Abstract
A new isolate of the solvent-producing Clostridium acetobutylicum YM1 was used to produce butanol in batch culture fermentation. The effects of glucose concentration, butyric acid addition and C/N ratio were studied conventionally (one-factor-at-a-time). Moreover, the interactions between glucose concentration, butyric acid addition and C/N ratio were further investigated to optimize butanol production using response surface methodology (RSM). A central composite design was applied, and a polynomial regression model with a quadratic term was used to analyze the experimental data using analysis of variance (ANOVA). ANOVA revealed that the model was highly significant (p < 0.0001) and the effects of the glucose and butyric acid concentrations on butanol production were significant. The model validation experiment showed 13.82 g/L butanol was produced under optimum conditions. Scale up fermentation in optimized medium resulted in 17 g/L of butanol and 21.71 g/L of ABE. The experimental data of scale up in 5 L bioreactor and flask scale were fitted to kinetic mathematical models published in the literature to estimate the kinetic parameters of the fermentation. The models used gave the best fit for butanol production, biomass and glucose consumption for both flask scale and bioreactor scale up.
Keywords: Butanol, Fermentation, Clostridium acetobutylicum YM1, Optimization, Scale-up, Kinetics
1. Introduction
Over the past decades butanol has gained the increasing interest as an alternative and renewable biofuel due to the expected depletion of the fossil fuels, the growing demand for energy and a high fluctuation in oil prices. Butanol shows many advantageous properties as a fuel compared to ethanol. It has a high energy content, has a low vapor pressure. Furthermore, butanol is less corrosive and can be used directly or blended with gasoline. On the other hand, butanol can be shipped through the existing pipelines and can be used in existing engines without any modifications (Dürre, 2007, Dürre, 2008, Lee et al., 2008). It has been found that butanol is the most similar to gasoline in its properties compared to other biofuels such as ethanol. Therefore, butanol can be considered the most suitable candidate for the next generation biofuel (Tashiro and Sonomoto, 2010).
Butanol has been produced biologically via anaerobic acetone–butanol–ethanol (ABE) fermentation using many solvent-producing clostridia strains (Formanek et al., 1997, Qureshi and Maddox, 1992). Considerable efforts have been devoted to the improvement of the butanol production, including microbial screening, development of efficient butanol fermentation systems, the enhancement of pretreatment methods for lignocellulosic materials to use as inexpensive substrates, using of molecular tools to improve the selectivity of butanol production, improvement of recovery systems and utilization of metabolic engineering. However, butanol fermentation still has many limitations, such as its low productivity due to the effect of produced butanol accumulating on the microorganisms, the price of the substrate and the cost of recovering butanol due to the presence of byproducts such as acetone, ethanol and acids.
One of the solutions to these problems is to screen new solvent-producing isolates that can produce the higher amounts of butanol and that are able to tolerate high concentrations of solvent. In addition, the optimization of the medium and fermentation conditions for attaining the maximum production of butanol has been found as a crucial factor (Al-Shorgani et al., 2016). The ratio of carbon to nitrogen (C/N) has been recognized as one of the most important factors in biological processes. In this view, microorganisms require proper nitrogen supplementation for use in metabolism during both growth and fermentation. An optimal C/N ratio for solvent-producing Clostridium strains is vital to maximize butanol production. Butyric acid has also been used as a precursor to enhance the production of butanol by Clostridia (Al-Shorgani et al., 2012a, Tashiro et al., 2007).
Response surface methodology (RSM) is one of the most useful statistical optimization techniques in biological and chemical processes (Ba-Abbad et al., 2013, Vishwanatha et al., 2010). RSM basically consists of the central composite design, the Box–Behnken design, the one-factor design, the D-Optimal design, the user-defined design and the historical data design. The most used RSM statistical methods are the central composite design (CCD) and the Box–Behnken design (BBD). For one numeric variable, CCD has 5 levels (−α, −1, 0, +1 and +α), whereas BBD only has 3 levels (−1, 0 and +1) (Bezerra et al., 2008). Furthermore, RSM is a time-saving method for carrying out the minimal number of experiments required and for making overall data analysis easier (Bezerra et al., 2008) because it provides an experimental model that predicts the correlation and interaction between a set of experimental variables and observed results, thus it determines optimized conditions for experiments (Zheng et al., 2008).
RSMs with different designs have been successfully used for optimization in numerous bioprocesses, such as butanol production (Lin et al., 2011, Ranjan et al., 2013b), growth of propionic acid bacteria (Liew et al., 2005), lactic acid production (Dey et al., 2012), α-amylase production (Sivakumar et al., 2012), ethanol production (Ratnam et al., 2003, Wang et al., 2008), acid dye decolonization (Olya et al., 2014), fluoride ion removal in drinking water (Fakhri, 2014), wastewater treatment (Gengec et al., 2012) and biohydrogen production (Mu et al., 2006, Pan et al., 2008).
In the present study, the aim was to optimize butanol production using RSM with CCD by investigating the effects of some of the medium components such as glucose concentration, C/N ratio and butyric acid as well as their interactions on butanol production using a new isolate of solvent-producing Clostridium acetobutylicum YM1. Moreover, butanol fermentation in the optimized medium was carried out in flask scale and in a 5 L bioreactor. The mathematical models reported in the literature were applied to describe the kinetics of this fermentation.
2. Materials and methods
2.1. Microorganism
The solvent-producing strain C. acetobutylicum YM1 was used in this study. This strain was isolated from the soil samples in Malaysia and showed a high ability to produce high concentrations of butanol from the various carbon sources. This strain was then identified as C. acetobutylicum using 16S rRNA (GenBank accession No. KC969670). The strain C. acetobutylicum YM1 was stored as spores in 50% glycerol at −30 °C. The strain YM1 was then grown in tryptone-yeast extract-acetate (TYA) medium agar to form bacterial colonies. The inoculum was prepared by transferring 3 loopfuls of the fresh single pure colonies of the YM1 strain in 100 mL of TYA medium and growing bacterial cells at 30 °C overnight. The clostridial culture obtained was used as fermentation inoculum.
2.2. Fermentation conditions
TYA medium used for butanol production contained (g/L); 6 tryptone, 2 yeast extract, 3 ammonium acetate, 0.001 FeSO4·7H2O and 0.3 MgSO4. The concentrations of glucose, tryptone, yeast extract and ammonium acetate in TYA medium were varied to investigate their effects on butanol production. Fermentations were carried out in 250 mL Duran Scott bottles with a working volume of 100 mL. Bioreactor scale up fermentation was carried out in a 5 L bioreactor (INFORS HT, Swiss) with a working volume of 3 L. Anaerobic conditions were established by flushing nitrogen gas for approximately one minute before inoculation. The amount of 10 % (v/v) of fresh inoculum (20 h culture) of the strain YM1 was used. The initial pH of the fermentation medium and incubation temperature were set at 6.5 and 30 °C, respectively.
2.3. Experimental design and statistical analysis
In this study, CCD was used to determine the optimum concentrations of glucose, butyric acid and C/N ratio as pivotal nutrient variables using batch culture for the new strain of C. acetobutylicum YM1. CCD has been widely applied as a statistical method for medium optimization and for process optimization of butanol. It is a nonlinear model and is used to establish the regression model equations and operating conditions for suitable experiments (Arulkumar et al., 2011). A minimum number of experiments are required for modeling to fit a second-order model using CCD (Ahmad et al., 2009, Tanyildizi, 2011).
The number of runs in a CCD depends on the number of variables. The number of experimental runs for a complete replicate of the design is shown by Eq. (1):
| (1) |
where n is the number of variables; 2n is the number of factorial runs (coded to the usual ± notation); 2n represents the number of axial runs (±α, 0, 0,…, 0), (0, ±α, 0, 0,…, 0), …, (0, 0, …, ±α); and nc is the number of center runs (six replicates, 0, 0, 0, …, 0).
Based on a second-order quadratic model for butanol, an empirical model was developed to analyze the impact of factor interactions, as shown by Eq. (2):
| (2) |
where Y is the predicted response, b′0 is the constant coefficient, bi is the linear coefficient, bij is the interaction coefficient, bii is the quadratic coefficient, and Xi and Xj are the coded values.
2.4. Kinetic modeling analysis
Experimental data of scale up fermentation and flask scale fermentation under optimized conditions obtained after optimization by RSM-CCD were fitted to proposed models using Polymath® Version 6.1 (CACHE Corp., USA) by nonlinear regression using the least-squares method.
Butanol production was mathematically modeled using the model proposed by Mercier et al. (1992) which was used for lactic acid production, biosurfactant and biobutanol production (Ranjan et al., 2013a, Rodrigues et al., 2006) (Eq. (3)).
| (3) |
where P is butanol production (g/L), Pmax is maximum concentration of butanol (g/L), P0 is initial butanol concentration (g/L), t denotes fermentation time (h) and Pt represents kinetic constant.
Biomass production of C. acetobutylicum YM1 was defined by the model equation proposed by Rodrigues et al. (2006) (Eq. (4)). This kinetic model was used to determine the biomass production in biosurfactant production by Lactobacillus strains.
| (4) |
where X is biomass concentration (g/L), Xm denotes the maximum concentration of biomass (g/L), X0 is the initial biomass concentration (g/L), t represents cultivation time (h) and μ0 (h−1) indicates specific growth rate of biomass formation. The model parameters X0, Xm and μ0 were calculated from the series of experimental data biomass concentration/time.
Substrate consumption (glucose) by C. acetobutylicum YM1 can be represented by the Eq. (5) which was used by Mercier et al. (1992).
| (5) |
where YP/S is the product yield for butanol based on glucose concentration (g/g), P denotes the final butanol concentration (g/L), P0 indicates the initial butanol concentrations (g/L), YX/S is the biomass yield based on glucose concentration (g/g), X represents the biomass growth, X0 shows the initial biomass growth, and S0 is the initial glucose concentration (g/L).
The model parameters YP/S and S0 (g/L) were calculated from the series of experimental data glucose concentration/time.
The three mathematical models mentioned above ((3), (4), (5)) were selected to fairly describe the fermentation kinetics of butanol production, biomass growth and glucose consumption. These modeled can be used to predict ABE fermentation results. Furthermore, the mathematical models can be applied to adjust the butanol production results with statistical significance of the parameters determined.
2.5. Analytical methods
Samples were collected at appropriate times and were centrifuged at 5000×g for 5 min. Subsequently, the resulting supernatant was used for the biochemical analysis tests. Solvents (acetone, butanol and ethanol) and organic acids (butyric acid and acetic acid) were determined using a gas chromatography system (7890A GC-System, Agilent Technologies, Palo Alto, CA, USA) equipped with a flame ionization detector (FID) and a 30-m capillary column (Equity1™; 30 m × 0.32 mm × 1.0 μm film thickness; Supelco Co, Bellefonate, PA, USA). The oven temperature was set to increase from 40 °C to 130 °C at a rate of 8 °C/min. The injector and detector temperatures were set at 250 °C and 280 °C, respectively. Helium was used as the carrier gas at a flow rate of 1.5 mL/min.
The residual glucose concentration was measured using a commercial glucose oxidase kit [GOD, (E.C. 1.1.34), Roche Ltd., Swiss]. The growth of C. acetobutylicum YM1 was detected based on the optical density measured at 600 nm using a UV–vis spectrophotometer (Genesys 10, Thermo Spectronic, USA). In addition, growth was also measured gravimetrically and expressed in terms of dry cell weight (DCW) per volume of culture (g/L). The DCW determination was carried out by centrifuging the samples at 5000×g for 10 min. The supernatant was discharged and the remaining pellet was washed twice with distilled water and recentrifuged. The pellet was resuspended in distilled water and filtered with preweighted Millipore filter membrane (0.2 μm pore size) and then dried at 80 °C for 24 h in an oven. After drying, the excess moisture was removed by a desiccator. The dried cells were then weighted and measured as DCW. The filters were weighted before and after drying and DCW was calculated.
3. Results and discussion
3.1. Effect of glucose concentration on butanol production
The effect of the glucose concentration on the production of butanol by the new isolate of C. acetobutylicum YM1 is shown in Table 1. The glucose concentrations were varied from 20 to 100 g/L, and the medium used was the TYA described previously. The fermentation conditions were an initial pH of 6.5, incubation temperature of 30 °C, inoculum size of 10% and anaerobic conditions. It was observed that a rise in the glucose concentrations from 20 g/L to 50 g/L increased the production of butanol and ABE. The highest concentrations of butanol and ABE were obtained with the values of 9.48 and 12.72 g/L, respectively where 50 g/L glucose was utilized. Increasing the glucose concentration beyond 50 g/L led to a decrease in butanol production. The butanol concentration obtained from 30 g/L glucose was 2-fold higher than that produced from 20 g/L glucose, whereas 50 g/L glucose produced a 3-fold higher amount of butanol than that achieved from 20 g/L glucose (Table 1).
Table 1.
Effect of glucose concentration on butanol production using C. acetobutylicum YM1.
| Glucose concentration (g/L) | Solvent (g/L) |
Acid (g/L) |
||||
|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ABE | Acetate | Butyrate | |
| 20 | 0.90 | 3.14 | 0.04 | 4.08 | 0.04 | 0.09 |
| 30 | 2.60 | 6.81 | 0.09 | 9.50 | 0.03 | 0.17 |
| 40 | 2.70 | 8.09 | 0.14 | 10.92 | 0.02 | 0.19 |
| 50 | 3.09 | 9.48 | 0.16 | 12.72 | 0.03 | 0.35 |
| 60 | 2.75 | 8.01 | 1.15 | 11.91 | 1.3 | 0.88 |
| 70 | 2.39 | 7.03 | 0.92 | 10.34 | 1.23 | 0.88 |
| 80 | 2.40 | 7.05 | 0.90 | 10.35 | 1.3 | 0.86 |
| 90 | 2.38 | 7.05 | 0.90 | 10.33 | 1.22 | 0.77 |
| 100 | 2.15 | 6.32 | 0.77 | 9.24 | 1.41 | 0.95 |
This strain as a wild type could produce 9.48 g/L butanol and 12.72 g/L of total ABE, which demonstrated the good potential of this strain to be used in butanol fermentation. It was previously reported that wild-types of Clostridium strains were able to produce 9–12 g/L butanol in a batch culture with 40–60 g/L glucose in the medium (Chua et al., 2013, Formanek et al., 1997, Monot et al., 1982). Recently, Razak et al. (2013) reported that C. acetobutylicum ATCC 824 produced 8.17 g/L butanol from 70 g/L glucose. Based on these results, it was suggested that the optimum concentration of glucose for butanol production using the new isolate of Clostridium YM1 was 50 g/L in batch fermentation.
Increasing the concentrations of glucose and xylose over the optimal concentration was found to cause a decrease in the glucose and xylose transport system activities (Ounine et al., 1985). It has been shown that the limited carbon source in the fermentation medium produces only acids and more than 10 g/L glucose was required to shift to solvent production in ABE fermentation by Clostridium (Long et al., 1984, Fond et al., 1985).
3.2. Effect of nitrogen source on butanol production
TYA medium, which was used as the fermentation medium, contains 3 types of nitrogen sources: tryptone, yeast extract and ammonium acetate at a normal ratio of 6:2:3. The effects of the nitrogen sources at various ratios (at constant total nitrogen content) were evaluated, as shown in Table 2.
Table 2.
Effect of nitrogen sources on butanol production and growth of YM1 isolate using TYA medium.
| Nitrogen source (g/L) |
Growth (OD at 600 nm) | Residual glucose (g/L) | Final pH | Solvents (g/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Tryptone | Yeast extract | Acetate salt (3 g/L) | Acetone | Butanol | Ethanol | ABE | |||
| 6 | 2 | Ammonium | 1.369 | 0.03 | 4.89 | 1.22 | 4.35 | 0.06 | 5.62 |
| 8 | 0 | Ammonium | 1.417 | 0.06 | 4.90 | 1.48 | 4.21 | 0.06 | 5.75 |
| 0 | 8 | Ammonium | 1.494 | 1.94 | 4.70 | 0.74 | 2.60 | 0.03 | 3.37 |
| 6 | 2 | Sodium | 1.437 | 0.24 | 4.70 | 1.04 | 3.47 | 0.04 | 4.55 |
| 4 | 4 | Ammonium | 1.652 | 0.09 | 4.81 | 1.06 | 3.55 | 0.04 | 4.66 |
| 2 | 6 | Ammonium | 1.520 | 3.61 | 4.66 | 0.64 | 2.61 | 0.03 | 3.28 |
As cab be found, in the absence of yeast extract and in the presence of 8 g/L tryptone, butanol production was slightly decreased to 4.21 g/L compared to the butanol production (4.35 g/L) obtained from the normal ratio of nitrogen sources in TYA medium. In addition, the growth of the bacteria was enhanced in the absence of yeast extract. Replacing the tryptone with yeast extract (maintaining the same nitrogen content) enhanced the growth of bacteria, but the production of butanol and ABE decreased significantly compared to the normal nitrogen ratio in TYA, with the concentrations of 2.6 vs. 4.35 g/L, respectively. The highest growth rate obtained was 1.652 OD at 600 nm when the concentrations of yeast extract and tryptone were similar (4 g/L for each), whereas butanol production was decreased to 3.55 g/L (Table 2).
In the presence of 2 g/L tryptone and 6 g/L yeast extract, butanol production was decreased to 2.61 g/L, which was a similar concentration to that produced from a medium containing only 8 g/L yeast extract (Table 2). Replacing ammonium acetate with sodium acetate reduced butanol production to 3.47 g/L, compared to 4.35 g/L from ammonium acetate, which indicated the importance of ammonium acetate as both a nitrogen source and as an acetate source. Ammonium acetate has been reported to be an essential component in the medium for the growth of C. acetobutylicum and ABE production (Ladisch, 1991).
Gu et al. (2009) concluded that the supplementation of cassava medium with ammonium acetate produced high amounts of acetic acid and butyric acid, and the released acids were then reutilized by C. acetobutylicum EA 2018 to produce higher concentrations of acetone and butanol compared to non-supplemented cassava medium.
These results indicated that tryptone was important and it should be present in higher concentrations than yeast extract to obtain high concentrations of butanol. The results also showed that the best tryptone, yeast extract and ammonium acetate ratio was 6:2:3 to achieve maximum butanol production.
3.3. Effect of C/N ratio on butanol production
In fermentation medium, the C/N ratio has a key role in butanol production using solvent-producing Clostridium. In this investigation, the C/N ratio of the fermentation medium was varied from 5 to 120 using nitrogen sources, namely tryptone, yeast extract and ammonium acetate in a ratio of 6:2:3.
The aforementioned results indicated that a glucose concentration of 50 g/L and a nitrogen source with a ratio of 6:2:3 were optimal for the production of butanol. Thus, in this part of the experiment, the C/N ratio was varied from 5 to 120 while the concentration of glucose was maintained constant at 50 g/L and the ratio of nitrogen was maintained as 6 tryptone, 2 yeast extract and 3 ammonium acetate.
Table 3 shows the effects of various C/N ratios on the production of butanol, acetone, ethanol and acids, as well as on bacterial growth.
Table 3.
Effect of C/N ratio on butanol production in batch culture of C. acetobutylicum YM1.
| C:N ratio | Growth DCW (g/L) | Residual glucose (g/L) | Final pH | Solvent production (g/L) |
Acid production (g/L) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ABE | Acetic | Butyric | T. Acids | ||||
| 5.00 | 1.65 | 10.36 | 5.20 | 2.21 | 5.20 | 0.67 | 8.09 | 2.70 | 0.38 | 3.08 |
| 10.00 | 1.39 | 3.79 | 5.27 | 3.27 | 9.05 | 1.65 | 13.98 | 1.23 | 0.22 | 1.45 |
| 12.80 | 1.34 | 6.24 | 5.15 | 2.61 | 9.21 | 1.70 | 13.52 | 0.86 | 0.02 | 0.87 |
| 25.00 | 1.20 | 8.82 | 6.67 | 1.59 | 7.38 | 0.90 | 9.88 | 0.92 | 0.46 | 1.38 |
| 30.00 | 1.17 | 14.36 | 4.51 | 1.40 | 6.84 | 0.73 | 8.98 | 1.00 | 0.65 | 1.65 |
| 45.00 | 1.12 | 14.21 | 4.38 | 1.31 | 6.24 | 0.63 | 8.18 | 0.93 | 0.69 | 1.62 |
| 65.00 | 1.07 | 18.30 | 4.20 | 1.32 | 6.61 | 0.65 | 8.59 | 0.86 | 0.67 | 1.52 |
| 75.00 | 1.05 | 16.15 | 4.12 | 1.25 | 6.08 | 0.57 | 7.91 | 0.76 | 0.67 | 1.44 |
| 90.00 | 1.06 | 17.12 | 4.11 | 1.24 | 6.01 | 0.56 | 7.81 | 0.75 | 0.63 | 1.38 |
| 120.00 | 1.06 | 17.91 | 4.08 | 1.17 | 5.55 | 0.51 | 7.23 | 0.68 | 0.60 | 1.28 |
In terms of butanol production, a C/N ratio of 12.8 was optimal, in which 9.21 g/L butanol was produced. Increasing the C/N ratio resulted in a gradual decrease in butanol and total solvent production. Lower C/N ratios resulted in the lower butanol and ABE production, whereas the highest acetic acid concentration (2.7 g/L) was produced at a C/N ratio of 5. Growth was found to be inversely related to the C/N ratios. Excess nitrogen enhanced the growth of the culture but negatively affected butanol and solvent production. The highest concentration of ABE (13.98 g/L) was obtained when a C/N ratio of 10 was employed, which also produced the highest concentration of acetone (3.27 g/L). These results showed that moderate C/N ratios were optimal for butanol and ABE production and the excess or low nitrogen did not increase butanol or ABE production compared to a C/N ratio of 12.8.
The butanol concentration was found to be reduced drastically when the C/N ratio was greater than 12.8 in the fermentation medium. When the C/N ratio was 5, very low butanol production was obtained (5.2 g/L), which was also similar to that obtained from the highest C/N ratio of 120.
A previous study using sago starch as the carbon source for C. acetobutylicum P262 showed that increasing the C/N ratio while maintaining the starch concentration constant at 50 g/L led to a linear decrease in ABE production. In contrast, when the nitrogen concentration was fixed, the elevated C/N ratio led to an increase in ABE production up to a C/N ratio of 20, followed by a decrease in solvent production above a C/N ratio of 20 (Madihah et al., 2001). Thus, to maximize butanol production, an optimal C/N ratio of 12.8 should be applied in the butanol fermentation medium.
3.4. Effect of butyric acid addition on butanol production
Butyric acid is a precursor for butanol production (Al-Shorgani et al., 2012a, Al-Shorgani et al., 2012b, Song et al., 2010, Tashiro et al., 2007). Various concentrations of butyric acid, from 0.5 to 10 g/L, were added to TYA medium containing 30 g/L glucose to determine whether the addition of butyric acid could improve butanol production.
The results showed that the increment of the amount of butyric acid added linearly increased the production of butanol and ABE (Table 4). Butanol production was enhanced significantly in the presence of butyric acid compared to a culture without butyric acid addition. The residual butyric acid analysis showed that butyric acid was utilized by the culture efficiently. The highest butanol production was 8.74 g/L when the culture of the strain YM1 was fed 10 g/L butyric acid. In this view, Bramono et al. (2011) reported that Clostridium BOH3 could produce 5.87 g/L butanol when the culture was supplemented with butyric acid (0.7 g/L) and 30 g/L glucose. However, our study observed higher butanol production compared to that reported by Bramono et al. (2011) (Table 4).
Table 4.
Effect of butyric acid on butanol production using C. acetobutylicumYM1 isolate.
| Initial butyric acid (g/L) | Solvent production (g/L) |
Residual glucose (g/L) | Residual butyric acid (g/L) | |||
|---|---|---|---|---|---|---|
| Acetone | Butanol | Ethanol | ABE | |||
| 0 | 1.94 | 5.93 | 0.08 | 7.94 | 0.13 | 0.90 |
| 0.5 | 1.82 | 6.41 | 0.08 | 8.31 | 0.12 | 1.90 |
| 1 | 1.91 | 6.56 | 0.08 | 8.56 | 0.15 | 2.46 |
| 2 | 1.82 | 6.86 | 0.08 | 8.76 | 2.39 | 1.64 |
| 3 | 1.93 | 7.29 | 0.08 | 9.30 | 0.21 | 1.81 |
| 4 | 1.84 | 7.57 | 0.08 | 9.48 | 0.88 | 2.34 |
| 5 | 1.91 | 8.02 | 0.09 | 10.02 | 0.00 | 2.13 |
| 10 | 1.94 | 8.74 | 0.09 | 10.77 | 1.52 | 3.84 |
| 12.5 | 1.92 | 8.06 | 0.07 | 10.05 | 2.20 | 5.23 |
Only negligible amounts of ethanol were produced from all fermentation experiments (0.08–0.09 g/L), and the acetone concentration remained approximately constant (1.82–1.94 g/L) even with the increases in the amount of butyric acid added. In contrast, it was previously reported that different combinations of glucose and butyric acid are responsible for influencing the ratio of ABE (Al-Shorgani et al., 2012a, Shinto et al., 2007).
As shown in Table 4, an increment in the butyric acid concentration increased the butanol production, whereas the acetone concentration remained nearly constant. These results may be attributed to the pathway of butyric acid conversion to butanol.
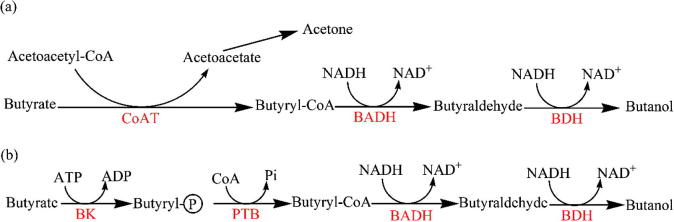
In this regard, butyrate can be converted to butanol through the CoA transferase pathway or through the butyrate kinase pathway (Fig. 1). In the CoA transferase pathway, acetone and butanol are the products (Fig. 1a). In the butyrate kinase pathway, only butanol is produced with no acetone formation. Our results showed that no effect on acetone concentration was observed when butyric acid was added (Table 4). This suggested that butanol production from butyric acid using strain YM1 was produced through the CoA transferase pathway (Fig. 1b). The production of butanol as a major product is preferred in butanol production; therefore, a decrease in the amount of byproduct produced would result in a decrease in the cost of recovery.
Figure 1.
Conversion of butyrate to butanol via two suggested mechanisms: (a) CoAT pathway and (b) butyrate kinase pathway. CoAT; CoA transferase, BADH; butylaldehyde dehydrogenase, BDH; butanol dehydrogenase, BK; butyrate kinase, PTB; phosphotransbutyrylase.
3.5. Response surface methodology (RSM)
Based on the above results, glucose concentration, butyric acid concentration and C/N ratio had significant effects on butanol production in the batch culture of the C. acetobutylicum strain YM1. As explained previously, the optimal values of glucose concentration, butyric acid concentration and C/N ratio were 50 g/L, 10 g/L and 12.8, respectively.
The interaction between these three factors (glucose concentration, butyric acid concentration and C/N ratio) was investigated using RSM with a central composite design (CCD) to optimize butanol production. The nitrogen sources were similar to those found in TYA medium (yeast extract, tryptone and ammonium acetate), and the nitrogen sources ratio was also maintained constant (3:6:2), with only the C/N ratio being varied. The levels of each variable are listed in Table 5.
Table 5.
Experimental design matrix using RSM with CCD and responses for butanol production.
| Run No. | Variables |
Butanol (g/L) |
|||
|---|---|---|---|---|---|
| Glucose (g/L) | C/N ratio | Butyric acid (g/L) | Actual value | Predicted value | |
| 1 | 20.0 | 10.0 | 0.0 | 4.85 | 4.37 |
| 2 | 80.0 | 10.0 | 0.0 | 10.66 | 11.03 |
| 3 | 20.0 | 120.0 | 0.0 | 3.01 | 2.67 |
| 4 | 80.0 | 120.0 | 0.0 | 11.11 | 11.15 |
| 5 | 20.0 | 10.0 | 10.0 | 7.25 | 7.02 |
| 6 | 80.0 | 10.0 | 10.0 | 8.94 | 9.73 |
| 7 | 20.0 | 120.0 | 10.0 | 6.64 | 6.71 |
| 8 | 80.0 | 120.0 | 10.0 | 10.95 | 11.24 |
| 9 | 5.0 | 00.0 | 5.0 | 0.90 | 1.55 |
| 10 | 100.5 | 65.0 | 5.0 | 11.50 | 10.69 |
| 11 | 50.0 | 5.0 | 5.0 | 11.18 | 10.07 |
| 12 | 50.0 | 157.5 | 5.0 | 9.00 | 8.97 |
| 13 | 50.0 | 65.0 | 0.0 | 9.23 | 9.56 |
| 14 | 50.0 | 65.0 | 13.4 | 10.41 | 10.55 |
| 15 | 50.0 | 65.0 | 5.0 | 10.89 | 10.72 |
| 16 | 50.0 | 65.0 | 10.0 | 12.16 | 10.94 |
| 17 | 50.0 | 65.0 | 5.0 | 10.64 | 10.72 |
| 18 | 50.0 | 65.0 | 5.0 | 10.53 | 10.72 |
| 19 | 50.0 | 65.0 | 5.0 | 9.90 | 10.72 |
| 20 | 50.0 | 65.0 | 5.0 | 10.08 | 10.72 |
3.6. Development of regression model analysis
In the present study, the software Design-Expert version 8.0 (DOE, Stat Ease, USA) was applied for performing CCD. The RSM was conducted using three independent variables that affected butanol production, namely glucose concentration (g/L), C/N ratio and butyric acid concentration (g/L). CCD analysis gave a total of 20 experiments that would be required to evaluate the coefficients of each model using linear regression analysis.
Table 5 shows the CCD design, the levels of each variable and the butanol production as the response.
Quadratic regression analysis using ANOVA was performed to estimate the significance of model coefficients. The significance of each coefficient was indicated by the p values, which also reflect the interaction strength between each independent variable.
The model in terms of actual variables and butanol production as the predicted response was as follows:
The ANOVA and the model regression of coefficients are listed in Table 6. The model was highly significant, and the R2 value was 0.96, which indicates a good correlation between the actual results and the predicted values of the response by the model (Weisberg, 2005). The R2 value of 0.96 indicated that the model could explain 96% of the variable content that positively affected the response, and only 4 % of the total variations were not explained by the model. The adjusted determination coefficient value (adj. R2 = 0.93) was within reasonable agreement of the predicted R2 of 0.96, and it also indicated the significance of the model.
Table 6.
ANOVA for response surface quadratic model for butanol production.
| Source | Sum of squares | DF | Mean square | F value | Prob > F |
|---|---|---|---|---|---|
| Model | 165.5974 | 9 | 18.39971 | 28.96829 | <0.0001 |
| A: glucose | 91.14471 | 1 | 91.14471 | 143.4972 | <0.0001 |
| B: C/N ratio | 0.026234 | 1 | 0.026234 | 0.041302 | 0.8430 |
| C: butyric acid | 5.238802 | 1 | 5.238802 | 8.247908 | 0.0166 |
| A2 | 32.26561 | 1 | 32.26561 | 50.7986 | <0.0001 |
| B2 | 2.983714 | 1 | 2.983714 | 4.697524 | 0.0554 |
| C2 | 1.864879 | 1 | 1.864879 | 2.936044 | 0.1174 |
| AB | 1.966217 | 1 | 1.966217 | 3.09559 | 0.1090 |
| AC | 7.808876 | 1 | 7.808876 | 12.2942 | 0.0057 |
| BC | 0.972474 | 1 | 0.972474 | 1.531052 | 0.2442 |
| Residual | 6.351673 | 10 | 0.635167 | ||
| Lack of fit | 5.680897 | 6 | 0.946816 | 5.64609 | 0.0579 |
| Pure error | 0.670776 | 4 | 0.167694 | ||
| Cor total | 171.9491 | 19 | |||
| Std. dev. = 0.796 | Mean = 8.99 | Adequate precision | 17.196 | ||
| R2 = 0.96 | Adj R2 = 0.93 | ||||
These results showed that the quadratic terms of the glucose concentration and butyric acid concentration had a significant effect on the production of butanol (p < 0.05).
ANOVA was performed to test the significance of the fit of the second-order polynomial equation to the actual values, as shown in Table 6. The ANOVA demonstrated that the fitting model was highly significant (p < 0.0001), whereas the lack of fit was not significant (p > 0.0579), which suggested that this model accurately represented the data in the experimental region and implied that the model equation could properly illustrate the effect of glucose concentration, C/N ratio and butyric acid concentration on the production of butanol using the new isolate of C. acetobutylicum YM1.
Adequate precision value evaluates the signal-to-noise ratio, and a value greater than 4 is desirable in supporting the fitness of the quadratic model. According to the results in Table 6, the adequate precision value of 17.2 indicated an adequate signal-to-noise ratio, which supported the fitness of the model.
Butanol production varied significantly from 0.9 to 12.16 g/L when the concentrations of glucose, butyric acid and C/N ratio were changed, as shown in Table 5. ANOVA of the model also showed that the quadratic effect of glucose concentration and butyric acid on butanol production was highly significant (p < 0.01), showing that these variables had a considerable effect on butanol production. However, the quadratic effect of the C/N ratio was not significant (p > 0.05), indicating that this factor had little effect on butanol production. Additionally, the interaction between glucose concentration and butyric acid concentration on butanol production was significant (p = 0.0057), whereas the interaction effect between the glucose concentration and C/N ratio (p = 0.109) and butyric acid concentration and C/N ratio (p = 0.244) were not significant as estimated by the quadratic model effect.
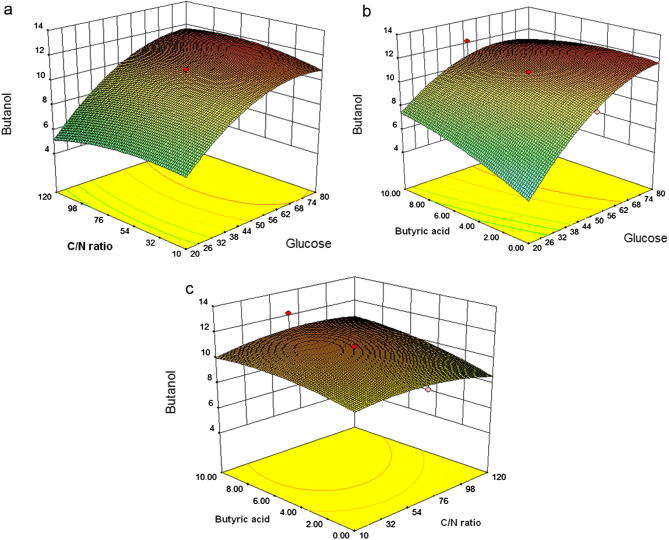
Three-dimensional plots (3D) of response surfaces were constructed based on the model equation (Eq. (3)) to investigate the interaction among factors and to verify the optimum concentration of each variable for maximum butanol production by C. acetobutylicum YM1. The response surfaces plots are shown in Fig. 2, which explain the interaction between the variables based on the final model equation. In these plots, one factor is constant at the optimum level, whereas the other two factors are varying within their experimental range.
Figure 2.
Response surface plot of butanol production from C. acetobutylicum YM1 showing the interaction between glucose concentration and C/N ratio (a), glucose concentration and butyric acid concentration (b), and butyric acid concentration and C/N ratio (c).
Fig. 2a displays the effects of varying the glucose concentration and C/N ratio on butanol production. It was found that the maximum butanol production was at a glucose concentration of 50 g/L and a C/N ratio of 65. Increasing the glucose concentration led to a continuous increase in butanol production, whereas the C/N ratio did not have any significant effect on butanol production.
Fig. 2b shows the conjugate effects of glucose and butyric acid on butanol production. The surface plot shows that when the C/N ratio is constant at 65, the maximum butanol was obtained at a glucose concentration of 50 g/L and a butyric acid concentration of 5 g/L.
Fig. 2c presents the effects of butyric acid and the C/N ratio on butanol production. The 3D plot shows that there is no significant interaction between butyric acid and C/N ratio, as shown in Table 6.
3.7. Validation of the model with the optimized conditions
The statistical model was verified in regards to butanol production by applying the optimized medium composition, as shown in Table 7. The highest butanol production obtained was 13.82 g/L from a medium containing 50 g/L of glucose, a C/N ratio of 65 and butyric acid of 8.7 g/L concentration. This result showed a good agreement with the predicted value of butanol production (12.16 g/L) and experimental butanol production (13.82 g/L), which verifies the model validity.
Table 7.
Model validation and effect of C/N ratio on butanol production under optimized conditions.
| Run No. | Variables |
Response (g/L) |
Growth (OD600 nm) | ABE ratio | |||||
|---|---|---|---|---|---|---|---|---|---|
| Glucose (g/L) | C:N ratio | Butyric acid (g/L) | Butanol | Acetone | Ethanol | ABE | |||
| 1 | 50 | 65 | 8.7 | 13.82 | 2.67 | 0.90 | 17.40 | 1.299 | 2.97:15.36:1 |
| 2 | 50 | 65 | 10 | 13.16 | 2.09 | 0.75 | 15.99 | 1.301 | 2.79:17.55:1 |
| 3 | 50 | 25 | 10 | 9.61 | 1.14 | 1.97 | 12.72 | 1.670 | 0.58:4.87:1 |
| 4 | 50 | 53 | 10 | 9.14 | 1.65 | 1.51 | 12.30 | 1.312 | 1.10:6.07:1 |
| 5 | 50 | 65 | 10 | 13.16 | 2.09 | 0.75 | 15.99 | 1.301 | 2.79:17.55:1 |
The optimal medium composition for maximum butanol production was found to be as follows: 50 g/L glucose, C/N ratio of 65 (tryptone 1.2 g/L, yeast extract 0.45 g/L and ammonium acetate 0.55 g/L) and 8.7 g/L butyric acid. Using the above optimized medium, the model predicted that the maximum butanol production that can be obtained is 12.16 g/L. These results demonstrated that RSM with CCD can be a useful method for improving butanol production through the optimization of the medium composition. The low residual values indicated a good model, which fitted well to experimental data (Bezerra et al., 2008).
The butanol production was 9.48 g/L from non-optimized medium, whereas a 31.3% increase in production was attained from optimized medium. These results showed the effectiveness of the quadratic model in this study.
In ABE fermentation with Clostridium, the normal ratio of acetone, butanol and ethanol is 3:6:1 (Formanek et al., 1997, Jones and Woods, 1986). In this study, it was demonstrated that under optimized conditions using strain YM1, the production of butanol over acetone and ethanol was favored, and the ABE ratio was 2.97: 15.36: 1, as shown in Table 7, run 1. The production of butanol as the major product is favorable due to the lower recovery cost. The change in the ABE ratio may be due to the presence of butyric acid in the medium, as reported previously (Al-Shorgani et al., 2012a, Martin et al., 1983).
Table 3 shows that the C/N ratio had an effect on butanol production and that the optimal C/N ratio for butanol production was 12.8. In contrast, RSM revealed that the C/N ratio was not significant in regards to butanol production in the presence of butyric acid. For further confirmation, three different C/N ratios were used to verify their effect while the glucose and butyric acid concentrations were constant at their optimal points (50 g/L and 10 g/L, respectively) (Table 7, runs No. 3, 4 and 5). The data showed that butanol was not highly produced when compared to the optimized conditions. The low C/N ratio (high nitrogen content) was not shown to support butanol production in the presence of butyric acid, which was not in agreement with the results reported in Table 3. These results are in agreement with those reported by Madihah et al. (2001), who stated that there was no clear relationship between the C/N ratio and butanol production. However, high C/N ratios (low nitrogen content) in the presence of butyric acid did not support cell growth when compared to low C/N ratios (Table 7). Butanol production was independent of cell growth, as reported by Al-Shorgani et al. (2012a). They found that butanol could be produced in significant amounts when washed cells of Clostridium saccharoperbutylacetonicum N1-4 were used in limited nutrient medium containing butyric acid and glucose with no growth observed (Al-Shorgani et al., 2012a). It was also reported that butanol and ABE production by C. acetobutylicum occurred only under nitrogen-limitation conditions (Roos et al., 1985). It was observed that feeding fermentation medium with butyrate did not stimulate ABE production in glucose-limited batch cultures (Al-Shorgani et al., 2012a) or continuous cultures (Jöbses and Roels, 1983).
Alvarado-Cuevas et al. (2013) reported that the selection of an adequate nitrogen source in hydrogen production is necessary to enhance the utilization of the carbon source and subsequently produce high contents of hydrogen.
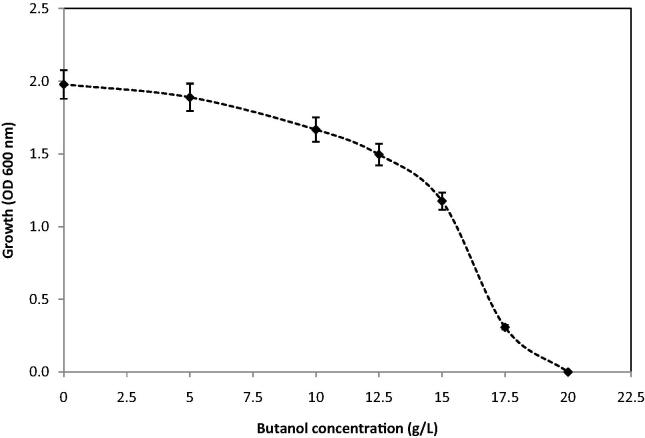
Based on butanol production by other wild-type solvent-producing Clostridia, the results presented in this study indicated that similar butanol production levels were achieved by wild-type C. acetobutylicum YM1. The limited butanol production of 13.8 g/L was due to the inhibitory effect of butanol on the culture (Fig. 3). It was reported that butanol toxicity directly controlled the capability of Clostridium in butanol production (Chen et al., 2012).
Figure 3.
Effect of butanol on growth of C. acetobutylicum YM1.
3.8. Scale up and kinetic modeling analysis
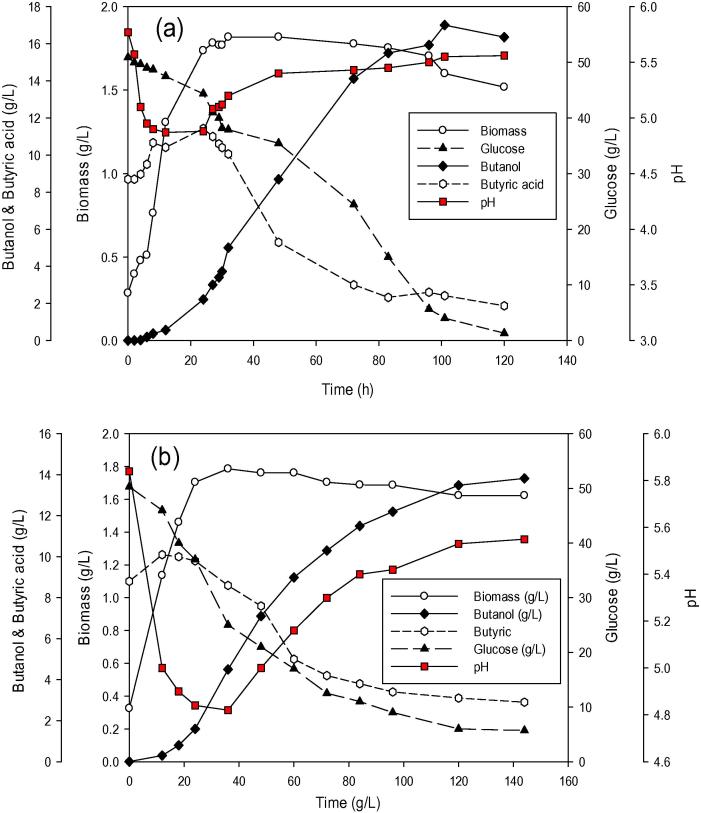
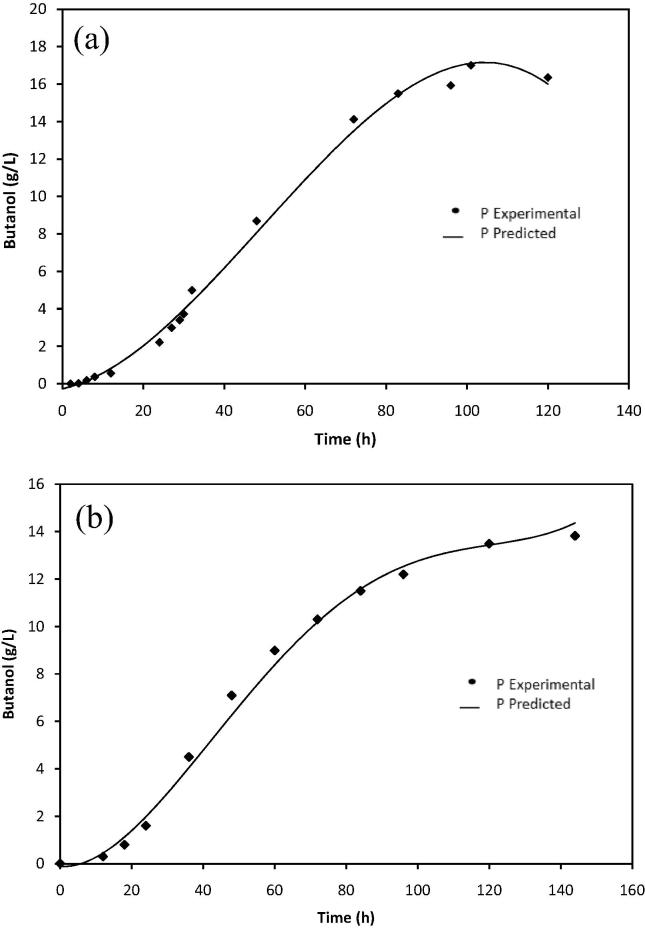
Scale up fermentation was carried out under optimized conditions using 5L fermentor with a working volume of 3L. The scale up fermentation resulted in 17 g/L butanol with total ABE of 21.71 g/L. The butanol and ABE yield were 0.363 and 0.463 g/g, respectively. Butanol and ABE productivity were 0.168 and 0.215 g/L h, respectively. Fig. 4a shows a time course of fermentation in scale up fermentor in optimized fermentation medium. The total ABE and butanol concentrations produced by scale up in 5L bioreactor were higher than that produced by flask scale fermentation using same optimized fermentation medium and under same conditions (30 °C, 10 % inoculum and initial pH at 6) as shown in Fig. 4.
Figure 4.
Butanol fermentation in optimized fermentation medium by batch culture of C. acetobutylicum YM1; (a) scale up fermentor experiment and (b) flask scale experiment.
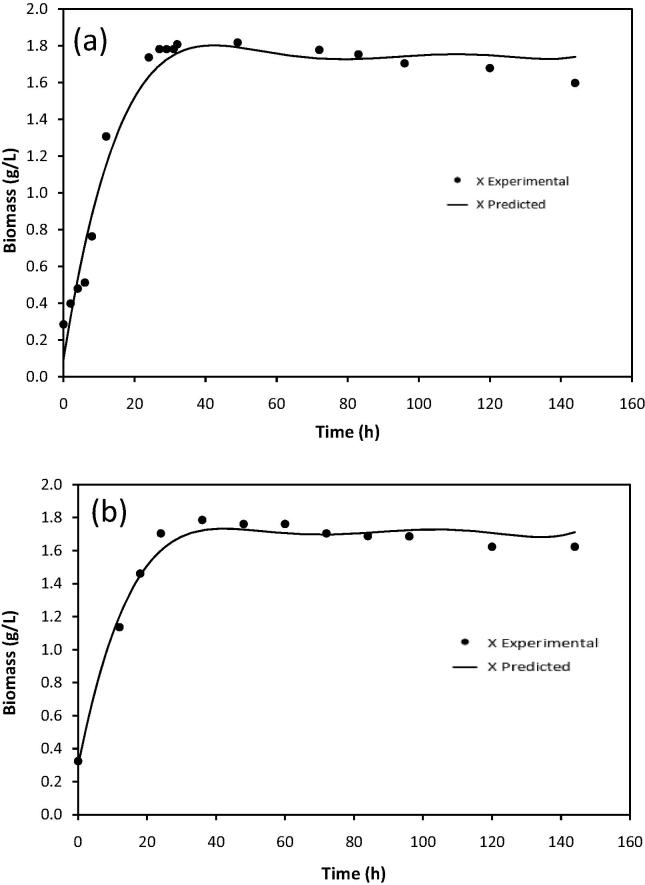
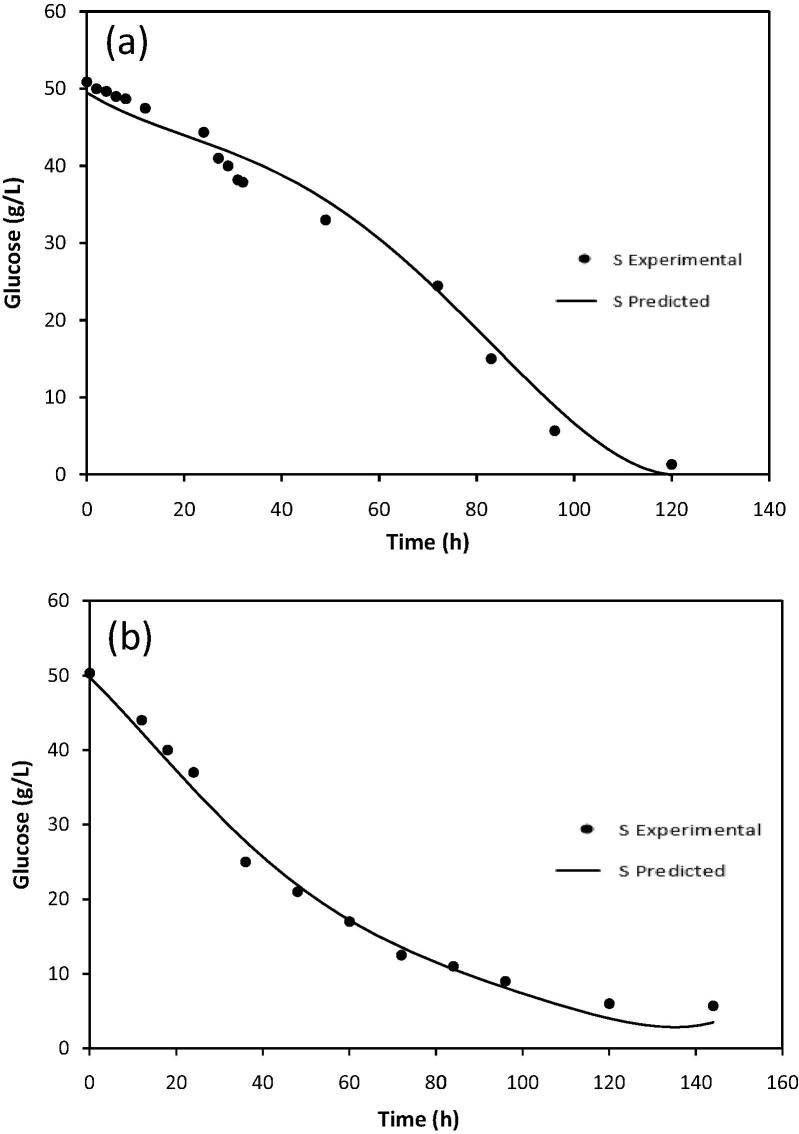
Kinetic constants of fermentation under optimized conditions for both flask scale experiment and scale up fermentation experiment in 5L bioreactor were described using Monod kinetic models reported in previous studies (Don and Shoparwe, 2010, Mercier et al., 1992, Rodrigues et al., 2006) as essentially unstructured logistic models originally to estimate the kinetics of biomass, product formation and substrate consumption. The models were fitted to the experimental data of biomass, glucose utilization and butanol production. The kinetic models of fermentation process were calculated using Polymath® Version 6.1 based on kinetic parameters and the experimental data were fitted. Table 6 shows the estimated values of the kinetic parameters which were obtained from applying models for both 5L bioreactor scale up and flask scale experiments.
Figure 5, Figure 6, Figure 7 show the experimental data along with predicted data profiles of butanol formation, biomass production and substrate consumption for butanol fermentation for both 5L bioreactor scale and flask scale. The kinetic models gave the best fit for butanol production, biomass production and substrate consumption in scale up fermentation in 5L bioreactor with regression coefficients (R2) of 0.999, 0.975 and 0.999, respectively. Similarly, the tested models also fit in flask scale fermentation for butanol production, biomass production and substrate utilization with R2 of 0.988, 0.981 and 0.999, respectively. In all tested parameters the R2 values were greater than 0.9 which indicate that the model prediction is fit with the experimental data.
Figure 5.
Fitting of the experimental data to the model describing butanol production over time in optimized fermentation medium; (a) bioreactor scale up experiment and (b) flask scale experiment.
Figure 6.
Fitting of the experimental data to the model describing biomass production over time in optimized fermentation medium; (a) bioreactor scale up experiment and (b) flask scale experiment.
Figure 7.
Fitting of the experimental data to the model describing glucose consumption over time in optimized fermentation medium; (a) bioreactor scale up experiment and (b) flask scale experiment.
Mean squares error (MSE) was used to evaluate the profiles from models simulation and experimental. This performance criterion was selected because they are easy to identify and have convenient mathematical properties (Kleijnen and Sargent, 2000). The MSE value can be calculated by Eq. (6).
| (6) |
where fi is the predicted data by the model, yi is the experimental data and nt is the length of actual data period.
In this study the MSE value for butanol production, biomass and substrate concentration were 0.0413, 0.0218 and 0.0456, respectively for scale up fermentation whereas the MSE value for flask scale fermentation were 0.1601, 0.0156 and 0.0037, respectively. Lower values of the MSE are also another indicator for goodness of the model fitting as presented in Table 8. Small MSE value of the model indicates that the data can be represented more accurately than the models with larger MSE value (Don and Shoparwe, 2010). The values of variance (σ) for both mode fermentations during the time course of fermentation also estimated between the predicted and experimental data. The σ values were very low as listed in Table 8 which indicates the models proposed adequately describe the fermentation process and it is valid to be used to represent the batch fermentation of butanol by C. acetobutylicum YM1. The predicted values of maximum butanol production (Pm) by the model for both scale up in bioreactor (16.92 g/L) and flask scale (13.80 g/L) agree well with the experimental values of 17.005 and 13.822 g/L, respectively.
Table 8.
Kinetic models applied for estimation of kinetic parameters and kinetic constants values for butanol production, biomass growth and substrate consumption for scale-up fermentation by 5L bioreactor and flask scale fermentation.
| Parameter estimation | Fermentation process |
Model | |
|---|---|---|---|
| Bioreactor scale up | Flask scale | ||
| Butanol production | |||
| P0 (g/L) | 0.001 | 0.009 | |
| Pmax (g/L) | 17.005 | 13.822 | |
| Pt | 0.0754 | 0.068 | |
| R2 (regression coefficient) | 0.999 | 0.988 | |
| δ (variance) | 0.0353 | 0.412 | |
| MSE | 0.0413 | 0.1601 | |
| Biomass | |||
| X0 | 0.205 | 0.303 | |
| Xm | 1.742 | 1.710 | |
| μ0 | 0.241 | 0.189 | |
| R2 (regression coefficient) | 0.975 | 0.981 | |
| δ (variance) | 0.0098 | 0.0039 | |
| MSE | 0.0218 | 0.0156 | |
| Glucose consumption | |||
| S0 | 50.80 | 50.30 | |
| YP/S | 0.363 | 0.310 | |
| R2 (regression coefficient) | 0.999 | 0.999 | |
| δ (variance) | 0.06826 | 0.02032 | |
| MSE | 0.04563 | 0.00378 | |
Mean squares error (MSE).
The specific growth rate (μ) of C. acetobutylicum YM1 cultivated in optimized medium in both bioreactor scale up and flask scale was similar as 0.06908 and 0.06909 h−1, respectively. This observation indicates that the effect of scale of fermentation on the specific growth rate becomes insignificant when optimized fermentation medium was used and similar observation was reported by Ranjan et al. (2013a).
The results of kinetics showed that the used mathematical models satisfactorily estimated the butanol production, biomass production and substrate consumption in butanol fermentation by C. acetobutylicum YM1 in optimized medium in two scales of batch fermentation; flask scale experiment and 5L bioreactor scale up. The models used also described accurately the behavior of the fermentation process and it can be used for prediction, design and economic estimation of butanol fermentation processes.
4. Conclusions
The results showed that glucose, butyric acid and the C/N ratio are important factors in the production of butanol by C. acetobutylicum YM1. Optimization of butanol production by manipulating the C/N ratio and glucose and butyric acid concentrations was successfully achieved using RSM. The quadratic model shows that glucose and butyric acid were highly significant in regards to butanol production compared to the C/N ratio. The optimum conditions to maximize butanol production are as follows: glucose concentration of 50 g/L, butyric acid concentration of 8.7 g/L and C/N ratio of 65. Statistical model validation by applying the optimized conditions resulted in 13.87 g/L butanol, which is in agreement with the predicted value and showed the validity and strength of this model.
Acknowledgments
This research was supported by grants “Dana Impak Perdana UKM” under code UKM-DIP-2012-30, DLP-2013-023 and “Dana Lonjakan Penerbitan UKM” under code UKM-DLP-2012-007.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Najeeb Kaid Nasser Al-Shorgani, Email: nag_nas2001@yahoo.com.
Hafiza Shukor, Email: ieza_72@yahoo.com.
Mohd Sahaid Kalil, Email: sahaid@eng.ukm.my.
Wan Mohtar Wan Yusoff, Email: wantar@ukm.edu.my.
Aidil Abdul Hamid, Email: aidilmikrob@gmail.com.
References
- Ahmad A.A., Hameed B.H., Ahmad A.L. Removal of disperse dye from aqueous solution using waste-derived activated carbon: optimization study. J. Hazard. Mater. 2009;170(2–3):612–619. doi: 10.1016/j.jhazmat.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Al-Shorgani N.K.N., Ali E., Kalil M.S., Yusoff W.M.W. Bioconversion of butyric acid to butanol by Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) in a limited nutrient medium. BioEnergy Res. 2012;5(2):287–293. [Google Scholar]
- Al-Shorgani N.K.N., Kalil M.S., Ali E., Yusoff W.M.W., Hamid A.A. Enhancement of biobutanol production by butyric acid addition using Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) Biotechnology. 2012;11(6):326–332. [Google Scholar]
- Al-Shorgani N.K.N., Isa M.H.M., Yusoff W.M.W., Kalil M.S., Hamid A.A. Isolation of a Clostridium acetobutylicum strain and characterization of its fermentation performance on agricultural wastes. Renewable Energy. 2016;86:459–465. [Google Scholar]
- Alvarado-Cuevas Z.D., Acevedo L.G.O., Salas J.T.O., De León-Rodríguez A. Nitrogen sources impact hydrogen production by Escherichia coli using cheese whey as substrate. New Biotechnol. 2013;30(6):585–590. doi: 10.1016/j.nbt.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Arulkumar M., Sathishkumar P., Palvannan T. Optimization of Orange G dye adsorption by activated carbon of Thespesia populnea pods using response surface methodology. J. Hazard. Mater. 2011;186(1):827–834. doi: 10.1016/j.jhazmat.2010.11.067. [DOI] [PubMed] [Google Scholar]
- Ba-Abbad M.M., Kadhum A.A.H., Bakar Mohamad A., Takriff M.S., Sopian K. The effect of process parameters on the size of ZnO nanoparticles synthesized via the sol–gel technique. J. Alloys Compd. 2013;550:63–70. [Google Scholar]
- Bezerra M.A., Santelli R.E., Oliveira E.P., Villar L.S., Escaleira L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta. 2008;76(5):965–977. doi: 10.1016/j.talanta.2008.05.019. [DOI] [PubMed] [Google Scholar]
- Bramono S.E., Lam Y.S., Ong S.L., He J. A mesophilic Clostridium species that produces butanol from monosaccharides and hydrogen from polysaccharides. Bioresour. Technol. 2011;102(20):9558–9563. doi: 10.1016/j.biortech.2011.07.077. [DOI] [PubMed] [Google Scholar]
- Chen B.-Y., Chuang F.-Y., Lin C.-L., Chang J.-S. Deciphering butanol inhibition to Clostridial species in acclimatized sludge for improving biobutanol production. Biochem. Eng. J. 2012;69:100–105. [Google Scholar]
- Chua T.K., Liang D.-W., Qi C., Yang K.-L., He J. Characterization of a butanol–acetone-producing Clostridium strain and identification of its solventogenic genes. Bioresour. Technol. 2013;135:372–378. doi: 10.1016/j.biortech.2012.08.085. [DOI] [PubMed] [Google Scholar]
- Dey P., Sikder J., Roy S., Pal P. Fermentative lactic acid production from a renewable carbon source under response surface optimized conditions without alkali addition: a membrane-based green approach. Clean Technol. Environ. Policy. 2012;14(5):827–835. [Google Scholar]
- Don M.M., Shoparwe N.F. Kinetics of hyaluronic acid production by Streptococcus zooepidemicus considering the effect of glucose. Biochem. Eng. J. 2010;49(1):95–103. [Google Scholar]
- Dürre P. Biobutanol: an attractive biofuel. Biotechnol. J. 2007;2(12):1525–1534. doi: 10.1002/biot.200700168. [DOI] [PubMed] [Google Scholar]
- Dürre P. Fermentative butanol production. Ann. N. Y. Acad. Sci. 2008;1125(1):353–362. doi: 10.1196/annals.1419.009. [DOI] [PubMed] [Google Scholar]
- Fakhri A. Application of response surface methodology to optimize the process variables for fluoride ion removal using maghemite nanoparticles. J. Saudi Chem. Soc. 2014;18(4):340–347. [Google Scholar]
- Fond O., Matta-Ammouri G., Petitdemange H., Engasser J.M. The role of acids on the production of acetone and butanol by Clostridium acetobutylicum. Appl. Microbiol. Biotechnol. 1985;22(3):195–200. [Google Scholar]
- Formanek J., Mackie R., Blaschek H.P. Enhanced butanol production by Clostridium beijerinckii BA101 grown in semidefined P2 medium containing 6 percent maltodextrin or glucose. Appl. Environ. Microbiol. 1997;63(6):2306–2310. doi: 10.1128/aem.63.6.2306-2310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengec E., Kobya M., Demirbas E., Akyol A., Oktor K. Electrochemical treatment of Baker’s yeast wastewater containing melanoidin: optimization through response surface methodology. Water Sci. Technol. 2012;65(12):2183–2190. doi: 10.2166/wst.2012.130. [DOI] [PubMed] [Google Scholar]
- Gu Y., Hu S., Chen J., Shao L., He H., Yang Y., Yang S., Jiang W. Ammonium acetate enhances solvent production by Clostridium acetobutylicum EA 2018 using cassava as a fermentation medium. J. Ind. Microbiol. Biotechnol. 2009;36:1225–1232. doi: 10.1007/s10295-009-0604-1. [DOI] [PubMed] [Google Scholar]
- Jöbses I.M.L., Roels J.A. Experience with solvent production by Clostridium beijerinckii in continuous culture. Biotechnol. Bioeng. 1983;25(4):1187–1194. doi: 10.1002/bit.260250426. [DOI] [PubMed] [Google Scholar]
- Jones D.T., Woods D.R. Acetone–butanol fermentation revisited. Microbiol. Rev. 1986;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleijnen J.P.C., Sargent R.G. A methodology for fitting and validating metamodels in simulation. Eur. J. Oper. Res. 2000;120(1):14–29. [Google Scholar]
- Ladisch M.R. Fermentation-derived butanol and scenarios for its uses in energy-related applications. Enzyme Microb. Technol. 1991;13:280–283. [Google Scholar]
- Lee S.Y., Park J.H., Jang S.H., Nielsen L.K., Kim J., Jung K.S. Fermentative butanol production by clostridia. Biotechnol. Bioeng. 2008;101(2):209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- Liew S.L., Ariff A.B., Raha A.R., Ho Y.W. Optimization of medium composition for the production of a probiotic microorganism, Lactobacillus rhamnosus, using response surface methodology. Int. J. Food Microbiol. 2005;102(2):137–142. doi: 10.1016/j.ijfoodmicro.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Lin Y., Wang J., Wang X., Sun X. Optimization of butanol production from corn straw hydrolysate by Clostridium acetobutylicum using response surface method. Chin. Sci. Bull. 2011;56(14):1422–1428. [Google Scholar]
- Long S., Jones D.T., Woods D.R. Initiation of solvent production, clostridial stage and endospore formation in Clostridium acetobutylicum P262. Appl. Microbiol. Biotechnol. 1984;20(4):256–261. [Google Scholar]
- Madihah M.S., Ariff A.B., Sahaid K.M., Suraini A.A., Karim M.I.A. Direct fermentation of gelatinized sago starch to acetone–butanol–ethanol by Clostridium acetobutylicum. World J. Microbiol. Biotechnol. 2001;17(6):567–576. doi: 10.1007/BF02818533. [DOI] [PubMed] [Google Scholar]
- Martin J.R., Petitdemange H., Ballongue J., Gay R. Effects of acetic and butyric acids on solvents production by Clostridium acetobutylicum. Biotechnol. Lett. 1983;5(2):89–94. [Google Scholar]
- Mercier P., Yerushalmi L., Rouleau D., Dochain D. Kinetics of lactic acid fermentation on glucose and corn by Lactobacillus amylophilus. J. Chem. Technol. Biotechnol. 1992;55(2):111–121. [Google Scholar]
- Monot F., Martin J.R., Petitdemange H., Gay R. Acetone and butanol production by Clostridium acetobutylicum in a synthetic medium. Appl. Environ. Microbiol. 1982;44(6):1318–1324. doi: 10.1128/aem.44.6.1318-1324.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu Y., Wang G., Yu H.-Q. Response surface methodological analysis on biohydrogen production by enriched anaerobic cultures. Enzyme Microb. Technol. 2006;38(7):905–913. [Google Scholar]
- Olya M.E., Vafaee M., Jahangiri M. Modelling of acid dye decolorisation by TiO2–Ag2O nano-photocatalytic process using response surface methodology. J. Saudi Chem. Soc. 2014;18(4):340–347. [Google Scholar]
- Ounine K., Petitdemange H., Raval G., Gay R. Regulation and butanol inhibition of d-xylose and d-glucose uptake in Clostridium acetobutylicum. Appl. Environ. Microbiol. 1985;49(4):874–878. doi: 10.1128/aem.49.4.874-878.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan C.M., Fan Y.T., Xing Y., Hou H.W., Zhang M.L. Statistical optimization of process parameters on biohydrogen production from glucose by Clostridium sp. Fanp2. Bioresour. Technol. 2008;99(8):3146–3154. doi: 10.1016/j.biortech.2007.05.055. [DOI] [PubMed] [Google Scholar]
- Qureshi N., Maddox I. Application of novel technology to the ABE fermentation process. Appl. Biochem. Biotechnol. 1992;34–35(1):441–448. [Google Scholar]
- Ranjan A., Mayank R., Moholkar V. Development of semi-defined rice straw-based medium for butanol production and its kinetic study. 3 Biotech. 2013;3(5):353–364. doi: 10.1007/s13205-013-0120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjan A., Mayank R., Moholkar V. Process optimization for butanol production from developed rice straw hydrolysate using Clostridium acetobutylicum MTCC 481 strain. Biomass Convers. Biorefin. 2013;3(2):143–155. [Google Scholar]
- Ratnam B.V.V., Narasimha Rao M., Damodar Rao M., Subba Rao S., Ayyanna C. Optimization of fermentation conditions for the production of ethanol from sago starch using response surface methodology. World J. Microbiol. Biotechnol. 2003;19(5):523–526. [Google Scholar]
- Razak M.N.A., Ibrahim M.F., Yee P.L., Hassan M.A., Abd-Aziz S. Statistical optimization of biobutanol production from oil palm decanter cake hydrolysate by Clostridium acetobutylicum ATCC 824. BioResources. 2013;8(2):1758–1770. [Google Scholar]
- Rodrigues L., Moldes A., Teixeira J., Oliveira R. Kinetic study of fermentative biosurfactant production by Lactobacillus strains. Biochem. Eng. J. 2006;28(2):109–116. [Google Scholar]
- Roos J.W., McLaughlin J.K., Papoutsakis E.T. The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol. Bioeng. 1985;27(5):681–694. doi: 10.1002/bit.260270518. [DOI] [PubMed] [Google Scholar]
- Shinto H., Tashiro Y., Yamashita M., Kobayashi G., Sekiguchi T., Hanai T., Kuriya Y., Okamoto M., Sonomoto K. Kinetic modeling and sensitivity analysis of acetone–butanol–ethanol production. J. Biotechnol. 2007;131(1):45–56. doi: 10.1016/j.jbiotec.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Sivakumar K., Karuppiah V., Sethubathi G., Thangaradjou T., Kannan L. Response surface methodology for the optimization of α-amylase production by Streptomyces sp. ML12 using agricultural byproducts. Biologia. 2012;67(1):32–40. [Google Scholar]
- Song H., Eom M.-H., Lee S., Lee J., Cho J.-H., Seung D. Modeling of batch experimental kinetics and application to fed-batch fermentation of Clostridium tyrobutyricum for enhanced butyric acid production. Biochem. Eng. J. 2010;53(1):71–76. [Google Scholar]
- Tanyildizi M.Ş. Modeling of adsorption isotherms and kinetics of reactive dye from aqueous solution by peanut hull. Chem. Eng. J. 2011;168(3):1234–1240. [Google Scholar]
- Tashiro Y., Shinto H., Hayashi M., Baba S.-I., Kobayashi G., Sonomoto K. Novel high-efficient butanol production from butyrate by non-growing Clostridium saccharoperbutylacetonicum N1-4 (ATCC 13564) with methyl viologen. J. Biosci. Bioeng. 2007;104(3):238–240. doi: 10.1263/jbb.104.238. [DOI] [PubMed] [Google Scholar]
- Tashiro Y., Sonomoto K. Advances in butanol production by clostridia. In: Méndez-Vilas A., editor. Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology, Microbiology Book Series: 1383–1394. FORMATEX; Spain: 2010. [Google Scholar]
- Vishwanatha K., Rao A., Singh S. Acid protease production by solid-state fermentation using Aspergillus oryzae MTCC 5341: optimization of process parameters. J. Ind. Microbiol. Biotechnol. 2010;37(2):129–138. doi: 10.1007/s10295-009-0654-4. [DOI] [PubMed] [Google Scholar]
- Wang Q., Ma H., Xu W., Gong L., Zhang W., Zou D. Ethanol production from kitchen garbage using response surface methodology. Biochem. Eng. J. 2008;39(3):604–610. [Google Scholar]
- Weisberg S. third ed. John Wiley & Sons INC; New York: 2005. Applied Linear Regression. [Google Scholar]
- Zheng Z.-M., Hu Q.-L., Hao J., Xu F., Guo N.-N., Sun Y., Liu D.-H. Statistical optimization of culture conditions for 1,3-propanediol by Klebsiella pneumoniae AC 15 via central composite design. Bioresour. Technol. 2008;99(5):1052–1056. doi: 10.1016/j.biortech.2007.02.038. [DOI] [PubMed] [Google Scholar]