Summary
Background
Alcohol and drug use can have negative consequences on the health, economy, productivity, and social aspects of communities. We aimed to use data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2016 to calculate global and regional estimates of the prevalence of alcohol, amphetamine, cannabis, cocaine, and opioid dependence, and to estimate global disease burden attributable to alcohol and drug use between 1990 and 2016, and for 195 countries and territories within 21 regions, and within seven super-regions. We also aimed to examine the association between disease burden and Socio-demographic Index (SDI) quintiles.
Methods
We searched PubMed, EMBASE, and PsycINFO databases for original epidemiological studies on alcohol and drug use published between Jan 1, 1980, and Sept 7, 2016, with out language restrictions, and used DisMod-MR 2.1, a Bayesian meta-regression tool, to estimate population-level prevalence of substance use disorders. We combined these estimates with disability weights to calculate years of life lived with disability (YLDs), years of life lost (YLLs), and disability-adjusted life-years (DALYs) for 1990–2016. We also used a comparative assessment approach to estimate burden attributable to alcohol and drug use as risk factors for other health outcomes.
Findings
Globally, alcohol use disorders were the most prevalent of all substance use disorders, with 100·4 million estimated cases in 2016 (age-standardised prevalence 1320·8 cases per 100 000 people, 95% uncertainty interval [95% UI] 1181·2–1468·0). The most common drug use disorders were cannabis dependence (22·1 million cases; age-standardised prevalence 289·7 cases per 100 000 people, 95% UI 248·9–339·1) and opioid dependence (26·8 million cases; age-standardised prevalence 353·0 cases per 100 000 people, 309·9–405·9). Globally, in 2016, 99·2 million DALYs (95% UI 88·3–111·2) and 4·2% of all DALYs (3·7–4·6) were attributable to alcohol use, and 31·8 million DALYs (27·4–36·6) and 1·3% of all DALYs (1·2–1·5) were attributable to drug use as a risk factor. The burden of disease attributable to alcohol and drug use varied substantially across geographical locations, and much of this burden was due to the effect of substance use on other health outcomes. Contrasting patterns were observed for the association between total alcohol and drug-attributable burden and SDI: alcohol-attributable burden was highest in countries with a low SDI and middle-high middle SDI, whereas the burden due to drugs increased with higher S DI level.
Interpretation
Alcohol and drug use are important contributors to global disease burden. Effective interventions should be scaled up to prevent and reduce substance use disease burden.
Funding
Bill & Melinda Gates Foundation and Australian National Health and Medical Research Council.
Introduction
Alcohol and other drugs have long been consumed for recreational purposes.1 So-called illicit drugs are substances for which extramedical use has been prohibited under international control systems.2 Illicit drugs include, but are not limited to, opioids including heroin, morphine, opium, and other pharmaceutical opioids; cannabis; amphetamines; and cocaine. Harms can also occur due to extramedical use of prescription drugs. In this Article, we will refer to all use of drugs as drug use.
Dependence on illicit and prescription drugs can develop among people who use them regularly over a sustained period, and is characterised by a loss of control over use and increased prominence of use of the substance in a person's life. The ICD 10th edition definition,3 which was broadly similar to the American Psychiatric Association's DSM-IV definition,4 requires that at least three of the following criteria are met: a strong desire to take the substance; impaired control over use; a withdrawal syndrome on ceasing or reducing use; tolerance to the effects of the drug; a disproportionate amount of time spent by the user obtaining, using, and recovering from drug use; and continuing to take drugs despite the problems that occur.
Substance use also carries risks of other adverse health outcomes. For example, injection of drugs carries risks if non-sterile injecting equipment is used, because of potential exposure to HIV and viral hepatitis, other infections, and other injection-related injuries and diseases such as sepsis, thrombosis, and endocarditis.5 Alcohol use increases the risk of unintentional and intentional injury, and both non-communicable (eg, cancer, gastrointestinal, and cardiovascular) and infectious (eg, tuberculosis and pneumonia) diseases.1, 6 Use of both alcohol and drugs can cause harm to others.7
Research in context.
Evidence before this study
We did a systematic review of PubMed, EMBASE, and PsycINFO for epidemiological studies of prevalence, incidence, remission, duration, and excess mortality associated with substance use and substance dependence published between Jan 1, 1980, and Sept 7, 2016, without language restrictions. Full search terms are listed in the appendix. We also searched grey literature, and supplemented our search through consultation with experts. Previous Global Burden of Disease (GBD) studies have provided evidence on overall burden attributable to alcohol and drug use and more detailed assessment of alcohol and drug use burden, but with each iteration of GBD, new data and improvements to methods provides better estimates of this burden. Other organisations, including WHO and the UN Office on Drugs and Crime, periodically produce estimates of health consequences of alcohol and drug use. This GBD study provides the first detailed peer-reviewed estimates of attributable burden due to both alcohol and drug use available for all locations between 1990 and 2016, directly contrasting the prevalence and burden due to these different substances.
Added value of this study
We provide clear comparative analysis of alcohol and drug epidemiology and attributable burden. The results of this study show that considerable geographical variation exists with regard to the magnitude and relative contribution of alcohol and drug use to disease burden. To the best of our knowledge, this study is the first to provide estimates of the association between alcohol and drug attributable burden and sociodemographic development. Our results show that burden attributable to alcohol and drug use is strongly associated with socioeconomic development, and its composition varied across Socio-demographic Index (SDI) quintiles. Other consequences of alcohol use were much larger causes of disease burden than alcohol use disorders, and many of these were much more common in countries with a lower SDI than those with higher SDIs. Drug-attributable burden was higher in countries with higher SDI than those with a lower SDI, and most of this burden was attributable to drug use disorder, rather than other consequences of drug use such as HIV/AIDS, acute hepatitis, liver cancer, cirrhosis and other liver disease due to hepatitis, or self-harm.
Implications of all the available evidence
Alcohol and drug use cause substantial disease burden globally, and the composition and extent of this burden varies between countries and is strongly associated with sociodemographic development. Since 1990, there has been a considerable increase in the number of people with alcohol and drug use disorders globally, driven by population growth and population ageing. Age-standardised prevalence also increased for opioid, cocaine, and amphetamine use disorders. The prevalence of substance use disorders varied substantially by substance and across countries, with clear differences between different geographical regions. Alcohol and drug use contribute substantially to the global burden of disease, not only through substance use disorders but also from other disease consequences resulting from use. For example, a high proportion of disease burden attributable to alcohol was due to other outcomes, including unintentional injuries and suicide, cancers, and cirrhosis, and the consequences of chronic hepatitis C infection (ie, cirrhosis, cancer) make a substantial contribution to the burden attributable to drug use. Interventions that reduce the prevalence of these other health outcomes are available and need to be scaled up, but this scaling remains a challenge even in high-resource settings.
Since 1993, estimates of the causes of global disease burden have used disability-adjusted life-years (DALYs),8 which combines measures of disease burden caused by premature mortality (years of life lost [YLLs]) and burden due to disability (years of life lived with disability [YLDs]). The comparative risk assessment approach developed for GBD provides a conceptual framework for population risk assessment of exposures to risk factors and their attributable health burden;9 alcohol and drugs are included as risk factors in this approach. Each iteration of GBD has updated estimates of modelled prevalence of alcohol and drug use disorders, burden due to those disorders, and burden attributable to alcohol and drug use. Improved methods are used in each iteration of GBD, with increased data coverage, and better strategies to inform the modelling that occurs in GBD. In this Article, we use data from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2016, to estimate the prevalence of alcohol and drug use disorders, and to calculate the burden attributable to alcohol and drug use globally and for 195 countries and territories within 21 regions and seven super-regions between 1990 and 2016. We present global and regional estimates of alcohol, amphetamine, cannabis, cocaine, and opioid use disorders; report disease burden attributable to each of these disorders in terms of YLDs, YLLs, and DALYs; summarise burden due to alcohol and drug use as risk factors for other health outcomes; and analyse the association between alcohol-attributable and drug-attributable burden and Socio-demographic Index (SDI) quintiles.
Methods
Overview
All GBD 2016 analyses adhered to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER).10 A suite of visualisation tools is available to explore GBD data inputs and outputs. Full details of the overall methods used to assess disorder prevalence,11 burden of disorders (YLDs),11 mortality (YLLs),12 overall substance use disorder burden (DALYs), calculated by the equation DALYs = YLLs + YLDs,13 and burden attributable to risk factors, including alcohol and drug use (comparative risk assessment)14 have been described previously.
Disease burden was quantified by geography, for 23 age groups (0–6 days to >95 years), both sexes, and six timepoints between 1990 and 2016. The GBD 2016 geographical hierarchy included 775 total geographies within 195 countries and territories, within 21 regions and seven super-regions. Comprehensive methods used in GBD 2016 for estimating YLDs, YLLs, and DALYs have been described previously, and the process used to estimate prevalence-based YLDs, YLLS, and DALYs is described in the appendix (p 2).
Case definition of substance use disorders
Substance use disorders were defined according to DSM-IV4 and ICD-10.3 Six substance use disorders were included: opioid dependence, cocaine dependence, amphetamine dependence, cannabis dependence, alcohol dependence, and fetal alcohol syndrome (a disorder that affects offspring due to maternal alcohol use during pregnancy).15 A residual category of other drug use disorders was also included.
YLLs
Input data on causes of death were obtained from vital registration, verbal autopsy, and surveillance databases from 1980 to 2016.16 Normative life tables were generated using data on the lowest death rates for each age group within geographies with total populations of more than 5 million. YLLs were then estimated by multiplying cause-specific deaths at a specific age by the standard life expectancy at that age obtained from normative life tables. Full details of all the modelling processes have been published previously.16
The Cause of Death Ensemble model (CODEm) strategy was used to model cause of death data by location, age, sex, and year for each substance use disorder.12 The CODEm outputs for all GBD causes were then rescaled to establish estimates consistent with all-cause mortality levels for each age, sex, year, and location. Deaths coded as alcohol and drug poisonings were attributed to the relevant alcohol and drug use disorders.
YLDs
We did systematic reviews of the literature to compile data on the prevalence, incidence, remission, and excess mortality associated with each disorder. We searched PubMed, EMBASE, and PsycINFO databases and grey literature sources in accordance with the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines.17 For each epidemiological parameter, eligible estimates were derived from studies published since 1980. Datapoints are summarised for each disorder in the appendix (p 5) and the data input tools are available elsewhere.
The epidemiological data obtained from our systematic literature reviews were modelled in DisMod-MR 2.1,18 a Bayesian meta-regression tool that pools datapoints from different sources and adjusts for known sources of variability (eg, differences in case definitions and sampling method) to produce internally consistent estimates of incidence, prevalence, remission, and excess mortality (appendix pp 6–31). Estimates are generated for locations where raw data are unavailable using the modelled output from surrounding regions. According to the GBD protocol, an uncertain estimate is preferable to no estimate, even when data are sparse or not available, because no estimate would result in no health loss from that condition in the location being estimated. DisMod-MR 2.1 also uses both study-level and location-level covariates to better inform the epidemiological models. Study-level covariates adjust suboptimal data toward those considered to be the gold standard (eg, adjusting data from population surveys of opioid dependence toward estimates from so-called indirect estimates of opioid dependence, which were preferred), whereas location-level covariates help DisMod-MR 2.1 better predict disorder distribution. DisMod-MR 2.1 analyses ran in a sequence of estimations at each level of the GBD geographic hierarchy (global, super-region, region, country, and if applicable, subnational locations) with consistency imposed between estimates at each level.
Although our inclusion criteria ensured minimum study quality, considerable variability was identified between studies that reflected the use of different methods and analyses.19 Data availability varied across disorders and regions (appendix p 5). Uncertainty in both the epidemiological data and in modelling was propagated to the final prevalence output used to calculate YLDs in addition to the uncertainty from fixed effects and random effects for country and regions.18
Disability weights
We used disability weights to quantify the severity of the health loss associated with a particular disease or injury, and disability weights for each injury or disease were applied to the prevalence of that condition.
In this study, we used disability weights generated by the general public, on the basis of the argument that their views are relevant in comparative assessments that inform public policy.20, 21
In GBD 2016, disability weights were obtained from population surveys in various different countries (Bangladesh, Indonesia, Peru, Tanzania, USA, Hungary, Italy, Sweden, and the Netherlands) and from an open-access survey available in multiple languages in which lay participants were presented with pairs of short non-clinical descriptions of the health states of two hypothetical individuals and asked to rate which they considered healthier.20, 22 Participant responses were scored on a scale ranging from 0 to 1 (0=no loss of health; 1=loss equivalent to death) using a series of questions comparing the benefits of lifesaving and disease-prevention programmes for a number of health states. The pair-wise comparisons showed the relative position of health states to each other, and this additional step in the analysis was necessary to anchor those relative positions as values on a 0 to 1 scale. Disability weights were generated for all sequelae of diseases and injuries included in GBD. Further details regarding the calculation of disability weights have been published previously.20, 22 Each country-specific, age-specific, sex-specific, and year-specific prevalence derived by DisMod-MR 2.1 was multiplied by a disorder-specific disability weight to estimate YLDs.
For each substance use disorder, we estimated the proportion of cases that were asymptomatic using data from the US National Epidemiological Survey on Alcohol and Related Conditions (NESARC) for the time periods 2000–01 and 2004–05,23 and the Australian Comorbidity and Trauma Study (CATS) for opioid dependence (2005–08).24, 25 These proportions were used to calculate a mean disability weight for each disorder across the different levels of severity in which asymptomatic cases were assigned a disability weight of 0. For all substance use disorders in GBD 2016, we removed the proportion of diagnosed individuals who reported no additional disability at the time of the survey. The remaining proportion of individuals represented so-called asymptomatic cases (ie, people with substance use disorders who experienced no disability due to their disorder).11
Comorbidity
The burden due to each cause in the GBD study was estimated separately. Since individuals might have more than one disease or injury at a specific timepoint, a simulation method was used to adjust for presence of comorbidity. The co-occurrence of different diseases and injuries was estimated by simulating populations of 40 000 individuals in each GBD location stratified by age, sex, and year. Hypothetical individuals within each population were exposed to the independent probability of having any combination of sequelae included in GBD 2016. The probability of being exposed to a sequela corresponded to its prevalence in the population. A combined disability weight to account for individuals with more than one condition was calculated by combining the disability weights, with the health loss associated with two disability weights multiplied together and then a weighted average of each constituent disability weight was calculated. The so-called GBD comorbidity correction was the difference between the average disability weight estimated for individuals with one sequela and the combined disability weight estimated for those with multiple sequelae. The average comorbidity correction estimated for each sequela was applied to the respective location-specific, age-specific, sex-specific, and year-specific YLDs. Although the probability of two sequelae co-occurring might be dependent, insufficient data were available to confidently estimate all dependent probabilities by age and sex in the GBD study. Thus, all probabilities of comorbidity were modelled as independent.
DALYs
We estimated burden by aggregating substance-use-disorder-specific epidemiological data and disability weights to calculate prevalent YLDs; multiplying substance-use-disorder-specific estimates of mortality by standard life expectancy at the age of death to calculate YLLs; summing YLDs and YLLs to generate substance use disorder-specific DALYs; and estimating YLDs, YLLs, and DALYs attributable to alcohol and drug use as a risk factor for other health outcomes (comparative risk assessment).
DALYs were derived as the sum of YLD and YLLs for each disorder, location, age group, sex, and year. Age-standardised prevalence, deaths, YLLs, YLDs, and DALYs were estimated using the GBD world population age standard. Uncertainty was derived for all estimates by simulating 1000 draws from each estimate's posterior distribution, to calculate uncertainty arising from primary inputs, sample sizes in the data collected, adjustments made to the data during modelling, and model estimation. For YLLs, uncertainty estimates reflected uncertainty due to study sample sizes, adjustments made to the all-cause mortality data, and model estimation.
Comparative risk assessment
GBD 2016 also quantified burden attributable to alcohol and drug use as risk factors for other health outcomes in the comparative risk assessment.26 The comparative risk assessment method estimated the burden from a risk factor attributable to an exposure compared with an alternative (counterfactual) exposure distribution.9 For drugs, the counterfactual exposure distribution was no use of the substance in the population; for alcohol it was between 0–0·8 standard daily drinks. Literature reviews were done to estimate relative risks (RR) for dimensions of substance use as a risk factor for other health outcomes to which alcohol and drug use was considered causally linked for dimensions of alcohol and drug use as a risk factor for other health outcomes. Disease burden associated with the characteristics of alcohol consumption patterns has been explored in detailed elsewhere.27 Causality was established by standard epidemiological criteria with an attempt to be comparatively similar across all risk factors included in the GBD comparative risk assessment. On the basis of exposure and relative risk (RR), population attributable fractions (PAFs) were calculated, which denote the burden of disease that could have been avoided if individuals were not exposed to substances.28 The substance-attributable burden was calculated by multiplying the attributable fractions with the respective burden estimates.
The alcohol and drug use risk factor outcome pairings included in GBD 2016 are summarised in the appendix (p 32). RR estimates were used together with a mixed effect meta-regression with age-integration from DisMod ordinary differential equations to calculate PAFs. PAFs were multiplied by relevant cause-specific DALYs to calculate attributable burden. Details of the comparative risk assessment modelling process have been published in full elsewhere.26
Comparative risk assessment requires exposure and relative risk for pairings that have been defined as causally linked. Alcohol exposure was modelled as a continuous risk factor for the dimension of level of consumption, and for injury and coronary heart disease outcomes, patterns of drinking (binge drinking) were added as an additional dimension. Exposure to alcohol was based on a triangulation of survey and sales data,29 as described previously.14, 27, 30 For opioid, amphetamine, and cocaine as risk factors for suicide, dependent use of these substances (as modelled in GBD 2016) was the defined exposure.14 For injecting drug use as a risk factor for HIV, we extracted data on the proportion of notified HIV cases by transmission route from global HIV surveillance agencies.14, 31 For injecting drug use as a risk factor for hepatitis C and hepatitis B viruses, we used a cohort method, estimating the accumulated risk of individuals having incident hepatitis B and C due to injecting drug use. We pooled data on injecting drug use in DisMod-MR 2.1, did a meta-analysis of hepatitis B and hepatitis C incidence among people who inject drugs, and estimated the population-level incidence of hepatitis B and C since 1960.14, 31
Estimating association between burden and SDI
The SDI is the geometric mean of total fertility rate, income per capita, and mean years of education among individuals aged 15 years and older, which was included as a composite measure of developmental status in GBD 2016. The index is similar to the human development index. SDI scores range from 0 to 1 (0=highest fertility, lowest income, and lowest education; 1=highest income, highest education, and lowest fertility). To calculate the SDI, these three attributes were rescaled whereby 0 was the lowest value observed between 1980 and 2016, and 1 was the highest observed value. Each GBD location was allocated an SDI score for each year. In this study, we investigate the association between SDI and DALYs attributable to alcohol and drug use.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. All authors had full access to the data in the study and final responsibility for the decision to submit for publication.
Results
Prevalence of substance use disorders
Globally, alcohol dependence was the most prevalent of the substance use disorders (table 1), with 100·4 million estimated cases in 2016 (age-standardised prevalence 1320·8 cases per 100 000 people, 95% uncertainty interval [UI] 1181·2–1468·0). The most common drug use disorders in 2016 were cannabis dependence (22·1 million cases; age-standardised prevalence 289·7 cases per 100 000 people, 95% UI 248·9–339·1) and opioid dependence (26·8 million cases; age-standardised prevalence 353·0 cases per 100 000 people, 309·9–405·9). Amphetamine and cocaine dependence were less common, with cocaine dependence the least common. Across all substance use disorders, age-standardised prevalence was significantly higher for men than women, with the exception of other drug use disorders.
Table 1.
Global prevalence and age-standardised prevalence of substance use disorders in 1990 and 2016
|
1990 |
2016 |
Percentage change in prevalence from 1990 to 2016 (%) | Percentage change in age-standardised prevalence from 1990 to 2016 (%) | |||
|---|---|---|---|---|---|---|
| Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | |||
| Alcohol use disorders | ||||||
| Total | 69 618·9 (61 748·5–77 915·2) | 1407·7 (1260·8–1558·1) | 100 389·4 (89 591·7–111 658·6) | 1320·8 (1181·2–1468·0) | 44·2% | −6·2% |
| Women | 21 145·8 (18 554·1–24 049·8) | 858·8 (757·6–968·9) | 29 516·0 (25 833·0–33 485·2) | 783·1 (685·4–888·2) | 39·6% | −8·8% |
| Men | 48 473·1 (43 065·2–54 175·1) | 1954·9 (1763·8–2156·0) | 70 873·4 (63 453·2–78 522·9) | 1853·3 (1666·2–2052·8) | 46·2% | −5·2% |
| Amphetamine use disorders | ||||||
| Total | 4044·7 (2924·8–5395·7) | 70·4 (51·8–92·7) | 4955·2 (3693·4–6490·5) | 64·7 (48·3–84·8) | 22·5% | −8·0% |
| Women | 1439·0 (1037·8–1901·6) | 51·0 (37·5–66·8) | 1701·1 (1273·6–2223·4) | 45·4 (33·9–59·5) | 18·2% | −10·9% |
| Men | 2605·7 (1874·4–3493·9) | 89·2 (65·3–117·9) | 3254·0 (2438·5–4279·8) | 83·2 (62·4–109·3) | 24·9% | −6·7% |
| Cannabis use disorders | ||||||
| Total | 17 584·8 (14 846·9–20 983·9) | 311·7 (267·2–367·3) | 22 094·5 (18 964·7–25 855·5) | 289·7 (248·9–339·1) | 25·6% | −7·1% |
| Women | 5714·2 (4798·1–6778·1) | 207·0 (175·7–243·3) | 7026·2 (6011·5–8188·0) | 187·8 (160·6–219·0) | 23·0% | −9·3% |
| Men | 11 870·6 (9971·3–14 194·7) | 413·6 (353·5–486·0) | 15 068·4 (12 887·9–17 649·6) | 387·5 (331·9–455·4) | 26·9% | −6·3% |
| Cocaine use disorders | ||||||
| Total | 4180·7 (3781·7–4653·3) | 82·6 (75·2–91·0) | 5840·3 (5321·7–6473·4) | 77·6 (70·7–85·9) | 39·7% | −6·0% |
| Women | 1337·7 (1205·7–1495·3) | 53·2 (48·3–58·9) | 1845·3 (1667·5–2051·6) | 49·4 (44·7–54·9) | 37·9% | −7·2% |
| Men | 2843·0 (2575·2–3151·4) | 112·1 (102·2–123·1) | 3995·0 (3638·0–4414·3) | 105·5 (96·3–116·3) | 40·5% | −5·8% |
| Opioid use disorders | ||||||
| Total | 18 218·7 (16 148·9–20 800·5) | 360·8 (322·5–406·9) | 26 834·5 (23 563·1–30 952·0) | 353·0 (309·9–405·9) | 47·3% | −2·2% |
| Women | 7330·2 (6439·8–8505·1) | 290·8 (256·6–333·4) | 10 408·6 (9023·7–12 193·6) | 275·8 (239·1–322·8) | 42·0% | −5·2% |
| Men | 10 888·6 (9684·8–12 291·6) | 431·3 (387·6–481·3) | 16 425·9 (14 490·4–18 696·7) | 429·6 (380·2–487·5) | 50·9% | −0·4% |
| Other drug use disorders | ||||||
| Total | 2514·2 (2258·7–2781·2) | 51·9 (46·7–57·1) | 3944·0 (3536·6–4361·6) | 52·1 (46·9–57·6) | 56·9% | 0·6% |
| Women | 1356·9 (1216·9–1501·5) | 56·3 (50·5–62·1) | 2146·3 (1923·4–2375·0) | 56·9 (51·0–62·9) | 58·2% | 1·0% |
| Men | 1157·3 (1040·2–1278·3) | 47·2 (42·6–51·9) | 1797·7 (1621·0–1981·4) | 47·3 (42·7–52·0) | 55·3% | 0·2% |
95% UI=95% uncertainty interval.
Between 1990 and 2016, the global prevalence of all substance use disorders increased for both men and women (table 1). Conversely, age-standardised prevalence decreased for all substance use disorders, with the exception of the other drug use disorders group. The estimated decrease in age-standardised prevalence was greater among women than men for all substance use disorders. For all ages, the increase in prevalence of disorders was greater among men than women, with the exception of other drug use disorders, whereby the increase in prevalence was greater for women than men (table 1).
Substantial regional variations were observed in the estimated prevalence of substance use disorders (table 2). Australasia was among the regions with the highest age-standardised prevalence across all drug use disorders, and age-standardised prevalence of amphetamine dependence was highest in this region. High-income North America had the highest prevalence of cannabis, cocaine, and opioid dependence. The prevalence of alcohol use disorders was highest in Eastern Europe.
Table 2.
Modelled estimates of prevalence and age-standardised prevalence per 100 000 people of substance use disorders by GBD region, 2016
|
Alcohol |
Amphetamines |
Cannabis |
Cocaine |
Opioids |
Other drugs |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95%UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 per people (95% UI) | Prevalence, in thousands (95% UI) | Age-standardised prevalence per 100 000 people (95% UI) | |
| Global | 100 389·4 (89 591·7–111 658·6) | 1320·8 (1181·2–1468·0) | 4955·2 (3693·4– 6490·5) | 64·7 (48·3–84·8) | 22 094·5 (18 964·7– 25 855·5) | 289·7 (248·9–339·1) | 5840·3 (5321·7– 6473·4) | 77·6 (70·7–85·9) | 26 834·5 (23 563·1– 30 952·0) | 353·0 (309·9–405·9) | 3944·0 (3536·6– 4361·6) | 52·1 (46·9–57·6) |
| East Asia | 17 126·8 (15 327·6–19 115·7) | 1023·8 (915·5–1143·6) | 1195·7 (820·8– 1658·7) | 85·4 (57·9–120·0) | 5309·9 (4469·0– 6321·7) | 375·9 (310·7–453·2) | 418·3 (364·4– 483·1) | 25·6 (22·3–29·8) | 4654·3 (4131·1– 5292·9) | 285·8 (252·9–326·8) | 901·5 (808·8– 1000·8) | 54·7 (49·1–60·5) |
| Southeast Asia | 5464·9 (4789·9–6183·0) | 806·1 (710·7–907·4) | 890·7 (593·9– 1254·1) | 126·4 (84·3–177·9) | 2535·6 (2091·0– 3071·1) | 362·5 (299·3–438·8) | 99·5 (84·6– 117·6) | 14·8 (12·6–17·3) | 1345·3 (1138·6– 1603·4) | 200·7 (171·3–237·1) | 263·8 (233·8– 295·7) | 39·1 (34·7–43·5) |
| Oceania | 88·7 (76·2– 101·9) | 860·0 (751·2–976·7) | 7·7 (4·9–11·1) | 61·6 (40·3–88·0) | 50·0 (40·3–61·3) | 408·2 (334·8–495·8) | 1·6 (1·4–1·9) | 15·8 (13·5–18·5) | 18·2 (14·6–22·5) | 178·2 (146·0–216·9) | 3·7 (3·3–4·2) | 37·6 (33·4–41·9) |
| Central Asia | 1852·8 (1644·8– 2070·0) | 2023·9 (1810·1–2251·4) | 68·5 (47·5–95·6) | 70·4 (48·7–98·1) | 223·4 (183·5– 268·7) | 236·4 (194·9–286·1) | 65·0 (56·9–74·8) | 71·7 (62·8–82·1) | 241·6 (207·5– 285·0) | 262·5 (227·4–305·4) | 53·4 (47·8–59·0) | 59·7 (53·5–65·8) |
| Central Europe | 2225·5 (2017·8– 2450·5) | 1730·3 (1553·1–1919·4) | 121·6 (96·2– 152·8) | 121·4 (94·2–154·0) | 315·9 (272·3– 367·1) | 307·7 (259·3–363·7) | 75·3 (66·0– 85·8) | 63·6 (54·9–73·4) | 191·9 (166·1– 223·2) | 151·3 (130·0–180·0) | 76·9 (69·0–84·9) | 58·6 (52·2–65·0) |
| Eastern Europe | 10 217·7 (9199·5– 11 293·4) | 4245·6 (3799·9– 4739·6) | 237·5 (178·1– 312·5) | 122·4 (88·6–163·0) | 509·6 (433·7– 595·4) | 270·1 (223·6–323·8) | 238·1 (211·7– 267·9) | 110·5 (97·2–125·7) | 1188·5 (1058·0– 1336·0) | 497·8 (438·6–562·6) | 231·3 (211·9– 252·6) | 96·0 (87·7–105·2) |
| High-income Asia Pacific | 1689·2 (1485·7– 1906·6) | 967·7 (834·6–1105·1) | 147·1 (106·1– 198·5) | 100·6 (69·9–140·3) | 546·0 (462·6– 639·5) | 367·5 (303·0–437·2) | 265·9 (235·7– 298·7) | 148·7 (129·8–169·7) | 462·5 (392·8– 540·7) | 250·5 (207·8–302·0) | 124·4 (110·2– 139·4) | 62·8 (55·0–70·9) |
| Australasia | 384·4 (332·8– 439·0) | 1305·4 (1120·5– 1498·4) | 154·9 (131·1– 178·5) | 574·2 (483·7–664·7) | 204·4 (173·8– 239·0) | 747·9 (628·5–882·3) | 68·2 (59·5–77·1) | 234·9 (204·1–267·6) | 123·5 (107·4– 141·5) | 414·7 (358·6–479·4) | 46·9 (42·9–51·2) | 154·6 (141·0–169·8) |
| Western Europe | 5177·2 (4632·9– 5727·3) | 1203·1 (1053·8–1353·5) | 387·3 (302·6– 500·6) | 110·2 (84·3–146·6) | 1586·2 (1405·3– 1771·5) | 450·8 (391·5–509·2) | 673·5 (609·7– 744·2) | 162·8 (146·1–181·2) | 1149·3 (1048·0– 1264·7) | 267·1 (242·5–297·9) | 402·8 (365·4– 441·2) | 89·5 (80·9–98·6) |
| Southern Latin America | 1187·3 (1021·4– 1366·3) | 1775·6 (1526·8–2044·2) | 117·4 (82·6– 164·5) | 179·3 (126·3–252·0) | 262·6 (216·1– 316·2) | 402·0 (330·0–485·7) | 172·6 (151·8– 196·6) | 259·6 (227·7–295·8) | 177·8 (147·5– 214·9) | 262·3 (217·1–317·9) | 43·4 (38·5–48·3) | 63·5 (56·2–70·8) |
| High-income North America | 6947·1 (6438·5– 7526·1) | 1880·4 (1734·7–2046·8) | 816·0 (695·3– 967·8) | 240·5 (202·5–287·2) | 2958·3 (2608·0– 3360·2) | 884·3 (772·7–1013·2) | 1905·2 (1767·8– 2057·5) | 524·1 (484·6–567·0) | 4199·6 (3812·1– 4584·3) | 1168·3 (1051·4–1282·5) | 486·7 (448·7– 527·1) | 129·2 (118·9– 140·3) |
| Caribbean | 744·4 (657·7– 834·7) | 1602·7 (1416·2–1794·8) | 4·5 (3·3–5·9) | 9·7 (7·0–12·6) | 125·3 (105·0– 150·5) | 267·6 (224·8–321·1) | 59·4 (51·6– 68·4) | 127·8 (111·2–147·2) | 117·9 (99·1– 141·9) | 256·5 (215·8–308·4) | 20·1 (17·9–22·3) | 43·5 (38·8–48·2) |
| Andean Latin America | 844·3 (731·3– 960·4) | 1421·3 (1246·6–1607·3) | 59·4 (39·6– 84·5) | 91·3 (61·4–129·2) | 96·0 (80·1– 113·7) | 153·0 (128·5–180·0) | 78·7 (68·3– 90·4) | 130·3 (113·6–149·4) | 152·7 (127·4– 185·5) | 263·8 (223·0–316·5) | 24·0 (21·3–27·0) | 41·7 (37·1–46·5) |
| Central Latin America | 4202·1 (3764·3– 4674·1) | 1602·5 (1442·5–1769·7) | 228·8 (167·4– 308·6) | 81·8 (60·0–109·6) | 292·0 (253·9– 337·5) | 107·5 (93·9–123·4) | 249·7 (217·2– 286·8) | 95·4 (83·3–109·3) | 601·6 (506·7– 721·4) | 229·5 (194·6–273·0) | 106·6 (94·5– 119·3) | 41·8 (37·2–46·6) |
| Tropical Latin America | 6210·4 (5502·5– 6914·4) | 2656·5 (2357·7– 2956·5) | 272·7 (190·9– 372·9) | 118·4 (82·5–161·9) | 622·0 (523·5– 731·8) | 268·8 (226·4–316·9) | 678·3 (601·5– 767·2) | 294·6 (261·5–333·4) | 517·8 (445·2– 606·1) | 223·4 (192·9–260·5) | 86·2 (76·3–96·5) | 37·4 (33·3–41·8) |
| North Africa and Middle East | 3484·0 (2948·1– 4048·9) | 593·0 (507·9– 683·0) | 81·5 (60·8– 105·3) | 13·1 (9·9–16·7) | 937·9 (779·0– 1128·2) | 151·4 (126·4–180·5) | 266·6 (228·4– 312·9) | 45·9 (39·6–53·3) | 4164·7 (3507·7– 4977·3) | 705·4 (603·3–832·9) | 235·4 (208·7– 264·3) | 41·7 (37·1–46·5) |
| South Asia | 21 024·0 (18 583·8– 23 815·0) | 1252·3 (1116·0– 1407·6) | 82·7 (59·4– 111·4) | 4·6 (3·4–6·1) | 3813·4 (3162·1– 4567·3) | 204·1 (171·1–242·8) | 405·9 (350·9– 474·7) | 23·9 (20·8–27·8) | 5435·1 (4610·0– 6346·6) | 308·7 (265·2–357·8) | 567·4 (499·5– 637·1) | 34·0 (30·2–37·9) |
| Central sub-Saharan Africa | 1320·0 (1122·8– 1533·0) | 1413·3 (1225·6– 1618·6) | 7·7 (5·2–10·4) | 6·7 (4·6–9·2) | 201·4 (166·9– 244·6) | 179·1 (151·1–212·9) | 13·6 (11·4–16·4) | 14·6 (12·4–17·2) | 222·0 (181·6– 273·1) | 240·1 (201·5–288·9) | 28·9 (25·3–32·9) | 33·6 (29·7–37·8) |
| Eastern sub-Saharan Africa | 5176·7 (4389·9– 5926·1) | 1611·0 (1410·9– 1810·8) | 24·5 (16·5–33·8) | 6·2 (4·3–8·4) | 810·8 (651·8– 1002·1) | 206·8 (170·3–249·6) | 44·4 (36·7–53·8) | 13·8 (11·7–16·4) | 664·9 (538·5– 839·1) | 212·1 (176·1–258·2) | 100·8 (88·1– 114·2) | 34·0 (30·0–38·0) |
| Southern sub-Saharan Africa | 1178·3 (1010·2– 1364·0) | 1515·0 (1324·1–1719·2) | 25·0 (17·8–33·4) | 27·3 (19·8–35·9) | 180·9 (151·0– 217·3) | 204·0 (172·4–241·9) | 14·7 (12·6–17·4) | 20·0 (17·3–23·1) | 305·7 (261·5– 359·6) | 376·8 (325·6–436·1) | 29·3 (26·0–32·8) | 40·2 (36·0–44·4) |
| Western sub-Saharan Africa | 3843·4 (3247·2– 4471·2) | 1168·1 (1012·8–1335·2) | 24·0 (16·2–32·5) | 6·1 (4·2–8·2) | 513·0 (429·0– 610·7) | 133·4 (113·5–155·9) | 45·9 (38·3–55·4) | 14·4 (12·3–16·9) | 899·5 (753·2– 1088·7) | 276·4 (235·8–327·9) | 110·4 (96·5– 125·6) | 36·2 (31·9–40·5) |
95% UI=95% uncertainty interval. GBD=Global Burden of Disease.
The disease burden of substance use disorders varied substantially by region (appendix pp 34, 35) and country (appendix pp 36–46), reflecting variations in prevalence.
Burden due to substance use as risk factors for injuries and diseases
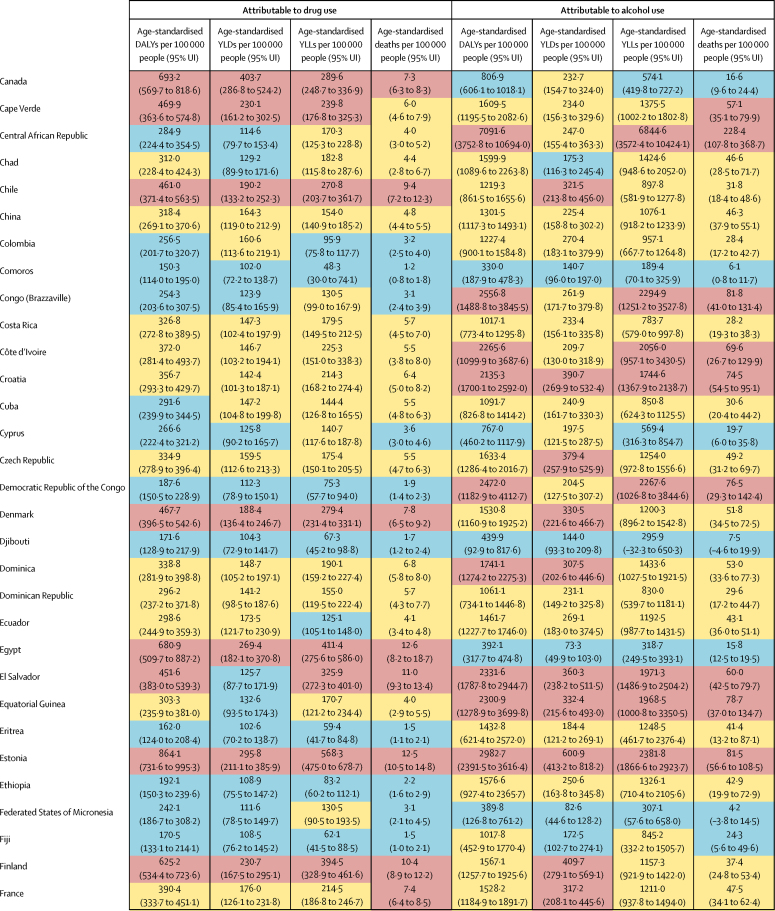
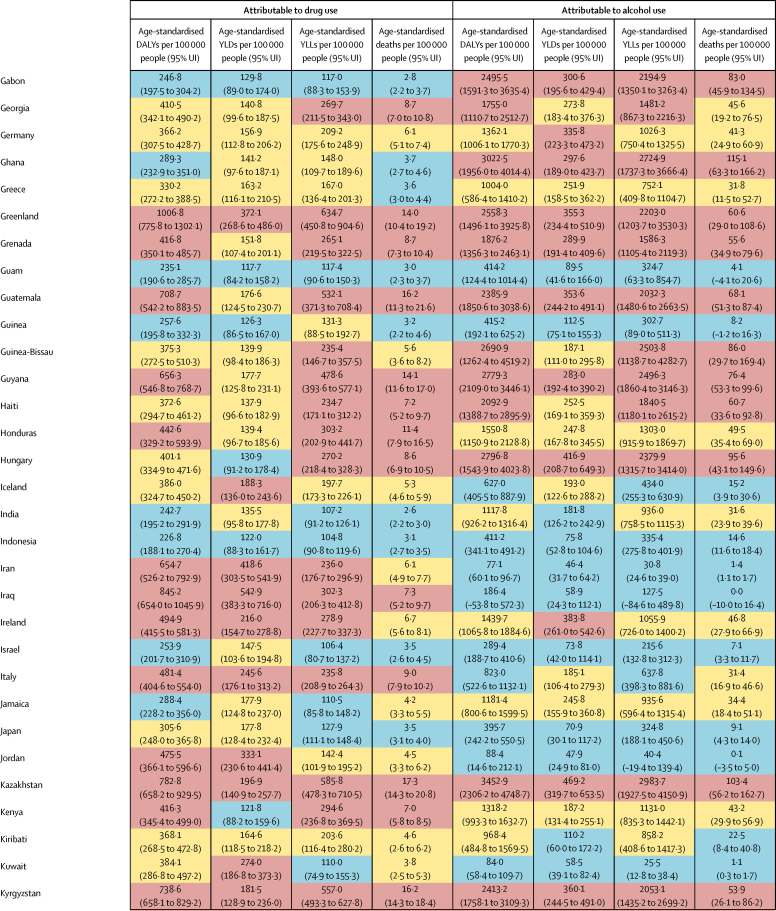
Global DALYs attributable to alcohol use were highest for injuries (21·0 million DALYs, 95% UI 15·9–26·3), cardiovascular diseases (20·8 million DALYs, 14·9–27·1), and cancers (14·8 million DALYs, 13·5–16·1; table 3; appendix p 47). Drug-attributable DALYs were highest for drug use disorders (20·4 million DALYs, 95% UI 16·2–24·7), cancers (1·6 million DALYs, 1·4–1·9) and cirrhosis (4·8 million DALYs, 4·2–5·5) driven by chronic hepatitis C infection due to injecting drug use, and HIV (3·2 million DALYs, 2·6–3·9; table 4; appendix p 49). There were similar patterns for deaths, YLLs, and YLDs (table 3, table 4). Overall, 2·8 million deaths (95% UI 2·4–3·3) were attributed to alcohol use, and 452 000 deaths (420 000–487 000) were attributed to drug use.
Table 3.
DALYs, deaths, YLLs, and YLDs attributed to alcohol use, globally, in 2016
| DALYs, in thousands | Age-standardised DALYs per 100 000 people | Deaths, in thousands | Age-standardised DALYs per 100 000 people | YLLs, in thousands | Age-standardised DALYs per 100 000 people | YLDs, in thousands | Age-standardised YLDs per 100 000 people | |||
|---|---|---|---|---|---|---|---|---|---|---|
| All causes | 99 204·9 | 1352·0 | 2814·6 | 40·4 | 81 959·3 | 1120·1 | 17 245·6 | 231·9 | ||
| Communicable, maternal, neonatal, and nutritional diseases | 12 868·9 | 174·6 | 394·1 | 5·6 | 12 156·2 | 164·9 | 712·7 | 9·7 | ||
| HIV/AIDS and tuberculosis | 10 169·5 | 136·8 | 280·5 | 3·9 | 9479·7 | 127·4 | 689·8 | 9·4 | ||
| Tuberculosis | 10 169·5 | 136·8 | 280·5 | 3·9 | 9479·7 | 127·4 | 689·8 | 9·4 | ||
| HIV/AIDS | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| HIV/AIDS resulting in other diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Diarrhoea, lower respiratory, and other common infectious diseases | 2699·4 | 37·8 | 113·6 | 1·7 | 2676·5 | 37·5 | 22·9 | 0·3 | ||
| Lower respiratory infections | 2699·4 | 37·8 | 113·6 | 1·7 | 2676·5 | 37·5 | 22·9 | 0·3 | ||
| Other communicable, maternal, neonatal, and nutritional diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Hepatitis | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Hepatitis B | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Hepatitis C | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Non-communicable diseases | 65 381·3 | 900·4 | 1982·3 | 28·9 | 51 421·6 | 713·3 | 13 959·8 | 187·1 | ||
| Neoplasms | 14 750·5 | 207·8 | 606·5 | 9·0 | 14 374·6 | 202·3 | 375·9 | 5·4 | ||
| Lip and oral cavity cancer | 1769·4 | 24·5 | 66·2 | 1·0 | 1715·9 | 23·7 | 53·5 | 0·8 | ||
| Nasopharynx cancer | 843·7 | 11·5 | 28·4 | 0·4 | 826·1 | 11·2 | 17·5 | 0·2 | ||
| Other pharynx cancer | 1285·1 | 17·7 | 46·3 | 0·7 | 1259·3 | 17·3 | 25·8 | 0·4 | ||
| Oesophageal cancer | 3052·6 | 43·3 | 130·6 | 1·9 | 3017·9 | 42·8 | 34·7 | 0·5 | ||
| Colon and rectum cancer | 2544·9 | 36·6 | 116·8 | 1·8 | 2452·2 | 35·2 | 92·7 | 1·4 | ||
| Liver cancer | 2924·5 | 41·8 | 129·2 | 1·9 | 2890·9 | 41·3 | 33·6 | 0·5 | ||
| Liver cancer due to hepatitis B | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Liver cancer due to hepatitis C | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Liver cancer due to alcohol use | 2924·5 | 41·8 | 129·2 | 1·9 | 2890·9 | 41·3 | 33·6 | 0·5 | ||
| Larynx cancer | 764·4 | 10·7 | 29·8 | 0·4 | 741·5 | 10·4 | 22·8 | 0·3 | ||
| Breast cancer | 1565·9 | 21·8 | 59·2 | 0·9 | 1470·7 | 20·4 | 95·2 | 1·4 | ||
| Cardiovascular diseases | 20 833·0 | 293·9 | 797·9 | 11·8 | 18 975·8 | 266·8 | 1857·3 | 27·1 | ||
| Ischaemic heart disease | 1084·0 | 12·2 | −24·2 | −0·7 | 1076·6 | 12·2 | 7·4 | 0 | ||
| Cerebrovascular disease | 13 888·4 | 198·0 | 581·9 | 8·8 | 12 807·2 | 182·4 | 1081·3 | 15·7 | ||
| Ischaemic stroke | 2931·0 | 43·1 | 124·2 | 1·9 | 2297·7 | 33·9 | 633·2 | 9·2 | ||
| Haemorrhagic stroke | 10 957·5 | 155·0 | 457·7 | 6·8 | 10 509·4 | 148·5 | 448·1 | 6·5 | ||
| Hypertensive heart disease | 2547·3 | 37·4 | 131·9 | 2·1 | 2337·8 | 34·3 | 209·5 | 3·1 | ||
| Cardiomyopathy and myocarditis | 2590·3 | 35·2 | 83·3 | 1·2 | 2493·5 | 33·8 | 96·8 | 1·4 | ||
| Atrial fibrillation and flutter | 722·9 | 11·2 | 25·0 | 0·4 | 260·6 | 4·2 | 462·2 | 7·0 | ||
| Cirrhosis and other chronic liver diseases | 9748·7 | 133·4 | 334·7 | 4·8 | 9435·2 | 129·1 | 313·5 | 4·4 | ||
| Cirrhosis and other chronic liver diseases due to hepatitis B | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cirrhosis and other chronic liver diseases due to hepatitis C | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cirrhosis and other chronic liver diseases due to alcohol use | 9748·7 | 133·4 | 334·7 | 4·8 | 9435·2 | 129·1 | 313·5 | 4·4 | ||
| Digestive diseases | 1196·6 | 16·2 | 37·3 | 0·5 | 1174·8 | 15·9 | 21·8 | 0·3 | ||
| Pancreatitis | 1196·6 | 16·2 | 37·3 | 0·5 | 1174·8 | 15·9 | 21·8 | 0·3 | ||
| Neurological disorders | 1903·2 | 25·5 | 22·0 | 0·3 | 876·7 | 11·6 | 1026·5 | 13·8 | ||
| Epilepsy | 1903·2 | 25·5 | 22·0 | 0·3 | 876·7 | 11·6 | 1026·5 | 13·8 | ||
| Mental and substance use disorders | 16 237·2 | 214·3 | 173·8 | 2·4 | 6211·8 | 82·6 | 10 025·4 | 131·8 | ||
| Alcohol use disorders | 16 237·2 | 214·3 | 173·8 | 2·4 | 6211·8 | 82·6 | 10 025·4 | 131·8 | ||
| Drug use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Opioid use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cocaine use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Amphetamine use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cannabis use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other drug use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Diabetes, urogenital, blood, and endocrine diseases | 712·2 | 9·3 | 10·1 | 0·1 | 372·8 | 5·0 | 339·4 | 4·3 | ||
| Diabetes mellitus | 712·2 | 9·3 | 10·1 | 0·1 | 372·8 | 5·0 | 339·4 | 4·3 | ||
| Injuries | 20 954·6 | 277·1 | 438·3 | 5·9 | 18 381·5 | 241·9 | 2573·1 | 35·1 | ||
| Transport injuries | 9294·3 | 123·3 | 182·2 | 2·5 | 7611·9 | 100·2 | 1682·4 | 23·0 | ||
| Road injuries | 8577·2 | 113·7 | 170·3 | 2·3 | 7118·2 | 93·7 | 1459·0 | 20·0 | ||
| Pedestrian road injuries | 2791·7 | 37·2 | 66·2 | 0·9 | 2485·5 | 33·0 | 306·3 | 4·2 | ||
| Cyclist road injuries | 647·1 | 8·7 | 10·1 | 0·1 | 371·2 | 4·9 | 275·9 | 3·8 | ||
| Motorcyclist road injuries | 1857·7 | 24·4 | 32·4 | 0·4 | 1522·0 | 19·8 | 335·7 | 4·6 | ||
| Motor vehicle road injuries | 3120·5 | 41·2 | 60·0 | 0·8 | 2675·4 | 35·1 | 445·1 | 6·1 | ||
| Other road injuries | 160·2 | 2·2 | 1·6 | 0 | 64·1 | 0·8 | 96·0 | 1·3 | ||
| Other transport injuries | 717·1 | 9·5 | 11·9 | 0·2 | 493·8 | 6·5 | 223·3 | 3·0 | ||
| Unintentional injuries | 1824·2 | 24·4 | 33·9 | 0·5 | 1339·3 | 17·7 | 485·0 | 6·6 | ||
| Drowning | 632·3 | 8·4 | 15·2 | 0·2 | 618·3 | 8·2 | 14·0 | 0·2 | ||
| Fire, heat, and hot substances | 386·4 | 5·2 | 7·0 | 0·1 | 226·6 | 3·1 | 159·8 | 2·2 | ||
| Poisonings | 150·1 | 2·0 | 3·4 | 0 | 129·3 | 1·7 | 20·7 | 0·3 | ||
| Exposure to mechanical forces | 87·0 | 1·1 | 1·6 | 0 | 70·0 | 0·9 | 16·9 | 0·2 | ||
| Unintentional firearm injuries | 87·0 | 1·1 | 1·6 | 0 | 70·0 | 0·9 | 16·9 | 0·2 | ||
| Other unintentional injuries | 568·5 | 7·6 | 6·8 | 0·1 | 295·0 | 3·9 | 273·5 | 3·8 | ||
| Self-harm and interpersonal violence | 9836·1 | 129·5 | 222·1 | 3·0 | 9430·3 | 124·0 | 405·8 | 5·5 | ||
| Self-harm | 6499·1 | 85·8 | 160·7 | 2·2 | 6406·5 | 84·6 | 92·6 | 1·3 | ||
| Interpersonal violence | 3337·0 | 43·6 | 61·5 | 0·8 | 3023·8 | 39·4 | 313·2 | 4·2 | ||
95% uncertainty intervals are reported in the appendix (pp 47, 48). DALYs=disability-adjusted life-years. YLLs= years of life lost. YLDs=years of life lived with disability.
Table 4.
DALYs, deaths, YLLs, and YLDs attributed to drug use, globally, in 2016
| DALYs, in thousands | Age-standardised DALYs per 100 000 people | Deaths, in thousands | Age-standardised DALYs per 100 000 people | YLLs, in thousands | Age-standardised DALYs per 100 000 people | YLDs, in thousands | Age-standardised YLDs per 100 000 people | |||
|---|---|---|---|---|---|---|---|---|---|---|
| All causes | 31 836·3 | 421·0 | 451·8 | 6·2 | 16 782·3 | 223·1 | 15 053·9 | 197·9 | ||
| Communicable, maternal, neonatal, and nutritional diseases | 3223·6 | 42·1 | 64·6 | 0·8 | 2973·2 | 38·8 | 250·4 | 3·3 | ||
| HIV/AIDS and tuberculosis | 3193·8 | 41·7 | 63·8 | 0·8 | 2944·6 | 38·5 | 249·2 | 3·3 | ||
| Tuberculosis | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| HIV/AIDS | 3193·8 | 41·7 | 63·8 | 0·8 | 2944·6 | 38·5 | 249·2 | 3·3 | ||
| HIV/AIDS resulting in other diseases | 2670·8 | 34·9 | 53·2 | 0·7 | 2460·8 | 32·1 | 210·0 | 2·8 | ||
| Diarrhoea, lower respiratory, and other common infectious diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Lower respiratory infections | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other communicable, maternal, neonatal, and nutritional diseases | 29·8 | 0·4 | 0·8 | 0 | 28·6 | 0·4 | 1·2 | 0 | ||
| Hepatitis | 29·8 | 0·4 | 0·8 | 0 | 28·6 | 0·4 | 1·2 | 0 | ||
| Hepatitis B | 11·5 | 0·2 | 0·3 | 0 | 11·1 | 0·1 | 0·4 | 0 | ||
| Hepatitis C | 18·2 | 0·2 | 0·5 | 0 | 17·5 | 0·2 | 0·7 | 0 | ||
| Non-communicable diseases | 26 806·4 | 355·3 | 349·9 | 4·9 | 12 025·7 | 161·0 | 14 780·7 | 194·3 | ||
| Neoplasms | 1636·6 | 22·8 | 65·0 | 0·9 | 1617·5 | 22·6 | 19·1 | 0·3 | ||
| Lip and oral cavity cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Nasopharynx cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other pharynx cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Oesophageal cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Colon and rectum cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Liver cancer | 1636·6 | 22·8 | 65·0 | 0·9 | 1617·5 | 22·6 | 19·1 | 0·3 | ||
| Liver cancer due to hepatitis B | 78·1 | 1·1 | 2·6 | 0 | 77·2 | 1·0 | 0·8 | 0 | ||
| Liver cancer due to hepatitis C | 1558·5 | 21·8 | 62·5 | 0·9 | 1540·3 | 21·5 | 18·3 | 0·3 | ||
| Liver cancer due to alcohol use | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Larynx cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Breast cancer | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cardiovascular diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Ischaemic heart disease | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cerebrovascular disease | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Ischaemic stroke | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Haemorrhagic stroke | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Hypertensive heart disease | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cardiomyopathy and myocarditis | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Atrial fibrillation and flutter | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cirrhosis and other chronic liver diseases | 4784·9 | 64·2 | 141·2 | 1·9 | 4622·0 | 62·0 | 162·9 | 2·2 | ||
| Cirrhosis and other chronic liver diseases due to hepatitis B | 82·8 | 1·1 | 2·4 | 0 | 80·1 | 1·1 | 2·7 | 0 | ||
| Cirrhosis and other chronic liver diseases due to hepatitis C | 4702·2 | 63·1 | 138·7 | 1·9 | 4542·0 | 60·9 | 160·2 | 2·2 | ||
| Cirrhosis and other chronic liver diseases due to alcohol use | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Digestive diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Pancreatitis | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Neurological disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Epilepsy | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Mental and substance use disorders | 20 384·8 | 268·3 | 143·7 | 2·0 | 5786·1 | 76·5 | 14 598·7 | 191·8 | ||
| Alcohol use disorders | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Drug use disorders | 20 384·8 | 268·3 | 143·7 | 2·0 | 5786·1 | 76·5 | 14 598·7 | 191·8 | ||
| Opioid use disorders | 14 782·0 | 194·2 | 86·2 | 1·2 | 3656·1 | 48·1 | 11 125·8 | 146·1 | ||
| Cocaine use disorders | 1153·6 | 15·3 | 8·8 | 0·1 | 356·9 | 4·7 | 796·7 | 10·6 | ||
| Amphetamine use disorders | 881·4 | 11·5 | 5·2 | 0·1 | 224·2 | 2·9 | 657·2 | 8·6 | ||
| Cannabis use disorders | 646·5 | 8·5 | ·· | ·· | ·· | ·· | 646·5 | 8·5 | ||
| Other drug use disorders | 2921·4 | 38·8 | 43·5 | 0·6 | 1548·9 | 20·7 | 1372·5 | 18·1 | ||
| Diabetes, urogenital, blood, and endocrine diseases | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Diabetes mellitus | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Injuries | 1806·2 | 23·6 | 37·3 | 0·5 | 1783·4 | 23·3 | 22·8 | 0·3 | ||
| Transport injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Pedestrian road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Cyclist road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Motorcyclist road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Motor vehicle road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other road injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other transport injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Unintentional injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Drowning | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Fire, heat, and hot substances | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Poisonings | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Exposure to mechanical forces | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Unintentional firearm injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Other unintentional injuries | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
| Self-harm and interpersonal violence | 1806·2 | 23·6 | 37·3 | 0·5 | 1783·4 | 23·3 | 22·8 | 0·3 | ||
| Self-harm | 1806·2 | 23·6 | 37·3 | 0·5 | 1783·4 | 23·3 | 22·8 | 0·3 | ||
| Interpersonal violence | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ·· | ||
95% uncertainty intervals are reported in the appendix (p 49). DALYs=disability-adjusted life-years. YLLs=years of life lost. YLDs=years of life lived with disability.
The estimated number of deaths, YLLs, YLDs, and DALYs attributed to alcohol and drug use varied considerably between regions (table 5; appendix pp 50, 51). The highest alcohol-attributable burdens were in Eastern Europe (4730·9 age-standardised DALYs per 100 000 people [95% UI 3591·3–6104·9]) and Southern sub-Saharan Africa (3178·8 age-standardised DALYs per 100 000 people [2599·8–3759·5]). The highest drug-attributable burdens were in Eastern Europe (1252·3 age-standardised DALYs per 100 000 people [95% UI 1029·6–1498·4]) and high-income North America (1380·3 age-standardised DALYs per 100 000 people [1173·9–1579·0]). In terms of absolute burden, the largest number of alcohol-attributable DALYs were in East Asia, South Asia, Eastern Europe, and Tropical Latin America, and the largest number of drug-attributable DALYs were in East Asia, high-income North America, South Asia, and Eastern Europe.
Table 5.
Regional data on number, age-standardised rate and percentage of all DALYs attributed to alcohol and drug use, 2016
|
Burden attributable to alcohol |
Burden attributable to drugs |
|||||
|---|---|---|---|---|---|---|
| DALYs, in thousands (95% UI) | Age-standardised DALYs per 100 000 people (95% UI) | Percentage of DALYs (95% UI) | DALYs, in thousands (95% UI) | Age-standardised DALYs per 100 000 people (95% UI) | Percentage of DALYs (95% UI) | |
| Andean Latin America | 595·4 (482·9–727·2) | 1115·9 (897·5–1372·3) | 4·4% (3·6–5·3) | 167·5 (135·1–203·2) | 293·4 (238·0–352·0) | 1·2% (1·0–1·4) |
| Australasia | 346·3 (265·5–435·2) | 1017·0 (799·8–1253·6) | 5·4% (4·1–6·7) | 202·9 (169·2–238·1) | 686·4 (567·8–814·4) | 3·1% (2·7–3·5) |
| Caribbean | 620·4 (509·4–744·9) | 1348·0 (1105·6–1617·3) | 4·4% (3·6–5·2) | 156·2 (132·6–179·9) | 339·4 (288·4–391·3) | 1·1% (1·0–1·3) |
| Central Asia | 1780·3 (1402·8–2195·7) | 2151·6 (1632·1–2745·4) | 6·6% (5·3–8·2) | 516·1 (455·4–573·3) | 596·6 (529·7–660·1) | 1·9% (1·7–2·1) |
| Central Europe | 3576·7 (2697·8–4466·8) | 2229·6 (1713·1–2745·0) | 9·4% (7·0–11·8) | 470·8 (402·2–547·9) | 339·6 (291·0–394·4) | 1·2% (1·1–1·4) |
| Central Latin America | 3789·5 (3293·4–4370·2) | 1596·0 (1372·6–1843·3) | 6·4% (5·5–7·4) | 1050·8 (917·0–1199·0) | 423·1 (368·9–480·4) | 1·8% (1·6–2·0) |
| Central sub-Saharan Africa | 1849·9 (1246·9–2585·7) | 2733·4 (1738·6–3961·7) | 2·9% (1·9–4·2) | 180·5 (144·3–220·3) | 199·3 (161·2–240·5) | 0·3% (0·2–0·4) |
| East Asia | 22 213·1 (19 192·0–25 298·4) | 1295·8 (1115·8–1485·4) | 6·1% (5·2–7·0) | 5315·2 (4530·8–6123·9) | 321·8 (272·4–373·3) | 1·5% (1·3–1·7) |
| Eastern Europe | 12 349·8 (9271·6–16 063·8) | 4730·9 (3591·3–6104·9) | 13·8% (11·3–16·7) | 3005·5 (2477·8–3590·1) | 1252·3 (1029·6–1498·4) | 3·4% (3·0–3·7) |
| Eastern sub-Saharan Africa | 4687·4 (3827·3–5571·1) | 2010·6 (1582·4–2467·0) | 2·7% (2·2–3·2) | 726·3 (564·8–913·4) | 251·4 (194·3–320·2) | 0·4% (0·3–0·5) |
| High-income Asia Pacific | 1144·8 (708·4–1599·0) | 534·4 (364·5–713·8) | 2·5% (1·5–3·4) | 592·7 (492·9–703·0) | 305·2 (247·6–371·4) | 1·3% (1·1–1·5) |
| High-income North America | 4588·4 (3472·1–5788·8) | 1139·3 (874·3–1414·8) | 4·5% (3·4–5·8) | 5146·5 (4410·5–5852·5) | 1380·3 (1173·9–1579·0) | 5·1% (4·5–5·6) |
| North Africa and Middle East | 1311·8 (1055·6–1625·3) | 264·6 (204·8–342·2) | 0·8% (0·7–1·0) | 2881·6 (2299·6–3522·1) | 512·3 (414·7–619·2) | 1·7% (1·4–2·1) |
| Oceania | 159·0 (95·3–229·6) | 1706·4 (941·4–2599·4) | 3·3% (2·0–4·7) | 20·5 (16·3–25·4) | 190·5 (150·4–233·3) | 0·4% (0·3–0·5) |
| South Asia | 14 501·1 (12 413·8–16 876·2) | 967·0 (810·3–1135·3) | 2·5% (2·1–2·8) | 4408·8 (3568·7–5339·6) | 253·3 (206·2–305·4) | 0·7% (0·6–0·9) |
| Southeast Asia | 7575·6 (6499·4–8644·9) | 1255·7 (1072·4–1439·0) | 4·1% (3·6–4·7) | 2235·1 (1918·1–2601·2) | 329·9 (284·0–382·0) | 1·2% (1·1–1·4) |
| Southern Latin America | 792·2 (549·1–1059·1) | 1159·8 (819·5–1540·8) | 4·8% (3·4–6·4) | 327·2 (275·8–384·4) | 482·3 (405·9–567·6) | 2·0% (1·7–2·2) |
| Southern sub-Saharan Africa | 2054·4 (1710·3–2409·4) | 3178·8 (2599·8–3759·5) | 5·1% (4·3–6·0) | 434·6 (359·9–522·3) | 584·0 (490·1–692·2) | 1·1% (0·9–1·3) |
| Tropical Latin America | 12 433·3 (10 547·1–14 476·7) | 2075·5 (1701·3–2495·9) | 7·4% (6·1–8·7) | 724·6 (582·9–875·5) | 312·4 (251·5–378·2) | 1·2% (1·0–1·4) |
| Western Europe | 4347·6 (3601·0–5101·9) | 1921·8 (1578·9–2267·7) | 6·3% (5·3–7·5) | 1994·5 (1745·2–2248·7) | 425·2 (366·5–486·3) | 1·8% (1·6–2·0) |
| Western sub-Saharan Africa | 7079·6 (5951·2–8252·9) | 1166·0 (997·1–1346·1) | 1·7% (1·4–2·1) | 1278·5 (1045·3–1568·9) | 410·5 (341·3–492·1) | 0·6% (0·5–0·7) |
| Global | 99 204·9 (88 310·4–111 168·3) | 1352·0 (1198·4–1521·4) | 4·2% (3·7–4·6) | 31 836·3 (27445·9–36580·0) | 421·0 (363·7–483·1) | 1·3% (1·2–1·5) |
DALYs=disability-adjusted life-years. 95% CI UI=95% uncertainty interval.
Globally, in 2016, 99·2 million DALYs (95% UI 88·3–111·2) and 4·2% of all DALYs (3·7–4·6) were attributable to alcohol use, and 31·8 million DALYs (27·4–36·6) and 1·3% of all DALYs (1·2–1·5) were attributable to drug use (table 5). Globally, alcohol accounted for around three-quarters (76%) of all substance-use-attributable DALYs. Drugs accounted for a higher percentage of substance-use-attributable DALYs than alcohol in two regions: high-income North America (53% of total alcohol and drug attributable DALYs) and North Africa and the Middle East (69% of total alcohol and drug attributable DALYs).
The proportion of DALYs attributable to alcohol and drug use, and the contribution of each to overall DALYs, varied substantially, both in the absolute and relative data, at the regional level. Alcohol attributable burden was largest for Eastern Europe (12·3 million DALYs, 95% UI 9·3–16·1; 13·8% of all DALYs, 95% UI 11·3–16·7) and Central Europe (3·6 million DALYs, 2·7–4·5; 9·4% of all DALYs, 7·0–11·8), whereas alcohol attributable burden was smallest for North Africa and the Middle East (1·3 million DALYs, 1·1–1·6; 0·8% of all DALYs, 0·7–1·0) and Western sub-Saharan Africa (7·1 million DALYs, 5·9–8·3; 1·7% of all DALYs, 1·4–2·1).
The regions where drug use accounted for the highest proportion of DALYs were high-income North America (5·2 million DALYs, 95% UI 4·4–5·9; 5·1% of all DALYs, 4·5–5·6), Eastern Europe (3·0 million DALYs, 2·5–3·6; 3·4% of all DALYs, 3·0–3·7), and Australasia (203 000 DALYs (169 000–238 000; 3·1% of all DALYs, 2·7–3·6). Drugs accounted for the smallest percentage of total DALYs in several regions in sub-Saharan Africa (table 5).
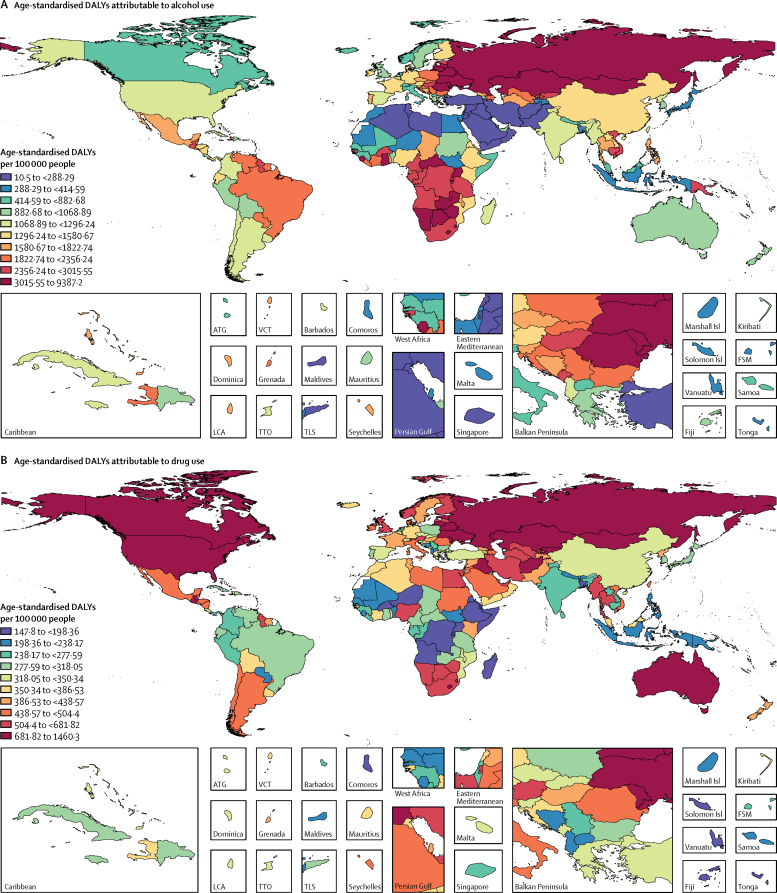
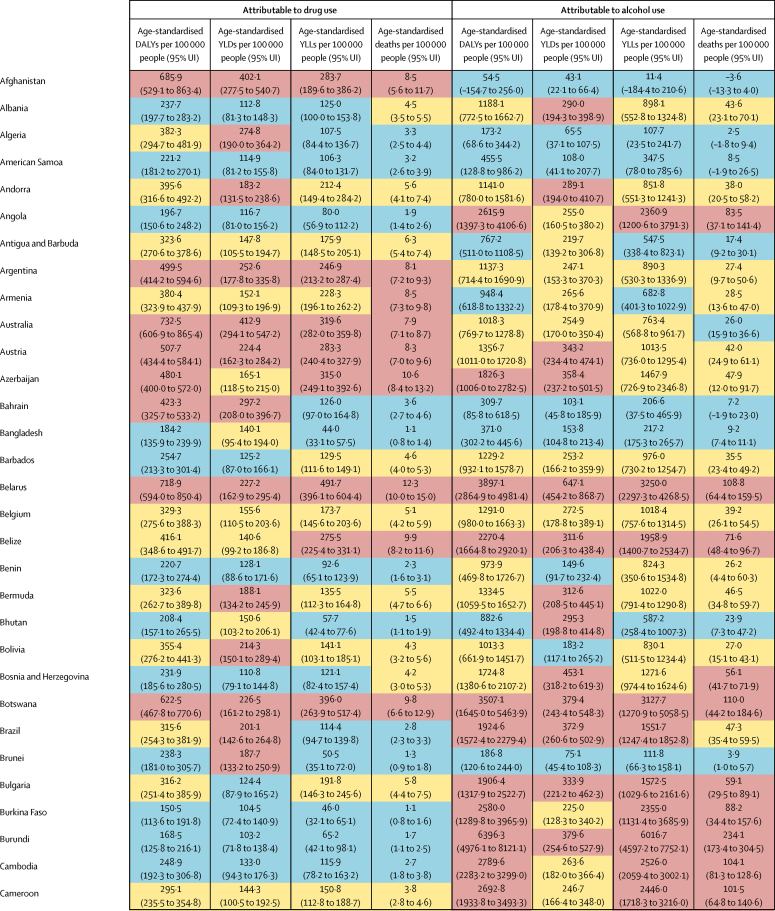
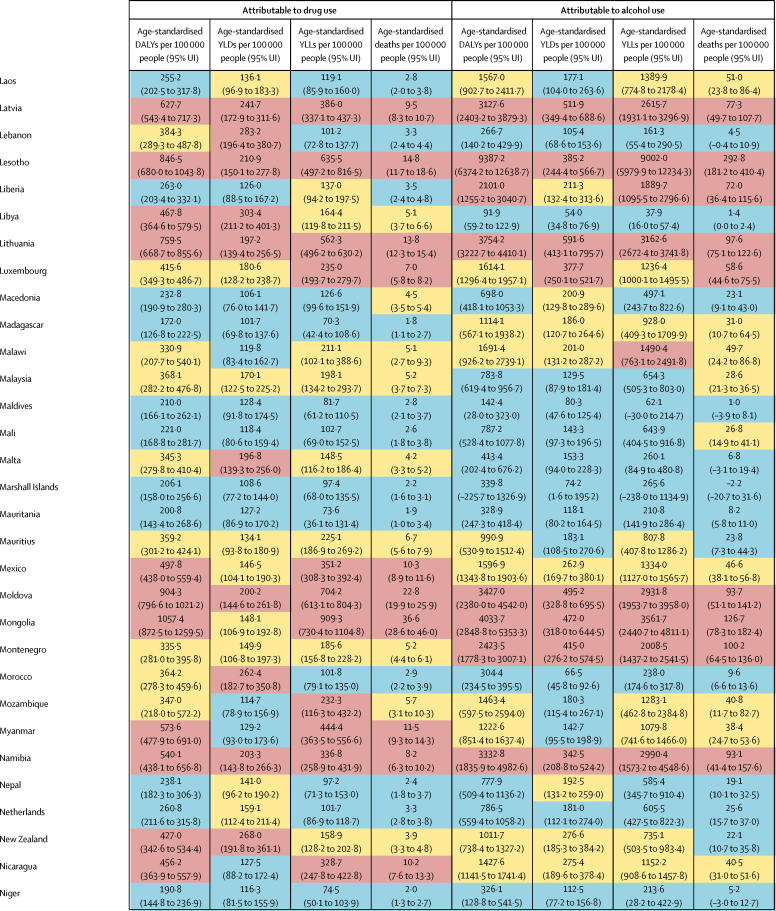
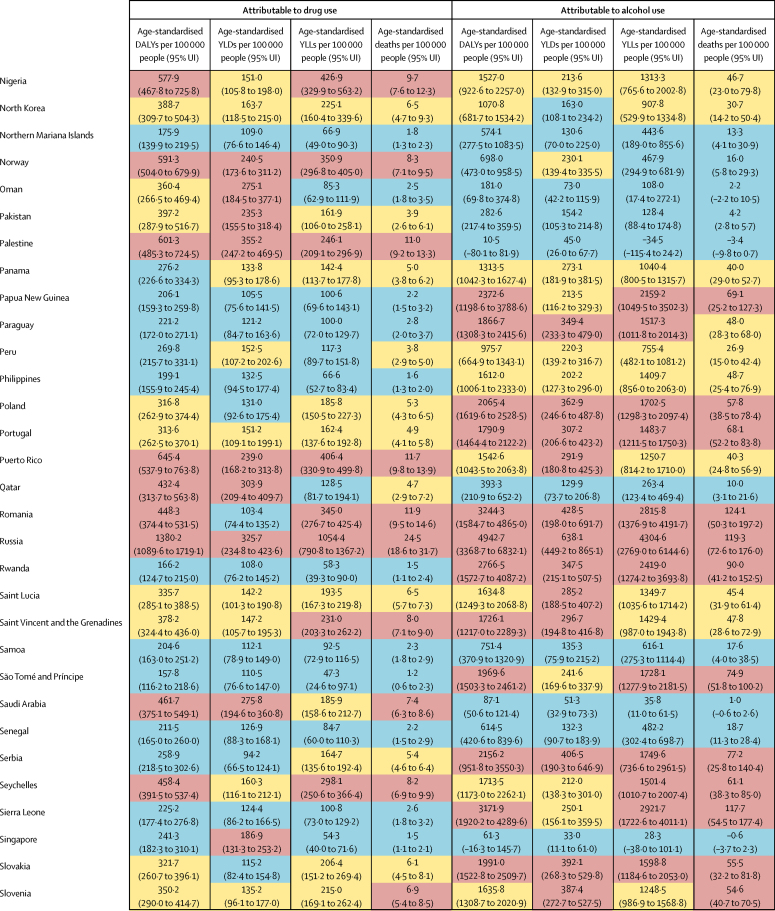
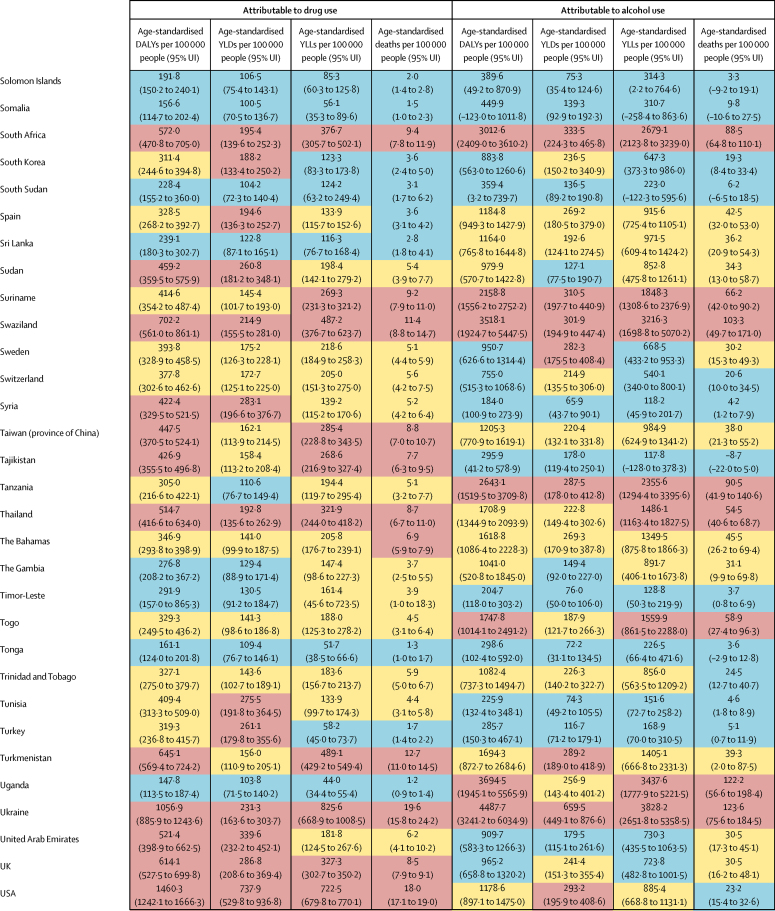
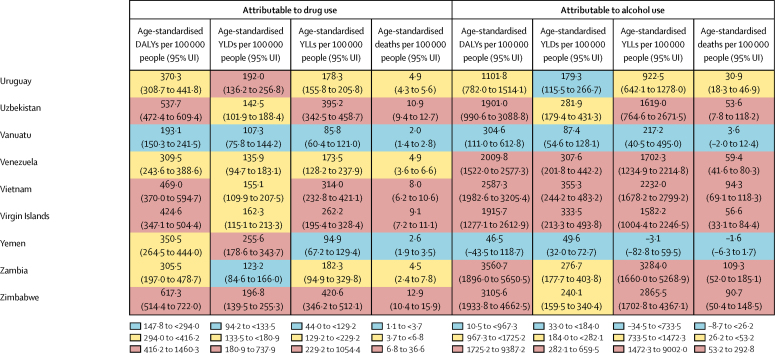
The overall disease burden attributable to alcohol and drugs (age-standardised DALY rates per 100 000 population) varied substantially between countries (figure 1, figure 2). The countries with the highest alcohol-attributable age-standardised DALYs per 100 000 people were in Eastern Europe (Russia [4942·7 DALYs, 95% UI 3368·7 to 6832·1], Ukraine [4487·7 DALYs, 3241·2 to 6034·9], and Belarus [3897·1 DALYs, 2864·9 to 4981·4), and sub-Saharan Africa (Lesotho [9387·2 DALYs, 6374·2 to 12 638·7], Central African Republic [7091·6 DALYs, 3752·8 to 10 694·0], and Burundi [6396·3 DALYs, 4976·1 to 8121·1). Countries with the highest age-standardised DALYs per 100 000 people attributable to drug use were the USA (1460·3 DALYs, 1242·1 to 1666·3), Russia (1380·2 DALYs, 1089·6 to 1719·1), and Mongolia (1057·4 DALYs, 872·5 to 1259·5).
Figure 1.
Age-standardised DALYs per 100 000 people attributable to alcohol and drug use for both sexes by country in 2016, in 195 locations
(A) DALYs attributed to alcohol use. (B) DALYs attributable to drug use. DALYs=disability-adjusted life-years. ATG=Antigua and Barbuda. FSM=Federated States of Micronesia. Isl=Islands. LCA=Saint Lucia. TLS=Timor-Leste. TTO=Trinidad and Tobago. VCT=Saint Vincent and the Grenadines.
Figure 2.
Age-standardised DALYs, YLDs, YLLs, and deaths per 100 000 people attributed to drug and alcohol use, in 2016, by country in 2016
Blue indicates mild severity, yellow indicates moderate severity, and red indicates high severity. 95% UI=95% uncertainty intervals. DALYs=disability-adjusted life-years. YLDs=years of life lived with disability. YLLs=years of life lost.
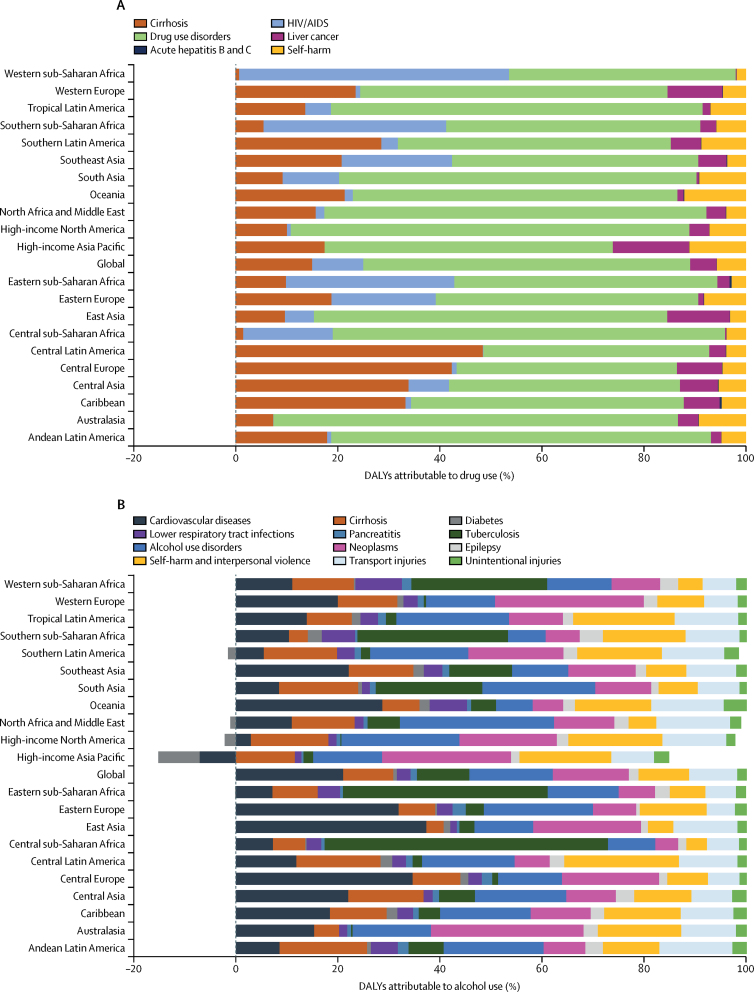
The distribution of diseases or injuries that contributed to alcohol and drug-attributable burden varied by GBD region (figure 3). HIV accounted for a large proportion of disease burden attributable to drug use in African regions. Drug use disorders were the largest contributor to drug-attributable burden in almost all regions, particularly Australasia, high-income North America, and North Africa and the Middle East. Alcohol burden was attributed to a wider variety of diseases and injuries than drug burden (figure 3).
Figure 3.
Regional variation in DALYs attributed to drug and alcohol use in 2016
(A) DALYs attributable to drug use. (B) DALYs attributable to alcohol. DALYs=disability-adjusted life-years.
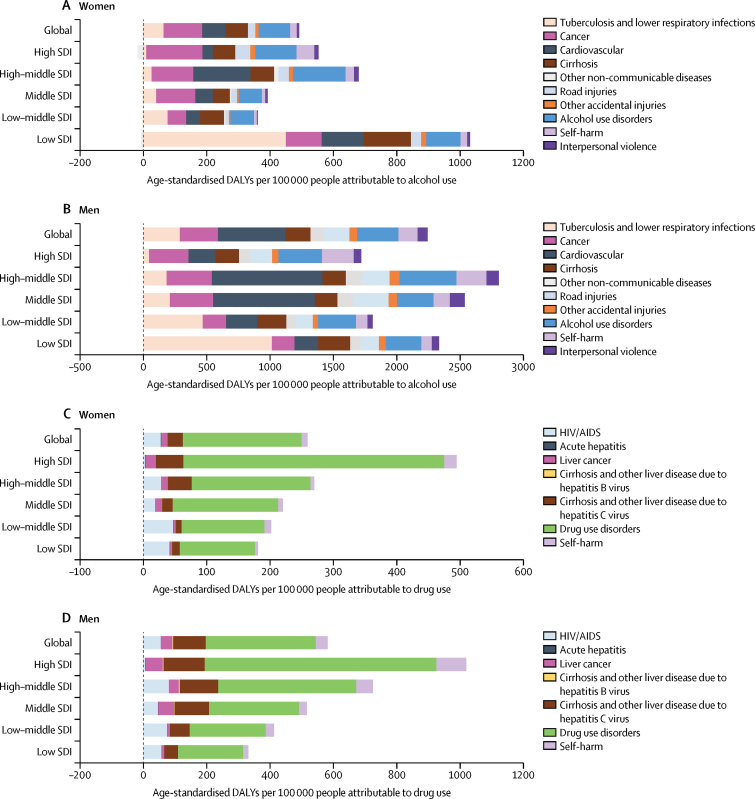
Disease burden attributable to alcohol and drug use was strongly associated with socioeconomic development, and burden composition varied across SDI quintiles (figure 4). Alcohol use disorder was a much smaller cause of disease burden than other consequences of alcohol use, and many of these were much more common in countries with a low SDI (figure 4A, figure 4B). Tuberculosis and lower respiratory infections were considerable causes of burden due to alcohol use in low SDI countries, whereas middle SDI and high-middle SDI countries had larger alcohol-attributable cardiovascular disease burden. Drug-attributable burden was higher in countries with higher SDI (figure 4C, figure 4D), and most of this burden was due to drug use disorder. HIV/AIDS was a larger cause of drug-attributable burden in low SDI countries than high-middle SDI countries, whereas the consequences of chronic hepatitis C virus infection (ie, cirrhosis and liver cancer) were greater in countries with higher SDI (figure 4C, figure 4D).
Figure 4.
Estimated burden attributable to alcohol and drug use for both sexes by SDI quintile, in 2016
(A) Burden attributable to alcohol in women (A) and men (B), and burden attributable to drug use in women (C) and men (D). The data on alcohol burden and its association with SDI by age group have been published previously.27 SDI=Socio-demographic Index. DALYs=disability-adjusted life-years.
Discussion
Since 1990, the number of people with alcohol and drug use disorders has increased substantially, driven by population growth and population ageing. Age-standardised prevalence also increased for opioid, cocaine, and amphetamine use disorders. The prevalence of substance use disorders varied markedly by substance and across countries, with clear differences between different geographic regions.
Substance use disorders were not the only conditions that contributed to the global burden of disease attributed to alcohol and drug use. A high proportion of the disease burden attributable to alcohol was due to increased risk of other health outcomes, including unintentional injuries and suicide, cancers, and cirrhosis, and the consequences of chronic hepatitis C infection (ie, cirrhosis, cancer) make a substantial contribution to the disease burden attributable to drug use.
Disease burden attributable to alcohol and drugs and the composition of this burden varied substantially across geographical locations. Eastern Europe had the highest age-standardised attributable burden for alcohol, followed by southern sub-Saharan Africa, and the highest age-standardised attributable burden for drug use was in high-income North America. The association between geographical differences in attributable burden and SDI varied for alcohol and drugs. Countries in low SDI and middle SDI quintiles had the highest alcohol-attributable burdens, whereas countries in high SDI quintiles had the highest drug-attributable burden.
Globally, large variation in attributable burden has been observed within countries with the highest SDIs.32 In GBD 2013, in Finland, overall age-standardised alcohol-attributable DALYs were 1567 per 100 000 in 2013, whereas in Norway, with the lowest burden, corresponding estimates were 698 per 100 000; DALYs in Denmark were 1530 per 100 000 and in Sweden 950 per 100 000. All countries had a similar attributable disease pattern and the majority of alcohol-attributed DALYs were due to YLLs, mainly from alcohol use disorder, cirrhosis, transport injuries, self-harm, and violence.32
The high attributable burden, even in high-income countries, where a substantially higher proportion of health budgets are spent to address these issues, deserves attention. Multiple factors might contribute to this burden, including low treatment rates, delays in initiating treatment, and stigma associated with alcohol and substance use disorders.33, 34, 35 A longstanding problem in most countries36 is also the poor availability of highly effective interventions that can address HIV and hepatitis C virus among people who inject drugs, such as needle and syringe programmes, HIV and hepatitis C virus treatment, and opioid substitution therapy.
The emergence of alcohol-attributable burden in Southern sub-Saharan Africa reflects the changing strategies of the alcohol industry, which has started to target Africa and other low-income and middle-income countries37, 38, 39 in the past few years to avoid the stricter regulation of the market and public health initiatives in high-income countries, where consumption has been steadily falling. This calls for the global health community to respond adequately to accelerate efforts toward development of a framework convention for alcohol control,40 similar to that which has been implemented to counter the harmful effects for tobacco consumption.
Many of the causes of alcohol and drug burden can be prevented or treated. Taxation and regulation of availability and marketing can substantially reduce harms associated with alcohol.41 Additionally, reducing the alcoholic strength of beverages and minimum pricing show promise in reducing alcohol-attributable harm.42, 43 Transport injuries are an important consequence of alcohol use that can be prevented via a range of interventions (seat belts, helmets, and implementation of blood alcohol limits for drivers and roadside alcohol testing of drivers).
Treatment and brief interventions have been shown to be effective with a potential public health impact,35 but of all mental health disorders, alcohol use disorder has the lowest treatment rates globally.44 Medications for alcohol dependence such as naltrexone have shown efficacy, but uptake and adherence are very low; for example, in Australia, only around 0·5% of people who are alcohol-dependent are estimated to have been prescribed naltrexone or acamprosate for the recommended 3 month duration.45 Psychosocial interventions might assist people with cannabis and psychostimulant use disorders.46, 47 Opioid substitution therapy reduces opioid use and injecting risk, improves physical and mental wellbeing, and reduces mortality.48, 49, 50
Opioid overdose might also be reduced by distributing the opioid antagonist naloxone to reverse overdoses.51 Much of the burden due to infectious disease among people who inject drugs could be averted by scaling up needle and syringe programmes, opioid agonist therapy, and HIV antiretroviral therapy.52, 53, 54, 55 However, coverage of these interventions remains low.56 The development of highly effective hepatitis C virus treatments has the capacity to increase rates of hepatitis C virus treatment among people who inject drugs,57 and might produce secondary prevention benefits, similar to those observed with HIV treatment.57 One of the biggest barriers to the scale up of hepatitis C virus treatment will be the high cost of these medications. It is also crucial to acknowledge that for people who inject drugs, without coverage of blood borne virus prevention interventions, such as needles and syringe programmes and opioid agonist therapy, the preventive effects of hepatitis C virus treatment will be limited.
The limitations of the GBD approach have been described previously.11, 12, 13, 14, 16 Such limitations include gaps in data, variable data quality, and controversies with regard to disability weights used to estimate non-fatal disease burden. Although this study modelled results where data were not available, the gaps in data for many countries result in uncertainty around the modelled estimates, which can only be reduced by improved epidemiological evidence.
Alcohol consumption consists of recorded and unrecorded consumption data and is subject to uncertainties. The absence of a gold standard method in measurement of the prevalence of drug use poses major challenges for cross-national comparisons.
An important limitation of all substance use cause of death estimates was variation in ICD-codes used to classify overdoses across countries (ie, some countries have additional codes that permit more accurate attribution of cause of death to specific substances and others do not), which will be improved in the next GBD.
The distribution of substance use disorders across levels of severity was informed by analyses of data from the USA (NESARC) and Australia (CATS). The extent to which the severity distribution of substance use disorder cases is consistent across countries is an important question. For example, in countries where patterns of use or effects of use on functioning are more severe, a greater proportion of disorders might be classified as severe, and potentially fewer people are classified as having no disability. Thus, our estimates might have underestimated burden associated with use disorders (or indeed overestimated such burden). Studies that investigate not only the prevalence of substance use disorders but also levels of severity of those cases across countries that vary culturally, economically, and socially are needed to ascertain whether the severity distribution of substance use disorder cases has been incorrectly estimated, and if so, the magnitude of the error.
The GBD uses the ICD-10 classification system for injuries and diseases. The introduction of the American Psychiatric Association's DSM-5 included a shift from DSM-IV's abuse and dependence,4 to a category of use disorder (with levels of severity defined as mild, moderate, and severe).58 There has been some discussion regarding the consistency of substance use disorder definitions used in DSM-5 compared with other classification systems, with results suggesting that moderate agreement exists between moderate to severe DSM-5 substance use disorders and ICD-10 dependence,59, 60, 61 but prevalence of DSM-5 moderate to severe substance use disorder might be higher than that of DSM-IV and ICD-10 dependence,59, 62 implying that estimates of substance use disorder burden might have been higher if DSM-5 prevalence estimates were used.
Research examining the methodology used in GBD to generate disability weights, namely paired comparison responses, has revealed the method suggests that the approach of simultaneous estimation of cardinal severity values from a pooled dataset with a combination of responses to chronic and temporary paired comparisons is a reasonable methodological choice.20, 21 Disability weights can be generated by health-care professionals, individuals with the disorder, or the general public. Arguments can be made for each of these groups; these have been discussed in detail previously.20, 21 The disability weights used in GBD 1996 were generated by health-care professionals on the basis that they would have knowledge of a diverse set of health states and would be able to make comparative judgments. Individuals in a health state have the most intimate knowledge of the reductions in function associated with that state, but they will be less able to make comparisons with other health states. Such individuals might have adapted to their health loss, and therefore not appreciate the extent to which their functioning has been impaired relative to a completely healthy individual.
Our comparative risk assessment of burden attributable to drug use is conservative because a range of potential health outcomes of drug use were not included. First, although unintentional injuries and homicide are often among the most prevalent causes of death among people who are dependent upon opioids, cocaine, and amphetamines,48 they have not yet been included as outcomes of these forms of drug dependence. We aim to present evidence that will warrant their inclusion in future iterations of GBD. Second, the evidence for a causal association between drug use and a range of possible outcomes (such as cannabis use and intentional injuries) was weak. However, the inclusion of these outcomes might be reconsidered in future iterations of GBD, since a 2016 WHO monograph63 on the health effects of cannabis use reported increasing evidence for a causal link between cannabis use and road traffic accidents. Third, many putative consequences of drug use exist, for which we did not attempt to quantify the magnitude of possible associations because the level of evidence was too low.2 These consequences included a range of health outcomes that are increased among people with drug dependence, such as mental disorders, myocardial infarction, and cardiovascular pathology.64 Well designed prospective studies are needed to estimate the risks of these consequences of drug use while controlling for confounding factors.
Finally, in GBD, the concept of disability is intended to only capture the health loss of an individual. Thus, disability does not include social or other impacts on non-drug users such as the family or the social and economic consequences of mental and substance use disorders. To that extent, our estimates of disease burden due to alcohol and drugs are partial estimates of the adverse impact of substance use on society.
Alcohol and drug use cause substantial disease burden globally, and the composition and extent of this burden varies substantially between countries, and is strongly associated with social development. Existing interventions that are known to reduce the varied causes of burden exist. These interventions need to be scaled up, which remains a challenge even in high-resource settings.
For more on visualisation tools see http://www.healthdata.org/gbd
For more on data input tools see http://ghdx.healthdata.org/gbd-2016/data-input-sources and https://vizhub.healthdata.org/epi/
For more on the human development index see http://hdr.undp.org/en/content/human-development-index-hdi
Acknowledgments
Acknowledgments
LD is supported by an Australian National Health and Medical Research Council (NHMRC) Senior Principal Research Fellowship (APP1135991). HE is a recipient of an NHMRC Early Career Fellowship (APP1137969). AF is supported by an Australian NHMRC Early Career Fellowship (APP1121516), and is also affiliated with the Queensland Centre for Mental Health Research, supported by the Queensland Department of Health. SIH is supported by grants from the Bill & Melinda Gates Foundation. JM was supported by an NHMRC John Cade Fellowship (APP1056929) and a Niels Bohr Professorship from the Danish National Research Foundation. JS is the recipient of an Australian NHMRC Practitioner Fellowship Grant (APP1105807).
GBD 2016 Alcohol and Drug Use Collaborators
Louisa Degenhardt, Fiona Charlson, Alize Ferrari, Damian Santomauro, Holly Erskine, Ana Mantilla-Herrara, Harvey Whiteford, Janni Leung, Mohsen Naghavi, Max Griswold, Jürgen Rehm, Wayne Hall, Benn Sartorius, James Scott, Stein Emil Vollset, Ann Kristin Knudsen, Josep Maria Haro, George Patton, Jacek Kopec, Deborah Carvalho Malta, Roman Topor-Madry, John McGrath, Juanita Haagsma, Peter Allebeck, Michael Phillips, Joshua Salomon, Simon Hay, Kyle Foreman, Stephen Lim, Ali Mokdad, Mari Smith, Emmanuela Gakidou, Christopher Murray, Theo Vos.
Affiliations
National Drug and Alcohol Research Centre, University of New South Wales, Sydney, NSW, Australia (Prof L Degenhardt PhD, J Leung PhD); Queensland Centre For Mental Health Research, University of Queensland, Brisbane, QLD, Australia (F Charlson PhD, A Ferrari PhD, D Santomauro PhD, H Erskine PhD, A Mantilla-Herrara MHEcon, H Whiteford PhD, W Hall PhD, J Scott PhD, J McGrath MD) Institute of Health Metrics and Evaulation, University of Washington, Seattle, WA, USA (M Naghavi PhD, M Griswold, S Hay DPhil, K Foreman PhD, S Lim PhD, A Mokdad PhD, M Smith MBA, E Gakidou PhD, C Murray DPhil, T Vos PhD); Centre for Addiction and Mental Health, Toronto, ON, Canada (J Rehm PhD); University of KwaZulu-Natal, Durban, South Africa (B Sartorius PhD); Norwegian Institute of Public Health, Oslo, Norway (S E Vollset PhD, A K Knudsen PhD); Parc Sanitari Sant Joan de Déu (CIBERSAM), Barcelona, Spain (J M Haro MD); University of Melbourne, Melbourne, VIC, Australia (G Patton MD); University of British Columbia, Vancouver, BC, Canada (Jacek Kopec); Ministério da Saúde do Brasil, Brasília, Brazil (D Carvalho Malta); Jagiellonian University Medical College, Krakow, Poland (R Topor-Madry PhD); Erasmus University Medical Center, Rotterdam, Netherlands (J Haagsma PhD); Karolinska Institutet, Solna, Sweden (P Allebeck MD); Shanghai Jiao Tong University, Shanghai, China (M Phillips MD); and Stanford University, Stanford, CA, USA (J Salomon PhD).
Contributors
LD conceived the study and provided overall guidance. LD, FC, AF, DS, HE, and JL prepared the first draft. DS, JL, LD, AF, HE, and FC extracted data. All other authors provided data, developed models, reviewed results, initiated modelling infrastructure, or reviewed and contributed to the final manuscript.
Declaration of interests
LD reports grants from Mundipharma, Indivior, and Seqirus, outside the submitted work. All other authors declare no competing interests.
Contributor Information
GBD 2016 Alcohol and Drug Use Collaborators:
Louisa Degenhardt, Fiona Charlson, Alize Ferrari, Damian Santomauro, Holly Erskine, Ana Mantilla-Herrara, Harvey Whiteford, Janni Leung, Mohsen Naghavi, Max Griswold, Jürgen Rehm, Wayne Hall, Benn Sartorius, James Scott, Stein Emil Vollset, Ann Kristin Knudsen, Josep Maria Haro, George Patton, Jacek Kopec, Deborah Carvalho Malta, Roman Topor-Madry, John McGrath, Juanita Haagsma, Peter Allebeck, Michael Phillips, Joshua Salomon, Simon Hay, Kyle Foreman, Stephen Lim, Ali Mokdad, Mari Smith, Emmanuela Gakidou, Christopher Murray, and Theo Vos
Supplementary Material
References
- 1.Rehm J, Gmel GE, Sr, Gmel G. The relation between different dimensions of alcohol consumption and burden of disease: an update. Addiction. 2017;112:968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet. 2012;379:55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 3.WHO . World Health Organization; Geneva: 1993. The ICD-10 classification of mental and behavioural disorders. Diagnostic criteria for research. [Google Scholar]
- 4.American Psychiatric Association . 4th edn. American Psychiatric Association; Washington DC: 2000. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) [Google Scholar]
- 5.Larney S, Peacock A, Mathers BM, Hickman M, Degenhardt L. A systematic review of injecting-related injury and disease among people who inject drugs. Drug Alcohol Depend. 2017;171:39–49. doi: 10.1016/j.drugalcdep.2016.11.029. [DOI] [PubMed] [Google Scholar]
- 6.Rehm J, Baliunas D, Borges GL. The relation between different dimensions of alcohol consumption and burden of disease: an overview. Addiction. 2010;105:817–843. doi: 10.1111/j.1360-0443.2010.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nutt D, King L, Phillips L. Drug harms in the UK: a multicriteria decision analysis. Lancet. 2010;376:1558–1565. doi: 10.1016/S0140-6736(10)61462-6. [DOI] [PubMed] [Google Scholar]
- 8.World Bank . Oxford University Press; New York: 1993. World Development Report 1993: investing in health. [Google Scholar]
- 9.Murray CJ, Lopez AD. On the comparable quantification of health risks: lessons from the Global Burden of Disease Study. Epidemiology. 1999;10:594–605. [PubMed] [Google Scholar]
- 10.Stevens GA, Alkema L, Black RE. Guidelines for Accurate and Transparent Health Estimates Reporting: the GATHER statement. Lancet. 2016;388:e19–e23. doi: 10.1016/S0140-6736(16)30388-9. [DOI] [PubMed] [Google Scholar]
- 11.Vos T, Abajobir AA, Abate KH. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. doi: 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naghavi M, Abajobir AA, Abbafati C. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hay SI, Abajobir AA, Abate KH. Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1260–1344. doi: 10.1016/S0140-6736(17)32130-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gakidou E, Afshin A, Abajobir AA. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popova S, Lange S, Shield K. Comorbidity of fetal alcohol spectrum disorder: a systematic review and meta-analysis. Lancet. 2016;387:978–987. doi: 10.1016/S0140-6736(15)01345-8. [DOI] [PubMed] [Google Scholar]
- 16.Wang H, Abajobir AA, Abate KH. Global, regional, and national under-5 mortality, adult mortality, age-specific mortality, and life expectancy, 1970–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1084–1150. doi: 10.1016/S0140-6736(17)31833-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Flaxman AD, Vos DT, Murray CJ. University of Washington Press; Seattle: 2015. An integrative metaregression framework for descriptive epidemiology. [Google Scholar]
- 19.Degenhardt L, Bucello C, Calabria B. What data are available on the extent of illicit drug use and dependence globally? Results of four systematic reviews. Drug Alcohol Depend. 2011;117:85–101. doi: 10.1016/j.drugalcdep.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 20.Salomon JA, Haagsma JA, Davis A. Disability weights for the Global Burden of Disease 2013 study. Lancet Glob Health. 2015;3:e712–e723. doi: 10.1016/S2214-109X(15)00069-8. [DOI] [PubMed] [Google Scholar]
- 21.Salomon JA, Vos T, Hogan DR. Common values in assessing health outcomes from disease and injury: disability weights measurement study for the Global Burden of Disease Study 2010. Lancet. 2012;380:2129–2143. doi: 10.1016/S0140-6736(12)61680-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haagsma JA, De Noordhout CM, Polinder S. Assessing disability weights based on the responses of 30,660 people from four European countries. Popul Health Metr. 2015;13:10. doi: 10.1186/s12963-015-0042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hasin DS, Grant BF. The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) waves 1 and 2: review and summary of findings. Soc Psychiatry Psychiatr Epidemiol. 2015;50:1609–1640. doi: 10.1007/s00127-015-1088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shand F, Degenhardt L, Nelson E, Mattick R. Predictors of social anxiety in an opioid dependent sample and a control sample. J Anxiety Disord. 2010;24:49–54. doi: 10.1016/j.janxdis.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shand F, Slade T, Degenhardt L, Baillie A, Nelson E. Opioid dependence latent structure: two classes with differing severity? Addiction. 2011;106:590–598. doi: 10.1111/j.1360-0443.2010.03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullman N, Barber RM, Abajobir AA. Measuring progress and projecting attainment on the basis of past trends of the health-related Sustainable Development Goals in 188 countries: an analysis from the Global Burden of Disease Study 2016. Lancet. 2017;390:1423–1459. doi: 10.1016/S0140-6736(17)32336-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018 doi: 10.1016/S0140-6736(18)31310-2. published online Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray CJ, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S. Comparative quantification of health risks: conceptual framework and methodological issues. Popul Health Metr. 2003;1:1. doi: 10.1186/1478-7954-1-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rehm J, Kehoe T, Gmel G, Stinson F, Grant B, Gmel G. Statistical modeling of volume of alcohol exposure for epidemiological studies of population health: the US example. Popul Health Metr. 2010;8:3. doi: 10.1186/1478-7954-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shield KD, Rylett M, Gmel G, Gmel G, Kehoe-Chan TA, Rehm J. Global alcohol exposure estimates by country, territory and region for 2005—a contribution to the Comparative Risk Assessment for the 2010 Global Burden of Disease Study. Addiction. 2013;108:912–922. doi: 10.1111/add.12112. [DOI] [PubMed] [Google Scholar]
- 31.Degenhardt L, Charlson F, Stanaway J. Estimating the burden of disease attributable to injecting drug use as a risk factor for HIV, hepatitis C, and hepatitis B: findings from the Global Burden of Disease Study 2013. Lancet Infect Dis. 2016;16:1385–1398. doi: 10.1016/S1473-3099(16)30325-5. [DOI] [PubMed] [Google Scholar]
- 32.Agardh EE, Danielsson AK, Ramstedt M. Alcohol-attributed disease burden in four Nordic countries: a comparison using the Global Burden of Disease, Injuries and Risk Factors 2013 study. Addiction. 2016;111:1806–1813. doi: 10.1111/add.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang PS, Angermeyer M, Borges G. Delay and failure in treatment seeking after first onset of mental disorders in the World Health Organization's World Mental Health Survey Initiative. World Psychiatry. 2007;6:177. [PMC free article] [PubMed] [Google Scholar]
- 34.Degenhardt L, Glantz M, Evans-Lacko S. Estimating treatment coverage for people with substance use disorders: an analysis of data from the World Mental Health Surveys. World Psychiatry. 2017;16:299–307. doi: 10.1002/wps.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rehm J, Shield K, Gmel G, Rehm M, Frick U. Modeling the impact of alcohol dependence on mortality burden and the effect of available treatment interventions in the European Union. Eur Neuropsychopharmacology. 2013;23:89–97. doi: 10.1016/j.euroneuro.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 36.Larney S, Peacock A, Leung J. Gobal, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Glob Health. 2017;5:e1208–e1220. doi: 10.1016/S2214-109X(17)30373-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jernigan DH, Babor TF. The concentration of the global alcohol industry and its penetration in the African region. Addiction. 2015;110:551–560. doi: 10.1111/add.12468. [DOI] [PubMed] [Google Scholar]
- 38.Bakke Ø, Endal D. Vested interests in addiction research and policy alcohol policies out of context: Drinks industry supplanting government role in alcohol policies in sub-Saharan Africa. Addiction. 2010;105:22–28. doi: 10.1111/j.1360-0443.2009.02695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanefeld J, Hawkins B, Knai C, Hofman K, Petticrew M. What the InBev merger means for health in Africa. BMJ Glob Health. 2016;1:e000099. doi: 10.1136/bmjgh-2016-000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.The Lancet A framework convention on alcohol control. Lancet. 2007;370:1102. doi: 10.1016/S0140-6736(07)61486-X. [DOI] [PubMed] [Google Scholar]
- 41.Anderson P, Chisholm D, Fuhr DC. Effectiveness and cost-effectiveness of policies and programmes to reduce the harm caused by alcohol. Lancet. 2009;373:2234–2246. doi: 10.1016/S0140-6736(09)60744-3. [DOI] [PubMed] [Google Scholar]
- 42.Rehm J, Lachenmeier DW, Llopis EJ, Imtiaz S, Anderson P. Evidence of reducing ethanol content in beverages to reduce harmful use of alcohol. Lancet Gastroenterol Hepatol. 2016;1:78–83. doi: 10.1016/S2468-1253(16)30013-9. [DOI] [PubMed] [Google Scholar]
- 43.Purshouse RC, Meier PS, Brennan A, Taylor KB, Rafia R. Estimated effect of alcohol pricing policies on health and health economic outcomes in England: an epidemiological model. Lancet. 2010;375:1355–1364. doi: 10.1016/S0140-6736(10)60058-X. [DOI] [PubMed] [Google Scholar]
- 44.Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82:858–866. [PMC free article] [PubMed] [Google Scholar]
- 45.Morley KC, Logge W, Pearson S-A, Baillie A, Haber PS. National trends in alcohol pharmacotherapy: Findings from an Australian claims database. Drug Alcohol Depend. 2016;166:254–257. doi: 10.1016/j.drugalcdep.2016.06.027. [DOI] [PubMed] [Google Scholar]
- 46.Gates PJ, Sabioni P, Copeland J, Le Foll B, Gowing L. Psychosocial interventions for cannabis use disorder. Cochrane Database Syst Rev. 2016;5 doi: 10.1002/14651858.CD005336.pub4. CD005336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Minozzi S, Saulle R, De Crescenzo F, Amato L. Psychosocial interventions for psychostimulant misuse. Cochrane Database Syst Rev. 2016;9 doi: 10.1002/14651858.CD011866.pub2. CD011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Degenhardt L, Bucello C, Mathers B. Mortality among regular or dependent users of heroin and other opioids: a systematic review and meta-analysis of cohort studies. Addiction. 2011;106:32–51. doi: 10.1111/j.1360-0443.2010.03140.x. [DOI] [PubMed] [Google Scholar]
- 49.Mattick RP, Breen C, Kimber J, Davoli M. Methadone maintenance therapy versus no opioid replacement therapy for opioid dependence. Cochrane Database Syst Rev. 2009;3 CD002209. [Google Scholar]
- 50.Mattick RP, Kimber J, Breen C, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2008;2 doi: 10.1002/14651858.CD002207.pub3. CD002207. [DOI] [PubMed] [Google Scholar]
- 51.McDonald R, Campbell ND, Strang J. Twenty years of take-home naloxone for the prevention of overdose deaths from heroin and other opioids-Conception and maturation. Drug Alcohol Depend. 2017;178:176–187. doi: 10.1016/j.drugalcdep.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 52.MacArthur GJ, Minozzi S, Martin N. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. doi: 10.1136/bmj.e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gowing L, Farrell MF, Bornemann R, Sullivan LE, Ali R. Oral substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2011;8 doi: 10.1002/14651858.CD004145.pub4. CD004145. [DOI] [PubMed] [Google Scholar]
- 54.Degenhardt L, Mathers B, Vickerman P, Rhodes T, Latkin C, Hickman M. Prevention of HIV infection for people who inject drugs: why individual, structural, and combination approaches are needed. Lancet. 2010;376:285–301. doi: 10.1016/S0140-6736(10)60742-8. [DOI] [PubMed] [Google Scholar]
- 55.Turner KM, Hutchinson S, Vickerman P. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction. 2011;106:1978–1988. doi: 10.1111/j.1360-0443.2011.03515.x. [DOI] [PubMed] [Google Scholar]
- 56.Degenhardt L, Mathers BM, Wirtz AL. What has been achieved in HIV prevention, treatment and care for people who inject drugs, 2010–2012? A review of the six highest burden countries. Int J Drug Policy. 2014;25:53–60. doi: 10.1016/j.drugpo.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 57.Martin NK, Vickerman P, Dore GJ, Hickman M. The hepatitis C virus epidemics in key populations (including people who inject drugs, prisoners and MSM): the use of direct-acting antivirals as treatment for prevention. Curr Opin HIV AIDS. 2015;10:374–380. doi: 10.1097/COH.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Psychiatric Association . 5th edn. American Psychiatric Association; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 59.Lago L, Bruno R, Degenhardt L. Concordance of ICD-11 and DSM-5 definitions of alcohol and cannabis use disorders: a population survey. Lancet Psychiatry. 2016;3:673–684. doi: 10.1016/S2215-0366(16)00088-2. [DOI] [PubMed] [Google Scholar]
- 60.Goldstein RB, Chou SP, Smith SM. Nosologic comparisons of DSM-IV and DSM-5 alcohol and drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions-III. J Stud Alcohol Drugs. 2015;76:378–388. doi: 10.15288/jsad.2015.76.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lundin A, Hallgren M, Forsman M, Forsell Y. Comparison of DSM-5 classifications of alcohol use disorders with those of DSM-IV, DSM-III-R, and ICD-10 in a general population sample in Sweden. J Stud Alcohol Drugs. 2015;76:773–780. doi: 10.15288/jsad.2015.76.773. [DOI] [PubMed] [Google Scholar]
- 62.Degenhardt L, Bharat C, Bruno R, et al. Concordance between the diagnostic guidelines for alcohol and cannabis use disorders in the draft ICD-11 and other classification systems: analysis of data from the WHO's World Mental Health Surveys. Addiction (in press). [DOI] [PMC free article] [PubMed]
- 63.WHO . World Health Organization; Geneva: 2016. The health and social effects of nonmedical cannabis use. [Google Scholar]
- 64.Darke S, Kaye S, McKetin R, Duflou J. Major physical and psychological harms of methamphetamine use. Drug Alcohol Rev. 2008;27:253–262. doi: 10.1080/09595230801923702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.