Abstract
Percutaneous coronary intervention (PCI) with a drug coated balloon (DCB) is a novel treatment which seeks to acutely dilate a coronary stenosis and deliver an anti-proliferative drug to the vessel wall (reducing the risk of re-stenosis), without implanting a drug eluting stent (DES). In this study, we performed a systematic review of stentless DCB-only angioplasty in de novo coronary artery disease. We identified 41 studies examining the effects of DCB-only PCI in a variety of clinical scenarios including small vessels, bifurcations, calcified lesions, and primary PCI. DCB-only PCI appears to be associated with comparable clinical outcomes to DESs and superior angiographic outcomes to plain-old balloon angioplasty. Although current data are promising, there is still a need for further long-term randomized control trial data comparing a DCB-only approach specifically against a second- or third-generation DES. A 4-week period of dual antiplatelet therapy provides a real advantage for the DCB-only PCI approach, which is not possible with most DESs. Since rates of adverse clinical outcomes are very low for all PCI procedures attention should be turned to the development of robust endpoints with which to compare DCB-only PCI approaches to the standard treatment with a DES.
Electronic supplementary material
The online version of this article (10.1007/s40119-018-0121-2) contains supplementary material, which is available to authorized users.
Keywords: Coronary artery disease, De novo, Drug-coated balloon, Drug-eluting balloon, Percutaneous coronary intervention
Introduction
Percutaneous coronary intervention (PCI) is the commonest procedure used in the invasive treatment of coronary artery disease (CAD) [1]. Historically, this has involved plain-old balloon angioplasty (POBA, limited by elastic recoil, dissection and restenosis) and the bare metal stent (BMS, limited by in-stent restenosis/ISR and stent thrombosis/ST requiring the prophylactic use of dual anti-platelet therapy/DAPT) [2, 3]. Currently, the mainstay of coronary revascularization with PCI is with the drug-eluting stent (DES). This enables the local delivery of an anti-proliferative drug via a polymer and has a considerably lower incidence of ISR [4, 5]. The second- and third-generation DESs have further reduced the incidence of ISR and ST and are now preferred over first-generation devices [6]. However, DES use is still suboptimal in small vessel disease (SVD); which occurs in 20–30% of patients with symptomatic CAD [7, 8]. Furthermore, there still remains a small but significant risk of ST. Late ST (> 30 days) and very late ST (> 12 months) have been especially problematic due to delayed stent endothelialization [9]. This necessitates the use of long-term prophylactic DAPT, which is associated with an increased risk of bleeding complications and mortality in the elderly as well as being an economic burden [10].
The drug-coated balloon (DCB) is a semi-compliant balloon coated with an anti-proliferative drug, which is rapidly released via an excipient upon inflation [11]. The vast majority of DCBs are coated with 3 μg/mm2 of paclitaxel. The use of DCBs for the treatment of ISR has class Ia recommendation from the European Society of Cardiology [4]. However, their role in de novo coronary disease is still not clear. The DCB proposes certain advantages over the DES such as a reduced duration of DAPT and immediate homogenous drug release without the presence of a metal and polymer, which have been shown to provoke inflammatory reactions in vessels [12]. Table 1 summarizes the DCBs used in human de novo CAD studies that use DCB-only PCI. DCB-only PCI (also referred to as DCB-only angioplasty and the DCB-only approach) describes the inflation of a DCB (usually for 30–60 s) following acceptable pre-dilatation of a coronary lesion with a cutting/non-compliant balloon and where provisional/bailout stenting is reserved only in cases of an unsatisfactory result [13]. The 2013 German Consensus Group recommendations define this as residual stenosis > 30%; ≥ type C National Heart, Lung and Blood Institute (NHLBI) coronary dissection or a Thrombolysis In Myocardial Infarction (TIMI) flow < 3 [14]. This review aims to provide a comprehensive summary of the published data regarding the use of DCB-only PCI for the treatment of de novo CAD.
Table 1.
Overview of the DCBs used in de novo DCB-only CAD studies
| DCB name | Manufacturer | Excipient |
|---|---|---|
| Dior I | Eurocor (Bonn, Germany) | Dimethyl sulfate |
| Dior II | Eurocor (Bonn, Germany) | Shellac |
| Elutax SV | Aachen Resonance (Aachen, Germany) | Dextran |
| Restore | Cardionovum (Milan, Italy) | Shellac |
| Pantera Lux | Biotronik AG (Buelach, Switzerland, Germany) | Butyryl-tri-hexyl-citrate |
| IN.PACT Falcon | Medtronic-Invatec (Frauenfeld, Switzerland) | Urea |
| SeQuent Please | B. Braun Melsungen AG (Berlin, Germany) | Iopromide |
DCB drug-coated balloon, CAD coronary artery disease
Methods
This was a systematic review conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. MEDLINE, EMBASE, and Cochrane databases were searched (see Appendix A in supplementary material). Included were randomized and observational human de novo CAD studies that employed a DCB-only approach, reporting a clinical outcome of any kind. Exclusion criteria included studies that only employed routine stenting and those that did not separately report results for de novo CAD lesions. This article is based on previously conducted studies and does not involve any new studies with human participants or animal subjects performed by any of the authors.
Preferred clinical outcomes were target lesion revascularization (TLR, defined as any repeat revascularization within the DCB/stented region, either clinically driven or due to > 50% restenosis at follow-up) and major adverse cardiovascular events (MACE, a composite outcome of all-cause mortality, TLR, and myocardial infarction/MI). There is some variability in the way studies define MACE and these have been highlighted (Appendix B in supplementary material). Angiographic data, where reported, were also extracted with the majority of studies reporting late luminal loss (LLL), measured in millimetres. This is defined as the vessel minimal luminal diameter (MLD) post-procedurally, subtracted from the MLD at follow-up. In studies that provided information on where the LLL was taken from, the in-balloon/stent LLL was preferred. Where only an in-segment LLL was reported, this was explicitly mentioned.
In studies that did not report these specific outcomes, other endpoints were instead extracted. Examples include: periprocedural MI (defined as 5 × the 99th percentile upper reference limit of normal for creatine kinase-myocardial band or troponin T, occurring within 48 h after PCI), target lesion thrombosis (TLT, an angiographic occlusion with an acute clinical presentation in a previously treated region), target vessel failure (TVF, a composite outcome of cardiac death, target vessel-related MI and TLR) and device-oriented adverse cardiovascular events (DOCE, a composite outcome of cardiac death, target vessel MI, stroke, and TLR). Other angiographic outcomes include: percentage diameter stenosis (%DS, defined as 100 multiplied by the difference between the reference vessel diameter/RVD and the MLD divided by the RVD), binary restenosis (defined as the presence of a %DS of ≥ 50%) and MLD post-procedure and at follow-up (where a LLL is not provided).
Results
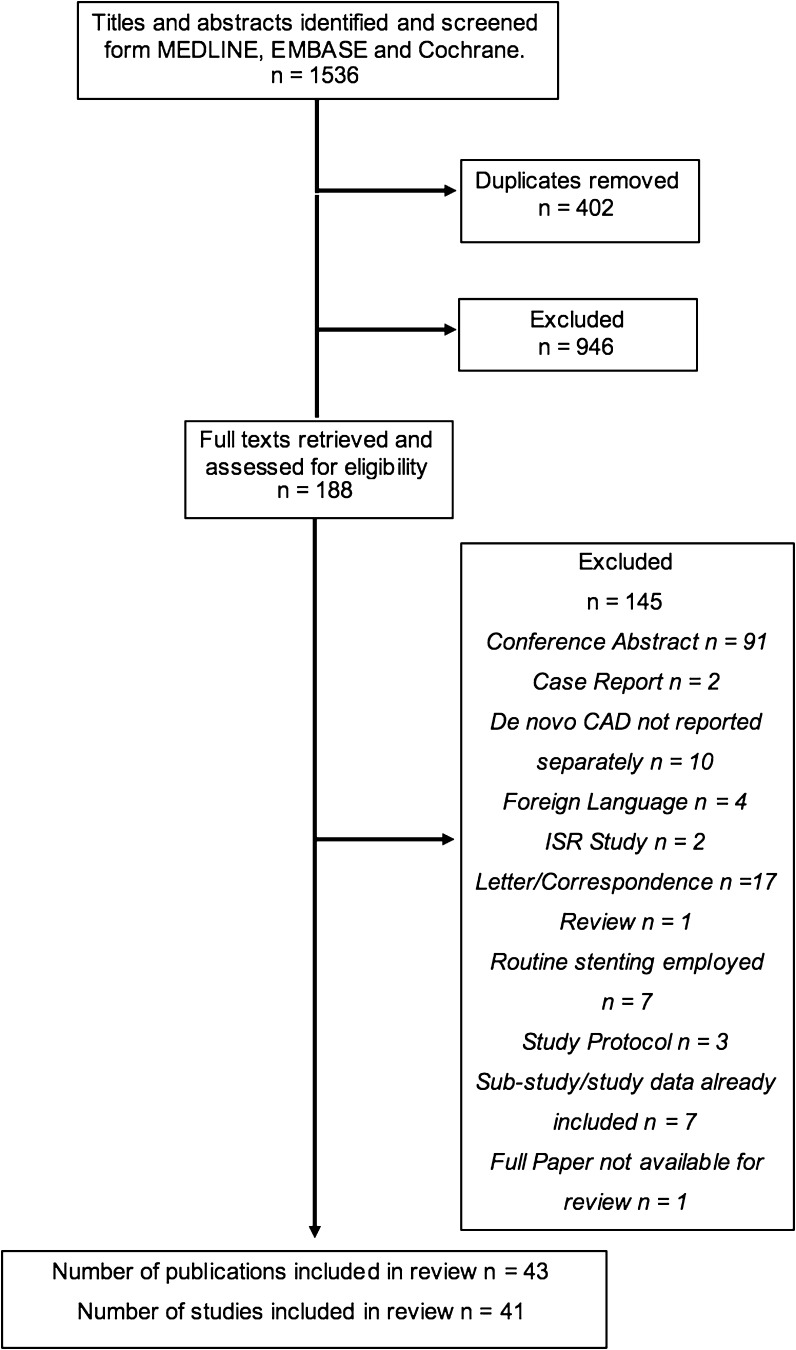
Databases were searched up to 13/03/2018 and identified 1535 results. Forty-one studies (reported over 43 publications) were included in the final review (Fig. 1). These either investigated the general use of DCB-only angioplasty or focused specifically on bifurcating lesions, primary PCI (PPCI, for acute coronary syndrome/ACS), calcified lesions or chronic total occlusions (CTOs). It should be noted that one paper, a registry of the MagicTouch (Concept Medicals Inc.) Sirolimus DCB, fulfilled the inclusion criteria but was not available at the time of writing this review.
Fig. 1.
PRISMA flowchart outlining the study selection process from the initial search download to title and abstract screening to full text analysis. Reasons for removal of full texts are provided
Patient Characteristics
As shown in Table 2, the majority of the 6586 patients enrolled in studies were male (82%) with a mean age of 65. Where registries report patient characteristics for both de novo CAD and ISR, this has been explicitly shown. Among the classical cardiovascular risk factors, hypertension and dyslipidemia were most frequently observed. Temporal inconsistencies existed between studies with regards to smoking habits. The mean percentage of diabetics seen was 37% although, overall, the PPCI studies showed a low prevalence of diabetic patients. The use of DCB-only PCI in de novo CAD was almost exclusively investigated in small vessels (< 2.8 mm). The exceptions to this cut-off value reported a mean vessel diameter (MVD) only marginally greater than 2.8 mm. The majority of studies report a %bailout stenting of below 25%, however certain studies do exceed this; reasons for this are later discussed.
Table 2.
Summary of the patient characteristics of studies investigating the use of DCBs alone in de novo coronary artery disease
| Author | Number of patients | Mean vessel diameter (mm) | Bail out (%) | Mean age | Male N (%) | Diabetes N (%) | Smokers N (%) | HTN N (%) | Dyslip. N (%) |
|---|---|---|---|---|---|---|---|---|---|
| General de novo lesions—randomised studies | |||||||||
| Cortese et al. (2010) [15] | 60 (29 DCB, 31 DES) | 2.54 (DCB), 2.58 (DES) | 36% | 67 | 44 (73%) | 24 (42%) | NR | 41 (72%) | 30 (53%) |
| Latib et al. (2012, 2015) [19, 20] | 182 (90 DCB, 92 DES) | 2.15 (DCB), 2.25 (DES) | 20% | 65 | 143 (79%) | 74 (41%) | 25 (14%) | 147 (81%) | 144 (79%) |
| Nishiyama et al. (2016) [23] | 60 (DCB 27, DES 33) | 2.88 (DCB), 2.72 (DES) | 0%* | 69 | 44 (73%) | 25 (42%) | 36 (60%) | 50 (83%) | 47 (78%) |
| Funatsu et al. (2017) [28] | 133 (DCB 92, POBA 41) | 2.04 (DCB), 1.99 (POBA) | 3% | 68 | 100 (75%) | 57 (43%) | NR | 107 (80%) | 104 (78%) |
| General de novo lesions—comparative observational studies | |||||||||
| Her et al. (2016) [29] | 72 (DCB 49, POBA 23) | 2.3 (DCB), 2.1 (POBA) | 0%* | 63 | 49 (68%) | 25 (35%) | 21 (29%) | 45 (63%) | 43 (60%) |
| Shin et al. (2016) [24] | 66 (DCB 44, BMS/DES 22) | 2.69 (DCB), 2.92 (DES/BMS) | 0%* | 60 | 50 (76%) | 18 (27%) | 25 (38%) | 32 (48%) | 27 (41%) |
| Sinaga et al. (2016) [25] | 335 (172 DCB, 163 DES) | 2.22 (DCB) vs. 2.44 (DES) | 0%* | 57 | 249 (74%) | 168 (50%) | 125 (37%) | 238 (71%) | 238 (71%) |
| Giannini et al. (2017) [22] | 181 (90 DCB, 91 DES) | 2.15 (DCB), DES NR (100% < 2.8) | 20% | 66 | 143 (79%) | 76 (42%) | 69 (38%) | 146 (81%) | 142 (78%) |
| Her et al. (2017) [27] | 104 (DCB 52, DES 52) | 2.3 (DCB), 2.2 (DES) | 0%* | 60 | 34 (33%) | 44 (42%) | 37 (36%) | 60 (58%) | 47 (45%) |
| Venetsanos et al. (2018) [26] | 1648 (DCB 824, 824 DES) | NR (82% < 2.5) | 8% | 68 | 1724 (72%) | 698 (29%) | NR | 1588 (66%) | 1413 (59%) |
| General de novo lesions—observational studies | |||||||||
| Unverdorben et al. (2010, 2013) [17, 18] | 118 | 2.35 | 27% | 68 | 85 (72%) | 51 (43%) | NR | 103 (87%) | 95 (80%) |
| Cuculi et al. (2012) [44] | 79 | 2.8 | 5% | 69 | 63 (80%) | 19 (24%) | 17 (21%) | 56 (71%) | 53 (67%) |
| Woehrle et al. (2012) [35] | 491 | 2.56 | 21% | NR | 379 (77%) | 166 (34%) | 192 (39%) | 408 (83%) | 348 (71%) |
| Calé et al. (2013) [40] | 74 de novo (156 total) | NR (86% < 2.8) | 3% | 66 | 114 (73%) | 68 (44%) | 78 (50%) | 129 (83%) | 120 (77%) |
| Waksman et al. (2013) [47] | 103 | 2.4 | 12% | 63 | 82 (80%) | 29 (28%) | 37 (36%) | 86 (84%) | 61 (60%) |
| Basavarajah et al. (2014) [45] | 79 de novo (184 total) | NR (70% < 2.5) | 22% | 66 | 160 (87%) | 64 (35%) | 99 (54%) | 132 (72%) | 130 (71%) |
| Toelg et al. (2014) [49] | 105 | 2.5 | 23% | 65 | 74 (71%) | 38 (36%) | 71 (68%) | 81 (77%) | 70 (67%) |
| Zeymer et al. (2014) [36] | 447 | 2.14 | 6% | 66 | 324 (73%) | 164 (37%) | 169 (38%) | 360 (80%) | 308 (69%) |
| Kleber et al. (2015) [30] | 56 | 2.58 | 0%* | 67 | 46 (82%) | 19 (34%) | 37 (66%) | 49 (88%) | 46 (82%) |
| Vaquerizo et al. (2015) [48] | 104 | 1.95 | 7% | 65 | 78 (75%) | 45 (43%) | 34 (33%) | 74 (71%) | 68 (65%) |
| Cortese et al. (2015) [31] | 156 | 2.83 | 3% | 61 | 106 (68%) | 55 (35%) | NR | 91 (58%) | 95 (61%) |
| Ann et al., FFR and OCT (2016) [33] | 20 | 2.68 | 0%* | 59 | 13 (65%) | 4 (20%) | 7 (35%) | 11 (55%) | 9 (45%) |
| Ann et al., FFR and IVUS (2016) [32] | 27 | 2.53 | 0%* | 59 | 18 (64%) | 7 (25%) | 9 (32%) | 15 (54%) | 13 (46%) |
| Benezet et al. (2016) [41] | 53 | 2.4 | 25% | 66 | 35 (63%) | 28 (50%) | 24 (43%) | 41 (73%) | 30 (54%) |
| Uhlemann et al. (2016) [42] | 76 | NR (100% < 2.5) | 4% | 70 | 60 (79%) | 33 (45%) | 15 (20%) | 73 (96%) | 55 (72%) |
| Hee et al. (2017) [43] | 65 | NR | 10% | 66 | 56 (86%) | 24 (37%) | 30 (46%) | NR | NR |
| Poerner et al. (2017) [34] | 46 | 2.32 | 6% | 67 | 29 (63%) | 18 (39%) | 17 (37%) | 40 (87%) | 14 (30%) |
| Zivelonghi et al. (2017) [46] | 35 de novo (143 total) | 2.28 | 12% | 67 | 120 (84%) | 56 (39%) | 29 (20%) | 120 (84%) | 118 (83%) |
| Cortese et al. DCB-RISE (2018) [50] | 238 de novo (544 total) | 2.84 | 12% | 67 | 388 (71%) | 177 (32%) | 217 (40%) | 413 (76%) | NR |
| Primary PCI (de novo lesions) | |||||||||
| Gobic et al. (2017) [51] | 75 (DCB 38, DES 37) | 2.6 (DCB), 3.04 (DES) | 0%* | 55 | 54 (72%) | 6 (8%) | 37 (49%) | 19 (25%) | 11 (14%) |
| Nijhoff et al. (2015) [52] | 190 (DCB 40, BMS 51, DCB + BMS 50, DES 49) | 2.83 (DCB), 2.84 (DCB + BMS), 2.84 (BMS), 2.78 (DES) | 10% | 58 | 150 (79%) | 16 8%) | 87 (46%) | 64 (34%) | 47 (25%) |
| Ho et al. (2015) [53] | 89 | 2.4 | 4% | 59 | 74 (83%) | 25 (28%) | 50 [56] | 49 (55%) | 25 (28%) |
| Vos et al. (2014) [54] | 100 | 3.02 | 41% | 60 | 74 (74%) | 11 (11%) | 51 (51%) | 29 (29%) | 10 (10%) |
| Bifurcating lesions | |||||||||
| Kleber et al. (2016) [55] | 64 (DCB 32, POBA 32) | DCB 2.38, POBA 2.41 | 9% | 67 | 47 (73%) | 23 (36%) | 36 (56%) | NR | 23 (36%) |
| Schulz et al. (2014) [56] | 38 | NR | 13% | 71 | 23 (61%) | 17 (45%) | NR | 35 (92%) | 20 (53%) |
| Bruch et al. (2016) [57] | 127 | MB: 2.98, SB: 2.34 | 45% | 66 | 102 (80%) | 40 (32%) | 43 (34%) | 116 (91%) | 96 (76%) |
| Vaquerizo et al. (2016) [58] | 49 | 2.18 | 14% | 62 | 38 (78%) | 20 (41%) | 22 (45%) | 26 (53%) | 30 (61%) |
| Her et al. (2016) [59] | 16 | MB: 2.72, SB: 1.25 | 0% | 60 | 11 (68%) | 4 (25%) | 6 (38%) | 7 (44%) | 8 (50%) |
| Other clinical scenarios (calcifications and chronic total occlusions) | |||||||||
| Ito et al. (2017) [60] | 81 (calcified 46, non-calcified 35) | 2.22 calcified, 2.22 non-calcified | 0%* | 70 | 59 (73%) | 49 (60%) | 11 (14%) | 60 (74%) | 61 (75%) |
| Rissanen et al. (2017) [61] | 65 | NR | 10% | 72 | 44 (68%) | 24 (37%) | 25 (38%) | 49 (75%) | 58 (89%) |
| Köln et al. (2017) [62] | 34 | 2.27 | 0%* | 59 | 26 (73%) | 8 (24%) | 5 (15%) | 25 (74%) | 19 (56%) |
DCB drug-coated balloon, DES drug-eluting stent, POBA plain-old balloon angioplasty, BMS bare metal stent, HTN hypertension, Dyslip. dyslipidemia, N number of patients, NR not reported, MB main branch, SB side branch
*Indicates studies were 0% bailout by design, i.e., they excluded patients receiving a bailout stent
DCB-Only Angioplasty in General De Novo CAD
The use of DCB-only PCI in non-specific clinical scenarios, mainly in SVD, forms the main focus of the current literature. Full details of all study outcomes are provided in Table 3.
Table 3.
DCB-only angioplasty in general de novo coronary lesions
| Author | DCB used (comparator) | Angiographic outcome (FU, %FU) | Clinical outcome (FU, %FU) |
Duration of DAPT |
|---|---|---|---|---|
| Randomized studies | ||||
| Cortese et al. PICCOLETO Study (2010) [15] | Dior I (1st-Gen DES) | %DS: DCB 43.6% vs. DES 24.3%, p = 0.029 (6 months, 95%) |
MACE: DCB 35.7% vs. DES 13.8%, p = 0.054; TLR: DCB 32.1% vs. DES 10.3%, p = 0.15 (9 months, 95%) |
DCB 1 month, Bailout BMS 3 months, DES 12 months |
| Latib et al. BELLO Study (2012, 2015) [19, 20] | IN.PACT Falcon (1st-Gen DES) | In-stent/balloon LLL: DCB 0.08 ± 0.38 vs. DES 0.29 ± 0.44 p < 0.001 (6 months, 89.6%) |
MACE*: DCB 14.8% vs. DES 25.3%, p = 0.08 TLR: DCB 6.8% vs. DES 12.1%, p = 0.23 (24 months, 98.4%) |
DCB 1 months, bailout BMS 3 months, DES 12 months |
| Nishiyama et al. (2016) [23] | SeQuent Please (2nd-Gen DES) | LLL: DCB 0.25 ± 0.25 vs. DES 0.37 ± 0.40 p = 0.185 (8 months, 100%) |
MACE: DCB 0% vs. DES 6.1% TLR: DCB 0% vs. DES 6.1%, p = 0.193 (8 months, 100%) |
DCB and DES 8 months |
| Funatsu et al. (2017) [28] | SeQuent Please (POBA) | In-balloon LLL: DCB 0.01 ± 0.31 vs. POBA 0.32 ± 0.34), p < 0.01 (6 months, 95%) |
TVF: DCB 3.4% vs. POBA 10.3%, p = 0.2 TLR: DCB 2.3% vs. POBA 10.3%, p = 0.07 (6 months, 95%) |
3 months |
| Comparative observational studies | ||||
| Her et al. (2016) [29] | SeQuent Please (POBA) | LLL: DCB − 0.12 ± 0.30 vs. POBA 0.25 ± 0.50 p < 0.001 (9 months, 100%) | TLR: DCB 0% vs. POBA 4.3%, p = 0.229 (9 months, 100%) | 1.5 months |
| Shin et al. (2016) [24] | SeQuent Please (2nd Gen DES/BMS) | LLL: DCB 0.05 ± 0.27 vs. DES/BMS 0.40 ± 0.54 p = 0.022 (9 months, 79%) |
MACE: DCB 0% vs. DES/BMS 9%, p N.S. TLR: DCB 0% vs. DES/BMS 5%, p N.S. (12 months, 100%) |
DCB 1.5 months, bailout BMS 6 months, DES 12 months |
| Sinaga et al. (2016) [25] | SeQuent Please (2nd/3rd-Gen DES) | NR |
MACE: DCB 11.6% vs. DES 11.7%, p = 1.000 TLR: DCB 5.2% vs. DES 3.7%, p = 0.601 (12 months, 100%) |
DCB 6 months, DES 12 months |
| Giannini et al. (2017) [22] | IN.PACT Falcon (2nd-Gen DES) | NR |
MACE*: DCB 12.2% vs. DES 15.4%, p = 0.538 TLR: DCB 5.6% vs. DES 4.4%, p = 0.720 (12 months, 100%) |
DCB 1 month, Bailout BMS 3 months, DES 12 months |
| Her et al. (2017) [27] | SeQuent Please (1st/2nd Gen DES) | NR |
Pericprocedural MI: DCB 1.9% vs. DES 23.1% p = 0.002 TLR: DCB 1% vs. DES 0%, p = 1.00 (12 months, 100%) |
DCB 1.5 months, DES 12 months |
| Venetsanos et al. (2018) [26] | SeQuent Please, Pantera Lux, IN.PACT Falcon (2nd/3rd-Gen DES) | NR |
TLR: DCB 0.2% vs. DES 1.1%, HR: 1.05; (95% CI 0.72–1.53) TLT: DCB 7.0% vs. DES 6.2%, HR: 0.18 (95% CI 0.04–0.82) (30 months, 100%) |
DCB 1 month, DES 6 months |
| Single-armed observational studies | ||||
| Unverdorben et al. PEPCAD I (2010, 2013) [17, 18] | SeQuent Please | In-Segment LLL: 0.28 ± 0.53 (6 months, 89%) |
MACE: 15.3% TLR: 11.9% (36 months, 100%) |
DCB 1 month, bailout BMS 3 months |
| Cuculi et al. (2012) [44] | IN.PACT Falcon | NR | TLR: 4.8% (12 months, 95%) | 1.5 months |
| Woehrle et al. SeQuent Please World Wide Registry (2012) [35] | SeQuent Please | NR |
MACE: 2.6% TLR: 1.0% (9 months, 100%) |
1 month |
| Calé et al. (2013) [40] | SeQuent Please | NR |
MACE: 14.7% TLR: 4.0% (12 months, 100%) |
3 months |
| Waksman et al. Valentines II (2013) [47] | Dior II | In-Balloon LLL: 0.38 ± 0.39 (7.5 months, 34%) |
MACE: 8.7% TLR: 2.9% (6–9 months, 100%) |
DCB 3 months, bailout BMS NR |
| Basavarajah et al. (2014) [45] | IN.PACT Falcon | NR |
MACE*: 16.5% TLR: 17.7% (15 months, 100%) |
DCB 1 month, Bailout BMS 3 months, DES 12 months |
| Toelg et al. DELUX Registry (2014) [49] | Pantera Lux | NR |
MACE*: 9.4% TLR: 3.1% (12 months, 91%) |
DCB 3 months |
| Zeymer et al. SeQuent Please Small Vessel ‘PCB Only’ Registry (2014) [36] | SeQuent Please | NR |
MACE: 4.7% TLR: 3.6% (9 months, 100%) |
1 month |
| Kleber et al. (2015) [30] | SeQuent Please, IN.PACT Falcon | In-balloon MLD: PP 1.73 ± 0.55 vs. FU 1.86 ± 0.5, p = 0.012 (4 months, 100%) |
MACE: 1.8% TLR: 0% (4 months, 100%) |
1 month |
| Vaquerizo et al., Spanish Dior Registry (2015) [48] | Dior I/II | In-stent/balloon LLL: 0.31 ± 0.2 (6–8 months, 84%) |
MACE: 6.7% TLR: 2.9% (12 months, 100%) |
DCB 1 month, bailout BMS NR |
| Cortese et al. (2015) [31] | Restore Elutax SV | Dissection cohort LLL: 0.14 ± 0.28 (6 months, 100%) |
MACE: 7.2. % TLR: 5.3% (9 months, 100%) |
DCB 1 month, bailout stent 6 months |
| Ann et al. FFR and OCT (2016) [33] | SeQuent Please | In-balloon LLL: 0.01 ± 0.21 (9 months, 100%) |
MACE: 0% TLR: 0% (9 months, 100%) |
NR |
| Ann et al. FFR and IVUS (2016) [32] | SeQuent Please | In-balloon LLL: 0.02 ± 0.27 (9 months, 100%) |
MACE: 0% TLR: 0% (9 months, 100%) |
1.5 months |
| Benezet et al. (2016) [41] | SeQuent Please | NR |
MACE*: 8.9% TLR: 5.4% (36 months, 100%) |
DCB 1 months, bailout BMS 6 months |
| Uhlemann et al. Leipzig Registry (2016) [42] | SeQuent Please | NR |
MACE*: 13% TLR: 0% (27 months, 100%) |
3 months |
| Hee et al. (2017) (2017) [43] | SeQuent Please | NR |
MACE*: 1% TLR: 0% (16 months, 100%) |
DCB 3 months, bailout BMS 6 months, bailout DES 12 months |
| Poerner et al. OCTOPUS II (2017) [34] | SeQuent Please | LLL: − 0.13 ± 0.44 (6 months, 85%) |
MACE: 6.5% TLR: 4.3% (12 months, 100%) |
DCB 1 month |
| Zivelonghi et al. (2017) [46] | IN.PACT Falcon | NR |
MACE*: 14.3% TLR: 11.4% (48 months, 100%) |
DCB 1 month, bailout DES 6 months |
| Cortese et al. Italian Elutax SV rEgistry-DCB-RISE (2018) [50] | Elutax SV | NR |
DOCE: 2.6% TLR: 2.6% (13 months, 93.2) |
3 months |
DCB drug-coated balloon, DES drug-eluting stent, POBA plain-old balloon angioplasty, BMS bare metal stent, Gen generation, FU follow-up, %FU percentage follow-up, DAPT dual anti-platelet therapy, %DS percentage diameter stenosis, LLL late luminal loss, TLR target lesion revascularization, MACE major adverse cardiovascular events, MI myocardial infarction, TLT target lesion thrombosis, MLD minimum luminal diameter, DOCE device-orientated adverse cardiovascular events, TVR target vessel revascularization, PP post procedure, HR hazard ratio, NS non-significant, NR not reported
*Indicates studies that adopted a different definition for the composite outcome of MACE and these are elaborated upon in Appendix B
DCB vs. DES
PICCOLETO compared the Dior I DCB to the 1st-generation DES, Taxus Liberté [15]. It was stopped prematurely due to clear superiority of the DES. However, certain factors explain these discouraging results. Above all, the Dior I DCB has been shown to elute lower concentrations of paclitaxel compared to subsequent DCB generations. Also, adequate preparation of lesions with POBA before DCB application was only performed in 25% of lesions. Pre-dilatation with POBA and the use of cutting balloons has been shown to further facilitate intimal and medial drug delivery via the formation of microdissections [16]. Moreover, a high rate of bailout stenting was seen and may be attributed to use in type B dissections, which later recommendations do not advocate [14]. Furthermore ‘geographical mismatch’ was not taken into account. This describes stented areas of the vasculature in bailout (with a BMS) that have not been previously treated with a DCB. They are particularly prone to restenosis and are associated with poorer outcomes. PEPCAD I previously also identified this [17, 18]. Geographical mismatch can be avoided through the use of a shorter stent implanted within the DCB treated area. A DES may be used in these cases or a BMS where long-term DAPT is contraindicated [14].
The BELLO study compared the IN.PACT Falcon DCB against the Taxus Liberté DES. It showed a smaller LLL in DCB-treated patients with comparable clinical outcomes to the DES [19, 20]. A sub-analysis of diabetic patients showed similar results [21]. However, issues arise when using LLL to compare stenting strategies with a balloon-only approach as stent placement, by nature, will result in greater acute luminal gain and consequently a greater LLL. Moreover, only a 1st-generation DES was the comparator. Giannini et al. showed comparable clinical outcomes in the BELLO DCB group when propensity score matched against patients treated with a 2nd-generation DES (Xience V or Promus) [22].
Nishiyama et al. [23] report comparable clinical outcomes between patients randomized to receive either SeQuent Please or a 2nd-generation DES (Xience Prime or Xpedition). This was largely due to a lack of adverse events seen in both groups over the short follow-up and the exclusion of DCB patients who received bailout stenting. The use of a Lacrosse non-slip element (NSE) balloon for pre-dilatation in addition to the use of intravascular ultrasound (IVUS) for the evaluation of an optimal result before DCB application, may have also contributed to the good outcomes. Shin et al. [24] compared SeQuent Please against a 2nd-generation DES (Xience Prime or Resolute Integrity). The investigators used a fractional flow reserve (FFR)-guided approach. Following POBA pre-dilatation, if a good FFR was seen (> 0.85) a DCB was used; otherwise a DES was implanted. Excellent clinical, angiographic, and functional results were seen. However, given the reservation of DES for more complex lesions, comparison is limited.
Sinaga et al. [25] retrospectively compared cohorts of SeQuent Please and 2nd/3rd-generation DES (Resolute Integrity, Xience, Promus Element, Biomatrix or Nobori)-treated patients. Comparable clinical outcomes were seen. However, the DCB-only treated group showed a significantly smaller MVD with DES use being associated with more proximal lesions of the major epicardial arteries. This could have confounded clinical outcomes with stenosis of smaller vessels perhaps having a less significant impact. Venetsanos et al. compared large propensity score-matched populations receiving a DCB (SeQuent Please, IN.PACT Falcon or Pantera Lux) against a 2nd/3rd-generation DES (Xience, Promus, Synergy, Resolute, Orsiro or Nobori). DCB treatment was notably associated with a significantly lower occurrence of TLT. This may be related to early discontinuation of DAPT as a minimum of only 6 months was required in the DES group [26]. DCB use was also seen in significantly less complex lesions, despite propensity score matching. A DES was used in the majority of bailout cases with good outcomes. DCB investigators were initially hesitant to do this due to concerns about the vascular effects of combining two drug-eluting devices. Her et al. showed a significantly lower incidence of periprocedural MI in propensity score-matched SeQuent Please treated patients when compared to 1st/2nd-generation DESs (Cypher, Taxus Express and Endeavor). However, 88.5% of DES patients were given only a first-generation device, which may explain the poorer outcomes seen. Periprocedural MI is a complication of up to 30% of DES procedures and troponin release after PCI is classically associated with worse outcomes [27].
DCB vs. POBA
Funatsu et al. report a smaller LLL in SeQuent Please-treated patients when compared to POBA (with no significant difference in adverse outcomes) [28]. Sub-analysis showed significantly lower adverse clinical outcomes in patients pre-dilated with a Lacrosse NSE. This may be related to significantly less bailout procedures being performed in this subgroup. A short follow-up period may also explain why POBA results were better than expected. Furthermore, bailout stenting was not indicated in residual stenosis up to 50% (as opposed 30%), with authors commenting that the German Consensus Group recommendations would have resulted in too aggressive a pre-dilatory approach, leading to a higher %bailout. The long-term effects of such an approach are unclear. Her et al. [29] also similarly report superior angiographic and comparable clinical outcomes when comparing SeQuent Please to POBA.
Moreover, in both studies, luminal enlargement was observed in over 50% of patients at follow-up. In a single-armed study, Kleber et al. [30] also found this in 69% of patients. This was most pronounced in areas where plaque burden was highest and was attributed to possible positive vessel remodelling, vascular healing and plaque regression. Cortese et al. [31] found that 94% of patients left dissection (type A–C) had later healed. To better characterize these positive remodeling processes, Ann et al. [32, 33] used IVUS histology and optical coherence tomography (OCT) in two cohorts of DCB-treated patients with a FFR-guided approach. Both studies showed good angiographic, functional, and clinical results. IVUS histological analysis showed the conversion of four thin-cap fibroatheromas to a thick-cap, suggesting possible plaque stabilization. OCT showed an increased mean luminal diameter and volume at follow-up in addition to the sealing of 66% of dissections. The OCTOPUS II study again through an FFR-guided approach with OCT analysis also showed positive luminal gain, healing of dissections and a lack of thin-cap fibroatheromas [34].
Registry Studies
Various registries exist to monitor the safety and efficacy of DCBs in the ‘real world’. The majority of these have used SeQuent Please. The SeQuent Please worldwide registry is the largest of these and showed low MACE and TLR rates [35]. Bailout stenting was not associated with adverse outcomes however; diabetes was a significant predictor of TLR. The SeQuent Please Small Vessel PCB-only Registry reported slightly higher (but nonetheless good) MACE and TLR rates [36]. This was attributed to a smaller cohort MVD. Sub-analyses of this registry comparing elderly patients (> 75 years), patients with ACS and Asian versus Western populations have been published. Similar outcomes and %bailout was seen across all groups, despite the presence of more comorbidities in elderly patients and on average smaller vessels with longer lesions in Asian patients [37–39].
Other smaller SeQuent Please registries include Calé et al. [40], which showed a relatively high incidence of MACE and TLR despite a low frequency of bailout stenting. The authors attributed this to a high-risk population. Benezet et al., The Leipzig Registry, and Hee et al. all show favorable long-term MACE and TLR rates [41–43]. Cuculi et al. [44] report an IN.PACT Falcon DCB registry showing favorable %bailout, MACE, and TLR rates. Basavarajah et al’s IN.PACT Falcon registry report a relatively high MACE and TLR rate. This was attributed to the presence of diffuse CAD (> 20 mm) in 80% of patients [45]. Zivelonghi et al. provide the longest follow-up seen in any DCB registry, showing good long-term MACE and TLR rates. Over half the cohort presented with ACS and this was associated with a higher incidence of adverse outcome [46].
Registries of the Dior II DCB include Valentines II. Here, angiographic follow-up was only performed in 34% of patients and showed a relatively high LLL (although low MACE and TLR rates were still seen) [47]. This may be due to recommended pre-dilatation being performed in only 85% of patients. The Spanish Dior Registry similarly reported a high LLL. However, 54% of patients received the less effective Dior I balloon. Again, favorable clinical outcomes were reported and BMS bailout was a predictor of adverse events [48]. DELUX was a Pantera Lux DCB registry which showed comparable outcomes to other DCB registries, with BMS bailout again predicting poorer outcome [49]. The Italian Elutax SV Registry-DCB Rise is a registry of the Elutax SV DCB. It found low DOCE and TLR rates [50].
DCB-Only Angioplasty in PPCI
Study outcomes focusing on the use of DCB-only angioplasty for patients presenting with ACS are summarized in Table 4. In PPCI, the risk of ST occurrence with DES is greater than in elective cases due factors such as incomplete stent apposition and delayed tissue coverage, making the role of a DCB-only approach for this indication interesting to consider. Gobic et al. conducted a randomized trial, in ST elevation MI (STEMI) patients comparing SeQuent Please to the 3rd-generation DES Biomime. They showed a superior LLL in DCB-treated patients (although issues with LLL in stent versus balloon studies have been discussed) and comparable short-term clinical outcomes [51]. Luminal enlargement was also observed. However, DCB patients requiring BMS bailout were excluded from analysis. DCB patients also had a significantly smaller MVD compared to the DES group despite randomization. Nijhoff et al. [52] report an observational comparison of a DCB-only cohort against a previous three-armed randomized trial, DEB-AMI. DCB-only PCI exhibited a comparable LLL versus BMS and DCB + routine BMS, but was inferior to the Taxus Liberté DES. Despite this, comparable clinical outcomes were seen across all four groups. Coronary endothelial dysfunction (tested using acetylcholine) was also least pronounced in the DCB-only group. A lack of acute and late thrombosis indicates DCB-only may be viable in STEMI patients with a long-term DAPT contraindication. PAPPA was a feasibility study of the DCB-only approach (using Pantera Lux) in STEMI showing favorable TLR and MACE rates. However, a very high bailout rate of 41% was seen, due to a high rate of dissections, which may be related to the use of a slightly oversized balloon for pre-dilatation [53]. Ho et al. [54] report acceptable short-term clinical outcomes in STEMI patients treated with SeQuent Please. It should be noted that DCB-only PPCI investigators have commented on the importance of thrombus aspiration where relevant before DCB application to avoid reduced paclitaxel transfer by an interposed mural thrombus.
Table 4.
DCB-only angioplasty in primary PCI for de novo lesions
| Author | Design | DCB used | Angiographic outcome (FU, %FU) | Clinical outcome (FU, %FU) |
|---|---|---|---|---|
| Gobic et al. (2017) [51] | Randomized trial, DCB vs. 3rd-Gen DES | SeQuent Please |
LLL: DCB − 0.09 ± 0.09 vs. DES 0.1 ± 0.19, p < 0.05 (6 months, 84%) |
MACE*: DCB 5.3% vs. DES 5.4%, p NS TLR: 0% DCB vs. 5.4% DES, p NS (6 months, 100%) |
| Nijhoff et al. DEB-AMI (2015) [52] | Comparative observational study, DCB only vs. DCB + BMS vs. BMS vs. 1st-Gen DES | Dior II |
In Balloon/Stent LLL: DCB 0.51 ± 0.59 vs. DCB + BMS 0.64 ± 0.56 p = 0.33 vs. BMS 0.74 ± 0.32 p = 0.08 vs. DES 0.2 1 ± 0.32 p < 0.01 (6 months, 90%) |
MACE*: DCB 17.5% vs. DCB + BMS 23.9% vs. BMS 25.0% vs. DES 4.4% p NS TLR: DCB 12.5% vs. DCB + BMS 23.9% vs. BMS 19.1% vs. DES 2.2%, p NS (12 months, 100%) |
| Vos et al. PAPPA (2014) [53] | Single-armed observational study | Pantera Lux | NR |
MACE*: 5% TLR: 3% (12 months, 100%) |
| Ho et al. (2015) [54] | Single-armed observational study | SeQuent Please | NR |
MACE: 4.5% TVR: 0% 1 month (100%) |
DCB drug-coated balloon, DES drug-eluting stent, BMS bare metal stent, Gen generation, FU follow-up, %FU percentage follow-up, LLL late luminal loss, TLR target lesion revascularization, MACE major adverse cardiovascular events, TVR target vessel revascularization, NS non-significant, NR not reported
*Indicates studies that adopted a different definition for the composite outcome of MACE and these are elaborated upon in Appendix B
DCB-Only Angioplasty in Bifurcation
Table 5 provides a summary of the DCB-only studies which focus on bifurcating lesions. Bifurcation represents around 20% of PCI procedures and is associated with a higher risk of restenosis and complications. Both the interventional approach and operator factors are highly important for a successful procedure. DCB-only PCI in bifurcation theoretically provides certain advantages over stenting strategies. These include the maintenance of a natural flow distribution and avoidance of plaque and carina shift due to the absence of a stent overstretching and straightening the distal vessel, which predisposes to side-branch occlusion or narrowing, leading to adverse clinical events. PEPCAD-BIF compared SeQuent Please against POBA in a randomized study. The DCB group showed a significantly smaller LLL, however comparable clinical outcomes were nonetheless seen [55]. Bifurcating lesions involving the proximal main-branch (Medina class 1,X,X) were, however, notably excluded. Various single-armed studies have also been conducted reporting acceptable MACE and TLR rates [56, 57]. Bruch et al. report a cohort where 75% of patients presented with true bifurcation (Medina 1,1,1, 1,0,1, or 0,1,1). Although not associated with worse clinical outcomes, a high %bailout of 45% was seen and this was associated with lesions of the left anterior descending (LAD) artery and the presence of B2/C lesions. Vaquerizo et al. report clinical and angiographic outcomes in ostial side-branch (SB) lesions (Medina 0,0,1) treated with Dior II and found high MACE and TLR rates [58]. PCI of ostial side-branch lesions is naturally associated with a smaller vessel diameter, greater incidence of recoil, lower acute luminal gain and thus a higher rate of complications. Her et al. found late luminal gain (confirmed on OCT analysis) in both the main-branch (MB) and SB of bifurcating lesions treated with SeQuent Please [59]. No adverse events were reported. Lesions that showed poor image quality due to dissection or artifact on OCT were notably excluded.
Table 5.
DCB-only angioplasty in de novo coronary bifurcating lesions
| Author | Design | DCB used | Angiographic outcome (FU, %FU) | Clinical outcome (FU, %FU) |
Duration of DAPT |
|---|---|---|---|---|---|
| Kleber et al. PEPCAD-BIF (2016) [55] | Randomized trial, DCB vs. POBA | SeQuent Please | In-Segment LLL: DCB 0.08 ± 0.31 vs. POBA 0.47 ± 0.61 p = 0.006 (9 months, 75%) |
MACE: DCB 3.1% vs. POBA 12.5%, p N.S TLR: DCB 3.1% vs. POBA 9.4%, p N.S (9 months, 100%) |
1 month, bailout BMS/DES 12 months |
| Schulz et al. (2014) [56] | Single-armed observational study | SeQuent Please, IN.PACT Falcon | Binary restenosis: 10% (4 months, 77%) |
MACE: 7.7% TLR: 7.7% (4 months, 100%) |
1 month |
| Bruch et al. (2016) [57] | Single-armed observational study | SeQuent Please | NR |
MACE*: 6.1% TLR: 4.5% (9 months, 100%) |
1 month, bailout BMS 6 months |
| Vaquerizo et al. (2016) [58] | Single-armed observational study | Dior II | In-balloon LLL: 0.32 ± 0.7 (7–8 months, 63%) |
MACE*: 16.3%, TLR: 14.3% (12 months, 82%) |
1 month |
| Her et al. (2016) [59] | Single-armed observational study | SeQuent Please | MB LLL: − 0.01 ± 0.18, SB LLL: − 0.02 ± 0.22 (9 months, 100%) | MACE: 0% (9 months, 100%) | 1.5 months |
DCB drug-coated balloon, DES drug-eluting stent, POBA plain-old balloon angioplasty, BMS bare metal stent, Gen generation, FU follow-up, %FU percentage follow-up, DAPT dual anti-platelet therapy, LLL late luminal loss, TLR target lesion revascularization, MACE major adverse cardiovascular events, MB main branch, SB side branch, NS non-significant, NR not reported
*Indicates studies that adopted a different definition for the composite outcome of MACE and these are elaborated upon in Appendix B
DCB-Only Angioplasty in Other Clinical Scenarios
Table 6 outlines the studies focusing on DCB-only angioplasty in other clinical scenarios, namely in calcification and CTO. Heavily calcified CAD is associated with poorer clinical outcomes due to the difficulty of adequately deploying a stent due to incomplete stent expansion and strut apposition. Calcification is especially problematic in patients with chronic kidney disease. Ito et al. [60] show acceptable MACE and TLR rates in a feasibility study. Preparation of the calcified lesion required rotational atherectomy in 80% of patients. Chronic hemodialysis (seen in 21% of patients) was associated with an increased risk of adverse events. Comparable clinical and angiographic results were seen when compared to patients with non-calcified lesions. These favorable results may be explained by the exclusion of patients with significant residual stenosis and dissection following lesion preparation as well as the use of IVUS and OCT to aid the procedure and the use of a Lacrosse NSE for pre-dilatation. Rissanen et al. [61] report outcomes in 65 patients with calcified lesions treated with SeQuent Please, following rotational atherectomy. It is thought that rotational atherectomy prior to DCB treatment reduces calcific burden, thus enhancing the penetration of paclitaxel into the vessel wall. This technique has already been established in calcified femoro-popliteal lesions. CTOs are defined as a coronary occlusion without anterograde flow that has been present for at least 3 months. It has a reported incidence of up to 30% and is associated with higher rates of ISR and ST in addition to being technically challenging for interventionalists. In Köln et al.’s [62] feasibility study, favorable angiographic results were seen. A lack of MI, vessel thrombosis, or death at follow-up are also significant findings. However, this study excluded patients who did not achieve satisfactory pre-dilatation and only included patients requiring an anterograde interventional approach.
Table 6.
DCB-only angioplasty in other clinical scenarios
| Author | Design | DCB used | Angiographic outcome (FU, %FU) | Clinical outcome (FU, %FU) |
Duration of DAPT |
|---|---|---|---|---|---|
| Calcified lesions | |||||
| Ito et al. (2017) [60] | Comparative observational calcified vs. non-calcified lesions | SeQuent Please |
LLL: Calcified 0.03 vs. non-calcified − 0.18, p = 0.093 (6 months, 73%) |
MACE: 18.6% calcified vs. 11.5% non-calcified, p = 0.57 TLR 14.7% vs. 6.6%, p = 0.64 (24 months 100%) |
3 months |
| Rissanen et al. (2017) [61] | Single-armed observational study | SeQuent Please | NR |
MACE*: 20% TLR: 3.1% (24 months, 100%) |
1 month |
| Chronic total occlusions | |||||
| Köln et al. (2017) [62] | Single-armed observational study | SeQuent Please, IN.PACT Falcon | MLD: PP 1.69 ± 0.31 vs. FU 1.59 ± 0.57 p = 0.954 (8 months, 100%) |
MACE: 17.6%, TLR: 17.6% (8 months, 100%) |
1 month |
DCB drug-coated balloon, DES drug-eluting stent, BMS bare metal stent, FU follow-up, %FU percentage follow-up, DAPT dual anti-platelet therapy, LLL late luminal loss, TLR target lesion revascularization, MACE major adverse cardiovascular events, MLD minimal luminal diameter, PP post procedure, NR not reported
*Indicates studies that adopted a different definition for the composite outcome of MACE and these are elaborated upon in Appendix B
Discussion
This was a systematic review of 41 studies employing a DCB-only approach for the treatment of de novo CAD. These consisted of randomized trials and comparative observational studies that compared DCB-only against DES or POBA, in addition to single-armed observational studies (mostly registries). The majority of studies investigate DCB-only angioplasty in all patients with de novo CAD, however some studies focus on specific interventional scenarios. The vast majority of all DCB-only studies have been conducted in small vessels (< 2.8 mm) as DES therapy for this indication is currently suboptimal.
DCB-Only Angioplasty in General De Novo CAD
Comparison of the DCB-only approach against the DES is essential to consider, given the DES forms the current mainstay in de novo CAD treatment. With the exception of the PICCOLETO study, DCB-only and DES PCI show similar clinical outcomes [15]. Despite these encouraging results, the literature is largely lacking in data from randomized trials that compare to a 2nd- or 3rd-generation DES, with Nishiyama et al. [22] providing the only such general de novo CAD study. Longer-term data is also needed. The finding of a reduced risk of thrombosis and peri-procedural MI in comparative observational studies is also of interest [26, 27]. Although reasons for why these results were seen have been speculated, further investigation in a randomized setting would be of use.
Furthermore, deriving conclusions on the angiographic superiority of a DCB-only approach versus the DES is difficult, as the majority of studies used LLL, which naturally favors DCB-only PCI. LLL as an angiographic endpoint should no longer be used due to the larger acute luminal gain seen in DESs, rather studies can use %DS (which is less influenced by this) or focus on clinical MACE and TLR as primary endpoints. This being said, given the low rate of adverse clinical outcomes occurring in all PCI procedures, comparison between a DCB-only approach and DES may be difficult to characterize based on these alone. As such, angiographic data still have an important role to play. Additionally, the emergence of studies using intravascular imaging such as OCT and IVUS has been useful in further characterizing the benefits of a DCB-only approach on the vasculature. Moreover, their use to guide the DCB-only procedure and to ensure a satisfactory result has been associated with improved outcomes [32–34]. Future research should continue to adopt these techniques to supplement angiographic data where possible.
Studies comparing the use of DCB-only PCI versus POBA have shown superior angiographic outcomes as expected, however MACE and TLR rates were largely similar and this may be attributed to a short follow-up period in these studies. Although studies comparing POBA are useful to characterize the additional benefits of drug elution, their scope for influencing clinical practice is limited. As such, there should be less emphasis placed on the importance of further such investigation.
There is a wealth of registry data regarding the use of a DCB-only approach for the treatment of de novo CAD. However, their rates of clinical events are highly heterogeneous. This may be attributed to a large variation in follow-up period, patient characteristics, rates of bail-out stenting and experiential and operator factors. A lack of true consensus for the definition of the composite clinical outcome MACE may also be of importance.
DCB-Only Angioplasty in Specific Clinical Scenarios
Data regarding the use of a DCB-only approach in PPCI are promising, with Gobic et al. [51] showing comparable short-term clinical outcomes to a 3rd-generation DES in a randomized study. Additional longer-term studies are required to further characterize this. Conversely, the use of a DCB-only approach in bifurcation is limited by a lack of data in lesions involving the proximal MB in addition to a lack of randomised studies comparing to DES therapy of any kind. The current strategic mainstay of bifurcation PCI is through MB stenting with provisional stenting of the SB. Ideally, randomized studies specifically comparing these two strategies are needed. The use of DCB-only PCI in the treatment of calcified lesions and CTOs is still in its infancy, with only a small number of single-armed studies that at best point towards possible feasibility as opposed to efficacy.
Duration of DAPT
As expected, due to a lack of foreign body placement in the vasculature, a DCB-only approach was associated with a shorter duration of DAPT of 1–3 months when compared to DESs, which typically required a minimum of 12 months. Given that the majority of studies show comparable clinical outcomes between DCB-only and DES PCI, the shorter duration of DAPT appears to be well tolerated. This presents a key advantage of a DCB-only strategy, especially in cases where long-term DAPT is contraindicated. It should be noted that for the treatment of ACS, DAPT is given for 12 months according to European guidelines, thus the benefit of reducing DAPT for the purposes of PPCI has not been seen [4].
Future Perspectives
Although current data are promising, there is still a need for further long-term randomized control trial data comparing a DCB-only approach against a 2nd/3rd-generation DES. An example of such a study, BASKET-SMALL 2, has recently been published. It compared SeQuent Please to the 2nd-generation DESs Xience and Taxus Element in 758 patients. Comparable, low MACE rates at 12 months of 7.5% (DCB) vs. 7.3% (DES) were seen, showing non-inferiority of the DCB-only approach [63]. Future, emerging areas of interest include the use of FFR to guide intervention in DCB-only angioplasty and the use of the Lacrosse NSE for pre-dilatation, which have both shown good results. The randomized REVELATION study will compare 120 STEMI patients treated with DCB-only angioplasty versus DES using FFR with a primary endpoint of MACE at 5 years [64]. Its findings are awaited with great interest. Furthermore, DCBs using drugs other than paclitaxel are also beginning to be seen, with the MagicTouch Sirolimus-coated balloon recently gaining approval. These may prove to be superior to the current paclitaxel DCBs and characterization of this will be of great significance.
Conclusions
The treatment of de novo CAD using a DCB-only approach has shown promising data in SVD, with comparable clinical outcomes to DESs specifically in general de novo CAD and STEMI. Drug elution to a vascular lesion in the absence of a foreign-body placement, such as a stent, poses certain advantages over the DES such as positive remodeling and of even greater clinical relevance; a shorter duration of DAPT therapy, favoring use in those with a contraindication to long-term DAPT. Areas where further research should proceed have been identified and there is a specific need for longer-term randomized trials that compare DCB-only PCI against a 2nd/3rd-generation DES. DCB-only angioplasty is also beginning to see use in other challenging interventional scenarios such as bifurcation, CTOs, and calcified lesions, although further evidence for these specific indications is needed.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
No funding or sponsorship was received for this study or publication of this article. Article processing charges were funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Hasan Mohiaddin, Tamar DFK Wong, Anne Burke-Gaffney, and Richard Bogle have nothing to disclose.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not involve any new studies with human participants or animal subjects performed by any of the authors.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Open Access
This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Footnotes
Richard G. Bogle and Anne Burke-Gaffney are equally contributed.
Enhanced digital features
To view enhanced digital features for this article go to 10.6084/m9.figshare.7157111.
References
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakatani M, Takeyama Y, Shibata M, Yorozuya M, Suzuki H, Koba S, et al. Mechanisms of restenosis after coronary intervention: difference between plain old balloon angioplasty and stenting. Cardiovasc Pathol. 2003;12(1):40–48. doi: 10.1016/S1054-8807(02)00135-7. [DOI] [PubMed] [Google Scholar]
- 3.Venkitachalam L, Kip KE, Selzer F, Wilensky RL, Slater J, Mulukutla SR, et al. Twenty-year evolution of percutaneous coronary intervention and its impact on clinical outcomes: a report from the national heart, lung, and blood institute-sponsored, multicenter 1985–1986 PTCA and 1997–2006 dynamic registries. Circ Cardiovasc Interv. 2009;2(1):6–13. doi: 10.1161/CIRCINTERVENTIONS.108.825323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolh P, Windecker S, Alfonso F, Collet J-P, Cremer J, Falk V, et al. 2014 ESC/EACTS guidelines on myocardial revascularization. Eur J Cardio-Thoracic Surg. 2014;46(4):517–592. doi: 10.1093/ejcts/ezu366. [DOI] [PubMed] [Google Scholar]
- 5.Stefanini GG, Holmes DR. Drug-eluting coronary-artery stents. N Engl J Med. 2013;368(3):254–265. doi: 10.1056/NEJMra1210816. [DOI] [PubMed] [Google Scholar]
- 6.Kalra A, Rehman H, Khera S, Thyagarajan B, Bhatt DL, Kleiman NS, et al. New-generation coronary stents: current data and future directions. Curr Atheroscler Rep. 2017;19(3):14. doi: 10.1007/s11883-017-0654-1. [DOI] [PubMed] [Google Scholar]
- 7.Schunkert H, Harrell L, Palacios IF. Implications of small reference vessel diameter in patients undergoing percutaneous coronary revascularization. J Am Coll Cardiol. 1999;34(1):40–48. doi: 10.1016/S0735-1097(99)00181-3. [DOI] [PubMed] [Google Scholar]
- 8.Ismail MD, Ahmad WAW, Leschke M, Waliszewski M, Boxberger M, Abidin IZ, et al. The outcomes of patients with very small coronary artery disease treated with thin strut cobalt chromium bare metal stents: an observational study. Springerplus. 2016;5(1):1668. doi: 10.1186/s40064-016-3350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habib A, Finn AV. Endothelialization of drug-eluting stents and its impact on dual anti-platelet therapy duration. Pharmacol Res. 2015;93:22–27. doi: 10.1016/j.phrs.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reejhsinghani R, Lotfi AS. Prevention of stent thrombosis: challenges and solutions. Vasc Health Risk Manag. 2015;11:93–106. doi: 10.2147/VHRM.S43357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Poerner TC, Otto S, Gassdorf J, Nitsche K, Janiak F, Scheller B, et al. Stent coverage and neointimal proliferation in bare metal stents postdilated with a paclitaxel-eluting balloon versus everolimus-eluting stents: prospective randomized study using optical coherence tomography at 6-month follow-up. Circ Cardiovasc Interv. 2014;7(6):760–767. doi: 10.1161/CIRCINTERVENTIONS.113.001146. [DOI] [PubMed] [Google Scholar]
- 12.Picard F, Doucet S, Asgar AW. Contemporary use of drug-coated balloons in coronary artery disease: where are we now? Arch Cardiovasc Dis. 2017;110(4):259–272. doi: 10.1016/j.acvd.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Wickramarachchi U, Eccleshall S. Drug-coated balloon-only angioplasty for native coronary disease instead of stents. Interv Cardiol. 2016;11(2):110–115. doi: 10.15420/icr.2016:17:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleber FX, Rittger H, Bonaventura K, Zeymer U, Wöhrle J, Jeger R, et al. Drug-coated balloons for treatment of coronary artery disease: updated recommendations from a consensus group. Clin Res Cardiol. 2013;102(11):785–797. doi: 10.1007/s00392-013-0609-7. [DOI] [PubMed] [Google Scholar]
- 15.Cortese B, Micheli A, Picchi A, Coppolaro A, Bandinelli L, Severi S, et al. Paclitaxel-coated balloon versus drug-eluting stent during PCI of small coronary vessels, a prospective randomised clinical trial. The PICCOLETO Study. Heart. 2010;96(16):1291–1296. doi: 10.1136/hrt.2010.195057. [DOI] [PubMed] [Google Scholar]
- 16.Belkacemi A, Agostoni P, Nathoe HM, Voskuil M, Shao C, Van Belle E, et al. First results of the DEB-AMI (drug-eluting balloon in acute ST-segment elevation myocardial infarction) trial. J Am Coll Cardiol. 2012;59(25):2327–2337. doi: 10.1016/j.jacc.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 17.Unverdorben M, Kleber FX, Heuer H, Figulla H-R, Vallbracht C, Leschke M, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99(3):165–174. doi: 10.1007/s00392-009-0101-6. [DOI] [PubMed] [Google Scholar]
- 18.Unverdorben M, Kleber F, Heuer H, Figulla H, Vallbracht C, Leschke M, et al. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter in the PEPCAD I study: are lesions clinically stable from 12 to 36 months? EuroIntervention. 2013;9(5):620–628. doi: 10.4244/EIJV9I5A99. [DOI] [PubMed] [Google Scholar]
- 19.Latib A, Colombo A, Castriota F, Micari A, Cremonesi A, De Felice F, et al. A randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels. J Am Coll Cardiol. 2012;60(24):2473–2480. doi: 10.1016/j.jacc.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 20.Naganuma T, Latib A, Sgueglia GA, Menozzi A, Castriota F, Micari A, et al. A 2-year follow-up of a randomized multicenter study comparing a paclitaxel drug-eluting balloon with a paclitaxel-eluting stent in small coronary vessels the BELLO study. Int J Cardiol. 2015;184:17–21. doi: 10.1016/j.ijcard.2015.01.080. [DOI] [PubMed] [Google Scholar]
- 21.Giannini F, Latib A, Jabbour RJ, Costopoulos C, Chieffo A, Carlino M, et al. Comparison of paclitaxel drug-eluting balloon and paclitaxel-eluting stent in small coronary vessels in diabetic and nondiabetic patients—results from the BELLO (balloon elution and late loss optimization) trial. Cardiovasc Revascul Med. 2017;18(1):4–9. doi: 10.1016/j.carrev.2016.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Giannini F, Latib A, Ancona MB, Costopoulos C, Ruparelia N, Menozzi A, et al. A propensity score matched comparative study between paclitaxel-coated balloon and everolimus-eluting stents for the treatment of small coronary vessels. Catheter Cardiovasc Interv. 2017;90(3):380–386. doi: 10.1002/ccd.26929. [DOI] [PubMed] [Google Scholar]
- 23.Nishiyama N, Komatsu T, Kuroyanagi T, Fujikake A, Komatsu S, Nakamura H, et al. Clinical value of drug-coated balloon angioplasty for de novo lesions in patients with coronary artery disease. Int J Cardiol. 2016;222:113–118. doi: 10.1016/j.ijcard.2016.07.156. [DOI] [PubMed] [Google Scholar]
- 24.Shin E-S, Ann SH, Balbir Singh G, Lim KH, Kleber FX, Koo B-K. Fractional flow reserve-guided paclitaxel-coated balloon treatment for de novo coronary lesions. Catheter Cardiovasc Interv. 2016;88(2):193–200. doi: 10.1002/ccd.26257. [DOI] [PubMed] [Google Scholar]
- 25.Sinaga DA, Ho HH, Watson TJ, Sim A, Nyein TT, Jafary FH, et al. Drug-coated balloons: a safe and effective alternative to drug-eluting stents in small vessel coronary artery disease. J Interv Cardiol. 2016;29(5):454–460. doi: 10.1111/joic.12333. [DOI] [PubMed] [Google Scholar]
- 26.Venetsanos D, Lawesson SS, Panayi G, Todt T, Berglund U, Swahn E, et al. Long-term efficacy of drug-coated balloons compared with new generation drug-eluting stents for the treatment of de novo coronary artery lesions. Catheter Cardiovasc Interv; 2018; Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&PAGE=reference&D=medp&NEWS=N&AN=29481718. [DOI] [PubMed]
- 27.Her AY, Cho KI, Singh GB, Garg S, Kim YH, Koo BK, et al. A comparison of peri-procedural myocardial infarction between paclitaxel-coated balloon and drug-eluting stent on de novo coronary lesions. Yonsei Med J. 2017;58(1):99–104. doi: 10.3349/ymj.2017.58.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Funatsu A, Nakamura S, Inoue N, Nanto S, Nakamura M, Iwabuchi M, et al. A multicenter randomized comparison of paclitaxel-coated balloon with plain balloon angioplasty in patients with small vessel disease. Clin Res Cardiol. 2017;106:824–832. doi: 10.1007/s00392-017-1126-x. [DOI] [PubMed] [Google Scholar]
- 29.Her A-Y, Ann SH, Singh GB, Kim YH, Yoo S-Y, Garg S, et al. Comparison of paclitaxel-coated balloon treatment and plain old balloon angioplasty for de novo coronary lesions. Yonsei Med J. 2016;57(2):337. doi: 10.3349/ymj.2016.57.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kleber FX, Schulz A, Waliszewski M, Hauschild T, Böhm M, Dietz U, et al. Local paclitaxel induces late lumen enlargement in coronary arteries after balloon angioplasty. Clin Res Cardiol. 2015;104(3):217–225. doi: 10.1007/s00392-014-0775-2. [DOI] [PubMed] [Google Scholar]
- 31.Cortese B, Silva Orrego P, Agostoni P, Buccheri D, Piraino D, Andolina G, et al. Effect of drug-coated balloons in native coronary artery disease left with a dissection. JACC Cardiovasc Interv. 2015;8(15):2003–2009. doi: 10.1016/j.jcin.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 32.Ann SH, Balbir Singh G, Lim KH, Koo B-K, Shin E-S. Anatomical and physiological changes after paclitaxel-coated balloon for atherosclerotic de novo coronary lesions: serial IVUS-VH and FFR study. PLoS One. 2016;11(1):e0147057. doi: 10.1371/journal.pone.0147057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ann SH, Her A-Y, Singh GB, Okamura T, Koo B-K, Shin E-S. Serial morphological and functional assessment of the paclitaxel-coated balloon for de novo lesions. Rev Española Cardiol. 2016;69(11):1026–1032. doi: 10.1016/j.recesp.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 34.Poerner TC, Duderstadt C, Goebel B, Kretzschmar D, Figulla HR, Otto S. Fractional flow reserve-guided coronary angioplasty using paclitaxel-coated balloons without stent implantation: feasibility, safety and 6-month results by angiography and optical coherence tomography. Clin Res Cardiol. 2017;106(1):18–27. doi: 10.1007/s00392-016-1019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wöhrle J, Zadura M, Möbius-Winkler S, Leschke M, Opitz C, Ahmed W, et al. SeQuent please world wide registry. J Am Coll Cardiol. 2012;60(18):1733–1738. doi: 10.1016/j.jacc.2012.07.040. [DOI] [PubMed] [Google Scholar]
- 36.Zeymer U, Waliszewski M, Spiecker M, Gastmann O, Faurie B, Ferrari M, et al. Prospective ‘real world’ registry for the use of the ‘PCB only’ strategy in small vessel de novo lesions. Heart. 2013;100(4):311–316. doi: 10.1136/heartjnl-2013-304881. [DOI] [PubMed] [Google Scholar]
- 37.Sinaga D, Ho H, Zeymer U, Waliszewski M, Jafary F, Ooi Y, et al. Drug-coated balloon angioplasty in elderly patients with small vessel coronary disease. Ther Adv Cardiovasc Dis. 2015;9(6):389–396. doi: 10.1177/1753944715598714. [DOI] [PubMed] [Google Scholar]
- 38.Mahmood Zuhdi A, Zeymer U, Waliszewski M, Spiecker M, Ismail M, Boxberger M, et al. The use of paclitaxel-coated balloon (PCB) in acute coronary syndrome of small vessel de novo lesions: an analysis of a prospective ‘real-world’ registry. SpringerPlus. 2016;5(1):373. doi: 10.1186/s40064-016-2014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ong P, Zeymer U, Waliszewski M, Tan J, Ho H. Differences in clinical and angiographic profiles between Asian and Western patients with coronary artery disease: insights from the prospective “real-world” paclitaxel-coated balloon registry. Int J Cardiol. 2014;175(1):199–200. doi: 10.1016/j.ijcard.2014.04.239. [DOI] [PubMed] [Google Scholar]
- 40.Calé R, Sousa PJ, Pereira E, et al. One-year clinical outcomes of percutaneous treatment with drug-eluting balloons: results from a multicenter registry. Rev Port Cardiol. 2013;32:361–369. doi: 10.1016/j.repc.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 41.Benezet J, Gutiérrez-Barrios A, Agarrado A, et al. Paclitaxel-coated balloon angioplasty for de novo coronary lesions: a long-term follow-up study. Minerva Cardioangiol. 2016;64:15–22. [PubMed] [Google Scholar]
- 42.Uhlemann M, Möbius-Winkler S, Adam J, et al. The Leipzig prospective drug-eluting balloon-registry—outcome of 484 consecutive patients treated for coronary in-stent restenosis and de novo lesions using paclitaxel-coated balloons. Circ J. 2016;80:379–386. doi: 10.1253/circj.CJ-14-1352. [DOI] [PubMed] [Google Scholar]
- 43.Hee L, Terluk A, Thomas L, Hopkins A, Juergens CP, Lo S, French JK, Mussap CJ. Late clinical outcomes for SeQuent please paclitaxel-coated balloons in PCI of in-stent restenosis and de novo lesions: a single-center, real-world registry. Cathet Cardiovasc Interv. 2017;89:375–382. doi: 10.1002/ccd.26546. [DOI] [PubMed] [Google Scholar]
- 44.Cuculi F, Young M, Beeler R, Schoenenberger A, Erne P. Good efficacy of drug-eluting balloons in a mixed population of patients with coronary artery disease. J Invasive Cardiol. 2012;24(4):151–153. [PubMed] [Google Scholar]
- 45.Basavarajaiah S, Latib A, Shannon J, et al. Drug-eluting balloon in the treatment of in-stent restenosis and diffuse coronary artery disease: real-world experience from our registry. J Interv Cardiol. 2014;27:348–355. doi: 10.1111/joic.12129. [DOI] [PubMed] [Google Scholar]
- 46.Zivelonghi C, Ghione M, Benfari G, Cuman M, Fede A, Lunardi M, et al. Drug-coated balloon: long-term outcome from a real world three-center experience. J Interv Cardiol. 2017;30(4):318–324. doi: 10.1111/joic.12391. [DOI] [PubMed] [Google Scholar]
- 47.Waksman R, Serra A, Loh JP, et al. Drug-coated balloons for de novo coronary lesions: results from the Valentines II trial. EuroIntervention. 2013;9:613–619. doi: 10.4244/EIJV9I5A98. [DOI] [PubMed] [Google Scholar]
- 48.Vaquerizo B, Miranda-Guardiola F, Fernández E, et al. Treatment of small vessel disease with the paclitaxel drug-eluting balloon: 6-month angiographic and 1-year clinical outcomes of the Spanish multicenter registry. J Interv Cardiol. 2015;28:430–438. doi: 10.1111/joic.12227. [DOI] [PubMed] [Google Scholar]
- 49.Toelg R, Merkely B, Erglis A, et al. Coronary artery treatment with paclitaxel-coated balloon using a BTHC excipient: clinical results of the international real-world DELUX registry. EuroIntervention. 2014;10:591–599. doi: 10.4244/EIJV10I5A102. [DOI] [PubMed] [Google Scholar]
- 50.Cortese B, D’Ascenzo F, Fetiveau R, Balian V, Blengino S, Fineschi M, et al. Treatment of coronary artery disease with a new-generation drug-coated balloon. J Cardiovasc Med. 2018;19(5):247–252. doi: 10.2459/JCM.0000000000000632. [DOI] [PubMed] [Google Scholar]
- 51.Gobić D, Tomulić V, Lulić D, Židan D, Brusich S, Jakljević T, et al. Drug-coated balloon versus drug-eluting stent in primary percutaneous coronary intervention: a feasibility study. Am J Med Sci. 2017;354(6):553–560. doi: 10.1016/j.amjms.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 52.Nijhoff F, Agostoni P, Belkacemi A, et al. Primary percutaneous coronary intervention by drug-eluting balloon angioplasty: the nonrandomized fourth arm of the DEB-AMI (drug-eluting balloon in ST-segment elevation myocardial infarction) trial. Catheter Cardiovasc Interv. 2015;86(Suppl 1):S34–S44. doi: 10.1002/ccd.26060. [DOI] [PubMed] [Google Scholar]
- 53.Vos NS, Dirksen MT, Vink MA, et al. Safety and feasibility of a paclitaxel-eluting balloon angioplasty in primary percutaneous coronary intervention in Amsterdam (PAPPA): one-year clinical outcome of a pilot study. EuroIntervention. 2014;10:584–590. doi: 10.4244/EIJV10I5A101. [DOI] [PubMed] [Google Scholar]
- 54.Ho HH, Tan J, Ooi YW, et al. Preliminary experience with drug-coated balloon angioplasty in primary percutaneous coronary intervention. World J Cardiol. 2015;7:311–314. doi: 10.4330/wjc.v7.i6.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schulz A, Hauschild T, Kleber FX. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin Res Cardiol. 2014;103:451–456. doi: 10.1007/s00392-014-0671-9. [DOI] [PubMed] [Google Scholar]
- 56.Schulz A, Hauschild T, Kleber FX. Treatment of coronary de novo bifurcation lesions with DCB only strategy. Clin Res Cardiol. 2014;103:451–456. doi: 10.1007/s00392-014-0671-9. [DOI] [PubMed] [Google Scholar]
- 57.Bruch L, Zadura M, Waliszewski M, Platonic Z, Eränen J, Scheller B, et al. Results from the international drug-coated balloon registry for the treatment of bifurcations. Can a bifurcation be treated without stents? J Interv Cardiol. 2016;29(4):348–356. doi: 10.1111/joic.12301. [DOI] [PubMed] [Google Scholar]
- 58.Vaquerizo B, Fernández-Nofreiras E, Oategui I, de Suarez Lezo J, Rumoroso J, Martín P, et al. Second-generation drug-eluting balloon for ostial side branch lesions (001-bifurcations): mid-term clinical and angiographic results. J Interv Cardiol. 2016;29(3):285–292. doi: 10.1111/joic.12292. [DOI] [PubMed] [Google Scholar]
- 59.Her A, Ann S, Singh G, Kim Y, Okamura T, Garg S, et al. Serial morphological changes of side-branch ostium after paclitaxel-coated balloon treatment of de novo coronary lesions of main vessels. Yonsei Med J. 2016;57(3):606. doi: 10.3349/ymj.2016.57.3.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito R, Ueno K, Yoshida T, Takahashi H, Tatsumi T, Hashimoto Y, et al. Outcomes after drug-coated balloon treatment for patients with calcified coronary lesions. J Interv Cardiol. 2017;31(4):436–441. doi: 10.1111/joic.12484. [DOI] [PubMed] [Google Scholar]
- 61.Rissanen T, Uskela S, Siljander A, Kärkkäinen J, Mäntylä P, Mustonen J, et al. Percutaneous coronary intervention of complex calcified lesions with drug-coated balloon after rotational atherectomy. J Interv Cardiol. 2017;30(2):139–146. doi: 10.1111/joic.12366. [DOI] [PubMed] [Google Scholar]
- 62.Köln P, Scheller B, Liew H, Rissanen T, Ahmad W, Weser R, et al. Treatment of chronic total occlusions in native coronary arteries by drug-coated balloons without stenting—a feasibility and safety study. Int J Cardiol. 2016;225:262–267. doi: 10.1016/j.ijcard.2016.09.105. [DOI] [PubMed] [Google Scholar]
- 63.Jeger RV, Farah A, Ohlow M-A, Mangner N, Möbius-Winkler S, Leibundgut G, et al. Drug-coated balloons for small coronary artery disease (BASKET-SMALL 2): an open-label randomised non-inferiority trial. Lancet. 2018;392(10150):849–856. doi: 10.1016/S0140-6736(18)31719-7. [DOI] [PubMed] [Google Scholar]
- 64.Vos NS, van der Schaaf RJ, Amoroso G, Herrman J-PR, Patterson MS, Slagboom T, et al. REVascularization with paclitaxEL-coated balloon angioplasty versus drug-eluting stenting in acute myocardial infarcTION—A randomized controlled trial: Rationale and design of the REVELATION trial. Catheter Cardiovasc Interv. 2016;87(7):1213–1221. doi: 10.1002/ccd.26241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.