Abstract
In this work, an efficient method for the immobilization of L-proline on magnetic nanoparticles was offered and evaluated as a recoverable magnetic nanocatalyst for synthesis of 2,4,6-triarylpyridines through one-pot three-component reaction of acetophenone, aryl aldehydes and ammonium acetate. This article is the first report of the catalytic application of L-proline functionalized magnetic nanoparticles in organic reactions as a magnetic nanocatalyst. This novel magnetic nanocatalyst proved to be effective and provided the products in high to excellent yield under solvent-free conditions. The structure of obtained nanoparticles was characterized by Fourier transform infrared spectrophotometry (FT-IR), field-emission scanning electron microscopy (FE-SEM), thermogravimetric analysis (TGA) and energy-dispersive X-ray spectroscopy (EDX). TGA result revealed that it is stable up to 200 °C for using as a catalyst in organic reactions. FE-SEM image of the synthesized nanocatalyst showed that it has nearly core-shell spherical shape and uniform size distribution with an average size about 80 nm. Moreover, the catalyst could be easily recovered by facile separation by magnetic forces and recycled for several times without significant loss of its catalytic activity. The benefits of this study are simplicity, nontoxicity, low cost, simple workup, and an environmentally benign nature.
Introduction
In the recent years, organocatalysts have attracted increasing interest in organic synthesis particularly from the green chemistry points of view1–4. Organocatalysts are metal-free small organic molecules that are able to function as efficient and selective catalysts for a wide range of organic reactions. Among them, L-proline and its derivatives have considered as powerful organocatalysts5. L-Proline has been successfully applied in many reactions, such as Robinson annulations, aldol reactions, Mannich reactions, Michael reactions, direct electrophilic α-aminations, Diels–Alder reactions, Baylis–Hillman reactions, aza-Morita-Baylis–Hillman reactions, α-selenenylation, oxidation, chlorination, and others6–11. Recently, immobilization and recycling of L-proline have received considerable concerns and there are several types of supports for the immobilizations of proline and its derivatives such as polymer, silica, ionic liquid, cyclodextrin, and magnetite12–16. Furthermore, magnetic nanoparticles (MNPs) have recently considered as a new type of catalyst support for organocatalysts due to their price, high dispersion, good stability, easy synthesis and functionalization method, high surface area and facile separation by using external magnetic fields17–49.
Multicomponent reactions (MCRs) as an important organic synthesis strategy are one-pot process in which three or more accessible substrate react to produce a more complex molecule that essentially includes most or all atoms of the starting materials50. Pyridines are nitrogen-containing heterocyclic compounds which have received significant attention because of their various medicinal, biological and pharmaceutical activities such as hypoglycemic activity, hypolipidemic activity, fungicidal activity, antimicrobial agent, dopamine transporter inhibitors and anti-inflammatory agents51–53. In addition, pyridines are used in supramolecular chemistry due to their π-stacking ability54. Among all of the pyridine derivatives, 2,4,6-triarylpyridines have received much interest by organic chemists due to their importance in medicinal chemistry. Because of significant features of these heterocyclic scaffolds, many efficient protocols were developed to more efficient synthesis of 2,4,6-triarylpyridines, for example solid-phase synthesis55, one-pot synthesis under microwave irradiation56, and solvent-free reaction between acetophenones, benzaldehydes, and ammonium acetate in the presence of various catalyst such as nanoparticles57, heteropolyacid58, HClO4–SiO259, and ionic liquid60. However, most of these methods suffer from drawbacks such as long reaction time, harsh reaction conditions, the use of volatile organic solvents, low yields, high catalyst loading, thermal conditions and expensive or difficult procedures of catalyst preparation. Therefore, design and development of mild and efficient methods with more environmentally-friendly catalysts is in of prime importance.
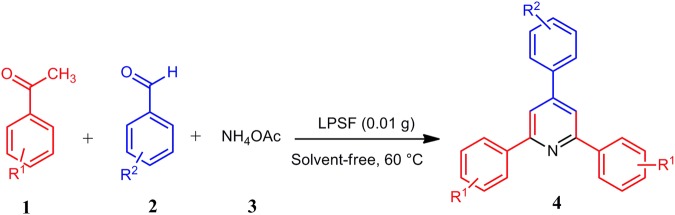
In continuation of our research on the introduction of recoverable catalysts in organic synthesis61–63, herein, we report a convenient and facile one pot synthesis of 2,4,6- triarylpyridines from acetophenones 1 (2 mmol), aromatic aldehydes 2 (1 mmol) and NH4OAc 3 (1.5 mmol) in the presence of Fe3O4\SiO2\propyltriethoxysilane\L-proline nanoparticles (LPSF) nanoparticle, as heterogeneous catalyst at 60 °C under solvent-free conditions (Fig. 1). To the best of our knowledge, this synthesized nanocatalyst was synthesized and applied as a novel, efficient and eco-friendly nanocatalyst in chemical reactions, especially in the synthesis of 2,4,6- triarylpyridines 4a–k.
Figure 1.
Synthesis of 2,4,6-triarylpyridines 4a–k in the presence of LPSF nanocatalyst.
Results and Discussion
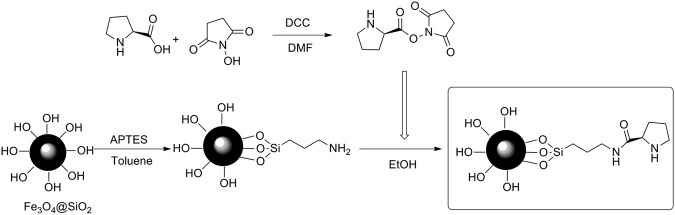
In this work, we have synthesized a novel nanomagnetic organocatalyst Fe3O4\SiO2\propyltriethoxysilane\L-proline (LPSF) and applied for the synthesis of 2,4,6-triarylpyridines. As can be seen in Fig. 2, LPSF nanocatalyst was prepared after several steps. At first, L-proline N-hydroxysuccinimide ester was prepared from L-proline and N-hydroxy succinimide (NHS) in the presence of N,N’-dicyclohexylcarbodiimide (DCC). After that, the synthesized Fe3O4\SiO2 was treated by (3-aminopropyl)triethoxysilane (APTES) to synthesize Fe3O4\SiO2\3-aminopropyltriethoxysilane. Finally, the synthesize Fe3O4\SiO2\3-aminopropyltriethoxysilane was treated by NHS-L-proline to synthesize the aimed LPSF magnetic nanocatalyst. Then, the characterizations of the prepared nanocomposite were investigated by several analyses methods which will be discussed further.
Figure 2.
Preparation of LPSF magnetic nanocatalyst.
Characterization of the prepared Fe3O4\SiO2\propyltriethoxysilane\L-proline (LPSF)
FT-IR spectra
As can be seen in Fig. 3, the FT-IR spectrum of the LPSF magnetic nanocatalyst can verify the preparation of the expected product. The bending vibration band at 585 cm−1 is indicated Fe–O vibration. In addition, the sharp bands appearing at 1084 and 1120 cm−1 are attributed to Si–O–Si asymmetric stretching vibration confirmatory to the SiO2 formation. The asymmetric and symmetric aromatic C–H stretching vibrations are appeared at 2920 and 2852 cm−1. Furthermore, the asymmetric stretching vibrations of O–H and N–H groups observed at 3401 cm−1. Furthermore, we have characterized the recycled LPSF magnetic nanocatalyst. As shown in Fig. S1, there was no considerable deformation or leaching after seven times reusing.
Figure 3.
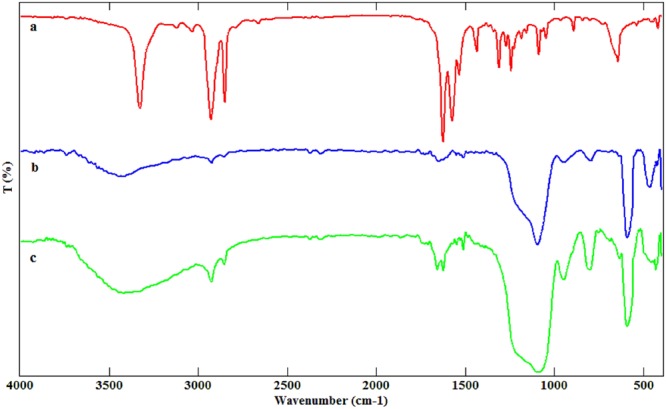
FT-IR spectra of: (a) NHS-L-proline, (b) Fe3O4\SiO2\3-aminopropyltriethoxysilane, (c) LPSF magnetic nanocatalyst.
FE-SEM images
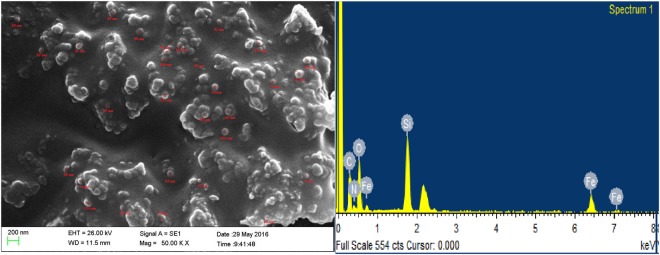
Field-emission scanning electron microscopy (FE-SEM) images are used to investigate the surface structure of the nanocomposite. As it is seen in Fig. 4a, FE-SEM images show that the LPSF nanocatalyst has nearly spherical shape and uniform size distribution with an average size of 80 ± 40 nm.
Figure 4.
(a) FE-SEM image and (b) EDX analysis of LPSF magnetic nanocatalyst.
EDX analysis
The result of the EDX analysis of LPSF magnetic nanocatalyst is illustrated in Fig. 4b. It confirms the presence of C, Fe, N, Si and O atoms elements in the nanocatalyst.
Thermal analysis
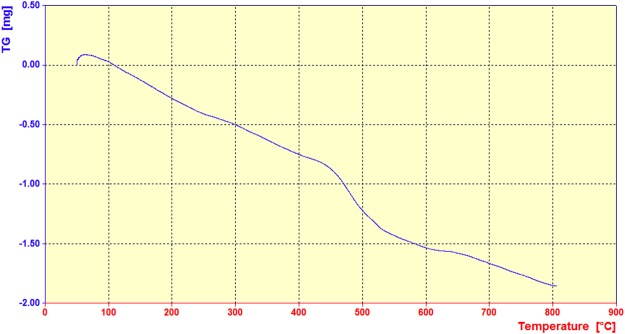
As can be seen in Fig. 5, the thermal behaviour of the prepared nanocomposite was evaluated by thermogravimetric analysis (TGA) over the temperature range of 20–800 °C at air atmosphere. The first weight loss between 0–100 °C was due to evaporation of adsorbed water in the sample. After that, the weight loss from 200 to 600 is related to the destruction of the organic compounds.
Figure 5.
TG curve of LPSF nanocatalyst.
Catalytic application of Fe3O4\SiO2\propyltriethoxysilane\L-proline (LPSF) in the synthesis of 2,4,6-triarulpyridines
The catalytic activity of the synthesized magnetic nanocatalyst was investigated in a one-pot three-component synthesis of 2,4,6-triarylpyridine derivatives. Initially, to optimize the reaction conditions, we evaluated the reaction of acetophenone, 4-chloro-benzaldehyde and ammonium acetate in the presence of different catalytic amounts of LPSF magnetic nanocatalyst at 60 °C under solvent-free conditions, as a model reaction to yield 4b. It was observed that 0.01 g of catalyst was enough to catalyze the reaction to produce high yields of products (Table S1 in Supplementary Information file). To study of the solvent effect and comparing the efficiency of ethanol, the model reaction was performed in several solvents with different polarities in the presence of LPSF magnetic nanocatalyst. As can be seen in Table S1 (Entries 6–9), the efficiency and the yield of the model reaction under solvent-free conditions were higher than those obtained in other solvents.
In addition, a comparison was done between the present work and others earlier reports for the synthesis of 4b. The results summarized in Table S2 in Supplementary Information file clearly demonstrate the superiority of the present work in saving energy, high yields of the products and the reusability of the nanocatalyst.
Finally, in order to examine the generality of this nanocatalyst for the synthesis of 2,4,6-triarylpyridine derivatives, a number of aromatic aldehydes and acetophenones with electron-withdrawing and electron-releasing substitutions, were employed and a variety of products were synthesized under the optimized conditions the results are summarized in Table 1.
Table 1.
Synthesis of 2,4,6-triarylpyridines 4a–k by using LPSF magnetic nanocatalyst.
| Entry | R1 | R2 | Product | Time (min) | Yielda (%) | Mp (°C) | |
|---|---|---|---|---|---|---|---|
| Observed | Literature | ||||||
| 1 | H | H | 4a | 60 | 88 | 134–137 | 135–13764 |
| 2 | H | 4-Cl | 4b | 60 | 94 | 121–123 | 123–12465 |
| 3 | H | 4-NO2 | 4c | 60 | 91 | 193–194 | 196–19864 |
| 4 | H | 4-OH | 4d | 60 | 88 | 199–200 | 196–19865 |
| 5 | H | 4-Me | 4e | 60 | 83 | 117–120 | 119–12056 |
| 6 | H | 4-Br | 4f | 60 | 90 | 162–163 | 165–16665 |
| 7 | H | 4-OMe | 4g | 60 | 83 | 98–100 | 97–9866 |
| 8 | 4-Cl | H | 4h | 60 | 84 | 180–181 | 175–17856 |
| 9 | 4-Cl | 4-OMe | 4i | 60 | 88 | 188–189 | 190–19167 |
| 10 | 4-Me | 4-Cl | 4j | 60 | 89 | 162–164 | 159–16064 |
| 11 | H | furan-2-carbaldehyde | 4k | 60 | 75 | 110–112 | 112–11568 |
aIsolated yield.
Mechanistic evaluation
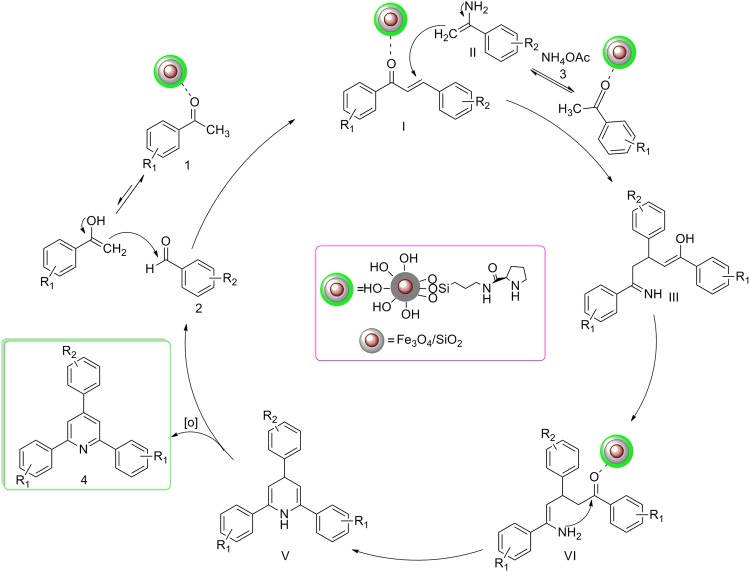
The plausible mechanism for the formation of 2,4,6-triarylpyridines is shown in Fig. 6. The first step is formation of an intermediate I, formed via Aldol condensation of an aromatic aldehyde 2 and acetophenones 1 in the presence of LPSF magnetic nanocatalyst. After that, enamine II is formed via condensation of the other molecule of acetophenones and ammonium acetate III. In continuation of the reaction, a Michael addition is occurred between intermediate I and enamine II to afford intermediate III. Then, cyclization of intermediate IV leads to produce dihydropyridine V. Finally, oxidation takes place in the presence of LPSF magnetic nanocatalyst to afford final product 4.
Figure 6.
Plausible mechanism for the formation of 2,4,6-triarylpyridines (4a–k).
Recyclability of LPSF magnetic nanocatalyst
In order to investigate the possibility of several recycling runs for LPSF magnetic nanocatalyst, the solid catalyst was separated from the reaction mixture by using an external magnet. It was washed two times with ethanol and water, dried and reused in subsequent reactions. The catalyst can be reused seven times without any significant decrease in yield of the products (Fig. S1). Finally, as can be seen in Figs S2 and S3, we have characterized the recycled nanocatalyst by FT-IR spectroscopy and FE-SEM image which showed suitable retention of its structure and morphology.
Experimental
General
All the solvents, chemicals and reagents were purchased from Merck, Sigma and Aldrich. Melting points were measured on an Electro thermal 9100 apparatus and are uncorrected. Fourier transforms infrared spectroscopy (FT-IR) spectra were recorded on a Shimadzu IR-470 spectrometer by the method of KBr pellet. 1H and 13C NMR spectra were recorded on a Bruker DRX-300 Avance spectrometer at 500 and 125 MHz, respectively. Field-emission scanning electron micrograph (FE-SEM) images were taken with Sigma-Zeiss microscope with attached camera. Elemental analysis of the nanocatalyst was carried out by energy-dispersive X-ray (EDX) analysis recorded Numerix DXP-X10P. Thermal analysis was taken by Bahr-STA 504 instrument under argon atmosphere.
Preparation of Fe3O4\SiO2\propyltriethoxysilane\L-proline nanoparticles
Preparation of compound L-proline N-hydroxysuccinimide ester
In the first step, 0.24 g L-proline and 0.45 g N-hydroxy succinimide (NHS) were mixed in 35 mL of DMF and vigorously stirred under 35 °C until the two components were completely dissolved. Then, 0.75 g N,N’-dicyclohexylcarbodiimide (DCC) was added gradually and the reaction mixture were stirred for 24 h. After that, white precipitate L-proline N-hydroxysuccinimide ester (NHS-L-proline) was separated after drying solvent by rotary and washed with diethyl ether.
Preparation of Fe3O4 nanoparticles
The Fe3O4 nanoparticles were synthesized via the coprecipitation of FeCl3·6H2O and FeCl2·4H2O at a molar ratio of 2: 1 in the presence of ammonia. Typically, 2.82 g of FeCl3·6H2O and 1.72 g of FeCl2·4H2O were mixed in 80 mL of distilled water and vigorously stirred at 80 °C with a mechanical stirrer. After the temperature had reached 80 °C, 10 mL ammonia was added drop wise to the mixture. The mixture was then stirred for another 40 min and then cooled to room temperature. The black precipitate was collected using an external magnet and washed several times with ethanol and distilled water. The black product was dried at 80 °C in an oven.
Preparation of Fe3O4\SiO2 nanoparticles
Initially, 45 mg of Fe3O4 nanoparticles were dispersed in 16 mL of deionized water by using an ultrasonic water bath, after that 2 mL of aqueous ammonia solution (25 wt%) and 80 mL of ethanol were added to reaction mixture. Next, 0.8 mL of tetraethyl orthosilicate (TEOS) was added drop wise into the Fe3O4 nanoparticle solution under vigorous stirring at room temperature. The mixture was then stirred for 24 h at room temperature. The products were separated by an external magnet and washed several times with distilled water. The final product was collected and dried at 50 °C.
Preparation of Fe3O4\SiO2\3-aminopropyltriethoxysilane nanoparticles
At first, 1 g obtained Fe3O4@SiO2 nanocomposite was added in 5 mL of toluene and ultrasonicated for 10 min. Then, 2 mL (3-aminopropyl)triethoxysilane (APTES) was added to this solution and refluxed for 18 h. The obtained amino-substituted nanocomposites were separated by an external magnet and washed two times with toluene and washed. After that, collected nanocomposite was extracted and washed in toluene using a Soxhlet apparatus in toluene for removing unreacted starting materials. The precipitation was dried at 60 °C for 12 h.
Preparation of Fe3O4\SiO2\propyltriethoxysilane\L-proline nanoparticles (LPSF)
The obtained Fe3O4@SiO2@OSi(CH2)3NH2 (1 g) was dispersed in with 15 mL ethanol. Then NHS-L-proline (1 g) was added into the above solution under vigorous stirring. The obtained mixture was stirred for 6 h at room temperature. After completion of the reaction, the products were separated by an external magnet and washed several times with ethanol. The precipitation was dried at 50 °C for 12 h.
General procedure for preparing 2,4,6-triarylpyridines
A mixture of acetophenones (2 mmol), aromatic aldehyde (1.0 mmol), ammonium acetate (1.5 mmol) and 0.01 g LPSF nanocatalyst was stirred at 60 °C under solvent-free conditions for an appropriate time. The completion of the reaction was monitored by thin layer chromatography (TLC). After completion of the reaction, hot ethanol was added to the mixture added to the mixture and the catalyst was separated easily by an external magnet. The pure products were obtained from the reaction mixture by recrystallization from hot EtOH and no more purification was required. All the product were known compounds which were identified by characterization of their melting point with those authentic literature samples and also in some cases their 1H and 13C NMR spectral data.
Selected Spectral data
4-(4-Nitrophenyl)-2,6-diphenylpyridine (4c): White solid; 1H NMR (500 MHz, CDCl3): δH (ppm) = 7.47–7.57 (8 H, m, H-Ar), 7.70 (2 H, d, J = 8.0 Hz, H-Ar), 7.86 (2 H, s, H-Ar), 8.23 (4 H, d, J = 6.3 Hz, H-Ar); 13C NMR (125 MHz, CDCl3): δC (ppm) = 117.2, 127.6, 128.8, 129.2, 129.6, 129.8, 135.6, 137.9, 139.8, 149.4, 158.1.
Conclusions
In summary, LPSF magnetic nanocatalyst was prepared and used as a novel, green, magnetically recyclable, environmentally-friendly and efficient composite nanocatalyst for the synthesis of chemically and biologically important 2,4,6-triarylpyridines by a simple, clean, eco-friendly and inexpensive method. The novel magnetic nanocatalyst can be easily separated by an external magnet and recycled for several times without any significant loss of activity. We used FT-IR, EDX, TGA and FE-SEM to confirm that the nanocomposite was formed, and 1H and 13C NMR analyses were performed for the confirmation of the synthesized products. TGA result revealed that it is stable up to 200 °C for using as a catalyst in organic reactions. FE-SEM image of the synthesized nanocatalyst showed that it has nearly core-shell spherical shape and uniform size distribution with an average size about 80 nm. This study is the first report on design, synthesis, functionalization and characterization of the novel magnetic nanocomposite and also performance as a heterogeneous catalyst in organic reactions.
Electronic supplementary material
Acknowledgements
The authors gratefully acknowledge the partial support from the Research Council of the Iran University of Science and Technology.
Author Contributions
A.M. have designed the study, participated in discussing results and revised the manuscript. R.F.-H. have designed, carried out the literature study, performed the assay, conducted the optimization, purification of compounds and prepared the manuscript. Furthermore, performed the related analyses. All authors read and approved the final manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-35676-x.
References
- 1.Guillena G, Ramón DJ. Enantioselective α-heterofunctionalisation of carbonyl compounds: organocatalysis is the simplest approach. Tetrahedron Asymm. 2006;17:1465. doi: 10.1016/j.tetasy.2006.05.020. [DOI] [Google Scholar]
- 2.Dalko PI, Moisan L. In the golden age of organocatalysis. Angew. Chem. Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]
- 3.Duthaler RO. Proline‐Catalyzed Asymmetric α‐Amination of Aldehydes and Ketones—An Astonishingly Simple Access to Optically Active α‐Hydrazino Carbonyl Compounds. Angew. Chem. Int. Ed. 2003;42:975. doi: 10.1002/anie.200390283. [DOI] [PubMed] [Google Scholar]
- 4.Dondoni A, Massi A. Asymmetric organocatalysis: from infancy to adolescence. Angew. Chem. Int. Ed. 2008;47:4638. doi: 10.1002/anie.200704684. [DOI] [PubMed] [Google Scholar]
- 5.List B. Proline-catalyzed asymmetric reactions. Tetrahedron. 2002;58:5573. doi: 10.1016/S0040-4020(02)00516-1. [DOI] [Google Scholar]
- 6.List B, Lerner RA, Barbas CF., III Proline-catalyzed direct asymmetric aldol reactions. J. Am. Chem. Soc. 2000;122:2395. doi: 10.1021/ja994280y. [DOI] [Google Scholar]
- 7.Sakthivel K, Notz W, Bui T, Barbas CF., III Amino acid catalyzed direct asymmetric aldol reactions: a bioorganic approach to catalytic asymmetric carbon− carbon bond-forming reactions. J. Am. Chem. Soc. 2001;123:5260. doi: 10.1021/ja010037z. [DOI] [PubMed] [Google Scholar]
- 8.Grondal C, Enders D. Direct asymmetric organocatalytic de novo synthesis of carbohydrates. Tetrahedron. 2006;62:329. doi: 10.1016/j.tet.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 9.Notz W, et al. The direct organocatalytic asymmetric Mannich reaction: unmodified aldehydes as nucleophiles. J. Org. Chem. 2003;68:9624. doi: 10.1021/jo0347359. [DOI] [PubMed] [Google Scholar]
- 10.Chowdari NS, Ramachary DB, Barbas CF., III Organocatalysis in ionic liquids: highly efficient L-proline-catalyzed direct asymmetric Mannich reactions involving ketone and aldehyde nucleophiles. Synlett. 2003;12:1906. [Google Scholar]
- 11.List B, Pojarliev P, Martin HJ. Efficient proline-catalyzed Michael additions of unmodified ketones to nitro olefins. Org. Lett. 2001;3:2423. doi: 10.1021/ol015799d. [DOI] [PubMed] [Google Scholar]
- 12.Zhong GA. Facile and Rapid Route to Highly Enantiopure 1, 2‐Diols by Novel Catalytic Asymmetric α‐Aminoxylation of Aldehydes. Angew. Chem. Int. Ed. 2003;42:4247. doi: 10.1002/anie.200352097. [DOI] [PubMed] [Google Scholar]
- 13.Gruttadauria M, Giacalone F, Marculescu AM, Notoa R. Novel Prolinamide‐Supported Polystyrene as Highly Stereoselective and Recyclable Organocatalyst for the Aldol Reaction. Adv. Synth. Catal. 2008;350:1397. doi: 10.1002/adsc.200800090. [DOI] [Google Scholar]
- 14.Gruttadauria M, Salvo AMP, Giacalone F, Agrigento P, Noto R. Enhanced activity and stereoselectivity of polystyrene‐supported proline‐based organic catalysts for direct asymmetric aldol reaction in water. Eur. J. Org. Chem. 2009;31:5437. doi: 10.1002/ejoc.200900829. [DOI] [Google Scholar]
- 15.Calogero S, et al. Supported l-proline on zirconium phosphates methyl and/or phenyl phosphonates as heterogeneous organocatalysts for direct asymmetric aldol addition. J. Catal. 2011;282:112. doi: 10.1016/j.jcat.2011.06.004. [DOI] [Google Scholar]
- 16.Doyagüez EG, Calderon F, Sanchez F, Fernndez-Mayoralas A. Asymmetric aldol reaction catalyzed by a heterogenized proline on a mesoporous support. The role of the nature of solvents. J. Org. Chem. 2007;72:9353. doi: 10.1021/jo070992s. [DOI] [PubMed] [Google Scholar]
- 17.Sharma RK, et al. An efficient copper-based magnetic nanocatalyst for the fixation of carbon dioxide at atmospheric pressure. Sci. Rep. 2018;8:1901. doi: 10.1038/s41598-018-19551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang J, Zhang X, Armstrong DW. Highly efficient asymmetric direct stoichiometric aldol reactions on/in water. Angew. Chem. Int. Ed. 2007;46:9073. doi: 10.1002/anie.200703606. [DOI] [PubMed] [Google Scholar]
- 19.Mu B, Tang J, Zhang L, Wang A. Facile fabrication of superparamagnetic graphene/polyaniline/Fe3O4 nanocomposites for fast magnetic separation and efficient removal of dye. Sci. Rep. 2017;7:5347. doi: 10.1038/s41598-017-05755-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Liu YH, Shang ZR, Hu HC, Zhang ZH. Supported molybdenum on graphene oxide/Fe3O4: An efficient, magnetically separable catalyst for one-pot construction of spiro-oxindole dihydropyridines in deep eutectic solvent under microwave irradiation. Catal. Commun. 2017;88:39. doi: 10.1016/j.catcom.2016.09.028. [DOI] [Google Scholar]
- 21.Zhang M, et al. Magnetically separable graphene oxide anchored sulfonic acid: a novel, highly efficient and recyclable catalyst for one-pot synthesis of 3, 6-di (pyridin-3-yl)-1 H-pyrazolo[3,4-b]pyridine-5-carbonitriles in deep eutectic solvent under microwave irradiation. RSC Adv. 2016;6:106160. doi: 10.1039/C6RA19579B. [DOI] [Google Scholar]
- 22.Maleki A, Azadegan S. Preparation and characterization of silica-supported magnetic nanocatalyst and application in the synthesis of 2-amino-4H-chromene-3-carbonitrile derivatives. Inorg. Nano-Met. Chem. 2017;47:917. doi: 10.1080/24701556.2016.1241266. [DOI] [Google Scholar]
- 23.Gupta VK, Jain R, Nayak A, Agarwal S, Shrivastava M. Removal of the hazardous dye—tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C. 2011;31:1062. doi: 10.1016/j.msec.2011.03.006. [DOI] [Google Scholar]
- 24.Maleki A, Azadegan S. Amine-functionalized silica-supported magnetic nanoparticles: preparation, characterization and catalytic performance in the chromene synthesis. J. Inorg. Organomet. Polym. 2017;27:714. doi: 10.1007/s10904-017-0514-z. [DOI] [Google Scholar]
- 25.Maleki A, Ghassemi M, Firouzi-Haji R. Green multicomponent synthesis of four different classes of six-membered N-containing and O-containing heterocycles catalyzed by an efficient chitosan-based magnetic bionanocomposite. Pure Appl. Chem. 2018;90:387. doi: 10.1515/pac-2017-0702. [DOI] [Google Scholar]
- 26.Maleki A, Movahed H, Ravaghi P. Magnetic cellulose/Ag as a novel eco-friendly nanobiocomposite to catalyze synthesis of chromene-linked nicotinonitriles. Carbohydr. Polym. 2017;156:259. doi: 10.1016/j.carbpol.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Saleh TA, Gupta VK. Processing methods, characteristics and adsorption behavior of tire derived carbons: a review. Adv. Colloid Interface Sci. 2014;211:93. doi: 10.1016/j.cis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gupta VK, Kumar R, Nayak A, Saleh TA, Barakat MA. Adsorptive removal of dyes from aqueous solution onto carbon nanotubes: a review. Adv. Colloid Interface Sci. 2013;193:24. doi: 10.1016/j.cis.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Maleki A, Rahimi J, Demchuk OM, Wilczewska AZ, Jasiński R. Green in water sonochemical synthesis of tetrazolopyrimidine derivatives by a novel core-shell magnetic nanostructure catalyst. Ultrason. Sonochem. 2018;43:262. doi: 10.1016/j.ultsonch.2017.12.047. [DOI] [PubMed] [Google Scholar]
- 30.Maleki A, Movahed H, Ravaghi P, Kari T. Facile in situ synthesis and characterization of a novel PANI/Fe3O4/Ag nanocomposite and investigation of catalytic applications. RSC Adv. 2016;6:98777. doi: 10.1039/C6RA18185F. [DOI] [Google Scholar]
- 31.Rajendran S, et al. Ce3+-ion-induced visible-light photocatalytic degradation and electrochemical activity of ZnO/CeO2 nanocomposite. Sci. Rep. 2016;6:31641. doi: 10.1038/srep31641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saravanan R, Joicy S, Gupta VK, Narayanan V, Stephen A. Visible light induced degradation of methylene blue using CeO2/V2O5 and CeO2/CuO catalysts. Mater. Sci. Engin. C. 2013;33:4725. doi: 10.1016/j.msec.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Maleki A, Aghaei M. Ultrasonic assisted synergetic green synthesis of polycyclic imidazo (thiazolo) pyrimidines by using Fe3O4@clay core-shell. Ultrason. Sonochem. 2017;38:585. doi: 10.1016/j.ultsonch.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 34.Gupta VK, Saleh TA. Sorption of pollutants by porouscarbon, carbon nanotubes and fullerene-An overview. Environ. Sci. Poll. Res. 2013;20:2828. doi: 10.1007/s11356-013-1524-1. [DOI] [PubMed] [Google Scholar]
- 35.Maleki A. Fe3O4/SiO2 nanoparticles: an efficient and magnetically recoverable nanocatalyst for the one-pot multicomponent synthesis of diazepines. Tetrahedron. 2012;68:7827. doi: 10.1016/j.tet.2012.07.034. [DOI] [Google Scholar]
- 36.Maleki A. One-pot multicomponent synthesis of diazepine derivatives using terminal alkynes in the presence of silica-supported superparamagnetic iron oxide nanoparticles. Tetrahedron Lett. 2013;54:2055. doi: 10.1016/j.tetlet.2013.01.123. [DOI] [Google Scholar]
- 37.Maleki A. One-pot three-component synthesis of pyrido[2′,1′:2,3]imidazo[4,5-c]isoquinolines using Fe3O4@SiO2 –OSO3H as an efficient heterogeneous nanocatalyst. RSC Adv. 2014;4:64169. doi: 10.1039/C4RA10856F. [DOI] [Google Scholar]
- 38.Maleki A. Green oxidation protocol: Selective conversions of alcohols and alkenes to aldehydes, ketones and epoxides by using a new multiwall carbon nanotube-based hybrid nanocatalyst via ultrasound irradiation. Ultrason. Sonochem. 2018;40:460. doi: 10.1016/j.ultsonch.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Saleh TA, Gupta VK. Synthesis and characterization of alumina nano-particles polyamide membrane with enhanced flux rejection performance. Sep. Pur. Tech. 2012;89:245. doi: 10.1016/j.seppur.2012.01.039. [DOI] [Google Scholar]
- 40.Maleki A. Synthesis of imidazo[1,2-a]pyridines using Fe3O4@SiO as an efficient nanomagnetic catalyst via a one-pot multicomponent reaction. Helv. Chim. Acta. 2014;97:587. doi: 10.1002/hlca.201300244. [DOI] [Google Scholar]
- 41.Saravanan R, et al. ZnO/Ag nanocomposite: an efficient catalyst for degradation studies of textile effluents under visible light. Mater. Sci. Eng. C. 2013;33:2235. doi: 10.1016/j.msec.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 42.Maleki A, Kari T, Aghaei M. Fe3O4@SiO2@TiO2-OSO3H: an efficient hierarchical nanocatalyst for the organic quinazolines syntheses. J. Porous Mater. 2017;24:1481. doi: 10.1007/s10934-017-0388-z. [DOI] [Google Scholar]
- 43.Maleki A, Ghalavand R, Firouzi-Haji R. A novel and eco-friendly o-phenylendiamine stabilized on silicacoated magnetic nanocatalyst for the synthesis of indenoquinoline derivatives under ultrasonicassisted solventfree conditions. Iran. J. Catal. 2018;8:221. [Google Scholar]
- 44.Saravanan R, et al. ZnO/Ag/Mn2O3 nanocomposite for visible light-induced industrial textile effluent degradation, uric acid and ascorbic acid sensing and antimicrobial activity. RSC Adv. 2015;5:34645. doi: 10.1039/C5RA02557E. [DOI] [Google Scholar]
- 45.Maleki A, Rahimi J. Synthesis of dihydroquinazolinone and octahydroquinazolinone and benzimidazoloquinazolinone derivatives catalyzed by an efficient magnetically recoverable GO-based nanocomposite. J. Porous Mater. 2018;25:1789. doi: 10.1007/s10934-018-0592-5. [DOI] [Google Scholar]
- 46.Ghaedi M, et al. Modeling of competitive ultrasonic assisted removal of the dyes–Methylene blue and Safranin-O using Fe O nanoparticles. Chem. Eng. J. 2015;268:28. doi: 10.1016/j.cej.2014.12.090. [DOI] [Google Scholar]
- 47.Maleki A, Kari T. Novel leaking-free, green, double core/shell, palladium-loaded magnetic heterogeneous nanocatalyst for selective aerobic oxidation. Catal. Lett. 2018;148:2929. doi: 10.1007/s10562-018-2492-3. [DOI] [Google Scholar]
- 48.Asfaram A, Ghaedi M, Agarwal S, Tyagi I, Gupta VK. Removal of basic dye Auramine-O by ZnS: Cu nanoparticles loaded on activated carbon: optimization of parameters using response surface methodology with central composite design. RSC Adv. 2015;5:18438. doi: 10.1039/C4RA15637D. [DOI] [Google Scholar]
- 49.Gupta VK, Atar N, Yola ML, Üstündağ Z, Uzun L. A novel magnetic Fe@Au core–shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2014;48:210. doi: 10.1016/j.watres.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 50.Boukis AC, Reiter K, Frölich M, Hofheinz D, Meier MA. Multicomponent reactions provide key molecules for secret communication. Nat. Commun. 2018;9:1439. doi: 10.1038/s41467-018-03784-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim BY, et al. Synthesis and biological activity of novel substituted pyridines and purines containing 2, 4- thiazolidinedione. Eur. J. Med. Chem. 2004;1:433. doi: 10.1016/j.ejmech.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 52.Tian L, Song J, Wang J, Liu B. Synthesis and bioactivity of N-cyclopropanecarboxyl-N′-pyridin-2-yl thiourea derivatives and related fused ring compounds. Chin. Chem. Lett. 2009;20:288. doi: 10.1016/j.cclet.2008.11.027. [DOI] [Google Scholar]
- 53.Allen RD, Johnston GAR. Synthetic analogs for the study of GABA as a neurotransmitter. Med. Res. Rev. 1983;3:91. doi: 10.1002/med.2610030202. [DOI] [PubMed] [Google Scholar]
- 54.Constable EC, et al. Development of supramolecular structure through alkylation of pendant pyridyl functionality. J. Chem. Soc., Dalton Trans. 2000;13:2219. doi: 10.1039/b000940g. [DOI] [Google Scholar]
- 55.Montazeri N, Mahjoob S. Highly efficient and easy synthesis of 2,4,6-triarylpyridines catalyzed by pentafluorophenylammonium triflate (PFPAT) as a new recyclable solid acid catalyst in solvent-free conditions. Chin. Chem. Lett. 2012;23:419. doi: 10.1016/j.cclet.2012.01.035. [DOI] [Google Scholar]
- 56.Safari J, Gandomi-Ravandi S, Borujeni M. Green and solvent-free procedure for microwave-assisted synthesis of 2,4,6-triarylpyridines catalysed using MgAl2O4 nanocrystals. Chem. Sci. 2013;125:1063. doi: 10.1007/s12039-013-0477-8. [DOI] [Google Scholar]
- 57.Safari J, Zarnegar Z, Borujeni M. Mesoporous nanocrystalline MgAl O : A new heterogeneous catalyst for the synthesis of 2,4,6-triarylpyridines under solvent-free conditions. Chem. Pap. 2013;67:688. doi: 10.2478/s11696-013-0361-5. [DOI] [Google Scholar]
- 58.Heravi MM, Bakhtiari K, Daroogheha Z, Bamoharram FF. An efficient synthesis of 2,4,6-triarylpyridines catalyzed by heteropolyacid under solvent-free conditions. Catal. Commun. 2007;8:1991. doi: 10.1016/j.catcom.2007.03.028. [DOI] [Google Scholar]
- 59.Nagarapu L, Peddiraju AR, Apuri S. HClO4–SiO2 as a novel and recyclable catalyst for the synthesis of 2,4,6-triarylpyridines under solvent-free conditions. Catal. Commun. 2007;8:1973. doi: 10.1016/j.catcom.2007.08.003. [DOI] [Google Scholar]
- 60.Davoodnia A, Bakavoli M, Moloudi R, Tavakoli-Houseini N, Khashi M. Highly efficient, one-pot, solvent-free synthesis of 2,4,6-triarylpyridines using a Brønsted-acidic ionic liquid as reusable catalyst. Monatsh. Chem. 2010;141:867. doi: 10.1007/s00706-010-0329-x. [DOI] [Google Scholar]
- 61.Maleki A, Ghamari N, Kamalzare M. Chitosan-supported Fe3O4 nanoparticles: a magnetically recyclable heterogeneous nanocatalyst for the syntheses of multifunctional benzimidazoles and benzodiazepines. RSC Adv. 2014;4:9416. doi: 10.1039/c3ra47366j. [DOI] [Google Scholar]
- 62.Maleki A, Haji RF, Ghassemi M, Ghafuri H. Preparation and application of a magnetic organic-inorganic hybrid nanocatalyst for the synthesis of α-aminonitriles. J. Chem. Sci. 2017;129:457. doi: 10.1007/s12039-017-1253-y. [DOI] [Google Scholar]
- 63.Maleki A, Hajizadeh Z, Firouzi-Haji R. Eco-friendly functionalization of magnetic halloysite nanotube with SO3H for synthesis of dihydropyrimidinones. Microporous Mesoporous Mater. 2018;259:46. doi: 10.1016/j.micromeso.2017.09.034. [DOI] [Google Scholar]
- 64.Kamali M. One-pot, solvent-free, and efficient synthesis of 2,4,6-triarylpyridines using CoCl2. 6H2O as a recyclable catalyst. Cogent. Chem. 2016;2:1171123. doi: 10.1080/23312009.2016.1171123. [DOI] [Google Scholar]
- 65.Tabrizian E, et al. One-pot, solvent-free and efficient synthesis of 2,4,6-triarylpyridines catalyzed by nano-titania-supported sulfonic acid as a novel heterogeneous nanocatalyst. Chin. Chem. Lett. 2015;26:1278. doi: 10.1016/j.cclet.2015.06.013. [DOI] [Google Scholar]
- 66.Wang M, Yang Z, Song Z, Wang Q. Three‐component one‐pot synthesis of 2,4,6‐triarylpyridines without catalyst and solvent. J. Heterocycl. Chem. 2015;52:907. doi: 10.1002/jhet.2132. [DOI] [Google Scholar]
- 67.Zarnegar Z, Safari J, Borjian-borujeni M. Ultrasound-mediated synthesis of 2,4,6-triaryl-pyridines using MgAl2O4 nanostructures. Chem. Heterocycl. Compd. 2015;50:1863. doi: 10.1007/s10593-015-1638-0. [DOI] [Google Scholar]
- 68.Han J, et al. One‐pot synthesis of benzene and pyridine derivatives via copper‐catalyzed coupling reactions. Adv. Synth. Catal. 2017;359:2676. doi: 10.1002/adsc.201700053. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.