Abstract
Background
The aim of the study was to evaluate whether the use of chemotherapy in combination with naringin, a dietary plant polyphenolic flavonoid, could enhance the therapeutic efficacy of paclitaxel treatment in human prostate cancer (PCa) cells.
Materials and methods
DU145, PC3, and LNCaP cells were treated with various concentrations of paclitaxel, naringin, and their combinations. Methylthiazolyldiphenyl-tetrazolium bromide (MTT), image-based cytometer, quantitative reverse transcription PCR (RT-qPCR), Western blot, and transwell assay were used to evaluate cell viability, apoptosis and cell cycle, the mRNA expression, protein expression, and cell migration, respectively.
Results
Naringin treatment inhibited cell survival in a dose- and time-dependent manner by inducing apoptosis and cell cycle arrest in G1 phase. Among the pathways evaluated, naringin (150 μM) significantly induced the mRNA expressions of BAX, BID, caspase 3, cytochrome c, p53, p21Cip1, and p27Kip1 and downregulated the expressions of survivin and livin in DU145 cells. The combination of naringin and paclitaxel treatments synergistically increased the cytotoxic effects of paclitaxel in androgen-independent DU145 and PC3 cells, as well as in androgen-sensitive LNCaP cells. The combination of naringin with docetaxel has almost the same inhibitory effect on cell proliferation as the paclitaxel combination in androgen-independent cells, whereas there is no similar effect in LNCaP cells. Naringin exhibits significant inhibitory effects on the cell migration ability. The flavonoid either alone or in combination with paclitaxel therapy resulted in an increase in tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome 10) protein expression and decrease in nuclear factor-κB p50 protein level in DU145 cells.
Conclusion
In conclusion, naringin acts as a chemosensitizer which synergistically strengths the cytotoxic effect of paclitaxel in PCa cells. Therefore, naringin therapy alone or in combination with paclitaxel may be useful in the treatment of PCa. However, there is a need for more detailed in vivo studies of the mechanism of action.
Keywords: Apoptosis, Cell migration, Chemotherapy, Naringin, Prostate cancer
1. Introduction
The main therapeutic option of advanced prostate cancer (PCa) is androgen deprivation therapy with limited clinical outcomes. However, the therapeutic benefit does not last long and most patients develop metastatic castration-resistant prostate cancer (CRPC).1 After the development of PCa into CRPC, there are several drugs that prolong life, including taxanes such as paclitaxel, docetaxel, and cabazitaxel and new androgen receptor targeting agents such as abiraterone acetate and enzalutamide.2 Microtubule-disrupting agents such as taxanes and vinca alkaloids trigger apoptosis by aberrant mitosis or by subsequent multinucleated G1-like state related to mitotic slippage, depends on cell type and drug schedule.3 Though taxane chemotherapy is standard first-line therapy for recurrent metastatic CRPC, relapse eventually occurs because of the development of drug resistance.4 Therefore, there is an essential need for novel effective approaches in the development of novel therapeutic targets and molecular regulatory agents for the treatment of CRPC.
Naringin (4′,5,7-trihydroxy flavanone-7-rhamnoglucoside) is a natural glycoside known as bioflavonoid derived from grapefruit and other citrus fruits. Studies have shown that naringin possesses many beneficial pharmacological properties, such as antiinflammatory, antioxidant,5 and anticancer6, 7, 8 activities in vivo and in vitro.9 It has been shown that naringin not only suppresses cancer cell proliferation, but also protects against cisplatin-induced kidney injury through antiapoptotic and antiinflammatory effects.10 Strategies for combination therapy of cancer with chemotherapeutics and natural polyphenols may enhance the potential of the treatment and help to decrease the side effects of standard antitumor therapies.11
The phosphatidylinositol 3′ kinase (PI3K)/Akt signaling pathway is an actively pursued therapeutic target in oncology.12 The activation of this axis is emerging as a central feature of epithelial–mesenchymal transition (EMT). EMT has been associated with the invasiveness and the distant metastasis of PCa. Transcription factors such as Twist and Snail are involved in the downregulation of EMT activation.13 In this study, we examined the effects of naringin, paclitaxel, or their combination treatment on the cell migration of the PCa cell lines in vitro. Loss or alteration of at least one PTEN (phosphatase and tensin homolog deleted on chromosome 10) allele occurs in 70–80% of primary PCa.14 PTEN, a critical tumor and metastasis suppressor gene negatively regulates cell growth, proliferation, migration, and angiogenesis via the PI3K/Akt pathway.15, 16 Inhibition of nuclear factor-κB (NF-κB) activity could suppress PCa invasion and metastasis.15, 17 Recent studies have demonstrated that naringin inhibits tumor growth via inhibiting NF-κB7 and downregulating PI3K/Akt pathway in HeLa cells and human AGS gastric cancer cells,18 respectively. Thus, preventing PI3K/Akt signaling and inhibiting NF-κB provide potential targets for tumor therapeutic strategies.
The dietary bioflavonoid naringin has not been adequately studied as a chemosensitizer in the treatment of PCa. The underlying mechanisms of naringin and in combination with paclitaxel (and vincristine) in the treatment of PCa cells remain to be fully elucidated.
2. Material and methods
2.1. Cell culture and reagents
The human PCa cell lines DU145, PC3, and LNCaP were purchased from the ATCC (Manassas, VA, USA). The cells were seeded in complete medium Dulbecco's Modified Eagle Medium/Nutrient Mixture F-12K (DMEM/F-12K) medium (DMEM/Ham's F-12 Mix 50/50; Winsent, Quebec, Canada) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Carlbad, CA) and maintained at 37°C in a humidified incubator at 5% CO2. Paclitaxel (Sindaxel, Actavis, Italy) and naringin (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in 100% dimethyl sulfoxide (DMSO) and stored at −20°C until use. Paclitaxel and naringin were concomitantly administrated to the cultures. The final concentration of DMSO did not exceed 0.1%, and controls received the same volume of vehicle.
2.2. Cell proliferation assay
To quantify the effects of naringin, paclitaxel, or their combination on cell survival, DU145 cells were placed on 96-well plates (104 cells/well) for overnight incubation. The cells were then treated with different concentrations of paclitaxel (0–100 nM) and naringin (0–500 μM) or 5 nM paclitaxel with 150 μM naringin for 72 hour in complete medium. Each treatment was repeated three times. After the incubation period cells were incubated with MTT (Sigma–Aldrich, St. Louis, MO, USA) solution [(1 mg/mL)/well] for 3 hour. The medium was removed, MTT tests were performed, and absorbance at 570 nm was measured using a plate reader (Multiskan GO, Thermo Scientific, Vantaa, Finland).
The efficacy of naringin and paclitaxel or docetaxel (Cayman, MI, USA) combination therapy on cell survival was performed in PC3 and LNCaP lines cells.
2.3. Detection of apoptotic cells
To evaluate the degree of apoptosis, the Tali image-based cytometer apoptosis kit—annexin V Alexa Fluor 488 and propidium iodide—was used according to the manufacturer's instructions (Invitrogen/Life Technologies, Carlsbad, CA, USA). In brief, DU145 and PC3 were incubated at a density of 2.5 × 105 in a 6-well plate for 16 hour and then treated with paclitaxel (5 nM), naringin (150 μΜ), and their combination. The cells were collected 72 hour posttreatment and washed twice with ice-cold phosphate buffer saline (PBS) and were evaluated by Tali image-based cytometer within 30 minutes.
2.4. Analysis of cell cycle
DU145 and PC3 cells were treated with 150 μM of naringin, 5 nM of paclitaxel, 5 nM of vincristine (Kocak Farma, Istanbul, Turkey) or the combinations for 72 hour, harvested, fixed in 70% prechilled ethanol (−20°C) and were set at 4°C overnight. Cells were resuspended in propidium iodide (PI) buffer (50 g/mL PI and 100 μg/mL RNase) and incubated at room temperature for 30 minutes in the dark. Cells were then washed twice with 1 × PBS and subjected to Tali image-based cytometer.
2.5. RNA extraction, cDNA synthesis, and RT-qPCR
Total RNA was purified using RNA purification kit, and RNAs were then subjected to cDNA synthesis using reverse transcription cDNA synthesis kit (Thermo Fisher Scientific, Vilnius, Lithuania). The resultant cDNAs were used for real-time PCR analysis with gene-specific primer pairs. mRNA expression analyses were performed on a Step One Plus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA). The PCR cycling conditions included an initial denaturation at 95ºC for 5 minutes followed by 36 cycles at 95ºC for 15 seconds and 60ºC for 60 seconds. The oligonucleotide primers (Table 1) were synthesized by PZR Biotech (Ankara, Turkey). Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) expressions were used as an internal reference, and all reactions were run in triplicate, and performed three times on separate occasions using different RNA.
Table 1.
Primer sequences used in this study RT-qPCR was performed with co-amplification of the genes with a reference gene by use of the cDNA template and corresponding gene-specific primer sets.
| Genes and accession number | Primers |
|---|---|
|
BAX NM_001291428.1 |
F: 5′-TTGCTTCAGGGTTTCATCCA-3′ |
| R: 5′-CAGCCTTGAGCACCAGTTTG-3′ | |
|
BID NM_001244572.1 |
F: 5′- CCTACCCTAGAGACATGGAGAAG-3′ |
| R: 5′- TTTCTGGCTAAGCTCCTCACG-3′ | |
|
Bcl-2 NM_000633.2 |
F: 5′-ATGTGTGTGGAGAGCGTCAA-3′ |
| R: 5′-ACAGTTCCACAAAGGCATCC-3′ | |
|
Caspase 3 NM_001354779.1 |
F: 5′-GGCATTGAGACAGACAGTGG-3′ |
| R: 5′-CATGGAATCTGTTTCTTTGC-3′ | |
|
Caspase 8 XM_005246894.3 |
F: 5′-CTGCTGGGGATGGCCACTGTG-3′ |
| R: 5′-TCGCCTCGAGGACATCGCTCTC-3′ | |
|
Cytochrome c NM_018947.5 |
F: 5′-AGTGGCTAGAGTGGTCATTCATTTACA-3′ |
| R: 5′-TCATGATCTGAATTCTGGTGTATGAGA-3′ | |
|
Caspase 9 NM_032996.3 |
F: 5′-GAGTCAGGCTCTTCCTTTG-3′ |
| R: 5′-CCTCAAACTCTCAAGAGCAC-3′ | |
|
Survivin NM_001168.2 |
F: 5′-GACGACCCCATAGAGGAACA-3′ |
| R: 5′-GACAGAAAGGAAAGCGCAAC-3′ | |
|
Livin NM_022161.3 |
F: 5′-TGGCCTCCTTCTATGACTGG-3′ |
| R: 5′- ACCTCACCTTGTCCTGATGG-3′ | |
|
p21Cip1 NM_001291549.1 |
F: 5′-GGCGTTTGGAGTGGTAGAAA-3′ |
| R: 5′-GACTCTCAGGGTCGAAAACG-3′ | |
|
p27Kip1 NM_004064.4 |
F: 5′-CCGGCTAACTCTGAGGACAC-3′ |
| R: 5′-TTGCAGGTCGCTTCCTTATT-3′ | |
|
p53 NM_001126118.1 |
F: 5′-GAGGTTGGCTCTGACTGTACC-3′ |
| R: 5′-TCCGTCCCAGTAGATTACCAC-3′ | |
|
Snail NM_005985.3 |
F: 5′-AGACCCACTCAGATGTCAA-3′ |
| R: 5′-CATAGTTAGTCACACCTCGT-3′ | |
|
Twist NM_000474.3 |
F: 5′- GGGAGTCCGCAGTCTTAC-3′ |
| R: 5′- CCTGTCTCGCTTTCTCTTT-3′ | |
|
c-Myc NM_002467.5 |
F: 5′- AGCGACTCTGAGGAGGAACAAG-3′ |
| R: 5′- CCTGCCTCTTTTCCACAGAAA-3′ | |
|
GAPDH NM_001289745.2 |
F: 5′-TTGGTATCGTGGAAGGACTCA-3′ |
| R: 5′-TGTCATCATATTTGGCAGGTTT-3′ |
2.6. Transwell migration assays
To test the effect of naringin and its combination with paclitaxel on cell migration activity, transwell cell invasion assay was performed.19 Briefly the bottom chambers (Corning, Kennebunk, ME, USA) were filled with 750 μl of DMEM/F-12K medium with 10% FBS supplemented with or without the agents. Following 22 hour of migration, the DU145 cells were fixed with 4% paraformaldehyde and washed with PBS. After staining with 0.1% crystal violet, pictures of the migrated cells were taken using a microscope with a 10× objective (total magnification 100×).
2.7. Immunoblot analysis
Confluent cells were lysed in RIPA lysis buffer containing protease inhibitor cocktail (Thermo Fisher Scientific, Rockford, IL, USA). After addition of reducing agent, the samples were boiled and then loaded directly to an appropriate well of a NuPAGE® Bis-Tris polyacrylamide gel (10%) immersed in NuPAGE® MOPS SDS running buffer. Separated proteins were transferred to iBlot® Transfer Stack, polyvinylidene difluoride (PVDF) membranes (Life Technologies, Carlsbad, CA, USA). Membranes were incubated with monoclonal NF-κB p50 and β-actin (Novus Biologicals, Littleton, CO, USA), and monoclonal PTEN (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) primary antibodies overnight, followed by incubation with secondary antibodies for 1 hour. The immunoreactive bands were identified using enhanced chemiluminescence (Chemi detection system Mouse-EA, Waltham, MA, USA) by gel imaging system.
2.8. Analysis of drug combinations
The interaction between paclitaxel and naringin was estimated by the isobologram method and the median effect method defined by Chou and Talalay.20 CompuSyn software was used for analysis of combination data in the synergistic studies.
2.9. Statistical analyses
The half maximal inhibitory concentration (IC50) values were calculated using sigmoidal nonlinear regression analyses of cell inhibition as a ratio of the control using GraphPad Prism, version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Each experiment was performed in triplicate and was repeated at least twice; each data point represents the mean ± standard deviation. Statistical significance was compared between the various treatment groups and the controls using one-way analysis of variance followed by Duncan's multiple range test for multiple comparisons. A P < 0.05 was considered to indicate a statistically significant difference (SPSS, v19.0, Chicago, IL).
3. Results
3.1. Naringin inhibits cell survival
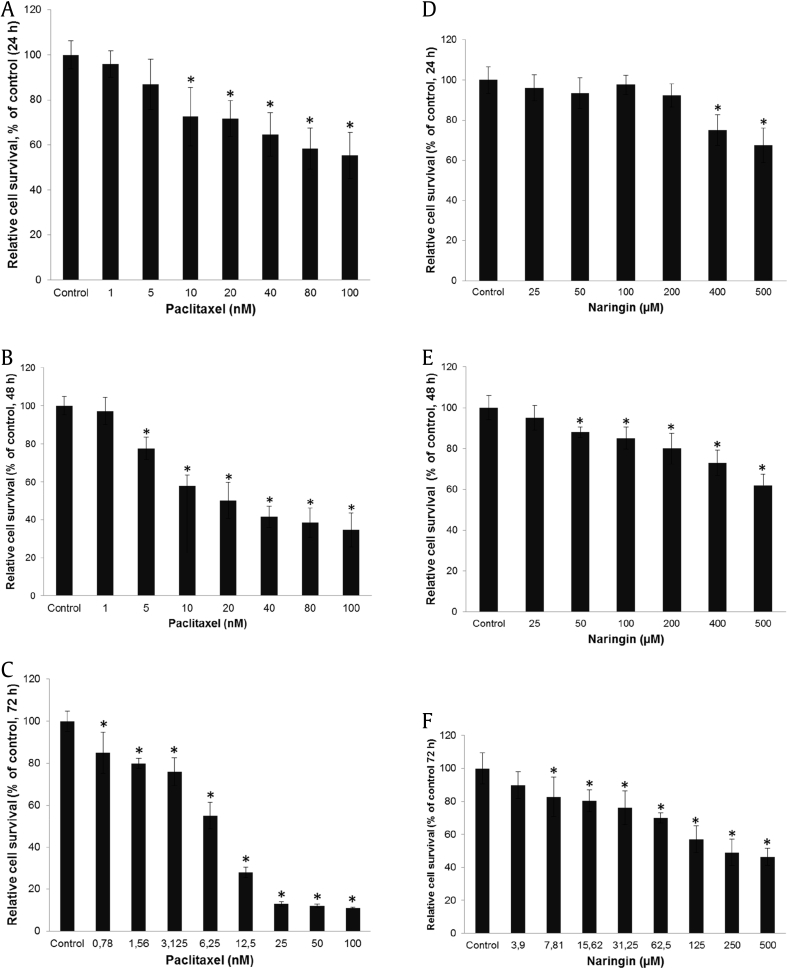
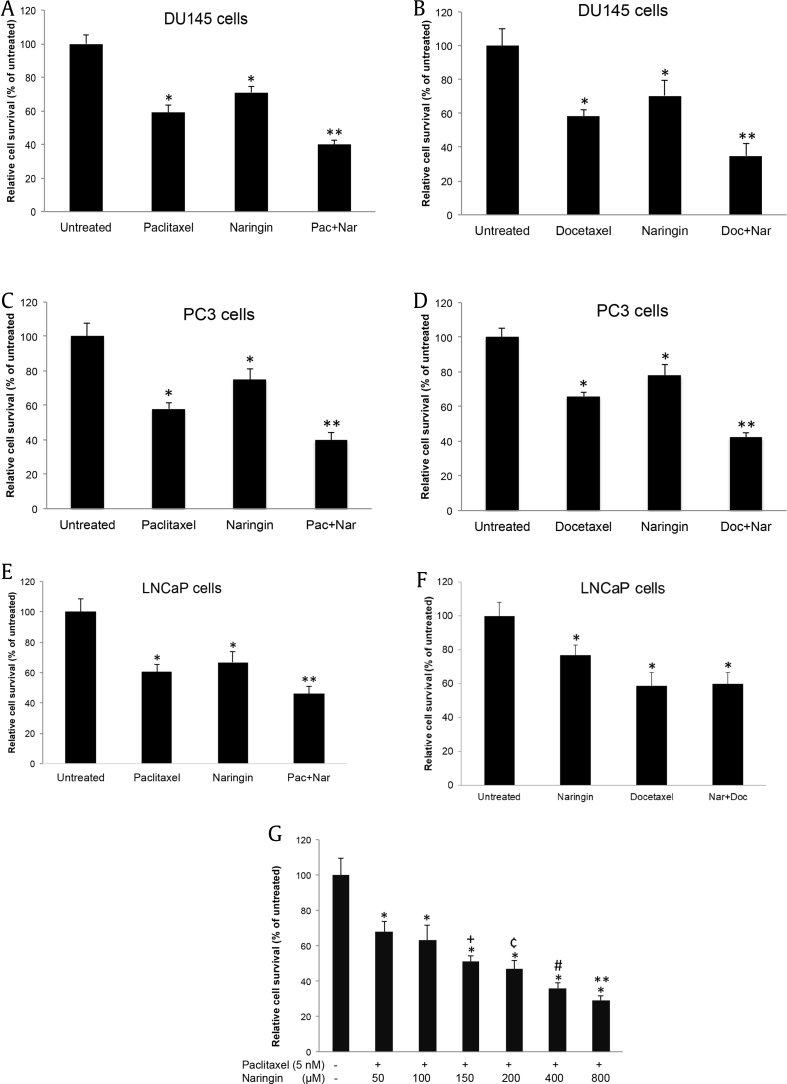
To assess the sensitivity of DU145 cells to the cytotoxic/cytostatic effects of naringin (3.9 μM–500 μM) and paclitaxel (0.78 nM–100 nM), the cells were seeded into 96-well microplates and incubated with different concentrations of agents as mentioned in Section 2. As shown in Fig. 1, paclitaxel (Fig. 1A–C) and naringin (Fig. 1D–F) reduced the viability of cells in a dose- and time-dependent manner. The IC50 values for DU145 cells were 5 nM for paclitaxel and 150 μM for naringin, at 72 hour treatment. Androgen-sensitive prostate cancer LNCaP cells were found to be more resistant to naringin treatment with the IC50 value of 260 μM, while PC3 cells were determined to be more sensitive to naringin therapy with an IC50 value of 101 μM (data not shown). To reveal possible synergy or additive effects, combinations of 5 nM paclitaxel and 150 μM naringin were administrated to the cells for 72 hour. The combined treatment did significantly strengthen paclitaxel's cell cytotoxicity effect in DU145 (Fig. 2A), PC3 (Fig. 2C) and LNCaP (Fig. 2E) cells. An intermediate synergism was detected with naringin and paclitaxel in DU145 cells where combination index < 0.72. Dose escalation of naringin (50–800 μM) with constant paclitaxel (5 nM) resulted in a dose-dependent decrease of DU145 cell survival (Fig. 2G).
Fig. 1.
Naringin inhibits the proliferation of human DU145 prostate cancer cell viability. Survival rate of the cells treated with different concentrations of paclitaxel (A–C) and naringin (D–F) for indicated hours treatment. Control cells were treated with DMSO-alone. *P < 0.05 versus untreated. Cell survival was determined following treatment and analyzed by MTT assay. The results were expressed as a percentage of the control value (mean ± SD of three experiments performed in octuplicate wells). SD, standard deviation.
Fig. 2.
Efficacy of combination therapy and survival rate in castration-sensitive and -resistant prostate cancer cell lines. DU145 (A, B), PC3 (C, D) and LNCaP (E, F) cells were treated with 5 nM paclitaxel or 5 nM docetaxel, 150 μM naringin or their combinations for 72 h, respectively. Untreated cells were incubated with DMSO-alone. *P < 0.05 versus untreated; **P < 0.05 versus paclitaxel or docetaxel and untreated group. (G) Increased concentrations of naringin with stable dose of paclitaxel (5 nM) significantly enhances the cytotoxic effect of paclitaxel in DU145 cell, treated for 72 h, *P < 0.001 versus untreated, **P < 0.05 versus 50–800, #P < 0.05 versus 50–200, ¢P < 0.05 versus 50–100, +P < 0.05 versus 50 and 100. Cell survival was determined following therapy, and analyzed by MTT test. The results were expressed as a percentage of the untreated value (mean ± SD of three experiments performed in octuplicate wells). Pac + Nar: paclitaxel and naringin; SD, standard deviation.
We also analyzed whether the combination of naringin with another microtubule stabilizer, docetaxel, could show similar effect with paclitaxel. To test this, DU145 (Fig. 2B), PC3 (Fig. 2D) and LNCaP cells (Fig. 2F) were incubated with 5 nM docetaxel, 150 μM naringin, and a combination of the two for 72 hours. Although combination of docetaxel with naringin had a similar effect to paclitaxel in androgen-independent cells, it did not show similar synergy in androgen-sensitive LNCaP cells.
3.2. Naringin induces apoptosis of DU145 and PC3 cells
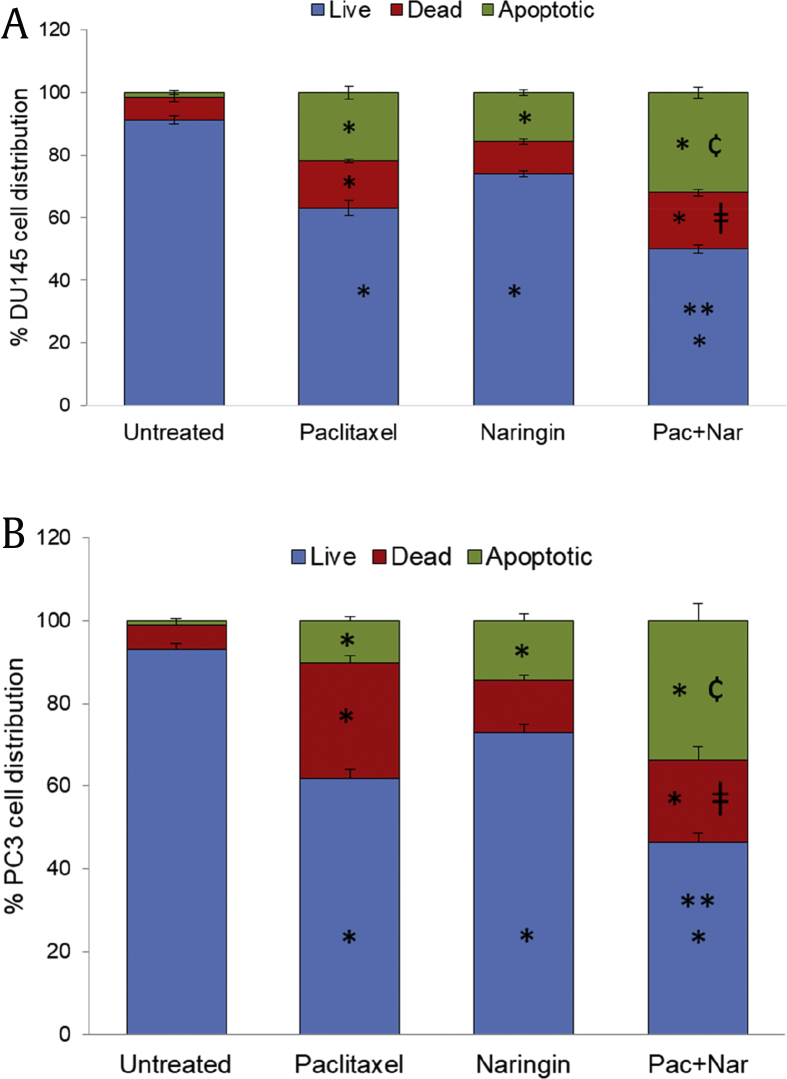
To assess whether naringin induces apoptosis, image-based cytometric evaluation of DU145 and PC3 cells were examined following exposure to 150 μM naringin and 5 nM paclitaxel or their combination for 72 hour. Annexin V and PI staining demonstrated an increase in the percentage of both cell types undergoing apoptosis (Fig. 3A, B) compared with untreated controls. As shown in Fig. 3A, B, the combined treatment did significantly raise the levels of apoptosis in both cell types when compared with individual treatments.
Fig. 3.
Percentage of viable, dead, and apoptotic cells after treatment with 5 nM paclitaxel or 150 μM naringin or both for 72 h. (A) DU145 cells and (B) PC3 cells. Untreated cells contained vehicle only. The cells were stained with annexin V/PI as determined by Tali image-based cytometer. All data were performed in triplicate and values are means of the two different experiments ± SD. For the all cell populations; *P < 0.01 versus untreated. For the live cell populations; **P < 0.01 versus paclitaxel and naringin. For dead cell populations; ǂP<0.05 versus untreated and naringin. For the apoptotic cell populations; ¢P < 0.001 versus all other groups. Pac + Nar: paclitaxel and naringin; SD, standard deviation.
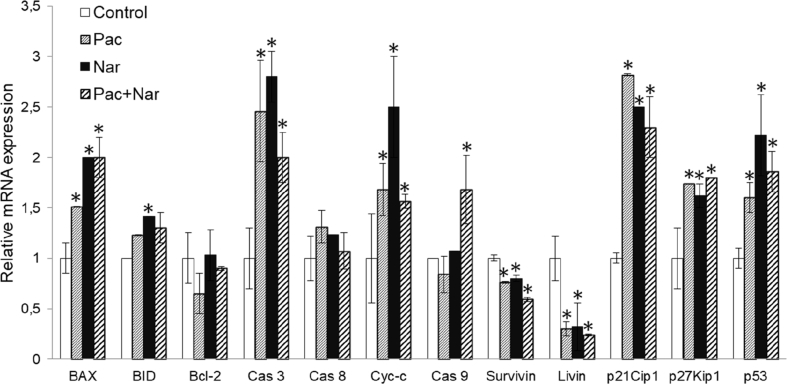
The apoptosis was also analyzed at the molecular level by RT-qPCR using DU145 cell RNA. Naringin treatment significantly upregulated BAX, BID, caspase 3, and cytochrome c (Cyt-c) expressions while did not change caspase 8, caspase 9, and Bcl-2 mRNA expressions (Fig. 4). Treatment of the cells with paclitaxel induced BAX, caspase 3, and Cyt-c, while it did not alter BID, caspase 8, caspase 9, and Bcl-2 expressions (Fig. 4). The combination of naringin with paclitaxel did upregulate the mRNA expression of caspase 9 (Fig. 4). It is demonstrating that naringin induces intrinsic apoptosis pathway in DU145 cells. However, the combination therapy did markedly downregulate the expression of livin and survivin mRNA (Fig. 4).
Fig. 4.
Regulation of genes related to apoptosis and cell cycle in DU145 cells. Cells were treated with 150 μM naringin, 5 nM paclitaxel, or their combination for 72 h. The target gene expressions were normalized to that of GAPDH. The data presented here are the means ± SD of three different experiments for each group performed in triplicate. *P < 0.05 versus untreated. Nar, naringin; Pac, paclitaxel; Pac + Nar, combination of paclitaxel and naringin; SD, standard deviation.
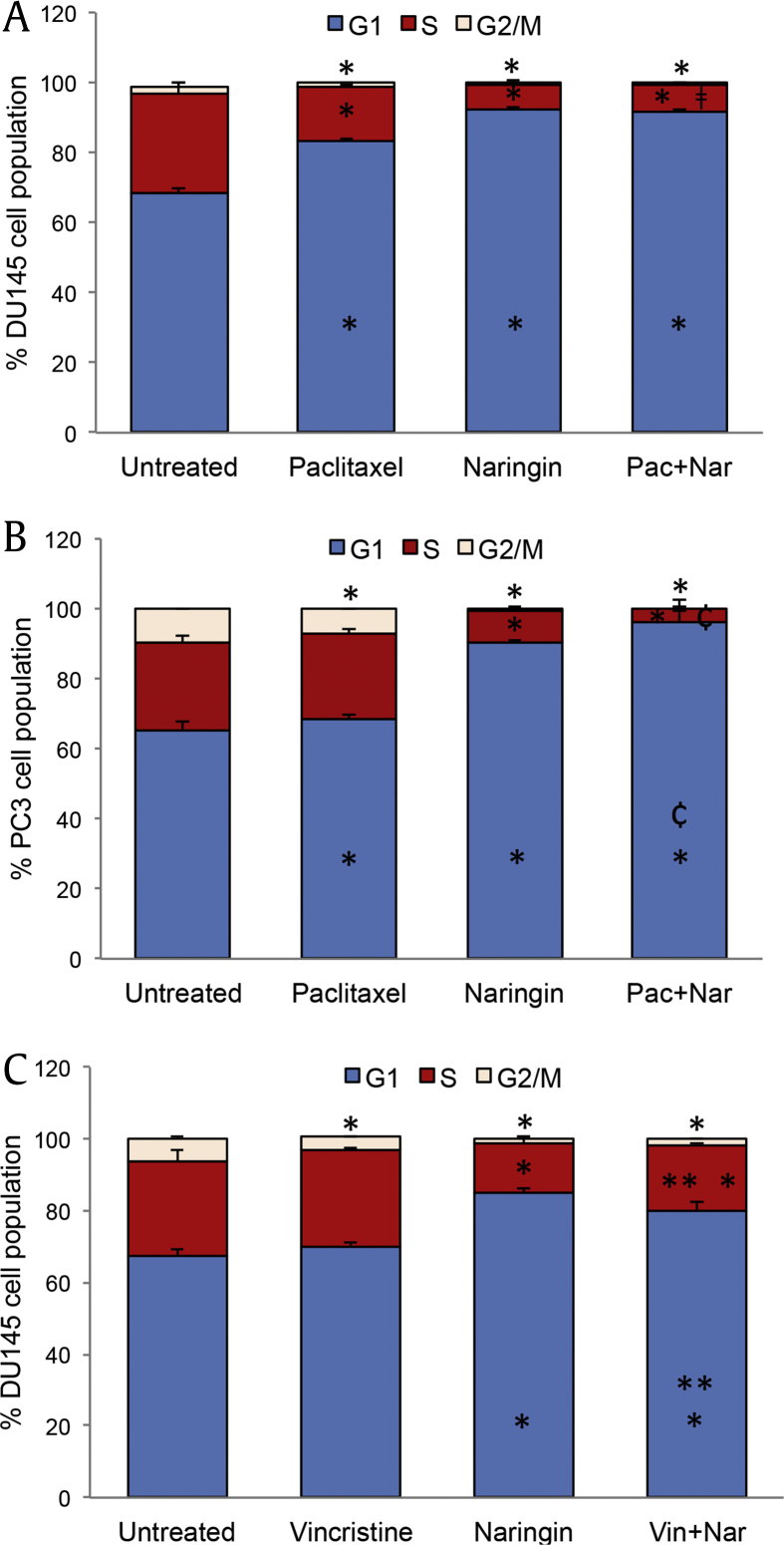
3.3. Naringin increases cell cycle arrest in DU145 and PC3 cells
To determine whether naringin regulates the cell cycle, we used image-based cytometry. Treatment with 150 μM naringin significantly increased the number of PC3 and DU145 cells at the G1 phase from 65.5% to 90.3% and 68.5% to 92.0%, respectively (Fig. 5A, B). G1 cell cycle arrest caused by paclitaxel was much less than those seen in naringin treatment. Combination therapy of naringin with paclitaxel significantly enhanced paclitaxel effect in PC3 cells. We also analyzed the effect of naringin with vincristine combination on cell cycle progression of DU145 cells. In contrast to the paclitaxel effect, vincristine did not alter cell cycle and combination with naringin is less effective than those seen in naringin alone (Fig. 5C).
Fig. 5.
Naringin treatment causes cell cycle arrest at G1 phase of cells. (A) DU145 and (B) PC3 cells. The cells were treated with 150 μM naringin, 5 nM paclitaxel, 5 nM vincristine or their combination for 72 h. Cell cycle distribution was examined using a Tali cell cycle kit. *P < 0.05 between the untreated, ¢P < 0.01 versus paclitaxel or vincristine and naringin, ǂP<0.01 versus paclitaxel. **P < 0.05 between vincristine, paclitaxel, naringin, . Pac + Nar: combination of paclitaxel and naringin, Vin + Nar: combination of vincristine and naringin.
We also examined the expressions of several genes related to cell cycle progress using DU145 cells. Given the essential role of p53, p21, and p27 in growth arrest after DNA damage, we evaluated the influence of naringin on the induction of p53, p21Cip1, and p27Kip1 by performing RT-qPCR. mRNA expression analyses revealed that naringin treatment causes an upregulation of p53 (2.2 fold), p21Cip1 (2.5 fold), and p27Kip1 (1.6 fold) when compared to controls (Fig. 4). A similar effect was observed in paclitaxel treatment. However, induction of p53, p21Cip1, and p27Kip1 expressions was not supported by a combination of naringin and paclitaxel treatment.
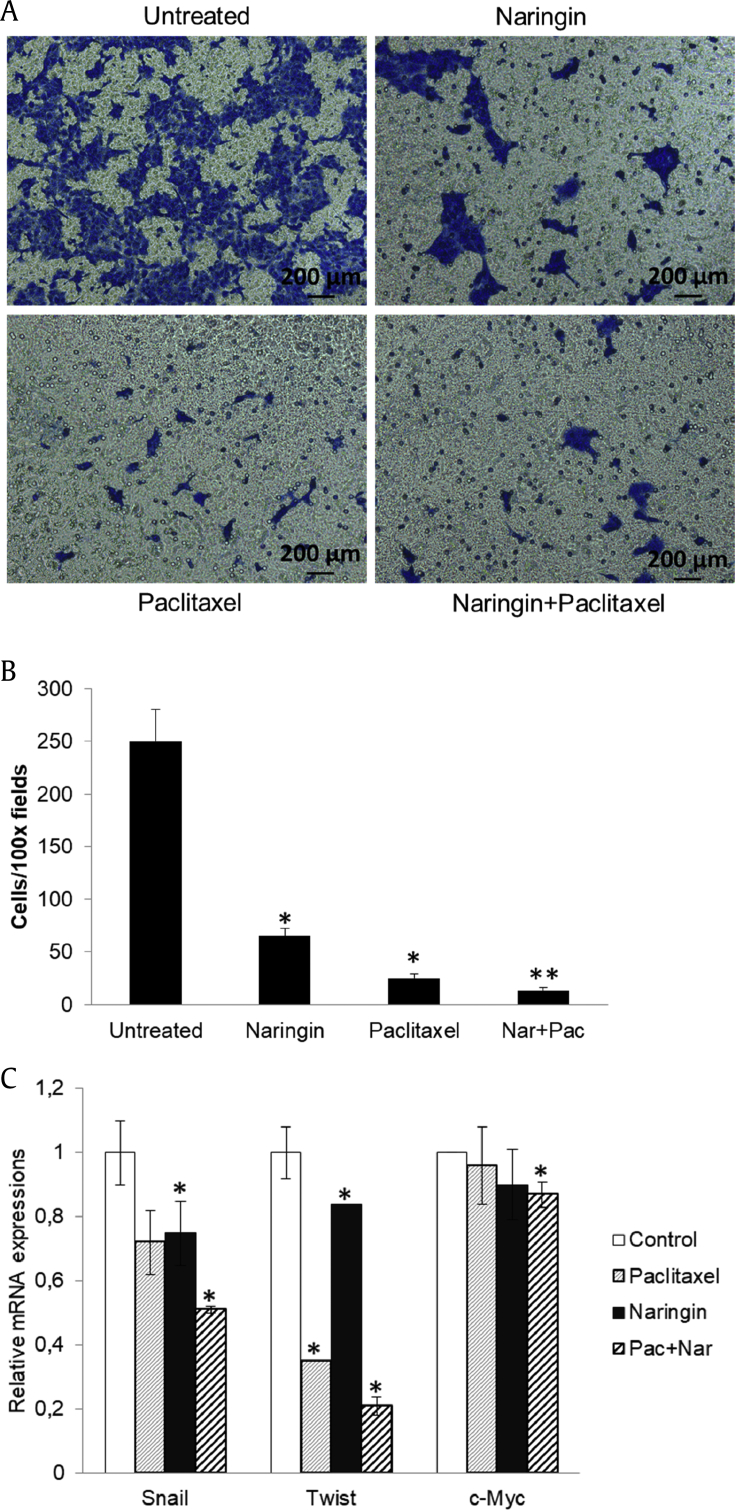
3.4. Naringin inhibits cell migration
To investigate whether naringin could inhibit the migration of PCa cells, we performed transwell cell migration assays. The results demonstrated that naringin (150 μM) and paclitaxel (5 nM) markedly decreased DU145 cell migration (Fig. 6A, B). Quantification analysis of transwell assay results indicated that the inhibition rate of naringin, paclitaxel, and their combination were approximately 74%, 87%, and 95%, respectively (Fig. 6B). Combined therapy significantly enhanced the downregulation of Snail, Twist and c-Myc mRNA expressions (Fig. 6C).
Fig. 6.
Naringin suppressed cell migration by down-regulating Snail and Twist expressions. (A) Twenty-four hour prestarved DU145 cells were treated with naringin (150 μM), paclitaxel (5 nM), or their combination and being subjected to transwell cell migration assay. (B) The quantitative results for migration data. (C) RT-qPCR mRNA expressions of Snail, Twist and c-Myc in DU145 cells were analyzed 72 h after agent treatments. *P < 0.05 versus untreated/control, **P < 0.05 versus paclitaxel and naringin. Each column represents the mean ± SD. NF-κB, nuclear factor-κB; PTEN, phosphatase and tensin homolog deleted on chromosome 10; Pac + Nar: paclitaxel and naringin; SD, standard deviation.
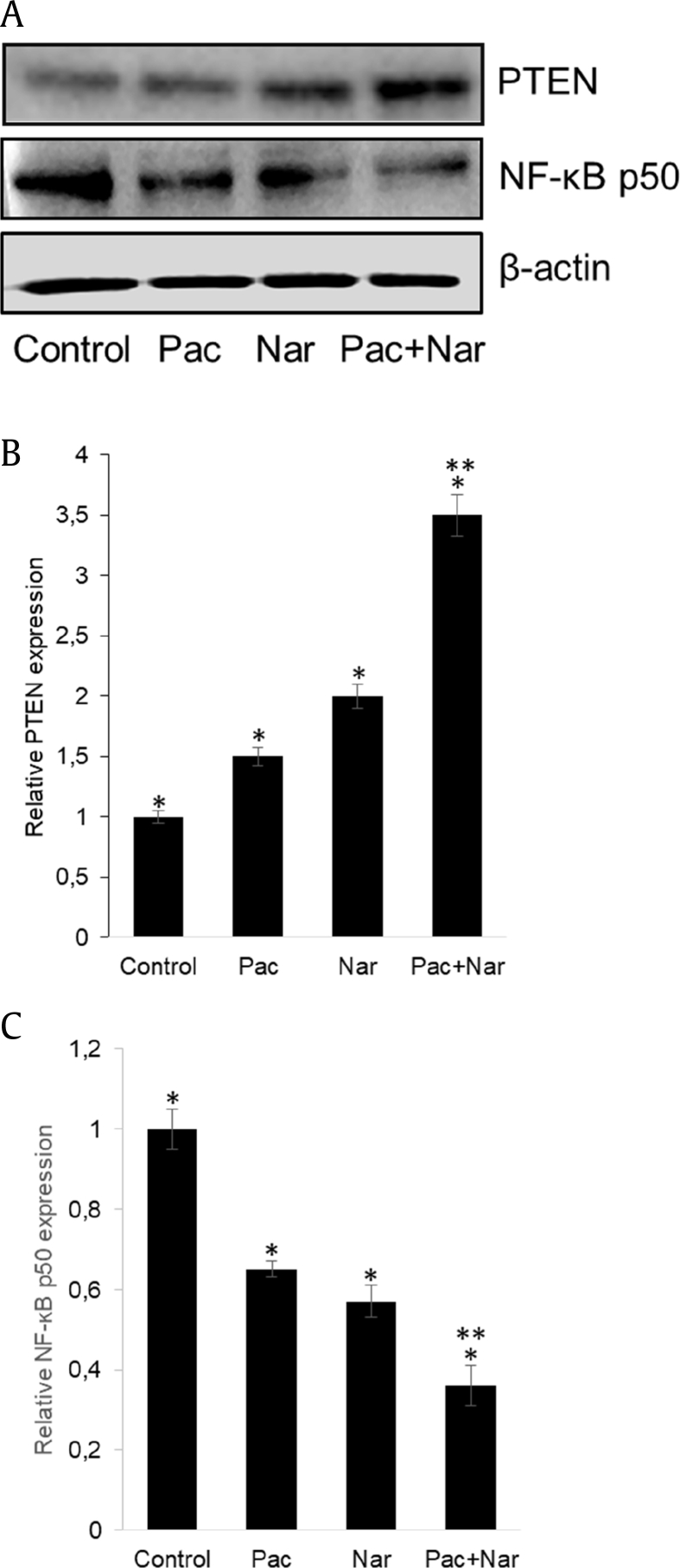
3.5. Naringin upregulates PTEN and inhibits NF-κB signaling
PTEN is a major negative regulator of the PI3K/Akt signaling pathway, which regulates proliferation, survival, and growth of PCa. While naringin treatment elevated PTEN protein expression, the combination with paclitaxel significantly increased this effect (Fig. 7A, B). Treatment of cells with naringin markedly inhibited the expression level NF-κB p50 protein (Fig. 7A, C). Paclitaxel treatment similarly inhibited the expression of NF-κB and upregulated PTEN expression. Combination treatment markedly strengthened paclitaxel (Fig. 7A–C).
Fig. 7.
Naringin regulates the protein expressions of PTEN and NF-κB. (A) Western blot analysis of PTEN and NF-κB p50 in homogenates isolated from DU145 cells treated with 5 nM paclitaxel, or 150 μM naringin and in combination with the two agents for 72 h. Intensities of immunoreactive bands on Western blots were quantified by densitometric analysis. (B) PTEN. (C) NF-κB, nuclear factor-κB. *P < 0.05 versus untreated, **P < 0.01 versus paclitaxel (Pac) and naringin (Nar). NF-κB, nuclear factor-κB; PTEN, phosphatase and tensin homolog deleted on chromosome 10; Pac + Nar: paclitaxel and naringin.
4. Discussion
Natural flavonoids such as apigenin, luteolin, quercetin, naringin, and epigallocatechin may play a role in the prevention of cancer.7, 15, 18, 21 Investigations have shown that these flavonoids possess inhibition activity on certain cancer growth through diverse mechanisms. Although published data are available regarding anticarcinogenic effects of naringin in different cancer models, there has been only very limited data related to the effect of naringin in androgen-dependent and independent PCa cells and its combination therapy with taxanes. Therefore, we have investigated the properties of naringin in combination with paclitaxel on apoptosis, proliferation, and migration of DU145, PC3, and LNCaP human PCa cells.
Previous reports have shown that naringin weakens the mitotic checkpoint and inhibits cell growth and induces apoptosis through inhibiting NF-κB expression7 in human cervical cancer cells, modulating glycogen synthase kinase-3 (GSK-3β) and adenomatous polyposis coli (APC)/β-catenin signaling in colonic cancer in mice.22 This current study demonstrates that naringin treatment significantly decreases PCa cell survival in a dose- and time-dependent manner, which is comparable to the existing literature. The presence of the anticancer agent at an optimal concentration is crucial for inducing a pharmacodynamic effect.11 For this reason, the cellular response of DU145, PC3, and LNCaP to naringin, paclitaxel, and vincristine combinations was investigated. The combination index analysis in DU145 cells demonstrated that the combination effect of naringin and paclitaxel was synergistic in these cells. Moreover, increased concentrations of naringin with stable dose of paclitaxel resulted in a dose-dependent decrease in cell survival. It is suggesting that combination therapy of paclitaxel with naringin might have a wide mixing ratio window for exerting its antitumor activity effectively. It has previously been revealed that the PI3K/Akt and NF-κB signaling pathways are involved in cell survival, migration, and the development of chemoresistance.12 Our study has shown that naringin alone induces tumor suppressor PTEN, a negative regulator of the PI3K-Akt pathway, and also enhances the paclitaxel effect. It has also been shown here that naringin treatment inhibited NF-κB expression and increased the inhibitory potency of paclitaxel in combination therapy.
To investigate whether suppression of cell growth was caused by an increase in apoptosis, image-based cytometer analysis was performed on DU145 and PC3 cells exposed to naringin. Indeed, naringin significantly induces apoptosis through upregulating BAX, BID, caspases 3, and Cyt-c expressions that are in agreement with the previous results.23, 24 The levels of Cyt-c and caspase 3 mRNA expressions were lower in the combined treatment group than in the single treatment groups. The reason for this decline may be explained by the fact that the expression of the induced mRNA may have returned to its initial state, depending on the length of the treatment. Our findings therefore show that naringin treatment prevents the growth of PCa cells by at least increasing the expression of some genes associated with apoptosis, thus inhibiting prostate carcinogenesis.
Our study shows that combination of 150 μM naringin and 5 nM paclitaxel leads to a synergistic inhibitory effect on PCa cell survival through induction of cell death by apoptosis and cell cycle arrest. A comparable effect of naringin was also previously observed in Ehrlich ascites tumor–bearing mice.25 The authors have reported that naringin exhibits synergistic antitumor activity in combination with the cytotoxic chemotherapeutic drug irinotecan. Moreover, naringin enhances the bioavailability of paclitaxel after oral administration of paclitaxel in rats.26 Our data suggest that naringin potentially inhibits cell survival, and combination therapy sensitizes androgen-independent cells to paclitaxel as well as docetaxel treatment. Naringin combination therapy also sensitizes androgen-dependent cells to paclitaxel, but this does not apply to docetaxel.
Naringin treatment causes p21-mediated G1 cell cycle arrest and causes cell survival inhibition in human triple-negative breast cancer cells6 by targeting β-catenin signaling. Our data show that a significant upregulation in p53, p21Cip1 and p27Kip1 occurred during G1-phase arrest in PCa cells treated with naringin, which are consistent with several previous reports.6, 27, 28 These data led to the hypothesis that naringin has potential as an anticancer compound in PCa cells. In addition, our studies sought to verify whether the combination treatment could synergistically increase cell cycle arrest; image-based cytometer analysis demonstrated that naringin treatment significantly elevates paclitaxel-induced cell cycle arrest in G1 phase. The ability of p21 to stimulate cell cycle inhibition may also depend on its capacity to mediate p53-dependent gene repression, as p21 is both necessary and adequate for p53-dependent repression of genes regulating cell cycle progression.29 We have shown that, naringin has similar potency to that of paclitaxel in the cell cycle with another microtubule-targeting drug vincristine. The result clearly shows that naringin effectively inhibits the cell cycle in the G1 phase, but the combination with microtubule–actin drugs does not increase its efficacy.
We analyzed whether either naringin alone or in combination with paclitaxel would inhibit DU145 and PC3 cell migration. Our data show that naringin attenuate the metastatic behavior of both cells via downregulating Snail and Twist expressions. Snail and Twist are invasion-associated genes, and they have crucial roles in EMT regulation, invasion, and metastasis of cancer cells.13 These genes are regulated by NF-κB, Akt and extracellular signal-regulated kinase (ERK) signaling.12 The tumor suppressor gene PTEN has a negative regulatory role for PI3K/Akt pathway30 that restrains and fine-tunes the PI3K signaling pathway. Our data have revealed that naringin treatment upregulated PTEN expression levels and decreased NF-κB expression. Inhibition of NF-κB signaling by naringin treatment may lead to attenuation of Snail and Twist expressions, which cause suppression of the migration.31, 32 Phuong et al33 have demonstrated that aberrant methylation and consequent silencing of the PTEN gene cause the activation of the PI3K/Akt and ERK pathways and subsequently promotes cancer cell proliferation and progression of tumor. Certain polyphenols such as hesperetin, quercetin, naringin, and apigenin modulate DNA methylation by indirectly regulating DNA methyltransferase activity through regulating the ratio of adenosylmethionine and S-adenosylhomocystein.34 These results indicate that naringin may provide a promising strategy for controlling metastasis and the invasiveness of tumors.
5. Conclusions
Taken together, we showed that the bioflavonoid naringin induces apoptosis and cell cycle arrest and reduces the migration ability of PCa cells. The combination of naringin with paclitaxel significantly enhanced the antitumor activity compared with either treatment alone. In addition, naringin sensitizes PCa cells to docetaxel, though ineffective in androgen-dependent PCa cells. These enhanced antiproliferative efficacies functioned by inducing cell apoptosis, arresting cell cycle, and inhibiting cell migration. The present findings may prove useful in further explorations of the potential application of this combined approach in the treatment of malignant cancer.
Conflicts of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgments
This study was funded by the Trakya University Research Project Foundation (Project no: 2016-45), Edirne/Turkey. We would like to thank R. Serttas for his technical assistance.
References
- 1.Eisenberger M.A., Blumenstein B.A., Crawford E.D., Miller G., McLeod D.G., Loehrer P.J. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. New Engl J Med. 1998;339(15):1036–1042. doi: 10.1056/NEJM199810083391504. [DOI] [PubMed] [Google Scholar]
- 2.Kapoor A., Wu C., Shayegan B., Rybak A.P. Contemporary agents in the management of metastatic castration-resistant prostate cancer. Can Urol Assoc J. 2016;10(11–12):E414–E423. doi: 10.5489/cuaj.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nature Reviews Cancer. 2004;4(4):253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 4.Zhu Y.Z., Liu C.F., Nadiminty N., Lou W., Tummala R., Evans C.P. Inhibition of ABCB1 expression overcomes acquired docetaxel resistance in prostate cancer. Mol Cancer Ther. 2013;12(9):1829–1836. doi: 10.1158/1535-7163.MCT-13-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xianchu L., Lan P.Z., Qiufang L., Yi L., Xiangcheng R., Wenqi H. Naringin protects against lipopolysaccharide-induced cardiac injury in mice. Environ Toxicol Pharmacol. 2016;48:1–6. doi: 10.1016/j.etap.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Li H., Yang B., Huang J., Xiang T., Yin X., Wan J. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting beta-catenin signaling pathway. Toxicol Lett. 2013;220(3):219–228. doi: 10.1016/j.toxlet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 7.Zeng L., Zhen Y., Chen Y., Zou L., Zhang Y., Hu F. Naringin inhibits growth and induces apoptosis by a mechanism dependent on reduced activation of NFkappaB/COX2caspase-1 pathway in HeLa cervical cancer cells. Int J Oncol. 2014;45(5):1929–1936. doi: 10.3892/ijo.2014.2617. [DOI] [PubMed] [Google Scholar]
- 8.Lewinska A., Siwak J., Rzeszutek I., Wnuk M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol in Vitro. 2015;29(3):417–425. doi: 10.1016/j.tiv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Bharti S., Rani N., Krishnamurthy B., Arya D.S. Preclinical evidence for the pharmacological actions of naringin: a review. Planta Med. 2014;80(6):437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- 10.Chtourou Y., Aouey B., Aroui S., Kebieche M., Fetoui H. Anti-apoptotic and anti-inflammatory effects of naringin on cisplatin-induced renal injury in the rat. Chem Biol Interact. 2016;243:1–9. doi: 10.1016/j.cbi.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 11.Fantini M., Benvenuto M., Masuelli L., Frajese G.V., Tresoldi I., Modesti A. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: perspectives on cancer treatment. Int J Mol Sci. 2015;16(5):9236–9282. doi: 10.3390/ijms16059236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sever R., Brugge J.S. Signal transduction in cancer. Cold Spring Harb Perspect Med. 2015;5(4) doi: 10.1101/cshperspect.a006098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Smith B.N., Odero-Marah V.A. The role of Snail in prostate cancer. Cell Adhes Migr. 2012;6(5):433–441. doi: 10.4161/cam.21687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whang Y.E., Wu X.Y., Suzuki H., Reiter R.E., Tran C., Vessella R.L. Inactivation of the tumor suppressor PTEN/MMAC1 in advanced human prostate cancer through loss of expression. Proc Natl Acad Sci USA. 1998;95(9):5246–5250. doi: 10.1073/pnas.95.9.5246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Erdogan S., Doganlar O., Doganlar Z.B., Serttas R., Turkekul K., Dibirdik I. The flavonoid apigenin reduces prostate cancer CD44(+) stem cell survival and migration through PI3K/Akt/NF-kappaB signaling. Life sciences. 2016;162:77–86. doi: 10.1016/j.lfs.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Georgescu M.M. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1(12):1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Q., Lai Y., Wang C., Xu G., He Z., Shang X. Matrine inhibits the proliferation, invasion and migration of castration-resistant prostate cancer cells through regulation of the NF-kappaB signaling pathway. Oncol Rep. 2016;35(1):375–381. doi: 10.3892/or.2015.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raha S., Yumnam S., Hong G.E., Lee H.J., Saralamma V.V., Park H.S. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of MAPK pathways in AGS cancer cells. Int J Oncol. 2015;47(3):1061–1069. doi: 10.3892/ijo.2015.3095. [DOI] [PubMed] [Google Scholar]
- 19.Justus C.R., Leffler N., Ruiz-Echevarria M., Yang L.V. In vitro cell migration and invasion assays. J Vis Exp. 2014;(88) doi: 10.3791/51046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chou T.C. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 21.Lin D., Kuang G., Wan J., Zhang X., Li H., Gong X. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of beta-catenin expression. Oncol Rep. 2017;37(2):895–902. doi: 10.3892/or.2016.5311. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y.S., Li Y., Wang Y., Sun S.Y., Jiang T., Li C. Naringin, a natural dietary compound, prevents intestinal tumorigenesis in Apc (Min/+) mouse model. J Cancer Res Clin Oncol. 2016;142(5):913–925. doi: 10.1007/s00432-015-2097-9. [DOI] [PubMed] [Google Scholar]
- 23.Ramesh E., Alshatwi A.A. Naringin induces death receptor and mitochondria-mediated apoptosis in human cervical cancer (SiHa) cells. Food Chem Toxicol. 2013;51:97–105. doi: 10.1016/j.fct.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Banjerdpongchai R., Wudtiwai B., Khawon P. Induction of human hepatocellular carcinoma HepG2 cell apoptosis by naringin. Asian Pac J Cancer Prev. 2016;17(7):3289–3294. [PubMed] [Google Scholar]
- 25.Knezevic A.H., Dikic D., Lisicic D., Kopjar N., Orsolic N., Karabeg S. Synergistic effects of irinotecan and flavonoids on Ehrlich ascites tumour-bearing mice. Basic Clin Pharmacol Toxicol. 2011;109(5):343–349. doi: 10.1111/j.1742-7843.2011.00735.x. [DOI] [PubMed] [Google Scholar]
- 26.Choi J.S., Shin S.C. Enhanced paclitaxel bioavailability after oral coadministration of paclitaxel prodrug with naringin to rats. Int J Pharm. 2005;292(1-2):149–156. doi: 10.1016/j.ijpharm.2004.11.031. [DOI] [PubMed] [Google Scholar]
- 27.Lee E.J., Moon G.S., Choi W.S., Kim W.J., Moon S.K. Naringin-induced p21WAF1-mediated G(1)-phase cell cycle arrest via activation of the Ras/Raf/ERK signaling pathway in vascular smooth muscle cells. Food Chem Toxicol. 2008;46(12):3800–3807. doi: 10.1016/j.fct.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Kim D.I., Lee S.J., Lee S.B., Park K., Kim W.J., Moon S.K. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis. 2008;29(9):1701–1709. doi: 10.1093/carcin/bgn055. [DOI] [PubMed] [Google Scholar]
- 29.Roy S., Singh R.P., Agarwal C., Siriwardana S., Sclafani R., Agarwal R. Downregulation of both p21/Cip1 and p27/Kip1 produces a more aggressive prostate cancer phenotype. Cell Cycle. 2008;7(12):1828–1835. doi: 10.4161/cc.7.12.6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ciuffreda L., Falcone I., Incani U.C., Del Curatolo A., Conciatori F., Matteoni S. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul. 2014;56:66–80. doi: 10.1016/j.jbior.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 31.Qin Y., Zhao D., Zhou H.G., Wang X.H., Zhong W.L., Chen S. Apigenin inhibits NF-kappaB and snail signaling, EMT and metastasis in human hepatocellular carcinoma. Oncotarget. 2016;7(27):41421–41431. doi: 10.18632/oncotarget.9404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shukla S., Gupta S. Suppression of constitutive and tumor necrosis factor alpha-induced nuclear factor (NF)-kappa B activation and induction of apoptosis by apigenin in human prostate carcinoma PC-3 cells: correlation with down-regulation of NF-kappa B-responsive genes. Clin Cancer Res. 2004;10(9):3169–3178. doi: 10.1158/1078-0432.ccr-03-0586. [DOI] [PubMed] [Google Scholar]
- 33.Phuong N.T., Kim S.K., Lim S.C., Kim H.S., Kim T.H., Lee K.Y. Role of PTEN promoter methylation in tamoxifen-resistant breast cancer cells. Breast Cancer Res Treat. 2011;130(1):73–83. doi: 10.1007/s10549-010-1304-2. [DOI] [PubMed] [Google Scholar]
- 34.Fang M.Z., Chen D.P., Yang C.S. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137(1):223s–228s. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]