Abstract
The effects of magnesium (Mg) supplementation on the growth performance, oxidative damage, DNA damage, and photosynthetic pigment synthesis, as well as on the activity level of carbonic anhydrase (CA), ribulose-1,5-bisphosphate carboxylase (Rubisco), and antioxidant enzymes were studied in Vicia faba L. plants exposed to heat stress (HS) and non-heat-stress (non-HS) conditions. Seeds were grown in pots containing a 1:1 mixture of sand and peat, with Mg treatments. The treatments consisted of (i) 0 Mg and non-HS (ambient temperature; control); (ii) 50 mM Mg; (iii) HS (38 °C); and (iv) 50 mM Mg and HS (38 °C). HS was imposed by placing potted plants in an incubator at 38 °C for 48 h. Growth attributes, total chlorophyll (Total Chl), and CA, and Rubisco activity decreased in plants subjected to HS, whereas accumulation of organic solutes [proline (Pro) and glycine betaine (GB)]; superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT) activity; DNA damage; electrolyte leakage (EL); and malondialdehyde (MDA) and hydrogen peroxide (H2O2) content all increased. Application of Mg, however, significantly enhanced further proline (Pro), glycinebetaine (GB), SOD, POD, and CAT activity, and decreased DNA damage, EL, and MDA and H2O2 concentrations. These results suggest that adequate supply of Mg is not only essential for plant growth and development, but also improves plant tolerance to HS by suppressing cellular damage induced by reactive oxygen species through the enhancement of the accumulation of Pro and GB, and the actions of antioxidant enzymes.
Keywords: Cellular damage, DNA damage, Faba bean, Heat stress, Magnesium
1. Introduction
Rising mean ambient temperatures have become serious threats to global food-crop production. According to the Intergovernmental Panel on Climate Change (IPCC 2013) the global mean surface temperature will increase by 1.5–4.0 °C by the end of the 21st century—a consequence of the increasing levels of greenhouse gases generated by human activity.
Plants are generally unable to escape from the adverse effects of high temperature, and perform differently when subjected to heat stress (HS), with some species more tolerant than others (Siddiqui et al., 2015). Plants frequently experience high temperature stress, which alters plant growth metabolism by disturbing protein stability, enzymatic activity in the chloroplast and mitochondria, proline (Pro) translocation, carbohydrate metabolism, and hormonal balance (Mittler et al., 2012, Hemantaranjan et al., 2014, Lavania et al., 2015, Sangu et al., 2015). The process of photosynthesis is extremely heat sensitive, and may even stop entirely if temperatures rise above threshold levels. High ambient temperatures may also lead to the overproduction of reactive oxygen species (ROS) in some plants, which results in the disruption of chloroplast activity, inhibition of chlorophyll (Chl) synthesis, and inactivation of enzymes, and under extreme circumstances even the breakdown of cellular organization (Cui et al., 2006, Chen et al., 2012, Siddiqui et al., 2015).
Plants have developed an assortment of osmotic and cellular mechanisms to cope with HS, including the accumulation of organic solutes (osmoprotectants), such as Pro and glycine betaine (GB) (Siddiqui et al., 2008). Pro, an especially potent universal osmoprotectant, counters the effects of abiotic stress by acting as an antioxidant, and as an important source of energy, nitrogen, and carbon, as well as a reducing equivalent in the Krebs cycle and oxidative phosphorylation, resulting in the formation of NADH and ATP (Matysik et al., 2002, Verslues and Sharma, 2010). It insulates plants from environmental stress by protecting biological membranes, and by stabilizing enzymes and proteins. Proline biosynthesis is catalyzed by the Δ1-pyrroline-5-carboxylate (P5C) reductase (P5CR) enzyme, which reduces P5C to Pro. GB, a quaternary ammonium compound, aids in the development of various tissues, and also increases the abundance and area of mesophyll cells, as well as the abundance of vascular bundles, in plants (Rasheed et al., 2011).
Magnesium (Mg) is essential for optimal physiological and biochemical functioning of plants. According to Cakmak and Yazici (2010), however, it is an oft-overlooked element, despite its shortage adversely affecting plant growth and development; for example, Mg-deficient plants often exhibit low rates of photosynthesis because Mg is a key component of the chlorophyll molecule and activates numerous key chloroplast enzymes, including ribulose-1,5-bisphosphate (RuBP) carboxylase, the precursor enzyme of photosynthesis (Cakmak and Yazici, 2010, Waraich et al., 2012, Senbayram et al., 2015). Approximately 20% of total Mg is found in the chloroplast, with the balance is present in mobile forms (Marschner, 2012). Due to its mobility in phloem, photosynthates are transported from source photosynthetic organs to sink roots; as such, carbohydrates tend to accumulate in source leaves of Mg-deficient plants, which also display altered photosynthetic carbon metabolic activity and decreased CO2 fixation. Impairment of such plant metabolic processes may lead to the overproduction of ROS from O2 because of excess accumulation of non-utilized electrons and absorbed energy (Cakmak, 2014). Under HS, ROS are formed constantly in the chloroplast, mitochondria, and peroxisomes due to impairment of their various metabolic pathways (Waraich et al., 2012). Moreover, Mg is also involved in transferring energy from photosystem II to NADP+ and provides protection to the thylakoid membrane, resulting in decreased excitation energy and higher oxidative damage (Waraich et al., 2012, Mengutay et al., 2013).
Given the projected changes in future global temperature and climate, research on the effects of nutrients, particularly Mg, on plants under HS conditions is becoming increasingly important. To the best of our knowledge, no previous studies have focused on the interaction of Mg and heat stress in the bean Vicia faba. As with other crops, faba bean crops are increasingly challenged with HS-induced oxidative damage during growth phases; understanding how an adequate supply of Mg influences the morphological, physiological, and biochemical parameters of faba bean plants subjected to HS conditions are therefore critical. Thus, the goal of our research was to measure the changes in various growth parameters, photosynthetic pigment accumulation, and activity of carbonic anhydrase (CA) and ribulose-1,5-bisphosphate carboxylase (Rubisco), and the antioxidant enzymes superoxide dismutase (SOD), peroxidase (POD) and catalase activity, in faba bean plants under conditions of HS and adequate Mg.
2. Materials and methods
2.1. Plant growth conditions and treatments
Individuals of the V. faba genotype C5 were collected from a market in Riyadh, Saudi Arabia, the seeds of which were sown in a growth chamber under controlled climatic conditions (25 ± 3 °C, relative humidity 50–60%, light 90 μmol of photons m−2 s−1, 6/8-h light/dark cycle). Seeds were grown in pots containing a 1:1 mixture of sand and peat, with Mg treatments. The treatments consisted of (i) 0 Mg and non-HS (ambient temperature; control); (ii) 50 mM Mg; (iii) HS (38 °C); and (iv) 50 mM Mg and HS (38 °C). HS was imposed by placing potted plants in an incubator at 38 °C for 48 h.
The experimental pots were arranged in a simple randomized design, with five replicates per treatment. Surface sterilized seeds (1% sodium hypochlorite) were sown in sand and peat-filled pots supplied with Raukura’s nutrient solution (Smith et al., 1983). The salts used to make up the nutrient solution were composed of macronutrient stock solution A (16 gL−1 Ca[NO3]2·4H2O, 8.48 gL–1 NH4NO3, 2.28 gL–1 KNO3) and macronutrient stock solution B (2.67 gL−1 of KH2PO4, 1.64 gL–1 K2HPO4, 6.62 gL–1 K2SO4, 0.60 gL–1 Na2SO4, 0.33 gL–1 NaCl), along with a micronutrient supplement (128.80 mgL−1 H3BO3, 4.84 mgL−1 CuCl2·2H2O, 81.10 mgL−1 MnCl2·4H2O, 0.83 mgL−1 [NH4]6Mo7O24·4H2O, 23.45 mgL−1 ZnCl2, 809.84 mgL−1 ferric citrate pentahydrate). Magnesium was supplied in the form of MgSO4·7H2O, at a concentration (50 mM) based on that used in previous experiments (Neuhaus et al., 2014). The solution was diluted prior to application by mixing 200 mL of each of the macronutrient stock solutions A and B with 100 mL of the micronutrient supplement and 4.5 L of DDW, Raukura’s nutrient solution.
2.2. Determination of the morphological characteristics of plants
Growth parameters of faba bean plants were measured after 10 days of HS treatments. Growth performance was evaluated in terms of plant height (PH), fresh and dry weight (FW and DW), and leaf area, which was measured directly using a leaf area meter (LI-COR Inc., USA).
2.3. Physiological and biochemical characteristics
2.3.1. Chlorophyll
Chlorophyll concentrations in the leaves of the faba bean plants were determined following dimethylsulfoxide (DMSO) extraction using the method described by Barnes et al. (1992). Chlorophyll in the extract was measured with a UV–vis spectrophotometer (SPEKOL 1500; Analytik Jena AG, Jena, Germany).
2.3.2. Proline
Proline concentration was estimated colorimetrically, in accordance with the procedures described by Bates et al. (1973) based on ninhydrin and Pro reactions.
2.3.3. Glycinebetaine
GB content was measured following the method described by Grieve and Grattan (1983). GB concentrations were determined spectrophotometrically at 365 nm, using aqueous extracts of dry-ground leaf material after reaction with KI2–I2.
2.3.4. Damage to leaf DNA in plants under HS
Heat-stress-induced DNA damage in leaves was measured using the comet assay (also known as single cell gel electrophoresis), as described by Lin et al. (2007). After washing, leaves from plants in each treatment were used in the comet assay immediately following drying, which was performed under dim or yellow light to avoid DNA damage induced by UV radiation. Each leaf sample was gently cut into pieces with a razor blade into a petri dish containing chilled phosphate-buffered saline (PBS; 130 mM NaCl, 7 mM Na2HPO4, 3 mM NaH2PO4, 50 mM EDTA 50, pH 7.5). Nuclei were collected from the buffer solution and used in the comet assay. The cell suspension was mixed with 75 μL of 0.5% low-melting-point agarose and layered on slides precoated with 1% normal melting agarose. After solidification of agarose, 75 μL of 0.5% low-melting-point agarose was layered. The prepared slides were steeped in lysis solution with a high salt concentration (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, and 1% sodium laurylsarcosine; 1% Triton X-100 and 10% DMSO) for 1 h at 4 °C. Equilibration for 3 × 5 min in 1× TBE buffer on ice was followed by electrophoresis at room temperature in the same buffer for 6 min at 15–17 mA (Koppen et al., 1999). Each slide was stained with ethidium bromide and viewed using a fluorescent microscope (Eclipse Ni-U, Nikon, Tokyo, Japan) with an excitation filter of 515–560 nm and a barrier filter of 590 nm. Comet images were taken with a DS-Ri1 camera.
2.3.5. Malondialdehyde
Malondialdehyde (MDA) concentration was estimated colorimetrically following the procedures described by Heath and Packer (1968). Leaf samples were collected and homogenized in a solution containing 10% trichloroacetic acid and 0.65% 2-thiobarbituric acid. The homogenates were heated at 95 °C for 60 min, then cooled to room temperature. After centrifugation at 10,000g for 10 min, absorbance of the supernatant was taken at 532 nm and 600 nm against a reagent blank.
2.3.6. Electrolyte leakage
Electrolyte leakage (EL) was measured following the method of Lutts et al. (1995). After washing leaf samples, leaf disks were placed in sealed vials containing 10 mL of DDW, followed by incubation on a rotary shaker for 24 h, after which the electrical conductivity of the solution (EC1) was measured. Leaf disks were then autoclaved at 120 °C for 20 min, with conductivity measured (EC2) once more after the solution had cooled to room temperature.
2.3.7. Determination of enzyme activity
Carbonic anhydrase (EC 4.2.1.1) activity was measured using the method described by Dwivedi and Randhawa (1974), and expressed as μmol (CO2) kg–1 (FW) s–1.
Rubisco (EC 4.1.1.39) activity was determined using a UV–vis spectrophotometer (SPEKOL 1500; Analytik Jena AG, Jena, Germany) by measuring NADH oxidation at 340 nm (Usuda, 1985). After washing, leaf samples were homogenized using a chilled mortar and pestle with ice-cold extraction buffer solution (0.05 M MgCl2, 250 mM Tris–HCl, pH 7.8), 2.5 mM EDTA, and 37.5 mg DTT. The homogenate was centrifuged at 10,000g for 10 min at 4 °C, with the supernatant used for the enzyme assay. The reaction mixture contained 100 mM Tris–HCl (pH 8.0), 40 mM NaHCO3, 10 mM MgCl2, 0.2 mM NADH, 4 mM ATP, 0.2 mM EDTA, 5 mM DTT, 1 U of glyceraldehyde 3-phosphodehydrogenase, and 1 U of 3-phosphoglycerate kinase. NADH oxidation was initiated following the addition of the enzyme extract and 0.2 mM ribulose-1,5-bisphosphate (RuBP), and the reaction read for 1 min once the rate of reaction was stopped. Enzyme activity was expressed as μmol CO2 fixed min−1 mg−1 protein. Protein content was quantified following the procedures set out by Bradford (1976) using bovine serum albumin as a standard.
To determine the activity of the antioxidant enzymes, leaves from plants in each treatment were homogenized in extraction buffer containing 5% Triton X-100 and 1% polyvinylpyrrolidone in 100 mM potassium phosphate buffer (pH 7.0) with a chilled mortar and pestle. Each homogenate was centrifuged at 15,000g for 20 min at 4 °C. The supernatant was used as a source for the enzymatic assays.
The level of SOD (EC 1.15.1.1) activity was estimated based on the inhibition of nitroblue tetrazolium (NBT) photochemical, in accordance with the method described by Giannopolitis and Ries (1977). The reaction solution consisted of 50 mM NBT, 1.3 mM riboflavin, 13 mM methionine, 75 μM ethylenediamine tetraacetic acid (EDTA), 50 mM phosphate buffer (pH 7.8), and 20–50 mL of enzyme extract. The reaction solution was irradiated under fluorescent light at 75 μM m–2 s–1 for 15 min. The absorbance of each reaction solution was read at 560 nm against a blank (non-irradiated reaction solution). One unit of SOD activity was defined as the amount of enzyme that inhibited 50% of NBT photoreduction.
POD (EC 1.11.1.7) activity was determined following the method of Chance and Maehly (1955), in which a mixture of 5 mL of enzyme reaction solution containing phosphate buffer (pH 6.8), 50 M pyrogallol, 50 mM H2O2, and 1 mL of 20-times diluted enzyme extract was used in the assay. Following incubation for 5 min at 25 °C, the reaction was stopped by the addition of 0.5 mL of 5% (v/v) H2SO4. The formation of purpurogallin was measured spectrophotometrically at 420 nm. One unit of POD activity was defined as the amount of purpurogallin formed per milligram of protein per minute.
We followed the techniques described by Aebi (1984) to determine catalase (CAT) (EC 1.11.1.6) activity. The decomposition of H2O2 was measured as the decrease in absorbance at 240 nm. For this assay, 50 mM phosphate buffer (pH 7.8) and 10 mM H2O2 were used in the reaction solution.
Data were expressed as mean ± standard error and were analyzed using SPSS ver. 17 statistical software; means were compared using Duncan’s multiple-range test at the p < 0.05 levels.
3. Results
3.1. Mg stimulates plant growth
In order to evaluate the involvement of Mg in plant tolerance to HS, we examined plant height, shoot fresh and dry weight, and leaf area under non-heat-stress and HS conditions, and how these parameters were affected by Mg application. Most of these attributes increased significantly when plants were supplied with Mg (Table 1). Under no-stress conditions, treatment with Mg significantly increased shoot fresh and dry weight but did not have any significant effect on plant height and leaf area. Application of Mg increased shoot fresh weight by 24.17%, shoot dry weight by 36.36% and leaf area by 33.74% in comparison to respective controls under non-HS. Plants performed most poorly under HS conditions (Table 1), but application of Mg improved plant height, shoot fresh and dry weight, and leaf area under HS. Application of Mg increased plant height by 48.80%, shoot fresh weight by 23.57%, shoot dry weight by 69.44% and leaf area by 53.14% under HS condition.
Table 1.
Effect of Mg on the growth performance of faba bean plants.
| Treatments | Plant height (cm) | Shoot FW (g) | Shoot DW (g) | Area leaf−1 (cm2) |
|---|---|---|---|---|
| Control | 87.93 ± 6.22a | 4.80 ± 0.096b | 0.55 ± 0.026b | 16.48 ± 1.73bc |
| Mg | 93.56 ± 6.73a | 5.96 ± 0.080a | 0.75 ± 0.020a | 22.04 ± 1.60ab |
| Control + HS | 38.69 ± 4.41c | 2.80 ± 0.096d | 0.36 ± 0.026c | 15.47 ± 1.80c |
| Mg + HS | 57.57 ± 4.50b | 3.46 ± 0.061c | 0.61 ± 0.017b | 23.69 ± 1.71a |
Bars followed by the same letter are not significantly different at P < 0.05 (Duncan Multiple Range Test). Average of five determinations are presented with bars indicating S.E.
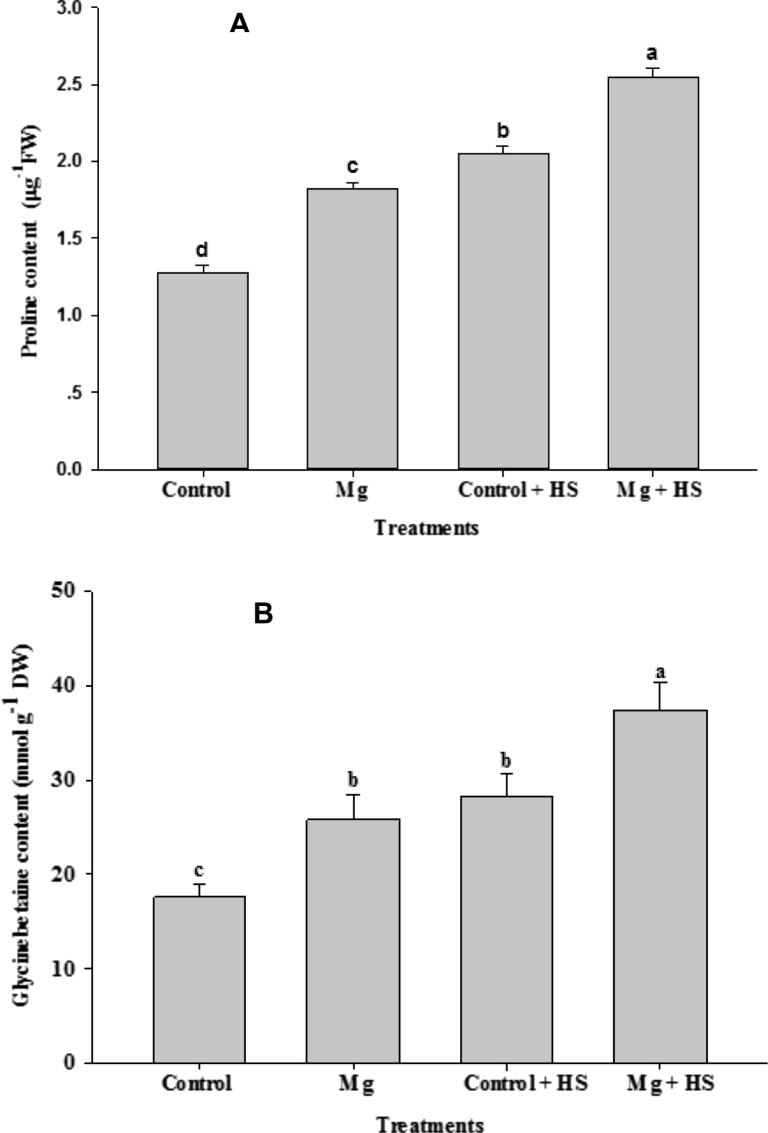
3.2. Mg involves in the accumulation of osmoprotectants in plant
Under non-HS conditions, both Pro and GB were enhanced by the application of Mg (Fig. 1A and B); application of Mg increased Pro by 43.31% and GB by 47.10% in comparison to non-stress controls; however, HS triggered the accumulation of osmoprotectants as compared to the control and Mg treatments, and application of Mg enhanced both Pro and GB in plants under HS conditions. Treatment of Mg increased Pro by 24.39% and GB by 32.53% in comparison to HS.
Figure 1.
Proline and glycinebetaine content in Vicia faba L. Plants were grown with/without magnesium nutrients under non-heat stress and heat stress conditions. Bars followed by the same letter are not significantly different at P < 0.05 (Duncan Multiple Range Test). Average of five determinations are presented with bars indicating S.E.
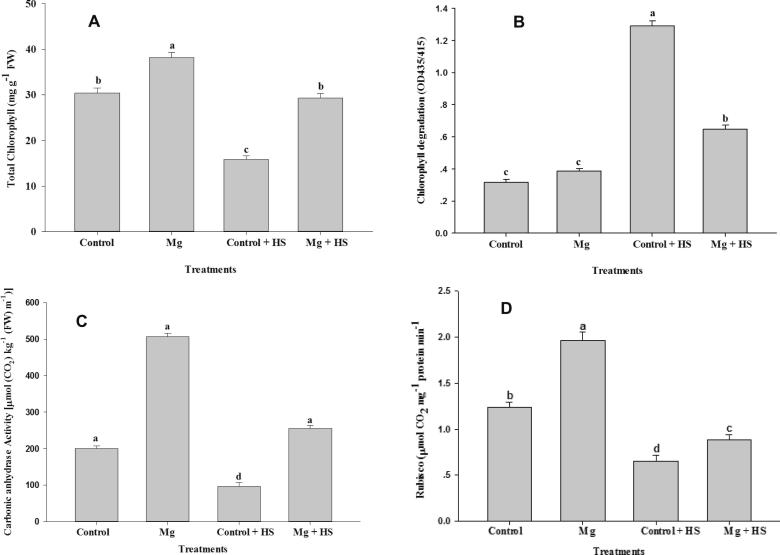
3.3. Influence of Mg on total Chl, Chl degradation, and CA and ribulose-1,5-bisphosphate carboxylase activity
Application of Mg significantly enhanced Total Chl, as well as CA and Rubisco activity (except Chl degradation) under both non-stress and HS conditions (Fig. 2A–D). However, Chl degradation increased under HS, whereas Total Chl as well as CA and Rubisco activity were all significantly reduced. Under not-HS condition, application of Mg increased Total Chl by 25.62%, CA activity by 152.92% and Rubisco by 35.89%; however, Chl degradation was increased by 303.13% in comparison to control under HS. Under HS, application of Mg increased Total Chl by 91.92% and CA activity by 163.23%, and decreased Chl degradation by 49.61%.
Figure 2.
Total chlorophyll content (A), chlorophyll degradation (B), and activity of carbonic anhydrase (C) and Rubisco (D) in Vicia faba L. Plants were grown with/without magnesium nutrients under non-heat stress and heat stress conditions. Bars followed by the same letter are not significantly different at P < 0.05 (Duncan Multiple Range Test). Average of five determinations are presented with bars indicating S.E.
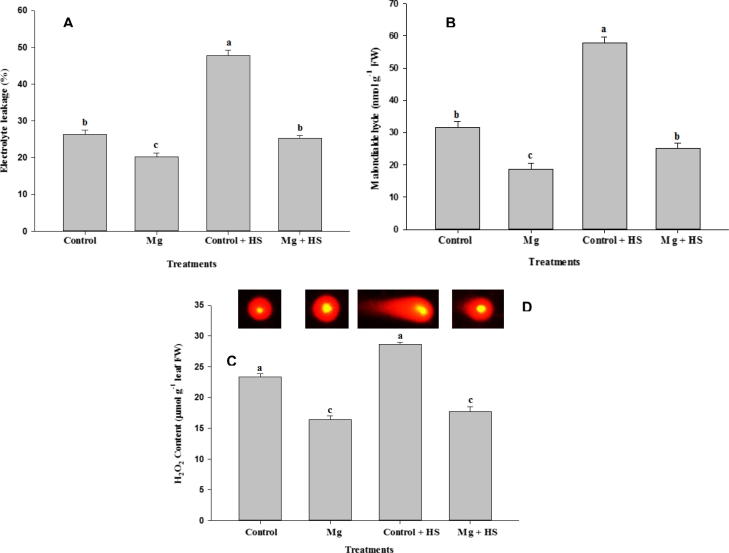
3.4. Application of Mg reduces HS-induced oxidative stress
The role of Mg in alleviating HS-induced oxidative stress was examined by determining EL, MDA, and H2O2 concentrations, and DNA damage (Fig. 3A–D). Application of Mg proved effective in alleviating oxidative damage by reducing EL and MDA levels, along with DNA damage, under HS. Under HS conditions, application of Mg decreased MDA by 56.72%, H2O2 by 38.17%, and EL by 47.07%.
Figure 3.
Electrolyte leakage (A), malondialdehyde and H2O2 content and DNA damage in Vicia faba L. Plants were grown with/without magnesium nutrients under non-heat stress and heat stress conditions. Bars followed by the same letter are not significantly different at P < 0.05 (Duncan Multiple Range Test). Average of five determinations are presented with bars indicating S.E.
We observed a greater degree of DNA damage in HS-stressed faba bean plants than in non-HS plants (Fig. 3D). The control plants and Mg-treated plants exhibited similar signals for DNA damage under non-HS conditions, but Mg-treated plants under HS displayed fewer DNA damage signals than did HS-stressed plants. The application of Mg was found to be effective in lessening the adverse effects of HS through enhancement of DNA protection.
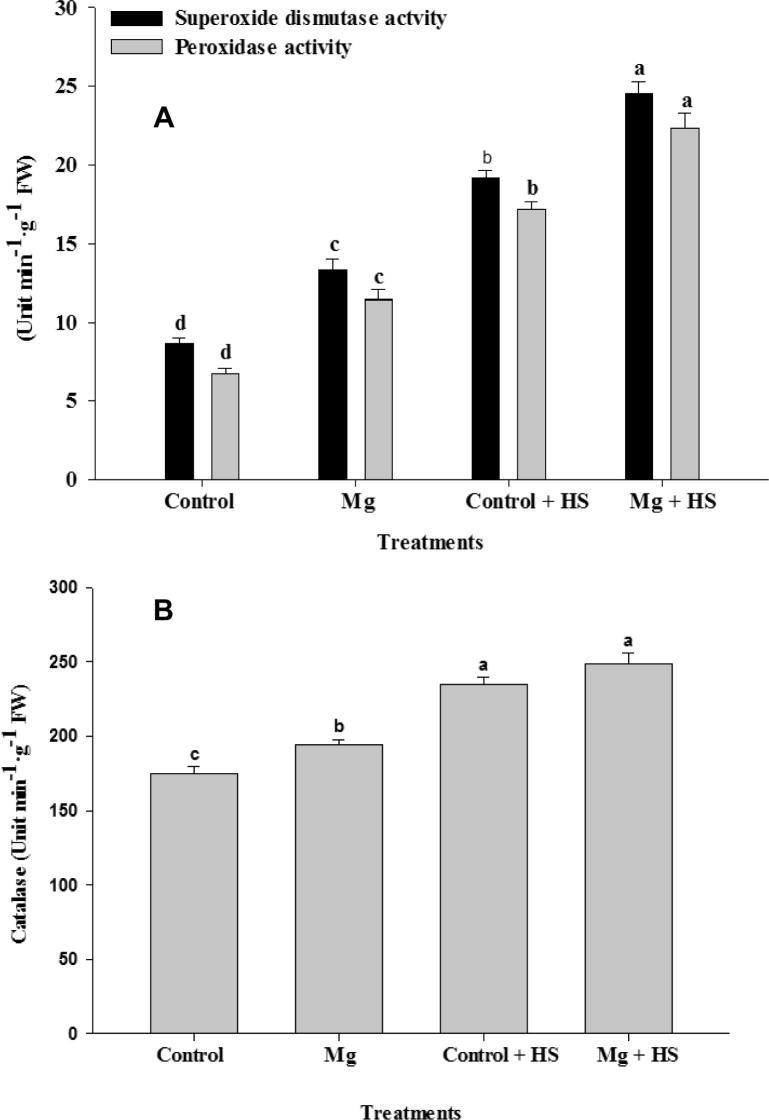
3.5. Mg enhances HS tolerance by improving antioxidant enzyme activity
The influence of Mg and HS on antioxidant enzyme activity in V. faba plants is shown in Fig. 4A and B. Application of Mg significantly increased SOD, POD, and CAT activity compared to control plants; moreover, Mg application was effective in promoting further enzymatic activity in plants under HS conditions. Application of Mg enhanced SOD by 28.10%, POD by 30.21%, and CAT by 5.87% in comparison to the HS treatment.
Figure 4.
Activity of Superoxide dismutase and peroxidase (A), and catalase (B) in Vicia faba L. Plants were grown with/without magnesium nutrients under non-heat stress and heat stress conditions. Bars followed by the same letter are not significantly different at P < 0.05 (Duncan Multiple Range Test). Average of five determinations are presented with bars indicating S.E.
4. Discussion
The application of Mg to faba bean plants significantly enhanced growth (Table 1), as well as physiological and biochemical characteristics (Figure 1, Figure 2, Figure 3, Figure 4); however, HS affected all growth parameters.
Supplying Mg to faba plants improved growth characteristics, most likely because of the important roles Mg plays in plant growth and development, most particularly in regard to photosynthesis and chlorophyll biosynthesis, and the activation of numerous key chloroplast enzymes (Cakmak and Yazici, 2010, Waraich et al., 2012, Senbayram et al., 2015). Control plants exhibited similar plant height regardless of the presence of supplemental Mg under non-stress conditions, but both fresh and dry weights were higher in plants supplied with Mg, a result consistent with those of previous research, in which it was reported that dry matter production was enhanced in response to adequate supply of Mg (Mengutay et al., 2013). It is well established that Mg, an important plant element, aids in the protection of plants to HS by enlarging the leaf area (Table 1) available to harvest solar energy, resulting in increased production of dry matter. Decreasing plant growth as a consequence of HS may be due to the impairment of several plant mechanisms; for instance, high temperatures are known to induce water stress, which causes reduced cell division (Stewart et al., 2016).
The accumulation of organic solutes enhances plant survival under different environmental conditions; among such solutes, Pro and GB are known to be important osmoprotectants (Siddiqui et al., 2008). Here, we found that accumulations of both Pro and GB were higher in HS-stressed and Mg-treated plants than in control plants (Fig. 1A and B), but with the highest accumulation of organic solutes occurring in Mg-treated HS plants. Under stress, the enhanced accumulation of Pro in Mg-treated plants may confer improved HS-stress resistance to plants because it acts as an antioxidant, and scavenges ROS by maintaining a GSH redox state (Siripornadulsil et al., 2002). Similarly, Mg-treated plants had the highest concentrations of GB under HS. Accumulation of GB provides biochemical adaptation in plants and is associated with stabilizing the quaternary structure of proteins that lead to the better enzymatic activity in plants, resulting in enhanced PS11 repair under stress (Giri, 2011).
Although total Chl increased following application of Mg in plants under both stress and non-stress conditions, total Chl decreased and Chl degradation increased in HS plants (Fig. 2A and B). Moreover, control plants with no Mg supplementation had lower total Chl and higher Chl degradation, indicating that Mg plays an important role in the biosynthesis of photosynthetic pigments. HS-induced Chl synthesis and degradation may be due to alterations in the structure and function of photosynthetic apparatus (Cui et al., 2006).
Carbonic anhydrase and Rubisco activity were lowest in plants under HS and in control plants not subjected to Mg treatment (Fig. 2C and D); this may be due to a destabilization of protein native structure, leading to the inhibition of enzymatic activity (Seregin and Kozhevnikova, 2006). The decreased levels of CA and Rubisco activities under HS may trigger overproduction of ROS because HS disturbs utilization of absorbed light energy, resulting in excess excitation energy in the chloroplast (Mengutay et al., 2013). However, application of Mg enhanced the activity of both enzymes under HS and non-stress conditions. Carbonic anhydrase is a key enzyme in ion exchange, acid–base balance, and in the reversible conversion of CO2 to bicarbonate that activates Rubisco activity (Lorimer and Miziorko, 1980). The improved activity of CA may help in hydration of CO2 and maintain its constant supply to Rubisco that could enhance photosynthesis by improving Chl synthesis. Magnesium is a key player in the activation and regulation of Rubisco (Lorimer and Miziorko, 1980, Andersson, 2008); as such, application of Mg is not only important for plant growth, it also contributes to the alleviation of HS by improving photosynthetic enzymes.
The enhanced EL, MDA concentration, and H2O2 in leaves (Fig. 3A–C) suggests that HS causes lipid peroxidation, resulting in cell death, as reported previously by Siddiqui et al. (2015). However, application of Mg decreased EL, MDA concentrations, and H2O2 in leaves of faba bean plants, most likely due to accumulation of antioxidant enzymes, organic solutes (Figure 1, Figure 4) that induce the detoxification of ROS and reduces oxidative cellular damage, resulting in better plant growth (Table 1). In addition, Mg reduces the formation of ROS via activation of CA and Rubisco (Fig. 2c,d) by maintaining the accumulation and translocation of carbohydrates and CO2 fixation in plants (Cakmak, 2014).
DNA damage occurs as a result of various environmental stresses, and is considered one of the key biomarkers of genotoxicity in plants. In this study, plants exposed to HS exhibited higher rates of DNA strand breakage than did control plants (Fig. 3D), possibly due to the overproduction of ROS (Fig. 3C), which induced DNA damage in leaves by reacting with cell components, resulting in cell death (Potters et al., 2010). This result agrees with the findings of Cvjetko et al. (2014). However, application of Mg significantly inhibited DNA damage, most likely because of the accumulation of Pro and GB, and the enhanced antioxidant enzyme activity (Figure 1, Figure 2), which mitigates the effects of ROS on leaf nuclear DNA (Gichner et al., 2008). In addition, Mg maintains genomic stability as it regulates DNA processing and helps in the removal of DNA damage induced by environmental stresses (Hartwig, 2001).
In the present study, SOD, POD, and CAT were enhanced in HS plants (Fig. 4A and B) as compared to control plants, indicating a higher requirement of these enzymes for scavenging the overproduction of ROS in plants. However, application of Mg further enhanced such enzymatic activity. Under different adverse conditions, plants require nutrients to maintain normal functioning, thereby reducing oxidative stress by suppressing ROS. The increase in antioxidant enzyme activity following Mg supplementation may be due to the involvement of Mg in uptake and utilization of nutrients, which are essential components of many metabolic processes and activated antioxidant systems in plants (Siddiqui et al., 2012, Senbayram et al., 2015).
5. Conclusion
Application of Mg to faba bean plants improved plant growth and tolerance to HS. Under both HS and non-HS conditions, application of Mg improved plant growth attributes by enhancing biosynthesis of Chl, Pro, and GB, as well as CA and Rubisco activity; moreover, application of Mg reduced DNA damage in plants subjected to HS by reducing EL, malondialdehyde, and H2O2 content through the enhancement of antioxidant enzymes.
Conflict of interest
The authors declare that they have no conflicts of interest.
Acknowledgments
Financial support was provided by the Deanship of Scientific Research of King Saud University, Riyadh, Saudi Arabia, to Research Group No. RGPVPP-153.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Andersson I. Catalysis and regulation in Rubisco. J. Exp. Bot. 2008;59:1555–1568. doi: 10.1093/jxb/ern091. [DOI] [PubMed] [Google Scholar]
- Barnes J.D., Balaguer L., Manrique E., Elvira S., Davison A.W. A reappraisal of the use of DMSO for the extraction and determination of chlorophylls a and b in lichens and higher plants. Environ. Exp. Bot. 1992;32:85–100. [Google Scholar]
- Bates L.S., Waldren R.P., Teare I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205–207. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak, I. 2014. Major functions of calcium and magnesium in crop plants. In: 16th World Fertilizer Congress of CIEC, Rio de Janeiro, RJ, Brazil.
- Cakmak I., Yazici A.M. Magnesium: a forgotten element in crop production. Better Crops. 2010;94:23–25. [Google Scholar]
- Chance B., Maehly A.C. Assay of catalase and peroxidases. Methods Enzymol. 1955;2:764–775. [Google Scholar]
- Chen W.R., Zheng J.S., Li Y.Q., Guo W.D. Effects of high temperature on photosynthesis, chlorophyll fluorescence, chloroplast ultrastructure, and antioxidant activities in Fingered Citron. Russ. J. Plant Physiol. 2012;59:732–740. [Google Scholar]
- Cui L., Li J., Fan Y., Xu S., Zhang Z. High temperature effects on photosynthesis, PSII functionality and antioxidant activity of two Festuca arundinacea cultivars with different heat susceptibility. Bot. Stud. 2006;47:61–69. [Google Scholar]
- Cvjetko P., Balen B., Štefanić P.P., Debogović L., Pavlica M., Klobučar G.I.V. Dynamics of heat-shock induced DNA damage and repair in senescent tobacco plants. Biol. Plant. 2014;58:71–79. [Google Scholar]
- Dwivedi R.S., Randhawa N.S. Evaluation of rapid test for hidden hunger of zinc in plants. Plant Soil. 1974;40:445–451. [Google Scholar]
- Giannopolitis C.N., Ries S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gichner T., Patková Z., Száková J., Žnidarc I., Mukherjee A. DNA damage in potato plants induced by cadmium, ethyl methanesulphonate and γ-rays. Environ. Exp. Bot. 2008;62:113–119. [Google Scholar]
- Giri J. Glycinebetaine and abiotic stress tolerance in plants. Plant Sig. Behav. 2011;6:1746–1751. doi: 10.4161/psb.6.11.17801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieve C.M., Grattan S.R. Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil. 1983;70:303–307. [Google Scholar]
- Hartwig A. Role of magnesium in genomic stability. Mut. Res. 2001;475:113–121. doi: 10.1016/s0027-5107(01)00074-4. [DOI] [PubMed] [Google Scholar]
- Heath R.L., Packer L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Hemantaranjan A., Bhanu A.N., Singh M.N., Yadav D.K., Patel P.K., Singh R., Katiyar D. Heat stress responses and thermotolerance. Adv. Plants Agric. Res. 2014;3:1–10. [Google Scholar]
- Koppen G., Toncelli L.M., Triest L., Verschaeve L. The comet assay: a tool to study alteration of DNA integrity in developing plant leaves[J] Mech. Ageing Dev. 1999;110:13–24. doi: 10.1016/s0047-6374(99)00038-x. [DOI] [PubMed] [Google Scholar]
- Lavania D., Dhingra A., Siddiqui M.H., Al-Whaibi M.H., Grover A. Current status of the production of high temperature tolerant transgenic crops for cultivation in warmer climates. Plant Physiol. Biochem. 2015;86:100–108. doi: 10.1016/j.plaphy.2014.11.019. [DOI] [PubMed] [Google Scholar]
- Lin A.J., Zhang X.H., Chen M.M., Qing C.A.O. Oxidative stress and DNA damages induced by cadmium accumulation. J. Environ. Sci. 2007;19:596–602. doi: 10.1016/s1001-0742(07)60099-0. [DOI] [PubMed] [Google Scholar]
- Lorimer G.H., Miziorko H.M. Carbamate formation on the epsilon.-amino group of a lysyl residue as the basis for the activation of ribulosebisphosphate carboxylase by CO2 and magnesium 2+ Biochemistry. 1980;19:5321–5328. doi: 10.1021/bi00564a027. [DOI] [PubMed] [Google Scholar]
- Lutts S., Kinet J.M., Bouharmont J. Changes in plant response to NaCl during development of rice (Oryza sativa L) varieties differing in salinity resistance. J. Exp. Bot. 1995;46:1843–1852. [Google Scholar]
- Marschner H. third ed. Academic Press; London: 2012. Marschner’s Mineral Nutrition of Higher Plants. [Google Scholar]
- Matysik J., Bhalu A.B., Mohanty P. Molecular mechanisms of quenching of reactive oxygen species by proline under stress in plants. Curr. Sci. 2002;82:525–532. [Google Scholar]
- Mengutay M., Ceylan Y., Kutman U.B., Cakmak I. Adequate magnesium nutrition mitigates adverse effects of heat stress on maize and wheat. Plant Soil. 2013;368:57–72. [Google Scholar]
- Mittler R., Finka A., Goloubinoff P. How do plants feel the heat? Trends Biochem. Sci. 2012;37:118–125. doi: 10.1016/j.tibs.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Neuhaus C., Geilfus C.M., Mühling K.H. Increasing root and leaf growth and yield in Mg-deficient faba beans (Vicia faba) by MgSO4 foliar fertilization. J. Plant Nutr. Soil Sci. 2014;177:741–747. [Google Scholar]
- Potters G., Horemans N., Jansen M.A.K. The cellular redox state in plant stress biology – a charging concept. Plant Physiol. Biochem. 2010;48:292–300. doi: 10.1016/j.plaphy.2009.12.007. [DOI] [PubMed] [Google Scholar]
- Rasheed R., Wahid A., Farooq M., Hussain I., Basra S.M.A. Role of proline and glycinebetaine pretreatments in improving heat tolerance of sprouting sugarcane (Saccharum sp) buds. Plant Grow Regul. 2011;65:35–45. [Google Scholar]
- Sangu E., Tibazarwa F.I., Nyomora A., Symonds R.C. Expression of genes for the biosynthesis of compatible solutes during pollen development under heat stress in tomato (Solanum lycopersicum) J. Plant Physiol. 2015;178:10–16. doi: 10.1016/j.jplph.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Senbayram M., Gransee A., Wahle V., Thiel H. Role of magnesium fertilisers in agriculture: plant–soil continuum. Crop Past. Sci. 2015;66:1219–1229. [Google Scholar]
- Seregin I.V., Kozhevnikova A.D. Physiological role of nickel and its toxic effects on higher plants. Russ. J. Plant Physiol. 2006;53:257–277. [Google Scholar]
- Siddiqui M.H., Khan M.N., Mohammad F., Khan M.M.A. Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J. Agron. Crop Sci. 2008;194:214–224. [Google Scholar]
- Siddiqui M.H., Mohammad F., Khan M.M.A., Al-Whaibi M.H. Cumulative effect of nitrogen and sulphur on Brassica juncea L genotypes under NaCl stress. Protoplasma. 2012;249:139–153. doi: 10.1007/s00709-011-0273-6. [DOI] [PubMed] [Google Scholar]
- Siddiqui M.H., Al-Khaishany M.Y., Al-Qutami M.A., Al-Whaibi M.H., Grover A., Ali H.M., Al-Wahibi M.S. Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J. Biol. Sci. 2015;22:656–663. doi: 10.1016/j.sjbs.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siripornadulsil S., Traina S., Verma D.P.S., Sayre R.T. Molecular mechanisms of proline-mediated tolerance to toxic heavy metals in transgenic microalgae. Plant Cell. 2002;14:2837–2847. doi: 10.1105/tpc.004853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G.S., Johnston C.M., Cornforth I.S. Comparison of nutrient solutions for growth of plants in sand culture. New Phytol. 1983;94:537–548. [Google Scholar]
- Stewart J.J., Demmig-Adams B., Cohu C.M., Wenzl C.A., Muller O., Adams W.W., III Growth temperature impact on leaf form and function in Arabidopsis thaliana ecotypes from northern and southern Europe. Plant Cell Environ. 2016 doi: 10.1111/pce.12720. [DOI] [PubMed] [Google Scholar]
- Usuda H. The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. 1985;91:1455–1463. [Google Scholar]
- Verslues P.E., Sharma S. Proline metabolism and its implications for plant environment interaction. Arabidopsis Book. 2010;8:e0140. doi: 10.1199/tab.0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waraich E.A., Ahmad R., Halim A., Aziz T. Alleviation of temperature stress by nutrient management in crop plants: a review. J. Soil Sci. Plant Nutr. 2012;12:221–244. [Google Scholar]