Abstract
Background
Androgen deprivation therapy in addition to radiation therapy (RT + ADT) has shown benefits in local control and progression-free survival compared with RT alone for patients with locally advanced prostate cancer in Radiation Therapy Oncology Group 85-31. However, the survival gain may be diluted with increased toxicity of ADT. The aim of the study is to compare quality-adjusted life years (QALYs) values between two groups.
Methods
We developed “quality-adjusted survival analysis using duration” (QASAD) and “quality-adjusted survival analysis using probability” (QASAP) to estimate the quality-adjusted survival time. The QASAD uses the median duration in each health state to weight the utilities, whereas the QASAP uses the proportional probability of being in each state for weighting. The survival and complication rates were reconstructed based on published Kaplan–Meier survival curves, and the utility values for states were obtained from the previous literature.
Results
QALYs values for RT + ADT were generally higher than those for RT. The QASAD resulted in a QALY value of 4.93 [95% bootstrapped confidence interval (CI) = 4.12–5.71] for RT and of 5.60 (95% CI = 4.30–6.48) for RT + ADT. QASAP resulted in a QALY value of 4.85 (95% CI = 4.16–5.39) for RT and 4.96 (95% CI = 3.73–5.78) for RT + ADT.
Conclusions
We showed that RT + ADT provided slightly better quality-adjusted survival outcome than RT alone. The QASAD and QASAP methods may help the decision of optimal treatment balancing between survival gain and unfavorable quality of life.
Keywords: Decision support techniques, Quality-adjusted life years, Quality-adjusted survival analysis, Quality of life, Survival analysis
1. Introduction
Radiation Therapy Oncology Group (RTOG) trial 85-31 showed that long-term androgen deprivation therapy (ADT) along with standard radiation therapy (RT) improved local control and progression-free survival among patients with locally advanced prostate cancer (clinical stage T3 or N1) compared with RT alone. In adjuvant ADT group, goserelin started during the last week of RT and continued indefinitely or until signs of progression.1 Similar clinical trials which differed only in hormonal manipulation (RTOG 86-10 and 92-02) also showed clinical benefit of additional ADT.2, 3 RTOG 86-10 compared short-term ADT (2 months before and during RT) + RT with RT alone, and RTOG 92-02 evaluated short-term (4 months) versus long-term (4 months + additional 2 years) ADT during and after RT. By secondary analysis of these two trials, additional ADT showed cost-effectiveness.4, 5 However, to the best of our knowledge, no decision analysis has been performed for RTOG 85-31. Because duration of ADT in RTOG 85-31 was the longest among these three trials, the survival gain may be significantly diluted with increased toxicity of prolonged ADT. Therefore, decision analysis based on quality-adjusted survival comparison of RTOG 85-31 might be most challenging among these three trials. Initially, we aimed to compare quality-adjusted life years (QALYs) values between two groups.
The QALY has become one of the most widely recognized survival outcome measurements for decision analysis. The QALY is a composite outcome measure in which the patient's survival time is scaled down according to his quality of life. A simple way to calculate the QALY is to multiply the length of life by a quality adjustment fraction, namely utility.6 Despite concerns regarding methodological and practical difficulties in calculating the QALY,7 it has gained popularity as a tool for evaluating clinical strategies.8 Furthermore, it could be applied to coverage and reimbursement decision-making based on cost-effectiveness analysis.
To compare composite outcomes such as QALYs, several methods have been developed.9 In the Markov method,10 a finite number of health states and movements between the states are defined. Because the rates of movement of individuals between the states are quantified by transition probabilities, it is possible to model a stochastic process and to evaluate the long-term survival outcomes by incorporating diverse health states and time-dependent factors. However, an investigator needs to conduct extensive evidence synthesis or make clinical assumptions to estimate transition probabilities.10 Furthermore, current modeling strategies are often complicated or involve prespecified functions such as exponential survival function.11, 12
The quality-adjusted survival method computes an overall quality outcome by combining the length and quality of the patient's survival into a single value. “Quality-adjusted time without symptoms or toxicity” (Q-TWiST) has been proposed.9, 13 The Q-TWiST calculates a utility-weighted sum of time spent without disease symptoms or toxicity of treatment. The mean health state durations are usually estimated from the areas between the partitioned curves.13 Although the Q-TWiST delivers a single measure of quantity (length) and quality of life, which can be extended to a parametric approach, it is cumbersome to define the TWiST states and the corresponding utility weights because various timelines and toxicities may exist.13 Furthermore, time-dependent complication probabilities or utilities are not considered in this method.
We found that current analytic methods are not satisfactory for complex clinical scenario such as RTOG 85-31. Therefore, we decided to develop new strategies to take various health states and persistent complications into account in the quality-adjusted survival analysis. Furthermore, we evaluated the quality-adjusted survival benefit of adjuvant ADT for patients who were treated with radiation for locally advanced prostate cancer using our new decision analysis methods.
2. Materials and methods
The newly developed methods, quality-adjusted survival analysis using duration (QASAD) of the states and quality-adjusted survival analysis using probability (QASAP) of the states, are fully described in Supplementary Methods.
We evaluated patient-centered outcomes with published data from RTOG 85-31 by the QASAD and QASAP, applying time-varying complications within various clinical health states.
The following assumptions were made: four health states, i.e., remission, biochemical recurrence (BCR), recurrence (radiological progression, local recurrence, or metastasis), and death, were considered. Remission included both during tumor response and no evidence of disease. In the RT group, ADT was applied only from occurrence of BCR to recurrence. In the RT + ADT group, ADT was applied from the start of treatment until recurrence. The upper time limit was 9 years as this was the follow-up time for which published data were available. The types of toxicity were RT itself and grade-2 or higher gastrointestinal or genitourinary morbidity, where the time frame of RT was retained within 2.4 months in both groups. The BCR-free, recurrence-free, and overall survival rates for both groups were available from the study by Lawton et al.1 The complication rates used in this study were taken from the pooled data of similar trials (RTOG 85-31, 86-10, and 92-02) reported in the study by Lawton et al.14 Survival and toxicity rates were indirectly extracted from reported survival curves using the DigitizeIt software (version 2.0.5, Braunschweig, Germany).15 X (year) and Y (survival proportion) coordinates were digitally converted from Kaplan–Meier curve figures of RTOG 85-31 report. We used this estimated data set for analysis. Utility values were obtained from the Cost-Effectiveness Analysis Registry (available at: www.cearegistry.org) and previous publications (Table 1).4, 11
Table 1.
Utility assumptions and median durations of the states.
| Health conditions | Utilities (ranges) |
Median duration |
Reference | ||
|---|---|---|---|---|---|
| RT | RT + ADT | RT | RT + ADT | ||
| States | |||||
| Remission | 0.92 (0.92–1) | 0.793 (0.643–0.943) | 1.59 | 0.79 | Konski et al (2005)4 |
| BCR | 0.793 (0.643–0.943) | 0.793 (0.643–0.943) | 2.25 | 5.15 | Konski et al (2005)4 |
| Recurrence | 0.42 (0.33–0.51) | 0.42 (0.33–0.51) | 3.59 | 0.75 | Konski et al (2005)4 |
| Complications | |||||
| RT | 0.81 (0.678–0.942) | 0.81 (0.678–0.942) | 0.2 | 0.2 | Konski et al (2005)4 |
| Grade 2 + GU | 0.85 (0.775–0.925) | 0.85 (0.775–0.925) | — | — | Cooperberg et al (2013)11 |
| Grade 2 + GI | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | — | — | Cooperberg et al (2013)11 |
BCR, biochemical recurrence; GI, gastrointestinal; GU, genitourinary; RT, radiation therapy; RT + ADT, radiation therapy plus androgen deprivation therapy.
As one-way sensitivity analyses, we calculated the QALY values for a range of plausible utility values (Table 2). Probabilistic sensitivity analysis was carried out using Monte Carlo simulation with 1,000 simulated samples. The beta distribution for utilities and the mean and standard deviation of each parameter are shown in S1 Table.16 The 95% confidence interval (CI) was estimated by bootstrapping.17
Table 2.
Application of quality-adjusted survival analysis using duration (QASAD) and quality-adjusted survival analysis using probability (QASAP) on RTOG 85-31.
| Analytic conditions | QASAD |
QASAP |
||||||
|---|---|---|---|---|---|---|---|---|
| QALY values (multiplicative) |
QALY values (minimum) |
QALY values (multiplicative) |
QALY values (minimum) |
|||||
| RT | RT + ADT | RT | RT + ADT | RT | RT + ADT | RT | RT + ADT | |
| Overall QALY value | 4.9 | 5.57 | 4.92 | 5.6 | 4.83 | 4.93 | 4.89 | 5.02 |
| One-way sensitivity analysis | ||||||||
| States | ||||||||
| Remission = 1 | 5.04 | 5.57 | 5.04 | 5.6 | 5.06 | 4.93 | 5.11 | 5.02 |
| BCR = 0.943 | 5.24 | 6.48 | 5.25 | 6.49 | 5.14 | 5.79 | 5.19 | 5.82 |
| BCR = 0.643 | 4.57 | 4.66 | 4.58 | 4.69 | 4.58 | 4.08 | 4.64 | 4.16 |
| Recurrence = 0.51 | 5.23 | 5.74 | 5.24 | 5.77 | 5 | 5.03 | 5.07 | 5.12 |
| Recurrence = 0.33 | 4.58 | 5.41 | 4.59 | 5.44 | 4.66 | 4.84 | 4.72 | 4.93 |
| Toxicity | ||||||||
| RT = 0.942 | 4.93 | 5.59 | 4.94 | 5.6 | 4.85 | 4.96 | 4.92 | 5.02 |
| RT = 0.678 | 4.88 | 5.55 | 4.89 | 5.58 | 4.81 | 4.91 | 4.87 | 5 |
| Grade 2 + GU = 0.775 | — | — | — | — | 4.81 | 4.92 | 4.88 | 5.01 |
| Grade 2 + GU = 0.925 | — | — | — | — | 4.85 | 4.95 | 4.9 | 5.02 |
| Grade 2 + GI = 0.7 | — | — | — | — | 4.82 | 4.92 | 4.88 | 5.01 |
| Grade 2 + GI = 0.9 | — | — | — | — | 4.85 | 4.95 | 4.9 | 5.02 |
| Probabilistic sensitivity analysis (95% CI) | 4.93 (4.12–5.71) | 5.60 (4.30–6.48) | 4.94 (4.14–5.72) | 5.62 (4.33–6.49) | 4.85 (4.16–5.39) | 4.96 (3.73–5.78) | 4.91 (4.24–5.44) | 5.04 (3.79–5.82) |
BCR, biochemical recurrence; CI, confidence interval; GI, gastrointestinal; GU, genitourinary; QALY, quality-adjusted life year; RTOG, Radiation Therapy Oncology Group; RT, radiation therapy; RT + ADT, radiation therapy plus androgen deprivation therapy. “Multiplicative” and “minimum” refer to the method used to calculate the QALY values (see Supplementary data in Online Resource 1).
The QASAD and QASAP calculations and sensitivity analyses were performed to integrate survival data extracted from RTOG 85-31 and plausible utilities using code developed in-house (available on request from the corresponding author) in the R programming language (http://www.R-project.org). For the combined health states and complications, the common utility estimates were obtained using the multiplicative or minimum methods.18
3. Results
The overall QALY values obtained using multiplicative QASAD were 0.81 × 0.92 × 0.2 [under RT] + 0.92 × 1.59 [remission] + 0.79 × 2.25 [BCR] + 0.42 × 3.59 [recurrence] = 0.15 [under RT] + 1.46 [remission] + 1.78 [BCR] + 1.51 [recurrence] = 4.90 for the RT group and 0.81 × 0.793 × 0.2 [under RT] + 0.79 × 5.15 [remission] + 0.79 × 0.75 [BCR] + 0.42 × 1.82 [recurrence] = 0.13 [under RT] + 4.08 [remission] + 0.59 [BCR] + 0.76 [recurrence] = 5.57 for the RT + ADT group. Similarly, the QALY values obtained by using minimum QASAD were 4.92 for the RT group and 5.6 for the RT + ADT group. The overall QALY values obtained using multiplicative QASAP were 2.73 [remission] + 1.30 [BCR] + 0.80 [recurrence] = 4.83 and 4.16 [remission] + 0.34 [BCR] + 0.43 [recurrence] = 4.93 for the RT group and the RT + ADT group, respectively, and the QALY values obtained by using minimum QASAP were 2.75 [remission] + 1.32 [BCR] + 0.82 [recurrence] = 4.89 and 4.24 [remission] + 0.34 [BCR] + 0.44 [recurrence] = 5.02 (Table 2). In the RT + ADT group, the QALY value for the remission state was noticeably higher than that in the RT group because patients stay much longer in this state than those in the RT group.
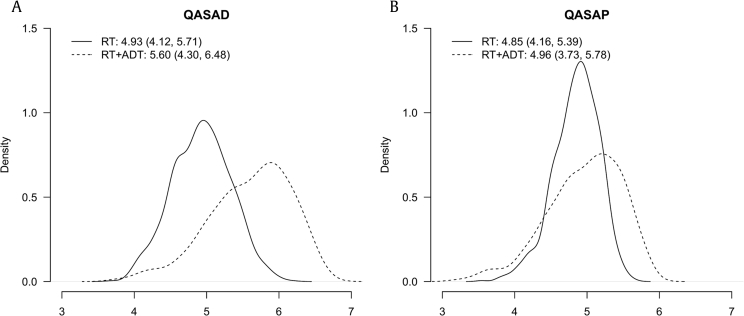
Sensitivity analyses showed that the overall QALY values in the RT + ADT group were generally higher than those in the RT group in both the QASAD and QASAP analyses. However, the overall differences were subtle, and the differences across various health states were diverse (Table 2). The highest values across all states for the one-way sensitivity analysis of the QASAD were noted for the RT + ADT group, indicating the superiority of this treatment. In the QASAP, RT was found to be the favorable treatment when the utility value of remission approached perfect health. The probabilistic sensitivity analysis resulted in QALY values of 4.93 (95% CI = 4.12–5.71) for the RT group and 5.6 (95% CI = 4.30–6.48) for the RT + ADT group when using the multiplicative QASAD method. The QALYs based on the multiplicative QASAP method were 4.85 (95% CI = 4.16–5.39) for the RT group and 4.96 (95% CI = 3.73–5.78) for the RT + ADT group. Fig. 1 illustrates the distributions of the QALY values obtained from the probabilistic sensitivity analyses.
Fig. 1.
Probabilistic sensitivity analyses. (A) Distribution of the QALY values obtained from quality-adjusted survival analysis using duration (QASAD). (B) Distribution of the QALY values obtained from quality-adjusted survival analysis using probability (QASAP).
ADT, androgen deprivation therapy; QALY, quality-adjusted life year; RT, radiation therapy.
4. Discussion
Most guidelines support the combination of RT with ADT based on high level evidence.19, 20 Typically, ADT begins either at the beginning of RT or 2–3 months before, but the accompanying component is critical to the potency of RT. Long-term ADT, from 2 years to 3 years is recommended for locally advanced prostate cancer rather than short term (6 months). Nowadays, the role of concomitant ADT with RT is expanding as evidence is getting accumulated. Recent the Groupe d’Etude des Tumeurs Uro-Génitales (GETUG)-the Association Française d’Urologie (AFU) 16 and RTOG 9601 trials demonstrated that adding ADT during salvage RT benefits men with BCR after radical prostatectomy.21, 22 However, actual combination of RT with ADT is not common in daily practice. Only 32.1% of salvage RT patients received ADT in the United States.23 The most likely reason is that ADT can cause many adverse effects ranging from hot flush, sexual dysfunction, diabetes, osteoporosis, and cardiovascular disease to depression and cognitive decline.24 Therefore, long-term use of ADT can badly affect the quality of life. Clinicians must balance the benefits and harms of ADT; therefore, we need reliable decision analysis based on quality-adjusted survival comparison.
Application of our models to RTOG trial 85-31 demonstrated that adding ADT to RT improved the quality of life. Under some extreme conditions such as assuming a perfect utility of the Remission state, adding ADT to RT does not seem to be improved the QALY values. However, the QASAD showed 14% improvements and QASAP showed 2–3% improvements in overall QALY values and probabilistic sensitivity analyses in the RT + ADT group. Our results were in agreement with those obtained in previous decision analyses using the Markov method, in which short-term ADT before and during RT resulted in a higher mean QALY value than RT alone (6.43 vs. 5.48) in RTOG 86-10,4 and 2-year additional ADT had a better mean QALY than short-term ADT + RT (4.13 vs. 3.68) in RTOG 92-02.5 Because the purpose of the Markov model is to incorporate all stages,25 the number of parameters increases with the number of stages. For this reason, a Markov model with limited number of stages is sometimes constructed.12 Moreover, heterogeneity and bias may be introduced when estimating parameters and performing model calibration from limited data sources.26 In contrast to the Markov method, in which many simulated estimates for transition probabilities are obtained from different data sources, our method is suitable for direct estimation of the QALY from cohort data. Another merit of our method is that it allows easy visualization of the models using decision tree diagrams. Furthermore, the QASAD and QASAP can cope with dynamic modeling components, assuming time-varying complication probabilities, and they can include discounting factors in the utilities. In our opinion, the QASAD is appropriate for diseases where the median survival time is obtainable for every state, and survival curves are characterized by an exponential shape. One such example is cancer metastasis, where the spread of cancer and corresponding stages are well defined. The QASAP is applicable to cases with long-term outcomes and possibly to more complex transitions over time-dependent complications. Because QASAP allows more flexibility in modeling perspectives than QASAD, QASAP may be more suitable for treatment comparisons in general situation. Alzheimer's disease is such an example where patients progress through exacerbating stages of the disease and require a long-term treatment with persisting risk of complications over time. When applying Q-TWiST to RTOG 95-31 trial, there were several uncertain areas in defining states and utilities. Defining duration with toxicity (TOX) was not straightforward because adverse event could be occurred during any course of RT or ADT, and it was similar in defining TWiST because symptom- or toxicity-free period was not plausible in RT + ADT groups. Q-TWiST assumed the same utilities in BCR progression-free and recurrence-free states and could not incorporate long-term toxicity such as gastrointestinal or genitourinary.
Our new methods naturally have several limitations. First, we assumed that the states are ordered, and backward movements are not allowed in the QASAD, which is a strong assumption of the Q-TWiST method. The Q-TWiST method restricts the health states to three time frames: “time having subjective toxic side effects,” “TWiST,” and “time following systemic relapse.” In contrast, our models require no further assumptions for any health states that are naturally ordered. In the QASAP, even the constraints on irreversible states are relaxed. Furthermore, we utilized the marginal probabilities of states, whereas the Markov model intends to estimate the transition probabilities between states. As our strategy reduces the number of parameters to be estimated, the number of parameters in the QASAD and QASAP increases linearly, while the number of parameters in a first-order Markov method is quadratic in the number of states. Second, the QASAD is reliable only if the total follow-up period is long enough to estimate the median duration. If the median duration does not correctly reflect the overall survival pattern, the restricted mean duration of survival time is a possible estimate, and the QASAP is a more appropriate alternative. Third, whereas a Markov process can be defined based on an extended time horizon, our methods involve marginal movements within the states that are characterized over a fixed period for a given cohort of patients. Fourth, both the QASAD and QASAP require reliable survival statistics from cohort data. Unless high-quality survival outcome data are available, our methods are not applicable, whereas the Markov method can still be used in such a case to conduct comparisons based on conjecture or simulation. Fifth, although complication probabilities tend to be dependent, we assumed them to be independent for practical reasons as the complication probabilities for each state were not obtainable for most trials.
Our method allows for elaboration. In our clinical example, as a nonparametric approach, we extracted patient data from published Kaplan–Meier survival curves. If survival is a known parametric function of time, a comparison can be made over various upper limits T.27 If certain baseline covariates are known to be associated with different prognoses, we can extend our methods using a regression model such as the proportional hazards model4 or the additive risks model28 to account for the effect of covariates on the hazard function. To account for uncertainty of the parameter settings, it is important to address variations such as probabilistic sensitivity analyses or bootstrapped confidence intervals. When covariate information is available which provides useful information about survival patterns, one may perform subgroup analyses to explore different patterns. Finally, our method can be extended to cost analysis if utility is replaced with cost.
5. Conclusions
We developed the QASAD and QASAP as alternative quality-adjusted survival analysis methods. Our methods are readily applicable for diseases that require survival outcome and quality-of-life assessment. Both methods represent an improvement in patient survival over various states accounting for life quality. The merit of our methods is that they incorporate time-dependent complication rates and utilities for joint health states into existing quality-adjusted survival analysis. We also demonstrated that adding long-term ADT to RT provided slightly better quality-adjusted survival outcome than RT alone using the QASAD and QASAP.
Conflicts of interest
All authors have no conflicts of interest to declare.
Acknowledgments
The authors are indebted to J. Patrick Barron, Professor Emeritus, Tokyo Medical University, and Adjunct Professor, Seoul National University Bundang Hospital, for his pro bono editing of this manuscript. The authors would like to express their gratitude to Jang Eun Jin, Ph.D., for suggestions and comments. A preliminary version of this study was presented as a poster at the Annual Congress of the Korean Prostate Society, Republic of Korea, March 2014, and the fourth Congress of Asian Pacific Prostate Society, Japan, March 2014.
Footnotes
Chang Wook Jeong received a grant from Seoul National University Bundang Hospital Research Fund (02-2012-019), and Soyeon Ahn received a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2013R1A1A3012306). Minjung Lee received a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2016R1C1B1010294). The funding agreement ensured the authors' independence in designing the study, interpreting the data, writing, and publishing the report.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.prnil.2018.01.002.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Lawton C.A., Winter K., Murray K., Machtay M., Mesic J.B., Hanks G.E. Updated results of the phase III Radiation Therapy Oncology Group (RTOG) trial 85-31 evaluating the potential benefit of androgen suppression following standard radiation therapy for unfavorable prognosis carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;49:937–946. doi: 10.1016/s0360-3016(00)01516-9. [DOI] [PubMed] [Google Scholar]
- 2.Pilepich M.V., Winter K., John M.J., Mesic J.B., Sause W., Rubin P. Phase III radiation therapy oncology group (RTOG) trial 86-10 of androgen deprivation adjuvant to definitive radiotherapy in locally advanced carcinoma of the prostate. Int J Radiat Oncol Biol Phys. 2001;50:1243–1252. doi: 10.1016/s0360-3016(01)01579-6. [DOI] [PubMed] [Google Scholar]
- 3.Lawton C.A.F., Lin X., Hanks G.E., Lepor H., Grignon D.J., Brereton H.D. Duration of androgen deprivation in locally advanced prostate cancer: long-term update of NRG oncology RTOG 9202. Int J Radiat Oncol Biol Phys. 2017;98:296–303. doi: 10.1016/j.ijrobp.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konski A., Sherman E., Krahn M., Bremner K., Beck J.R., Watkins-Bruner D. Economic analysis of a phase III clinical trial evaluating the addition of total androgen suppression to radiation versus radiation alone for locally advanced prostate cancer (Radiation Therapy Oncology Group protocol 86-10) Int J Radiat Oncol Biol Phys. 2005;63:788–794. doi: 10.1016/j.ijrobp.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Konski A., Watkins-Bruner D., Brereton H., Feigenberg S., Hanks G. Long-term hormone therapy and radiation is cost-effective for patients with locally advanced prostate carcinoma. Cancer. 2006;106:51–57. doi: 10.1002/cncr.21575. [DOI] [PubMed] [Google Scholar]
- 6.Hsieh M.H., Meng M.V. Decision analysis and Markov modeling in urology. J Urol. 2007;178:1867–1874. doi: 10.1016/j.juro.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 7.Kind P., Lafata J.E., Matuszewski K., Raisch D. The use of QALYs in clinical and patient decision-making: issues and prospects. Value Health. 2009;12(Suppl 1):S27–S30. doi: 10.1111/j.1524-4733.2009.00519.x. [DOI] [PubMed] [Google Scholar]
- 8.Neumann P.J. What next for QALYs? JAMA. 2011;305:1806–1807. doi: 10.1001/jama.2011.566. [DOI] [PubMed] [Google Scholar]
- 9.Billingham L.J., Abrams K.R., Jones D.R. Methods for the analysis of quality-of-life and survival data in health technology assessment. Health Technol Assess. 1999;3:1–152. [PubMed] [Google Scholar]
- 10.Naimark D., Krahn M.D., Naglie G., Redelmeier D.A., Detsky A.S. Primer on medical decision analysis: Part 5–working with Markov processes. Med Decis Making. 1997;17:152–159. doi: 10.1177/0272989X9701700205. [DOI] [PubMed] [Google Scholar]
- 11.Cooperberg M.R., Ramakrishna N.R., Duff S.B., Hughes K.E., Sadownik S., Smith J.A. Primary treatments for clinically localised prostate cancer: a comprehensive lifetime cost-utility analysis. BJU Int. 2013;111:437–450. doi: 10.1111/j.1464-410X.2012.11597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elliott S.P., Wilt T.J., Kuntz K.M. Projecting the clinical benefits of adjuvant radiotherapy versus observation and selective salvage radiotherapy after radical prostatectomy: a decision analysis. Prostate Cancer Prostatic Dis. 2011;14:270–277. doi: 10.1038/pcan.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelber R.D., Goldhirsch A. A new endpoint for the assessment of adjuvant therapy in postmenopausal women with operable breast cancer. J Clin Oncol. 1986;4:1772–1779. doi: 10.1200/JCO.1986.4.12.1772. [DOI] [PubMed] [Google Scholar]
- 14.Lawton C.A., Bae K., Pilepich M., Hanks G., Shipley W. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: analysis of radiation therapy oncology group studies 85-31, 86-10, and 92-02. Int J Radiat Oncol Biol Phys. 2008;70:437–441. doi: 10.1016/j.ijrobp.2007.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyot P., Ades A.E., Ouwens M.J., Welton N.J. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. 2012 Feb 1;12:9. doi: 10.1186/1471-2288-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrew Briggs M.S., Claxton Karl. Oxford University Press; 2006. Decision modelling for health economic evaluation. [Google Scholar]
- 17.Efron B., Tibshirani R.J. Chapman and Hall/CRC; 1994. An introduction to the bootstrap (Chapman & Hall/CRC monographs on statistics & applied probability) [Google Scholar]
- 18.Bo H., Fu A.Z. Predicting utility for joint health states: a general framework and a new nonparametric estimator. Med Decis Making. 2010;30:E29–E39. doi: 10.1177/0272989X10374508. [DOI] [PubMed] [Google Scholar]
- 19.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Mohler J.L., Armstrong A.J., Bahnson R.R., D'Amico A.V., Davis B.J., Eastham J.A. Prostate cancer, version 1.2016. J Natl Compr Canc Netw. 2016;14:19–30. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 21.Carrie C., Hasbini A., de Laroche G., Richaud P., Guerif S., Latorzeff I. Salvage radiotherapy with or without short-term hormone therapy for rising prostate-specific antigen concentration after radical prostatectomy (GETUG-AFU 16): a randomised, multicentre, open-label phase 3 trial. Lancet Oncol. 2016;17:747–756. doi: 10.1016/S1470-2045(16)00111-X. [DOI] [PubMed] [Google Scholar]
- 22.Shipley W.U., Seiferheld W., Lukka H.R., Major P.P., Heney N.M., Grignon D.J. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med. 2017;367:417–428. doi: 10.1056/NEJMoa1607529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang D.D., Muralidhar V., Mahal B.A., Nezolosky M.D., Labe S.A., Vastola M.E. Low rates of androgen deprivation therapy use with salvage radiation therapy in patients with prostate cancer after radical prostatectomy. Urol Oncol. 2017;35:542.e25–542.e32. doi: 10.1016/j.urolonc.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Rhee H., Gunter J.H., Heathcote P., Ho K., Stricker P., Corcoran N.M. Adverse effects of androgen-deprivation therapy in prostate cancer and their management. BJU Int. 2015;115(Suppl 5):3–13. doi: 10.1111/bju.12964. [DOI] [PubMed] [Google Scholar]
- 25.Sonnenberg F.A., Beck J.R. Markov models in medical decision making: a practical guide. Med Decis Making. 1993;13:322–338. doi: 10.1177/0272989X9301300409. [DOI] [PubMed] [Google Scholar]
- 26.Welton N.J., Ades A.E. Estimation of markov chain transition probabilities and rates from fully and partially observed data: uncertainty propagation, evidence synthesis, and model calibration. Med Decis Making. 2005;25:633–645. doi: 10.1177/0272989X05282637. [DOI] [PubMed] [Google Scholar]
- 27.Cole B.F., Gelber R.D., Anderson K.M. Parametric approaches to quality-adjusted survival analysis. International Breast Cancer Study Group. Biometrics. 1994;50:621–631. [PubMed] [Google Scholar]
- 28.Cole B.F., Gelber R.D., Goldhirsch A. Cox regression models for quality adjusted survival analysis. Stat Med. 1993;12:975–987. doi: 10.1002/sim.4780121009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.