Abstract
Bitter (T2R) and sweet (T1R) taste receptors have been implicated in sinonasal innate immunity and in the pathophysiology of chronic rhinosinusitis (CRS). Taste receptors are expressed on several sinonasal cell types including ciliated epithelial cells and solitary chemosensory cells. Bitter agonists released by pathogenic microbes elicit a T2R dependent signaling cascade which induces the release of bactericidal nitric oxide, increases mucociliary clearance, and promotes secretion of antimicrobial peptides. Genetic variation conferred by polymorphisms in T2R related genes is associated with differential CRS susceptibility, symptomatology and post-treatment outcomes. More recently, based on our understanding of T1R and T2R function, investigators have discovered novel potential therapeutics in T2R agonists and T1R antagonists. This review will discuss bitter and sweet taste receptor function in sinonasal immunity, explore the emerging diagnostic and therapeutic implications stemming from the most recent findings, and suggest directions for future research.
Keywords: Chronic rhinosinusitis, Taste Receptor Family 1 (T1R), Taste Receptor Family 2 (T2R), Sweet taste receptors, Bitter taste receptors, Innate immunity, Solitary chemosensory cells, Polymorphism
Introduction
Chronic rhinosinusitis (CRS) is a complex and heterogeneous syndrome with significant societal and financial burden. Affecting up to 16% of the United States population, healthcare costs total up to $13 billion per year.1, 2, 3 Patients suffering from CRS often endorse poorer quality of life scores than patients with other chronic diseases like chronic obstructive pulmonary disease and congestive heart failure.4 Outpatient visits for CRS account for more than 20% of antibiotic prescriptions, contributing to the concern for mounting antibiotic resistance.5 Despite its impact on both a societal and individual level, the etiology of CRS remains incompletely understood. In recent years, research has focused on the interactions of host and environmental factors that determine susceptibility to CRS. These factors ultimately result in defective mucociliary clearance and are thought to include inflammatory, infectious and genetic components.
More recently, a growing body of literature has implicated a role for bitter and sweet taste receptors in sinonasal innate immunity and the genetically heritable dysfunction of these receptors is thought to contribute at least in part to the pathogenesis of CRS.6, 7, 8, 9 This review will discuss the role of bitter and sweet taste receptors in upper airway immunity, explore the most recent advances in our understanding of the role of taste receptors in diseases of upper airway inflammation, and summarize the emerging diagnostic and therapeutic implications based on these discoveries.
Bitter and sweet taste receptor function
Bitter and sweet taste perception
The sense of taste in humans is mediated primarily by the perception of five basic taste modalities – salty, sour, umami, bitter and sweet. G protein-coupled receptors (GPCR), originally identified in taste bud type Ⅱ cells, govern oral bitter, umami, and sweet taste perception.10, 11
Sweet compounds such as glucose, fructose and sucrose as well as multiple amino acids such as isoleucine, leucine, phenylalanine and glutamate are detected by heterodimeric complexes formed by members of the Taste Receptor Family 1 (T1R) subtypes 1, 2 and 3.10, 12, 13 Similarly, bitter taste perception is mediated by a family of approximately 25 bitter taste receptors called Taste Receptor Family 2 (T2Rs), which respond to a variety of bitter compounds such as phenylthiocarbamide, denatonium benzoate, strychnine, quinine and caffeine.14, 15 While some receptors are specifically activated by a narrow range of bitter substances, some members of the T2R family display a broad spectrum of activation, stimulated by a variety of bitter compounds sharing a similar moiety.16
Bitter and sweet taste receptor signaling
Despite activation by differing sensory modalities, T1R and T2R receptors share a common signaling pathway. Briefly, activation of T1R or T2R receptors by a bitter or sweet ligand triggers the detachment of the GPCR α-subunit from the β- and γ-subunits that compose the remainder of the heterotrimeric protein. This triggers a downstream signaling cascade that involves activation of phospholipase C isoform β2 (PLCβ2) that increases inositol 1,4,5-trisphosphate (IP3) production. IP3 triggers calcium (Ca2+) release from the endoplasmic reticulum via stimulation of the IP3 receptor.17 Concurrent with this process, GPCR activation also activates phosphodiesterases (PDEs) which, via their reduction of cAMP levels, cause a decrease in protein kinase A (PKA) activity. When disinhibited, PKA inhibits the IP3 receptor, thus removal of this inhibition further augments Ca2+ efflux.18 Ca2+ activates the transient cation channel TRPM5 which via depolarization of the cell membrane activates voltage-gated sodium channels, generating an action potential that results in ATP release through the CALHM1 ion channel. This ATP release activates taste cell and sensory fiber receptors, propagating the transmission of taste sensation to the central nervous system.18, 19, 20, 21
Though originally thought to be limited to the oral and lingual surfaces, more recently bitter and sweet taste receptors have been discovered in many extra-oral tissues including the thyroid, brain, testes, fallopian tubes, pancreas and throughout both the respiratory and GI tracts.22, 23, 24, 25, 26 The discovery of extra-oral taste receptors has led to further investigation of their physiological role beyond taste perception.
Bitter and sweet taste receptors in the upper airway
In the airway, bitter and sweet receptors are present on a variety of cell types and have been found to play a vital role in the innate immune defense against invading pathogens. Ciliated sinonasal epithelial cells are an essential component of the first line of defense in upper airway immunity. Effective mucociliary clearance (MCC) requires the coordinated ciliary driven movement of airway surface liquid, composed of mucus-trapped pathogenic organisms and debris, in order to maintain a healthy sinonasal tract. When MCC is impaired, stasis of sinonasal secretions and resultant local inflammation can occur, and these can be inciting factors in increasing susceptibility to bacterial infection and the development of sinusitis. Beyond their role in MCC, ciliated airway cells also function as a source of antimicrobial compounds including lactoferrins, defensins, lysozyme, reactive oxygen species, nitric oxide (NO) and several epithelial-derived cytokines.27, 28, 29, 30, 31
These complementary immunoprotective mechanisms are triggered by the recognition of microbial pathogens, which occurs via activation of several receptor types. Toll-like receptors (TLRs) recognize conserved pathogen-associated molecular patterns including lipopolysaccharide (a component of gram-negative bacterial outer membranes), lipoteichoic acid (a constituent of gram-positive cell walls) and flagellin (found on motile organisms).28 TLR activation elicits a downstream and gradual immune response generated by changes in gene expression, but this effect can take up to 12 hours.32 In contrast, bitter taste receptors have been found to be able to recognize bacterial pathogens and elicit downstream responses within a matter of minutes and the mechanisms by which this response occurs in the sinonasal epithelium has been a topic of investigation for the past decade.
Taste receptors and ciliated epithelial cells
After demonstrating that several T2Rs are expressed on motile cilia and their activation by bitter agonists elicits a Ca2+ induced increase in ciliary beat frequency (CBF), investigators turned to identifying the bacterial components capable of acting as T2R ligands and characterizing the full spectrum of downstream effects after T2R activation.33 T2R38, perhaps the most extensively studied isoform of the T2R family, provided a prototype from which we began to expand our understanding of the role of bitter taste receptors in sinonasal immunity.
T2R38 is expressed by ciliated sinonasal epithelial cells and has a ligand–specific response to bitter bacterial compounds known as acyl-homoserine lactones (AHLs). AHLs are released by gram-negative bacteria like Pseudomonas aeruginosa and function as quorum sensing molecules, enabling communication between bacterial organisms about microbial density, and encouraging the formation of antibacterial resistant biofilm communities once a critical mass is reached.34, 35, 36 Quinolones are another example of quorum sensing molecules released by P. aeruginosa.37 Detection of these quorum sensing molecules by bitter taste receptors provides a means of immunologic surveillance and the opportunity for the immune system to intervene to ward off invading pathogens.
The innate immune responses elicited via activation of T2R38 include Ca2+ driven NO production. NO induces damage to the intracellular components of infectious microbes and, via its action on protein kinase G and guanylyl cyclase, increases CBF thereby increasing MCC (Fig. 1).8, 28, 38, 39 This increase in CBF accelerates the removal of mucus trapped bacteria and the dispersion of other antimicrobial compounds produced in response to bacterial pathogens.28, 40 Though much of past research has focused on AHLs released by gram-negative bacteria, gram-negative bacteria make up only a subset of causative bacterial agents cultured from CRS patients. Hariri et al41 sought to determine whether bacterial metabolites from other bacteria can serve as agonists to T2Rs. Using calcium based functional assays they found that a range of volatile bacterial metabolites including acetone, 2-butanone, 2-pentanone, 2-methylpropanal, γ-butyrolactone, dimethylsuflide and methylmercaptan which are produced by several pathogens including Staphylococcus aureus, Pseudomonas aeruginosa, and Streptococcus pneumoniae, elicit a T2R dependent NO response.42 T2R activation by Bacillus Cereus has also demonstrated a similar T2R dependent NO response.38 These findings lend further credence to a broader role of T2Rs in microbial detection and local immune response. Beyond T2R38, recent studies have investigated other T2Rs including T2R4, -14 and -16 with similar findings of ubiquitous expression in human ciliated sinonasal epithelium and a bitter ligand-dependent, Ca2+ mediated NO production.41, 43
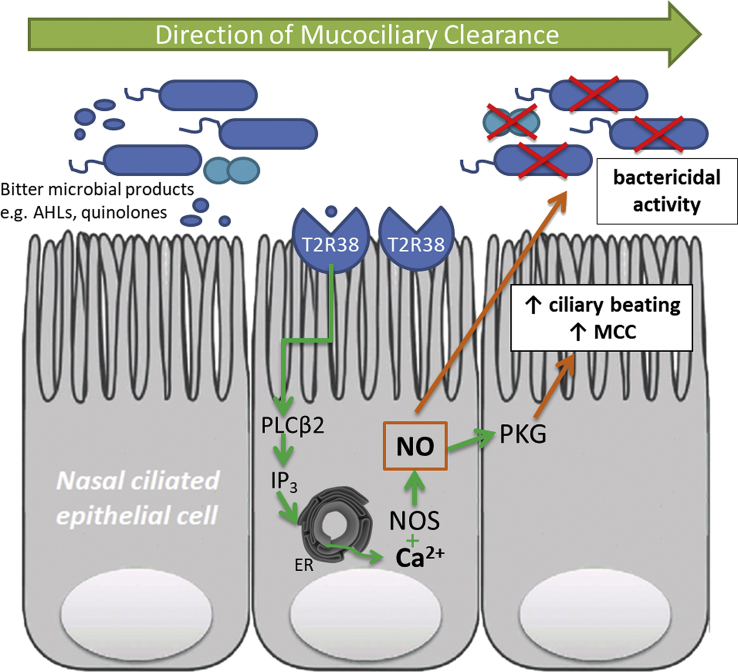
Fig. 1.
T2R38-mediated regulation of sinonasal immunity. Bacterial species like P. aeruginosa release quorum sensing molecules like acyl-homoserine lactones (AHLs) and quinolones. These bitter microbial products activate T2R38 and elicit a local immune response via the canonical taste-signaling pathway. This response involves activation of PLCβ2 and IP3 production. IP3 triggers the release of calcium (Ca2+) from the endoplasmic reticulum (ER). Increase in intracellular Ca2+ concentration activates nitric oxide (NO) formation via nitric oxide synthase (NOS). NO increases ciliary beat frequency via activation of protein kinase G and diffuses into the airway surface liquid to directly kill bacteria.
Taste receptors and solitary chemosensory cells
Taste receptors in the upper airway are not limited to ciliated epithelial cells. Solitary chemosensory cells (SCCs) are rare, non-ciliated, epithelial cells that express both T1R2/3 sweet and T2R bitter receptors. Taste receptor expression on SCCs was first detected in mouse nasal mucosa. Stimulation of murine SCCs by bitter or sweet ligands elicited acetylcholine-mediated stimulation of the trigeminal nerve with resultant protective reflexes including decreased respiratory rate, presumably to decrease the inspiration of airborne pathogens, and the release of inflammatory antimicrobial mediators.44, 45, 46 SCCs were found to be present in the human sinonasal tract at low density, accounting for approximately 1 in every 100 epithelial cells in the sinonasal cavity.47, 48 Despite their rarity in number, SCCs play an important role in immune function mediated by taste receptor activation.
Bitter and sweet taste receptors present on SCCs act in an antagonistic manner to affect the innate immune response to inspired microbes (Fig. 2). While T2R stimulation on ciliated epithelial cells elicits a Ca2+ dependent NO response, stimulation of SCC T2Rs results in the propagation of Ca2+ across gap junctions into ciliated cells triggering them to release AMPs including beta defensins 1 and 2 which are capable of permeabilizing bacterial cell membranes.7, 44, 48 Conversely, stimulation of T1Rs by sweet compounds antagonizes the signal transduction of SCC T2Rs; the addition of glucose and sucrose to murine and human sinonasal epithelial cultures inhibits the denatonium induced Ca2+ response and this inhibition is released with the addition of antagonists of the T1R2/3 receptors like lactisole and amiloride.7, 13, 49, 50, 51
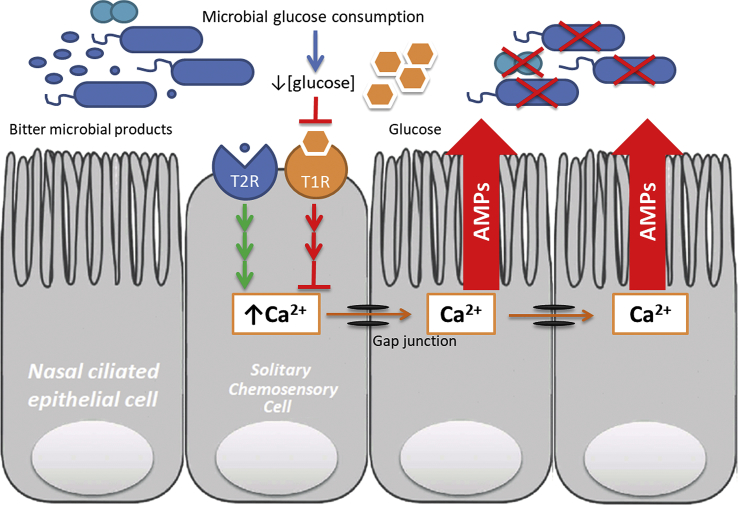
Fig. 2.
Bitter and sweet taste receptors on solitary chemosensory cells act antagonistically to regulate innate immunity. Activation of bitter T2R receptors leads to downstream increase in intracellular Ca2+ concentration. Ca2+ spreads to neighboring ciliated cells via gap junctions and activates the release of anti-microbial peptides (AMPs), directly killing pathogenic microbes. Activation of the sweet receptor T1R (dimer of T1R2 and T1R3) by sweet tasting compounds like glucose inhibits this Ca2+ mediated response. Microbial consumption of glucose decreases stimulation of the T1R receptor thereby disinhibiting the T2R response to microbial bitter products.
During pathogenic infection, microbes consume glucose for energy leading to a rapid depletion of airway mucus glucose. This reduction in glucose concentration interrupts the tonic activation of T1R2/3 which disinhibits the signal transduction and downstream effects of T2R activation, augmenting the immune response to microbial bitter products.
More recently SCCs have also been found to participate in innate mucosal immunity by releasing epithelial-derived cytokines involved in the type Ⅱ inflammatory cascade (Fig. 3). SCCs are the predominant source of epithelial IL-25 which stimulates local group-2 innate lymphoid cells (ILC-2s).31, 52 Activated ILC-2s initiate a localized type 2 inflammatory response which includes massive secretion of the cytokine IL-13, which in a feed-forward loop induces SCC proliferation.30, 31 To that end, SCCs are present in greater number in inflamed versus non-inflamed tissue, inducing higher levels of IL-25 production in a manner that is increased in the presence of IL-13 and decreased with steroid exposure.30 The exact stimulus driving the initial release of IL-25 has yet to be determined.
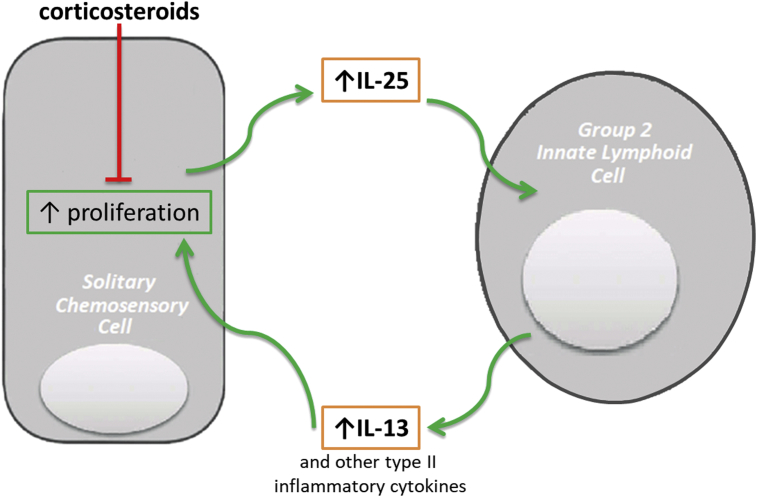
Fig. 3.
SCCs produce IL-25 with SCC proliferation and increased IL25 levels evident in nasal polyps. IL-25, in conjunction with other epithelial derived cytokines (IL-33 and TSLP), stimulates an eosinophilic type 2 inflammatory response via the activation and proliferation of group-2 innate lymphoid cells (ILC-2s). IL-13 released by ILC2s induces SCC proliferation in a feed-forward fashion while this IL-13 induced SCC proliferation is inhibited by corticosteroid exposure.
Clinical relevance of genetic variation in taste receptor function
Due to a wide range of polymorphisms that modulate receptor function and sensitivity, genetic variation in T1R and T2R taste receptors manifest in a variety of ways, the most obvious being in individual preference or aversion to sweet or bitter tastants. More recently, genetic polymorphisms of bitter taste receptors have been implicated in CRS susceptibility, severity and outcomes.
Three single nucleotide polymorphisms (SNPs) in the gene that encodes T2R38, TAS2R38, confer two common haplotypes including the functional variant PAV (proline-alanine-valine) and the nonfunctional variant AVI (alanine-valine-isoleucine). Homozygotes for the functional allele (PAV/PAV) perceive T2R38 agonists like phenylthiocarbamide (PTC) and propylthiouracil as intensely bitter, while homozygotes for the non-functional allele (AVI/AVI) are unable to perceive these compounds. Heterozygotes (PAV/AVI) demonstrate a wide range of bitter taste perception depending on the level of expression of the non-functional and functional alleles.16, 53 Sinonasal epithelial cells cultured from AVI/AVI individuals compared to cells cultured from PAV/PAV individuals also demonstrate reduced NO release in response to AHL stimulation with a resultant decrease in CBF, MCC and bactericidal activity. Compared to PAV/PAV CRS patients, AVI/AVI patients also demonstrate increased susceptibility to upper respiratory infections caused by gram-negative organisms, increased burden of biofilm formation, and the presence of culturable bacteria in mucus isolated from sinonasal swabs.8, 54, 55
T2R38 genotype also correlates with sinonasal symptomatology; AVI/AVI healthy adults and cystic fibrosis patients endorse poorer quality of life compared to their PAV/PAV counterparts. AVI/AVI CRS patients demonstrate a greater degree of inflammation on computerized tomography imaging than PAV/PAV patients. Additionally, AVI homozygotes with CRS are more likely to require surgical intervention for medically recalcitrant sinusitis.55, 56, 57, 58, 59, 60 Furthermore, PAV/PAV patients with non-polypoid CRS have better quality of life outcomes 6 months after sinus surgery when compared to heterozygotes and AVI homozygous patients. More recently, genome-wide association tests have identified other less well-studied bitter and sweet receptor genes that are associated with CRS including TAS2R13 and TAS2R49 suggesting that we have only begun to scratch the surface in our understanding of how taste related genes play a role in the pathogenesis of CRS.61
Diagnostic and therapeutic implications
Diagnostic implications
Though differential genotypic classification of CRS patients is associated with differential disease susceptibility, symptomatology, and treatment outcomes, genotyping has limited ability to assess receptor phenotype or sensitivity. More recently, researchers have explored the use of oral taste sensitivity as a proxy for extra-oral taste receptor function and hypothesize that receptor sensitivity as assessed with a simple taste test can reveal differences in sinonasal mucosal immunity.
Indeed, increased sensitivity to PTC confers protection from frequent sinonasal infections in healthy patients and is associated with increased post-surgical quality of life and decreased in vitro biofilm burden in non-polypoid CRS.54, 56, 60, 62 CRS patients also endorse lower taste intensity ratings for bitter compounds like denatonium benzoate (a T2R38 agonist) and quinine hydrochloride (a broad T2R agonist) compared to matched healthy controls. Additionally, compared to healthy controls CRS patients also rate T1R agonists like sucrose as higher in intensity, yet perceive salt at comparable intensity as non-CRS individuals.63, 64 Taken together, these results suggest that CRS patients have hyporesponsive bitter taste receptors and hyperresponsive sweet taste receptors and thus have reduced ability to mount a T2R dependent sinonasal innate immune response upon exposure to pathogenic microbes.
Given that differences in taste sensitivities appear to portend corresponding functional differences in upper airway immune response, simple and affordable taste tests may be useful to stratify CRS patients in hopes of identifying those susceptible to severe disease who may fail medical therapy and require more aggressive treatment.
Therapeutic implications
Our understanding of the role of bitter and sweet taste receptors also has potential implications for novel therapeutic applications in the treatment of CRS. Recently, studies have turned to investigating compounds that harness the immunomodulatory mechanisms triggered by bitter and sweet taste receptors.
Bitter taste receptor agonists
Flavones are a subclass of secondary plant metabolites with antibacterial and inflammatory effects that augment the T2R response. Some flavones stimulate T2R14 eliciting a Ca2+ dependent resultant increase in NO production and CBF.41 Although alone flavones demonstrate only low-level antibacterial activity, they have been found to synergistically function against P. aeruginosa with antibiotics or recombinant human lysozyme by encouraging lysozyme-mediated bacterial lysis and enhancing the function of AMPs released by ciliated epithelial cells stimulated by other T2R agonists.65 Flavones have already demonstrated therapeutic potential in several experiments. The flavonoid-based herbal extract mix Sinupret™ and a similar flavonoid, Quercetin, have been found to enhance CBF upon apical application on cultured human and murine primary sinonasal epithelial cells. This effect maybe via increased airway surface liquid hydration, through a T2R mediated NO release, or a combination of the two mechanisms. Sinupret™ has also demonstrated clinical efficacy in several randomized controlled trials as an adjunct therapy to antibiotics and decongestants in patients with rhinosinusitis perhaps through its action on T2R14.66, 67, 68, 69
Alkaloids represent another class of naturally occurring compounds with therapeutic potential. Recent work has investigated quinine, an alkaloid isolated from the bark of the cinchona tree, and its derivative chloroquine. Quinine and chloroquine are prototypical bitter compounds that share structural similarity with quinolones, the quorum sensing molecules released by P. aeruginosa.37 They are broad T2R agonists with quinine activating nine T2Rs (T2R4, −7, −10, −14, −39, −40, −43, −44, and −46) and chloroquine activating five (T2R3, −7, −10, −14, and −39).16, 70, 71 Like quinolones, quinine elicits a T2R-dependent NO release from ciliated sinonasal epithelial cells.37 Similar to studies with T2R38 agonist phenylthiocarbamide (PTC), phenotypic differences in quinine taste perception reveal a correlation between reduced taste sensitivity to quinine and CRS disease status, suggesting that the reduced function of quinine-responsive T2Rs may play role in CRS susceptibility.64 These differences are due at least in part to polymorphisms in a bitter receptor cluster on chromosome 12 where the gene encoding T2R14 is located.72 While the immunomodulatory effects of these bitter agonists and their respective T2R responses are yet to be fully understood, preliminary findings suggest a potential role for bitter agonists as topical therapies in diseases of sinonasal inflammation.
Sweet taste receptor antagonists
Given that stimulation of sweet taste receptors attenuates the bitter taste receptor cascade in response to microbial pathogens, T1R antagonists may have potential as therapeutic adjuncts in the treatment of CRS. Sinonasal epithelial cultures isolated from mice who lack the T1R2/3 receptors show no inhibition of denatonium induced T2R response in the presence of T1R agonists sucrose and glucose.7 The T1R mediated downregulation of the T2R SCC immunomodulatory response can also be blocked by the addition of T1R antagonists like lactisole and amiloride.49, 50, 51 d-amino acids produced by some bacterial species including Staphylococcus can serve as T1R agonists increasing the chance of bacterial survival and propagation by impairing the host's T2R mediated immunologic surveillance and AMP release. Further studies are required to determine whether T1R antagonists can be useful adjuncts to treatment, especially in Staphylococcal driven CRS.
Future directions
To date, research on the role of bitter and sweet taste receptors indicates that variation in taste receptor genotype and phenotype plays a significant role in upper airway immunity. Though our understanding of how taste receptor dysfunction may contribute to the development of CRS has increased over the past decade, there are still many outstanding questions and opportunities for further research. Much of our understanding of T2R function comes from studying T2R38 and its ligands and future studies should focus on elucidating how as yet uncharacterized bitter and sweet taste receptor isoforms and their unidentified ligands contribute to CRS. Whether polymorphisms of these T1Rs and T2Rs reveal differential susceptibility to CRS will also be an important consideration. Uncovering the full spectrum of bitter and sweet taste receptors expressed in the sinonasal epithelium will allow us to identify potential combinations of T1R agonist and T2R antagonists that will harness the power of endogenous immune mechanisms to maximize therapeutic benefit, either as adjunct or alternative therapies for patients with CRS. Identifying how bitter and sweet agonists and other endogenous triggers affect IL-25 release from SCCs and the resultant downstream inflammatory cascade could reveal potential therapeutic targets for a subset of CRS patients with predominantly type Ⅱ inflammation. Clinically, inexpensive and simple taste testing with the appropriate bitter and sweet agonists at the most informative concentrations may enable detection of CRS patients most at risk of recalcitrant disease, and may inform a more personalized regimen of conventional, alternative, and surgical management of each patient's disease.
Declarations of interest
IWM and ADW have no relevant conflicts to disclose. NAC has a patent pending “Therapy and Diagnostics for Respiratory Infection.”
Acknowledgements
This work was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under award number R01DC013588-04S2 (to IWM).
Edited by Yi Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.DeConde A.S., Soler Z.M. Chronic rhinosinusitis: epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30:134–139. doi: 10.2500/ajra.2016.30.4297. [DOI] [PubMed] [Google Scholar]
- 2.Halawi A.M., Smith S.S., Chandra R.K. Chronic rhinosinusitis: epidemiology and cost. Allergy Asthma Proc. 2013;34:328–334. doi: 10.2500/aap.2013.34.3675. [DOI] [PubMed] [Google Scholar]
- 3.Rudmik L. Economics of chronic rhinosinusitis. Curr Allergy Asthma Rep. 2017;17:20. doi: 10.1007/s11882-017-0690-5. [DOI] [PubMed] [Google Scholar]
- 4.Gliklich R.E., Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngol Head Neck Surg. 1995;113:104–109. doi: 10.1016/S0194-59989570152-4. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharyya N., Kepnes L.J. Assessment of trends in antimicrobial resistance in chronic rhinosinusitis. Ann Otol Rhinol Laryngol. 2008;117:448–452. doi: 10.1177/000348940811700608. [DOI] [PubMed] [Google Scholar]
- 6.Carey R.M., Adappa N.D., Palmer J.N., Lee R.J., Cohen N.A. Taste receptors: regulators of sinonasal innate immunity. Laryngoscope Investig Otolaryngol. 2016;1:88–95. doi: 10.1002/lio2.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee R.J., Kofonow J.M., Rosen P.L. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124:1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee R.J., Xiong G., Kofonow J.M. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122:4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Workman A.D., Palmer J.N., Adappa N.D., Cohen N.A. The role of bitter and sweet taste receptors in upper airway immunity. Curr Allergy Asthma Rep. 2015;15:72. doi: 10.1007/s11882-015-0571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y., Hoon M.A., Chandrashekar J. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell. 2003;112:293–301. doi: 10.1016/s0092-8674(03)00071-0. [DOI] [PubMed] [Google Scholar]
- 11.Iwata S., Yoshida R., Ninomiya Y. Taste transductions in taste receptor cells: basic tastes and moreover. Curr Pharm Des. 2014;20:2684–2692. doi: 10.2174/13816128113199990575. [DOI] [PubMed] [Google Scholar]
- 12.Nelson G., Hoon M.A., Chandrashekar J., Zhang Y., Ryba N.J., Zuker C.S. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 13.Lee R.J., Hariri B.M., McMahon D.B. Bacterial d-amino acids suppress sinonasal innate immunity through sweet taste receptors in solitary chemosensory cells. Sci Signal. 2017;10 doi: 10.1126/scisignal.aam7703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brockhoff A., Behrens M., Massarotti A., Appendino G., Meyerhof W. Broad tuning of the human bitter taste receptor hTAS2R46 to various sesquiterpene lactones, clerodane and labdane diterpenoids, strychnine, and denatonium. J Agric Food Chem. 2007;55:6236–6243. doi: 10.1021/jf070503p. [DOI] [PubMed] [Google Scholar]
- 15.Hansen J.L., Reed D.R., Wright M.J., Martin N.G., Breslin P.A. Heritability and genetic covariation of sensitivity to PROP, SOA, quinine HCl, and caffeine. Chem Senses. 2006;31:403–413. doi: 10.1093/chemse/bjj044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meyerhof W., Batram C., Kuhn C. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses. 2010;35:157–170. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci D.R., Groblewski G.E., Sneyd J., Yule D.I. Targeted phosphorylation of inositol 1,4,5-trisphosphate receptors selectively inhibits localized Ca2+ release and shapes oscillatory Ca2+ signals. J Biol Chem. 2000;275:33704–33711. doi: 10.1074/jbc.M004278200. [DOI] [PubMed] [Google Scholar]
- 18.Taruno A., Matsumoto I., Ma Z., Marambaud P., Foskett J.K. How do taste cells lacking synapses mediate neurotransmission? CALHM1, a voltage-gated ATP channel. Bioessays. 2013;35:1111–1118. doi: 10.1002/bies.201300077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kinnamon S.C. Taste receptor signalling - from tongues to lungs. Acta Physiol (Oxf) 2012;204:158–168. doi: 10.1111/j.1748-1716.2011.02308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Z., Zhao Z., Margolskee R., Liman E. The transduction channel TRPM5 is gated by intracellular calcium in taste cells. J Neurosci. 2007;27:5777–5786. doi: 10.1523/JNEUROSCI.4973-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taruno A., Vingtdeux V., Ohmoto M. CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature. 2013;495:223–226. doi: 10.1038/nature11906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clark A.A., Dotson C.D., Elson A.E. TAS2R bitter taste receptors regulate thyroid function. FASEB J. 2015;29:164–172. doi: 10.1096/fj.14-262246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu J., Cao J., Iguchi N., Riethmacher D., Huang L. Functional characterization of bitter-taste receptors expressed in mammalian testis. Mol Hum Reprod. 2013;19:17–28. doi: 10.1093/molehr/gas040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kojima I., Nakagawa Y., Ohtsu Y., Medina A., Nagasawa M. Sweet taste-sensing receptors expressed in pancreatic β-cells: sweet molecules act as biased agonists. Endocrinol Metab (Seoul Korea) 2014;29:12–19. doi: 10.3803/EnM.2014.29.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Depoortere I. Taste receptors of the gut: emerging roles in health and disease. Gut. 2014;63:179–190. doi: 10.1136/gutjnl-2013-305112. [DOI] [PubMed] [Google Scholar]
- 26.Laffitte A., Neiers F., Briand L. Functional roles of the sweet taste receptor in oral and extraoral tissues. Curr Opin Clin Nutr Metab Care. 2014;17:379–385. doi: 10.1097/MCO.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sleigh M.A., Blake J.R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988;137:726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- 28.Parker D., Prince A. Innate immunity in the respiratory epithelium. Am J Respir Cell Mol Biol. 2011;45:189–201. doi: 10.1165/rcmb.2011-0011RT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kato A., Schleimer R.P. Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity. Curr Opin Immunol. 2007;19:711–720. doi: 10.1016/j.coi.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel N.N., Kohanski M.A., Maina I.W. Solitary chemosensory cells producing interleukin-25 and group-2 innate lymphoid cells are enriched in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rhinol. 2018 doi: 10.1002/alr.22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kohanski M.A., Workman A.D., Patel N.N. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018 doi: 10.1016/j.jaci.2018.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hume D.A., Underhill D.M., Sweet M.J., Ozinsky A.O., Liew F.Y., Aderem A. Macrophages exposed continuously to lipopolysaccharide and other agonists that act via toll-like receptors exhibit a sustained and additive activation state. BMC Immunol. 2001;2(11):11. doi: 10.1186/1471-2172-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shah A.S., Ben-Shahar Y., Moninger T.O., Kline J.N., Welsh M.J. Motile cilia of human airway epithelia are chemosensory. Science (80-) 2009;325(5944):1131–1134. doi: 10.1126/science.1173869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parsek M.R., Greenberg E.P. Acyl-homoserine lactone quorum sensing in gram-negative bacteria: a signaling mechanism involved in associations with higher organisms. Proc Natl Acad Sci U S A. 2000;97:8789–8793. doi: 10.1073/pnas.97.16.8789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jimenez P.N., Koch G., Thompson J.A., Xavier K.B., Cool R.H., Quax W.J. The multiple signaling systems regulating virulence in Pseudomonas aeruginosa. Microbiol Mol Biol Rev. 2012;76:46–65. doi: 10.1128/MMBR.05007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Z., Nair S.K. Quorum sensing: how bacteria can coordinate activity and synchronize their response to external signals? Protein Sci. 2012;21:1403–1417. doi: 10.1002/pro.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freund J.R., Mansfield C.J., Doghramji L.J. Activation of airway epithelial bitter taste receptors by Pseudomonas aeruginosa quinolones modulates calcium, cyclic-AMP, and nitric oxide signaling. J Biol Chem. 2018;293:9824–9840. doi: 10.1074/jbc.RA117.001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey R.M., Workman A.D., Yan C.H. Sinonasal T2R-mediated nitric oxide production in response to Bacillus cereus. Am J Rhinol Allergy. 2017;31:211–215. doi: 10.2500/ajra.2017.31.4453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barraud N., Hassett D.J., Hwang S.H., Rice S.A., Kjelleberg S., Webb J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J Bacteriol. 2006;188:7344–7353. doi: 10.1128/JB.00779-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salathe M. Regulation of mammalian ciliary beating. Annu Rev Physiol. 2007;69:401–422. doi: 10.1146/annurev.physiol.69.040705.141253. [DOI] [PubMed] [Google Scholar]
- 41.Hariri B.M., McMahon D.B., Chen B. Flavones modulate respiratory epithelial innate immunity: anti-inflammatory effects and activation of the T2R14 receptor. J Biol Chem. 2017;292:8484–8497. doi: 10.1074/jbc.M116.771949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbeurgt C., Veithen A., Carlot S. The human bitter taste receptor T2R38 is broadly tuned for bacterial compounds. PLoS One. 2017;12 doi: 10.1371/journal.pone.0181302. e0181302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan C.H., Hahn S., McMahon D. Nitric oxide production is stimulated by bitter taste receptors ubiquitously expressed in the sinonasal cavity. Am J Rhinol Allergy. 2017;31:85–92. doi: 10.2500/ajra.2017.31.4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosimann B.L., White M.V., Hohman R.J., Goldrich M.S., Kaulbach H.C., Kaliner M.A. Substance P, calcitonin gene-related peptide, and vasoactive intestinal peptide increase in nasal secretions after allergen challenge in atopic patients. J Allergy Clin Immunol. 1993;92:95–104. doi: 10.1016/0091-6749(93)90043-f. [DOI] [PubMed] [Google Scholar]
- 45.Finger T.E., Böttger B., Hansen A., Anderson K.T., Alimohammadi H., Silver W.L. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci U S A. 2003;100:8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tizzano M., Gulbransen B.D., Vandenbeuch A. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci U S A. 2010;107:3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saunders C.J., Christensen M., Finger T.E., Tizzano M. Cholinergic neurotransmission links solitary chemosensory cells to nasal inflammation. Proc Natl Acad Sci U S A. 2014;111:6075–6080. doi: 10.1073/pnas.1402251111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barham H.P., Cooper S.E., Anderson C.B. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3:450–457. doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang P., Cui M., Zhao B. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J Biol Chem. 2005;280:15238–15246. doi: 10.1074/jbc.M414287200. [DOI] [PubMed] [Google Scholar]
- 50.Cui M., Jiang P., Maillet E., Max M., Margolskee R.F., Osman R. The heterodimeric sweet taste receptor has multiple potential ligand binding sites. Curr Pharm Des. 2006;12:4591–4600. doi: 10.2174/138161206779010350. [DOI] [PubMed] [Google Scholar]
- 51.Imada T., Misaka T., Fujiwara S., Okada S., Fukuda Y., Abe K. Amiloride reduces the sweet taste intensity by inhibiting the human sweet taste receptor. Biochem Biophys Res Commun. 2010;397:220–225. doi: 10.1016/j.bbrc.2010.05.088. [DOI] [PubMed] [Google Scholar]
- 52.Boita M., Bucca C., Riva G., Heffler E., Rolla G. Release of type 2 cytokines by epithelial cells of nasal polyps. J Immunol Res. 2016;2016:2643297. doi: 10.1155/2016/2643297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bufe B., Breslin P.A., Kuhn C. The molecular basis of individual differences in phenylthiocarbamide and propylthiouracil bitterness perception. Curr Biol. 2005;15:322–327. doi: 10.1016/j.cub.2005.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adappa N.D., Truesdale C.M., Workman A.D. Correlation of T2R38 taste phenotype and in vitro biofilm formation from nonpolypoid chronic rhinosinusitis patients. Int Forum Allergy Rhinol. 2016;6:783–791. doi: 10.1002/alr.21803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rom D.I., Christensen J.M., Alvarado R., Sacks R., Harvey R.J. The impact of bitter taste receptor genetics on culturable bacteria in chronic rhinosinusitis. Rhinol J. 2017;55:90–94. doi: 10.4193/Rhin16.181. [DOI] [PubMed] [Google Scholar]
- 56.Farquhar D.R., Kovatch K.J., Palmer J.N., Shofer F.S., Adappa N.D., Cohen N.A. Phenylthiocarbamide taste sensitivity is associated with sinonasal symptoms in healthy adults. Int Forum Allergy Rhinol. 2015;5:111–118. doi: 10.1002/alr.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adappa N.D., Workman A.D., Hadjiliadis D. T2R38 genotype is correlated with sinonasal quality of life in homozygous ΔF508 cystic fibrosis patients. Int Forum Allergy Rhinol. 2016;6:356–361. doi: 10.1002/alr.21675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dżaman K., Zagor M., Sarnowska E., Krzeski A., Kantor I. The correlation of TAS2R38 gene variants with higher risk for chronic rhinosinusitis in Polish patients. Otolaryngol Pol. 2016;70:13–18. doi: 10.5604/00306657.1209438. [DOI] [PubMed] [Google Scholar]
- 59.Adappa N.D., Zhang Z., Palmer J.N. The bitter taste receptor T2R38 is an independent risk factor for chronic rhinosinusitis requiring sinus surgery. Int Forum Allergy Rhinol. 2014;4:3–7. doi: 10.1002/alr.21253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cantone E., Negri R., Roscetto E. In vivo biofilm formation, gram-negative infections and TAS2R38 polymorphisms in CRSw NP patients. Laryngoscope. 2018 doi: 10.1002/lary.27175. [DOI] [PubMed] [Google Scholar]
- 61.Mfuna E.L., Filali-Mouhim A., Boisvert P., Boulet L.P., Bossé Y., Desrosiers M. Genetic variations in taste receptors are associated with chronic rhinosinusitis: a replication study. Int Forum Allergy Rhinol. 2014;4:200–206. doi: 10.1002/alr.21275. [DOI] [PubMed] [Google Scholar]
- 62.Adappa N.D., Farquhar D., Palmer J.N. TAS2R38 genotype predicts surgical outcome in nonpolypoid chronic rhinosinusitis. Int Forum Allergy Rhinol. 2016;6:25–33. doi: 10.1002/alr.21666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Workman A.D., Brooks S.G., Kohanski M.A. Bitter and sweet taste tests are reflective of disease status in chronic rhinosinusitis. J Allergy Clin Immunol Pract. 2018;6(3):1078–1080. doi: 10.1016/j.jaip.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Workman A.D., Maina I.W., Brooks S.G. The role of quinine-responsive Taste Receptor Family 2 in airway immune defense and chronic rhinosinusitis. Front Immunol. 2018;9:624. doi: 10.3389/fimmu.2018.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hariri B.M., McMahon D.B., Chen B. Plant flavones enhance antimicrobial activity of respiratory epithelial cell secretions against Pseudomonas aeruginosa. PLoS One. 2017;12 doi: 10.1371/journal.pone.0185203. e0185203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo R., Cantery P.H., Ernst E. Herbal medicines for the treatment of rhinosinusitis: a systematic review. Otolaryngol Neck Surg. 2006;135:496–506. doi: 10.1016/j.otohns.2006.06.1254. [DOI] [PubMed] [Google Scholar]
- 67.Neubauer N., März R.W. Placebo-controlled, randomized double-blind clinical trial with Sinupret® sugar coated tablets on the basis of a therapy with antibiotics and decongestant nasal drops in acute sinusitis. Phytomedicine. 1994;1:177–181. doi: 10.1016/S0944-7113(11)80061-9. [DOI] [PubMed] [Google Scholar]
- 68.Jund R., Mondigler M., Steindl H., Stammer H., Stierna P., Bachert C. Clinical efficacy of a dry extract of five herbal drugs in acute viral rhinosinusitis. Rhinology. 2012;50:417–426. doi: 10.4193/Rhino.12.015. [DOI] [PubMed] [Google Scholar]
- 69.Jund R., Mondigler M., Stammer H., Stierna P., Bachert C. Herbal drug BNO 1016 is safe and effective in the treatment of acute viral rhinosinusitis. Acta Otolaryngol. 2015;135:42–50. doi: 10.3109/00016489.2014.952047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Levit A., Nowak S., Peters M. The bitter pill: clinical drugs that activate the human bitter taste receptor TAS2R14. FASEB J. 2014;28:1181–1197. doi: 10.1096/fj.13-242594. [DOI] [PubMed] [Google Scholar]
- 71.Lossow K., Hübner S., Roudnitzky N. Comprehensive analysis of mouse bitter taste receptors reveals different molecular receptive ranges for orthologous receptors in mice and humans. J Biol Chem. 2016;291:15358–15377. doi: 10.1074/jbc.M116.718544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reed D.R., Zhu G., Breslin P.A. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Hum Mol Genet. 2010;19:4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]