Abstract
Sinonasal respiratory epithelium is a highly regulated barrier that employs mucociliary clearance (MCC) as the airways first line of defense. The biological properties of the airway surface liquid (ASL), combined with coordinated ciliary beating, are critical components of the mucociliary apparatus. The ASL volume and viscosity is modulated, in part, by the cystic fibrosis transmembrane conductance regulator (CFTR). The CFTR is an anion transporter of chloride (Cl−) and bicarbonate (HCO3−) that is located on the apical surface of respiratory epithelium and exocrine glandular epithelium. Improved understanding of how dysfunction or deficiency of CFTR influences the disease process in both genetically defined cystic fibrosis (CF) and acquired conditions has provided further insight into potential avenues of treatment. This review discusses the latest data regarding acquired CFTR deficiency and use of CFTR specific treatment strategies for CRS and other chronic airway diseases.
Keywords: CFTR, Cystic fibrosis, CFTR deficiency, CFTR dysfunction, Chronic sinusitis, Chronic rhinosinusitis, Chronic obstructive pulmonary disease, Tobacco, Hypoxia, Resveratrol, l-ascorbate
Introduction
The sinonasal respiratory epithelium is a highly regulated barrier that facilitates normal cilia function and mucociliary clearance (MCC). This barrier is made up of a mucus layer and is part of the innate immune system, representing an interface between the host and the environment. The mucus layer functions to trap inhaled particles (i.e. allergens and pollutant particulate matter) and moves in a coordinated fashion via ciliary beat out of the nose and sinuses into the pharynx where the mucus is ingested and degraded. The inherent biological properties of the respiratory epithelium include coordinated ciliary beating, and the airway surface liquid (ASL), both are essential components to ensure a functional MCC. The ASL is composed of a thin, low viscosity, periciliary layer (sol layer) that envelops the shafts of the cilia and a thick, more viscous layer (gel layer) that rides on the cilia and periciliary layer.1 The ASL volume and viscosity is modulated, in part, by the cystic fibrosis transmembrane conductance regulator (CFTR). This apical anion channel regulates anion (Cl−and HCO3−) secretion and is located on the apical surface of respiratory epithelium and exocrine glandular epithelium. Genetic mutations in the CFTR can result in cystic fibrosis (CF) – a disease that impacts multiple organ systems, including the sinuses.
CF is the most common lethal inherited disease among Caucasians and affects up to 30 000 people in the United States.2 A large number of mutations in the gene encoding CFTR can lead to poor Cl− and HCO3− transport and excessive absorption of sodium and water. The ASL becomes dehydrated under these conditions, resulting in viscous mucus.3 The cilia cannot function normally, and thus compromises MCC. The chronic stasis of inspissated mucus provides bacteria an ideal environment to propagate within the airways. Increased understanding regarding the role of CFTR in both genetically defined CF, and acquired conditions has provided a platform for newly defined treatment strategies that target the basic defect. A number of environmental insults including, cigarette smoke, hypoxia/high altitude, inflammation and infectious agents contribute to the pathogenesis of chronic rhinosinusitis (CRS), chronic obstructive pulmonary disease (COPD), chronic bronchitis, asthma, and pancreatitis by inducing acquired CFTR dysfunction.4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17
CFTR dysfunction and CRS
Numerous studies have shown associations between CFTR dysfunction and chronic rhinosinusitis (CRS).12, 18, 19, 20 Sinusitis is a consistent feature of patients with CF, and mutations that cause severe CFTR dysfunction or deficiency result in universal inflammatory paranasal sinus disease. Studies suggest that healthy patients heterozygous for CFTR mutation have an increased likelihood of developing CRS with and without nasal polyposis.21, 22 Carriers with a CFTR mutation have a higher prevalence of CRS compared with the general population.6 Furthermore, Wang et al23 demonstrated mild dysfunction of the CFTR might be associated with the development of CRS in the general population, with 7% of CRS patients identified to have a CF mutation, which was significantly higher than 2% of the control group. In addition, pediatric patients with CRS that did not meet the criteria for the diagnosis of CF were screened and identified to have a higher prevalence of CF variant mutations than would be expected in the general population.24 Using ex vivo sinonasal tissue Ussing study, Cho et al25 noted decreased CFTR-mediated Cl− secretion across human sinonasal epithelia from CRS sinonasal tissues compared to those from normal controls.
Acquired CFTR dysfunction and tobacco smoke
Tobacco smoke has a significant impact on the upper and lower airways. In addition to the mutagenic aspects of toxins in cigarette smoke, inhaled byproducts of tobacco combustion are known to impact the clearance of mucus. One method by which tobacco may confer its deleterious effects on sinonasal epithelium is by impacting the function of CFTR.26 Acrolein and cadmium are implicated in cigarette smoke as the key toxins affecting CFTR function.27 Prior to the discovery of CFTR, Welsh28 reported that cigarette smoke extract decreased Cl− secretion across canine airway epithelium. Over two decades later, Kreindler et al29 identified that cigarette smoke extract decreased CFTR-dependent Cl− secretion in human bronchoepithelium. Subsequent studies have demonstrated smoking impairs CFTR function. Cigarette smoke decreases the expression of the CFTR gene, causes protein destabilization, and gated channel dysfunction in vitro.26, 29, 30, 31 In 2018, Christensen et al32 performed a systematic review of the association between cigarette smoke exposure and CRS. This review further validated the body of evidence that active smoking increases the incidence of CRS in smokers. This was shown in a dose-dependent manner, and that previous smoker status and passive smoke exposure may be associated with an increased risk of developing CRS.32, 33, 34, 35, 36 They supported previous studies that confirmed children exposed to passive cigarette smoke in a household did significantly worse following endoscopic sinus surgery than those not exposed to smoke.37, 38, 39
Studies of healthy non-smokers without CFTR genetic mutations, exposed to cigarette smoke have also exhibited CFTR deficiency. Administration of cigarette smoke to the nares of healthy smokers caused an acute blockade of CFTR activity, as measured by NPD, suggesting exposure to cigarette smoke rapidly inhibits CFTR activity in vivo, as well as reduced ASL hydration in vitro.11, 26 Furthermore, cigarette smoke condensate inhibits transepithelial chloride secretion (through CFTR and calcium activated chloride channel TMEM16A) and ciliary beat frequency in upper and lower respiratory airway epithelial cells in vitro.15, 40
Data also suggests CFTR dysfunction from smoking is not limited to the airway. Extrapulmonary manifestations of smoking and subsequent CFTR dysfunction may include pancreatitis, cachexia and male infertility, also characteristic of CF. It is thought that mediators related to tobacco smoke circulate to cause systemic CFTR dysfunction.17, 26, 27
Acquired CFTR dysfunction and COPD
Evidence suggests that CFTR dysfunction may play a role in COPD, non-atopic asthma, and non-CF bronchiectasis.41 The chronic bronchitis phenotype of COPD has a pathophysiological association and clinical similarity to CF airway disease. Chronic bronchitis, like CF, is characterized by overproduction, poor clearance, and accumulation of hyperviscous mucus within the small airways.30, 42, 43 Sloane et al31 revealed reduced CFTR activity measured by nasal potential difference (NPD) was also predictive of the severity of bronchitis symptoms, even when controlled for cigarette smoking, indicating a significant association with the chronic bronchitis phenotype. Individuals with smoking related COPD exhibited a decreased CFTR expression (mRNA levels) and reduced activity, (approximately 50%) measured by NPD in the upper and lower airway.31 Research has shown that CFTR expression can be modulated,26, 44 and reversibility has been demonstrated in the upper airway in studies of patients with COPD. Patients who no longer smoke have normal Cl− transport on NPD assay.45
Acquired CFTR dysfunction and hypoxia
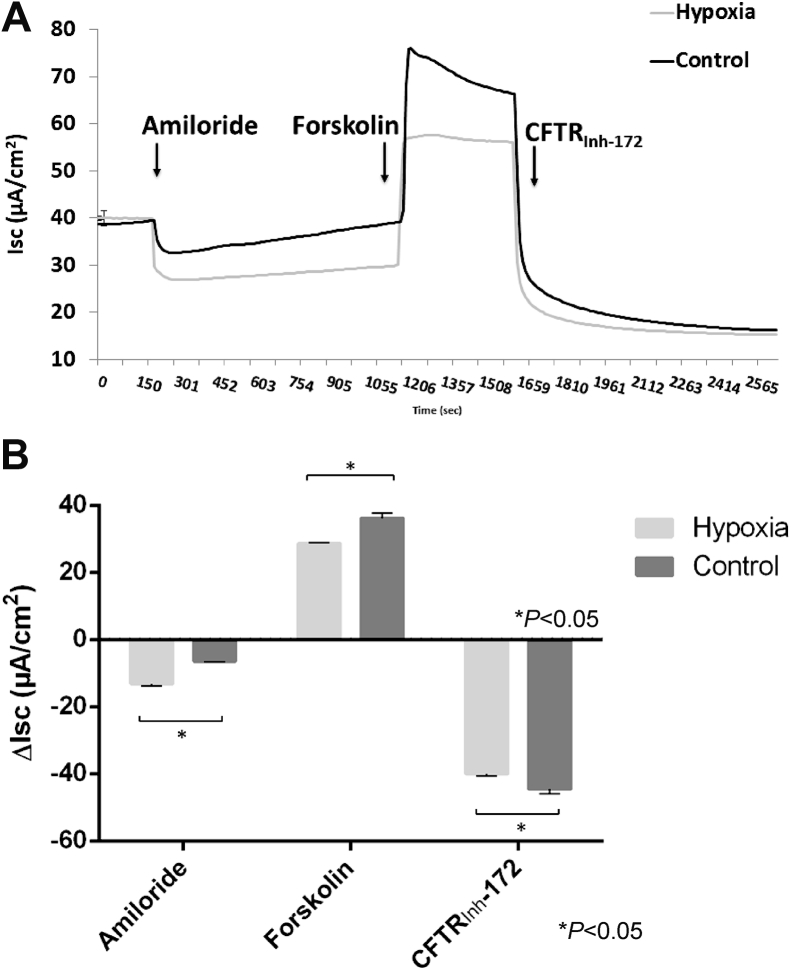
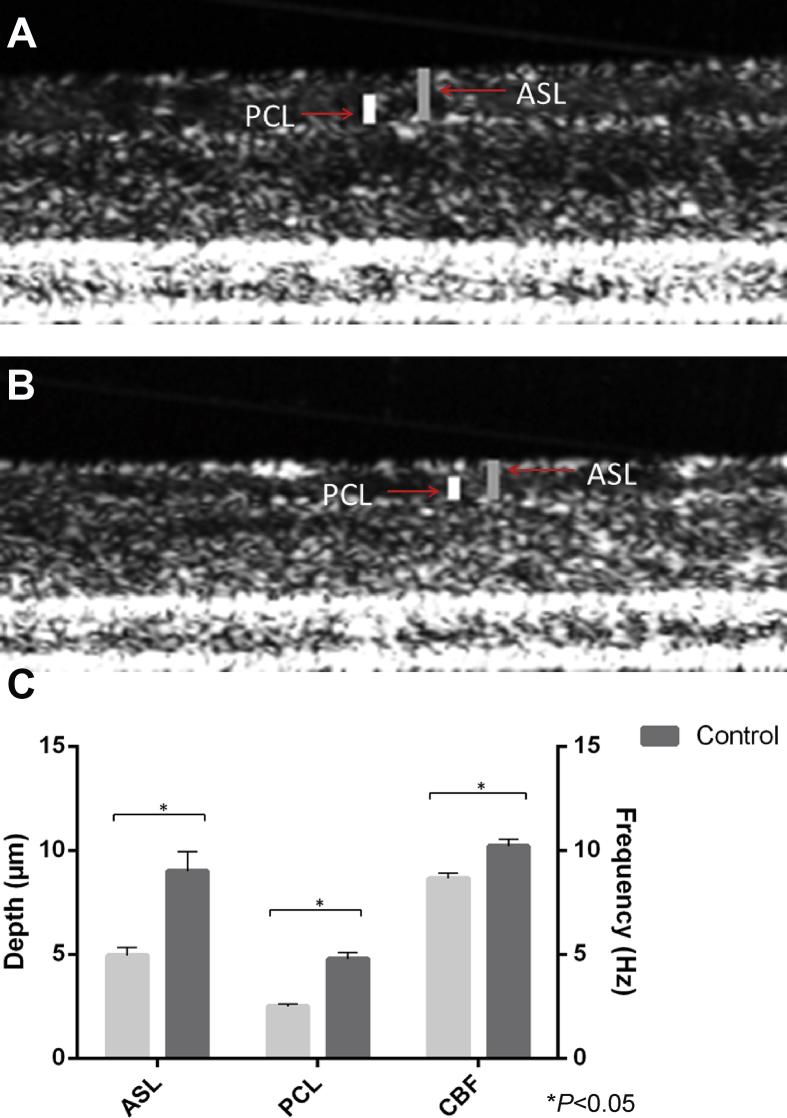
Hypoxia has been suggested to play a significant role in the pathophysiology of CRS among non-CF individuals.46, 47 Obstruction of the sinus ostia can lead to reduced oxygen tension in the sinus mucosal tissue48 and release of inflammatory mediators, thereby causing stasis of hyperviscous mucus. This promotes an ideal environment for bacterial growth.47 An oxygen-restricted environment in individuals with CRS could create regions of localized acquired CFTR deficiency and may initiate a cascade effect of ongoing abnormalities of fluid and electrolytes within the sinuses. In vitro experiments of hypoxia on ion transport physiology in both murine nasal septal epithelial (MNSE) and human sinonasal epithelial (HSNE) cultures, revealed an impaired transepithelial ion transport related to reduced CFTR function.8 HSNE incubated in hypoxic environment has a globally decreased transepithelial Cl− secretion and increased sodium absorption. These findings indicate that persistent hypoxia may lead to acquired defects in sinonasal Cl−transport in a fashion likely to confer mucociliary dysfunction in CRS. Blount et al49 established sinonasal epithelial CFTR and TMEM16A-mediated Cl− transport and mRNA expression were robustly decreased in an oxygen-depleted environment. This was subsequently identified to reduce ASL and CBF in hypoxic epithelium as measured by micro optical coherence tomography (Fig. 1, Fig. 2).50 Other studies have investigated hypoxia related to COPD and confirmed hypoxia causes a reduction in cell surface CFTR by decreasing steady state CFTR mRNA.45, 51 In mice subjected to hypoxia in vivo, CFTR mRNA expression in airways, gastrointestinal tissues, and the liver were repressed. Therefore environmental factors that induce hypoxic signaling regulate CFTR mRNA and epithelial Cl−transport in vitro and in vivo.51
Fig. 1.
Hypoxic incubation of human sinonasal epithelial cells for 12 h at 1% O2 increases amiloride-sensitive short-circuit current (ΔISC) while decreasing forskolin-stimulated ISC(A) Representative Ussing chamber current tracings and (B) summary of short-circuit current measurements from hypoxia-induced and control HSNE cultures after administration of amiloride, forskolin, and CFTRInh-172. By convention, a positive deflection in the tracing (ΔISC) represents movement of anion in the serosal to mucosal direction. Significant findings are indicated with a bracketed asterisk. Error bars represent standard error of the mean. CFTRInh-172 = cystic fibrosis transmembrane conductance regulator inhibitor 172; HSNE = human sinonasal epithelial cells (Adapted from 50).
Fig. 2.
Hypoxia reduces ASL, PCL, and CBF when measured by micro optical coherence tomography (μOCT). Images demonstrating ASL and PCL thickness (in μm) in control (A) and hypoxia-induced (B) HSNE cultures. ASL = airway surface liquid; PCL = periciliary fluid. Summary data of μOCT measurements (C). (CBF in Hertz (Hz)) (Adapted from 50).
Targeting acquired CFTR dysfunction in CRS
Acquired CFTR dysfunction likely plays a key role in many of the pathways associated with the pathogenesis of CRS and COPD. It has provided a rationale for extension of traditional CF treatment to patients with non-CF and represents a potential focus for the development of novel therapeutic approaches. The fundamentals of CFTR pharmacological modulation is correction of the underlying defects in the cellular processing and Cl− channel function of CFTR. Whilst current therapies treat disease manifestations, such as anti-inflammatory medication, hypertonic saline, mucolytics, antibiotics, and pancreatic enzyme replacement therapy, CFTR potentiators and correctors target the underlying CFTR anion channel defect. CFTR potentiator compounds increase the activity of dysfunctional CFTR at the cell surface, whereas corrector compounds improve defective protein processing and trafficking to the cell surface.18
Ivacaftor
Ivacaftor is an oral drug that has a well-established safety profile and FDA approval for use in CF. It increases Cl− secretion by potentiating CFTR, actively augmenting channel gating. This lends itself to pharmacological application in COPD, asthma, and CRS. The first clinical study of ivacaftor for COPD with chronic bronchitis phenotype in 2016 showed promising results with improved CFTR function following administration.52 Studies have shown that Ivacaftor augments ASL depth, accelerates MCC, and pharmacologically reverses acquired CFTR dysfunction due to cigarette smoke exposure.24, 47
Resveratrol
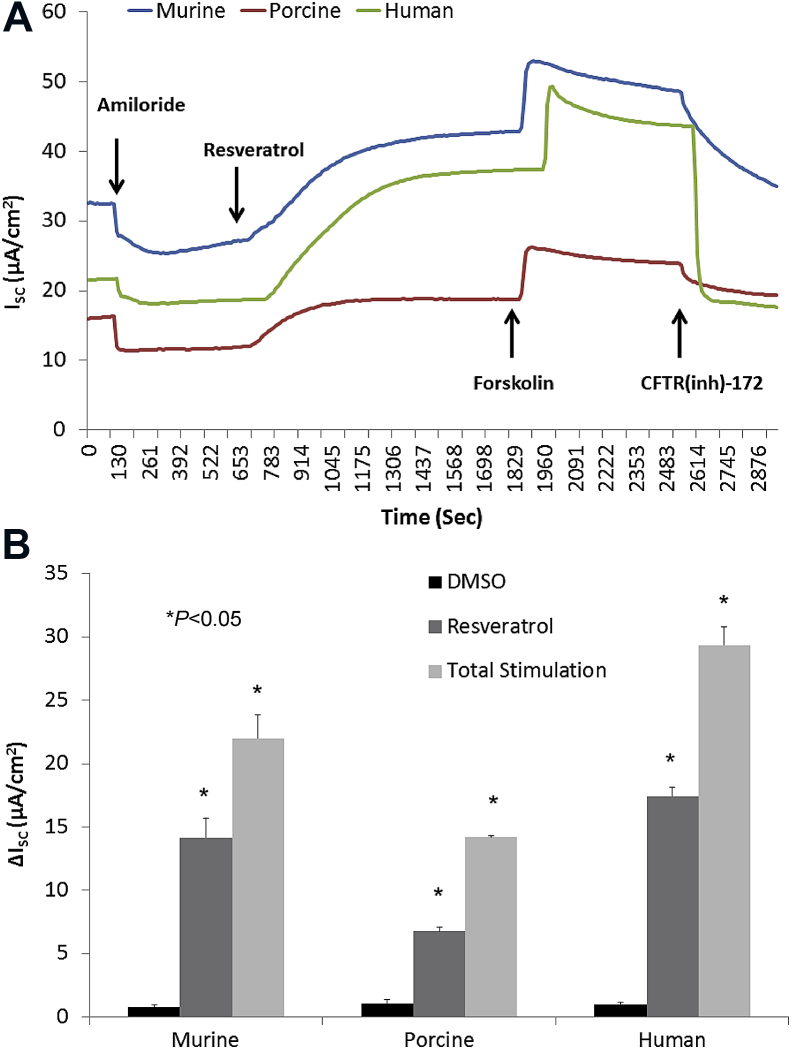
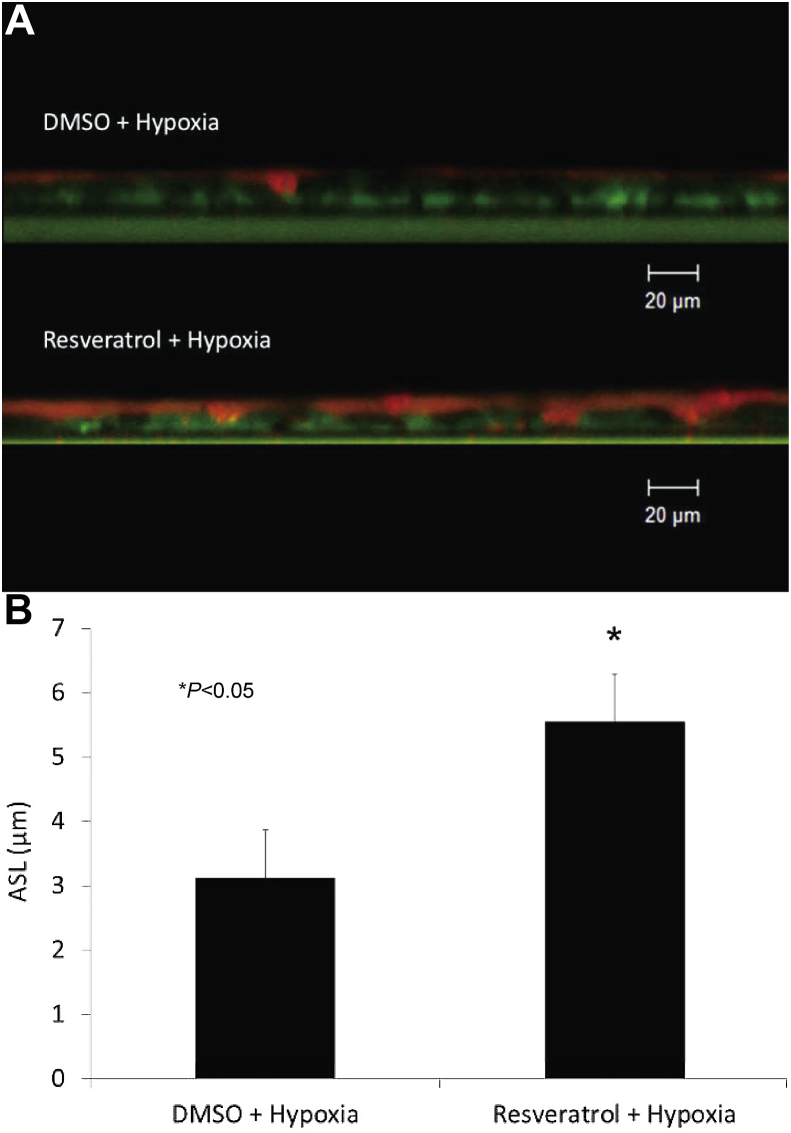
Resveratrol is an organic polyphenol found in many plants and vegetables, including the skins of red fruits, trees, some flowering plants, and peanuts.53, 54 Resveratrol is an immune modulator enhancing CFTR-dependent Cl−secretion in human sinonasal epithelium to an extent comparable to Ivacaftor.8 The drug can stimulate Cl− secretion in multiple species both in vitro and in vivo.55, 56 A 30-minute apical application of resveratrol increased ASL depth in normal epithelium and furthermore, hypoxia-induced abnormalities of fluid and electrolyte secretion in sinonasal epithelium were restored with resveratrol treatment (Fig. 3, Fig. 4). CFTR activation with a leading edge Cl− secretagogue such as resveratrol represents another innovative approach to overcoming acquired CFTR defects in sinus and nasal airway disease.
Fig. 3.
Resveratrol is a robust activator of CFTR-mediated Cl− secretion in primary sinonasal epithelial cell cultures from several mammalian species. (A) Representative Ussing chamber tracings demonstrate resveratrol activation of Cl− transport in murine, human, and porcine sinonasal epithelial cell cultures. (B) Summary data of resveratrol (100 μmol/L) stimulation vs. total stimulation [resveratrol (100 μmol/L) + forskolin (20 μmol/L)] for transepithelial Cl− conductance in murine, human, and porcine primary nasal epithelial cultures. Significant stimulation (P < 0.05) of Cl− transport was demonstrated in primary sinonasal cultures as compared to untreated vehicle controls in all species. Resveratrol (as a percentage of total stimulation) consistently activated between 50% and 70% of total CFTR-mediated anion transport (Adapted from 56).
Fig. 4.
Resveratrol restores hypoxia-depleted ASL Depth. Murine nasal epithelial cultures exhibit similar reduction in ASL with hypoxia as human sinonasal epithelium. Cultures were incubated for 24 h in 1% oxygen and followed by 30 min incubation with resveratrol or DMSO control vehicle and measured by confocal laser scanning microscopy (Panel A represents confocal images, cells – green, ASL - red). Resveratrol treatment significantly mitigated hypoxia-induced reductions in ASL depth (3.13 ± 0.17 μm (DMSO + hypoxia) vs. 5.55 ± 0.74 μm (resveratrol + hypoxia) P < 0.05) (B). (Adapted from 8).
l-ascorbate (commonly known as vitamin C)
L-ascorbatehas been identified as a robust Cl− secretagogue. Application of l-ascorbate to the surface of freshly excised sinonasal tissue stimulated Cl− secretion in a sustained fashion to 70% (in sinus epithelium from CRS) and 53.6% (in nasal epithelium from CRS) of maximally stimulated Cl−currents.25 Insufficient dietary intake of l-ascorbate, environmental pollutants, and a number of inflammatory disorders of the airways are known to severely deplete the pools of this vitamin in ASL or plasma.57 l-ascorbate levels needed for CFTR stimulation exceed plasma concentrations even at high oral doses.58 Topical delivery of l-ascorbate to the paranasal sinuses yields a higher concentration at the target tissues and may be an effective therapeutic modality for loosening thick mucus secretions and improving MCC within the paranasal sinuses.59
Sinupret
Sinupret® has been utilized extensively throughout Europe for more than 70 years as treatment of airway diseases associated with inadequate MCC, and has an excellent safety profile.45 The medication is registered as a regulated phyto-pharmaceutical in Germany and has been available in the United States for 9 years where it is considered an herbal supplement (not regulated by the FDA).45 A randomized, placebo-controlled trial demonstrated Sinupret either alone or in combination with antibiotics for CRS demonstrated significant improvement in radiologic outcomes and headache.60 Studies using upper airway epithelial cells demonstrated that Sinupret likely derives its clinical benefit, through stimulating transepithelial chloride secretion, CBF, and MCC.45, 61
Conclusion
The role of acquired CFTR deficiency in diseases of dysfunctional MCC in non-CF individuals has only recently been elucidated. Targeting acquired CFTR dysfunction in non-CF airway disease with Cl− secretagogues represents a novel treatment strategy that may benefit a larger patient population afflicted with respiratory diseases.
Conflicts of interest
Bradford A. Woodworth, MD is a consultant for Cook Medical, which is not affiliated with this work.
Acknowledgement
This work was supported by National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute (1 R01 HL133006-03) and National Institute of Diabetes and Digestive and Kidney Diseases (5P30DK072482-04, CF Research Center Pilot Award) to B.A.W., and John W. Kirklin Research and Education Foundation Fellowship Award and UAB Faculty Development Research Award to D.Y.C.
Edited by Yu-Xin Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Wanner A., Salathe M., O'Riordan T.G. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–1902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- 2.Knowles M., Gatzy J., Boucher R. Relative ion permeability of normal and cystic fibrosis nasal epithelium. J Clin Invest. 1983;71:1410–1417. doi: 10.1172/JCI110894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rowe S.M., Miller S., Sorscher E.J. Cystic fibrosis. N Engl J Med. 2005;352:1992–2001. doi: 10.1056/NEJMra043184. [DOI] [PubMed] [Google Scholar]
- 4.Alexander N.S., Blount A., Zhang S. Cystic fibrosis transmembrane conductance regulator modulation by the tobacco smoke toxin acrolein. Laryngoscope. 2012;122:1193–1197. doi: 10.1002/lary.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Azbell C., Zhang S., Skinner D., Fortenberry J., Sorscher E.J., Woodworth B.A. Hesperidin stimulates cystic fibrosis transmembrane conductance regulator-mediated chloride secretion and ciliary beat frequency in sinonasal epithelium. Otolaryngol Head Neck Surg. 2010;143:397–404. doi: 10.1016/j.otohns.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Kim J., McWilliams R., Cutting G.R. Increased prevalence of chronic rhinosinusitis in carriers of a cystic fibrosis mutation. Arch Otolaryngol Head Neck Surg. 2005;131:237–240. doi: 10.1001/archotol.131.3.237. [DOI] [PubMed] [Google Scholar]
- 7.Pinto J.M., Hayes M.G., Schneider D., Naclerio R.M., Ober C. A genomewide screen for chronic rhinosinusitis genes identifies a locus on chromosome 7q. Laryngoscope. 2008;118:2067–2072. doi: 10.1097/MLG.0b013e3181805147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodworth B.A. Resveratrol ameliorates abnormalities of fluid and electrolyte secretion in a hypoxia-Induced model of acquired CFTR deficiency. Laryngoscope. 2015;125(Suppl 7) doi: 10.1002/lary.25335. S1-1S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohn J.A., Friedman K.J., Noone P.G., Knowles M.R., Silverman L.M., Jowell P.S. Relation between mutations of the cystic fibrosis gene and idiopathic pancreatitis. N Engl J Med. 1998;339:653–658. doi: 10.1056/NEJM199809033391002. [DOI] [PubMed] [Google Scholar]
- 10.Cantin A.M. Cystic fibrosis transmembrane conductance regulator. Implications in cystic fibrosis and chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2016;13(Suppl 2):S150–S155. doi: 10.1513/AnnalsATS.201509-588KV. [DOI] [PubMed] [Google Scholar]
- 11.Clunes L.A., Davies C.M., Coakley R.D. Cigarette smoke exposure induces CFTR internalization and insolubility, leading to airway surface liquid dehydration. FASEB J. 2012;26:533–545. doi: 10.1096/fj.11-192377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gentile V.G., Isaacson G. Patterns of sinusitis in cystic fibrosis. Laryngoscope. 1996;106:1005–1009. doi: 10.1097/00005537-199608000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen J.E., Sheridan J.T., Polk W., Davies C.M., Tarran R. Cigarette smoke-induced Ca2+ release leads to cystic fibrosis transmembrane conductance regulator (CFTR) dysfunction. J Biol Chem. 2014;289:7671–7681. doi: 10.1074/jbc.M113.545137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomberger J.M., Ye S., Maceachran D.P. A Pseudomonas aeruginosa toxin that hijacks the host ubiquitin proteolytic system. Plos Pathog. 2011;7:e1001325. doi: 10.1371/journal.ppat.1001325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen N.A., Zhang S., Sharp D.B. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. [DOI] [PubMed] [Google Scholar]
- 16.Londino J.D., Lazrak A., Noah J.W. Influenza virus M2 targets cystic fibrosis transmembrane conductance regulator for lysosomal degradation during viral infection. FASEB J. 2015;29:2712–2725. doi: 10.1096/fj.14-268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raju S.V., Solomon G.M., Dransfield M.T., Rowe S.M. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis and other diseases of mucus clearance. Clin Chest Med. 2016;37:147–158. doi: 10.1016/j.ccm.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Illing E.A., Woodworth B.A. Management of the upper airway in cystic fibrosis. Curr Opin Pulm Med. 2014;20:623–631. doi: 10.1097/MCP.0000000000000107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaaban M.R., Walsh E.M., Woodworth B.A. Epidemiology and differential diagnosis of nasal polyps. Am J Rhinol Allergy. 2013;27:473–478. doi: 10.2500/ajra.2013.27.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaaban M.R., Kejner A., Rowe S.M., Woodworth B.A. Cystic fibrosis chronic rhinosinusitis: a comprehensive review. Am J Rhinol Allergy. 2013;27:387–395. doi: 10.2500/ajra.2013.27.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irving R.M., McMahon R., Clark R., Jones N.S. Cystic fibrosis transmembrane conductance regulator gene mutations in severe nasal polyposis. Clin Otolaryngol Allied Sci. 1997;22:519–521. doi: 10.1046/j.1365-2273.1997.00058.x. [DOI] [PubMed] [Google Scholar]
- 22.Varon R., Magdorf K., Staab D. Recurrent nasal polyps as a monosymptomatic form of cystic fibrosis associated with a novel in-frame deletion (591del18) in the CFTR gene. Hum Mol Genet. 1995;4:1463–1464. doi: 10.1093/hmg/4.8.1463. [DOI] [PubMed] [Google Scholar]
- 23.Wang X., Moylan B., Leopold D.A. Mutation in the gene responsible for cystic fibrosis and predisposition to chronic rhinosinusitis in the general population. JAMA. 2000;284:1814–1819. doi: 10.1001/jama.284.14.1814. [DOI] [PubMed] [Google Scholar]
- 24.Raman V., Clary R., Siegrist K.L., Zehnbauer B., Chatila T.A. Increased prevalence of mutations in the cystic fibrosis transmembrane conductance regulator in children with chronic rhinosinusitis. Pediatrics. 2002;109:E13. doi: 10.1542/peds.109.1.e13. [DOI] [PubMed] [Google Scholar]
- 25.Cho D.Y., Hwang P.H., Illek B. Effect of L-ascorbate on chloride transport in freshly excised sinonasal epithelia. Am J Rhinol Allergy. 2009;23:294–299. doi: 10.2500/ajra.2009.23.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantin A.M., Hanrahan J.W., Bilodeau G. Cystic fibrosis transmembrane conductance regulator function is suppressed in cigarette smokers. Am J Respir Crit Care Med. 2006;173:1139–1144. doi: 10.1164/rccm.200508-1330OC. [DOI] [PubMed] [Google Scholar]
- 27.Rab A., Rowe S.M., Raju S.V., Bebok Z., Matalon S., Collawn J.F. Cigarette smoke and CFTR: implications in the pathogenesis of COPD. Am J Physiol Lung Cell Mol Physiol. 2013;305:L530–L541. doi: 10.1152/ajplung.00039.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsh M.J. Cigarette smoke inhibition of ion transport in canine tracheal epithelium. J Clin Invest. 1983;71:1614–1623. doi: 10.1172/JCI110917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kreindler J.L., Jackson A.D., Kemp P.A., Bridges R.J., Danahay H. Inhibition of chloride secretion in human bronchial epithelial cells by cigarette smoke extract. Am J Physiol Lung Cell Mol Physiol. 2005;288:L894–L902. doi: 10.1152/ajplung.00376.2004. [DOI] [PubMed] [Google Scholar]
- 30.Dransfield M.T., Wilhelm A.M., Flanagan B. Acquired cystic fibrosis transmembrane conductance regulator dysfunction in the lower airways in COPD. Chest. 2013;144:498–506. doi: 10.1378/chest.13-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloane P.A., Shastry S., Wilhelm A. A pharmacologic approach to acquired cystic fibrosis transmembrane conductance regulator dysfunction in smoking related lung disease. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Christensen D.N., Franks Z.G., McCrary H.C., Saleh A.A., Chang E.H. A systematic review of the association between cigarette smoke exposure and chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2018;158:801–816. doi: 10.1177/0194599818757697. [DOI] [PubMed] [Google Scholar]
- 33.Reh D.D., Higgins T.S., Smith T.L. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol. 2012;2:362–369. doi: 10.1002/alr.21054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hur K., Liang J., Lin S.Y. The role of secondhand smoke in sinusitis: a systematic review. Int Forum Allergy Rhinol. 2014;4:22–28. doi: 10.1002/alr.21232. [DOI] [PubMed] [Google Scholar]
- 35.Benninger M.S. The impact of cigarette smoking and environmental tobacco smoke on nasal and sinus disease: a review of the literature. Am J Rhinol. 1999;13:435–438. doi: 10.2500/105065899781329683. [DOI] [PubMed] [Google Scholar]
- 36.Tamashiro E., Cohen N.A., Palmer J.N., Lima W.T. Effects of cigarette smoking on the respiratory epithelium and its role in the pathogenesis of chronic rhinosinusitis. Braz J Otorhinolaryngol. 2009;75:903–907. doi: 10.1016/S1808-8694(15)30557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramadan H.H., Hinerman R.A. Smoke exposure and outcome of endoscopic sinus surgery in children. Otolaryngol Head Neck Surg. 2002;127:546–548. doi: 10.1067/mhn.2002.129816. [DOI] [PubMed] [Google Scholar]
- 38.Ramadan H.H. Surgical management of chronic sinusitis in children. Laryngoscope. 2004;114:2103–2109. doi: 10.1097/01.mlg.0000149441.28231.0c. [DOI] [PubMed] [Google Scholar]
- 39.Kim H.Y., Dhong H.J., Chung S.K., Chung Y.J., Min J.Y. Prognostic factors of pediatric endoscopic sinus surgery. Int J Pediatr Otorhinolaryngol. 2005;69:1535–1539. doi: 10.1016/j.ijporl.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Virgin F.W., Azbell C., Schuster D. Exposure to cigarette smoke condensate reduces calcium activated chloride channel transport in primary sinonasal epithelial cultures. Laryngoscope. 2010;120:1465–1469. doi: 10.1002/lary.20930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solomon G.M., Fu L., Rowe S.M., Collawn J.F. The therapeutic potential of CFTR modulators for COPD and other airway diseases. Curr Opin Pharmacol. 2017;34:132–139. doi: 10.1016/j.coph.2017.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fernandez F.E., De Santi C., De Rose V., Greene C.M. CFTR dysfunction in cystic fibrosis and chronic obstructive pulmonary disease. Expert Rev Respir Med. 2018;12:483–492. doi: 10.1080/17476348.2018.1475235. [DOI] [PubMed] [Google Scholar]
- 43.Hogg J.C., Paré P.D., Hackett T.L. The contribution of small airway obstruction to the pathogenesis of chronic obstructive pulmonary disease. Physiol Rev. 2017;97:529–552. doi: 10.1152/physrev.00025.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Church D.F., Pryor W.A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S., Skinner D., Hicks S.B. Sinupret activates CFTR and TMEM16A-dependent transepithelial chloride transport and improves indicators of mucociliary clearance. PLoS One. 2014;9:e104090. doi: 10.1371/journal.pone.0104090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pahl A., Szelenyi S., Brune K. Hypoxia induced chemokine expression in nasal epithelial cells: development of an in vitro model for chronic rhinosinusitis. ALTEX. 2006;23:59–63. [PubMed] [Google Scholar]
- 47.Steinke J.W., Woodard C.R., Borish L. Role of hypoxia in inflammatory upper airway disease. Curr Opin Allergy Clin Immunol. 2008;8:16–20. doi: 10.1097/ACI.0b013e3282f3f488. [DOI] [PubMed] [Google Scholar]
- 48.Matsune S., Kono M., Sun D., Ushikai M., Kurono Y. Hypoxia in paranasal sinuses of patients with chronic sinusitis with or without the complication of nasal allergy. Acta Otolaryngol. 2003;123:519–523. doi: 10.1080/0036554021000028113. [DOI] [PubMed] [Google Scholar]
- 49.Blount A., Zhang S., Chestnut M. Transepithelial ion transport is suppressed in hypoxic sinonasal epithelium. Laryngoscope. 2011;121:1929–1934. doi: 10.1002/lary.21921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tipirneni K.E., Grayson J.W., Zhang S. Assessment of acquired mucociliary clearance defects using micro-optical coherence tomography. Int Forum Allergy Rhinol. 2017;7:920–925. doi: 10.1002/alr.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guimbellot J.S., Fortenberry J.A., Siegal G.P. Role of oxygen availability in CFTR expression and function. Am J Respir Cell Mol Biol. 2008;39:514–521. doi: 10.1165/rcmb.2007-0452OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Solomon G.M., Raju S.V., Dransfield M.T., Rowe S.M. Therapeutic approaches to acquired cystic fibrosis transmembrane conductance regulator dysfunction in chronic bronchitis. Ann Am Thorac Soc. 2016;13(Suppl 2):S169–S176. doi: 10.1513/AnnalsATS.201509-601KV. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Donnelly L.E., Newton R., Kennedy G.E. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. Am J Physiol Lung Cell Mol Physiol. 2004;287:L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 54.Lee M., Kim S., Kwon O.K., Oh S.R., Lee H.K., Ahn K. Anti-inflammatory and anti-asthmatic effects of resveratrol, a polyphenolic stilbene, in a mouse model of allergic asthma. Int Immunopharmacol. 2009;9:418–424. doi: 10.1016/j.intimp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 55.Alexander N.S., Hatch N., Zhang S. Resveratrol has salutary effects on mucociliary transport and inflammation in sinonasal epithelium. Laryngoscope. 2011;121:1313–1319. doi: 10.1002/lary.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang S., Blount A.C., McNicholas C.M. Resveratrol enhances airway surface liquid depth in sinonasal epithelium by increasing cystic fibrosis transmembrane conductance regulator open probability. PLoS One. 2013;8 doi: 10.1371/journal.pone.0081589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kodavanti U.P., Costa D.L., Richards J., Crissman K.M., Slade R., Hatch G.E. Antioxidants in bronchoalveolar lavage fluid cells isolated from ozone-exposed normal and ascorbate-deficient guinea pigs. Exp Lung Res. 1996;22:435–448. doi: 10.3109/01902149609046034. [DOI] [PubMed] [Google Scholar]
- 58.Levine M., Conry-Cantilena C., Wang Y. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996;93:3704–3709. doi: 10.1073/pnas.93.8.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fischer H., Schwarzer C., Illek B. Vitamin C controls the cystic fibrosis transmembrane conductance regulator chloride channel. Proc Natl Acad Sci U S A. 2004;101:3691–3696. doi: 10.1073/pnas.0308393100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richstein A., Mann W. Treatment of chronic sinusitis with sinupret. Ther Ggw. 1980;119:1055–1060. [PubMed] [Google Scholar]
- 61.Virgin F., Zhang S., Schuster D. The bioflavonoid compound, sinupret, stimulates transepithelial chloride transport in vitro and in vivo. Laryngoscope. 2010;120:1051–1056. doi: 10.1002/lary.20871. [DOI] [PubMed] [Google Scholar]