Abstract
Chronic rhinitis and rhinosinusitis are among the most common conditions worldwide with significant morbidity and decreased quality of life. Although the pathogenesis of these conditions is multifactorial, there has been increasing evidence for the role of environmental factors such as aeroallergens and air pollutants as initiating or exacerbating factors. This review will outline the current literature focusing on the role of aeroallergens and air pollution in the pathogenesis of chronic sinonasal inflammatory conditions.
Keywords: Aeroallergens, Air pollutants, Inflammatory cytokines, Innate immunity, Particulate matter, Rhinitis
Introduction
Chronic sinonasal inflammatory diseases including chronic rhinosinusitis (CRS) and allergic rhinitis (AR) affect millions of Americans annually.1 CRS-related health care costs are far-reaching and estimated to be 22 billion USD in 2014.1, 2 Although CRS is commonly diagnosed in the population, the fundamental pathologic mechanisms of mucosal inflammation affecting CRS have been particularly challenging to elucidate. CRS is frequently divided into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), however, as our understanding of the disease has improved the disease process is gradually becoming divided as a collection of endotypes.3 While the role of aeroallergens in CRS pathogenesis is controversial, the negative impact of air pollutants in CRS is beginning to be more defined. Allergic rhinitis is another highly prevalent sinonasal inflammatory disorder and is divided based on seasonal versus perennial and intermittent versus persistent.4 Here, we discuss the current understanding of the impact of aeroallergens and air pollutants on chronic sinonasal inflammatory disorders.
Aeroallergens
Aeroallergens, otherwise understood as inhalant allergens, have long been hypothesized to play a role in the pathogenesis and resilience of CRS to therapy. Frequently tested aeroallergens ranging from molds, trees, weeds, grass, animal dander are usually secondary to the house dust mite (HDM) as the most frequent offender.5 While some studies have purported direct associations between CRS volunteers and allergic sensitization, there is not a definitive correlation between the two. For instance, in a study by Gutman 2004 of 48 voluntary participants with CRS and recurrent acute rhinosinusitis, 57.4% had a positive allergy test with the majority being sensitive to more than one allergen (most commonly perennial allergens, mold and dust mites).6 Similarly in a group of 200 patients with CRS who underwent functional endoscopic sinus surgery, 84% had tested positive for allergies.7 Larger data from the UK National Chronic Rhinosinusitis Epidemiology Study were able to show that in over 500 patients with CRSwNP and CRSsNP, the rates of self-reported aeroallergen was 20.3% and 31.0% respectively. Interesting, HDM allergy was significantly higher in CRSwNP (16%) than in CRSsNP (9%).8 In contrast, cross sectional studies in children have shown no significant difference between sensitization to aeroallergens and CRS when compared to the general population.9 It is thereby clear that our understanding of mucosal specific inflammatory pathways will elucidate the pathogenesis of CRS that cannot be explained by systemic immunoregulatory dysfunction alone.
Aeroallergens – pathogenesis
Through sensitization and other innate mechanisms, aeroallergens have been associated with mucosal inflammation in pathology ranging from reactive airway disease to allergic rhinitis. The current model of pathogenesis is that aeroallergens, regardless of the extent of penetration into sinuses, cause a systemic allergic response. Instead of behaving as the sole conductor, this response subsequently contributes to the greater orchestra of factors that compose rhinosinusitis. The specific drivers of mucosal inflammation can thus be separated into three basic mechanisms: (1) Deficiencies in host defenses and transepithelial permeability; (2) Triggers associated with Th2 pro-inflammatory cytokines; (3) Innate immune mechanisms.
A number of cytokines and innate immune mechanisms have been shown to be involved with mucosal inflammation and associated with CRS pathogenesis (Fig. 1). Over the past three decades, our understanding of these immune mechanisms were initially derived from understanding the association between immunodeficiency syndromes (Good syndrome, CVID, Selective IgA deficiency) and CRS.10, 11 Others have relied on murine and rabbit models of sinusitis to replicate the conditions of CRS and evaluate therapeutics on treatment arms and controls. Khalmuratova et al12 were able to develop of a mouse model of CRSwNP using nasally injected HDM co-administered with staphylococcus aureus enterotoxin B. Tharaken et al13 were also able to develop a murine model of eosinophilic rhinosinusitis following administration of intranasal papain with comparable Th2 cytokines and innate immune responses to CRS. While these models are essential for testing and understanding basic fundamental mechanisms, their clinical utility remains questionable. In a multicenter study across several continents, CRSwNP and CRSsNP has demonstrated a multiplicity of Th1/Th2/Th17 cytokine profiles which in part explain the heterogeneity of immune sensitivity seen in CRS globally.14 This diversity of cytokine profiles demonstrates the need to better categorize CRS as a collection of clinical subtypes rather than a catch-all diagnosis.
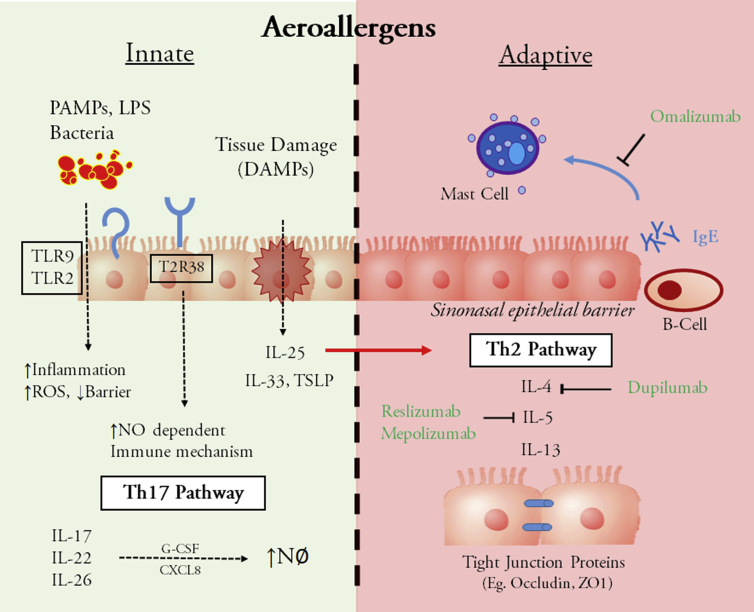
Fig. 1.
Innate immunity and adaptive immunity. Innate immunity (green): Pathogen-associated molecular patterns (PAMPs), lipopolysacharides (LPS) and bacteria act as stimuli to Toll like receptors (eg. TLR9, TLR2) resulting in increased reactive oxygen species (ROS), inflammation, and decreased sinonasal epithelial barrier function. Damage associated molecular patterns (DAMPs) directly stimulate the release of cytokines (IL-25, IL-33, TSLP) leading to Th2 activation. Taste receptor T2R38 has also been shown to be stimulated by LPS and in turn creates a nitrous oxide dependent immune response. Adaptive immunity (red): Activation of the Th2 pathway via IL-4, IL-5 and IL-13 results in increased epithelial barrier permeability in part through down regulation of tight junction proteins. An IgE mediated immune response results in mast cell degranulation.
As with any epithelium, nasal mucosa membrane penetration presents a key step in the translocation of aeroallergens, microbes and foreign particles such as pollutants. Up regulation of Th-17 cells, which serves to maintain mucosal barriers and facilitate pathogen clearance, and production of associated cytokines (IL-17, IL-22, and IL-26) have been shown to increase mucosal permeability and may contribute to the polypoid changes seen in CRS.15, 16 Via the Th2 pathway the cytokines IL-4, IL-5 and IL-13 have been key players in the generation of a number of alterations in host defense including changing nasal epithelium permeability. Using air-liquid cultured nasal epithelium of patients with HDM-induced allergic rhinitis, Steelant et al17 were able to lower levels of occludin and zonula occludens-1 expression, proteins involved with nasal epithelial tight junctions, in CRS tissue. Interestingly, they were also able to show that fluticasone could work as a countermeasure towards increasing barrier function.17 Other cytokines such as IL-25 have been recently identified as an early signal in the Th2 inflammatory cascade. Kohanski et al18 were able to demonstrate that IL-25, which can function as an early signal for the type 2 response in CRSwNP, is potentially derived from solitary chemosensory cells in CRSwNP but not in adjacent nasal turbinate tissue.
New targets within the innate immune pathway have become recently popularized in the CRS literature of the past decade. For instance, as part of the IL-1 superfamily, IL-33 is released from tissue damage/cellular stress and induces production of Th1/Th2 cytokines and facilitates neutrophil recruitment in patients with CRS. Treatment of allergen induced CRS in a murine model with anti-IL-33 antibody has showed reduced mucosa thickness, subepithelial collagen deposition, and neutrophil, but not eosinophil, infiltration vs. control mucosal tissue.19, 20 Toll-like receptor 9, which is present on antigen presenting cells, also been shown to play an important role in generating a pro-inflammatory cascade in response to PAMPs (Pathogen-associated molecular patterns) in CRS. Interestingly, TLR9 expression on culture primary nasal epithelial cells from CRSwNP patients was reduced by 50% when compared to control cells. Moreover, exposure to Th2 inflammatory cytokines down regulated TLR9 expression by about half.21 Other groups have shown that HDM-derived beta-glucans were critical for TLR-2 (nasal)/TLR-4 (lung) activation as well as production of dual oxidase-2 generated reactive oxygen species.22 This may serve a role in increasing sinonasal epithelial barrier dysfunction and permeability secondary to stimulants such as HDM. Indeed, activation of a cytoprotective pathway has been found to restore HDM-mediated disruption tight junction proteins and transepithelial resistance.23
Other new targets such as the bitter taste receptors (specifically T2R38) have been found in mature respiratory cilia and are thought to trigger early innate response via stimulation from acyl-homoserine lactones, gram negative quorum-sensing molecules and NO-dependent immune responses. Polymorphisms of these receptors are common and have been shown to be associated with CRS.24 Interestingly, phenyl thiocarbamide taste sensitivity has been associated with healthier sinuses based on symptoms than non-tasters.25 While it is still unclear how large of a role these receptors play in CRS, they may serve to better tailor therapies based on these genetic polymorphisms.
In a randomized, double-blinded, placebo-controlled study of patients with nasal polyps who received omalizumab (anti-IgE ab), there were significant reductions in airway symptoms (nasal congestion, anterior rhinorrhea, hyposmia, dyspnea) and quality of life scores vs controls.26 Other therapeutic antibodies are in development to target specific elements of the Th2 inflammatory pathway. Anti-IL-5 antibodies, namely Reslizumab and Mepolizumab, have been used in proof of concept and a phase III clinical trial, respectively, for CRS. While these studies showed a promising reduction in polyp rating scores and peripheral blood eosinophil counts, there were no significant improvement of symptoms in CRS patients.27, 28 Similarly, a phase Ⅱ study of 60 CRSwNP patients who received Dupilumab, a monoclonal antibody which targets the alpha chain of the IL-4 receptor, has shown promise in reducing endoscopic nasal polyp burden when used in combination with a nasal steroid spray.29, 30 Unfortunately, these immunotherapies are expensive and have also been associated with an increased risk of nasopharyngitis and injection site reactions. Given the roles of innate immunity and infection on CRS, a more comprehensive strategy that encompasses additional targets outside of the Th2 immune pathway alone is likely needed for comprehensive treatment.
Additional targeting strategies against innate immune checkpoints are now underway. Kim et al20 looked at the effects of anti-IL-33 treatment in a mouse model of allergic rhinitis. They found that the treatment group had significantly reduced the number of nose-scratching events and ameliorated skin denudation, decreased eosinophilic infiltration and decrease IL-4/IL-5 and IL-13 in BAL fluid. In a murine nasal polyp model, Shin et al showed that anti-IL-25 antibody treatment reduced the number of polyps, mucosal edema thickness, collagen deposition and infiltration of neutrophils and eosinophils while also inhibiting expression of IL-4/IFN-gamma.31 In addition to the anti-inflammatory effects, these studies show great promise in not only preventing, but reversing the mucosal changes inherent in CRS. While there remains a diversity of clinical phenotypes, which constitute the clinical diagnosis of CRS, by understanding the trends in immune response we can find common pharmacological targets to treat, and perhaps prevent, the associated inflammatory response and their sequelae.
Air pollutants and chronic sinonasal inflammatory disorders – cigarette smoke
Air pollution has well documented negative acute and chronic effects on human health including exacerbation of cardiovascular and pulmonary disease, increased risk of cancer, and premature death.32 The upper sinonasal airway acts as a first line of defense to inhaled environmental pollutant exposures including cigarette smoke, traffic-related air pollutants (TRAP) such as diesel exhaust particles, and particulate matter 2.5 (PM2.5) have been hypothesized to exacerbate chronic sinonasal inflammatory disorders (Fig. 2). Here we discuss what is known in relation to the clinical impact, pathophysiology, and dysregulatory function of these stimuli.
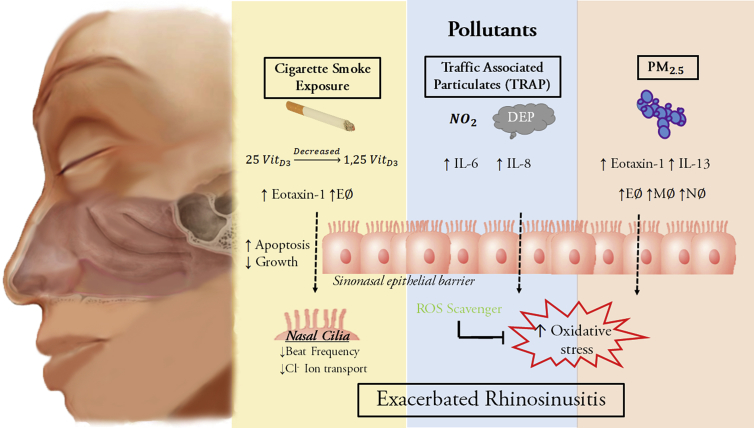
Fig. 2.
Cigarette smoke exposure (yellow) results in decreased conversion of 25 vitamin D3 to activated 1, 25 vitamin D3. The presence of smoke exposure results in increased Eotaxin-1 and eosinophil accumulation as well as increased apoptosis and reduced regeneration of the sinonasal epithelial barrier. Cigarette smoke also directly affects nasal cilia by reducing beat frequency and ion transport. Traffic associated particulates (blue), such as diesel exhaust particles (DEP), cause increased IL-6 and IL-8 activity and have likewise been shown to increase epithelial barrier permeability. The resulting effect is increased oxidative stress which can be combated with ROS (reactive oxygen species) scavengers. PM2.5 (particular matter < 2.5 microns in size, red) have been shown to increase immune cells response (E = eosinophils, M = macrophages, N = neutrophils). This likewise results in increased cellular oxidative stress. The cumulative effect from each pollutant is the exacerbation of rhinosinusitis.
Cigarette smoke is an environmental pollutant that may affect the sinonasal cavity through both primary- or second-hand exposure.33 A recent meta-analysis found that 11 of the 13 studies that met inclusion criteria demonstrated an association between primary smoke exposure and increased prevalence of CRS.34 For example, a recent population-based study by Hirsch et al35 mailed a CRS questionnaire to 23,700 primary care patients and found the odds of CRS was higher in current and former smokers compared to never smokers. A cross-sectional study performed by interviewing 10 636 patients using a standardized questionnaire found that tobacco smoking was associated with an increased risk of CRS and the negative impact generally increased with dose and duration of smoking.36 Thus active and former smoking may increase the risk of CRS and negatively impact sinonasal health.33, 34 A limitation of these studies, however, is that self-reported symptoms without documentation of inflammation on nasal endoscopy may lead to misclassification.37
The effect of second-hand or passive smoking exposure on CRS or rhinitis in adults is less clear as some studies report an increase while others report no association.34 In one case–control study, those with current or a history of second-hand smoke (SHS) had an increased risk of CRS as well as worse scores in nasal obstruction, nasal discharge, and headache.38 A second case–control study also found an increased odds of CRS in patients with a five year history of SHS at multiple independent venues including home, work, and private functions.39 In two cross-sectional surveys, however, while CRS was found to be associated with active smoking, no association was observed in SHS exposure.40, 41 Similar contrasting results between SHS exposure and rhinitis have been reported in adults.42 Interestingly the impact of SHS in children may be more consistent than in adults. One study using a combination of self-report and serum cotinine levels identified a strong association between second-hand smoke exposure and rhinitis in children.43 This finding is further supported by a recent study, which found an association between parental smoking and allergic rhinitis in children.44 Interestingly, in teenagers with perennial allergic rhinitis those with exposure to tobacco smoke demonstrated increased nasal mucosa eotaxin-1 and eosinophil counts compared to control.45 Thus second-hand smoke exposure may exacerbate rhinitis in children.
The pathophysiology and mechanism whereby cigarette smoke exposure disrupts sinonasal function is likely multi-factorial and may include disruption of ion transport, mucociliary clearance, vitamin D conversion, and sinonasal epithelial barrier function as well as increased oxidative stress and inflammatory mediators.34 The sinonasal epithelium regulates many of these functions, indeed, cigarette smoke extract (CSE) has been reported to impair sinonasal epithelial cell growth and promote apoptosis of normal nasal epithelial cells in vitro.46 One study reported that cigarette smoke condensate inhibited transepithelial chloride transport and ciliary beat frequency, two major components or mucociliary clearance, in primary murine and human sinonasal epithelial cultures in vitro.47 Consistent with these results, a case series found that parameters of decreased nasal mucociliary clearance including saccharin nasal transit time, ciliary movement, and additional microscopic parameters were reduced in active smokers.48 Indeed, poor mucociliary clearance is a common finding in CRS.49 Cigarette smoke has also been reported to decrease vitamin D3 conversion by human sinonasal epithelial cells resulting in increased sinonasal epithelial pro-inflammatory cytokine release which could be reversed by administration of exogenous 1, 25-dihydroxyvitamin D3.50 Cigarette smoke has also been demonstrated to increased reactive oxygen species in sinonasal tissue and CSE has been shown to disrupt sinonasal epithelial barrier function in vitro.51, 52 Thus the negative impact of cigarette smoke on the sinonasal cavity is likely multi-factorial.
Air pollutants and chronic sinonasal inflammatory disorders – TRAP
Traffic-related air pollution such as nitrogen dioxide (NO2) and diesel exhaust particles (DEP) have been associated with development of allergic asthma and exacerbation of lower airway disease.53, 54 However, the role of TRAP as a risk factor for CRS and allergic rhinitis is less well understood. One report found an increased risk of allergic rhinitis in adults residing within 100 m of a road with a high traffic intensity.53, 54 Another study found a positive correlation between the frequency of allergic rhinitis episodes and pollutant concentration and higher vehicular traffic.55 In contrast, a recent study estimated TRAP exposure based on proximity to the nearest major road as well as the density of major roads within 300 m from where children resided and found no association between TRAP exposure and risk of allergic rhinitis.56 A possible explanation for these conflicting results is the prevalence of genetic susceptibility in inflammatory genes in some patients to develop allergic rhinitis.57 Regardless, well-control prospective studies are necessary to determine the effect of air pollutants on clinical rhinitis symptoms.
The effect of DEP has been investigated in vivo where mice sensitized to ragweed pollen were challenged intranasally with ragweed pollen in the presence or absence of DEP. Mice that were treated with DEP were found to have increased frequency of sneezing, an indication of aggravation of allergic rhinitis.58 This group also found that DEP disrupted tight junction integrity, thereby disrupting the sinonasal epithelial barrier.58 Interestingly, these negative effects of DEP were suppressed by treatment with a reactive oxygen species scavenger.58, 59 A second possible mechanism of sinonasal inflammatory disease aggravation is through DEP-mediated induction of pro-inflammatory cytokines. Kim et al60 stimulated nasal fibroblasts with DEP and performed a cytokine and chemokine array where they found increased levels of interleukin-6 (IL-6) and interleukin-8 (IL-8). The effect of DEP on IL-6 and IL-8 expression was further confirmed by this group using inferior turbinate organ cultures ex vivo.
Air pollutants and chronic sinonasal inflammatory disorders – PM2.5
Particulate matter is an air pollutant with well described negative health consequences throughout the human body. The damaging effects of PM depend on the size of the particle, composition, and induction of oxidative stress.61 Multiple recent studies have begun to demonstrate the negative health consequences of PM2.5 exposure as it relates to chronic sinonasal inflammation. One group found this to be particularly applicable to CRSsNP patients where for each unit increase in PM2.5 exposure, there was a 1.89-fold increase in the proportion of CRSsNP who required further surgery.62 Another recent study found that an increase of 10 μg/m3 of the annual PM2.5 exposure was associated with an increased prevalence of allergic rhinitis in preschool children (odd ratio 1.20).63 These results have been corroborated by another study in Peruvian children where each 10 μg/m3 in PM2.5 exposure was associated with an increased odds of worsened rhinoconjunctivitis quality of life (odds ratio 1.83).64 In contrast, no association was reported in two European cohorts between an increase of 5 μg/m3 in PM2.5 exposure and rhinitis.65
The effects of chronic airborne PM2.5 exposure has recently been reported in mice. In the study by Ramanathan et al, mice were subjected to inhalation of concentrated PM2.5 at a mean concentration of 60.92 μg/m3 for 6 h a day, 5 days a week, for 16 weeks.66 An induction of sinonasal inflammatory cells including macrophages, neutrophils, and eosinophils was observed along with sinonasal epithelial barrier dysfunction, and an increase in expression of pro-inflammatory cytokines and chemokines including interleukin-1β, interleukin-13, and eotaxin-1.66 This results are supported by another study in which PM2.5 was instilled intranasally in rats exacerbated allergen-induced allergic rhinitis symptoms, eosinophil accumulation, and inflammatory cytokine expression.67 Several studies in vitro have demonstrated that PM2.5 exposure disrupts sinonasal epithelial barrier function and tight junction integrity.68, 69 Furthermore, these barrier destabilization effects were reduced through treatment with strategies aimed at reducing oxidative stress, which may represent a potential therapeutic approach for treating sinonasal inflammatory disease exacerbated by particulate matter exposure.68, 69 However, further pre-clinical testing in animal models will help to assess the potential applicability.
Conclusion
Chronic sinonasal inflammatory diseases including CRS and AR are highly prevalent and have far-reaching health care costs and decreased quality of life. Although the pathogenesis of these conditions is multifactorial, there has been increasing evidence for the role of environmental factors such as aeroallergens and air pollutants as initiating or exacerbating factors. Future studies may help to further elucidate disease mechanisms, contributing factors, and identify additional therapeutic options.
Edited by Yi Fang
Footnotes
Peer review under responsibility of Chinese Medical Association.
References
- 1.Benninger M.S., Ferguson B.J., Hadley J.A. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129:S1–S32. doi: 10.1016/s0194-5998(03)01397-4. [DOI] [PubMed] [Google Scholar]
- 2.Smith K.A., Orlandi R.R., Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125:1547–1556. doi: 10.1002/lary.25180. [DOI] [PubMed] [Google Scholar]
- 3.Bachert C., Zhang N., Hellings P.W., Bousquet J. Endotype-driven care pathways in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2018;141:1543–1551. doi: 10.1016/j.jaci.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Wise S.K., Lin S.Y., Toskala E. International consensus statement on allergy and rhinology: allergic rhinitis. Int Forum Allergy Rhinol. 2018;8:108–352. doi: 10.1002/alr.22070. [DOI] [PubMed] [Google Scholar]
- 5.Xu M., Ye X., Zhao F., He Y., Chen L. Allergogenic profile in patients with different subtypes of chronic rhinosinusitis with nasal polyps. ORL J Otorhinolaryngol Relat Spec. 2015;77:10–16. doi: 10.1159/000370121. [DOI] [PubMed] [Google Scholar]
- 6.Gutman M., Torres A., Keen K.J., Houser S.M. Prevalence of allergy in patients with chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2004;130:545–552. doi: 10.1016/j.otohns.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Emanuel I.A., Shah S.B. Chronic rhinosinusitis: allergy and sinus computed tomography relationships. Otolaryngol Head Neck Surg. 2000;123:687–691. doi: 10.1067/mhn.2000.110961. [DOI] [PubMed] [Google Scholar]
- 8.Philpott C.M., Erskine S., Hopkins C. Prevalence of asthma, aspirin sensitivity and allergy in chronic rhinosinusitis: data from the UK National Chronic Rhinosinusitis Epidemiology Study. Respir Res. 2018;19:129. doi: 10.1186/s12931-018-0823-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leo G., Piacentini E., Incorvaia C., Consonni D., Frati F. Chronic rhinosinusitis and allergy. Pediatr Allergy Immunol. 2018;18:19–21. doi: 10.1111/j.1399-3038.2007.00626.x. [DOI] [PubMed] [Google Scholar]
- 10.Frieri M. Good's syndrome, CVID, and selective antibody deficiency in patients with chronic rhinosinusitis. Curr Allergy Asthma Rep. 2014;14:438. doi: 10.1007/s11882-014-0438-4. [DOI] [PubMed] [Google Scholar]
- 11.Ocampo C.J., Peters A.T. Antibody deficiency in chronic rhinosinusitis: epidemiology and burden of illness. Am J Rhinol Allergy. 2013;27:34–38. doi: 10.2500/ajra.2013.27.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khalmuratova R., Lee M., Kim D.W., Park J.W., Shin H.W. Induction of nasal polyps using house dust mite and Staphylococcal enterotoxin B in C57BL/6 mice. Allergol Immunopathol (Madr) 2016;44:66–75. doi: 10.1016/j.aller.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Tharakan A., Dobzanski A., London N.R. Characterization of a novel, papain-inducible murine model of eosinophilic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8:513–521. doi: 10.1002/alr.22072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X., Zhang N., Bo M. Diversity of TH cytokine profiles in patients with chronic rhinosinusitis: a multicenter study in Europe, Asia, and Oceania. J Allergy Clin Immunol. 2016;138:1344–1353. doi: 10.1016/j.jaci.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 15.Ramezanpour M., Moraitis S., Smith J.L., Wormald P.J., Vreugde S. Th17 cytokines disrupt the airway mucosal barrier in chronic rhinosinusitis. Mediators Inflamm. 2016;2016 doi: 10.1155/2016/9798206. 9798206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miljkovic D., Psaltis A., Wormald P.J., Vreugde S. T regulatory and Th17 cells in chronic rhinosinusitis with polyps. Int Forum Allergy Rhinol. 2016;6:826–834. doi: 10.1002/alr.21742. [DOI] [PubMed] [Google Scholar]
- 17.Steelant B., Farré R., Wawrzyniak P. Impaired barrier function in patients with house dust mite-induced allergic rhinitis is accompanied by decreased occludin and zonula occludens-1 expression. J Allergy Clin Immunol. 2016;137 doi: 10.1016/j.jaci.2015.10.050. 1043-1053.e5. [DOI] [PubMed] [Google Scholar]
- 18.Kohanski M.A., Workman A.D., Patel N.N. Solitary chemosensory cells are a primary epithelial source of IL-25 in patients with chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2018;142 doi: 10.1016/j.jaci.2018.03.019. 460-469.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim D.K., Jin H.R., Eun K.M. The role of interleukin-33 in chronic rhinosinusitis. Thorax. 2017;72:635–645. doi: 10.1136/thoraxjnl-2016-208772. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y.H., Yang T.Y., Park C.S. Anti-IL-33 antibody has a therapeutic effect in a murine model of allergic rhinitis. Allergy. 2012;67:183–190. doi: 10.1111/j.1398-9995.2011.02735.x. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan M., Lee W.K., Spannhake E.W., Lane A.P. Th2 cytokines associated with chronic rhinosinusitis with polyps down-regulate the antimicrobial immune function of human sinonasal epithelial cells. Am J Rhinol. 2008;22:115–121. doi: 10.2500/ajr.2008.22.3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryu J.H., Yoo J.Y., Kim M.J. Distinct TLR-mediated pathways regulate house dust mite-induced allergic disease in the upper and lower airways. J Allergy Clin Immunol. 2013;131:549–561. doi: 10.1016/j.jaci.2012.07.050. [DOI] [PubMed] [Google Scholar]
- 23.London N.R., Tharakan A., Lane A.P., Biswal S., Ramanathan M. Nuclear erythroid 2-related factor 2 activation inhibits house dust mite-induced sinonasal epithelial cell barrier dysfunction. Int Forum Allergy Rhinol. 2017;7:536–541. doi: 10.1002/alr.21916. [DOI] [PubMed] [Google Scholar]
- 24.Cohen N.A. The genetics of the bitter taste receptor T2R38 in upper airway innate immunity and implications for chronic rhinosinusitis. Laryngoscope. 2017;127:44–51. doi: 10.1002/lary.26198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farquhar D.R., Kovatch K.J., Palmer J.N., Shofer F.S., Adappa N.D., Cohen N.A. Phenylthiocarbamide taste sensitivity is associated with sinonasal symptoms in healthy adults. Int Forum Allergy Rhinol. 2015;5:111–118. doi: 10.1002/alr.21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gevaert P., Calus L., Van Zele T. Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol. 2013;131 doi: 10.1016/j.jaci.2012.07.047. 110-116.e1. [DOI] [PubMed] [Google Scholar]
- 27.Weinstein S.F., Germinaro M., Bardin P., Korn S., Bateman E.D. Efficacy of reslizumab with asthma, chronic sinusitis with nasal polyps and elevated blood eosinophils. J Allergy Clin Immunol. 2016;137:AB86. [Google Scholar]
- 28.Gevaert P., Van Bruaene N., Cattaert T. Mepolizumab, a humanized anti–IL-5 mAb, as a treatment option for severe nasal polyposis. J Allergy Clin Immunol. 2011;128 doi: 10.1016/j.jaci.2011.07.056. 989-995.e8. [DOI] [PubMed] [Google Scholar]
- 29.Sastre J., Dávila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018;28:139–150. doi: 10.18176/jiaci.0254. [DOI] [PubMed] [Google Scholar]
- 30.Bachert C., Mannent L., Naclerio R.M. Effect of subcutaneous Dupilumab on nasal polyp burden in patients with chronic sinusitis and nasal polyposis: a randomized clinical trial. JAMA. 2016;315:469–479. doi: 10.1001/jama.2015.19330. [DOI] [PubMed] [Google Scholar]
- 31.Shin H.W., Kim D.K., Park M.H. IL-25 as a novel therapeutic target in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2015;135 doi: 10.1016/j.jaci.2015.01.003. 1476-1485.e7. [DOI] [PubMed] [Google Scholar]
- 32.Kampa M., Castanas E. Human health effects of air pollution. Environ Pollut. 2008;151:362–367. doi: 10.1016/j.envpol.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Reh D.D., Higgins T.S., Smith T.L. Impact of tobacco smoke on chronic rhinosinusitis: a review of the literature. Int Forum Allergy Rhinol. 2012;2:362–369. doi: 10.1002/alr.21054. http://www.ncbi.nlm.nih.gov/pubmed/22696460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Christensen D.N., Franks Z.G., McCrary H.C., Saleh A.A., Chang E.H. A systematic review of the association between cigarette smoke exposure and chronic rhinosinusitis. Otolaryngol Head Neck Surg. 2018;158:801–816. doi: 10.1177/0194599818757697. http://www.ncbi.nlm.nih.gov/pubmed/29460678 [DOI] [PubMed] [Google Scholar]
- 35.Hirsch A.G., Stewart W.F., Sundaresan A.S. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. 2017;72:274–281. doi: 10.1111/all.13042. http://www.ncbi.nlm.nih.gov/pubmed/27590749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi J.B., Fu Q.L., Zhang H. Epidemiology of chronic rhinosinusitis: results from a cross-sectional survey in seven Chinese cities. Allergy. 2015;70:533–539. doi: 10.1111/all.12577. http://www.ncbi.nlm.nih.gov/pubmed/25631304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sundaresan A.S., Hirsch A.G., Storm M. Occupational and environmental risk factors for chronic rhinosinusitis: a systematic review. Int Forum Allergy Rhinol. 2015;5:996–1003. doi: 10.1002/alr.21573. http://www.ncbi.nlm.nih.gov/pubmed/26077513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reh D.D., Lin S.Y., Clipp S.L., Irani L., Alberg A.J., Navas-Acien A. Secondhand tobacco smoke exposure and chronic rhinosinusitis: a population-based case-control study. Am J Rhinol Allergy. 2009;23:562–567. doi: 10.2500/ajra.2009.23.3377. [DOI] [PubMed] [Google Scholar]
- 39.Tammemagi C.M., Davis R.M., Benninger M.S., Holm A.L., Krajenta R. Secondhand smoke as a potential cause of chronic rhinosinusitis: a case-control study. Arch Otolaryngol Head Neck Surg. 2010;136:327–334. doi: 10.1001/archoto.2010.43. http://www.ncbi.nlm.nih.gov/pubmed/20403847 [DOI] [PubMed] [Google Scholar]
- 40.Lieu J.E., Feinstein A.R. Confirmations and surprises in the association of tobacco use with sinusitis. Arch Otolaryngol Head Neck Surg. 2000;126:940–946. doi: 10.1001/archotol.126.8.940. http://www.ncbi.nlm.nih.gov/pubmed/10922224 [DOI] [PubMed] [Google Scholar]
- 41.Lee W.H., Hong S.N., Kim H.J. Effects of cigarette smoking on rhinologic diseases: Korean national health and nutrition examination survey 2008-2011. Int Forum Allergy Rhinol. 2015;5:937–943. doi: 10.1002/alr.21553. [DOI] [PubMed] [Google Scholar]
- 42.Shargorodsky J. Secondhand smoke and rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2016;24:241–244. doi: 10.1097/MOO.0000000000000250. http://www.ncbi.nlm.nih.gov/pubmed/27054622 [DOI] [PubMed] [Google Scholar]
- 43.Shargorodsky J., Garcia-Esquinas E., Navas-Acien A., Lin S.Y. Allergic sensitization, rhinitis, and tobacco smoke exposure in U.S. children and adolescents. Int Forum Allergy Rhinol. 2015;5:471–476. doi: 10.1002/alr.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Singh S., Sharma B.B., Salvi S. Allergic rhinitis, rhinoconjunctivitis, and eczema: prevalence and associated factors in children. Clin Respir J. 2018;12:547–556. doi: 10.1111/crj.12561. [DOI] [PubMed] [Google Scholar]
- 45.Montaño-Velázquez B.B., Flores-Rojas E.B., García-Vázquez F.J. Effect of cigarette smoke on counts of immunoreactive cells to eotaxin-1 and eosinophils on the nasal mucosa in young patients with perennial allergic rhinitis. Braz J Otorhinolaryngol. 2017;83:420–425. doi: 10.1016/j.bjorl.2016.04.011. http://www.ncbi.nlm.nih.gov/pubmed/27287302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H.S., Kim J. Cigarette smoke inhibits nasal airway epithelial cell growth and survival. Int Forum Allergy Rhinol. 2013;3:188–192. doi: 10.1002/alr.21129. http://www.ncbi.nlm.nih.gov/pubmed/23281305 [DOI] [PubMed] [Google Scholar]
- 47.Cohen N.A., Zhang S., Sharp D.B. Cigarette smoke condensate inhibits transepithelial chloride transport and ciliary beat frequency. Laryngoscope. 2009;119:2269–2274. doi: 10.1002/lary.20223. http://www.ncbi.nlm.nih.gov/pubmed/19418539 [DOI] [PubMed] [Google Scholar]
- 48.Pagliuca G., Rosato C., Martellucci S. Cytologic and functional alterations of nasal mucosa in smokers: temporary or permanent damage? Otolaryngol Head Neck Surg. 2015;152:740–745. doi: 10.1177/0194599814566598. [DOI] [PubMed] [Google Scholar]
- 49.London N.R., Lane A.P. Innate immunity and chronic rhinosinusitis: what we have learned from animal models. Laryngoscope Investig Otolaryngol. 2016;1:49–56. doi: 10.1002/lio2.21. http://www.ncbi.nlm.nih.gov/pubmed/28459101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mulligan J.K., Nagel W., O'Connell B.P., Wentzel J., Atkinson C., Schlosser R.J. Cigarette smoke exposure is associated with vitamin D3 deficiencies in patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2014;134:342–349. doi: 10.1016/j.jaci.2014.01.039. http://www.ncbi.nlm.nih.gov/pubmed/24698317 [DOI] [PubMed] [Google Scholar]
- 51.Fordham M.T., Mulligan J.K., Casey S.E. Reactive oxygen species in chronic rhinosinusitis and secondhand smoke exposure. Otolaryngol Head Neck Surg. 2013;149:633–638. doi: 10.1177/0194599813496377. http://www.ncbi.nlm.nih.gov/pubmed/23838308 [DOI] [PubMed] [Google Scholar]
- 52.Tharakan A., Halderman A.A., Lane A.P., Biswal S., Ramanathan M. Reversal of cigarette smoke extract-induced sinonasal epithelial cell barrier dysfunction through Nrf2 Activation. Int Forum Allergy Rhinol. 2016;6:1145–1150. doi: 10.1002/alr.21827. http://www.ncbi.nlm.nih.gov/pubmed/27580429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lindgren A., Stroh E., Nihlén U., Montnémery P., Axmon A., Jakobsson K. Traffic exposure associated with allergic asthma and allergic rhinitis in adults. A cross-sectional study in southern Sweden. Int J Health Geogr. 2009;8:25. doi: 10.1186/1476-072X-8-25. http://www.ncbi.nlm.nih.gov/pubmed/19419561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jang A.S., Jun Y.J., Park M.K. Effects of air pollutants on upper airway disease. Curr Opin Allergy Clin Immunol. 2016;16:13–17. doi: 10.1097/ACI.0000000000000235. http://www.ncbi.nlm.nih.gov/pubmed/26658014 [DOI] [PubMed] [Google Scholar]
- 55.Nicolussi F.H., Santos A.P., André S.C., Veiga T.B., Takayanagui A.M. Air pollution and respiratory allergic diseases in schoolchildren. Rev Saude Publica. 2014;48:326–330. doi: 10.1590/S0034-8910.2014048004940. http://www.ncbi.nlm.nih.gov/pubmed/24897055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yi S.J., Shon C., Min K.D. Association between exposure to traffic-related air pollution and prevalence of allergic diseases in children, Seoul, Korea. Biomed Res Int. 2017;2017 doi: 10.1155/2017/4216107. 4216107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fuertes E., Brauer M., MacIntyre E. Childhood allergic rhinitis, traffic-related air pollution, and variability in the GSTP1, TNF, TLR2, and TLR4 genes: results from the TAG study. J Allergy Clin Immunol. 2013;132:342–352. doi: 10.1016/j.jaci.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 58.Fukuoka A., Matsushita K., Morikawa T., Takano H., Yoshimoto T. Diesel exhaust particles exacerbate allergic rhinitis in mice by disrupting the nasal epithelial barrier. Clin Exp Allergy. 2016;46:142–152. doi: 10.1111/cea.12597. http://www.ncbi.nlm.nih.gov/pubmed/26201369 [DOI] [PubMed] [Google Scholar]
- 59.Fukuoka A., Yoshimoto T. Barrier dysfunction in the nasal allergy. Allergol Int. 2018;67:18–23. doi: 10.1016/j.alit.2017.10.006. http://www.ncbi.nlm.nih.gov/pubmed/29150353 [DOI] [PubMed] [Google Scholar]
- 60.Kim J.A., Cho J.H., Park I.H., Shin J.M., Lee S.A., Lee H.M. Diesel exhaust particles upregulate interleukins IL-6 and IL-8 in nasal fibroblasts. PLoS One. 2016;11 doi: 10.1371/journal.pone.0157058. http://www.ncbi.nlm.nih.gov/pubmed/27295300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xing Y.F., Xu Y.H., Shi M.H., Lian Y.X. The impact of PM2.5 on the human respiratory system. J Thorac Dis. 2016;8:E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19. http://www.ncbi.nlm.nih.gov/pubmed/26904255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mady L.J., Schwarzbach H.L., Moore J.A., Boudreau R.M., Willson T.J., Lee S.E. Air pollutants may be environmental risk factors in chronic rhinosinusitis disease progression. Int Forum Allergy Rhinol. 2018;8:377–384. doi: 10.1002/alr.22052. http://www.ncbi.nlm.nih.gov/pubmed/29210519 [DOI] [PubMed] [Google Scholar]
- 63.Chen F., Lin Z., Chen R. The effects of PM2.5 on asthmatic and allergic diseases or symptoms in preschool children of six Chinese cities, based on China, Children, Homes and Health (CCHH) project. Environ Pollut. 2018;232:329–337. doi: 10.1016/j.envpol.2017.08.072. [DOI] [PubMed] [Google Scholar]
- 64.Bose S., Romero K., Psoter K.J. Association of traffic air pollution and rhinitis quality of life in Peruvian children with asthma. PLoS One. 2018;13 doi: 10.1371/journal.pone.0193910. http://www.ncbi.nlm.nih.gov/pubmed/29561906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burte E., Leynaert B., Bono R. Association between air pollution and rhinitis incidence in two European cohorts. Environ Int. 2018;115:257–266. doi: 10.1016/j.envint.2018.03.021. [DOI] [PubMed] [Google Scholar]
- 66.Ramanathan M., Jr., London N.R., Jr., Tharakan A. Airborne particulate matter induces nonallergic eosinophilic sinonasal inflammation in mice. Am J Respir Cell Mol Biol. 2017;57:59–65. doi: 10.1165/rcmb.2016-0351OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang Y.L., Gao W., Li Y., Wang Y.F. Concentration-dependent effects of PM2.5 mass on expressions of adhesion molecules and inflammatory cytokines in nasal mucosa of rats with allergic rhinitis. Eur Arch Otorhinolaryngol. 2017;274:3221–3229. doi: 10.1007/s00405-017-4606-8. http://www.ncbi.nlm.nih.gov/pubmed/28577221 [DOI] [PubMed] [Google Scholar]
- 68.London N.R., Jr., Tharakan A., Rule A.M., Lane A.P., Biswal S., Ramanathan M., Jr. Air-pollutant mediated disruption of sinonasal epithelial cell barrier function is reversed by activation of the Nrf2 pathway. J Allergy Clin Immunol. 2016;138:1736–1738. doi: 10.1016/j.jaci.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 69.Zhao R., Guo Z., Zhang R. Nasal epithelial barrier disruption by particulate matter ≤2.5 μm via tight junction protein degradation. J Appl Toxicol. 2018;38:678–687. doi: 10.1002/jat.3573. http://www.ncbi.nlm.nih.gov/pubmed/29235125 [DOI] [PubMed] [Google Scholar]