Abstract
Mycobacteriosis is an emerging zoonotic disease of domestic cats and timely, accurate diagnosis is currently challenging. To identify differential cytokine/chemokine concentrations in serum/plasma of cats, which could be diagnostic biomarkers of infection we analysed plasma/serum from 116 mycobacteria-infected cats, 16 healthy controls and six cats hospitalised for unrelated reasons was analysed using the Milliplex MAP Feline Cytokine Magnetic Bead multiplex assay. Three cytokines; sFAS, IL-13 and IL-4 were reduced while seven; GM-CSF, IL-2, PDGF-BB, IL-8, KC, RANTES and TNF-α were elevated in mycobacteria-infected cats compared to healthy controls. However, IL-8 and KC concentrations were not significantly different from cats hospitalised for other reasons. Elevations in TNF-α and PDGF-BB may have potential to identify M. bovis and M. microti infected cats specifically while GM-CSF, IL-2 and FLT3L were increased in MTBC infected cats. This study demonstrates potential use of feline tuberculosis as a spontaneously occurring model of this significant human disease. Cytokine profiling has clear diagnostic potential for mycobacteriosis of cats and could be used discriminate tuberculous from non-tuberculous disease to rapidly inform on zoonotic risk. Future work should focus on the in-field utility of these findings to establish diagnostic sensitivity and specificity of these markers.
Introduction
Tuberculosis (TB) has been the cause of considerable morbidity and mortality in the human population for centuries, and remains the leading cause of death from any single infectious agent (Mycobacterium (M.) tuberculosis) worldwide1–3. Historically, the significance of this disease has resulted in extensive biomedical research interest and, due to the growing threat of drug resistance, TB is a major focus of collaborative global healthcare investment4–8. Meanwhile, TB in domestic cats receives relatively little research attention, despite being more common and clinically significant than previously thought9–11. In the UK, approximately 1% of all feline biopsies submitted for routine histopathological analysis show changes consistent with mycobacteriosis and a third of these contain Ziehl-Neelsen (ZN) positive organisms when stained, with morphology indicative of the presence of mycobacteria10,12.
The various mycobacterial species that have been identified in companion animals, including cats, can be grouped into the same two major categories as human mycobacterial disease; those belonging to the Mycobacterium tuberculosis-complex (MTBC) and the non-tuberculous mycobacteria (NTM, also referred to as ‘atypical mycobacteria’ or ‘mycobacteria other than tuberculosis’, MOTT)11,13–19.
The MTBC consists of ten highly genetically related species of mycobacteria which are capable of causing TB in both man and other animals, and are some of the oldest recorded zoonotic diseases known to both human and veterinary medicine20–26. All member species of the complex share identical sequences across the 16s rRNA gene and 99.5% sequence homology across the remainder of the genome27. The most discriminating features between the species at the nucleotide level are genomic deletions, termed regions of difference (RD)28–31. The MTBC organisms which infect cats, Mycobacterium microti and Mycobacterium bovis, differ by RD112,32–34. This region is absent from M. microti but present in all other MTBC organisms28. It has been shown to encode a variety of molecules which act as virulence factors which are also immuno-dominant proteins such as; early secreted antigenic target-6KDa (ESAT-6) and culture filtrate protein-10KDa (CFP-10)35–37.
In companion animals, such as cats, MTBC infections pose a potential zoonotic risk to their owners; additionally these cases may act as a potential source of environmental contamination with mycobacteria such as M. bovis, a pathogen of major animal health significance in the UK and other countries as the causative agent of bovine tuberculosis (bTB)38–41.
In stark contrast to human TB cases, the majority of feline TB cases present with localised nodular cutaneous disease, frequently with a degree of ulceration and occasionally with a draining sinus tract9,13,14,34. The lesions are typically distributed around the face, extremities and tail base – the so-called “fight and bite sites”15,34. Skin lesions may be accompanied by a localised or even generalised lymphadenopathy, or lymphadenopathy (usually of the submandibular, pre-scapular or popliteal nodes) may be the only presenting sign, termed an incomplete primary complex14,33,34.
Pulmonary lesions do occur in cats, but rarely result from bacteria being inhaled and causing typical tubercle formation in the lungs and hilar lymph nodes9,14. Much more commonly, pulmonary disease is secondary to the putative haematogenous spread of bacteria from the site of inoculation in the skin12,14. This generates a diffuse interstitial pattern of disease which eventually becomes bronchial and is clinically observable as progressive dyspnoea followed eventually by a soft productive cough14,34. Radiographically this presents differently from primary pulmonary infection which more frequently causes the “classical” tuberculous cavitating lesions34. Disseminated disease can cause a range of clinical signs including hepato-splenomegaly, pleural and pericardial effusions, generalised lymphadenopathy, weight loss and pyrexia9,13,16,33,34.
With such variable clinical presentation and non-specific clinical signs, diagnosing feline mycobacterial infections rapidly and accurately is challenging. Mycobacterial culture conducted by the Animal and Plant Health Agency (APHA), is currently the recognised ‘gold standard test’ for the diagnosis of mycobacterial disease in UK companion animals; however, it has several limiting features. Firstly, it has been shown to have a relatively poor sensitivity of ~50%, secondly it requires weeks from the time that a contaminated tissue biopsy is submitted for results to be obtained e.g. the average culture time is 12–16 weeks for M. microti - the most frequently cultured organism9,34,42. During this time, if untreated, a patient will remain a potential source of infection to both its owner and the local environment.
As such, alternative diagnostic tests have been developed which aim to reduce the amount of time between clinical presentation and a definitive diagnosis being reached. Interferon-gamma (IFN-γ) release assays (IGRA) were developed on the principle of quantitatively evaluating IFN-γ production by peripherally circulating antigen-specific effector T-memory cells upon in vitro stimulation, in order to aid the diagnosis of bTB in cattle43–46. These have subsequently been adapted to identify both active and latent TB in human patients with both greater sensitivity and specificity than the previously utilised tuberculin skin test47–49. Where intra-dermal testing has been shown to be of unreliable clinical utility in the cat, the IGRA has been used successfully14,45.
IGRAs have several advantages over other diagnostic techniques; they are significantly quicker at generating results than culture and are cheaper than many commercially available PCR and subsequent sequencing methods, they are also relatively non-invasive requiring only a single peripheral blood sample45,48. Unlike intra-dermal skin testing, they can be repeated if necessary as conducting the assay does not alter the systemic immune response44,50,51. In 2008, an adapted methodology of the IGRA was validated for diagnostic use in cats; this assay has a reported 100% sensitivity to indicate MTBC infection45.
Recently, within the field of human diagnostics of mycobacterial diseases, and of TB in particular, there has been a marked increase in research focussed on the identification of circulating cytokine biomarkers for the diagnosis of both active and latent MTBC infections in humans52–57. The goal of such assays is to cheaply, sensitively and rapidly identify infected and infectious individuals, in order to combat what remains one of the most incident infectious diseases of man58. Such studies have utilised multiplex cytokine assays, most commonly Luminex xMAP technologies, with promising results54,59,60. Studies of multiple assays have found circulating cytokine concentrations and combinations of cytokines which can sensitively and specifically diagnose both active and latent TB in humans52–57. For example; CXCL10 and CCL2 plasma concentrations can be combined with circulating levels of IFN-γ to more accurately discriminate between active and latent TB states, whilst the quantification of vascular endothelial growth factor can help differentiate patients with TB pleural effusion from those with neoplastic pleural effusions57,61. Similar assays have also been used successfully in the study of a limited number of companion animal diseases, including canine lymphoma and feline cystitis62–65.
As the timely and accurate diagnosis of feline mycobacteriosis, and the identification of the causative species is currently challenging, the aim of this study was to evaluate whether cytokine profiling from peripheral blood samples of infected cats demonstrated the same clinical utility as has been shown for humans. We therefore hypothesised that a number of cytokines would be differentially detectable in cats infected with mycobacterial infections when compared to both healthy controls and sick cats hospitalised for other reasons. We further hypothesised that the cytokine response would differ according to the species of mycobacteria present in the patient.
Materials and Methods
Mycobacteria spp. infected cat blood sample collection
Blood samples analysed in this study consisted of either heparinised plasma or separated serum. Archived remnant samples obtained opportunistically by vets and donated by the cats’ owners were used in this project. Cats diagnosed with mycobacterial infection by histological identification of (pyo)granulomatous inflammation in lesion biopsy material with the presence of acid-fast bacilli morphologically indicative of mycobacteria were considered eligible for inclusion in this study. Private veterinary surgeons who contacted the Royal (Dick) School of Veterinary Studies for clinical assistance with case management were asked to retain any blood samples remaining after diagnostic procedures had been performed. With the owner’s consent, these samples were then sent directly to the University of Edinburgh where they were retained at −80 °C prior to analysis of chemokine and cytokine concentrations.
Cats were excluded from the study if, prior to blood sample collection, they had been known to be treated with immunomodulatory medications e.g. non-steroidal anti-inflammatories (NSAIDs), chemotherapeutic agents or corticosteroids within 14 days of sample collection. Cats were additionally excluded if they were pre-treated with antibiotics with efficacy against mycobacteria, including fluoroquinolones, macrolides/azides or doxycycline within the same time period. Cats were not excluded if they had been treated with antimicrobial agents if these would be ineffective against mycobacteria, such as a penicillin or cephalosporin. Similarly, cats were not excluded if they had been treated with non-immunomodulatory analgesic medications e.g. opioids.
This study was conducted following approval from the School of Veterinary Medicine Ethical Review Committee at the University of Edinburgh; all relevant guidelines and regulations were adhered to throughout.
Speciation of mycobacteria infecting case animals
All cats included in this study had positive histological diagnoses of mycobacterial infection as outlined above. Of these, cases which had undergone additional IGRA, PCR or culture testing were sub-classified by the species of infecting organism identified.
Cases were defined as being infected with an MTBC mycobacteria if the cat had an IGRA test which showed an antigen specific IFN-γ response biased to purified protein derivative from M. bovis (PPDB) above that generated in response to purified protein derivative from M. avium (PPDA). The MTBC cases which additionally responded to the ESAT-6/CFP-10 peptide combination were classified as M. bovis infected. Cats which did not respond to the ESAT-6/CFP-10 peptide cocktail but showed a PPDB biased response were defined as being infected with M. microti. Cats with a positive IGRA test that did not meet the criteria for MTBC infection were classified as being infected with non-tuberculous mycobacteria. Both the test method and interpretation have previously been described by Rhodes et al.45.
In addition to IGRA testing, the results of mycobacterial culture from tissue biopsies were used, where available, when conducted by APHA and/or PCR results were used to speciate infections when conducted by Leeds University Teaching Hospital Mycobacterial Reference Laboratory.
Collection of control cat blood samples
Remnant serum samples from healthy control cats were kindly gifted from the Waltham Centre for Pet Nutirtion. At the time of sampling each control animal underwent an annual routine health check comprising compete physical examination by the attending veterinary surgeon, plus full haematological testing and serum biochemical analyses. All cats were required to have findings for these tests within normal limits to be included as a control animal in this study. Separated serum were aliquoted, and sent directly to the University of Edinburgh and stored −80 °C prior to analysis of chemokine and cytokine concentrations.
Collection of hospitalised cat blood samples
Remnant serum was obtained from cats Hospital for Small Animals, at the Royal (Dick) School of Veterinary Studies, University of Edinburgh. These cats had no clinical signs consistent with mycobacterial disease and were only included if an alternative definitive diagnosis had been reached. Once obtained, samples were kept at 4 °C and assayed within 12 hours.
Cats were excluded from this group for any one or more of the following: a diagnosis of stage V neoplasia (i.e. bone marrow involvement was confirmed), myelodysplastic disorder, exogenous retroviral (FIV/FeLV) infection or if they had received treatment with any immunomodulatory medicines including NSAIDs, corticosteroids or chemotherapeutic agents.
Cytokine and chemokine measurements
Cytokine and chemokine concentrations were measured in all of the samples using a commercial, feline specific, antibody-coated microsphere-based multiplex cytokine immunoassay shown to be able to quantify 19 cytokines contemporaneously using 25 ul of each patient’s serum (FCTYOMAG-20K MILLIPLEX MAP Feline Cytokine/Chemokine Magnetic Bead Panel, Premix 19 Plex kit, MERCK Millipore Corporation, Billerica, MA, USA).
The following cytokines were measured: sFas, Flt-3 ligand, GM-CSF, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-12-(p40), IL-13, IL-18, KC, MCP-1, PDGF-BB, RANTES, SCF, SDF-1, TNF-α. All samples, standards and quality controls were assayed in accordance with the manufacturer’s instructions. All samples were assayed in duplicate and mean values analysed. Overnight incubation at 4 °C and a magnetic plate washer were utilised. The plates were read with a multiplex plate reader and companion software. All cytokine and chemokine concentrations are reported in pg/mL.
Statistical analysis
Statistical analysis was performed using commercially available statistical software (GraphPad Prism 7.0). Mean values of duplicate samples for each cat were used for analysis. For the purposes of statistical analysis, values that fell below the limit of detection of the assay were assigned a concentration of 0 pg/mL.
Initially, to compare all mycobacteria positive samples (n = 116) to healthy control cats (n = 16), the D’Agostino & Pearson omnibus test was used to confirm a Gaussian distribution of the control group data for each of the cytokines/chemokines measured. As no reference intervals currently exist for these biomarkers in the cat, these were generated from the healthy control cat population to include values 1.96 standard deviations above and below the mean of the control group (i.e. to encompass ~95% of the healthy population) for each cytokine/chemokine measured. The mean concentration, standard deviation and reference interval (RI) generated for each molecule measured are shown in Supplementary Table I.
For mycobacteria-infected cats, 95% confidence intervals around the mean were generated by logarithmic-transformation of the cytokine values so that the data conformed to a Gaussian distribution. On this basis, a 95% confidence interval was then generated for the transformed data which was then reverse transformed to give the confidence intervals provided in Supplementary Table 1.
Cytokine levels were considered to be statistically significantly different between mycobacteria-infected and healthy control cats if there was no overlap between the RI generated from the control data and confidence intervals of data generated from infected cats (shown in bold in Supplementary Table I).
A second comparison was made between mycobacteria-infected, healthy control cats and hospitalised cats. Confidence intervals for the cytokine levels of hospitalised cats were generated in the same way as described for mycobacteria-infected cats and are shown in Supplementary Table 1. Again, cytokine levels were considered to be significantly different between groups if there was no overlap between the RI generated from the control data and confidence intervals of data generated from sick cats or mycobacteria infected cats.
A sub-group analysis was conducted to compare healthy control (n = 16), M. bovis infected (n = 22), M. microti infected (n = 43) and NTM infected cats (n = 15) with each other. Of these four groups, only cytokine concentrations within the healthy control group followed a Gaussian distribution as assessed by D’Agostino & Pearson omnibus test. Therefore, a Kruskal-Wallis test by ranks was used to determine if differences existed between the groups for each cytokine measured. Where significant differences were found (defined as p < 0.05), each group was compared in a consecutive pairwise manner using Mann-Whitney U tests with post-hoc Bonferroni correction applied to accommodate for multiple comparisons. Resultantly, a value of p ≤ 0.003 was considered statistically significant for sub-group analyses. The median, range and 95% confidence interval for the cytokine concentrations measured within each sub-group of mycobacteria-infected cats are shown in Supplementary Table II.
Results
Patient characteristics
All cats included in this study were adult cats from the UK. In total, serum/plasma from 116 cases of feline mycobacterial disease met the inclusion criteria for the study, and serum from 16 healthy control cats and six hospitalised cats was also analysed.
Within the group of cats with clinical mycobacteriosis, the infecting organism was speciated based on IGRA, culture and/or PCR for 80 of the 116 (69.0%) cats. Of these, 22 (27.5%) were infected with M. bovis, 43 (53.8%) were infected with M. microti and the remaining 15 (18.7%) infected with NTM.
For the cats hospitalised for independent reasons the final diagnoses were established as hyperthyroidism (n = 2), congestive heart failure due to hypertrophic cardiomyopathy, hepatic lipidosis, lysosomal storage disease, chronic kidney disease and diabetes mellitus.
Cytokine and chemokine concentrations
All mycobacteria-infected cats compared to healthy control cats
The mean/median and standard deviation/range for each cytokine concentration for each of these groups is shown in Supplementary Table I. Three cytokines; sFAS, IL-13 and IL-4 were found to be significantly reduced in the mycobacteria-infected group compared to healthy control cats (Fig. 1). By contrast, seven cytokines; GM-CSF, IL-2, PDGF-BB, IL-8, KC, RANTES and TNF-α were present in significantly elevated concentrations within the peripheral circulation of mycobacteria infected cats when compared to healthy control animals (Fig. 2).
Figure 1.
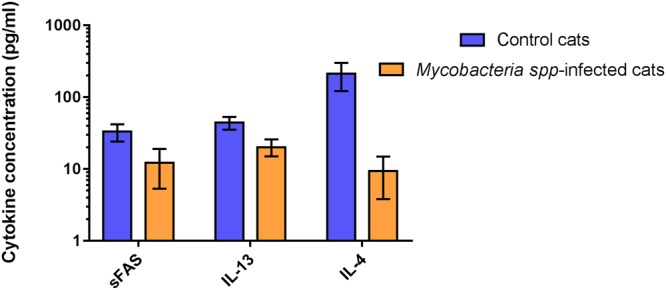
Cytokines found to have significantly reduced concentrations in the peripheral blood of cats with mycobacterial disease compared to healthy controls. Data are shown as the median for the group with error bars indicting the 95% confidence interval.
Figure 2.
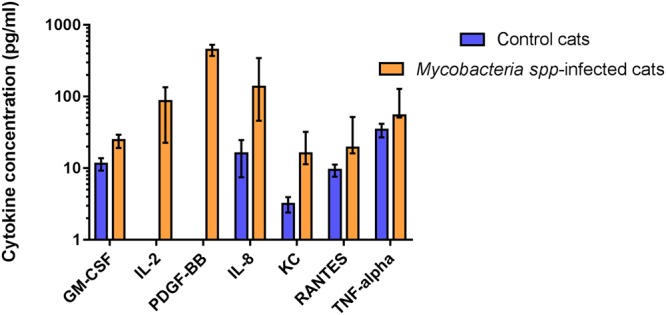
Cytokines found to have significantly increased concentrations in the peripheral blood of cats with mycobacterial disease compared to healthy controls. Data are shown as the median for the group with error bars indicting the 95% confidence interval.
There was no significant difference between the two groups in their circulating concentrations of Flt-3 ligand, IFN-γ, IL-1β, IL-12 (p40), IL-6, IL-18, SDF-1, MCP-1, or SCF.
Comparison of all mycobacteria-infected cats to hospitalised cats and controls
To begin to assess the specificity of changes in cytokine concentrations between feline mycobacteriosis patients and healthy controls, serum from six hospitalised cats was analysed and compared to both of these groups, data shown in Supplementary Table 1.
The cytokines GM-CSF, IL-2, PDGF-BB, RANTES and TNF-α were found at significantly elevated concentrations, whilst IL-4 and sFAS were detected at reduced concentrations in mycobacteria infected cats compared to both hospitalised non mycobacteria-infected cats and healthy controls (Fig. 3).
Figure 3.
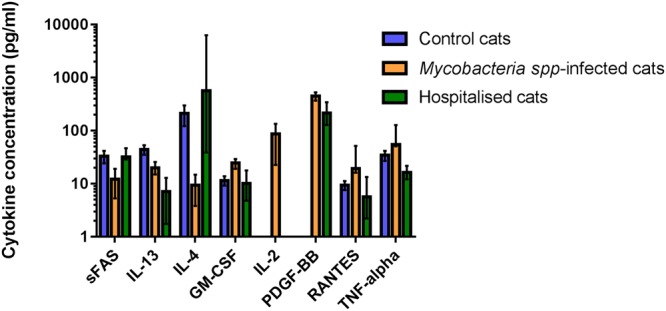
Cytokines found to have significantly altered concentrations in the peripheral blood of cats with mycobacterial disease compared to healthy controls and/or cats hospitalised for other reasons. Data are shown as the median for the group with error bars indicting the 95% confidence interval.
The concentrations of IL-13 and IL-1β were found to be significantly reduced in hospitalised cats when compared to both control cats and those with mycobacterial infections (Fig. 4). Whilst differences in the concentrations of IL-8 and KC were observed between healthy cats and those infected with mycobacteria, there was no difference in the concentrations of these cytokines between cats infected with mycobacteria and cats hospitalised for unrelated conditions (Fig. 4).
Figure 4.
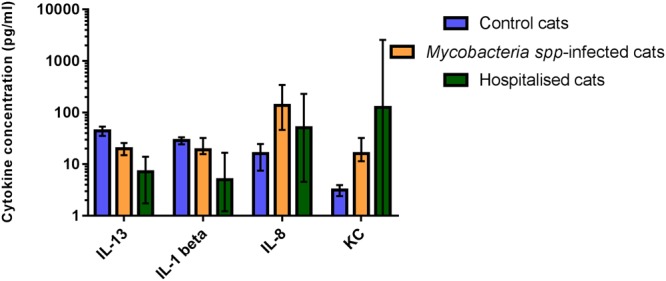
Cytokines found to have significantly altered concentrations in the peripheral blood of cats with mycobacterial disease compared to healthy controls and/or cats hospitalised for other reasons. Statistically significant differences between groups were determined by Kruskal-Wallis test by ranks (P < 0.05), each group was compared in a consecutive pairwise manner using Mann-Whitney U tests with post-hoc Bonferroni correction applied to accommodate for multiple comparisons (P ≤ 0.003). Data are shown as the median for the group with error bars indicting the 95% confidence interval.
Comparison of cats infected with different mycobacterial species
Of the 116 cats enrolled in the study, 80 had their mycobacterial infections definitively speciated to one of three causative agents, or group of causative agents; M. bovis, M. microti and NTM infections. These groups were compared to one another and also against the healthy control group. This analysis revealed the concentration of TNF-α to be significantly increased in the M. bovis-infected group when compared to all other groups; while the concentration of PDGF-BB was found to be significantly increased in cats infected with M. microti compared to all remaining groups (Fig. 5). Concentrations of GM-CSF, IL-2 and Flt3-L were significantly increased in both MTBC groups (M. bovis and M. microti) when compared to the remaining two groups (Fig. 6).
Figure 5.
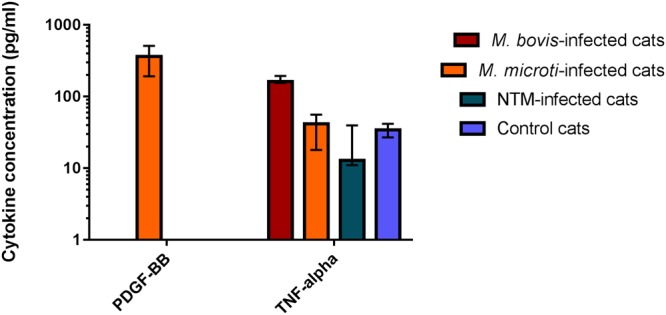
Cytokines found to have significantly altered concentrations in the peripheral blood of cats with mycobacterial disease due to M. bovis or M. microti, respectively, compared to healthy controls and cats infected with non-tuberculous mycobacterial (NTM) species. Data are shown as the median for the group with error bars indicting the 95% confidence interval.
Figure 6.
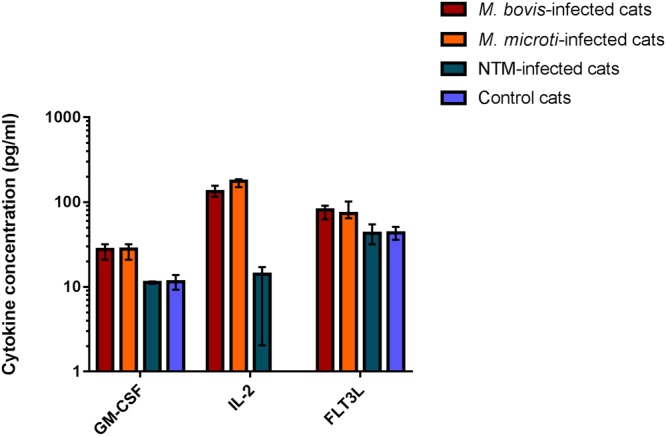
Cytokines found to have significantly altered concentrations in the peripheral blood of cats with mycobacterial disease due to TB-complex infections (M. bovis or M. microti respectively), compared to healthy controls and cats infected with non-tuberculous mycobacterial (NTM) species. Data are shown as the median for the group with error bars indicting the 95% confidence interval.
The group of cats infected with NTM species had a significant reduction in the concentration of MCP-1 in comparison to the healthy controls (data not shown).
Concentrations of cytokine IL-8 were increased in all three mycobacteria-infected groups when compared to healthy control animals (data not shown), with the greatest increase observed in the cats with NTM infections, though this trend was not statistically significant (p = 0.07).
Discussion
Current routine clinical methods for the diagnosis of feline mycobacterial disease, and tuberculosis in particular, have a number of drawbacks. Multiplex immunoassays have proven to be sensitive, specific, rapid, require very small sample volumes, and show a broad analytical and dynamic range66–70. The benefits of the multiplex assay approach used in this study include more rapid availability of results than currently achievable with culture or IGRA, and that this analysis requires only a small volume of serum or plasma to analyse (25 ul)66–70.
This population of cats consisted of adult cats resident in the UK; previous studies have shown that male cats are over-represented in the population of cats presenting with mycobacterial infections and cats are usually younger when they present with M. bovis compared to M. microti infections13,34. It was not possible for us to determine if any such bias existed within our study population as signalment data was infrequently reported to us by primary veterinary clinicians. The reasons for this are unclear but it may be a reflection of the predominately outdoor and semi-feral lifestyles of many of these cats meaning that there is limited information definitively known about them.
All samples used in this study were opportunistically obtained remnants collected as part of other diagnostic investigations and processed by primary veterinary clinicians. This approach meant that samples could be sourced ethically and provides demonstrable ‘proof of concept’ that such methodology is potentially practicable and achievable within a clinical setting. Due to the remnant nature of samples analysed in this study, one must be cognisant of the possibility that post-collection processing may not have been consistent across samples e.g. storage temperature, which may have influence on individual findings71,72. However, it seems reasonable to presume that such adverse effects would not impact any one group of cats in this study in particular, but occur randomly throughout the dataset generated and so the results obtained in this study are still (clinically) interpretable. Similarly, due to the opportunistic nature of sample collection, this study included two different types of sample media; serum and heparinised plasma. Previous work with the same commercially available multiplex assay kit has demonstrated strong correlation between cytokine/chemokine concentrations obtained in paired serum and heparinised plasma samples taken from the same cat70,73, and so again it would seem the results obtained in this study remain reliable and interpretable.
The inflammatory cascade induced by mycobacterial infection is complex and is poorly characterised in the cat. The hallmark lesion of mycobacterial infections, including TB, is the granuloma which is a compact, organised aggregate of immune cells formed as part of the host immune response to the persistent stimuli of the pathogen15,74–76. Upon phagocytosis, inhibition of phagosome-lysosome fusion enables the intracellular survival of mycobacteria within these phagocytes77. The internalised bacilli are then believed to stimulate the infected macrophage to invade the local tissues78,79. The stimulation of macrophage Toll-like receptors (TLRs) by mycobacteria induces the production of numerous cytokines, predominantly TNF-α, which drives the recruitment of mononuclear cells and neutrophils from surrounding blood vessels. In particular, there is recruitment of activated T-cells which interact with activated macrophages and secrete IFN-γ leading to mycobacterial killing80–84. Additionally, each group of recruited cells also release their own assortment of cytokines and chemokines which perpetuate the inflammatory cascade and the formation of a stable granuloma structure81,85.
In this study, we demonstrated that changes in the concentrations of eight critically important cytokines gave a sensitive and specific indication of mycobacterial infection in this study population. Elevated concentrations of the cytokines GM-CSF, IL-2, PDGF-BB, IL-8, KC, RANTES and TNF-α in mycobacteria infected cats with concurrent reductions in sFAS, IL-13 and IL-4 are suggestive of a pro-inflammatory process occurring in these patients, which is dominated by the recruitment and maturation of monocyte-macrophage lineage cells, the recruitment of cytotoxic T-cells, the proliferation of fibroblasts and the suppression of humoral immunity.
Granulocyte-macrophage colony-stimulating-factor (GM-CSF) is a critically important cytokine involved in the innate immune response against mycobacteria, specifically via influencing macrophage and dendritic cell function86–92. GM-CSF deficient mice are extremely susceptible to mycobacterial disease and their macrophages fail to effectively inhibit intracellular pathogen replication90. Similarly, humans with detectable titres of anti-GM-CSF auto-antibodies show an increased susceptibility to pulmonary tuberculosis compared to healthy controls, whilst GM-CSF immunotherapy can contribute to the resolution of disease due to drug-resistant M. tuberculosis93,94. GM-CSF activated cells, principally macrophages, go on to propagate the inflammatory cascade in particular via the production of TNF-α86.
GM-CSF has been shown by previous multiplex cytokine analyses to be critical in the early response and possible clearance of M. tuberculosis in humans and mice, so that the use of recombinant GM-CSF as an adjunctive therapy for TB has been posited; the findings of this study suggest that this may also be of benefit to feline patients.
TNF-α is an agonist of both nuclear factor (NF)-kB and mitogen-activated protein kinase (MAPK) which lead to downstream upregulation of pro-inflammatory immune functions including but not limited to; increased cell adhesion (e.g. giant cell formation) and macrophage apoptosis which are beneficial to the control of mycobacterial infections95–97. One such downstream effect is the increased expression and secretion of chemokines including RANTES and interleukin IL-898–100. These molecules are major drivers in the recruitment and migration of leukocytes towards an inflammatory focus98–100. Additionally, RANTES can combine synergistically with other locally secreted cytokines, such as IL-2 released from T-cells and IL-12(p40) released from pathogen-activated antigen-presenting cells e.g. mature dendritic cells, in order to stimulate the activation and proliferation of natural killer (NK) cells, which ultimately leads to a local increase in mycobactericidal activity98–100.
TNF-α, one of the most studied cytokines in the human mycobacterial response, has been repeatedly shown to be of significant diagnostic utility for mycobacterial infections in humans, with concentrations varying between TB and non-TB patients as well as being discriminatory between active and latent infections and generally being found to decline with successful therapy53–56. Similarly, a number of patients receiving TNF-α antagonists such as infliximab have been reported to contract and even die from mycobacterial infections101–103. In this study, it is notable that this cytokine was the only one to be uniquely indicative of infection with M. bovis. This is potentially due to the secretion of RD1 proteins during the course of these infections generating a more pro-inflammatory state in M. bovis infected cats. The possibility that cats infected with mycobacteria and with elevated TNF-α could have more severe or active disease and hence pose a more serious zoonotic threat to humans is one that warrants further investigation.
An essential step in the formation of an effective granuloma structure is fibroblast proliferation, mediated at least in part by the local production of PDGF-BB104. Similar to TNFα, it has recently been demonstrated that circulating levels of this cytokine are elevated in human patients with active pulmonary tuberculosis, and that these levels decline significantly over a six month course of therapy54. However, in contrast to TNFα, within our feline population, PDGF-BB was only detected at significant levels in cats infected with M. microti. This may indicate a difference in the immune response by cats to this species of mycobacteria, which was used as the human vaccine strain prior to the availability of M. bovis-BCG, and may therefore be considered attenuated105. Future analysis and comparison of the granuloma structures of M. microti and M. bovis infected cats may show physical manifestations of such differences, and may be useful to identify prognostic features of relevance to feline disease.
The Fas/Fas-ligand (FasL) system is an important molecule for immunological regulation; specifically it maintains T-cell homeostasis by the induction of apoptosis in order to limit T-cell expansion following antigenic stimulation106,107. It has been reported that mycobacteria exploit this system in order to subvert and evade the adaptive immune response of an infected animal by generating an immune-privileged niche106,108. Soluble Fas (sFAS) can antagonise cell surface FasL by competitive inhibition and so allow continued T-cell proliferation, a phenomenon shown to be significant in lymphoproliferative disorders such as non-Hodgkin’s lymphoma109.
This is the first time, to our knowledge, that evidence of such an extensively conserved systemic immune response to these infectious agents, directly comparable to that seen in many human and animal models of mycobacterial disease, has been demonstrated in the cat. This suggests that the occurrence of mycobacterial infections in this species could act as a naturally occurring model of human disease. This capacity could be particularly significant in overcoming the known shortcomings of murine models of mycobacterial disease, particularly as feline cases occur with relatively high clinical frequency and so could provide a naturally-occurring reliable source of study data.
Within the variety of mycobacterial species which are known to infect cats; those belonging to the MTBC, and M. bovis in particular, pose the greatest risk of zoonotic spread to in-contact humans39–41. Currently the gold standard diagnostic test to identify these organisms which can threaten human health is mycobacterial culture; however, M. bovis takes a minimum of 6–8 weeks to confirm by culture and M. microti requires even longer at 12–16 weeks9,14,42. Within the cytokines analysed in this study; Flt-3L, GM-CSF and IL-2 were all significantly elevated in cats with a diagnosis of infection with an MTBC organism compared to all other groups. Therefore, it may be possible that these cytokines in particular could be used to more rapidly identify those infections that pose zoonotic risk to owners and the general public, allowing for earlier intervention.
Cytokine profiling in companion animal medicine has been the subject of a number of recent studies62,64,65. One limitation of such studies to date is the study design to compare cytokine profiles in animals with the designated diagnosis of interest and apparently healthy animals as controls. In our investigations, we additionally included a small number of cats that had been hospitalised for reasons unrelated to mycobacterial disease. This allowed us to identify that the changes in concentration of the cytokines KC and IL-8 were not specific to mycobacterial infection, but rather may simply occur when cats were ill or stressed. These findings indicate that including such a group in experimental design is and will be important for the assessment of diagnostic accuracy, including sensitivity and specificity, of cytokine profiling in the future. Greater accuracy would be achieved by expanding on our small sample size (n = 6) and also by matching the diagnoses in this group to those with similar aetiopathogenesis to the diagnosis of interest. For example, such a study could include cats with other infectious granulomatous disease such as feline infectious peritonitis (FIP), where multiplex expression profile analysis of serum samples have also shown elevations in RANTES and GM-CSF concentrations64.
A limitation of the study we report here is its retrospective nature; this meant that it was not possible to have a standardised diagnostic assessment of each cat and samples from each cat did not undergo the gold standard test of mycobacterial culture. The majority of cats in this study were diagnosed by IGRA which has been shown to have a higher sensitivity for MTBC than NTM infections, which may have introduced some bias towards MTBC infected individuals in our population45. Similarly, the amount of time elapsed between the time of infection, the infective dose received, and the time of blood sampling was not known for these cats. It is therefore possible that the changes seen could reflect different stages or magnitude of the feline response to mycobacteria; for example, MTBC infections may be diagnosed earlier in the course of infection than NTM infections and so the differences seen may simply reflect an early immune response. It was therefore not possible for us to generate sensitivity or specificity data for these cytokines nor to assess their diagnostic capacity in combination as has been achieved in human medicine. To further investigate the potential for cytokine profiling in feline mycobacterial disease, prospective recruitment of cases would allow for additional control over the time of blood sampling in relation to patients being presented to a veterinary surgeon. Such a study would additionally further allow changes in cytokine concentrations to be tracked over time, and in response to treatment. This would allow much greater accuracy regarding the cessation of antimicrobial therapy in these cases, which is not currently possible.
Conclusion
In this study, we demonstrate a conserved immunological response to mycobacteria by domestic cats analogous to that of other species. Further research into feline tuberculosis as a spontaneously occurring model of a significant human disease is urgently required. These data show that cytokine profiling has the practical and immunological potential to be developed as a sensitive and specific diagnostic test for the presence of mycobacteria in feline infections, to readily speciate them and help inform on zoonotic risk to exposed humans. The Milliplex MAP Feline Cytokine Magnetic Bead multiplex assay was shown to be a practicable and reliable platform for these measurements and further work should focus on the prospective diagnostic utility of the findings reported in this study.
Electronic supplementary material
Acknowledgements
We would like to thank all of the owners and veterinary surgeons of the cats in this study for donating samples to us, in particular the Waltham Centre for Pet Nutrition who kindly gifted control cat sera to this study. We are very grateful to the BBSRC for ongoing financial support. Conor O’Halloran is supported by a CASE studentship BB/M0149894/1.
Author Contributions
O’Halloran, C. conducted the experiment, performed the data analysis and wrote the manuscript. McCulloch, L. & Rentoul, L. provided technical assistance conducting the assays. Alexander, J. provided the study with control animal sera. Hope, J.C. and Gunn-Moore, D.A. developed the concept and secured funding for the study.
Competing Interests
The authors declare no conflicts of interest. Mr Rentoul is an employee of Merck Millipore and Dr. Alexander is an employee of Waltham Centre for Pet Nutrition. The remaining authors have no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
J. C. Hope and D. A. Gunn-Moore jointly supervised this work.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35571-5.
References
- 1.Naghavi M, et al. Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. doi: 10.1016/S0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakroborty A. Drug-resistant tuberculosis: An insurmountable epidemic? Inflammopharmacology. 2011;19:131–137. doi: 10.1007/s10787-010-0072-2. [DOI] [PubMed] [Google Scholar]
- 5.Walter ND, et al. Translating basic science insight into public health action for multidrug- and extensively drug-resistant tuberculosis. Respirology. 2012;17:772–791. doi: 10.1111/j.1440-1843.2012.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dooley KE, et al. World health organization group 5 drugs for the treatment of drug-resistant tuberculosis: Unclear efficacy or untapped potential? J. Infect. Dis. 2013;207:1352–1358. doi: 10.1093/infdis/jis460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diel Roland, Loddenkemper Robert, Niemann Stefan, Meywald-Walter Karen, Nienhaus Albert. Negative and Positive Predictive Value of a Whole-Blood Interferon-γ Release Assay for Developing Active Tuberculosis. American Journal of Respiratory and Critical Care Medicine. 2011;183(1):88–95. doi: 10.1164/rccm.201006-0974OC. [DOI] [PubMed] [Google Scholar]
- 8.Velayati AA, Farnia P, Farahbod AM. Overview of drug-resistant tuberculosis worldwide. Int. J. Mycobacteriology. 2016;5:S161. doi: 10.1016/j.ijmyco.2016.09.066. [DOI] [PubMed] [Google Scholar]
- 9.O’Halloran C, Gunn-Moore D. Mycobacteria in cats: An update. In Pract. 2017;39:399–406. doi: 10.1136/inp.j4155. [DOI] [Google Scholar]
- 10.Gunn-Moore DA, Gaunt C, Shaw DJ. Incidence of mycobacterial infections in cats in great britain: Estimate from feline tissue samples submitted to diagnostic laboratories. Transbound. Emerg. Dis. 2013;60:338–344. doi: 10.1111/j.1865-1682.2012.01352.x. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien CR, et al. Feline leprosy due to Candidatus ‘Mycobacterium tarwinense’: Further clinical and molecular characterisation of 15 previously reported cases and an additional 27 cases. J. Feline Med. Surg. 2017;19:498–512. doi: 10.1177/1098612X17706467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Halloran C, Gunn-Moore D. Mycobacteria in cats: an update. In Pract. 2017;39:399–406. doi: 10.1136/inp.j4155. [DOI] [Google Scholar]
- 13.Gunn-Moore DA, et al. Mycobacterial disease in a population of 339 cats in Great Britain: II. Histopathology of 225 cases, and treatment and outcome of 184 cases. J. Feline Med. Surg. 2011;13:945–952. doi: 10.1016/j.jfms.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunn-Moore DA. Feline mycobacterial infections. Vet. J. 2014;201:230–238. doi: 10.1016/j.tvjl.2014.02.014. [DOI] [PubMed] [Google Scholar]
- 15.Malik Richard, Smits Bronwyn, Reppas George, Laprie Caroline, O’Brien Carolyn, Fyfe Janet. Ulcerated and nonulcerated nontuberculous cutaneous mycobacterial granulomas in cats and dogs. Veterinary Dermatology. 2013;24(1):146–e33. doi: 10.1111/j.1365-3164.2012.01104.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien CR, et al. Feline leprosy due to Mycobacterium lepraemurium: Further clinical and molecular characterisation of 23 previously reported cases and an additional 42 cases. J. Feline Med. Surg. 2017;19:737–746. doi: 10.1177/1098612X17706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien CR, et al. Feline leprosy due to Candidatus ‘Mycobacterium lepraefelis’: Further clinical and molecular characterisation of eight previously reported cases and an additional 30 cases. J. Feline Med. Surg. 2017;19:919–932. doi: 10.1177/1098612X17706470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porvaznik, I., Solovič, I. & Mokrý, J. Non-tuberculous mycobacteria: Classification, diagnostics, and therapy. Advances in Experimental Medicine and Biology944 (2017). [DOI] [PubMed]
- 19.Lockwood, D. N. Leprosy. BMJ Clin. Evid. 2007 (2007). [PMC free article] [PubMed]
- 20.Hingley-Wilson SM, Sambandamurthy VK, Jacobs WR. Survival perspectives from the world’s most successful pathogen, Mycobacterium tuberculosis. Nat. Immunol. 2003;4:949–955. doi: 10.1038/ni981. [DOI] [PubMed] [Google Scholar]
- 21.Donoghue Helen D., Spigelman Mark, O'Grady Justin, Szikossy Ildikó, Pap Ildikó, Lee Oona Y.-C., Wu Houdini H.T., Besra Gurdyal S., Minnikin David E. Ancient DNA analysis – An established technique in charting the evolution of tuberculosis and leprosy. Tuberculosis. 2015;95:S140–S144. doi: 10.1016/j.tube.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Lee Oona Y-C., Wu Houdini H. T., Donoghue Helen D., Spigelman Mark, Greenblatt Charles L., Bull Ian D., Rothschild Bruce M., Martin Larry D., Minnikin David E., Besra Gurdyal S. Mycobacterium tuberculosis Complex Lipid Virulence Factors Preserved in the 17,000-Year-Old Skeleton of an Extinct Bison, Bison antiquus. PLoS ONE. 2012;7(7):e41923. doi: 10.1371/journal.pone.0041923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crubézy Eric, Legal Luc, Fabas Ghislaine, Dabernat Henri, Ludes Bertrand. Pathogeny of archaic mycobacteria at the emergence of urban life in Egypt (3400 bc) Infection, Genetics and Evolution. 2006;6(1):13–21. doi: 10.1016/j.meegid.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Crubézy, E. et al. Identification of Mycobacterium DNA in an Egyptian Pott’s disease of 5400 years old | Identification d’ADN de Mycobacterium dans un mal de Pott egyptien de 5400 ans. Comptes Rendus l’Academie des Sci. - Ser. III321 (1998). [DOI] [PubMed]
- 25.Sylvester TT, et al. Prevalence and Risk Factors for Mycobacterium bovis Infection in African Lions (Panthera leo) in the Kruger National Park. J. Wildl. Dis. 2017;53:372–376. doi: 10.7589/2016-07-159. [DOI] [PubMed] [Google Scholar]
- 26.Viljoen IM, van Helden PD, Millar RP. Mycobacterium bovis infection in the lion (Panthera leo): Current knowledge, conundrums and research challenges. Vet. Microbiol. 2015;177:252–260. doi: 10.1016/j.vetmic.2015.03.028. [DOI] [PubMed] [Google Scholar]
- 27.Sheen, P. et al. A multiple genome analysis of Mycobacterium tuberculosis reveals specific novel genes and mutations associated with pyrazinamide resistance. BMC Genomics18, (2017). [DOI] [PMC free article] [PubMed]
- 28.Warren RM, et al. Differentiation of Mycobacterium tuberculosis complex by PCR amplification of genomic regions of difference. Int. J. Tuberc. Lung Dis. 2006;10:818–822. [PubMed] [Google Scholar]
- 29.Gey Van Pittius, N. C. et al. Evolution and expansion of the Mycobacterium tuberculosis PE and PPE multigene families and their association with the duplication of the ESAT-6 (esx) gene cluster regions. BMC Evol. Biol. 6 (2006). [DOI] [PMC free article] [PubMed]
- 30.Jiang Y, et al. Genetic diversity of immune-related antigens in Region of Difference 2 of Mycobacterium tuberculosis strains. Tuberculosis. 2017;104:1–7. doi: 10.1016/j.tube.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 31.Reddington K, et al. Novel multiplex real-time PCR diagnostic assay for identification and differentiation of Mycobacterium tuberculosis, Mycobacterium canettii, and Mycobacterium tuberculosis complex strains. J. Clin. Microbiol. 2011;49:651–657. doi: 10.1128/JCM.01426-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burthe, S. et al. Tuberculosis (Mycobacterium microti) in wild field vole populations. Parasitology135 (2008). [DOI] [PMC free article] [PubMed]
- 33.Laprie Caroline, Duboy Julie, Malik Richard, Fyfe Janet. Feline cutaneous mycobacteriosis: a review of clinical, pathological and molecular characterization of one case ofMycobacterium microtiskin infection and nine cases of feline leprosy syndrome from France and New Caledonia. Veterinary Dermatology. 2013;24(6):561–e134. doi: 10.1111/vde.12066. [DOI] [PubMed] [Google Scholar]
- 34.Gunn-Moore DA, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J. Feline Med. Surg. 2011;13:934–944. doi: 10.1016/j.jfms.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.El-Seedy FR, et al. The correlation between M. bovis isolation and ELISA using PPD-B and ESAT6-CFP10 mixture on the sera of tuberculin reactor cattle and buffaloes. J. Food, Agric. Environ. 2013;11:489–494. [Google Scholar]
- 36.Wang X-Y, Bao L, Zhao M-C, Zhang H-D, Long Y. Expression of the fusion protein CFP10-ESAT6 of Mycobacterium tuberculosis and the study of its immunogenicity. J. Sichuan Univ. (MedicalSci. Ed. 2006;37:353–356. [PubMed] [Google Scholar]
- 37.Guo S, et al. The CFP10/ESAT6 complex of Mycobacterium tuberculosis may function as a regulator of macrophage cell death at different stages of tuberculosis infection. Med. Hypotheses. 2012;78:389–392. doi: 10.1016/j.mehy.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 38.Sless R. Bovine TB: Mycobacterium bovis infection in cats and people. Vet. Rec. 2014;174:384. doi: 10.1136/vr.g2664. [DOI] [PubMed] [Google Scholar]
- 39.Talwar A, et al. A very strange tail. Thorax. 2014;69:1159–1160. doi: 10.1136/thoraxjnl-2014-206033. [DOI] [PubMed] [Google Scholar]
- 40.Ramdas KE, et al. Mycobacterium bovis infection in humans and cats in same household, texas, usa, 2012. Emerg. Infect. Dis. 2015;21:480–483. doi: 10.3201/eid2103.140715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Roberts T, O’Connor C, Nuñez-Garcia J, De La Rua-Domenech R, Smith NH. Unusual cluster of Mycobacterium bovis infection in cats. Vet. Rec. 2014;174:326. doi: 10.1136/vr.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith NH, Crawshaw T, Parry J, Birtles RJ. Mycobacterium microti: More diverse than previously thought. J. Clin. Microbiol. 2009;47:2551–2559. doi: 10.1128/JCM.00638-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ringshausen Felix C, Schablon Anja, Nienhaus Albert. Interferon-gamma release assays for the tuberculosis serial testing of health care workers: a systematic review. Journal of Occupational Medicine and Toxicology. 2012;7(1):6. doi: 10.1186/1745-6673-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pai Madhukar, Joshi Rajnish, Dogra Sandeep, Mendiratta Deepak K., Narang Pratibha, Kalantri Shriprakash, Reingold Arthur L., Colford John M., Riley Lee W., Menzies Dick. Serial Testing of Health Care Workers for Tuberculosis Using Interferon-γ Assay. American Journal of Respiratory and Critical Care Medicine. 2006;174(3):349–355. doi: 10.1164/rccm.200604-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rhodes SG, Gruffydd-Jones T, Gunn-Moore D, Jahans K. Interferon-γ test for feline tuberculosis. Vet. Rec. 2008;162:453–455. doi: 10.1136/vr.162.14.453. [DOI] [PubMed] [Google Scholar]
- 46.Bezos J, et al. Current ante-mortem techniques for diagnosis of bovine tuberculosis. Res. Vet. Sci. 2014;97:S44–S52. doi: 10.1016/j.rvsc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Thillai M, Pollock K, Pareek M, Lalvani A. Interferon-gamma release assays for tuberculosis: Current and future applications. Expert Rev. Respir. Med. 2014;8:67–78. doi: 10.1586/17476348.2014.852471. [DOI] [PubMed] [Google Scholar]
- 48.Jurcev-Savicevic A, Katalinić-Janković V, Miše K, Gudelj I. The role of interferon-gamma release assay in tuberculosis control. Arh. Hig. Rada Toksikol. 2012;63:49–59. doi: 10.2478/10004-1254-63-2012-2134. [DOI] [PubMed] [Google Scholar]
- 49.Pollock L, Basu Roy R, Kampmann B. How to use: Interferon γ release assays for tuberculosis. Arch. Dis. Child. Educ. Pract. Ed. 2013;98:99–105. doi: 10.1136/archdischild-2013-303641. [DOI] [PubMed] [Google Scholar]
- 50.Chung Woo Kyung, Zheng Zhen Long, Kim Hyung-Soo, Park Jin-Woong, Lee Hyun Joo, Chang Jae Hyun, Jeong Ji Yong, Kim Sejoong, Lee Hyun Hee, Yang Jaeseok. Serial testing of interferon-γ-release assays for the diagnosis of latent tuberculosis in hemodialysis patients. Journal of Infection. 2010;61(2):144–149. doi: 10.1016/j.jinf.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 51.Torres Costa José, Silva Rui, Sá Raul, Cardoso Maria João, Nienhaus Albert. Serial testing with the interferon-γ release assay in Portuguese healthcare workers. International Archives of Occupational and Environmental Health. 2010;84(4):461–469. doi: 10.1007/s00420-010-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yao X, et al. Multiplex analysis of plasma cytokines/chemokines showing different immune responses in active TB patients, latent TB infection and healthy participants. Tuberculosis. 2017;107:88–94. doi: 10.1016/j.tube.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, et al. Multiple cytokine responses in discriminating between active tuberculosis and latent tuberculosis infection. Tuberculosis. 2017;102:68–75. doi: 10.1016/j.tube.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Clifford V, et al. Mycobacteria-specific cytokine responses as correlates of treatment response in active and latent tuberculosis. J. Infect. 2017;75:132–145. doi: 10.1016/j.jinf.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Gomez-Pastrana D, Domínguez J. Diagnosis of tuberculosis in children using mycobacteria-specific cytokine responses: Are there reasons for hope? Am. J. Respir. Crit. Care Med. 2015;192:409–410. doi: 10.1164/rccm.201506-1186ED. [DOI] [PubMed] [Google Scholar]
- 56.Tebruegge M, et al. Mycobacteria-specific cytokine responses detect tuberculosis infection and distinguish latent from active tuberculosis. Am. J. Respir. Crit. Care Med. 2015;192:485–499. doi: 10.1164/rccm.201501-0059OC. [DOI] [PubMed] [Google Scholar]
- 57.Nonghanphithak D, Reechaipichitkul W, Namwat W, Naranbhai V, Faksri K. Chemokines additional to IFN-γ can be used to differentiate among Mycobacterium tuberculosis infection possibilities and provide evidence of an early clearance phenotype. Tuberculosis. 2017;105:28–34. doi: 10.1016/j.tube.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Dye C, Scheele S, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: Estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 59.Gourgouillon N, et al. TNF-α/IL-2 ratio discriminates latent from active tuberculosis in immunocompetent children: A pilot study. Pediatr. Res. 2012;72:370–374. doi: 10.1038/pr.2012.89. [DOI] [PubMed] [Google Scholar]
- 60.Sutherland JS, Adetifa IM, Hill PC, Adegbola RA, Ota MOC. Pattern and diversity of cytokine production differentiates between Mycobacterium tuberculosis infection and disease. Eur. J. Immunol. 2009;39:723–729. doi: 10.1002/eji.200838693. [DOI] [PubMed] [Google Scholar]
- 61.Kim H-R, et al. Diagnostic significance of measuring vascular endothelial growth factor for the differentiation between malignant and tuberculous pleural effusion. Tohoku J. Exp. Med. 2017;242:137–142. doi: 10.1620/tjem.242.137. [DOI] [PubMed] [Google Scholar]
- 62.Parys M, Yuzbasiyan-Gurkan V, Kruger JM. Serum Cytokine Profiling in Cats with Acute Idiopathic Cystitis. J. Vet. Intern. Med. 2018;32:274–279. doi: 10.1111/jvim.15032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gasparetto ND, et al. Density measurement of Demodex canis by qPCR and analysis of serum cytokine levels in dogs with different clinical forms of demodicosis. Vet. Parasitol. 2018;257:1–4. doi: 10.1016/j.vetpar.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 64.Safi, N. et al. Expression profiles of immune mediators in feline Coronavirus-infected cells and clinical samples of feline Coronavirus-positive cats. BMC Vet. Res. 13 (2017). [DOI] [PMC free article] [PubMed]
- 65.Calvalido J, et al. Comparison of serum cytokine levels between dogs with multicentric lymphoma and healthy dogs. Vet. Immunol. Immunopathol. 2016;182:106–114. doi: 10.1016/j.vetimm.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 66.Elshal MF, McCoy JP. Multiplex bead array assays: Performance evaluation and comparison of sensitivity to ELISA. Methods. 2006;38:317–323. doi: 10.1016/j.ymeth.2005.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Villar-Vázquez R, et al. Development of a novel multiplex beads-based assay for autoantibody detection for colorectal cancer diagnosis. Proteomics. 2016;16:1280–1290. doi: 10.1002/pmic.201500413. [DOI] [PubMed] [Google Scholar]
- 68.Young S-H, Antonini JM, Roberts JR, Erdely AD, Zeidler-Erdely PC. Performance evaluation of cytometric bead assays for the measurement of lung cytokines in two rodent models. J. Immunol. Methods. 2008;331:59–68. doi: 10.1016/j.jim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 69.Dunbar, S. A., Ritchie, V. B., Hoffmeyer, M. R., Rana, G. S. & Zhang, H. Luminex ® multiplex bead suspension arrays for the detection and serotyping of Salmonella spp. Methods in Molecular Biology1225 (2015). [DOI] [PubMed]
- 70.Gruen ME, et al. A comparison of serum and plasma cytokine values using a multiplexed assay in cats. Vet. Immunol. Immunopathol. 2016;182:69–73. doi: 10.1016/j.vetimm.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Skogstrand K, et al. Effects of blood sample handling procedures on measurable inflammatory markers in plasma, serum and dried blood spot samples. J. Immunol. Methods. 2008;336:78–84. doi: 10.1016/j.jim.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 72.Tvedt THA, Rye KP, Reikvam H, Brenner AK, Bruserud Ø. The importance of sample collection when using single cytokine levels and systemic cytokine profiles as biomarkers — a comparative study of serum versus plasma samples. J. Immunol. Methods. 2015;418:19–28. doi: 10.1016/j.jim.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Halpin RE, et al. Evaluation of a feline-specific multiplex, bead-based assay for detection of cytokines, chemokines, growth factors, and other immunologically active proteins in serum and plasma samples from cats. Am. J. Vet. Res. 2016;77:495–504. doi: 10.2460/ajvr.77.5.495. [DOI] [PubMed] [Google Scholar]
- 74.Dreher D, Nicod LP. Dendritic cells in the mycobacterial granuloma are involved in acquired immunity. Am. J. Respir. Crit. Care Med. 2002;165:1577–1578. doi: 10.1164/rccm.2204010. [DOI] [PubMed] [Google Scholar]
- 75.Shakila H, Jayasankar K, Ramanathan VD. The clearance of tubercle bacilli and mycobacterial antigen vis a vis the granuloma in different organs of guineapigs. Indian J. Med. Res. 1999;110:4–10. [PubMed] [Google Scholar]
- 76.Puissegur M-P, et al. An in vitro dual model of mycobacterial granulomas to investigate the molecular interactions between mycobacteria and human host cells. Cell. Microbiol. 2004;6:423–433. doi: 10.1111/j.1462-5822.2004.00371.x. [DOI] [PubMed] [Google Scholar]
- 77.Simeone Roxane, Bobard Alexandre, Lippmann Juliane, Bitter Wilbert, Majlessi Laleh, Brosch Roland, Enninga Jost. Phagosomal Rupture by Mycobacterium tuberculosis Results in Toxicity and Host Cell Death. PLoS Pathogens. 2012;8(2):e1002507. doi: 10.1371/journal.ppat.1002507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu C-W, et al. Invasion and persistence of Mycobacterium avium subsp. paratuberculosis during early stages of Johne’s disease in calves. Infect. Immun. 2007;75:2110–2119. doi: 10.1128/IAI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Middleton AM, et al. Investigation of mycobacterial colonisation and invasion of the respiratory mucosa. Thorax. 2003;58:246–251. doi: 10.1136/thorax.58.3.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Qu Hui-Qi, Fisher-Hoch Susan P., McCormick Joseph B. Molecular immunity to mycobacteria: knowledge from the mutation and phenotype spectrum analysis of Mendelian susceptibility to mycobacterial diseases. International Journal of Infectious Diseases. 2011;15(5):e305–e313. doi: 10.1016/j.ijid.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins FM. Cellular mechanisms of anti-mycobacterial immunity. Adv. Exp. Med. Biol. 1983;162:157–182. doi: 10.1007/978-1-4684-4481-0_16. [DOI] [PubMed] [Google Scholar]
- 82.Iseman MD, Fischer A. Tumor necrosis factor-α at the intersection of mycobacterial immunity and pathogenesis: An important new address in medicine. Clin. Infect. Dis. 2008;46:1741–1742. doi: 10.1086/587990. [DOI] [PubMed] [Google Scholar]
- 83.Cooke GS, Siddiqui MR. Host genetics and the dissection of mycobacterial immunity. Clin. Exp. Immunol. 2004;135:9–11. doi: 10.1111/j.1365-2249.2004.02353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ryffel B, et al. Innate immunity to mycobacterial infection in mice: Critical role for toll-like receptors. Tuberculosis. 2005;85:395–405. doi: 10.1016/j.tube.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 85.May RD, Fung M. Strategies targeting the IL-4/IL-13 axes in disease. Cytokine. 2015;75:89–116. doi: 10.1016/j.cyto.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 86.Becher B, Tugues S, Greter M. GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity. 2016;45:963–973. doi: 10.1016/j.immuni.2016.10.026. [DOI] [PubMed] [Google Scholar]
- 87.Denis M, Ghadirian E. Granulocyte-macrophage colony-stimulating factor restricts growth of tubercle bacilli in human macrophages. Immunol. Lett. 1990;24:203–206. doi: 10.1016/0165-2478(90)90049-V. [DOI] [PubMed] [Google Scholar]
- 88.Szeliga J, et al. Granulocyte-macrophage colony stimulating factor-mediated innate responses in tuberculosis. Tuberculosis. 2008;88:7–20. doi: 10.1016/j.tube.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zamanzadeh Z, et al. In Silico Perspectives on the Prediction of the PLP’s Epitopes involved in Multiple Sclerosis. Iran. J. Biotechnol. 2017;15:10–21. doi: 10.15171/ijb.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gonzalez-Juarrero M, et al. Disruption of granulocyte macrophage-colony stimulating factor production in the lungs severely affects the ability of mice to control Mycobacterium tuberculosis infection. J. Leukoc. Biol. 2005;77:914–922. doi: 10.1189/jlb.1204723. [DOI] [PubMed] [Google Scholar]
- 91.Higgins DM, et al. Relative levels of M-CSF and GM-CSF influence the specific generation of macrophage populations during infection with Mycobacterium tuberculosis. J. Immunol. 2008;180:4892–4900. doi: 10.4049/jimmunol.180.7.4892. [DOI] [PubMed] [Google Scholar]
- 92.Rothchild, A. C. et al. Role of granulocyte-macrophage colony-stimulating factor production by T cells during mycobacterium tuberculosis infection. MBio8 (2017). [DOI] [PMC free article] [PubMed]
- 93.Kim K, et al. Levels of anti-cytokine antibodies may be elevated in patients with pulmonary disease associated with non-tuberculous mycobacteria. Cytokine. 2014;66:160–163. doi: 10.1016/j.cyto.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Zhang YR, et al. Immunotherapy using IL-2 and GM-CSF is a potential treatment for multidrug-resistant Mycobacterium tuberculosis. Sci. China Life Sci. 2012;55:800–806. doi: 10.1007/s11427-012-4368-x. [DOI] [PubMed] [Google Scholar]
- 95.Lawlor C, et al. Treatment of mycobacterium tuberculosis-infected macrophages with poly(lactic-co-glycolic acid) microparticles drives NFKB and autophagy dependent bacillary killing. PLoS One. 2016;11:1DUMMY. doi: 10.1371/journal.pone.0149167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Toossi, Z., Salvekar, A., Hamilton, B. D. & Elloer, J. J. Activation of nuclear factor kappa b and its inhibitor, ikb/mad3, during tuberculosis and by ppd of mycobacterium tuberculosis. J. Investig. Med. 44 (1996).
- 97.Lancellotti M, Pereira RFC, Cury GG, De Hollanda LM. Pathogenic and opportunistic respiratory bacteria-induced apoptosis. Brazilian J. Infect. Dis. 2009;13:226–231. doi: 10.1590/S1413-86702009000300014. [DOI] [PubMed] [Google Scholar]
- 98.Murthy MK, Kaliappan T, Raja A. Cytokine and chemokine responses to selected early secreted antigenic target-6 and culture filtrate protein-10 peptides in tuberculosis. J. Interf. Cytokine Res. 2011;31:299–307. doi: 10.1089/jir.2010.0048. [DOI] [PubMed] [Google Scholar]
- 99.Rivas-Santiago, B., Vieyra-Reyes, P. & Araujo, Z. Cell immunity response in human pulmonary tuberculosis. Review | Respuesta de inmunidad celular en la tuberculosis pulmonar. Revisión. Invest. Clin. 46 (2005). [PubMed]
- 100.Saha PK, Sharma PK, Sharma SK, Singh A, Mitra DK. Recruitment of Th1 effector cells in human tuberculosis: Hierarchy of chemokine receptor(s) and their ligands. Cytokine. 2013;63:43–51. doi: 10.1016/j.cyto.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Gori Alessia, Fabroni Caterina, Prignano Francesca, Lotti Torello. Unusual presentation of tuberculosis in an infliximab-treated patient - which is the correct TB screening before starting a biologic? Dermatologic Therapy. 2010;23:S1–S3. doi: 10.1111/j.1529-8019.2009.01278.x. [DOI] [PubMed] [Google Scholar]
- 102.Sorrentino D, Avellini C, Zearo E. Colonic sarcoidosis, infliximab, and tuberculosis: A cautionary tale. Inflamm. Bowel Dis. 2004;10:438–440. doi: 10.1097/00054725-200407000-00018. [DOI] [PubMed] [Google Scholar]
- 103.Puri AS, Desai D, Sood A, Sachdeva S. Infliximab-induced tuberculosis in patients with UC: Experience from India—a country with high prevalence of tuberculosis. J. Gastroenterol. Hepatol. 2017;32:1191–1194. doi: 10.1111/jgh.13669. [DOI] [PubMed] [Google Scholar]
- 104.Hilhorst, M., Shirai, T., Berry, G., Goronzy, J. J. & Weyand, C. M. T cell-macrophage interactions and granuloma formation in vasculitis. Front. Immunol. 5 (2014). [DOI] [PMC free article] [PubMed]
- 105.Jones T. Vaccination against bovine tb with Mycobacterium microti. Vet. Rec. 2010;166:214–215. doi: 10.1136/vr.c373. [DOI] [PubMed] [Google Scholar]
- 106.Mustafa T, Mogga SJ, Mfinanga SGM, Mørkve O, Sviland L. Significance of Fas and Fas ligand in tuberculous lymphadenitis. Immunology. 2005;114:255–262. doi: 10.1111/j.1365-2567.2004.02080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Perosso J, et al. Alteration of sFAS and sFAS ligand expression during canine visceral leishmaniosis. Vet. Parasitol. 2014;205:417–423. doi: 10.1016/j.vetpar.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Mustafa T, Phyu S, Nilsen R, Bjune G, Jonsson R. Increased expression of Fas ligand on Mycobacterium tuberculosis infected macrophages: A potential novel mechanism of immune evasion by Mycobacterium tuberculosis? Inflammation. 1999;23:507–521. doi: 10.1023/a:1020286305950. [DOI] [PubMed] [Google Scholar]
- 109.Zoi-Toli O, et al. Expression of Fas and Fas-ligand in primary cutaneous T-cell lymphoma (CTCL): Association between lack of Fas expression and aggressive types of CTCL. Br. J. Dermatol. 2000;143:313–319. doi: 10.1046/j.1365-2133.2000.03656.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


