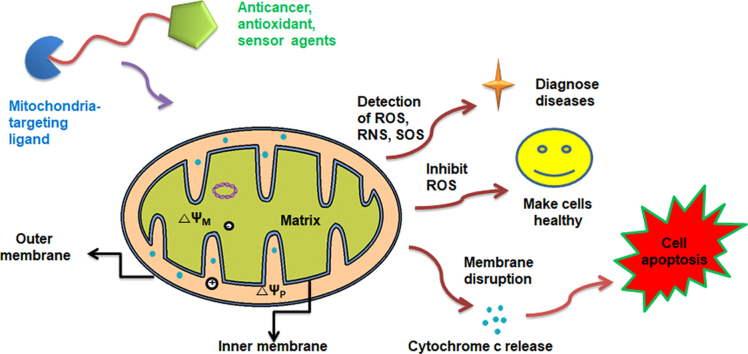
Mitochondria-targeted anticancer, antioxidant, and sensing agents can selectively accumulate in the mitochondria, where their modes of action occur. In most cases, lipophilic molecules intercalate into the mitochondrial membrane through lipophilic affinity and further move through the matrix owing to the membrane potential difference.
1. Introduction
Approximately two billion years ago, mitochondria arose from the engulfment of an α-proteobacterium by a precursor of the modern eukaryotic cell1, 2. Thousands of mitochondria are found in cells, and mitochondria account for approximately 40% of the cytoplasm. As essential organelles present in most eukaryotic cells, mitochondria play crucial roles in cellular energy metabolism and regulation of programmed cell death1, 2. First, mitochondria regulate energy production and are known as the “power house” of the cell, essential for all life. Second, during programmed cell death (i.e., apoptosis), numerous cell death-related signal transduction pathways are activated by mitochondria. Mitochondria have the ability to control the activation of programmed cell death by regulating the translocation of proapoptotic proteins from the mitochondrial intermediate space to the cytosol3. Apoptosis is triggered by a series of mitochondrial events, including the collapse of the intimal potential, mitochondrial swelling and opening of the permeability transition pores4. Thus, the main machinery and components in the mitochondria tightly regulate mitochondrial function to maintain cell homeostasis, viability and physiological functions.
However, various factors negatively influence the mitochondria, leading to mitochondrial malfunction and dysfunction, and successively initiate acute or chronic diseases. Dysfunctional mitochondria are strongly associated with cancerous traits, such as enhanced anabolism, limitless proliferative potential, insensitivity to antigrowth signals, impaired apoptosis and decreased autophagy5, 6, as well as type 2 diabetic characteristics, which include an increased intracellular glucose concentration and reduced insulin production7.
Thus, increasing interest in this subcellular organelle has triggered mitochondria-targeting pharmacological interventions, leading to the emergence of ‘mitochondria medicine’ as a new field of biomedical research. In particular, mitochondria-targeting medicines have focused on either promoting cell health or cytotoxicity or sensing mitochondria in cells.
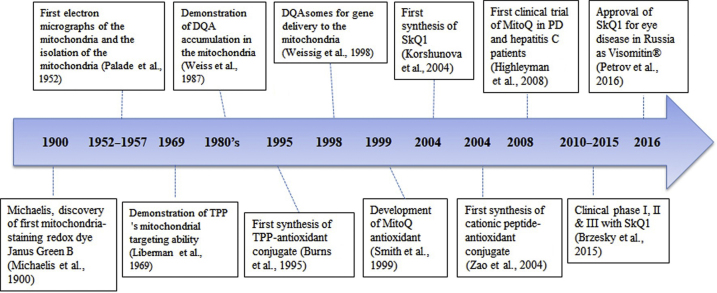
Interest in mitochondrial targeting began in the 1900s, and studies rapidly developed in 1960s due to the development of electron microscopy and mitochondrial targeting probes8, 9, 10, 11, 12, 13, 14. Several targeting moieties are available for mitochondrial targeting, such as the triphenylphosphonium (TPP), dequalinium (DQA) and cationic peptides, which are based on the mitochondrial membrane potential and lipophilicity. Two types of mitochondrial targeting strategies are available: one is a direct conjugation of a targeting moiety to a therapeutic molecule and the other involves attaching a mitochondrial targeting moiety to a nanocarrier. Direct conjugation of a thiol antioxidant with a mitochondrial targeting moiety (i.e., TPP) was initiated by Murphy׳s group in 199515. Since then, a number of studies have been performed to develop mitochondria-targeting anticancer drugs or antioxidants. Fig. 1 shows a brief summary of key historical studies.
Figure 1.
Historical progress of the development of mitochondrial-targeting drug and antioxidant molecules.
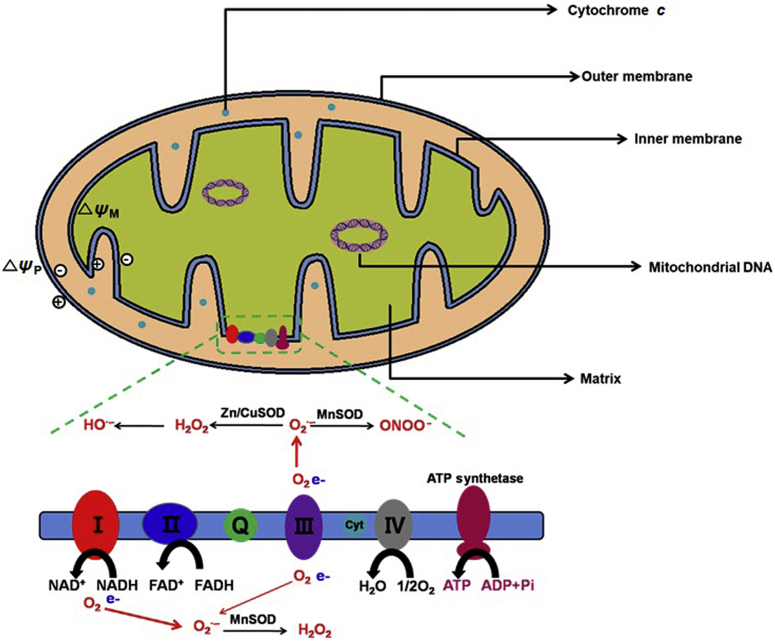
Unlike other subcellular organelles, the structure of the mitochondria is unusual and comprises four significant parts, including the outer mitochondrial membrane (OMM), inner mitochondrial membrane (IMM), intermembrane space (IMS) and matrix (Fig. 2). The specific functions of the parts are organically connected2. The structure and functions of cancer cells differ compared with normal cells5, 16. Hexokinase, the BCL-2 family, electron redox chain, voltage-dependent anion channel/adenine nucleotide translocase (VDAC/ANT) complex and mitochondrial DNA in the mitochondria all could represent targets for antitumor therapy17.
Figure 2.
Mitochondrial structure and general functions.
In this review, we summarize the commonly applied mitochondrial targeting moieties and their direct conjugations with cytotoxic agents or antioxidant agents to enhance cytotoxic or antioxidant effects, respectively. In addition, this review focuses on the conjugates of mitochondrial moieties and sensor molecules that are used for the diagnosis of mitochondria-mediated disorders.
2. Mitochondria: functions and disorders
Mitochondria are very complex organelles that harbor their own DNA and a double membrane structure with unusual lipid composition. Mitochondria have a high membrane potential of 150–180 mV (negative inside)18. Mitochondria are found in all eukaryotic cells, but the number of mitochondria per cell depends on the cell׳s specific energy requirements. These can vary due to cell type, cell-cycle stage, proliferative state and disease dysfunction. Various metabolically active organs, such as liver, brain and cardiac and skeletal muscles, contain up to several thousand mitochondria per cell, whereas stomach tissues with a low energy demand contain only a few dozen mitochondria. The shape, length, size and number of mitochondria in a cell are highly varied. Mitochondria range from small, individual spheres and short rods to long tubules and complex, interconnected, network-like structures19.
Mutations of mitochondrial DNA (mtDNA) are associated with multiple disorders, ranging from neurodegenerative diseases to cancers20. Cancer cells exhibit mitochondrial dysfunction, genetic instability with alterations (e.g., mutations, deletions or translocations) and highly glycolytic activities. Mitochondrial diseases result from functional and structural failure of the mitochondria21. Given that mitochondria generate energy, cell injury and even cell death can occur in the context of mitochondrial dysfunction. Various parts of the body (e.g., heart, brain, muscles and lungs) requiring the greatest amount of energy are strongly affected by mitochondrial energy production. Mitochondrial diseases are difficult to diagnose because each individual is differently affected by mitochondrial malfunctions and exhibit various symptoms (e.g., seizures, strokes, severe developmental delays and inability to walk, talk and see)22.
2.1. Energy production and metabolism
Mitochondria are special cellular organelles with their own genomic materials known as mitochondrial DNA (mtDNA), which may replicate independent of the nuclear DNA. The well-characterized functions of the mitochondria include energy metabolism, calcium homeostasis, redox regulation and apoptosis. As the main powerhouse of cells, mitochondria can utilize glucose, fatty acids, amino acids and other cellular materials to produce adenosine triphosphate (ATP) through a series of biochemical processes known as oxidative phosphorylation (OXPHOS), the citric acid cycle (Krebs cycle), and beta-oxidation. ATP is a complex organic molecule that is found in all forms of life. When ATP is consumed in metabolic processes, it is converted to its di- or monophosphate form (i.e., ADP or AMP, respectively). ATP consists of three components: a nitrogenous base (i.e., adenine), a sugar ribose, and a triphosphate. ATP is also a precursor of DNA and RNA and is used as a coenzyme (CoA) in cells23.
ATP is generated by a number of distinct cellular processes. The three main pathways in eukaryotes include glycolysis (pathway 1), the citric acid cycle/oxidative phosphorylation (pathway 2) and beta-oxidation (pathway 3). In aerobic environments, the overall processes of oxidizing glucose to carbon dioxide, which include pathways 1 and 2, are known as a cellular respiration and produce approximately 38 equivalents of ATP from each glucose24. In anaerobic conditions, glucose transforms to pyruvate in pathway 1 and then becomes lactate, which is finally pumped out from cells. During this process, one glucose produces 2 equivalents of ATP. Unlike glucose, fatty acids also generate ATP via beta-oxidation (pathway 3), and the number of ATP molecules produced is dependent on the number of carbons in fatty acids. Therefore, pathway 3 can generate considerably more ATP molecules compared with the other two pathways (pathways 1 and 2).
In addition, electron transport through the mitochondrial respiratory chain is an essential requirement for oxidative phosphorylation, and this process is associated with the generation of reactive oxygen species (ROS). In particular, when electrons leak from the respiratory complexes, ROS react with molecular oxygen to produce superoxide. Given that both a proper cellular ATP level and a proper redox balance are essential for cell viability and proliferation, mitochondrial dysfunction causes significant changes in cellular energy metabolism and ROS generation, thus profoundly affecting cellular fates and cellular responses against exogenously delivered drugs25.
2.2. Redox balance and reactive oxygen species (ROS)
The ROS is a general term for several reactive oxygen substrates, including superoxide ion (O2─), hydrogen peroxide (H2O2), hydroxyl radical (•OH) and singlet oxygen (1O2). ROS form through a reduction process of molecular oxygen (O2) in the presence of any inducer, such as irradiation. The excessive formation of ROS in mitochondria leads to several harmful consequences, including lipid oxidation, mtDNA/RNA damage, protein oxidation, calcium ion (Ca2+)-dependent activation of mitochondrial permeability transition pores and the release of cytochrome c, which results in the formation of apoptosomes and ultimately promotes apoptosis26. Both mitochondrial mutation and oxidative stress cause mitochondrial dysfunction that triggers cell death signaling cascades and results in organ failure and diseases2. The increased levels of ROS in cancer cells are associated with multiple changes in cellular functions, such as cell proliferation, migration, differentiation and apoptosis. Enhanced generation of ROS in cancer cells with mitochondrial dysfunction may make them more vulnerable to further oxidative stress compared with normal cells with lower ROS output. Interestingly, high levels of mitochondrial ROS generation in hypoxic cells are potentially associated with angiogenesis-related diseases, such as cancers and ischemic disorders27, 28. Oxidative phosphorylation is an essential cellular process that uses oxygen and simple sugars to create ATP.
2.3. Disorders
Mitochondrial dysfunction is associated with several chronic disorders, such as Alzheimer׳s disease (AD), Parkinson׳s disease (PD), cancers and ischemic diseases20, 22. AD is a chronic neurodegenerative disease that typically starts slowly and worsens over time29. The disease mechanism of AD could be explained by an amyloid hypothesis that traditionally points to the accumulation of beta amyloid (Aβ) peptides as the central event triggering neuron degeneration. Accumulation of aggregated amyloid fibrils, which are believed to be a toxic form of the protein responsible for disrupting the cell׳s calcium ion homeostasis, induces programmed cell death (apoptosis)30. Aβ selectively accumulates in mitochondria in the cells of Alzheimer׳s-affected brain and also inhibits certain enzyme functions and the utilization of glucose by neurons31. PD is a long-term degenerative disorder of the central nervous system that mainly affects the motor system. Mitochondrial dysfunction plays a central role in the pathogenesis of PD, and an inhibitor of complex I of the electron transport chain can induce Parkinsonism32.
Some studies suggest that mitochondrial dysfunction is the cause of type 2 diabetes, which increases intracellular glucose concentrations and reduces insulin production7. Lipid oxidation and increased acetyl CoA in the citrate molecules of mitochondria inhibit some enzymes (e.g., phosphofructokinase and pyruvate dehydrogenase) that use glucose molecules.
Cancer cells are the most rapidly growing cells; these cells require significant amounts of energy for continuous growth. Energy is mostly provided by mitochondrial respiration via ATP formation. On the other hand, mitochondrial dysfunction (e.g., ROS generation and membrane disruption) could promote apoptosis.
3. Mitochondria-targeting ligand conjugates
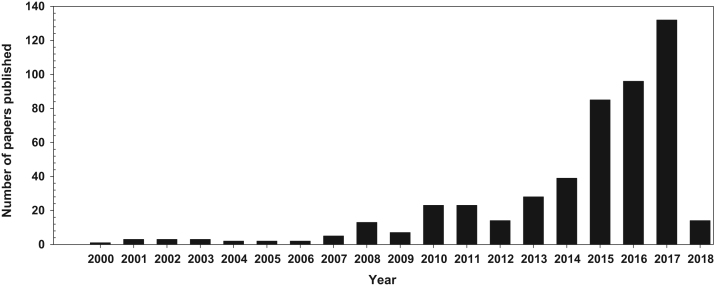
To improve therapeutic effects and reduce side effects of drugs, many researchers have become interested in subcellular targeting, especially mitochondria-targeting. The significant interest in mitochondria-targeting is underscored by the approximately 500 research articles published up to January 3, 2018 (Fig. 3). Although the number of publications is potentially inaccurate because the search was performed using several keywords (i.e., mitochondria, mitochondrial, targeting, moiety, moieties and delivery moiety) and suitable combinations are well-known in scientific databases (i.e., PubMed, Web of Science, Google Scholar and ScienceDirect), the recent dramatic increase in mitochondria-targeting studies indicate the strong value of this topic.
Figure 3.
The number of mitochondria-targeting research articles.
To date, numerous reports have revealed mitochondrial-targeting moieties and drug and antioxidant conjugates to enhance therapeutic effects. The advantage of direct conjugation of mitochondrial targeting moieties to therapeutic agents is that therapeutic agents could be localized more specifically to mitochondria, leading to rapid responses from delivering certain drugs. Based on a literature search, we can easily identify papers on TPP-anticancer drugs (doxorubicin, chlorambucil, porphyrin, proapoptotic peptides and coumarin)33, 34, 35, 36, 37, dequalinium (DQA)–drug conjugates38, peptide–anticancer drug conjugates39, 40 and (E)-4-(1H-indol-3-ylvinyl)-N-methylpyridinium iodide (F16)-anticancer drug conjugates41.
3.1. Mitochondria-targeting moieties and mechanisms
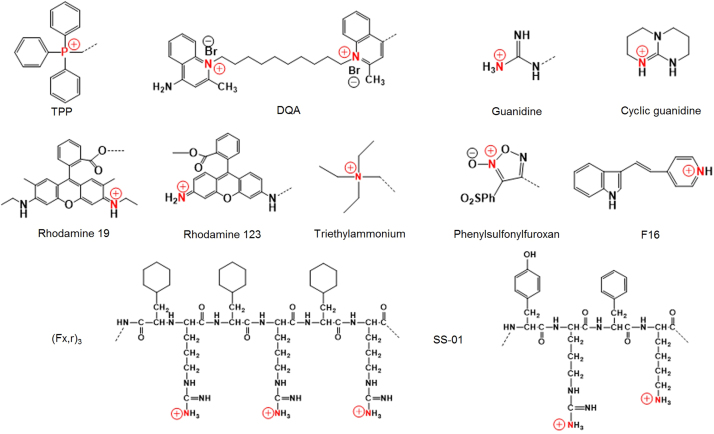
In several cases, the mitochondria of cancer cells and transformed cells exhibit significantly increased transmembrane potentials compared with normal cells42. This biological difference has been utilized as a basis to develop mitochondria-targeting compounds that may be preferentially accumulated within the mitochondria of cancer cells. Importantly, some commonly applied mitochondrial targeting moieties are available, including TPP, guanidinium, triethylammonium, pyridinium, 3-phenylsulfonylfuroxan, F16, 2,3-dimethylbenzothiazolium iodide, rhodamine 19, rhodamine 123 and DQA. Most of these agents are lipophilic cations (Fig. 4)43. These delocalized lipophilic cations (DLCs) accumulate in the mitochondria of tumor cells due to the highly negatively charged microenvironment within the mitochondrial matrix44. Herein, we briefly describe some of these agents.
-
i)
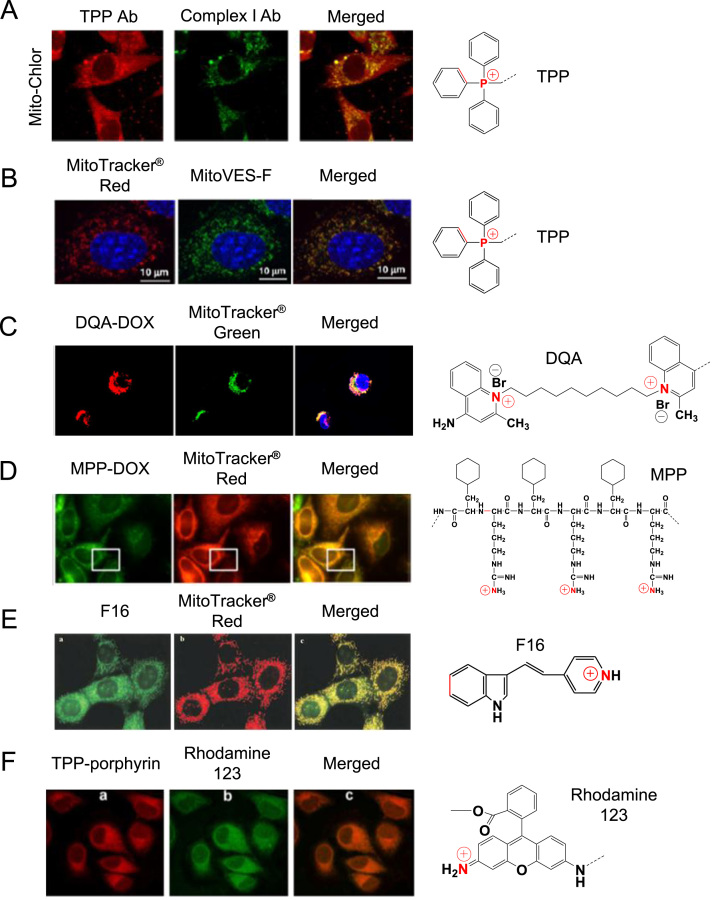
Triphenylphosphonium (TPP). TPP, a well-known mitochondrial targeting moiety, is a positively charged phosphorus atom surrounded by three hydrophobic phenyl groups that impart an extended hydrophobic surface45 (Fig. 4). TPP has been employed as a probe to explore the mitochondrial membrane potential for over 40 years. Consequently, its behavior and interaction with mitochondria are well-defined46. The relative TPP concentrations inside the negatively charged membrane compartments are increased by one order of magnitude for every 60 mV of negative membrane potential. Given the active transport of salt ions by membrane-bound pumps, the interior part of the plasma membrane is negatively charged compared with the exterior side. As a result, the plasma membrane potential generally ranges between –30 and –60 mV, which is sufficient to promote up to 10-fold accumulation of TPP inside the cell. Typical mitochondrial membrane potential is up to –180 mV, which facilitates a 1000-fold accumulation of TPP inside mitochondria47. Numerous examples are noted where TPP was used as a mitochondrial-targeting ligand33, 37, 48. For example, TPP was conjugated to chlorambucil, which is a DNA damaging anticancer agent. The cytotoxicity results of TPP–chlorambucil conjugates revealed that the conjugates exhibited approximately 12-fold reduced IC50 values compared with its counterpart (i.e., free chlorambucil) in a breast cancer cell line. The result was attributed to high accumulation of the conjugates in mitochondria as demonstrated by confocal microscopy (Fig. 5A)33. Another example involves various vitamin E analogues and TPP conjugates. Among them, TPP-vitamin E succinate (VES) conjugate (MitoVES) exhibited preferential mitochondrial accumulation37 (Fig. 5B).
-
ii)
Dequalinium (DQA). DQA is a lipophilic dication composed of two cationic quinolinium moieties linked via a 10-carbon alkyl chain (Fig. 4) that localizes in mitochondria of cancer cells (Fig. 5C)38. The compound exhibits antiproliferative activity against several cancer cell lines in vitro and antitumor effects in vivo10, 49. DQA׳s potential for delivering mitochondrial DNA to mitochondria has been investigated. In an aqueous medium, DQA single-chain bola-amphiphile molecules were self-associated and subsequently formed vesicles referred to as DQAsomes50. DQAsomes deliver pDNA to mitochondria without losing their pDNA load38.
-
iii)
Mitochondria-targeting/penetrating peptides. Recently, many cell penetrating peptides have demonstrated mitochondrial membrane permeability51. Among a variety of mitochondria-penetrating peptides (MPPs) are certain short mitochondria-permeable peptides harboring a repeating unit of lipophilic residues and cationic residues, such as (l-cyclohexyl alanine-d-arginine)3, called (Fx,r)3 (Fig. 4). These peptides exhibit mitochondrial accumulation with minimal toxicity in human cancer cells52 (Fig. 5D). A series of anticancer drug-MPP peptide conjugates have been reported39, 53. For example, MPP was linked to doxorubicin (DOX) via a succinate linkage. Szeto-Schiller (SS) peptides are another example of a peptide-targeting moiety. These peptides are small, water-soluble tetrapeptides composed of tyrosine (Tyr) or dimethyltyrosine (Dmt), arginine (Arg), phenylalanine (Phe) and lysine (Lys) residues, which selectively accumulate in the inner mitochondrial membrane, scavenge ROS, inhibit the opening of the mitochondrial permeability transition pores and conclusively inhibit the release of cytochrome c54. Using these amino acids, various SS peptides were prepared (SS-01 to SS-31). Among them, SS-01 (Tyr-d-Arg-Phe-Lys-NH2 shown in Fig. 4), SS-02 (Dmt-d-Arg-Phe-Lys-NH2) and SS-31 (d-Arg-Dmt-Lys-Phe-NH2) exhibit antioxidant efficacy due to the presence of aromatic amino acids, such as Dmt and Tyr, and subsequently scavenge ROS because unreactive tyrosyl or dityrosine radicals can react with superoxide radicals to form tyrosine hydroperoxide55. In addition, XJB Gramicidin S (Soviet) analogs are XJB peptides based on the sequence of the membrane-active gramicidin S (GS) antibiotics. Antioxidant properties of these agents are attributed to attachment to the stable free radical 4-amino-TEMPO (4-AT).
-
iv)
Guanidine. With functionalities bearing delocalized positive charges, guanidium and biguanidium groups (Fig. 4) are particularly interesting given their increased lipophilicity compared with certain molecules with localized charges. The amphiphilic porphyrin was conjugated with guanidium and biguanidium to improve mitochondrial targeting56. The conjugates exhibit high accumulation in the mitochondria and enhanced phototoxicity against cancer cells. The presence of guanidium groups caused lysosomal escape of the guanidium porphyrin conjugates via rupture of the lysosomal membrane probably via a proton sponge effect.
-
v)
(E)-4-(1H-Indol-3-ylvinyl)-N-methylpyridinium iodide (F16). F16 is also a delocalized cation and accumulates in the mitochondrial matrix (Fig. 5E). Interestingly, F16 was discovered during a high-throughput screen of a chemical library for antiproliferative compounds against neu-expressing mammary epithelial cells57. Mitochondrial accumulation of F16 caused depolarization of the mitochondrial membrane, disruption of the integrity of the mitochondrial structure and opening of the mitochondrial permeability transition pore. These successive events caused the release of cytochrome c and cell cycle arrest, subsequently leading to cell death57. Further, its anti-proliferative effects were noted in a variety of mouse mammary tumors and human breast cancer cell lines.
-
vi)
Rhodamine. Rhodamine derivatives are also mitochondrial-targeting agents given their binding affinity to the mitochondrial membranes, subsequently damaging the electron transport chain58. Preferential mitochondrial staining via rhodamine 123 was revealed in src-transformed cells43 (Fig. 5F). Mechanistically, its mitochondrial accumulation is caused by its lipophilic and cationic properties, which help it to cross the double mitochondrial membranes and remain within the negatively charged mitochondrial matrix59. Similar to rhodamine 123, rhodamine 19 is a potential mitochondrial-targeting moiety60. Its mitochondrial delivery capability was confirmed when rhodamine 19 effectively substituted TPP via chemical modification of TPP–drug conjugates to form rhodamine 19–drug conjugates61. In particular, rhodamine 19 can be considered as a mitochondria-targeted cationic uncoupler given its protonophorous uncoupling effect and its equilibration across the mitochondrial membranes in a Nernstian fashion60.
Figure 4.
Some common mitochondria-targeting moieties. Red color indicates cationic atoms and dot line indicates conjugation-formable parts with drug molecules.
Figure 5.
Evidence of mitochondrial accumulation of some mitochondrial targeting moieties monitored by a confocal microscopy: (A) Mito-Chlor (TPP–chloroambucil conjugates); adapted with permission from the reference33 (Copyright © 2013 American Chemical Society); (B) green fluorescence-labeled MitoVES (MitoVES-F) in NeuTL cells; reproduced with permission from the reference 37 (Copyright © 2011 Elsevier Inc.); (C) DQA–DOX conjugates in A549 cells; reproduced with permission from the reference43 (Copyright © 2015 Springer Nature); (D) (Fx,r)3–DOX (i.e., MPP–DOX) conjugates; adapted with permission from the reference39 (Copyright © 2013 American Chemical Society); (E) F16 (green); reproduced with permission from the reference57 (Copyright © 2002 CELL PRESS); and (F) TPP–porphyrin (red); reproduced with permission from the reference34 (Copyright © 2009 Elsevier, B.V.). Here, both an anti-Complex I antibody and anti-TPP antisera were used for Mito–Chlor; MitoTracker® Red was used for MitoVES–F, (Fx,r)3–DOX and F16; MitoTracker® Green was used for DQA–DOX and porphyrin conjugates; and TPP–porphyrin was used for rhodamine.
3.2. Applications of mitochondria-targeting drug conjugates
Drug candidates for mitochondrial targeting could be categorized based on their purposes and their modes of action in mitochondria. Regarding drug effects, the use of cytotoxic drugs, antioxidants or sensing/imaging molecules could be considered. Regarding mode of action, some drugs, such as doxorubicin, taxane derivatives, resveratrol, porphyrin and vitamin E43, exhibited their therapeutic activities in mitochondria. Certain drugs, including lonidamine, arsenite, betulinic acid, CD437 and proapoptotic peptides, act directly on mitochondrial membranes and/or the permeability transition pore complex (PTPC)62. In addition, the molecules are involved in the oxidation of thiols, generation of ROS and nitrogen oxide, depletion of ADP/ATP, alkalinization of the mitochondrial matrix, reduction of the mitochondrial transmembrane potential, production of ganglioside GD3, calcium ion release, activation of apical caspases and a translocation of proapoptotic BCL-2 family members62, 62.
3.2.1. Mitochondria-targeting cytotoxic drugs
To kill cancers and cells involved in other chronic disorders, mitochondria-targeting cytotoxic drugs could interact specifically with mitochondrial proteins and accumulate in the mitochondrial matrix via an electrochemical gradient. Numerous studies have applied TPP to anticancer drugs, and some studies have applied specific peptides to chlorambucil and doxorubicin39, 63. Here, we discuss some examples, and Figure 6, Figure 7 illustrate a summary of toxic mitochondrial-targeting moiety–drug conjugates.
Figure 6.
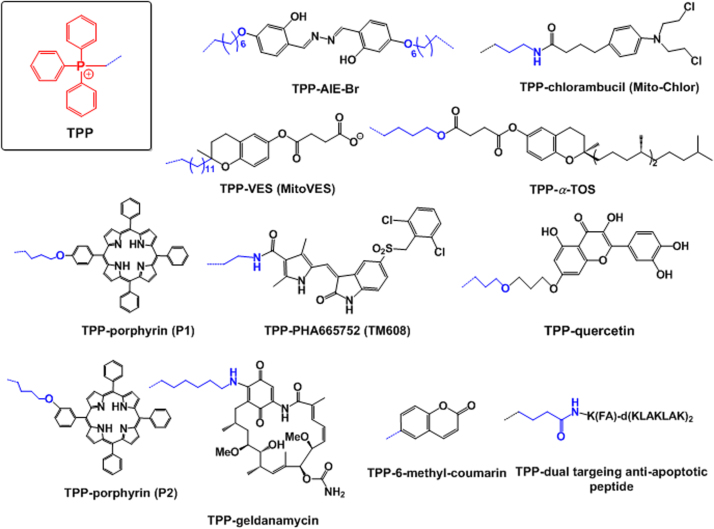
Some examples of cytotoxic drugs conjugated with the mitochondria-targeting moiety triphenylphosphonium (TPP). Mitochondria-targeting moieties, drugs and linkers are denoted by red, black and blue, respectively. The blue dotted lines indicate connections between a mitochondria-targeting moiety and linker.
Figure 7.
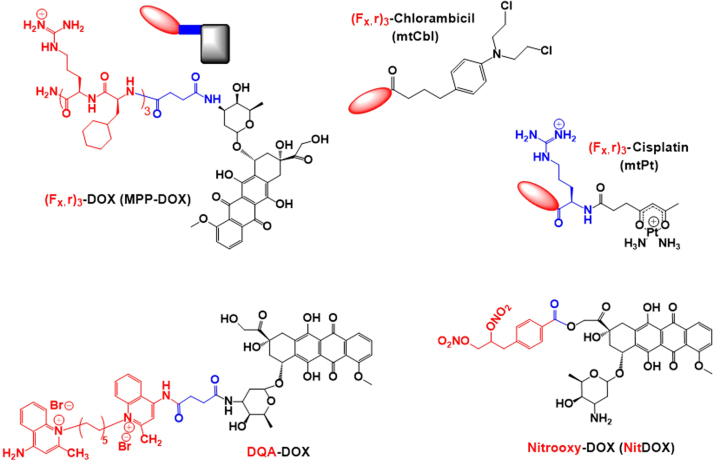
Some examples of cytotoxic drugs conjugated with the mitochondria-targeting moiety (Fx,r)3 and mitochondrial-targeting doxorubicin (DOX) conjugated with various targeting moieties, such as (Fx,r)3, DQA, nitrooxy (Nit) and 3-phenylsulfonylfuroxan (Fur). Mitochondria-targeting moieties, drugs and linkers are denoted in red, black and blue, respectively.
The most remarkable mitochondrial targeting moiety TPP has been chemically attached to a variety of cytotoxic drugs, such as salicylaldazine, chlorambucil, vitamin derivatives, porphyrin derivatives, and quercetin. First, an aggregation-induced emission (AIE) fluorophore called salicylaldazine was chemically linked with TPP, resulting in a synthesis of TPP–AIE–Br conjugates (i.e., AIE–mito–TPP, Fig. 6). AIE–mito–TPP conjugates, which were designed as image-guided mitochondrial targeting therapeutics for cancer treatment64, more quickly and more selectively accumulated in mitochondria of cancer cells compared with normal cells. Mitochondrial accumulation of AIE–mito–TPP conjugates reduced the mitochondrial membrane potential, induced ROS generation, inhibited ATP production and negatively affected essential cancer cell progress.
Second, chlorambucil from the nitrogen mustard class of DNA-alkylating agents was conjugated with TPP via an amide linkage, forming TPP–chlorambucil conjugates (i.e., Mito–Chlor, Fig. 6)33. Mito–Chlor exhibited increased anticancer efficacy compared with free chlorambucil in a pancreatic tumor in vivo. Although Mito–Chlor did not directly damage mtDNA to induce cell death, its mitochondrial accumulation and its indirect interaction with nuclear DNA (nDNA), which is the target of free chlorambucil, suggested that the mtDNA damage induced is sufficient to cause cell death. In addition, its mode of action is distinct from the mode of action of non-targeted chlorambucil.
Third, vitamin E succinate (VES) or alpha-tocopheryl succinate (αTOS), which can selectively induce apoptosis in cancer cells, were conjugated with TPP. As shown in Fig. 6, the resultant TPP–VES (MitoVES) or TPP–αTOS conjugates were synthesized37. In particular, mitochondrial accumulation of MitoVES conjugates generated increased ROS levels and more efficient apoptosis, leading to 10-fold greater antitumor efficacy compared with non-targeted αTOS in two different tumor models37.
Fourth, it is a challenge to exactly localize a photosensitizer (PS) at the target site, such as mitochondria, because light-induced PS generates very short living singlet oxygen molecules. Thus, TPP was directly conjugated with pheophorbide-a, a hydrophobic PS, to improve the photodynamic efficacy of pheophorbide-a through delivery to mitochondria65. TPP–pheophorbide-a conjugates exhibited enhanced phototoxic efficacies compared with the non-target counterpart (i.e., free pheophorbide-a) in both in vitro and in vivo studies. In addition, via reactions of meso-tetraphenylporphyrin with TPP and triethylammonium, two different types of TPP-porphyrin derivatives (Fig. 6) were synthesized34. Their mitochondrial localization was confirmed by overlapping the red fluorescence of the conjugates and the green fluorescence of rhodamine 123, which exhibits intrinsic mitochondrial targeting capability (Fig. 5F).
Fifth, MET (mesenchymal-epithelial transition) overexpression in cancer cells is associated with the localization of MET proteins in the mitochondria, leading to altered cellular metabolism, including enhanced glycolysis, increased tricarboxylic acid cycle activity, and increased oxidative phosphorylation66. Thus, TPP was attached to a selective MET kinase inhibitor PHA665752, and the resultant conjugate (i.e., TPP-PHA665752 conjugate, see Fig. 6) referred to as TM608 localized to mitochondria67. Rapid accumulation of TM608 in mitochondria suppressed MET activation in high MET-expressing, erlotinib-resistant cells.
Sixth, quercetin, which exhibits mitochondria-associated pharmacological activity, including antioxidation and anticancer activity68, was chemically linked with TPP via an nucleophilic substitution reaction to generate TPP–quercetin conjugates (shown in Fig. 6) to deliver the drug (i.e., quercetin) to mitochondria69. The conjugates triggered increased levels of ROS, sequentially activating depolarization of the mitochondrial membrane potential and the cytosolic release of cytochrome c from the mitochondria. Subsequent activation of the caspase cascades caused increased anticancer effects of the conjugates compared with free quercetin in vitro and in vivo.
Doxorubicin (DOX) is an anti-cancer drug that intercalates into DNA and inhibits the progression of Topoisomerase II, leading to cell death. The drug was modified with several types of mitochondria-targeting moieties, such as (Fx,r)3, nitrooxy, 3-phenylsulfonylfuroxan, and DQA (Fig. 7). For example, (Fx,r)3–DOX (mtDOX) conjugates, which were synthesized by a coupling reaction between (Fx,r)3 and DOX, delivered DOX into the mitochondria39 (Fig. 4E). Interestingly, mtDOX did not exhibit more effective cytotoxicity compared with free DOX in DOX-sensitive A2780 cells. However, mtDOX was not a substrate for a p-glycoprotein (P-gp) efflux pump in contrast to its parent drug DOX. Thus, mtDOX exhibited significantly greater therapeutic effects compared with free DOX in DOX-resistant A2780/ADR cells39. Using (Fx,r)3, mitochondria-targeting (Fx,r)3-platinum(II) (mtPt) conjugates and (Fx,r)3-chlorambucil (mtCbl) conjugates (Fig. 7) were also synthesized and exhibited mitochondrial accumulation40, 70. In addition, DOX was modified with 4-(2,3-dinitrooxypropyl)benzoic acid for enhanced mitochondria-targeting abilities. For the chemical conjugation of 4-(2,3-dinitrooxypropyl)benzoic acid with the free drug, DOX derivatives such as 14-bromo/14-chlorodaunorubicine hydrobromide were used, and the resultant mitochondria-targeting DOX conjugates were abbreviated as NitDOX71, 72 (Fig. 7). Compared to free DOX, NitDOX was preferentially accumulated in the mitochondria and then released NO in response to mitochondrial aldehyde dehydrogenase and P450 enzyme. The released NO further stimulated ROS and RNS generation for cytosolic release of cytochrome c in the mitochondria. Unlike DOX-mediated topoisomerase II inhibition in nucleus, the NO release of NitDOX caused apoptosis in the mitochondria, leading to improved cytotoxicity against drug-resistant cells. In general, mitochondria-targeting moieties have both hydrophilicity and a positive charge. However, although the introduction of the nitrooxy group into NitDOX made it more hydrophobic than free DOX, as confirmed by a hydrophobicity assay (i.e., an octane assay), the nitrooxy group was not positively charged. Thus, its driving force for selective mitochondrial accumulation was not clearly described. It seems possible that NitDOX could use the positive charge in DOX because the intracellular destinations of hydrophilic DOX derivatives (i.e., DOX·HCl) and hydrophobic DOX derivatives (i.e., DOX free base) are the nucleus and mitochondria, respectively73, 74. Also, regarding DQA-DOX conjugates (Fig. 7), the conjugate was further loaded into a polymeric micelle, and conjugate-loaded micelles suppressed drug resistant tumor growth38.
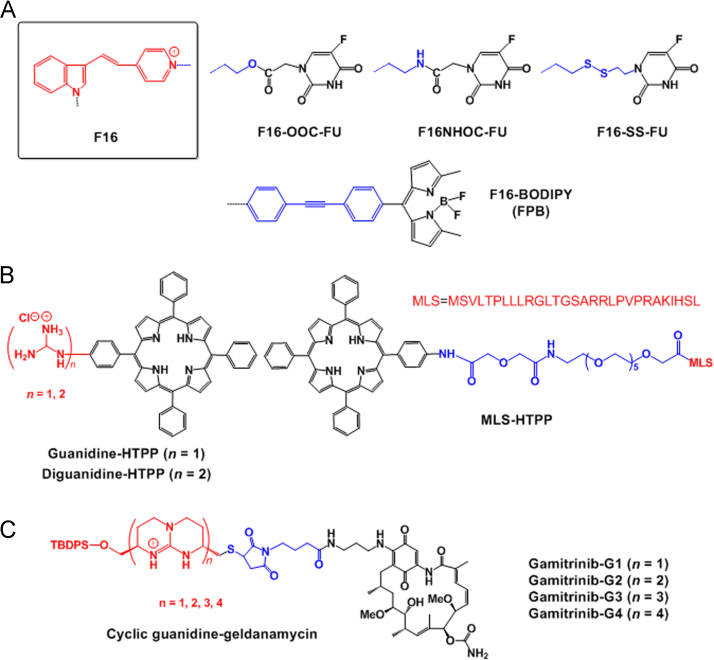
Fluorouracil (5-FU) has been conjugated with the selective mitochondria-localizing compound F16 to reduce its side effects and enhance its selectivity towards cancer cells. Specifically, the linkages in the F16–FU conjugates were altered via the introduction of a C–C bond, ester bond, amide bond and disulfide bond, and the resultant conjugates were referred to as F16–FU, F16–OOC–FU, F16–NHOC–FU and F16–SS–FU conjugates, respectively75 (Fig. 8A). F16–OOC–FU conjugates exhibited the highest cytotoxicity among the conjugates but exhibited cytotoxic activities similar to 5-FU in a tumor cell line. However, in a normal cell line, F16–OOC–FU conjugates exhibited reduced cytotoxic effects compared with 5-FU based on the former׳s significant mitochondrial targeting ability75. In addition, the mitochondrial targeting moiety F16 was introduced to boron–dipyrromethene (BODIPY) with a phenylethynyl linker, resulting in the synthesis of bifunctional mitochondria-targeting anticancer drug conjugates (F16–phenylethynyl–BODIPY conjugate; also called FPB, Fig. 8A). When FPB was administered to cancer cells and normal cells, its internalized amounts were higher in cancer cells (e.g., SGC-7901) than in normal cells (e.g., GES-1). However, the interesting results regarding the selection of tumor cells by the cationic FPB were not explained in the original paper41. These results could possibly be caused by different binding affinities because cationic nanoparticles bind to depolarized plasma membranes more than to polarized plasma membrane76. Furthermore, the plasma membranes of cancer cells are more depolarized than those of normal cells77, but this explanation has not been confirmed. After cancer cells internalize FPB, the F16 moiety in FPB preferentially guides FPB into mitochondria owing to both the hydrophobic and cationic characteristics of F1657. In the mitochondria of cancer cells, the delivered FPB increased superoxide levels, depolarized mitochondrial membrane potentials, disrupted mitochondrial structural integrity, opened mitochondrial permeability transition pores, arrested the cell cycle, induced the release of cytochrome c, generated ROS, and finally triggered cell death via apoptosis. As a result, FPB exhibited 2- to 20-fold increased cytotoxicity in cancer cells compared with normal cells. In particular, the fluorescence of both F16 and BODIPY indicates FPB as a potential conjugate for both mitochondria targeting and fluorescent imaging41.
Figure 8.
Some examples of (A) mitochondria-targeting cytotoxic drugs conjugated with the F16 moiety; (B) mitochondria-targeting porphyrin conjugates; and C) cyclic guanidine–geldanamycin conjugates.
PS has been used for photodynamic therapy (PDT), which represents an effective and minimally invasive therapeutic approach for treating cancers. Although some photosensitizers, such as protoporphyrin IX78, phthalocyanine Pc479 and pheophorbide a80, have been known to localize in the mitochondria for a long time, it is not observed for all PSs80. Thus, PSs must be targeted to specific organelles in cells to improve their activities. Among several mitochondria-targeting PSs developed34, 56, 65, 81, porphyrin derivatives, such as 5-(4-hydroxy-phenyl)-10,15,20-triphenylporphyrin (HTPP), were linked with various mitochondria-targeting moieties, including guanidine, diguanidine and mitochondria localization sequences (MLS), to synthesize guanidine–HTPP conjugates, diguanidine–HTPP conjugates and MLS–HTPP conjugates (Fig. 8B). Among three examples, guanidine–porphyrin conjugates exhibited increased mitochondrial accumulation and two-fold increased phototoxicity compared with the other two conjugates against cancer cells34. Specifically, these conjugates exhibited enhanced mitochondrial accumulation compared with MLS–porphyrin given the lysosomal escape properties of the guanidine group.
In addition, the small molecule gamitrinibs (also known as geldanamycin), which is an Hsp90ATPase inhibitor in mitochondria of tumor cells, was chemically linked with the mitochondria-targeting moiety, cyclic guanidinium for enhanced mitochondria targeting. Interestingly, monomer, dimers, trimers and tetramers of cyclic guanidinium were introduced to gamitrinib (i.e. gamitrinib–G1, gamitrinib–G2, gamitrinib–G3 and gamitrinib–G4 see Fig. 8C). gamitrinib–G3 and gamitrinib–G4 exhibited increased mitochondrial accumulation and 6- to 10-fold increased anticancer efficacy vs. gamitrinib–G1 and gamitrinib–G2, due to different membrane depolarization activities82.
3.2.2. Mitochondria-targeting antioxidant drugs
Antioxidants are drugs that can neutralize free radicals by accepting or donating electron(s) to eliminate the unpaired condition of the radical. Antioxidant drugs can directly react with certain reactive radicals and remove the radicals, whereas antioxidant-radicals may become new free radicals that are less active, longer lived and less dangerous than initial reactive radicals6. Generally, antioxidant chemicals have aromatic ring structures and delocalize unpaired electrons by donating a hydrogen atom toward free radicals formed during certain oxidation reactions83. Antioxidants exert these effects via several mechanisms: 1) scavenging the species that initiate peroxidation, 2) quenching singlet oxygen molecules, 3) chelating metal ions, 4) breaking chain reactions of free radicals and 5) reducing superoxides84. Another important function of antioxidants is to regulate ROS-related enzymes. Antioxidants can diminish the cellular level of free radicals either by inhibiting the activities and expression of free radical generating enzymes, such as NAD(P)H oxidase and xanthine oxidase (XO), or by enhancing the activities and expression of antioxidant enzymes, such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX)6. These antioxidant enzymes provide an important defense against free radicals. Representative antioxidants include vitamin C, vitamin E, uric acid, glutathione, phenolics (e.g., phenol and polyphenols), flavonoids, carotenoids, steroids and thiol compounds that are derived from a variety of natural sources, such as fruits, vegetables, spices, grains and herbs85.
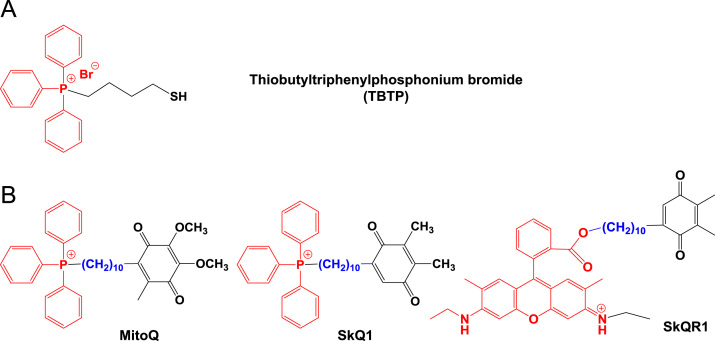
Cell proliferation in tumors is stimulated by ROS (e.g., superoxide ) and hydrogen peroxide [H2O2] at low picomolar levels86. Thus, some strategies to inhibit proliferation of cancer cells have mitigated intracellular ROS levels with mitochondria-targeting antioxidants in vitro and in vivo87, 88, 89, 90. In 1995, a thiol reagent was firstly modified with a mitochondria-targeting TPP moiety, leading to the synthesis of thiobutyltriphenylphosphonium bromide (Fig. 9A) to specifically deliver a thiol antioxidant into the mitochondria15. Since then, a series of TPP–antioxidant conjugates have been synthesized: TPP–ubiquinone conjugates (e.g., MitoQ), TPP–carboxy–proxyl conjugates (e.g., MitoCP), and TPP–vitamin E analogues conjugates (e.g., MitoE). Herein, we describe some examples of mitochondria-targeting antioxidants (Figure 9, Figure 10, Figure 11).
Figure 9.
(A) The first mitochondria-targeting antioxidant thiobutyltriphenylphosphonium bromide; and (B) mitochondria-targeting ubiquinone derivatives.
Figure 10.
Some examples of mitochondria-targeting antioxidants with (A) either pyrrolidine nitroxide or piperidin nitroxide derivatives; and (B) pyrroline nitroxide derivatives.
Figure 11.
Some examples of mitochondria-targeting antioxidants with (A) vitamin derivatives; (B) acidic derivatives; and (C) enzyme mimetics.
Ubiquinone is a mitochondrial respiratory chain component buried within the lipid core of the inner membrane where it is reduced by two electrons from complexes I or II. The resulting ubiquinol subsequently donates electrons to complex III (Fig. 2)91. Ubiquinone reduces the generation of lipid peroxyl radicals within mitochondria, similarly preventing lipid peroxidation92. However, reduced levels of ubiquinone in mitochondria could disrupt the mitochondrial reduction-oxidation balance, resulting in the loss of mitochondrial homeostasis. Thus, mitochondria-targeting TPP was covalently linked with a ubiquinone derivative via a bridge of an aliphatic carbon chain, and the resultant [10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl] triphenylphosphonium methane sulfonate was named MitoQ, one of the TPP-ubiquinone conjugates93 (Fig. 9B). MitoQ demonstrated its protective effects in normal and diseased tissues, such as colitis, cardiovascular diseases and neurodegenerative diseases, in in vitro and in vivo experiments and clinical trials94, 95, 96, 97, 98, 99, 100, 101. In addition, MitoQ was applied to manipulate and investigate the status and effects of mitochondrial antioxidants in mitochondrial oxidative damages in apoptotic cell death93. Since then, numerous studies have been performed using MitoQ102, 103, 104, 105, 106. In addition, the TPP–plastoquinone derivative SkQ1 (Fig. 9B) was synthesized107 and represents a successful mitochondria-antioxidant conjugate. In the name SkQ1, Sk refers to a penetrating cation of “Skulachev ion”, and Q refers to quinone61. SkQ1 exhibits two-fold increased hydrogen peroxide inhibition potency compared with its derivative MitoQ161. SkQ1 has been evaluated in vitro and in vivo and is currently approved as a droplet formulation (called Visomitin®) for eye diseases in Russia108.
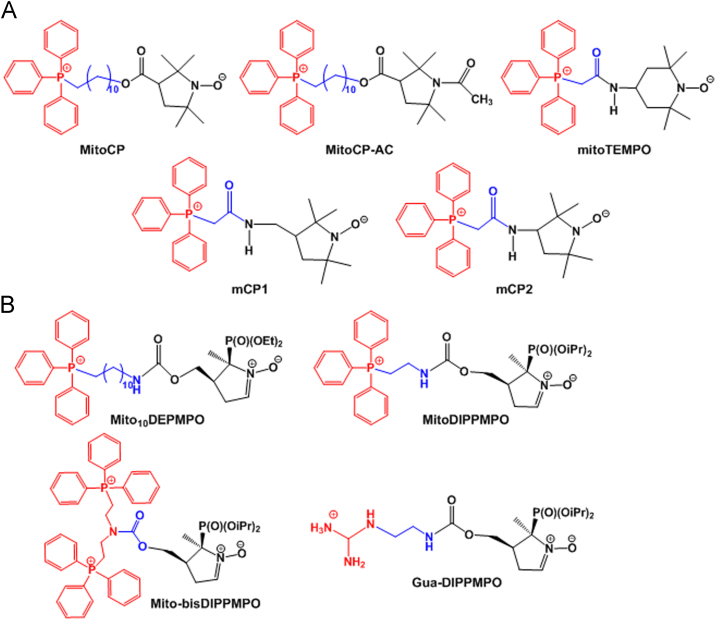
The antioxidant 3-carboxy proxyl, which contains a pyrrolidine nitroxide group, was chemically linked with mitochondria-targeting TPP, thus forming TPP-3-carboxy proxyl (MitoCP) (Fig. 10A). Its acetamide (Ac) derivative MitoCP-Ac (Fig. 10A) was synthesized by replacing the nitroxide in the MitoCP with Ac. Both MitoCP and MitoCP-Ac inhibited tumor growth via a superoxide dismutation-independent mechanism in mitochondria and activated AMPK and decreased FOXM1 expression via AKT/FOXO3/FOXM1 signaling pathways86. In addition, the modi-fied derivatives 3-[(2-(triphenyphosphonio)acetamido)methyl]-2,2,5,5-tetra-methylpyrrolidin-1-oxyl (mCP1) and 3-(2-(triphe-nyphosphonio)acetamido)-2,2,5,5-tetra-methylpyrrolidin-1-oxyl (mCP2) (Fig. 10A) were synthesized using both antioxidant and antihypertensive drugs109, 110. In vitro studies in human aortic endothelial cells showed that the cellular uptake of mCP1 and mCP2 was 1.37-fold increased vs. the known antioxidant (2-(2,2,6,6-tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium) [mitoTEMPO] (Fig. 10A) at high concentrations, indicating greater bioreduction resistance of the former proxyl nitroxide (i.e., mCP1 and mCP2). In contrast, in vivo studies in angiotensin II-infused mice revealed that mCP1 and mCP2 exhibited anti-hypertensive and antioxidant effects similar to mitoTEMPO. Thus, mitochondria-targeting proxyls could be effective antioxidants with anti-hypertensive activity because proxyls (i.e., the nitroxides of pyrrolidine series) exhibit increased resistant to bioreduction compared with 4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPOL)111. A library of TPP–nitroxide conjugates was prepared, and their radioprotective effects were assessed in γ-irradiated mouse embryo cells and human epithelial BEAS-2B cells111. From the library, [2-(1-oxyl-2,2,6,6-tetramethylpiperidin-4-ylimino)-ethyl]-triphenyl-phosphonium (TPEY–TEMPO) caused externalization of phosphatidylserine on the cell surface and inhibited the release of cytochrome c from mitochondria, leading to significant inhibition of radiation-induced apoptosis111.
Radical-trapping chemicals, such as 5-(diethylphosphono)-5-methyl-1-pyrroline N-oxide (DEPMPO) and 5-(diisopropoxyphosphoryl)-5-methyl-1-pyrroline-N-oxide (DIPPMPO), were modified by mitochondria-targeting TPP or guanidinium moieties (Fig. 10B)112. In mitochondrial uptake studies after a 30 min incubation, Mito10DEPMPO and MitoDIPPMPO exhibited 24-fold and 12-fold increased mitochondrial uptake compared with non-targeted DEPMPO and DIPPMPO, respectively. The lipophilic cation of the mitochondrial targeting moiety is thought to cause mitochondrial accumulation of the conjugates in a membrane potential-dependent manner. In addition, all TPP-modified derivatives (e.g., MitoDEPMPO and MitoDIPPMPO) efficiently reacted with superoxides and then formed stable adducts. In particular, the apparent half-life of the O2•− adducts were 22 min for Mito10DEPMPO and 73 min for MitoDIPPMPO, indicating that MitoDEPMPO and MitoDIPPMPO would be potential spin traps for mitochondrial superoxide.
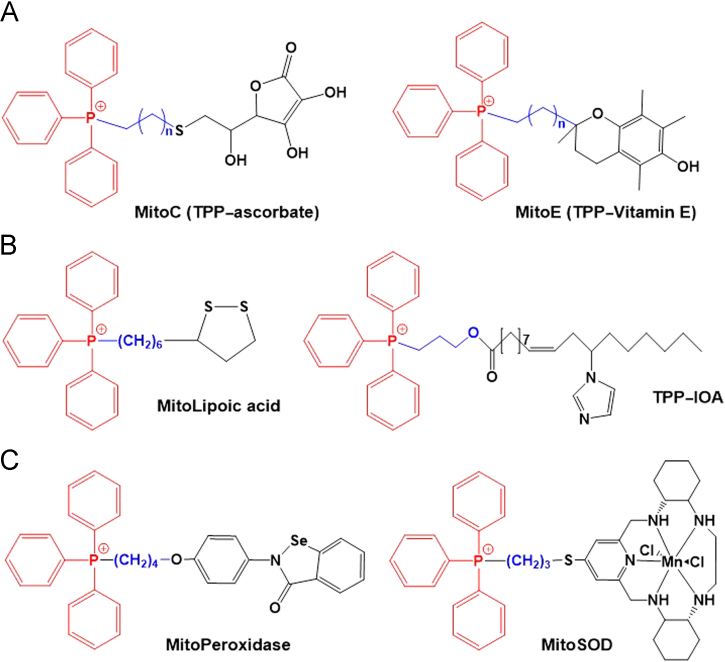
Vitamins, such as vitamin C and vitamin E, have also been designed for mitochondrial delivery. First, for hydrophilic ascorbate (i.e., vitamin C), TPP conjugates (i.e., TPP-ascorbates or MitoC analogues, Fig. 11A) were synthesized with various lipophilicities113. Among various MitoC analogues, MitoC with an 11-carbon linker accumulated in mitochondria and was continuously recycled to its active form for antioxidant activity by the mitochondrial glutathione and thioredoxin systems. MitoC was clearly shown to be a more effective antioxidant than ascorbate. Second, when conjugating vitamin E with a TPP moiety in the presence of alkyl linkers, TPP-vitamin E (MitoE) conjugates (Fig. 11A) were synthesized114. MitoE conjugates accumulated in mitochondria and exhibited increased prevention of lipid peroxidation, mitochondrial oxidative damage and mtDNA damage compared with non-targeted compounds.
Oleic acid, which is known as a mono-unsaturated omega-9-fatty acid, was modified by a substitution with an imidazole moiety. The resultant imidazole-substituted oleic acid (IOA) was conjugated with TPP in the presence of 3-hydroxypropyl linker, resulting in the formation of TPP-IOA conjugates (Fig. 11B). The TPP-IOA conjugates interacted directly with cytochrome c in mitochondria, preventing both the cytosolic release and peroxidase activity of cytochrome c, inhibiting oxidation of cardiolipin and consequently inhibiting apoptosis in SH-SY5Y cells115. As a specific inhibitor of cytochrome c, TPP–IOA conjugates could represent potential therapeutics to block cell death mediated by the mitochondrial mitophagy pathway. IOA positively affects oxidative phosphorylation, an important energy production pathway in proliferative cells (e.g., cancer cells) under hypoxic conditions. Therefore, the application of TPP-IOA conjugates for anti-apoptotic therapy in highly glycolytic cells might be limited.
Lipoic acid (LA) is a widely used antioxidant that protects mitochondria from oxidative damage in vivo based on the reduction of LA to dihydrolipoic acid (LAH2). To obtain effective mitochondrial protection, an α-lipoyl derivative was chemically linked with TPP for the synthesis of TPP–LA (MitoLA) conjugates116 (Fig. 11B). MitoLA exhibited several hundred-fold more rapid accumulation in mitochondria compared with its non-targeting counterpart (i.e., LA). The mitochondrial membrane potentials improved membrane penetration of lipophilic cations of MitoLA vs. the hydrophilic anions of LA. In particular, MitoLA conjugates were reduced to their active antioxidant form dihydro MitoLA (i.e., MitoLAH2) by thioredoxin and lipoamide dehydrogenase but not by thioredoxin reductase (TrxR).
To mimic oxidative stress scavenging enzymes in mitochondria, such as peroxidase and superoxide dismutase (SOD), certain chemical mimics were chemically modified to target their delivery in the mitochondria. First, 2-[4-(4-triphenylphosphoniobutoxy) phenyl]-1,2-benzisoselenazol-3(2H)-one iodide (MitoPeroxidase) was designed. Its co-delivery with ebselen reduced mitochondrial oxidative stress and then sequentially decreased oxidative stress-induced apoptosis117. Second, a superoxide ()-selective pentaaza macrocyclic Mn(II) SOD mimetic was conjugated to TPP to generate MitoSOD (Fig. 11C), a mitochondria-targeted SOD mimetic118. MitoSOD mimetics rapidly accumulated in the mitochondria by a driving force of extensive mitochondrial membrane potential, and the mitochondrial delivery of these compounds did not induce loss of Mn2+ and SOD biological activity. In particular, the designed MitoSOD mimetics selectively protected the mitochondria from superoxide damage, further indicating that the mimetics are effective alternatives to SOD.
3.2.3. Delivery of sensing/imaging molecules to mitochondria
Many chronic disorders are caused by the generation of mitochondrial ROS, reactive nitrogen species (RNS) and sulfur dioxide species (SOS) in the human body119. Thus, to understand and detect characteristics (e.g., concentration, lifetime, species, etc.) of ROS, RNS and SOS, the development of mitochondrial-targeted sensing materials for these reactive species is challenging. The detection of mitochondrial-targeted sensing materials is achieved via a fluorescence technique given the highly sensitivity and non-destructive characteristics of these compounds120, 121.
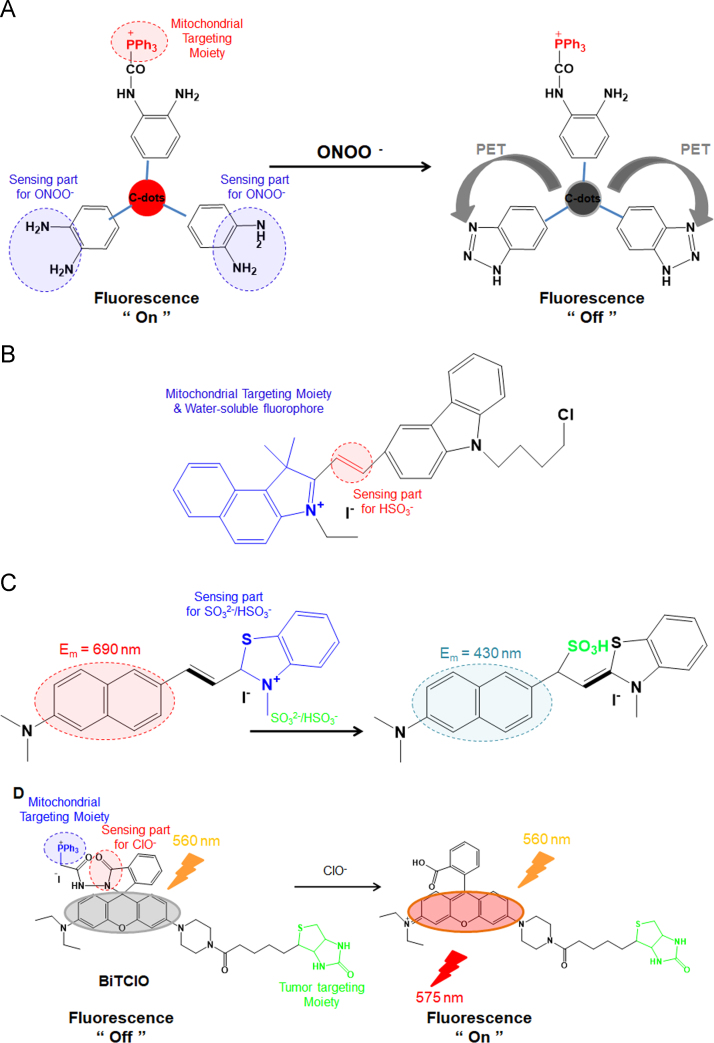
First, certain carbon dots (C-dots) were chemically modified with TPP, and the resultant TPP-C-dot conjugates were synthesized as mitochondria-targeting nanoprobes for detecting peroxynitrite (ONOO–)122. As shown in Fig. 12A, the o-diaminobenzene moiety on the surface of fluorescent TPP-C-dot conjugates selectively reacts with peroxynitrite and •NO2 (e.g., a kind of free radical intermediate obtained from the decomposition of ONOO−) and is then converted to a benzotriazole moiety by a photoinduced electron transfer (PET) process. During the reaction, the fluorescence of the TPP-C-dot conjugates is quenched. Using the two characteristics of TPP-guided mitochondrial accumulation and peroxynitrite-responsive fluorescence on/off switching, the fluorescent TPP-C-dot conjugates could be applied as mitochondrial nanoprobes for sensing the presence of ONOO− and •NO2 in the mitochondria and have the potential to be developed as intracellular diagnostic probes for detecting a variety of diseases related to ONOO− and •NO2.
Figure 12.
Mitochondrial-targeting sensor molecules and their activation. (A) Triphenylphosphonium carbon dot conjugate (TPP-C-dots) and its interaction with the peroxinitrite radical124; (B) design and sensing mechanism of the probe CZBI for bisulfite anion (HSO3–)121; (C) response mechanism of the mitochondrial-targeted near-infrared fluorescence probe NDMBT for HSO3–/SO32− sensing126; and (D) structure of BiTClO and its reaction mechanism with the hypochlorite anion (ClO–)128.
Both cytochrome c oxidase (COX; also called as complexes IV) and ATP synthase (complexes V) subunits are significant complexes of oxidative phosphorylation (OXPHOS) in mitochondrial respiration. Their mRNA levels could be negatively influenced by mitochondrial sulfur dioxide (SO2) derivatives, inducing various diseases, such as lung cancers and neurological disorders. Thus, their selective removal is significant. A mitochondria-targeted fluorescent probe CZBI (abbreviated from carbazole and benzo[e]indolium) was prepared via a condensation reaction of 9-(4-chlorobutyl)-9H-carbazole-3-carbaldehyde with 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indolium iodide. As shown in Fig. 12B, the nanoprobe conjugates with carbazole and benzo[e]indolium exhibited high sensitivity and high selectivity for SO2 derivatives compared with other sulfur-containing molecules, such as hydrogen sulfide, cysteine, homocysteine and glutathione, in aqueous solutions123. CZBI can be applied for the detection of intrinsically generated intracellular sulfur dioxide derivatives in the mitochondria by ratiometric fluorescence imaging in living cells.
In addition, for ultrafast and ratiometric detection of SO2 derivatives and live cell imaging, the mitochondrial-targeted near-infrared fluorescence probe NDMBT was synthesized by refluxing 6-(dimethylamino)-2-naphthaldehyde with 2,3-dimethylbenzothiazolium iodide in methanol124. The nanoprobe detects SO2 derivatives with a ultrafast response time (10 s) using a Michael addition reaction (Fig. 12C). In particular, NDMBT has large hypsochromic shift, high photostability, excellent selectivity and high sensitivity in aqueous media. The detection limit is 43 nmol/L for SO2 derivatives. Specifically, imaging results demonstrated that the NDMBT probe is mitochondria targeted and can be applied to imaging both exogenous and endogenous SO2 derivatives in live cells.
The peroxidation reaction by myeloperoxidase (MPO) chemically transforms hydrogen peroxide and chloride ions into hypochlorous acid (HClO)/hypochlorite (ClO–). The resultant HClO/ClO– species serve as powerful antimicrobial agents effective against a wide range of pathogens. However, the excessive generation of ClO– ions in the body causes numerous diseases, such as cardiovascular diseases, atherosclerosis, osteoarthritis, rheumatoid arthritis, lung injury and even cancers2, 125. Thus, the detection and sensing of ClO– ions in mitochondria is of great importance to understand the relationship between ClO– ions and related diseases. To sense the ion, a biotin–rhodamine–TPP conjugate called BiTClO was synthesized. Specifically, biotin and TPP were used in the conjugate to target the tumor and mitochondria, respectively126. When ClO– is absent in an aqueous solution supplemented with BiTClO, the nanoprobe does not emit fluorescence at 575 nm upon excitation at 560 nm. However, in response to the interaction between the ion and BiTClO, the nanoprobe emits strong fluorescence via ClO–-induced oxidation of the rhodamine in BiTClO (Fig. 12D). BiTClO senses the ClO– ion with great selectivity and high sensitivity compared with other ROS and metal ions.
3.2.4. Delivery of theranostics to mitochondria
Currently, interest in theranostics with the dual functions of therapeutic efficacy and diagnosis/imaging is growing. Additionally, when introducing mitochondrial targetability into theranostics, it is possible to design three-in-one conjugates equipped with treatment, imaging, and mitochondria-targeting abilities. Thus, some researchers have designed mitochondria-targeting theranostics by combining mitochondria-targeting molecules, therapeutic molecules, and imaging molecules and have sometimes selected certain molecules with dual or triple functions in one molecule127, 128, 129. Thus, mitochondria-targeting theranostics could be prepared with 1) a mitochondria-targeting moiety linked with a theranostic molecule (i.e., a therapeutic/imaging molecule); 2) a mitochondria-targeting/therapeutic molecule linked with an imaging molecule; 3) a mitochondria-targeting/imaging molecule linked with a therapeutic molecule; or 4) a mitochondrial targeting/imaging/therapeutic molecule.
First, examples of a mitochondrial-targeting moiety linked with a theranostic molecule including the mitochondria-targeting porphyrin derivatives previously mentioned in Figure 6, Figure 8. The conjugates have TPP, guanidine, or MTS for mitochondrial targeting. Interestingly, porphyrin derivatives not only could present therapeutic effects (i.e., mostly killing activities) in the presence of light and oxygen but also could be used as imaging agents due to their strong fluorescence emission properties. Thus, porphyrin derivatives have frequently been considered for use as theranostic agents in cancer34, 56, 65, 81.
Second, oridonin-linked coumarin derivatives are examples of mitochondria-targeting/imaging molecules linked with therapeutic molecules. Xu et al.129 chemically linked a neoplastic drug, oridonin, with highly fluorescent N,N-dialkyl-7-aminocoumarin derivatives and ultimately designed oridonin–coumarin conjugates as anticancer fluorescent probes. However, interestingly, the conjugates were preferentially accumulated in the mitochondria due to the lipophilic-cationic characteristics of N,N-dialkyl-7-aminocoumarin derivatives128 and their colocalization was confirmed by overlapping the fluorescence of both the coumarin derivatives and MitoTrackerTM Red. Moreover, the oridonin unit in the conjugates triggered the release of cytochrome c and induced apoptosis, leading to cell death.
F16 (Fig. 8) has three functions simultaneously in one molecule: 1) mitochondria-targeting ability due to its lipophilic and cationic characteristics; 2) therapeutic efficacy due to its killing activities against cancer cells; and 3) imaging abilities due to its intrinsic fluorescence41. Two F16–drug moieties (e.g., the F16-linked 5-FU conjugates and F16-linked porphyrin conjugates in Fig. 8), could be delivered in one conjugate to synergize their therapeutic effects. Additionally, by designing F16-imaging molecule conjugates (e.g., the F16-linked porphyrin conjugates and F16-linked BODIPY conjugates in Fig. 8), two imaging molecules could be delivered in one conjugate to synergize their imaging potentials. By designing F16-mitochondria-targeting molecule conjugates (e.g., F16-linked TPP conjugates), two mitochondria-targeting molecules could be delivered in one conjugate to achieve greater accumulation in the mitochondria of tumor cells than conjugates with one mitochondria-targeting moiety, which could cause increased cell-killing activities, indicating that this conjugate could be used in the image-guided therapy of cancer127.
4. Future perspective
A number of studies have assessed mitochondrial targeting of therapeutic agents, antioxidants and sensors. Regarding mitochondria-targeted anticancer agents, numerous promising results have been investigated, but few clinical studies have been reported. Successful mitochondria-targeting agents in the clinic should possess a variety of characteristics, including long circulation in the blood for drug exposure, selectivity for tumor tissues and tumor cells, and strong accumulation in the mitochondria3, 39, 130, 131. In addition, the currently applied mitochondria-targeting agents have some drawbacks. For example, DLC efficiently accumulates in the mitochondria due to the negative mitochondrial membrane potential; however, it is known to have intrinsic toxicity, limiting its clinical applications44, 132, 133, 134, 135. Some other targeting ligands such as peptides have drawbacks due to their bulky structures, solubility, intrinsically poor membrane permeability and extremely low serum stability3, 47. More studies should are needed to produce clinically applicable mitochondria-targeting agents.
It is still challenging to develop agents that preferentially target mitochondrial abnormalities in cancer cells without toxicity to normal cells. For this challenge, various factors (e.g., charge, size, anatomy, membrane potential, and extracellular specificity) and their combinations could be considered. First, the charges of agents affect their colloidal stability in the blood and cellular internalization. That is, anionic agents generally have colloidal stability in the blood, whereas cationic systems can aggregate with serum proteins and blood cells during blood circulation. Additionally, cationic agents are preferentially internalized in cancer cells over normal cells76 due to the greater depolarized plasma membrane potentials in cancer cells than in normal cells77. Thus, regarding charge, a design strategy for efficient anticancer agents could include agents with both a negative charge in the blood and a positive charge, which requires molecules with charge-conversion character. Second, regarding sizes, nanosized drug delivery system is preferentially accumulated in tumor tissue vs. small molecules owing to anatomical factors (e.g., the enhanced permeability and retention [EPR] effect). Thus, after the selective accumulation of negatively charged or hydrophilic polymer-stabilized nanosized DDS in tumor tissue, the surface characteristics of DDS would transform into cationic charges. The charge conversion could be chemically or physically triggered by tumor-specific stimuli such as extratumoral acidic pH values or enzymes (e.g., matrix metalloproteinases). For example, citraconic or aconitic amides with carboxylic acids could transform into amines after the acid-triggered detachment of citraconic or aconitic acids. The concept of charge-tunable nanosized DDS could allow selective accumulation in tumor cells over normal cells.
On the other hand, in many cases, directly linking a targeting moiety to therapeutic molecules reduced the efficacy of the parent drugs, which may be associated with the deactivation or inhibition of the active portion of therapeutic agents in certain diseases. This issue could be overcome by physical loading of drugs in nanosized drug carriers. In this field, many studies have been initiated during the last decade. As mentioned, the nanocarriers could be selectively accumulated in abnormal sites like cancers through enhanced permeability and retention (EPR) effect which occur due to leaky vasculature and reduced lymphatic drainage of tumor tissue.
Regarding mitochondrial targeting antioxidants, very promising clinical results have been reported, and the antioxidant SkQ1 (called as Visomitin®) was approved in Russia for the treatment of eye diseases. It is possible that this agent will be approved in the U.S. and other countries for eye diseases or other diseases.
5. Conclusions
The direct conjugation of mitochondrial-targeting moieties to various cytotoxic agents, antioxidants and sensing agents using various linkers was reviewed. In addition to the most widely used mitochondrial moiety, TPP, several other mitochondrial targeting moieties have been used to mitochondrially target drugs, antioxidants, and sensors. Several mitochondrial targeting moiety-anticancer drug conjugates have been studied in vitro and in vivo, yet clinical studies are lacking. Regarding mitochondrial targeting antioxidants, several clinical studies have been performed, especially studies on MitoQ and SkQ1 conjugates.
Acknowledgements
This study was supported by the National Research Foundation of Korea (NRF) funded by the Korean government (MSIT) (NRF-2017R1A4A1015036 and NRF-2015R1A1A05001459). Also, the study was supported by BK21PLUS grant of NRF funded by the Korean government (ME) (22A20130012250).
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association
References
- 1.Friedman J.R., Nunnari J. Mitochondrial form and function. Nature. 2014;505:335–343. doi: 10.1038/nature12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang Z., Guo W., Kuang X., Hou S., Liu H. Nanopreparations for mitochondria targeting drug delivery system: current strategies and future prospective. Asian J Pharm Sci. 2017;12:498–508. doi: 10.1016/j.ajps.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fulda S., Galluzzi L., Kroemer G. Targeting mitochondria for cancer therapy. Nat Rev Drug Discov. 2010;9:447–464. doi: 10.1038/nrd3137. [DOI] [PubMed] [Google Scholar]
- 4.Chipuk J.E., Bouchier-Hayes L., Green D.R. Mitochondrial outer membrane permeabilization during apoptosis: the innocent bystander scenario. Cell Death Differ. 2006;13:1396–1402. doi: 10.1038/sj.cdd.4401963. [DOI] [PubMed] [Google Scholar]
- 5.Modica-Napolitano J.S., Singh K.K. Mitochondrial dysfunction in cancer. Mitochondrion. 2004;4:755–762. doi: 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- 6.Galluzzi L., Morselli E., Kepp O., Vitale I., Rigoni A., Vacchelli E. Mitochondrial gateways to cancer. Mol Asp Med. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Lowell B.B., Shulman G.I. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 8.Ernster L., Schatz G. Mitochondria: a historical review. J Cell Biol. 1981;91:227s–255ss. doi: 10.1083/jcb.91.3.227s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liberman E.A., Topaly V.P., Tsofina L.M., Jasaitis A.A., Skulachev V.P. Mechanism of coupling of oxidative phosphorylation and the membrane potential of mitochondria. Nature. 1969;222:1076–1078. doi: 10.1038/2221076a0. [DOI] [PubMed] [Google Scholar]
- 10.Weiss M.J., Wong J.R., Ha C.S., Bleday R., Salem R.R., Steele G.D., Jr Dequalinium, a topical antimicrobial agent, displays anticarcinoma activity based on selective mitochondrial accumulation. Proc Natl Acad Sci U S A. 1987;84:5444–5448. doi: 10.1073/pnas.84.15.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michaelis L. Die vitale Färbung, eine Darstellungsmethode der Zellgranula. Arch Mikrosk Anat Enwicklmech. 1899;55:558–575. [Google Scholar]
- 12.Palade G.E. The fine structure of mitochondria. Anat Rec. 1952;114:427–451. doi: 10.1002/ar.1091140304. [DOI] [PubMed] [Google Scholar]
- 13.Lazarow A., Cooperstein S.J. Studies on the enzymatic basis for the Janus green B staining reaction. J Histochem Cytochem. 1953;1:234–241. doi: 10.1177/1.4.234. [DOI] [PubMed] [Google Scholar]
- 14.Palade G.E. The organization of living matter. Proc Natl Acad Sci U S A. 1964;52:613–634. doi: 10.1073/pnas.52.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burns R.J., Smith R.A., Murphy M.P. Synthesis and characterization of thiobutyltriphenylphosphonium bromide, a novel thiol reagent targeted to the mitochondrial matrix. Arch Biochem Biophys. 1995;322:60–68. doi: 10.1006/abbi.1995.1436. [DOI] [PubMed] [Google Scholar]
- 16.Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria in cancer cells: what is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Gorlach S., Fichna J., Lewandowska U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015;366:141–149. doi: 10.1016/j.canlet.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Galley H.F. Bench-to-bedside review: targeting antioxidants to mitochondria in sepsis. Crit Care. 2010;14:230. doi: 10.1186/cc9098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heller A., Brockhoff G., Goepferich A. Targeting drugs to mitochondria. Eur J Pharm Biopharm. 2012;82:1–18. doi: 10.1016/j.ejpb.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 20.Taylor R.W., Turnbull D.M. Mitochondrial DNA mutations in human disease. Nat Rev Genet. 2005;6:389–402. doi: 10.1038/nrg1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeviani M., Di Donato S. Mitochondrial disorders. Brain. 2004;127:2153–2172. doi: 10.1093/brain/awh259. [DOI] [PubMed] [Google Scholar]
- 22.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp Mol Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Törnroth-Horsefield S., Neutze R. Opening and closing the metabolite gate. Proc Natl Acad Sci U S A. 2008;105:19565–19566. doi: 10.1073/pnas.0810654106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rich P.R. The molecular machinery of Keilin׳s respiratory chain. Biochem Soc Trans. 2003;31:1095–1105. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- 25.Wen S., Zhu D., Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Med Chem. 2013;5:53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orrenius S., Gogvadze V., Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- 27.Kim K.H., Park J.Y., Jung H.J., Kwon H.J. Identification and biological activities of a new antiangiogenic small molecule that suppresses mitochondrial reactive oxygen species. Biochem Biophys Res Commun. 2011;404:541–545. doi: 10.1016/j.bbrc.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 28.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–591. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 29.Butterfield D.A. Amyloid β-peptide (1–42)-induced oxidative stress and neurotoxicity: implications for neurodegeneration in Alzheimer׳s disease brain. A review. Free Radic Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 30.Yankner B.A., Duffy L.K., Kirschner D.A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990;250:279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Yan S.D. Mitochondrial A beta: a potential cause of metabolic dysfunction in Alzheimer׳s disease. IUBMB Life. 2006;58:686–694. doi: 10.1080/15216540601047767. [DOI] [PubMed] [Google Scholar]
- 32.Winklhofer K.F., Haass C. Mitochondrial dysfunction in Parkinson׳s disease. Biochim Biophys Acta. 2010;1802:29–44. doi: 10.1016/j.bbadis.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Millard M., Gallagher J.D., Olenyuk B.Z., Neamati N. A selective mitochondrial-targeted chlorambucil with remarkable cytotoxicity in breast and pancreatic cancers. J Med Chem. 2013;56:9170–9179. doi: 10.1021/jm4012438. [DOI] [PubMed] [Google Scholar]
- 34.Lei W., Xie J., Hou Y., Jiang G., Zhang H., Wang P. Mitochondria-targeting properties and photodynamic activities of porphyrin derivatives bearing cationic pendant. J Photochem Photobiol B. 2010;98:167–171. doi: 10.1016/j.jphotobiol.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Chen W.H., Xu X.D., Luo G.F., Jia H.Z., Lei Q., Cheng S.X. Dual-targeting pro-apoptotic peptide for programmed cancer cell death via specific mitochondria damage. Sci Rep. 2013;3:3468. doi: 10.1038/srep03468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang H., Xu W. Mito-methyl coumarin, a novel mitochondria-targeted drug with great antitumor potential was synthesized. Biochem Biophys Res Commun. 2017;489:1–7. doi: 10.1016/j.bbrc.2017.05.116. [DOI] [PubMed] [Google Scholar]
- 37.Dong L.F., Jameson V.J., Tilly D., Prochazka L., Rohlena J., Valis K. Mitochondrial targeting of α-tocopheryl succinate enhances its pro-apoptotic efficacy: a new paradigm for effective cancer therapy. Free Radic Biol Med. 2011;50:1546–1555. doi: 10.1016/j.freeradbiomed.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 38.Song Y.F., Liu D.Z., Cheng Y., Liu M., Ye W.L., Zhang B.L. Dual subcellular compartment delivery of doxorubicin to overcome drug resistant and enhance antitumor activity. Sci Rep. 2015;5:16125. doi: 10.1038/srep16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chamberlain G.R., Tulumello D.V., Kelley S.O. Targeted delivery of doxorubicin to mitochondria. ACS Chem Biol. 2013;8:1389–1395. doi: 10.1021/cb400095v. [DOI] [PubMed] [Google Scholar]
- 40.Wisnovsky S.P., Wilson J.J., Radford R.J., Pereira M.P., Chan M.R., Laposa R.R. Targeting mitochondrial DNA with a platinum-based anticancer agent. Chem Biol. 2013;20:1323–1328. doi: 10.1016/j.chembiol.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.He H., Li D.W., Yang L.Y., Fu L., Zhu X.J., Wong W.K. A novel bifunctional mitochondria-targeted anticancer agent with high selectivity for cancer cells. Sci Rep. 2015;5:13543. doi: 10.1038/srep13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dairkee S.H., Hackett A.J. Differential retention of rhodamine 123 by breast carcinoma and normal human mammary tissue. Breast Cancer Res Treat. 1991;18:57–61. doi: 10.1007/BF01975444. [DOI] [PubMed] [Google Scholar]
- 43.Wang F., Ogasawara M.A., Huang P. Small mitochondria-targeting molecules as anti-cancer agents. Mol Asp Med. 2010;31:75–92. doi: 10.1016/j.mam.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Modica-Napolitano J.S., Aprille J.R. Delocalized lipophilic cations selectively target the mitochondria of carcinoma cells. Adv Drug Deliv Rev. 2001;49:63–70. doi: 10.1016/s0169-409x(01)00125-9. [DOI] [PubMed] [Google Scholar]
- 45.Cortes L.A., Castro L., Pesce B., Maya J.D., Ferreira J., Castro-Castillo V. Novel gallate triphenylphosphonium derivatives with potent antichagasic activity. PLoS One. 2015;10:e0136852. doi: 10.1371/journal.pone.0136852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang X.Y., Zhang P.Y. Mitochondria targeting nano agents in cancer therapeutics. Oncol Lett. 2016;12:4887–4890. doi: 10.3892/ol.2016.5302. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Yousif L.F., Stewart K.M., Kelley S.O. Targeting mitochondria with organelle-specific compounds: strategies and applications. Chembiochem. 2009;10:1939–1950. doi: 10.1002/cbic.200900185. [DOI] [PubMed] [Google Scholar]
- 48.Zielonka J., Joseph J., Sikora A., Hardy M., Ouari O., Vasquez-Vivar J. Mitochondria-targeted triphenylphosphonium-based compounds: syntheses, mechanisms of action, and therapeutic and diagnostic applications. Chem Rev. 2017;117:10043–10120. doi: 10.1021/acs.chemrev.7b00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christman J.E., Miller D.S., Coward P., Smith L.H., Teng N.N. Study of the selective cytotoxic properties of cationic, lipophilic mitochondrial-specific compounds in gynecologic malignancies. Gynecol Oncol. 1990;39:72–79. doi: 10.1016/0090-8258(90)90402-7. [DOI] [PubMed] [Google Scholar]
- 50.Weissig V., Lasch J., Erdos G., Meyer H.W., Rowe T.C., Hughes J. DQAsomes: a novel potential drug and gene delivery system made from dequaliniumTM. Pharm Res. 1998;15:334–337. doi: 10.1023/a:1011991307631. [DOI] [PubMed] [Google Scholar]
- 51.Szeto H.H. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Horton K.L., Stewart K.M., Fonseca S.B., Guo Q., Kelley S.O. Mitochondria-penetrating peptides. Chem Biol. 2008;15:375–382. doi: 10.1016/j.chembiol.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 53.Mourtada R., Fonseca S.B., Wisnovsky S.P., Pereira M.P., Wang X., Hurren R. Re-directing an alkylating agent to mitochondria alters drug target and cell death mechanism. PLoS One. 2013;8:e60253. doi: 10.1371/journal.pone.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szeto H.H. Mitochondria-targeted cytoprotective peptides for ischemia-reperfusion injury. Antioxid Redox Signal. 2008;10:601–619. doi: 10.1089/ars.2007.1892. [DOI] [PubMed] [Google Scholar]
- 55.Zhao K., Zhao G.M., Wu D., Soong Y., Birk A.V., Schiller P.W. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 56.Sibrian-Vazquez M., Nesterova I.V., Jensen T.J., Vicente M.G. Mitochondria targeting by guanidine-and biguanidine-porphyrin photosensitizers. Bioconjugate Chem. 2008;19:705–713. doi: 10.1021/bc700393u. [DOI] [PubMed] [Google Scholar]
- 57.Fantin V.R., Berardi M.J., Scorrano L., Korsmeyer S.J., Leder P. A novel mitochondriotoxic small molecule that selectively inhibits tumor cell growth. Cancer Cell. 2002;2:29–42. doi: 10.1016/s1535-6108(02)00082-x. [DOI] [PubMed] [Google Scholar]
- 58.Baracca A., Sgarbi G., Solaini G., Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim Biophys Acta. 2003;1606:137–146. doi: 10.1016/s0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- 59.Lampidis T.J., Hasin Y., Weiss M.J., Chen L.B. Selective killing of carcinoma cells "in vitro" by lipophilic-cationic compounds: a cellular basis. Biomed Pharmacother. 1985;39:220–226. [PubMed] [Google Scholar]
- 60.Antonenko Y.N., Avetisyan A.V., Cherepanov D.A., Knorre D.A., Korshunova G.A., Markova O.V. Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J Biol Chem. 2011;286:17831–17840. doi: 10.1074/jbc.M110.212837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Antonenko Y.N., Avetisyan A.V., Bakeeva L.E., Chernyak B.V., Chertkov V.A., Domnina L.V. Mitochondria-targeted plastoquinone derivatives as tools to interrupt execution of the aging program. 1. Cationic plastoquinone derivatives: synthesis and in vitro studies. Biochem (Mosc) 2008;73:1273–1287. doi: 10.1134/s0006297908120018. [DOI] [PubMed] [Google Scholar]
- 62.Costantini P., Jacotot E., Decaudin D., Kroemer G. Mitochondrion as a novel target of anticancer chemotherapy. J Natl Cancer Inst. 2000;92:1042–1053. doi: 10.1093/jnci/92.13.1042. [DOI] [PubMed] [Google Scholar]
- 63.Fonseca S.B., Pereira M.P., Mourtada R., Gronda M., Horton K.L., Hurren R. Rerouting chlorambucil to mitochondria combats drug deactivation and resistance in cancer cells. Chem Biol. 2011;18:445–453. doi: 10.1016/j.chembiol.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 64.Hu Q., Gao M., Feng G., Liu B. Mitochondria-targeted cancer therapy using a light-up probe with aggregation-induced-emission characteristics. Angew Chem Int Ed. 2014;53:14225–14229. doi: 10.1002/anie.201408897. [DOI] [PubMed] [Google Scholar]
- 65.Battogtokh G., Ko Y.T. Mitochondrial-targeted photosensitizer-loaded folate-albumin nanoparticle for photodynamic therapy of cancer. Nanomedicine. 2017;13:733–743. doi: 10.1016/j.nano.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 66.Guo T., Zhu Y., Gan C.S., Lee S.S., Zhu J., Wang H. Quantitative proteomics discloses MET expression in mitochondria as a direct target of MET kinase inhibitor in cancer cells. Mol Cell Proteom. 2010;9:2629–2641. doi: 10.1074/mcp.M110.001776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang T., Ng W.H., Chen H., Chomchopbun K., Huynh T.H., Go M.L. Mitochondrial-targeting MET kinase inhibitor kills erlotinib-resistant lung cancer cells. ACS Med Chem Lett. 2016;7:807–812. doi: 10.1021/acsmedchemlett.6b00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scarano W., Duong H.T., Lu H., De Souza P.L., Stenzel M.H. Folate conjugation to polymeric micelles via boronic acid ester to deliver platinum drugs to ovarian cancer cell lines. Biomacromolecules. 2013;14:962–975. doi: 10.1021/bm400121q. [DOI] [PubMed] [Google Scholar]
- 69.Xing L., Lyu J.Y., Yang Y., Cui P.F., Gu L.Q., Qiao J.B. pH-Responsive de-PEGylated nanoparticles based on triphenylphosphine-quercetin self-assemblies for mitochondria-targeted cancer therapy. Chem Commun (Camb) 2017;53:8790–8793. doi: 10.1039/c7cc04058j. [DOI] [PubMed] [Google Scholar]
- 70.Fonseca S.B., Kelley S.O. Peptide-chlorambucil conjugates combat pgp-dependent drug efflux. ACS Med Chem Lett. 2011;2:419–423. doi: 10.1021/ml1002663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chegaev K., Riganti C., Lazzarato L., Rolando B., Guglielmo S., Campia I. Nitric oxide donor doxorubicins accumulate into doxorubicin-resistant human colon cancer cells inducing cytotoxicity. ACS Med Chem Lett. 2011;2:494–497. doi: 10.1021/ml100302t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Riganti C., Rolando B., Kopecka J., Campia I., Chegaev K., Lazzarato L. Mitochondrial-targeting nitrooxy-doxorubicin: a new approach to overcome drug resistance. Mol Pharm. 2013;10:161–174. doi: 10.1021/mp300311b. [DOI] [PubMed] [Google Scholar]
- 73.Cho D.Y., Cho H., Kwon K., Yu M., Lee E., Huh K.M. Triphenylphosphonium-conjugated poly(ε-caprolactone)-based self-assembled nanostructures as nanosized drugs and drug delivery carriers for mitochondria-targeting synergistic anticancer drug delivery. Adv Funct Mater. 2015;25:5479–5491. [Google Scholar]
- 74.Moon S.Y., Choi Y.S., Cho J.K., Yu M., Lee E., Huh K.M. Intracellular thiol-responsive nanosized drug carriers self-assembled by poly(ethylene glycol)-b-poly(ε-caprolactone)-b-poly(ethylene glycol) having multiple bioreducible disulfide linkages in hydrophobic block. RSC Adv. 2016;6:15558–15576. [Google Scholar]
- 75.Wang J., Fan X.Y., Yang L.Y., He H., Huang R., Jiang F.L. Conjugated 5-fluorouracil with mitochondria-targeting lipophilic cation: design, synthesis and biological evaluation. MedChemComm. 2016;7:2016–2019. [Google Scholar]
- 76.Shin E.H., Li Y., Kumar U., Sureka H.V., Zhang X., Payne C.K. Membrane potential mediates the cellular binding of nanoparticles. Nanoscale. 2013;5:5879–5886. doi: 10.1039/c3nr01667f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang M., Brackenbury W.J. Membrane potential and cancer progression. Front Physiol. 2013;4:185. doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verma A., Facchina S.L., Hirsch D.J., Song S.Y., Dillahey L.F., Williams J.R. Photodynamic tumor therapy: mitochondrial benzodiazepine receptors as a therapeutic target. Mol Med. 1998;4:40–45. [PMC free article] [PubMed] [Google Scholar]
- 79.Trivedi N.S., Wang H.W., Nieminen A.L., Oleinick N.L., Izatt J.A. Quantitative analysis of Pc 4 localization in mouse lymphoma (LY-R) cells via double-label confocal fluorescence microscopy. Photochem Photobiol. 2000;71:634–639. doi: 10.1562/0031-8655(2000)071<0634:qaopli>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 80.Choi Y.S., Kwon K., Yoon K., Huh K.M., Kang H.C. Photosensitizer-mediated mitochondria-targeting nanosized drug carriers: subcellular targeting, therapeutic, and imaging potentials. Int J Pharm. 2017;520:195–206. doi: 10.1016/j.ijpharm.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 81.Zhang S., Yang L., Ling X., Shao P., Wang X., Edwards W.B. Tumor mitochondria-targeted photodynamic therapy with a translocator protein (TSPO)-specific photosensitizer. Acta Biomater. 2015;28:160–170. doi: 10.1016/j.actbio.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kang B.H., Plescia J., Song H.Y., Meli M., Colombo G., Beebe K. Combinatorial drug design targeting multiple cancer signaling networks controlled by mitochondrial Hsp90. J Clin Invest. 2009;119:454–464. doi: 10.1172/JCI37613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Williams R.J., Spencer J.P., Rice-Evans C. Flavonoids: antioxidants or signalling molecules? Free Radic Biol Med. 2004;36:838–849. doi: 10.1016/j.freeradbiomed.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 84.Murphy M.P. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 85.Lotito S.B., Frei B. Consumption of flavonoid-rich foods and increased plasma antioxidant capacity in humans: cause, consequence, or epiphenomenon? Free Radic Biol Med. 2006;41:1727–1746. doi: 10.1016/j.freeradbiomed.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 86.Cheng G., Zielonka J., McAllister D., Hardy M., Ouari O., Joseph J. Antiproliferative effects of mitochondria-targeted cationic antioxidants and analogs: role of mitochondrial bioenergetics and energy-sensing mechanism. Cancer Lett. 2015;365:96–106. doi: 10.1016/j.canlet.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Cheng G., Zielonka J., Dranka B.P., McAllister D., Mackinnon A.C., Jr, Joseph J. Mitochondria-targeted drugs synergize with 2-deoxyglucose to trigger breast cancer cell death. Cancer Res. 2012;72:2634–2644. doi: 10.1158/0008-5472.CAN-11-3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cheng G., Zielonka J., McAllister D.M., Mackinnon A.C., Jr, Joseph J., Dwinell M.B. Mitochondria-targeted vitamin E analogs inhibit breast cancer cell energy metabolism and promote cell death. BMC Cancer. 2013;13:285. doi: 10.1186/1471-2407-13-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jain M., Rivera S., Monclus E.A., Synenki L., Zirk A., Eisenbart J. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem. 2013;288:770–777. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Starenki D., Park J.I. Mitochondria-targeted nitroxide, Mito-CP, suppresses medullary thyroid carcinoma cell survival in vitro and in vivo. J Clin Endocrinol Metab. 2013;98:1529–1540. doi: 10.1210/jc.2012-3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Crane F.L. Hydroquinone dehydrogenases. Annu Rev Biochem. 1977;46:439–469. doi: 10.1146/annurev.bi.46.070177.002255. [DOI] [PubMed] [Google Scholar]
- 92.Oyewole A.O., Birch-Machin M.A. Mitochondria-targeted antioxidants. FASEB J. 2015;29:4766–4771. doi: 10.1096/fj.15-275404. [DOI] [PubMed] [Google Scholar]
- 93.Kelso G.F., Porteous C.M., Coulter C.V., Hughes G., Porteous W.K., Ledgerwood E.C. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 94.Dashdorj A., Jyothi K.R., Lim S., Jo A., Nguyen M.N., Ha J. Mitochondria-targeted antioxidant MitoQ ameliorates experimental mouse colitis by suppressing NLRP3 inflammasome-mediated inflammatory cytokines. BMC Med. 2013;11:178. doi: 10.1186/1741-7015-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gane E.J., Weilert F., Orr D.W., Keogh G.F., Gibson M., Lockhart M.M. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:L1019–L1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 96.Graham D., Huynh N.N., Hamilton C.A., Beattie E., Smith R.A., Cochemé H.M. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–328. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 97.Jauslin M.L., Meier T., Smith R.A., Murphy M.P. Mitochondria-targeted antioxidants protect friedreich ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 98.Lowes D.A., Thottakam B.M., Webster N.R., Murphy M.P., Galley H.F. The mitochondria-targeted antioxidant MitoQ protects against organ damage in a lipopolysaccharide-peptidoglycan model of sepsis. Free Radic Biol Med. 2008;45:1559–1565. doi: 10.1016/j.freeradbiomed.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 99.Oyewole A.O., Wilmot M.C., Fowler M., Birch-Machin M.A. Comparing the effects of mitochondrial targeted and localized antioxidants with cellular antioxidants in human skin cells exposed to UVA and hydrogen peroxide. FASEB J. 2014;28:485–494. doi: 10.1096/fj.13-237008. [DOI] [PubMed] [Google Scholar]
- 100.Ryan B.J., Hoek S., Fon E.A., Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson׳s: from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Snow B.J., Rolfe F.L., Lockhart M.M., Frampton C.M., O׳Sullivan J.D., Fung V. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson׳s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- 102.Miquel E., Cassina A., Martínez-Palma L., Souza J.M., Bolatto C., Rodríguez-Bottero S. Neuroprotective effects of the mitochondria-targeted antioxidant MitoQ in a model of inherited amyotrophic lateral sclerosis. Free Radic Biol Med. 2014;70:204–213. doi: 10.1016/j.freeradbiomed.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 103.Ghosh A., Chandran K., Kalivendi S.V., Joseph J., Antholine W.E., Hillard C.J. Neuroprotection by a mitochondria-targeted drug in a Parkinson׳s disease model. Free Radic Biol Med. 2010;49:1674–1684. doi: 10.1016/j.freeradbiomed.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rodriguez-Cuenca S., Cochemé H.M., Logan A., Abakumova I., Prime T.A., Rose C. Consequences of long-term oral administration of the mitochondria-targeted antioxidant MitoQ to wild-type mice. Free Radic Biol Med. 2010;48:161–172. doi: 10.1016/j.freeradbiomed.2009.10.039. [DOI] [PubMed] [Google Scholar]
- 105.Solesio M.E., Prime T.A., Logan A., Murphy M.P., Del Mar Arroyo-Jimenez M., Jordán J. The mitochondria-targeted anti-oxidant MitoQ reduces aspects of mitochondrial fission in the 6-OHDA cell model of Parkinson׳s disease. Biochim Biophys Acta. 2013;1832:174–182. doi: 10.1016/j.bbadis.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 106.Stucki D.M., Ruegsegger C., Steiner S., Radecke J., Murphy M.P., Zuber B. Mitochondrial impairments contribute to spinocerebellar ataxia type 1 progression and can be ameliorated by the mitochondria-targeted antioxidant MitoQ. Free Radic Biol Med. 2016;97:427–440. doi: 10.1016/j.freeradbiomed.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 107.Skulachev V.P. A biochemical approach to the problem of aging: "megaproject" on membrane-penetrating ions. The first results and prospects. Biochem (Mosc) 2007;72:1385–1396. doi: 10.1134/s0006297907120139. [DOI] [PubMed] [Google Scholar]
- 108.Novikova Y.P., Gancharova O.S., Eichler O.V., Philippov P.P., Grigoryan E.N. Preventive and therapeutic effects of SkQ1-containing Visomitin eye drops against light-induced retinal degeneration. Biochem (Mosc) 2014;79:1101–1110. doi: 10.1134/S0006297914100113. [DOI] [PubMed] [Google Scholar]
- 109.Dikalova A.E., Kirilyuk I.A., Dikalov S.I. Antihypertensive effect of mitochondria-targeted proxyl nitroxides. Redox Biol. 2015;4:355–362. doi: 10.1016/j.redox.2014.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ Res. 2010;107:106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jiang J., Stoyanovsky D.A., Belikova N.A., Tyurina Y.Y., Zhao Q., Tungekar M.A. A mitochondria-targeted triphenylphosphonium-conjugated nitroxide functions as a radioprotector/mitigator. Radiat Res. 2009;172:706–717. doi: 10.1667/RR1729.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hardy M., Poulhés F., Rizzato E., Rockenbauer A., Banaszak K., Karoui H. Mitochondria-targeted spin traps: synthesis, superoxide spin trapping, and mitochondrial uptake. Chem Res Toxicol. 2014;27:1155–1165. doi: 10.1021/tx500032e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Finichiu P.G., Larsen D.S., Evans C., Larsen L., Bright T.P., Robb E.L. A mitochondria-targeted derivative of ascorbate: mitoc. Free Radic Biol Med. 2015;89:668–678. doi: 10.1016/j.freeradbiomed.2015.07.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Jameson V.J., Cochemé H.M., Logan A., Hanton L.R., Smith R.A., Murphy M.P. Synthesis of triphenylphosphonium vitamin E derivatives as mitochondria-targeted antioxidants. Tetrahedron. 2015;71:8444–8453. doi: 10.1016/j.tet.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Maddalena L.A., Ghelfi M., Atkinson J., Stuart J.A. The mitochondria-targeted imidazole substituted oleic acid ׳TPP-IOA׳ affects mitochondrial bioenergetics and its protective efficacy in cells is influenced by cellular dependence on aerobic metabolism. Biochim Biophys Acta. 2017;1858:73–85. doi: 10.1016/j.bbabio.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 116.Brown S.E., Ross M.F., Sanjuan-Pla A., Manas A.R., Smith R.A., Murphy M.P. Targeting lipoic acid to mitochondria: synthesis and characterization of a triphenylphosphonium-conjugated α-lipoyl derivative. Free Radic Biol Med. 2007;42:1766–1780. doi: 10.1016/j.freeradbiomed.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 117.Filipovska A., Kelso G.F., Brown S.E., Beer S.M., Smith R.A., Murphy M.P. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J Biol Chem. 2005;280:24113–24126. doi: 10.1074/jbc.M501148200. [DOI] [PubMed] [Google Scholar]
- 118.Kelso G.F., Maroz A., Cochemé H.M., Logan A., Prime T.A., Peskin A.V. A mitochondria-targeted macrocyclic Mn(II) superoxide dismutase mimetic. Chem Biol. 2012;19:1237–1246. doi: 10.1016/j.chembiol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 119.Waris G., Ahsan H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J Carcinog. 2006;5:14. doi: 10.1186/1477-3163-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adegoke O.A., Orokotan O.A. Evaluation of directly observed treatment short courses at a secondary health institution in Ibadan, Oyo State, Southwestern Nigeria. Asian Pac J Trop Med. 2013;6:952–959. doi: 10.1016/S1995-7645(13)60170-4. [DOI] [PubMed] [Google Scholar]
- 121.Tian J., Chen H., Zhuo L., Xie Y., Li N., Tang B. A highly selective, cell-permeable fluorescent nanoprobe for ratiometric detection and imaging of peroxynitrite in living cells. Chemistry. 2011;17:6626–6634. doi: 10.1002/chem.201100148. [DOI] [PubMed] [Google Scholar]
- 122.Wu X., Sun S., Wang Y., Zhu J., Jiang K., Leng Y. A fluorescent carbon-dots-based mitochondria-targetable nanoprobe for peroxynitrite sensing in living cells. Biosens Bioelectron. 2017;90:501–507. doi: 10.1016/j.bios.2016.10.060. [DOI] [PubMed] [Google Scholar]
- 123.Xu J., Pan J., Jiang X., Qin C., Zeng L., Zhang H. A mitochondria-targeted ratiometric fluorescent probe for rapid, sensitive and specific detection of biological SO2 derivatives in living cells. Biosens Bioelectron. 2016;77:725–732. doi: 10.1016/j.bios.2015.10.049. [DOI] [PubMed] [Google Scholar]
- 124.Huang H., Liu W., Liu X.J., Kuang Y.Q., Jiang J.H. A novel mitochondria-targeted near-infrared fluorescence probe for ultrafast and ratiometric detection of SO2 derivatives in live cells. Talanta. 2017;168:203–209. doi: 10.1016/j.talanta.2017.03.043. [DOI] [PubMed] [Google Scholar]
- 125.Yap Y.W., Whiteman M., Cheung N.S. Chlorinative stress: an under appreciated mediator of neurodegeneration? Cell Signal. 2007;19:219–228. doi: 10.1016/j.cellsig.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 126.Li K., Hou J.T., Yang J., Yu X.Q. A tumor-specific and mitochondria-targeted fluorescent probe for real-time sensing of hypochlorite in living cells. Chem Commun (Camb) 2017;53:5539–5541. doi: 10.1039/c7cc01679d. [DOI] [PubMed] [Google Scholar]
- 127.Wu S., Cao Q., Wang X., Cheng K., Cheng Z. Design, synthesis and biological evaluation of mitochondria targeting theranostic agents. Chem Commun (Camb) 2014;50:8919–8922. doi: 10.1039/c4cc03296a. [DOI] [PubMed] [Google Scholar]
- 128.Guo D., Chen T., Ye D., Xu J., Jiang H., Chen K. Cell-permeable iminocoumarine-based fluorescent dyes for mitochondria. Org Lett. 2011;13:2884–2887. doi: 10.1021/ol200908r. [DOI] [PubMed] [Google Scholar]
- 129.Xu S., Luo S., Yao H., Cai H., Miao X., Wu F. Probing the anticancer action of oridonin with fluorescent analogues: visualizing subcellular localization to mitochondria. J Med Chem. 2016;59:5022–5034. doi: 10.1021/acs.jmedchem.6b00408. [DOI] [PubMed] [Google Scholar]
- 130.Buondonno I., Gazzano E., Jean S.R., Audrito V., Kopecka J., Fanelli M. Mitochondria-targeted doxorubicin: a new therapeutic strategy against doxorubicin-resistant osteosarcoma. Mol Cancer Ther. 2016;15:2640–2652. doi: 10.1158/1535-7163.MCT-16-0048. [DOI] [PubMed] [Google Scholar]
- 131.D׳Souza G.G., Wagle M.A., Saxena V., Shah A. Approaches for targeting mitochondria in cancer therapy. Biochim Biophys Acta. 2011;1807:689–696. doi: 10.1016/j.bbabio.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 132.Smith R.A., Porteous C.M., Gane A.M., Murphy M.P. Delivery of bioactive molecules to mitochondria in vivo. Proc Natl Acad Sci U S A. 2003;100:5407–5412. doi: 10.1073/pnas.0931245100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Murphy M.P. Selective targeting of bioactive compounds to mitochondria. Trends Biotechnol. 1997;15:326–330. doi: 10.1016/S0167-7799(97)01068-8. [DOI] [PubMed] [Google Scholar]
- 134.Galluzzi L., Zamzami N., de La Motte Rouge T., Lemaire C., Brenner C., Kroemer G. Methods for the assessment of mitochondrial membrane permeabilization in apoptosis. Apoptosis. 2007;12:803–813. doi: 10.1007/s10495-007-0720-1. [DOI] [PubMed] [Google Scholar]
- 135.Millard M., Pathania D., Shabaik Y., Taheri L., Deng J., Neamati N. Preclinical evaluation of novel triphenylphosphonium salts with broad-spectrum activity. PLoS One. 2010;5:e1313. doi: 10.1371/journal.pone.0013131. [DOI] [PMC free article] [PubMed] [Google Scholar]