Abstract
Background
The disadvantage of using total serum prostatic specific antigen (PSA) test for detection of prostate cancer is that it has a low specificity. The low specificity of total PSA (tPSA) test leads to unnecessary prostate biopsies. In this prospective study, we assessed the serum tPSA, free PSA, p2PSA, and the Prostate Health Index (PHI) in the detection of prostate cancer in men with a tPSA of 4–10 ng/mL and a negative digital rectal examination (DRE).
Materials and methods
101 male outpatients with a serum PSA of 4–10 ng/mL and nonsuspicious DRE for prostate cancer who underwent first transrectal ultrasound with a prostate biopsy were recruited. A blood sample to enable tPSA, free PSA, and p2PSA levels to be calculated was drawn before the prostate biopsy. The diagnosis and detection of high-grade cancer are correlated with the blood sample.
Results
Sixteen patients were positive for prostate cancer. All had significantly higher serum 2pPSA and PHI levels than patients with no cancer. A PHI level at 90% sensitivity (cutoff of 34.14) demonstrated a higher area under the receiver operating characteristic curve and more specificity in diagnosis and detection of high-grade prostate cancer than other tests.
Conclusions
The PHI in men with a PSA level of 4–10 ng/mL with negative DRE increased specificity in the detection of prostate cancer. This test is useful in discriminating between patients with or without cancer and also enables the detection of high-grade cancer avoiding unnecessary biopsies.
Keywords: PHI, PSA, Prostate biopsy
1. Introduction
The incidence of prostate cancer in Thailand has increased from the 6th to the 3rd most common cancer in Thai males in 10 years.1, 2 The incidence is lower, but the mortality to incidence ratio is higher in several countries in Asia than Caucasian ethnic groups.1 This means that early detection of prostate cancer is very important in Asian countries.
Prostatic specific antigen (PSA) testing in conjunction with digital rectal examination (DRE) is a screening tool in diagnosis of prostate cancer. PSA-based screening demonstrated 21% mortality risk reduction but leads to over diagnosis and over treatment.3 The indication for transrectal ultrasound with biopsy for the detection of prostate cancer is a total serum PSA level ≥4 ng/mL or abnormal findings from a DRE. The disadvantage of the PSA test is that although it has a high sensitivity, its specificity is low regarding the detection of prostate cancer as it is not prostate cancer specific.3, 4 An elevation of serum tPSA can also show a correlation with other causes, including infection, and prostate manipulation. This low specificity of the tPSA test leads to unnecessary prostate biopsies. It is recognized that prostate biopsies should be carried out only if necessary due to the significant morbidity after a prostate biopsy, especially the case of septic complications. Unnecessary prostate biopsies can also lead to over diagnosis, overtreatment, anxiety, and costs.
PSA derivatives such as free PSA (f PSA), PSA density, and PSA velocity have been used to increase the specificity. The p2PSA is the most specific isoform existing in the serum of men with prostate cancer.5, 6 The Prostate Heath Index (Beckman Coulter Inc, CA, USA) is the formulation of three biomarkers (p2PSA, tPSA, and f PSA). Systematic review and literature review demonstrated that the result of this calculation is the most reliable for the detection of a specific cancer.4, 7, 8, 9 The PHI is also useful in active surveillance of disease progression,10 prediction of early biochemical recurrence after radical prostatectomy,11, 12 and prediction of high-grade cancer.13 We carried out a prospective study into the rate of detection and aggressiveness of prostate cancer in Thai patients with a PSA between 4–10 ng/mL with a normal DRE.
2. Patients and methods
2.1. Patients
We prospectively evaluated 101 male outpatients who underwent first transrectal ultrasound with a prostate biopsy. All patients had a serum PSA of 4–10 ng/mL and nonsuspicious DRE of prostate cancer. All patients had no acute prostate or urinary tract infection, were not undergoing any treatment with a 5α-reductase inhibitor, and had had no previous treatment involving transurethral resection of the prostate and no prior androgen replacement therapy.
2.2. Study outcome
The primary outcome is the diagnosis of prostate cancer based on transrectal ultrasound with a prostate biopsy. The secondary outcome is the diagnosis of high-grade prostate cancer [Gleason score (GS) ≥ 7] based on the biopsy results.
The Institution Review Board of the Faculty of Medicine, Chiang Mai University approved this study.
2.3. Methods
In each patient, a blood sample for analysis of tPSA, f PSA, and p2PSA levels was drawn before the prostate biopsy. The blood sample was collected in Becton Dickinson SST II tubes, was centrifuged at 2,000 g for 4 minutes within 3 hours of collection, and was subsequently stored at –70°C until analysis. A transrectal ultrasound–guided biopsy was performed with 10–12 biopsy cores. The serum PHI was calculated using the formula: (p2PSA/f PSA @/PSA).
The prostate specimens were prepared and evaluated by experienced pathologists. The pathologists were blinded to the results of the laboratory tests. Identification and grading of any adenocarcinoma was carried out according to the definition included in the 2005 Consensus Conference of International Society of Urological Pathology.
2.4. Statistical analysis
The area under the receiver operating characteristic (AuROC) curve was used to estimate tPSA, f PSA, p2PSA, and the PHI using tPSA level as the standard. The sensitivity and specificity of each variable were calculated.
3. Results
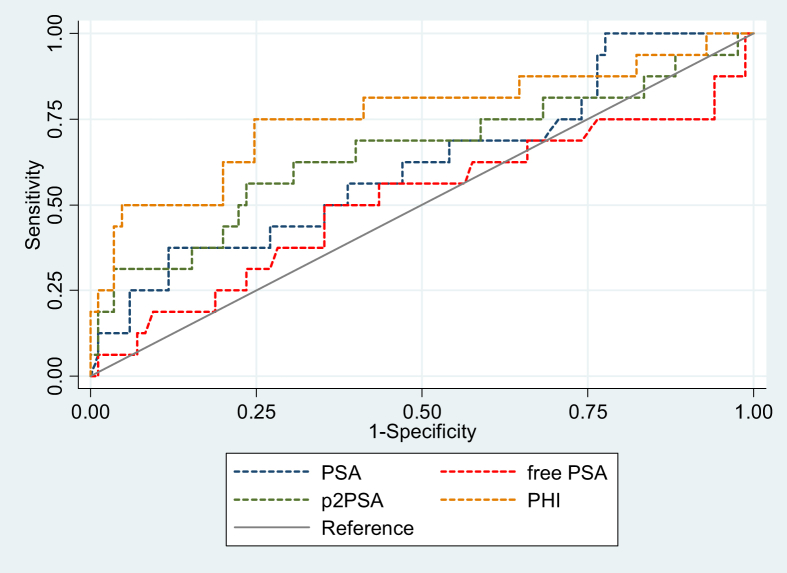
Sixteen of 101 patients who underwent transrectal ultrasound with biopsy were positive for prostate cancer. Prostate cancers identified with a sum GS of 6,7, and 8 were 6 (37.5%), 4 (25%), and 6 (37.5%), respectively. There was no significant difference in age, tPSA, and % f PSA level between cancer and no cancer groups. Patients with prostate cancer had significantly higher 2pPSA and PHI levels (Table 1). Sensitivity, specificity, Likelihood ratio LR+, and LR− of all PSA derivatives are shown in Table 2. The PHI cutoff at 34.14 (90% sensitivity) demonstrated the highest specificity compared with the tPSA, % f PSA, and 2pPSA. The PHI at this cutoff has highest AuROC compared with other PSA derivatives (Table 3 and Fig. 1). At the PHI cutoff of 34.14, only two cases had prostate cancer with a GS 3 + 4; there was no patient with GS 4 + 3 and ≥8 at this cutoff level (Table 4). The characteristics of studies for the PHI in Caucasian and Asian patients are shown in Table 5.
Table 1.
Characteristic of patients.
| Parameters | Cancer (%) N = 16 |
BPH (%) N = 85 |
P |
|---|---|---|---|
| Age | |||
| Mean (SD) | 67.0 (7.6) | 65.5 (6.6) | 0.406 |
| Range | 54–81 | 43–80 | |
| PSA | |||
| Mean (SD) | 7.5 (1.8) | 6.8 (1.8) | 0.141 |
| Range | 4.84–10 | 4.04–10 | |
| % Free PSA | |||
| Mean (SD) | 1.5 (1.1) | 1.4 (0.8) | 0.708 |
| Range | 0.32–4.67 | 0.28–6.01 | |
| p2PSA | |||
| Mean (SD) | 33.9 (45.9) | 15.8 (10.8) | 0.001∗ |
| Median | 19.2 | 12.4 | |
| Range | 4.1–192.5 | 2.6–65.5 | |
| PHI | |||
| Mean (SD) | 54.9 (35.6) | 28.8 (14.1) | <0.001∗ |
| Median | 39.7 | 26.3 | |
| Range | 13.6–129.2 | 8.1–93.6 | |
| Gleason score, n (%) | |||
| 6 | — | ||
| 7 | 6 (37.5) | n/a | |
| 8 | 4 (25) | n/a | |
| PHI: Prostate Health Index | 6 (37.5) | n/a | |
PHI, Prostate Health Index; PSA, prostate-specific antigen; BPH, Benign prostatic hyperplasia; SD, standard deviation.
Table 2.
Sensitivity, specificity, LR+, and LR− of PSA derivatives at 90% sensitivity for prediction of prostate cancer.
| PSA derivatives | Sensitivity (95% CI) | Specificity (95% CI) | LR+ (95% CI) | LR− (95% CI) |
|---|---|---|---|---|
| PSA ≥ 5.77 | 75 (66.6–83.4) | 29.41 (20.5–38.3) | 3.04 (1.9–4.84) | 0.33 (0.14–0.78) |
| % Free PSA ≥1.4 | 50 (40.2–59.8) | 64.71 (54.9–73.7) | 1.06 (0.78–1.46) | 0.85 (0.34–2.11) |
| p2PSA > 10.62 | 75 (47.6–92.7) | 41.18 (30.6–52.4) | NA | 1.01 (0.99–1.04) |
| PHI ≥ 34.14 | 75 (47.6–92.7) | 75.29 (64.7–84.0) | 1.27 (0.91–1.78) | 0.61 (0.25–1.47) |
CI, confidence interval; PHI, Prostate Health Index; PSA, prostate-specific antigen.
Table 3.
The area under the receiver operating characteristic curve (AuROC) of PSA derivatives at sensitivity 90%.
| PSA derivatives | AuROC | 95% CI |
|---|---|---|
| PSA | 0.615 | 0.5–0.8 |
| Free PSA | 0.509 | 0.5–0.8 |
| p2PSA | 0.651 | 0.3–0.7 |
| PHI | 0.758 | 0.6–0.9 |
AuROC, area under the receiver operating characteristic curve; CI, confidence interval; PHI, Prostate Health Index; PSA, prostate-specific antigen.
Fig. 1.
ROC for prostate cancer detection comparing PSA, p2PSA, free PSA, and PHI.
PHI, Prostate Health Index; PSA, prostate-specific antigen; ROC, receiver operating characteristic curve.
Table 4.
PHI level and Gleason score.
| PHI and Gleason core | Prostate cancer (cases) |
|---|---|
| PHI < 34.14 | 5 |
| ≥34.14 | 11 |
| GS score 6 | |
| PHI < 34.14 | 3 |
| ≥34.14 | 3 |
| GS score 7 | |
| PHI < 34.14 | 2 (all GS 3 + 4) |
| ≥34.14 | 2 (1 of GS 3 + 4, 1of GS 4 + 3) |
| GS score 8 | |
| PHI< 34.14 | 6 |
| ≥ 34.14 | 0 |
At PHI cutoff < 34.14.
Only two cases had significant prostate cancer (GS 3 + 4); no cancer ≥ GS 8.
GS, Gleason score; PHI, Prostate Health Index.
Table 5.
Characteristics of studies for PHI.
| First author (ref) | Year | Design | PSA level | Number | Prostate cancer (%) | AuROC total PSA | AuROC PHI | Percent of avoid biopsy | |
|---|---|---|---|---|---|---|---|---|---|
| Caucasian: | Loeb9 | 2015 | Pro | 4–10 | 334 | 145 (43.4) | 0.51 | 0.70 | N/A |
| Lazzeri15 | 2013 | Pro | 2–10 | 646 | 264 (40.8) | 0.50 | 0.67 | N/A | |
| Catalona16 | 2011 | Pro/Retro | 2–10 | 892 | 430 (48.2) | 0.52 | 0.70 | N/A | |
| Guazzoni17 | 2011 | Pro | 2–10 | 268 | 107 (39.9) | 0.53 | 0.76 | N/A | |
| Jansen18 | 2010 | Retro | 2–10 | 405 | 226 (55.8) | 0.58 | 0.75 | N/A | |
| Asian: | Tan19 | 2017 | Pro | 4–10 | 157 | 30 (19.1) | 0.47 | 0.79 | 49 |
| Chiu20 | 2016 | Pro | 4–10 | 569 | 62 (10.9) | 0.60 | 0.83 | N/A | |
| Chiu21 | 2016 | Pro | 10–20 | 312 | 53 (17.0) | 0.64 | 0.78 | 57.1 | |
| Yu22 | 2016 | Pro | 2–10 | 114 | 30 (26.05) | 0.55 | 0.78 | N/A | |
| Na23 | 2014 | Pro | >10 | 1538 | 710 (46.2) | 0.79 | 0.90 | 39 | |
| Na23 | 2014 | Retro | 4–10 | 230 | 21 (9.13) | 0.54 | 0.78 | 45.2 | |
| This series | 2017 | Pro | 4–10 | 101 | 16(15.8) | 0.61 | 0.75 | 31.2 |
AuROC, area under the receiver operating characteristic curve; PHI, Prostate Health Index; PSA, prostate-specific antigen.
4. Discussion
The disadvantage of PSA-based screening is the increasing number of men undergoing unnecessary prostate biopsies. PSA level can be elevated by benign conditions such as large benign prostate hyperplasia, prostatitis, and manipulation of the prostate. The specificity of a PSA level <10.0 ng/mL to the detection of prostate cancer was only 25%.5, 6, 7 PSA derivatives such as f PSA, PSA doubling time, and PSA velocity were used to improve the reliability of discriminating between men with and without cancer, but none of them have demonstrated a high specificity in clinical practice.
Pro-PSA is of greater concentration in malignant cells, whereas f PSA is higher in hyperplastic transition zone tissue. Four pro-PSA isoform, (−2) pro-PSA, (−4) pro-PSA, (−5) pro-PSA, and (−7) pro-PSA, exist in serum, but (−2) pro-PSA is the only form specific to prostate cancer.3, 4 (−2) pro-PSA has been studied in detail with the aim of enhancing the accuracy of diagnosis of prostate cancer and also assessing the aggressiveness of the tumor. The PHI resulting from a calculation involving the three biomarkers has a greater AuROC than % f PSA and p2PSA. The Food and Drug Administration approved the use of this test in 2012 for the detection of prostate cancer. The PHI can maintain high sensitivity, which was already possible with existing tests, but it has a higher specificity for prostate cancer.14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24 This test can avoid unnecessary biopsies by between 39% and 57%.19, 21, 23, 24 The PHI is used in the diagnosis of prostate cancer in gray zone PSA (2 ng/mL to 10 ng/mL, or 4 ng/mL to 10 ng/mL) at the first biopsy of standard or extended biopsy,14, 15, 16, 17, 18, 19, 20, 22, 23 active surveillance of disease progression,10 prediction of early biochemical recurrence after radical prostatectomy,11, 12 and prediction of high-grade cancer.13 Recently, data demonstrated that the PHI testing appears to be promising noninvasive biomarker for improvement of prostate cancer detection and providing prognostic information.4
Catalona et al compared the specificity and area under the cuve (AUC) of the PHI with the percentage of f PSA to detect overall incidence of prostate cancer and also prostate cancer with a GS ≥ 7. The risk of overall prostate cancer was increased 4.7-fold, and the risk of high-grade cancer (GS ≥7) was 1.61-fold for PHI and f PSA. AUC for PHI and % f PSA in the detection of prostate cancer with GS ≥4 + 3 were 0.724 and 0.670, respectively.16 The increasing use of the PHI and % p2PSA in active surveillance in the treatment of low-risk prostate cancer can enable the prediction of aggressive pathologies such as pT3 tumors and tumors with a GS ≥ 7 and tumor volume > 0.5 mL. This test may also be the most reliable test to use to identify nonaggressive cancer for active surveillance.10 Jansen et al confirmed the positive impact of the PHI and % p2PSA scores in increasing prostate cancer diagnosis but reported its limited value in the detection of high-grade cancer.18
Recently, the PHI was studied in the treatment of Asian patients. Tan et al studied the use of the PHI in the detection of prostate cancer in 157 Asian males with a tPSA of 4–10 ng/mL. The rate of detection was 19.1%. At the PHI cutoff level of 26.75, 49% of patients could have avoided prostate biopsies. At 90% sensitivity, the PHI was three times more useful at predicting prostate cancer in the initial biopsy. But at this PHI level, the diagnosis of prostate cancer was missed in three patients; two of them were at GS 3 + 3, and one at GS 4 + 3. At a PHI level of 55, 42.9% of patients had prostate cancer, and all of them were at GS > 6.19 Ng et al reported a retrospective study of the PHI in detection of prostate cancer in men with PSA 4–10 ng/mL with normal DRE.24 The detection rate was only 9% in our study, which was low, compared with that in the study by Tan et al, but use of PHI demonstrated the best performance in detection of prostate cancer (AUC of PHI: 0.781 and AUC of tPSA: 0.547).
Yu et al studied the performance of PHI in the detection of prostate cancer in patients of PSA ≥ 4 with a negative DRE and transrectal ultrasound. The AUC scores of PHI, % f PSA, and tPSA were 0.853, 0.751, and 0.598, respectively. The PHI was the best predictor of prostate biopsy results especially in the case of patients with tPSA 10.1–20 ng/mL. These data could be used to avoid unnecessary biopsies.22 Chiu et al extended use of PHI and {-2} pro-PSA in detection of prostate cancer in Chinese men with PSA 10–20 ng/mL and a normal DRE. Seventeen percent of patients were diagnosed with cancer on biopsy. This led to the finding that 57.1% of biopsies could be avoided with a PHI cutoff of 35.21 The PHI was the best predictor of prostate biopsy results especially in patients with a tPSA of 10.1–20 ng/mL to avoid unnecessary biopsies.
The PHI improved the cost-effective nature of prostate cancer detection with a 17% reduction in costs of diagnosis and a 1% reduction in the total costs for treatment of prostate cancer. These reductions were due to a decrease in the number of unnecessary biopsies.25, 26
A systematic review and meta-analysis study involving Caucasian patients demonstrated that % p2PSA and PHI could guide the decisions regarding the need for a prostate biopsy.5, 6 However, only two studies from Asia, which involved patients with a tPSA 4–10 ng/dl and a negative DRE, were included in this systematic review.5 Most patients in Western studies were patients with a PSA level between 2 ng/mL and 10 ng/mL, but in Asian patients, it was 4–10 ng/mL. The detection rate of cancer was higher in western studies, which was 40–55%; whereas it was 10–26% in Asian studies. Two studies from Asia in patients with PSA level >10 ng/mL showed the detection rate was high (46.2%). All studies with the PHI demonstrated that AuROC of tPSA was lower than AuROC of PHI in detection of prostate cancer; PHI testing can avoid unnecessary biopsy in 31–57% of patients.
The strengths of this study were the prospective collection of blood samples before the biopsy procedure, the performance of the transrectal ultrasound with biopsy by experienced urologists, and the interpretation of specimens by experienced pathologists. The limitation of the study is the relative small sample size and also the possibility of false negative biopsies.
5. Conclusion
The PHI increased the specificity in detection of prostate cancer in men with a PSA 4–10 ng/mL and negative DRE. This test also increased the detection rate of high-grade cancer. This test is a very useful and valid tool for discrimination between men with or without prostate cancer in clinical practice to avoid unnecessary prostate biopsy.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
- 1.Lojanapiwat B. Urologic cancer in Thailand. Jpn J Clin Oncol. 2015;45:1007–1015. doi: 10.1093/jjco/hyv125. [DOI] [PubMed] [Google Scholar]
- 2.Lojanapiwat B., Anutrakulchai W., Chongruksut W., Udomphot C. Correlation and diagnostic performance of the prostate-specific antigen level with the diagnosis, aggressiveness, and bone metastasis of prostate cancer in clinical practice. Prostate Int. 2014;2:133–139. doi: 10.12954/PI.14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cabarkapa S., Perera M., McGrath S., Lawrentschuk N. Prostate cancer screening with prostate-specific antigen: a guide to the guidelines. Prostate Int. 2016;4:125–129. doi: 10.1016/j.prnil.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath S., Christidis D., Perera M., Hong S.K., Manning T., Vela I. Prostate cancer biomarkers: are we hitting the mark? Prostate Int. 2016;4:130–135. doi: 10.1016/j.prnil.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mikolajczyk S.D., Catalona W.J., Evans C.L., Linton H.J., Millar L.S., Marker K.M. Proenzyme forms of prostate-specific antigen in serum improve the detection of prostate cancer. Clin Chem. 2004;50:1017–1025. doi: 10.1373/clinchem.2003.026823. [DOI] [PubMed] [Google Scholar]
- 6.Semjonow A., Kopke T., Eltze E., Pepping-Schefers B., Bürgel H., Darte C. Pre-analytical in-vitro stability of [-2] proPSA in blood and serum. Clin Biochem. 2010;43:926–928. doi: 10.1016/j.clinbiochem.2010.04.062. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Wang M., Wang L., Adams T.S., Tian Y., Xu J. Diagnostic ability of %p2PSA and Prostate Health Index for aggressive prostate cancer: a meta-analysis. Sci Rep. 2014;4:512. doi: 10.1038/srep05012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrate A., Lughezzani G., Gadda G.M., Lista G., Kinzikeeva E., Fossati N. Clinical use of [-2] proPSA (p2PSA) and its derivatives (%p2PSA and Prostate Health Index) for the detection of prostate cancer: a review of the literature. Korean J Urol. 2014;5:436–445. doi: 10.4111/kju.2014.55.7.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loeb S., Catalona W.J. The Prostate Health Index: a new test for the detection of prostate cancer. Ther Adv Urol. 2014;6:74–77. doi: 10.1177/1756287213513488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andreas D., Tosoian J.J., Landis P., Wolf S., Glavaris S., Lotan T.L. Elevated Prostate Health Index (phi) and biopsy reclassification during active surveillance of prostate cancer. Urol Case Rep. 2016;7:64–66. doi: 10.1016/j.eucr.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fossati N., Buffi N.M., Haese A., Stephan C., Larcher A., McNicholas T. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and Prostate Health Index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer: results from a multicentric European prospective study. Eur Urol. 2014;68:132–138. doi: 10.1016/j.eururo.2014.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Chiu P.K., Lai F.M., Teoh J.Y., Lee W.M., Yee C.H., Chan E.S. Prostate Health Index and %p2PSA predict aggressive prostate cancer pathology in Chinese patients undergoing radical prostatectomy. Ann Surg Oncol. 2016;23:2707–2714. doi: 10.1245/s10434-016-5183-6. [DOI] [PubMed] [Google Scholar]
- 13.de la Calle C., Patil D., Wei J.T., Scherr D.S., Sokoll L., Chan D.W. Multicenter evaluation of the Prostate Health Index (PHI) for detection of aggressive prostate cancer in biopsy-naive men. J Urol. 2015;194:65–72. doi: 10.1016/j.juro.2015.01.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loeb S., Sanda M.G., Broyles D.L., Shin S.S., Bangma C.H., Wei J.T. The Prostate Health Index selectively identifies clinically significant prostate cancer. J Urol. 2015;193:1163–1169. doi: 10.1016/j.juro.2014.10.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazzeri M., Haese A., de la Taille A., Palou Redorta J., McNicholas T., Lughezzani G. Serum isoform [-2]proPSA derivatives significantly improve prediction of prostate cancer at initial biopsy in a total PSA range of 2-10 ng ml-1: a multicentric European study. Eur Urol. 2013;63:986–994. doi: 10.1016/j.eururo.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 16.Catalona W., Partin A., Sanda M., Wei J.T., Klee G.G., Bangma C.H. A multicenter study of [-2] pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol. 2011;185:1650–1655. doi: 10.1016/j.juro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guazzoni G., Nava L., Lazzeri M., Scattoni V., Lughezzani G., Maccagnano C. Prostate-specific antigen (PSA) isoform p2PSA significantly improves the prediction of prostate cancer at initial extended prostate biopsies in patients with total PSA between 2.0 and 10 ng ml-1: results of a prospective study in a clinical setting. Eur Urol. 2011;60:214–222. doi: 10.1016/j.eururo.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 18.Jansen F.H., van Schaik R.H., Kurstjens J., Horninger W., Klocker H., Bektic J. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol. 2010;57:921–927. doi: 10.1016/j.eururo.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Tan L.G., Tan Y.K., Tai B.C., Tan K.M., Gauhar V., Tiong H.Y. Prospective validation of %p2PSA and the Prostate Health Index, in prostate cancer detection in initial prostate biopsies of Asian men, with total PSA 4-10 ng ml-1. Asian J Androl. 2017;19:286–290. doi: 10.4103/1008-682X.168687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu P.K., Roobol M.J., Teoh J.Y., Lee W.M., Yip S.Y., Hou S.M. Prostate health index (PHI) and prostate-specific antigen (PSA) predictive models for prostate cancer in the Chinese population and the role of digital rectal examination-estimated prostate volume. Int Urol Nephrol. 2016;48:1631–1637. doi: 10.1007/s11255-016-1350-8. [DOI] [PubMed] [Google Scholar]
- 21.Chiu P.K., Teoh J.Y., Lee W.M., Yee C.H., Chan E.S., Hou S.M. Extended use of Prostate Health Index and percentage of [-2]pro-prostate-specific antigen in Chinese men with prostate specific antigen 10-20 ng/mL and normal digital rectal examination. Investig Clin Urol. 2016;57:336–342. doi: 10.4111/icu.2016.57.5.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu G.P., Na R., Ye D.W., Qi J., Liu F., Chen H.T., Wu Y.S. Performance of the Prostate Health Index in predicting prostate biopsy outcomes among men with a negative digital rectal examination and transrectal ultrasonography. Asian J Androl. 2016;18:633–638. doi: 10.4103/1008-682X.172823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Na R., Ye D., Liu F., Chen H., Qi J., Wu Y., Zhang G. Performance of serum prostate-specific antigen isoform [-2]proPSA (p2PSA) and the Prostate Health Index (PHI) in a Chinese hospital-based biopsy population. Prostate. 2014;74:1569–1575. doi: 10.1002/pros.22876. [DOI] [PubMed] [Google Scholar]
- 24.Ng C.F., Chiu P.K., Lam N.Y., Lam H.C., Lee K.W., Hou S.S. The Prostate Health Index in predicting initial prostate biopsy outcomes in Asian men with prostate-specific antigen levels of 4-10 ng ml-1. Int Urol Nephrol. 2014;46:711–717. doi: 10.1007/s11255-013-0582-0. [DOI] [PubMed] [Google Scholar]
- 25.Nichol M.B., Wu J., An J.J., Huang J., Denham D., Frencher S. Budget impact analysis of a new prostate cancer risk index for prostate cancer detection. Prostate Cancer Prostatic Dis. 2011;14:253–261. doi: 10.1038/pcan.2011.16. [DOI] [PubMed] [Google Scholar]
- 26.Nichol M.B., Wu J., Huang J., Denham D., Frencher S.K., Jacobsen S.J. Cost-effectiveness of Prostate Health Index for prostate cancer detection. BJU Int. 2012;110:353–362. doi: 10.1111/j.1464-410X.2011.10751.x. [DOI] [PubMed] [Google Scholar]