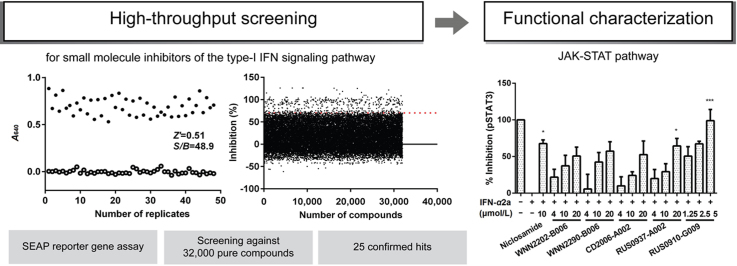
Abstract
Interferons (IFNs) are cytokines with fundamental roles in resistance to infections, cancer and other diseases. Type-I IFNs, interferon α (IFN-α) and interferon β (IFN-β), act through a shared receptor complex (IFNAR) comprised of IFNAR1 and IFNAR2 subunits. Binding of type-I IFN to IFNAR1 will robustly activate Janus activated kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway. Aberrant activation of the type-I IFN response results in a spectrum of disorders called interferonopathies. The purpose of this research is to develop an assay for high-throughput screening (HTS) of small molecule inhibitors of the type-I IFN signaling pathway. Inhibition of type-I IFN signaling can be beneficial in terms of therapeutic use and understanding the underlying mechanism of action. We report here a HTS campaign with the secreted embryonic alkaline phosphatase (SEAP) reporter gene assay against 32,000 compounds which yielded 25 confirmed hits. These compounds were subsequently characterized for their cytotoxicity, effects on STAT phosphorylation and activities in IFN regulatory factor (IRF) transcription.
Abbreviations: cDNA, complementary DNA; CV, coefficient of variation; DMEM, Dulbecco׳s modified Eagle׳s medium; DMSO, dimethyl sulfoxide; FRET, fluorescence resonance energy transfer; HEK, human embryonic kidney; HTS, high-throughput screening; IFN, interferon; IFNAR, IFN alpha receptor; IRF, IFN regulatory factor; ISGF3, IFN-stimulated gene factor 3; ISRE, IFN-stimulated response element; JAK, Janus activated kinase; pSTAT, phosphorylated STAT; S/B, signal to background ratio; SEAP, secreted embryonic alkaline phosphatase; STAT, signal transducer and activator of transcription; TYK, tyrosine kinase
KEY WORDS: High-throughput screening, Interferon α receptor, Secreted embryonic alkaline phosphatase, JAK-STAT, IFN regulatory factor, Inhibitor
Graphical abstract
A high-throughput screening campaign is described here for small molecule inhibitors of the type-I interferon signaling pathway utilizing the SEAP reporter gene assay. Screening against 32,000 pure compounds yielded 25 confirmed hits. Several of them were tested in SH-SY5Y cells and showed inhibition of STAT1/STAT3 phosphorylation and IRF7 mRNA transcription.
1. Introduction
Interferons (IFN) are pleiotropic cytokines involved in innate and adaptive immunity. Classified into types I, II and III, they play fundamental roles in resistance to infections, cancer, and other diseases1. Type-I IFNs comprise 14 IFN-αs, IFN-β, IFN-ε, and IFN-ω2. IFN-α and IFN-β are both used for treatment of a wide range of diseases: IFN-α2a for chronic hepatitis C, IFN-α2b for malignancies, and IFN-β1a for multiple sclerosis3, 4. Their side-effects include neurological and hematologic toxicities5. Aberrant activation of type-I IFN response results in a spectrum of disorders called interferonopathies, such as Aicardi-Goutieres syndrome, chronic autoinflammatory systemic lupus erythematosus, and cerebrovascular disease6, 7, 8.
Type-I IFN response occurs when IFN-α/β binds to their receptor complex, IFNAR. This receptor complex is comprised of two subunits: IFNAR1 and IFNAR2. The ligand–receptor complex is phosphorylated, presumably by pre-associated Janus activated kinases (JAKs) namely tyrosine kinase 2 (TYK2) on IFNAR1 and JAK1 on IFNAR2. The phosphorylated receptors are docking sites for signal transducers and activators of transcription (STAT) factors that dimerise and translocate to the nucleus. STATs 1, 2, 3, 4, and 5 are activated by type-I IFNs in many cell types. Other kinases (e.g., mitogen-activated protein kinases) and transcription factors (e.g., nuclear factor-κB) can also be activated in response to type-I IFNs. Multiple pathways and IFN-regulated genes are activated by IFNs, many of which remain unknown9.
Clearly, inhibition of IFN signaling can be beneficial in terms of therapeutic use and understanding the underlying molecular and cellular mechanisms. Several attempts have been tried to develop JAK or STAT inhibitors as well as monoclonal antibodies against IFN-α and IFN receptor antagonists10, 11. For instance, AstraZeneca-Medimmune developed anifrolumab (formerly known as MEDI-546), a fully human immunoglobulin G1κ monoclonal antibody directed against IFNAR1 for treatment of systemic lupus erythematosus which is currently undergoing phase 3 clinical trials12.
High-throughput screening (HTS) efforts in identifying JAK and STAT inhibitors have been made13, 14, 15, 16. Nonetheless, up to now there is only one reported small molecule inhibitor with clinical efficacy for this pathway17. Here we describe an HTS assay capable of discovering potential inhibitors of type-I IFN signaling. HTS was applied to screen 32,000 compounds which resulted in 25 confirmed hits. This was followed by characterization for compound cytotoxicity in stably engineered human embryonic kidney (HEK) and neuroblastoma SH-SY5Y cells. As type-I IFN is implicated in neuronal inflammation18, 19, SH-SY5Y cell line was thus chosen as a potential model for testing bioactivities. This cell line has also been shown to possess an active JAK-STAT signaling and the ability to provoke inflammatory reaction upon type-I IFN treatment20, 21. Functional effects of the hits on JAK-STAT signaling, including STAT phosphorylation and IFN regulatory factor (IRF) mRNA expression were studied in parallel.
2. Materials and methods
2.1. Compounds
The chemical compound library used for the screening of type-I interferon signaling pathway inhibitors consisted of 32,000 synthetic and natural products derived pure compounds. All the compounds come from the Chinese National Compound Library (www.cncl.org.cn). The structural diversity covers heterocycles, lactams, sulfonates, sulfonamides, amines and secondary amides. Compounds with the code of WNN are proprietary in our collection and the others are commercially available. They are stored at concentrations of 5 mg/mL and 1 mg/mL in 100% dimethyl sulfoxide (DMSO), respectively. Compounds were diluted in cell medium for each assay.
2.2. Chemicals and antibodies
Niclosamide (Selleck Chemicals, Houston, TX, USA) was initially diluted in 33% dimethylacetylamine and 67% PEG400 to 25 mmol/L stock concentration. Cells were treated with test compounds at a concentration of 10 μmol/L. Recombinant human IFN-α2a (Novoprotein, Shanghai, China) is produced by Escherichia coli expressing Cys24-Glu188. Interferon was dissolved in distilled water, aliquoted, and stored at a concentration of 2×104 IU/mL. Antibodies used were STAT1 (D1K9Y), Phospho-STAT1 (Tyr701) (58D6), STAT3 (79D7), Phospho-STAT3 (Tyr705) (D3A7) XP®, and β-actin made in rabbits; goat anti-rabbit IgG HRP-linked antibody (Cell Signaling Technology, Danvers, MA, USA), and GAPDH mouse mAb 39–8600 (ZG003) (ThermoFisher Scientific, Waltham, MA, USA), and goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The assay plates (SpectraPlate™-384 TC) were produced by PerkinElmer (Boston, MA, USA).
2.3. Cell lines
HEK-Blue IFNα/β cells, QUANTI-Blue, zeocin, and blasticidin were purchased from Invivogen (Carlsbad, CA, USA). HEK-Blue IFNα/β cells are specifically designed to monitor the activation of the JAK-STAT pathway induced by type-I IFNs. This cell line was maintained in Dulbecco׳s modified Eagle׳s medium (DMEM) with 10% fetal bovine serum, 30 µg/mL of blasticidin and 100 µg/mL of zeocin in a 37 °C, 5% CO2 incubator. Human neuroblastoma cells, SH-SY5Y, were cultured in DMEM with 10% fetal bovine serum, 50 U/mL penicillin and 50 μg/mL streptomycin. DMEM, fetal bovine serum, 0.25% Trypsin-EDTA were bought from Life Technologies (Carlsbad, CA, USA). DMSO was procured from Sigma (St Louis, MO, USA).
2.4. Treatment
HEK-Blue IFNα/β and SH-SY5Y cells were treated with IFN-α2a at concentrations of 150 IU/mL for 15 min and 300 IU/mL for 30 min, respectively. Compounds were added 15 min before IFN treatment in fresh culture medium for protein and RNA isolation.
2.5. High-throughput screening
Compounds (0.5 μL/well) were dissolved in DMSO and added into 384-well plates prior to seeding HEK-Blue IFNα/β (5,000/well). The mixture was kept in a 37 °C, 5% CO2 incubator for 5 h. IFN-α2a was then added to the plates to make final concentration of 150 IU/mL. The wells were incubated at 37 °C, 5% CO2 overnight. Seven microliters supernatant of each well was transferred to the new assay plate followed by addition of QUANTI-Blue (72 μL/well). The wells were incubated for 2 h at 37 °C. The absorbance of 640 nm was read by FlexStationIII (Molecular Device, Sunnyvale, CA, USA).
Samples dissolved in 100% DMSO were applied to the primary screening, with an average final concentration of 20 μmol/L for each compound. In each 384-well assay plate, 24 wells were used as positive control (1% DMSO with 10 μmol/L niclosamide and 150 IU/mL IFN-α2a), 24 wells as negative control (1% DMSO with 150 IU/mL IFN-α2a without any compounds), and 16 wells as blank control (1% DMSO without IFN-α2a and compounds). Compounds showing greater than 70% inhibition relative to negative controls were considered “hits”.
2.6. Dose response
Dose–response study was performed essentially as the same as described above except that test compounds were hand-picked (average stock concentration: 10 mmol/L) and diluted (1:5) seven times to give a total of eight serial diluents. The final compound concentration was ranging from 100 μmol/L to 1.28 nmol/L. Each compound was tested in triplicate.
2.7. Data analysis
Z′ factor was evaluated in 48 positive control wells, 48 negative control wells and 32 blank control wells before the HTS campaign as following:
| (1) |
where MPC means the mean value of positive control wells; SDPC means the standard deviation of positive control wells; MNC means the mean value of negative control wells; and SDNC means the standard deviation of negative control wells.
The equation of inhibition (%) is as following:
| (2) |
where Signaltest means the value of each compound test well; Signalblank means the mean value of blank control wells; and SignalNC means the mean value of negative control wells.
2.8. Cytotoxicity assay
Cells were cultured in 96-well plate until 60%–80% density was reached. Compounds were prepared to give five different final concentrations: 50, 25, 12.5, 6.25, and 3.125 μmol/L. Non-treated control and blank (medium only) control were placed in each plate. Twenty-four hour compound incubation was followed by addition of CCK8 solution (Dojindo, Kumamoto, Japan) to each well. Absorbance at 450 nm 3 h thereafter was measured by an Envision plate reader (PerkinElmer). Cell viability (%) was calculated compared to the non-treated and blank wells.
2.9. Protein extraction and western blot analysis
HEK-Blue IFNα/β and SH-SY5Y cells were grown for 48 h until reach confluence in 24-well plates. 5× SDS-PAGE loading buffer stock and 8% SDS-PAGE gel were prepared according to standard protocol. The material used were Tris base, tetramethylethylenediamine, ammonium persulfate (Sigma), SDS (OurChem, Guangdong, China), bromphenol blue, glycerol, hydrochloric acid (SCR, Shanghai, China), β-mercaptoethanol (Fluka Chemie, Buchs, Switzerland) and 30% acrylamide (Generay Biotech, Shanghai, China).
Protein was extracted by 1× SDS-PAGE loading buffer, 100 μL per well. Cells were scraped from the plate and put into chilled tube. Denaturation was carried out by boiling at 100 °C for 10 min. Protein extract was loaded into 1 mm thick gel, each well was given 45 μg, and run for stacking 30 V for 30 min and then 120 V for 1 h with 1× SDS running buffer (10× recipe is 30.2 g Tris-base, 188 g glycine from SCR, 10 g SDS, and distilled water to make 1 L). Protein was then transferred to Immobilon-P PVDF membrane (Merck, Darmstadt, Germany) for 1 h at 100 V on ice by wet transfer. Rapid Transfer Buffer (EpiZyme Biotech, Shanghai, China) was used with addition of 15% methanol. Membrane was blocked by 5% skim milk in TBST (EpiZyme Biotech) for 1 h. Primary antibodies were diluted according to the manual, incubated with membrane for 16 h at 4 °C. Membrane was washed three times in TBST. Incubation with secondary antibodies was for 1 h at room temperature. Signal was enhanced by Super Signal® West Dura Extended Duration Substrate (ThermoFisher Scientific). Images were analyzed with ImageJ software (National Institutes of Health, Bethesda, MA, USA). Percent inhibition of compound was calculated compared to non-treated controls (100% inhibition) and IFN treatment (no inhibition).
2.10. RNA isolation and quantitative PCR
Total RNA from confluent monolayer cells in 12-well plate was isolated with 500 μL/well TRIzol reagent (Ambion, Carlsbad, CA, USA) and the concentration measured by Nanodrop. Two μg of RNA was reverse transcribed into complementary DNA (cDNA) using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Primer pairs (Table 1) were ordered from Genewiz (Jiangsu, China). Quantitative PCR was performed with 100 ng cDNA using SYBR Select Master Mix (Applied Biosystems). Each sample was done in triplicate and repeated three times. Relative expression of the gene of interest was calculated by the delta-delta Ct method with GAPDH as endogenous control.
Table 1.
Primer pairs used for quantitative PCR (pga.mgh.harvard.edu).
| Gene | Position | Primer sequence |
|---|---|---|
| IRF3 | Forward | AGAGGCTCGTGAATGGTCAAG |
| Reverse | AGGTCCACAGTATTCTCCAGG | |
| IRF7 | Forward | GCTGGACGTGACCATCATGTA |
| Reverse | GGGCCGTATAGGAACGTGC | |
| GAPDH | Forward | GAAGGTGAAGGTCGGAGT |
| Reverse | GAAGATGGTGATGGGATTTC |
2.11. Statistical analysis
Dose--response curves and IC50 values were generated using log (inhibitor) vs. response equation in nonlinear regression analysis. Data are expressed as means±SEM. The significance was evaluated by a one-way analysis of variance (ANOVA) followed by Bonferroni post hoc test. Differences were considered significant when a P value was less than 0.05. All statistical analyses were performed with GraphPad Prism software version 5 (San Diego, CA, USA).
3. Results
3.1. Assay validation
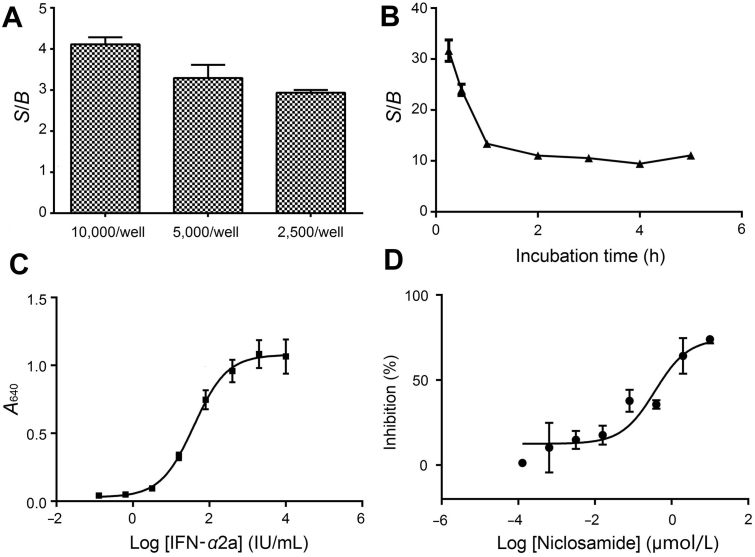
Different numbers of cells (2500/well, 5000/well and 10,000/well) were employed to assess the optimal number of cells for the screening assay. The number of cells for each well was determined to be 5000 (Fig. 1A). The result of incubation time evaluation of QUANTI-Blue course was shown in Fig. 1B. QUANTI-Blue is a solution which in the presence of any alkaline phosphatase changes color from pink to purple-blue. In combination with the secreted reporter, embryonic alkaline phosphatase (SEAP), it offers many advantages over intracellular reporters. Since the signal/background (S/B) ratio did not change significantly between 1 and 5 h, 2 h was selected. IFN-α2a titration was performed to choose the optimal concentration (Fig. 1C) and EC80 (150 IU/mL) was chosen for the assay. EC80 depicts the concentration which elicited 80% of the IFN-α2a response and signal represents the value of cells treated with IFN-α2a compared to blank control. Under these optimized conditions, the IC50 value of the positive molecule niclosamide was 0.37 μmol/L (Fig. 1D). It is in agreement with a previous study in which niclosamide dose-dependently inhibited STAT3 mediated luciferase reporter activity with an IC50 of 0.25 μmol/L in HeLa cells after 24 h incubation22.
Figure 1.
Optimization for the HTS assay. (A) Signals after treatment with IFN-α2a (200 IU/mL) with different number of cells were measured (n=3). (B) Activities of different incubation time with QUANTI-Blue (0–5 h) after treatment with IFN-α2a (200 IU/mL) were measured (n=3). (C) IFN-α2a titration (0.128 to 10,000 IU/mL, n=3). (D) Dose-response curve of niclosamide upon IFN-α2a treatment measured with the optimal assay conditions, from which IC50 value was calculated (n=3).
3.2. Assay performance
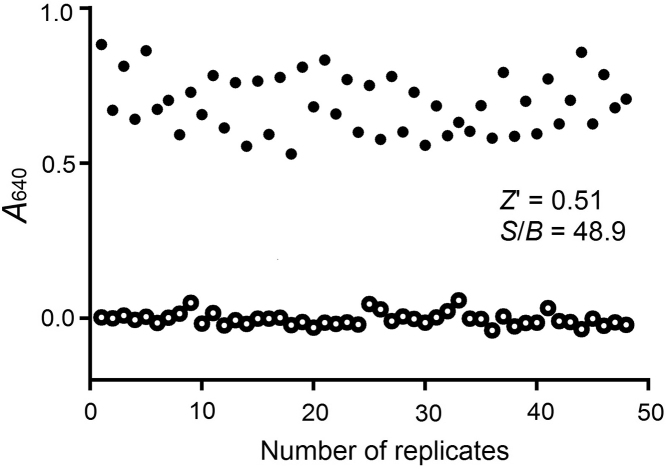
Both signals evoked by negative control (IFN-α2a stimulation) and by positive control (background) were studied. As shown in Fig. 2, the coefficient of variation (CV) value was 13.3% for signal of IFN-α2a stimulation and the Z′ factor was 0.51 with an S/B ratio of 48.9. These characteristics indicate that the system is of high quality and well-suited to HTS23.
Figure 2.
Z′ factor of the HTS assay was determined under the optimized conditions. Forty-eight replicates of signal (negative control, black circle) and background (positive control, white circles) were investigated.
3.3. Identification of inhibitors of type-I interferon signaling pathway
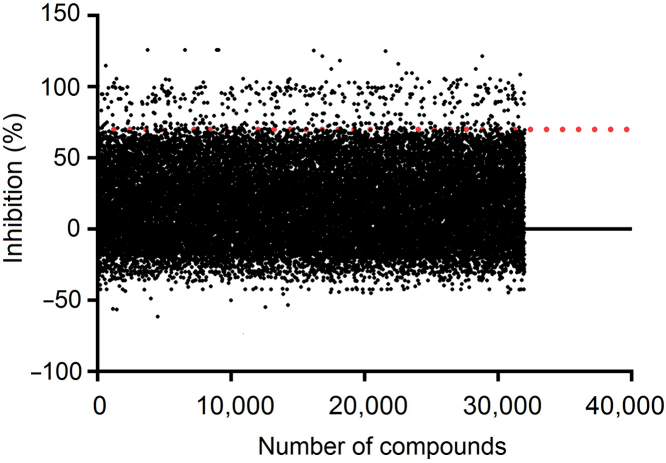
Results of the primary screening of type-I interferon signaling pathway against 32,000 compounds are shown in Fig. 3. 457 hits (1.4%) that displayed inhibitory activity higher than 70% were picked for confirmation. In the secondary screening (initial hits in duplicates), 25 compounds demonstrated consistent inhibition and dose-dependency at the two concentration studied (20 and 4 μmol/L; Table 2 and Supplementary Information Fig. S1).
Figure 3.
HTS campaign of 32,000 compounds using SEAP reporter assay. The results are expressed as percentage of inhibition of each sample on SEAP response induced by IFN-α2a. Dashed line shows the cut off at 70% inhibitory activity. 457 hits (1.4%) showed higher than 70% inhibition in the primary screening.
Table 2.
Structures of 25 confirmed hits and their IC50 values.
| Compd. | Structure | Chemical formula | MW | IC50 (μmol/L) |
|---|---|---|---|---|
| WNN2202-B006 | 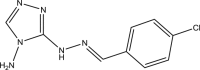 |
C9H9ClN6 | 236.661 | 10.48 |
| WNN2210-B007 | 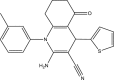 |
C21H19N3OS | 361.46 | NA |
| WNN2210-C004 | 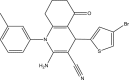 |
C21H18BrN3OS | 440.356 | 13.94 |
| WNN2290-B006 | 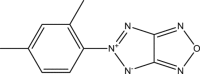 |
C10H9N5O | 215.211 | 6.522 |
| CD2001-B009 | 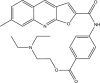 |
C26H27N3O4 | 445.51 | 15.69 |
| CD2006-A002 | 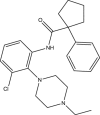 |
C24H30ClN3O | 411.967 | 3.39 |
| CD2015-G003 | 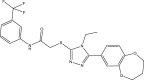 |
C22H21F3N4O3S | 478.487 | 5.478 |
| CD2020-G009 | 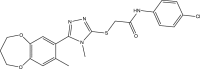 |
C21H21ClN4O3S | 444.934 | 3.195 |
| CD2047-G004 |  |
C26H22FN7O2 | 483.497 | 20.29 |
| CD2079-E003 |  |
C24H22ClN7O2S | 507.995 | 2.993 |
| CD2093-G007 |  |
C26H22Cl2N2O2S2 | 529.501 | 0.2976 |
| CD2100-B008 |  |
C24H26N6O | 414.503 | 43.34 |
| JK3038-D010 | 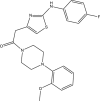 |
C22H23FN4O2S | 426.507 | 2.959 |
| JK3043-F003 | 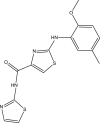 |
C15H14N4O2S2 | 346.427 | 5.88 |
| JK3049-H002 | 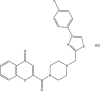 |
C24H21ClFN3O3S | 485.958 | 3.777 |
| JK3051-A002 | 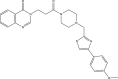 |
C26H27N5O3S | 489.589 | 4.194 |
| JK3090-H004 | 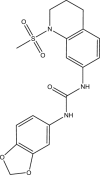 |
C18H19N3O5S | 389.426 | 3.316 |
| RUS0903-C006 | 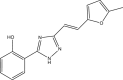 |
C15H13N3O2 | 267.283 | 1.492 |
| RUS0910-G009 | 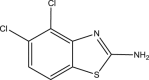 |
C7H4Cl2N2S | 219.091 | 3.144 |
| RUS0937-A002 | 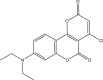 |
C16H14ClNO4 | 319.74 | 2.263 |
| RUS0948-D009 | 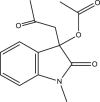 |
C14H15NO4 | 261.273 | 4.984 |
| RUS0966-F006 | 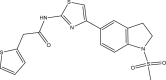 |
C18H17N3O3S3 | 419.541 | 7.88 |
| RUS0971-B008 | 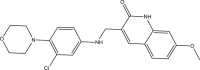 |
C21H22ClN3O3 | 399.871 | 4.917 |
| RUS0971-C008 |  |
C21H24N2O3 | 352.427 | 20.91 |
| RUS0998-A003 |  |
C17H19N3O2S | 329.417 | 4.883 |
NA, not active.
3.4. Preliminary characterization of confirmed hits
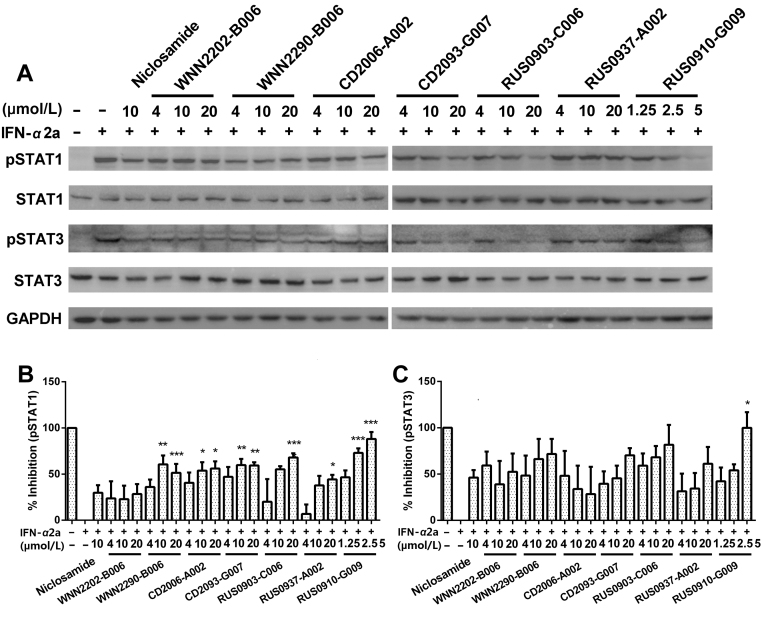
Twenty-five confirmed hits were assessed for their effects on phosphorylation of STAT1 and STAT3 (Supplementary Information Fig. S2). Of which, 7 compounds significantly reduced the phosphorylation level of STAT1 and/or STAT3 at 20 μmol/L. WNN2202-B006, WNN2290-B006, CD2093-G007 and CD2006-A002 distinctly inhibited pSTAT3, while RUS0903-C006, RUS0910-G009 and RUS0937-A002 suppressed pSTAT1.
3.5. Cytotoxicity measurement
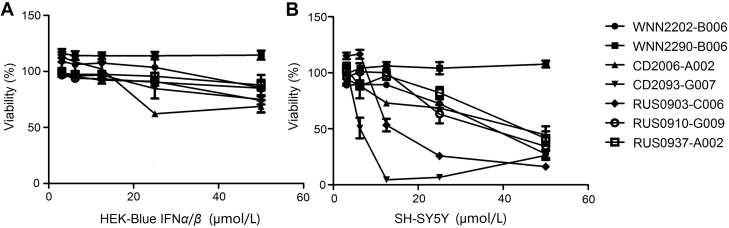
The above 7 confirmed hits were evaluated for cytotoxicity using the CCK8 assay kit. Treatment of SH-SY5Y cells with IFN-α2a up to 800 IU/mL did not affect cell viability (Data not shown). Among the seven, WNN2290-B006 showed no toxicity for both cell lines (Fig. 4). RUS0910-G009 and RUS0937-A002 were slightly toxic at the highest concentration in HEK cells while the rest demonstrated certain degree of toxicity. Neuroblastoma cell line SH-SY5Y was more sensitive towards the compounds. Six reduced cell viability by 50% or more at the highest concentration tested (50 μmol/L), whereas at 25 μmol/L only two hits (CD2093-G007 and RUS0903-C006) decreased viability to less than 50%.
Figure 4.
Viability of HEK-Blue IFNα/β (A) and SH-SY5Y (B) cells after 24 h incubation with hit compounds at 50, 25, 12.5, 6.25, and 3.125 μmol/L. Viability (%) was calculated compared to the non-treated control (100% viable) and blank (0% viable).
3.6. Effect on STAT1/STAT3 phosphorylation
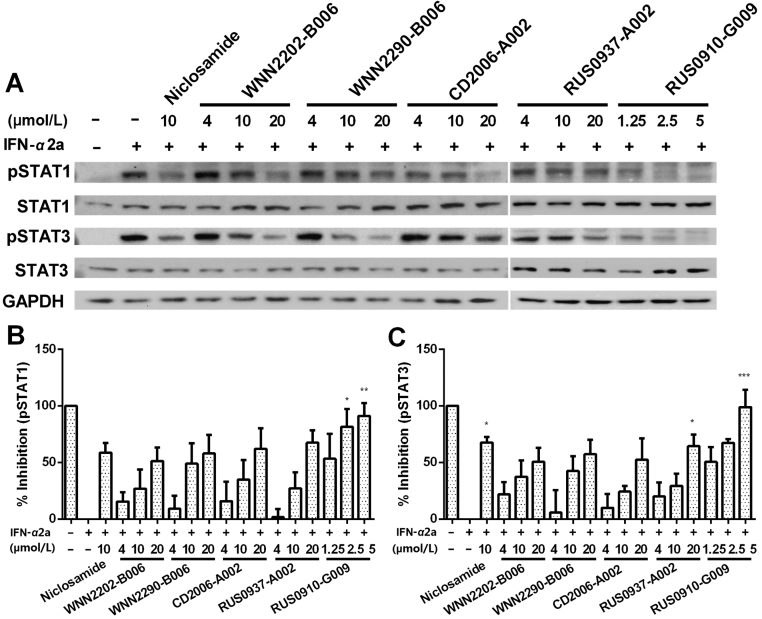
Binding of type-I IFN to IFNAR will activate STAT1 and STAT2. In addition, STAT3 and STAT5 are also known to be phosphorylated. STAT1 induces pro-inflammatory genes and inhibits cell growth while STAT3 homodimers indirectly suppress pro-inflammatory gene expression and stimulate growth24. Consistent with the literature21, 25, IFN-α2a induced robust phosphorylation of STAT1 and STAT3 in both cell lines as shown in Fig. S2, Figs. 5 and 6. Niclosamide was previously reported to only inhibit STAT3 phosphorylation at concentrations below 10 μmol/L22. Interestingly, in this study, a relatively high concentration (10 μmol/L) of niclosamide also inhibited STAT1 phosphorylation (Figs. 5B, 6B and Fig. S2).
Figure 5.
Some confirmed hits reduced STAT phosphorylation in response to IFN-α2a treatment (150 IU/mL, 15 min) in HEK-Blue IFNα/β cells. (A) Representative western blots of 7 hits in three different concentrations. (B) and (C) Inhibition (%) of pSTAT1 and pSTAT3 after treatment. Protein levels were determined by western blotting and normalized to GAPDH. Two SDS gels were used for each experiment. Data represent means±SEM from at least three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared to IFN-α2a treated cells.
Figure 6.
Some confirmed hits reduced STAT phosphorylation in response to IFN-α2a treatment (300 IU/mL 30 min) in SH-SY5Y cells. Representative western blots (A), inhibition (%) of pSTAT1 (B) and pSTAT3 (C) after treatment are shown. Protein levels were determined by western blotting and normalized to GAPDH. Two SDS gels were used for each experiment. Data represent means±SEM from at least three independent experiments. *P<0.05, **P<0.01, ***P<0.001 compared to IFN-α2a treated cells.
Seven hits were studied in three concentrations (20, 10 and 4 μmol/L) with HEK-Blue IFNα/β cells (Fig. 5). Four com-pounds (CD2006-A002, RUS0903-C006, RUS0937-A002 and RUS0910-G009) showed dose-dependent suppression on STAT1 and five compounds (WNN2290-B006, CD2093-G006, RUS0903-C006, RUS0937-A002 and RUS0910-G009) inhibited STAT3 phosphorylation. Of the seven, five relatively non-toxic hits were tested in three concentrations (20, 10 and 4 μmol/L) on SH-SY5Y cells (Fig. 6): they dose-dependently suppressed STAT1 and STAT3 phosphorylation at various potencies. Compound RUS0910-G009 showed the highest potency among them.
3.7. Effect on IRF7 mRNA transcription
IRF3 and IRF7 are essential IRFs required to induce IFN-α/β gene transcription. Moreover, IRF7 is markedly elevated in all cell types by type-I IFN26. In most cases, transcription of these regulatory factors is triggered by STAT1/STAT2/IRF9 heterodimeric complex, namely, IFN-stimulated gene factor 3 (ISGF3), through an IFN-stimulated response element (ISRE) binding site1, 27.
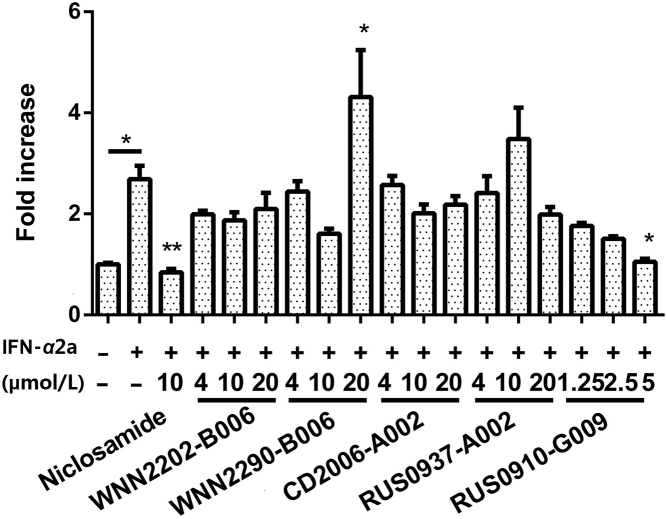
IFN-α2a treatment for 1 h increased IRF7 mRNA transcription level by 2.09±0.16 folds and 2.69±0.26 folds compared to untreated controls for HEK (Supplementary Information Fig. S3) and SH-SY5Y (Fig. 7) cells, respectively. However, the treatment did not significantly affect IRF3 mRNA levels in both cell lines (1.25±0.15 folds and 1.03±0.04 folds for HEK and SH-SY5Y, respectively). Positive control niclosamide suppressed IRF7 level to a level close to normal (0.84±0.07 folds) in SH-SY5Y cells.
Figure 7.
Effects of some confirmed hits on IRF7 transcription level in response to IFN-α2a treatment (1 h) in SH-SY5Y cells. Data represent mean expression fold±SEM relative to GAPDH, measured from three independent experiments, each in triplicates. *P<0.05, **P<0.01, and ***P<0.001 compared to IFN-α2a treated cells.
Seven hits were tested in HEK-Blue IFNα/β cells (Fig. S3B) while 5 relatively non-toxic hits were investigated in SH-SY5Y cells (Fig. 7) in three concentrations (20, 10 and 4 μmol/L): they displayed variable inhibitory properties on IRF7 mRNA transcription but only RUS0910-G009 exhibited dose-dependency suppression.
4. Discussion
Type-I IFNs primarily activate the JAK-STAT signaling pathway. The normal function of the IFN system is essential to human health and disease interventions28. Numerous attempts to find inhibitors of type-I IFN signal transduction are based upon the clinical importance of this pathway covering tumor growth29, 30, viral infection, rheumatoid arthritis10, 17, 31, systemic lupus erythematosus, Alzheimer׳s disease and neuroinflammatory diseases8.
In this study, we utilized SEAP reporter gene assay in specially engineered HEK-Blue IFNα/β cells to detect JAK-STAT pathway activities induced by type-I IFN. This cell line is stably transfected with STAT2, IRF9, and SEAP genes. Following activation by type-I IFN, ISGF3 complex will bind to ISRE in the promoter of IFN-stimulated genes (ISG). SEAP, attached to ISG54 promoter, will be then be easily expressed and secreted to the culture medium and measured spectrophotometrically by detection reagent. It has been commonly used for measurement of type-I IFN activity from cell culture supernatant32.
Application of ISRE to different screening systems has been reported previously, usually linked with a luciferase gene reporter14, 15, 16. In our case, cells were stimulated with recombinant human IFN-α2a, a key innate cytokine and essential to antiviral immune responses33. IFN-α2a was reported to robustly activate signaling pathways as fast as 5 min for TYK2 phosphorylation and 10 min for STAT phosphorylation at 30 ng/mL concentration21. We optimized the incubation time with QUANTI-Blue and the IFN concentration to enhance the signal readout. This validation process was successful as demonstrated by high quality assay parameters such as CV, Z׳ factor and S/B ratio. Thus, we have developed an HTS assay to search for novel inhibitors of IFN-α2a signaling. In the primary screening of 32,000 compounds, 457 initial hits (1.4%) were identified. Secondary screening and dose-response experiments resulted in 25 confirmed hits. Of which, 18 exhibited IC50 values below 10 μmol/L and 7 were capable of inhibiting phosphorylation of STAT1 Try701 and/or STAT3 Tyr705 at 20 μmol/L and two of them displayed consistent dose-dependent features. RUS0910-G009 appears most active in the two engineered cell lines used: it also caused notable IRF7 mRNA suppression.
In summary, by use of a SEAP reporter gene based HTS assay, we were able to discover 25 potential small molecule inhibitors of the IFN-α2a signaling pathway. Several of them were further characterized for cytotoxicity, inhibition on STAT phosphorylation and suppression of IRF7 transcription. It seems that this SEAP reporter gene assay is a viable approach to the discovery of small molecules interfering with type-I IFN action, especially via the JAK-STAT pathway. It provides an alternative to other screening systems such as thermofluor-13 and fluorescence resonance energy transfer (FRET)-based34 techniques.
Acknowledgments
We are indebted to Caihong Zhou, Ji Wu and Qiang Shen for technical assistance. This work was partially supported by grants from Shanghai Science and Technology Development Fund (to MWW: 15DZ2291600) and the Thousand Talents Program in China (to MWW: [2011]166). PJC was a recipient of an Australian Research Council Future Fellowship and CAS President׳s International Fellowship Initiative (PIFI). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation.
Footnotes
Peer review under responsibility of Institute of Materia Medica, Chinese Academy of Medical Sciences and Chinese Pharmaceutical Association.
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.apsb.2018.07.005.
Appendix A. Supplementary material
Supplementary material.
.
Supplementary material.
.
References
- 1.McNab F., Mayer-Barber K., Sher A., Wack A., O' Garra A. Type I interferons in infectious disease. Nat Rev Immunol. 2015;15:87–103. doi: 10.1038/nri3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng C.T., Mendoza J.L., Garcia K.C., Oldstone M.B. Alpha and beta type 1 interferon signaling: passage for diverse biologic outcomes. Cell. 2018;164:349–352. doi: 10.1016/j.cell.2015.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jonasch E., Haluska F.G. Interferon in oncological practice: review of interferon biology, clinical applications, and toxicities. Oncologist. 2001;6:34–55. doi: 10.1634/theoncologist.6-1-34. [DOI] [PubMed] [Google Scholar]
- 4.Capobianchi M.R., Uleri E., Caglioti C., Dolei A. Type I IFN family members: similarity, differences and interaction. Cytokine Growth Factor Rev. 2015;26:103–111. doi: 10.1016/j.cytogfr.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oie S., Ono M., Yano H., Maruyama Y., Terada T., Yamada Y. The up-regulation of type I interferon receptor gene plays a key role in hepatocellular carcinoma cells in the synergistic antiproliferative effect by 5-fluorouracil and interferon-α. Int J Oncol. 2006;29:1469–1478. [PubMed] [Google Scholar]
- 6.Mcglasson S., Jury A., Jackson A., Hunt D. Type I interferon dysregulation and neurological disease. Nat Rev Neurol. 2015;11:515–523. doi: 10.1038/nrneurol.2015.143. [DOI] [PubMed] [Google Scholar]
- 7.Bialas A.R., Presumey J., Das A., van der Poel C.E., Lapchak P.H., Mesin L. Microglia-dependent synapse loss in type I interferon-mediated lupus. Nature. 2017;546:539–543. doi: 10.1038/nature22821. [DOI] [PubMed] [Google Scholar]
- 8.Taylor J.M., Moore Z., Minter M.R., Crack P.J. Type-I interferon pathway in neuroinflammation and neurodegeneration: focus on Alzheimer׳s disease. J Neural Transm. 2018;125:797–807. doi: 10.1007/s00702-017-1745-4. [DOI] [PubMed] [Google Scholar]
- 9.Hervas-Stubbs S., Perez-Gracia J.L., Rouzaut A., Sanmamed M.F., Le Bon A., Melero I. Direct effects of type I interferons on cells of the immune system. Clin Cancer Res. 2011;17:2619–2627. doi: 10.1158/1078-0432.CCR-10-1114. [DOI] [PubMed] [Google Scholar]
- 10.Cornez I., Yajnanarayana S.P., Wolf A.M., Wolf D. JAK/STAT disruption induces immune-deficiency: rationale for the development of JAK inhibitors as immunosuppressive drugs. Mol Cell Endocrinol. 2017;451:88–96. doi: 10.1016/j.mce.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 11.Kirou K.A., Gkrouzman E. Anti-interferon alpha treatment in SLE. Clin Immunol. 2013;148:303–312. doi: 10.1016/j.clim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Chasset F., Arnaud L. Targeting interferons and their pathways in systemic lupus erythematosus. Autoimmun Rev. 2018;17:44–52. doi: 10.1016/j.autrev.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo E.D., Manaswiyoungkul P., Israelian J., Park J., Yuen K., Farhangi S. High-throughput thermofluor-based assays for inhibitor screening of STAT SH2 domains. J Pharm Biomed Anal. 2017;143:159–167. doi: 10.1016/j.jpba.2017.04.052. [DOI] [PubMed] [Google Scholar]
- 14.Patel D.A., Patel A.C., Nolan W.C., Zhang Y., Holtzman M.J. High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PLoS One. 2012;7:e36594. doi: 10.1371/journal.pone.0036594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tai Z.F., Zhang G.L., Wang F. Identification of small molecule activators of the Janus kinase/signal transducer and activator of transcription pathway using a cell-based screen. Biol Pharm Bull. 2012;35:65–71. doi: 10.1248/bpb.35.65. [DOI] [PubMed] [Google Scholar]
- 16.Wonganan O., He Y., Shen X., Wongkrajang K., Suksamrarn A., Zhang G. 6-Hydroxy-3-O-methyl-kaempferol 6-O-glucopyranoside potentiates the anti-proliferative effect of interferon α/β by promoting activation of the JAK/STAT signaling by inhibiting SOCS3 in hepatocellular carcinoma cells. Toxicol Appl Pharmacol. 2017;336:31–39. doi: 10.1016/j.taap.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz D.M., Kanno Y., Villarino A., Ward M., Gadina M., O׳Shea J.J. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov. 2017;16:843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 18.Taylor J.M., Minter M.R., Newman A.G., Zhang M., Adlard P.A., Crack P.J. Type-1 interferon signaling mediates neuro-inflammatory events in models of Alzheimer׳s disease. Neurobiol Aging. 2014;35:1012–1023. doi: 10.1016/j.neurobiolaging.2013.10.089. [DOI] [PubMed] [Google Scholar]
- 19.Minter M.R., Taylor J.M., Crack P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer׳s disease. J Neurochem. 2016;136:457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 20.Jiao J., Kaur N., Lu B., Reeves S.A., Halvorsen S.W. Initiation and maintenance of CNTF-Jak/STAT signaling in neurons is blocked by protein tyrosine phosphatase inhibitors. Mol Brain Res. 2003;116:135–146. doi: 10.1016/s0169-328x(03)00286-9. [DOI] [PubMed] [Google Scholar]
- 21.Dedoni S., Olianas M.C., Onali P. Interferon-β induces apoptosis in human SH-SY5Y neuroblastoma cells through activation of JAK-STAT signaling and down-regulation of PI3K/Akt pathway. J Neurochem. 2010;115:1421–1433. doi: 10.1111/j.1471-4159.2010.07046.x. [DOI] [PubMed] [Google Scholar]
- 22.Ren X., Duan L., He Q., Zhang Z., Zhou Y., Wu D. Identification of niclosamide as a new small-molecule inhibitor of the STAT3 signaling pathway. ACS Med Chem Lett. 2010;1:454–459. doi: 10.1021/ml100146z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J.-H., Chung T.D.Y., Oldenburg K.R. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 24.Main B.S., Zhang M., Brody K.M., Ayton S., Frugier T., Steer D. Type-1 interferons contribute to the neuroinflammatory response and disease progression of the MPTP mouse model of Parkinson׳s disease. Glia. 2016;64:1590–1604. doi: 10.1002/glia.23028. [DOI] [PubMed] [Google Scholar]
- 25.Minter M.R., Zhang M., Ates R.C., Taylor J.M., Crack P.J. Type-1 interferons contribute to oxygen glucose deprivation induced neuro-inflammation in BE(2) M17 human neuroblastoma cells. J Neuroinflamm. 2014;11:43. doi: 10.1186/1742-2094-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul F., Pellegrini S., Uzé G. IFNA2: the prototypic human alpha interferon. Gene. 2015;567:132–137. doi: 10.1016/j.gene.2015.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Molle C., Goldman M., Goriely S. Critical role of the IFN-stimulated gene factor 3 complex in TLR-mediated IL-27p28 gene expression revealing a two-step activation process. J Immunol. 2010;184:1784–1792. doi: 10.4049/jimmunol.0902005. [DOI] [PubMed] [Google Scholar]
- 28.Arimoto K., Löchte S., Stoner S.A., Burkart C., Zhang Y., Miyauchi S. STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat Struct Mol Biol. 2017;24:279–289. doi: 10.1038/nsmb.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chau M.N., Banerjee P.P. Development of a STAT3 reporter prostate cancer cell line for high throughput screening of STAT3 activators and inhibitors. Biochem Biophys Res Commun. 2008;377:627–631. doi: 10.1016/j.bbrc.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Page B.D.G., Ball D.P., Gunning P.T. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:65–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- 31.Thoma G., Drückes P., Zerwes H.-G. Selective inhibitors of the Janus kinase Jak3—Are they effective? Bioorg Med Chem Lett. 2014;24:4617–4621. doi: 10.1016/j.bmcl.2014.08.046. [DOI] [PubMed] [Google Scholar]
- 32.Dallari S., Macal M., Loureiro M.E., Jo Y., Swanson L., Hesser C. Src family kinases Fyn and Lyn are constitutively activated and mediate plasmacytoid dendritic cell responses. Nat Commun. 2017;8:14830. doi: 10.1038/ncomms14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horvath C.M. The Jak-STAT pathway stimulated by interferon α or interferon β. Sci STKE. 2004;2004:tr10. doi: 10.1126/stke.2602004tr10. [DOI] [PubMed] [Google Scholar]
- 34.Robers M.B., Horton R.A., Bercher M.R., Vogel K.W., Machleidt T. High-throughput cellular assays for regulated posttranslational modifications. Anal Biochem. 2008;372:189–197. doi: 10.1016/j.ab.2007.09.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Supplementary material.