Abstract
In the present study laccase production potential of a photosynthetic, non nitrogen fixing cyanobacteria Arthrospira maxima (SAE-25780) was investigated for their probable use in synthetic dye decolorization which poses environmental pollution problem in aquatic bodies. A. maxima (SAE-25780) showed a constitutive production of laccase which increased up to 80% in the presence of inducer guaiacol. The optimal condition for laccase was 30 °C, 10 mM sucrose as a carbon source, 10 mM sodium nitrate as a nitrogen source, and 2 mM copper as metal activator. The partially purified laccase showed 84% and 49% decolorization potential for the two anthroquinonic dyes-Reactive Blue 4 and Remazol Brilliant Blue R, respectively (RBBR) within 96 h without any mediator. Therefore the laccase extracted from A. maxima (SAE-25780) can be used efficiently in bioremediation of synthetic dyes from paper, pulp and textile industries.
Keywords: Laccase, Arthrospira maxima (SAE-25780), Optimization, Remazol Brilliant Blue R, Reactive Blue 4, Decolorization
1. Introduction
Textile industry is one of the greatest generators of liquid effluent containing synthetic dyes. Textile dyes are responsible for the deep color of the effluents. The color of water has adverse effects on O2 evolution by photosynthetic organism as reduced dissolved oxygen causes death of flora and fauna of the habitat. In the current time 10,000 synthetic dyes are frequently utilized in the textile industry representing an annual consumption of around 7 × 105 tonnes worldwide (Karthikeyan et al., 2010). These dyes may be classified based on their chromophores and can be divided into several groups such as azo, anthraquinone, sulfur, indigo, triphenylmethyl and phthalocyanine derivatives. Most of these are stable against light, temperature and biodegradation and therefore accumulate in the environment and form recalcitrant compound (Nozaki et al., 2008) and dyes are mutagenic and carcinogenic to the human population (Rindle and Troll, 1975). Thus industrial effluents with synthetic dyes need some tools and techniques to make them inactive before they are discharged into the environment. Physicochemical methods such as- (coagulation, flocculation, membrane filtration, and activated carbon adsorption) have high operating costs (Robinson et al., 2001). A number of biotechnological approaches have been suggested by recent research as of potential interest toward combating this pollution source in an ecoefficient manner. Biological decolorization has been considered as effective, specific, less energy intensive and environmentally benign, since it results in partial or complete bioconversion of synthetic dyes to stable nontoxic end products (Kuhad et al., 2004).
Cyanobacteria are ubiquitous in nature but their role in the decolorization and degradation of dyes are scanty. Parik and Madamwar (2005) reported textile dye decolorization using cyanobacteria. However, the cyanobacterial enzymes involved in the decolorization of synthetic dyes have not yet been characterized. Microbial sources may provide stable and reliable source of enzymes, due to the possibility of the production of large quantities of enzyme by them and ease of extraction (Desai and Nityananda, 2011).
Laccases (benzenediol:oxygen oxidoreductases, (EC:1.10.3.2) are a diverse group of multi-copper proteins that oxidize a surprisingly wide variety of organic and inorganic compounds, including diphenols, polyphenols, substituted phenols, diamines and aromatic amines, with concomitant a reduction of molecular oxygen to water (Thurston, 1994). Laccase is a dimeric or tetrameric glycoprotein and usually contains four copper atoms per monomer. To function, laccase depends on Cu atoms distributed among the three binding sites. Due to its low specificity for the reducing substrate, laccases have attracted increasing attention in industrial and environmental fields, such as pulp delignification, dye decolorization, environmental pollutant detoxification, biopolymer modification, and biotransformation (Hou et al., 2004, Leonowicz et al., 2001, Yaropolov et al., 1994). The production of active and large quantities of laccase at a relatively low cost is of major interest. Laccases can be either constitutive or inducible enzymes and their production occurs during secondary metabolism and is subject to complex regulation by nutrients (carbon, nitrogen, inducers, and copper) (Dittmer et al., 1997, Palmieri et al., 2000). These regulators affect the transcription levels of laccase and other genes (Collins and Dobson, 1997, Soares et al., 2001). Aromatic and phenolic compounds have been widely used to elicit enhanced laccase production by different organisms (De Souza et al., 2004, Leonowicz et al., 2001). The presence of inducers increase laccase synthesis by providing contact with compounds that may naturally elicit a stress response and further increase production. Laccases are mostly found in plants and fungi but also in some bacteria (Alexandre and Zhulin, 2000, Claus, 2004). One of the advantages associated with laccases is that they do not require H2O2 for substrate oxidation unlike peroxidases and, moreover, they have broad substrate specificity (Saito et al., 2003). Of late, the demand for the removal of synthetic dyes from industrial waste using laccases is being increased tremendously (Abadull et al., 2000, Zille et al., 2003). Therefore, search for potential laccases to cope with this demand is an important task. Due to phototrophic mode of nutrition, short generation time and easy mass cultivation, cyanobacteria may appear as good candidate for laccase production.
Previously various studies have been conducted on purification and decolorization of synthetic dyes from fungal sources but so far, in cyanobacteria only Phormidium valderianum BDU 30501 and Oscillatoria boryana are reported to have ligninolytic activity and decolorization ability (Palanisami and Uma, 2010). Considering the potential application of cyanobacterial laccase for solving environmental problems, it is essential to define the best conditions for the production of such enzymes. Thus, in the present study laccase production potential of Arthrospira maxima (SAE-25780), optimization of culture conditions for its highest yield and synthetic dye (Reactive Blue 4 and Remazol Brilliant Blue R) decolorization ability were investigated.
2. Material and methods
2.1. Chemicals and reagents
All chemicals used were of analytical grade and were obtained from Merck, India. Synthetic Dyes (Reactive Blue 4 and Remazol Brilliant Blue R) were purchased from Sigma–Aldrich Chemical Co, St. Louis, MO, USA. The structure of all dyes is shown in Table 1.
Table 1.
Structure and maximum visible wavelengths of dyes.
| Dye | λmax (nm) | Structure |
|---|---|---|
| Reactive Blue 4 | 595 | 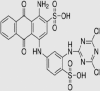 |
| Remazol Brilliant Blue R | 592 | 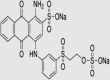 |
2.2. Culture condition and maintenance
The A. maxima (SAE-25780) was procured from the University of Chennai, Tamil Nadu; and was grown in 500 ml Erlenmeyer flask containing 200 ml Zarrouk’s medium (1996) containing (g/L) NaCl, 1; CaCl2, 0.04; NaNO3, 2.5; FeSO4, 0.01; EDTA, 0.08; K2SO4, 0.08; MgSO4, 0.2; NaHCO3, 16.8; K2HPO4, 0.5, Trace metal mix, 1 ml at light intensity 2000 ± 200 lux; photoperiod 12:12 h light: dark; temperature 30 ± 1 °C. The culture was induced with 100 μM guaiacol (dissolved in 50% ethanol) for laccase production. The culture without guaiacol was considered as control. The cultures were swirled twice a day for aeration and mixing of nutrients. Sample were collected by centrifugation at 8000g for 20 min at 4 °C and used for the enzyme assay.
2.3. Assay for laccase activity
Laccase activity was assayed at 25 °C using 2, 2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) as substrate. The assay mixture contained 2 mM ABTS and 0.1 M glycine HCl buffer, pH 2.5. Oxidation of ABTS was followed by an absorbance increase at 405 nm (ε = 36,000 M−1 cm−1). Activity was expressed in units: 1 U as μmol of oxidized product formed in a min. The blank contained all the constituents except the active enzyme.
2.4. Zymogram analysis of laccase on Native-PAGE
Partial purification of laccase was done for zymogram analysis. For this the culture was induced with guaiacol and the supernatant was obtained by centrifugation at 8000 rpm for 20 min. The protein was precipitated with chilled cold acetone at a volume of 1:4 incubated for 1.5 h at −20 °C in a deep freezer and then centrifuged for 30 min at 10,000g. The supernatant was discarded and the pellet was retained. The pellet was air dried to remove any residual acetone and served as the enzyme source.
Native PAGE was carried out at 15 ± 1 °C using tris-glycine as running buffer (pH 8.3) under non-denaturing and non-reducing conditions (Laemmli, 1970). A uniform amount of protein (500 μg, estimated by Lowry et al. (1951)) was loaded to each lane along with native sample buffer (devoid of sodium dodecyl sulfate and b-mercaptoethanol). Samples were then electrophoresed at 50 V through the stacking gel (6%) and at 100 V through the resolving gel (10%) using Power Supply-EPS 1001 (Amersham Pharmacia). Laccase activity was visualized in the gel with guaiacol (1 mM) as substrate in 0.5 M sodium citrate buffer (pH 4.5)
2.5. Decolorization of synthetic dyes
For finding out the decolorization potential of partially purified laccase a slightly modified protocol of Couto, 2007) was adopted. The dye solution [Reactive Blue 4 (50 mg/l) and Remazol Brilliant Blue R (50 mg/l)] were incubated with laccase (1.5 U/ml) in 50 mmol/L sodium citrate buffer, pH 6.0 at 30 °C. Samples were taken at 24 h interval and centrifuged at 8000 rpm for 20 min. The decrease of color intensity in supernatant was analyzed spectrophotometrically at λ = 495 nm for Reactive Blue 4 and at λ = 595 for Remazol Brilliant Blue R.
Percent dye decolorization was calculated according to the formula: D = 100(Aini − Aobs)/Aini, where D is decolorization (in%), Aini, initial absorbance and Aobs, observed absorbance.
2.6. Optimization of laccase production
To investigate optimal time for laccase production, the experimental cultures were incubated for 16 days and their laccase activity was measured after every 24 h. The effect of temperature on enzyme production was determined by incubating the inoculated flask at different temperatures (30–60 °C) under control and induced conditions. Similarly 10 mM of glucose, fructose and sucrose were tested individually for the effect of carbon source on laccase yield. Sodium nitrate, ammonium chloride, Urea at a concentration of (10 mM) each was tested as nitrogen source to examine their effect on laccase production. After 72 h of after inoculation copper as copper sulfate (0.1, 0.5, 1.0, 1.5 and 2.0, 2.5, 3.0 mM) was added in the culture medium and its effect on laccase production was determined at regular intervals.
2.7. Statistical analysis
All experiments were carried out in triplicates and the results were expressed as mean ± standard error. Dunnett’s multiple range test was applied and differences among the means were analyzed by one way ANOVA using Graph Pad Prism version-6.1 (Graph Pad Software, San Diago, CA, USA).
3. Results and discussions
3.1. Time course study
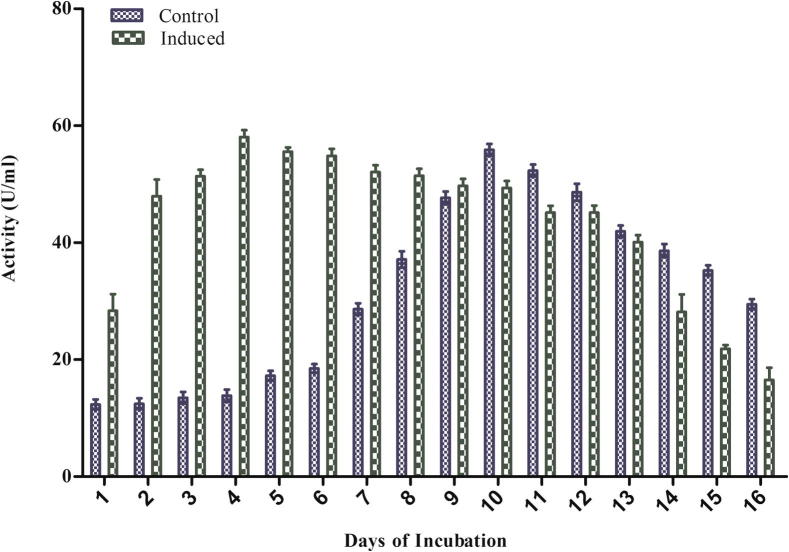
The main purpose of this study was to examine the laccase production potential of A. maxima (SAE-25780). The normal and guaiacol induced cultures (100 μM) were incubated for 16 days and laccase activity was measured at 24 h intervals. Under normal condition maximum laccase secretion started on the 2nd day of incubation and the maximum activity (54.671 U/ml) was achieved on the 10th day suggesting its constitutive production which was also reported by Scheel et al., 2000. From the growth curve study of A. maxima (SAE-25780) we found that during lag phase on the 2nd day biomass was (3.08 mg/g) then it increased exponentially during log phase and reached its maximum value (21.42 mg/g) on the 10th day and thereafter stationary phase started which suggested that total laccase activity increased proportionally with the biomass production. In our study we found that under the induced condition the average time production of laccase was decreased and highest activity was measured on the 4th day (56.894 U/ml) (Fig. 1). It was found that there was a 2–4 fold enhancement (ANOVA p < 0.0001) in the laccase activity in the presence of inducer guaiacol as compared to control. The activity ranged from (12.114–54.671 U/ml) in control condition to (25.632–56.685 U/ml) in induced condition (Fig. 1). Aromatic and phenolic compounds have been widely used to elicit enhanced laccase production by different organisms and the nature of the compound that induces laccase activity differs greatly with the species (De Souza et al., 2004, Leonowicz et al., 2001). Earlier we have reported the presence of laccase in Synechocystis NCCU-370 but its production was quite low under normal condition to (25.37 mU/ml) that peaked on the 7th day (Afreen and Fatma, 2013). A. maxima (SAE-25780) is better than Synechocystis NCCU-370 in laccase production as it produced (56.894 U/ml) on the 4th day in the presence of guaiacol. It is reported that guaiacol supplementation (1 mM) enhanced laccase production in different fungi eg. Phlebia spp., Pleurotus ostreatus, Coriolus hirsutus (Hou et al., 2004, Koroljova-Skorobogt’ko et al., 1998, Patel et al., 2014). Mongkolthanaruk et al. (2012) reported that 8 mM guaiacol acts as a good inducer. It induced laccase production from 33 to 56 mU/l after 48 h of incubation in bacteria viz Rhodococcus sp., Enterobacter sp., and Staphylococcus saprophyticus. It has been reported that marine cyanobacteria Phormidium tenue induced laccase production to various folds after the addition of 100 μM of guaiacol, tannic acid, caffeic acid, copper (Palanisami and Uma, 2010).
Figure 1.
Extracellular laccase production by Arthrospira maxima (SAE-25780) under controlled and guaiacol induced condition.
The presence of laccase in cyanobacteria A. maxima (SAE-25780) was further confirmed by native Page followed by zymogram analysis. The native PAGE result revealed a single laccase enzyme was detected by activity staining with guaiacol. The enzyme was able to oxidize the substrate (guaiacol) and developed reddish brown color which is due to the oxidative polymerization of guaiacol in the presence of laccase (Fig. 2).
Figure 2.

Confirmation of laccase production by zymogram analysis of Arthrospira maxima (SAE-25780) using guaiacol as substrate.
3.2. Effect of temperature on laccase production
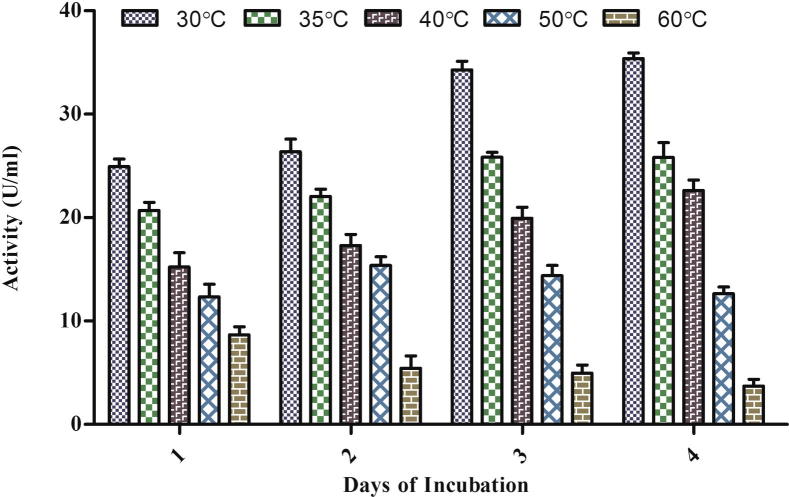
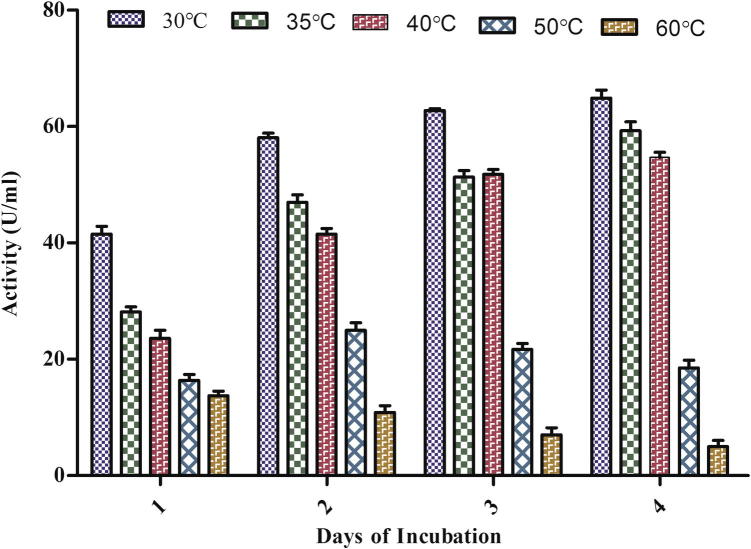
Temperature affects the properties of aqueous environment as well as all metabolic processes of the organism including nutrient availability and uptake. During optimization of culture condition for the highest yield of laccase from A. maxima it was found that temperature has a profound influence on enzyme activity that was significantly increased (ANOVA p < 0.0001) daywise and maximum activity was found on the 4th day at 30 °C under both normal and induced conditions (Figure 3a, Figure 3b). Results also indicated that, by increasing the incubation temperature above 35 °C, a gradual decrease in laccase production occurred. At 60 °C after the 2nd day the culture started to decay and became pale yellow. It produced only a small amount of enzyme (3.714 U/ml).
Figure 3a.
Effect of temperature on the laccase production in spent medium under control condition.
Figure 3b.
Effect of temperature on the laccase production in spent medium under guaiacol induced condition.
A previous study carried in our lab suggested that the optimal temperature for the better growth of this Spirulina strain was found to be 27 °C–33 °C (Jetley et al., 2004). Above 40 °C, both the growth and laccase production decreased. This suggested that proper growth of Spirulina is necessary for the optimal laccase production.
Nakamura et al. (1999) and Zadrazil et al. (1999) have reported maximum laccase activity at 30 °C in Pleurotus, Dichomitus squalens and Bjerkendera adusta at 30 °C. Farnet et al. (2000) and Pointing et al. (2000) have found 25 °C and 50 °C as optimal temperature in Marasmius quercophilus strain and Pycnoporus sanguineus.
3.3. Effect of carbon on laccase production
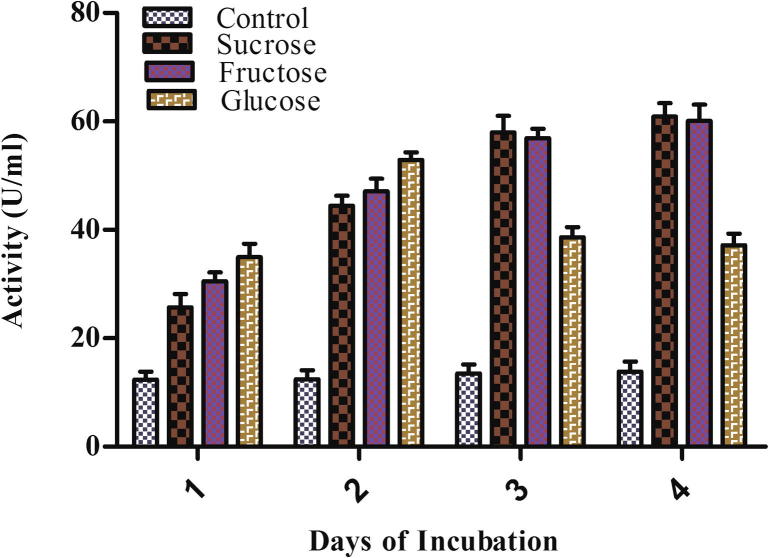
The concentration and nature of carbon source used in the medium showed a notable effect on enzyme production (Gawande and Kamat, 2000). Exogenous addition of fructose, glucose, and sucrose as different carbon sources increased the laccase production in A. maxima (Fig. 4). In terms of maximum activity during carbon supplementation experiment, sucrose and fructose showed a much higher activity than glucose being 60.914, 60.114 and 37.118 U/ml. Mansur et al. (1997) found a 100 fold increase in the specific laccase activity of basidiomycetes fungi with fructose. But on the 2nd day glucose supplementation showed the highest laccase production in comparison to sucrose and fructose. Early, enzyme enhancing response of glucose may be due to ready and quick utilization by organism (Fig. 4). The order of activity was 52.863, 47.083 and 44.457 U/ml in the presence of glucose, fructose and sucrose. Glucose supplementation also showed increased laccase activity in C. hirsutus (Koroljova-Skorobogt’ko et al., 1998) and Ganoderma sp. (Revankar et al., 2007).
Figure 4.
Effect of carbon sources on the laccase production by Arthrospira maxima (SAE-25780).
3.4. Effect of nitrogen on laccase production
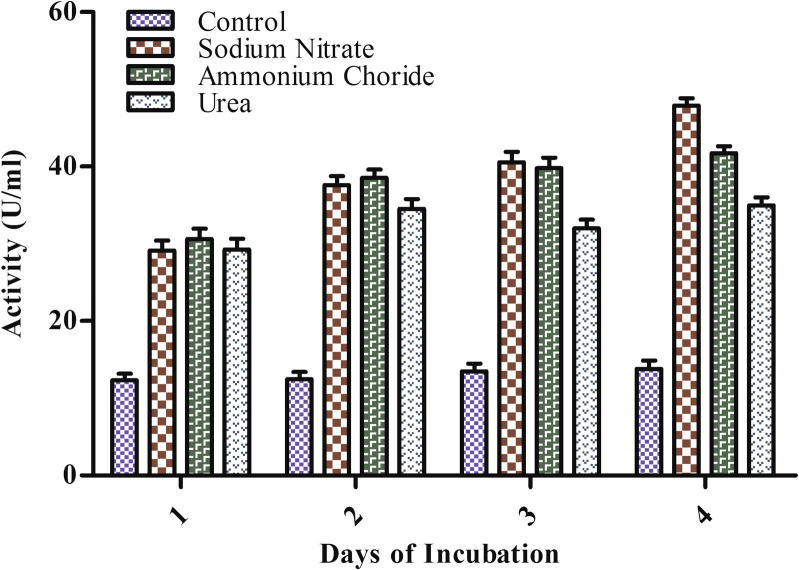
Nitrogen is involved in amino acid synthesis which makes up proteins and other value added substances. The effect of nitrogen sources on laccase production by different organisms appears to be greatly controversial (Collins and Dobson, 1997). Nitrogen sufficient media has been reported to enhance ligninolytic enzymes production in many fungi (Kaal et al., 1995) but in Phanerochaete chrysosporium laccase was limited by nitrogen sources (Buswell et al., 1992). In the present study the effect of different nitrogen sources viz urea, ammonium chloride, and sodium nitrate on laccase production was determined. All nitrogen sources (10 mM) tested stimulated the laccase production that ranged from 2.5–3.5-fold. Among the tested nitrogen sources on the 4th day maximum enzyme production (47.885 U/ml) was achieved with sodium nitrate as compared to control (13.485 U/ml) (Fig. 5) that may be due to higher growth of the organism (Anwer et al., 2012). Shraddha et al. (2011) also found sodium nitrate as the best nitrogen source for laccase production.
Figure 5.
Effect of nitrogen sources on laccase production by Arthrospira maxima (SAE-25780).
Ammonium chloride also significantly increased (ANOVA p < 0.0001) laccase activity on the 4th day of incubation. P. ostreatus fungi showed the highest laccase activity with ammonium chloride (Stajic et al., 2006). Culture supplemented with urea resulted in least laccase activity (34.971 U/ml) but still it was much higher than control (13.485 U/ml). Pleurotus sanguineus also showed contrary higher laccase activity with urea than sodium nitrate (Lee et al., 2006). The order of different nitrogen sources on laccase production was sodium nitrate > ammonium chloride > urea.
3.5. Effect of copper on laccase production
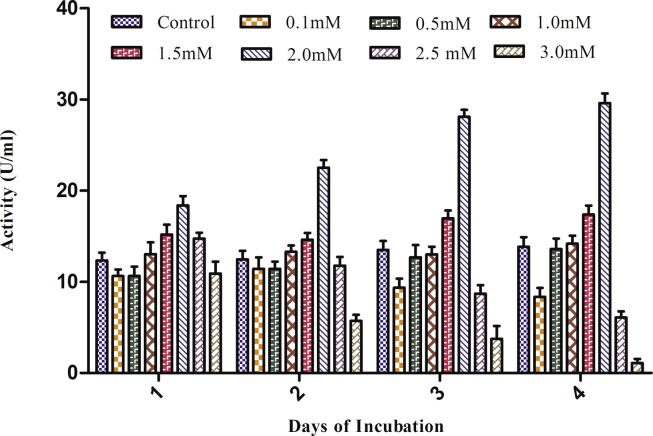
Laccase are multicopper oxidases therefore, copper as micronutrients has key role as metal activators. For optimizing copper concentration for higher laccase production by A. maxima, copper as CuSO4 (0.1–3.0 mM) was added in culture medium on the third day of inoculation and on the 4th day it showed a considerable increase in activity (ANOVA p < 0.0001) (Fig. 6). Enhanced laccase yield after the third day copper addition might attribute to the fact that organism was already adapted to the media conditions and exponential growth was starting, leading to enhancement of laccase production by the primary metabolism (Palmieri et al., 2000). On the 4th day lower CuSO4 concentration (0.1 mM–1.5 mM) does not affect significantly as compared to control. In 2.0 mM CuSO4 laccase activity increased and was the highest, whereas, above 2 mM CuSO4 (2.5 and 3.0 mM) its laccase activity decreased. The suppression at higher copper concentration may be due to downfall in the growth of organism due to metal toxicity that results from a low photosynthetic rate (Fathi et al., 2005). Copper has been reported to be a strong laccase inducer in the fungal species Trametes versicolor and P. chrysosporium (Collins and Dobson, 1997, Domınguez et al., 2007). Hao et al. (2007) also found 2.0 mM of CuSO4 as optimal concentration of copper in Pestalotiopsis sp. but Palmieri et al. (2000) found 150 μM of copper sulfate as the best concentration for laccase activity. The regulation of the synthesis of several laccase isoforms by copper occurs at the level of gene transcription (Galhaup et al., 2002, Palmieri et al., 2000).
Figure 6.
Effect of different concentration of CuSO4 on the laccase production by Arthrospira maxima (SAE-25780).
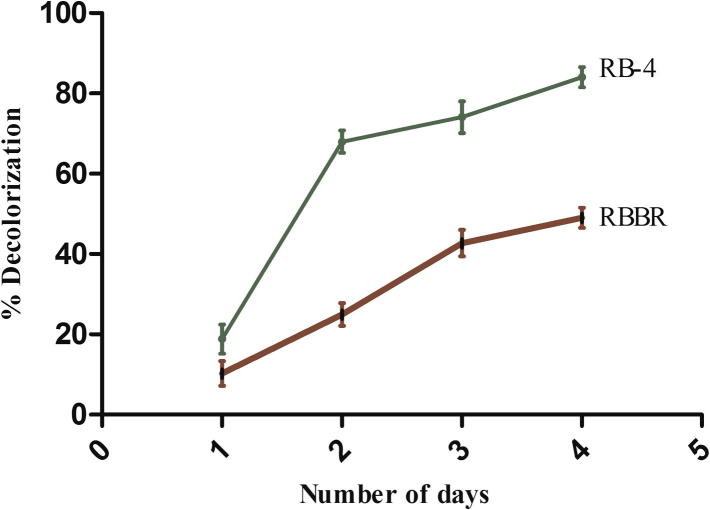
3.6. Decolorization of synthetic dyes
The dye decolorization ability of the partially purified laccase of A. maxima (SAE-25780) was assayed regularly against anthraquinonic synthetic dyes Reactive Blue 4 and RBBR (Fig. 7). From the results we found that the decolorization rate of Reactive Blue 4 (50 mg/l) was 84% and 49% for RBBR (50 mg/l) by laccase enzyme within 96 h of incubation. Both the dyes were not decolorized at the same extent that may be due to the difference of the redox potentials and the suitability of their steric structure with the active site of the enzyme (Tavares et al., 2008). The extent of decolorization activity depends on the source of the enzyme and the chemical structure of the dye (Abadull et al., 2000, Couto, 2007). It was reported that laccase hardly decolorized RBBR which might be due to the absence of mediator as suggested by Hamid et al. (2012). According to them crude laccase enzyme requires mediator like – 1-hydroxybenzotriazole (HBT) for efficient dye decolorization. The decolorization rate of Reactive dyes was increased from 3% to 70% by the addition of HBT as the mediator (Claus, 2004). Yemendzhiev et al. (2009) have also observed efficient decolorization of Reactive Blue 4 (50 mg/l) by fungus T. versicolor. Parik and Madamwar (2005) reported that the cyanobacteria Phormidium ceylanicum, Gloeocapsa pleurocapsoides and Chroococcus minutes showed 70–90% decolorization of dye (50 mg l) after 26 days of incubation. However in our study A. maxima laccase showed a rapid decolorization of Reactive Blue 4 (84%) and of RBBR (49%) without any mediator within 4 days.
Figure 7.
Decolorization of anthraquinonic textile dyes Reactive Blue 4 and RBBR by Arthrospira maxima (SAE-25780) laccase.
4. Conclusion
The results of this work disclose the potential of cyanobacteria A. maxima (SAE-25780) to enhance laccases production. It showed a constitutive production of laccase which increased up to 80% in the presence of inducer (guaiacol). Under normal condition laccase production was the highest on the 10th day but in the presence of inducer (guaiacol) the duration for maximum production of laccase was reduced to 4th day that will reduce cost production during its industrial application and the optimal condition for laccase production was 30 °C, 10 mM sucrose as a carbon source, 10 mM sodium nitrate as a nitrogen source, 2 mM copper as metal activator. The synthetic dyes viz RB4 & RBBR were efficiently decolorized 84% and 49%, respectively within four days of incubation with extracellular laccase. To the best of our knowledge this is the first report on laccase production from cyanobacteria [A. maxima (SAE-25780)] that has anthraquinonic dye (Reactive Blue 4 & RBBR) decolorization potential for the bioremediation of synthetic dyes from paper, pulp and textile industries. More efforts are needed to increase the laccase yield from A. maxima (SAE-25780) in order to make it more economical.
Acknowledgements
The authors are thankful to University of Chennai, Tamil Nadu for providing the test strains and University Grant Commission India, for providing financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abadull E., Robra K.H., Gübitz G.M., Silva L.M., Cavaco P.A. Enzymatic decolorization of textile dyeing effluents. Text. Res. J. 2000;70:409–414. [Google Scholar]
- Afreen S., Fatma T. Laccase production and simultaneous decolorization of synthetic dyes by cyanobacteria. Int. J. Innovative Res. Sci. Eng. Technol. 2013;2:3563–3568. [Google Scholar]
- Alexandre G., Zhulin I.B. Laccases are widespread in bacteria. Trends Biotechnol. 2000;18:41–42. doi: 10.1016/s0167-7799(99)01406-7. [DOI] [PubMed] [Google Scholar]
- Anwer R., Khursheed S., Fatma T. Detection of immunoactive insulin in Spirulina. J. Appl. Phycol. 2012;24:583–591. [Google Scholar]
- Buswell J.A., Arora D.K., Rai B., Mukerji K.G. Fungal degradation of lignin. In: Kundsen, editor. Handbook of Applied Mycology Soil and Plants. Marcel Dekker; New York: 1992. pp. 425–480. [Google Scholar]
- Claus H. Laccases: structure, reactions, distribution. Micron. 2004;35:93–96. doi: 10.1016/j.micron.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Collins P.J., Dobson A.D.W. Regulation of laccase gene transcription in Trametes versicolor. Appl. Environ. Microbiol. 1997;63:3444–3450. doi: 10.1128/aem.63.9.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto S.R. Decolouration of industrial azo dyes by crude laccase from Trametes hirsute. J. Hazard. Mat. 2007;148:768–770. doi: 10.1016/j.jhazmat.2007.06.123. [DOI] [PubMed] [Google Scholar]
- De Souza C.G.M., Tychanowicz G.K., De Souza D.F., Peralta R.M. Production of laccase isoforms by Pleurotus pulmonarius in response to presence of phenolic and aromatic compounds. J. Basic Microbiol. 2004;3:129–136. doi: 10.1002/jobm.200310365. [DOI] [PubMed] [Google Scholar]
- Desai S.S., Nityananda C. Microbial laccases and their applications: a review. Asian J. Biotechnol. 2011;2:98–124. [Google Scholar]
- Dittmer J.K., Patel N.J., Dhawale S.W., Dhawale S.S. Production of multiple laccase isoforms by Phanerochaete chrysosporium grown under nutrient sufficiency. FEMS Microbiol. 1997;149:65–70. [Google Scholar]
- Domınguez A., Gomez J., Lorenzo M., Sanroman A. Enhanced production of laccase activity by Trametes versicolor immobilized into alginate beads by the addition of different inducers. World J. Microbiol. Biotechnol. 2007;23:367–373. [Google Scholar]
- Farnet A.M., Criquet S., Tagger S., Gil G., LePetit J. Purification, partial, characterization, and reactivity with aromatic compounds of two laccases from Marasmiusquercophilus strain 17. Can. J. Microbiol. 2000;46:189–194. doi: 10.1139/w99-138. [DOI] [PubMed] [Google Scholar]
- Fathi A.A., Zaki F.T., Ibraheim H.A. Response of tolerant and wild type strains of Chlorella vulgaris to copper with special references to copper uptake system. Protistology. 2005;4:73–78. [Google Scholar]
- Galhaup C., Goller S., Peterbauer C.K., Strauss J., Haltrich D. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology. 2002;148:2159–2169. doi: 10.1099/00221287-148-7-2159. [DOI] [PubMed] [Google Scholar]
- Gawande P.V., Kamat M.Y. Production of xylanases by immobilized Aspergillus sp using lignocellulosic waste. World J. Microbiol. Biotechnol. 2000;16:111–112. [Google Scholar]
- Hamid F., Moezzi A., Khozani A.M., Mahmoudjanlou Y., Ameri A., Niknejad F., Faramarzi M.A. Synthetic dye decolorization by three sources of fungal laccase. Iranian J. Environ. Health Sci. Eng. 2012;9:27. doi: 10.1186/1735-2746-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J., Song F., Huang F., Yang C., Zhang Z., Zheng Y., Tian X. Production of laccase by a newly isolated deuteromycetes fungus Pestalotiopsis sp. and its decolorization of azo dye. J. Ind. Microbiol. Biotechnol. 2007;34:233–240. doi: 10.1007/s10295-006-0191-3. [DOI] [PubMed] [Google Scholar]
- Hou H., Zhou J., Wang J., Du C., Yan B. Enhancement of laccase production by Pleurotus ostreatus and its use. Process Biochem. 2004;39:1415–1419. [Google Scholar]
- Jetley U.K., Choudhary M., Fatma T. The impact of physical stresses on the growth of cyanobacterium Spirulina platensis-S5. J. Environ. Sci. Eng. 2004;46:303–311. [PubMed] [Google Scholar]
- Kaal E.E.J., Field J.A., Joyce T.W. Increasing ligninolytic enzyme activities in several white-rot basidiomycetes by nitrogen sufficient media. Bioresour. Technol. 1995;53:133–139. [Google Scholar]
- Karthikeyan K., Nanthakumar K., Shanthi K., Lakshmanaperumalsamy P. Response surface methodology for optimization of culture conditions for dye decolorization by a fungus, Aspergillus niger HM11 isolated from dye affected soil. Iranian J. Microbiol. 2010;2:213–222. [PMC free article] [PubMed] [Google Scholar]
- Koroljova-Skorobogt’ko O.V., Stepanova E.V., Gavrilova V.P., Morozova O.V., Lubimova N.V., Dzchafarova A.N., Jaropolov A.J., Makower A. Purification and characterization of the constitutive form of laccase from the basidiomycete Coriolus hirsutus and effect of inducers on laccase synthesis. Biotechnol. Appl. Biochem. 1998;28:47–54. [PubMed] [Google Scholar]
- Kuhad R.C., Sood N., Tripathi K.K., Singh A., Ward O.P. Developments in microbial methods for the treatments of dye effluents. Adv. Appl. Microbiol. 2004;56:185–213. doi: 10.1016/S0065-2164(04)56006-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y., Park C., Lee B., Han E., Kim T. Effect of nutrients on the production of extracellular enzymes for decolorization of reactive blue 19 and reactive black 5. J. Microbiol. Biotechnol. 2006;16:226–231. [Google Scholar]
- Leonowicz A., Cho N.S., Luterek J., Wilkolazka A., Wojtas Wasilewska M., Matuszewska A., Hofrich-ter M., Wesenberg D., Rogalski J. Fungal laccase: properties and activity on lignin. J. Basic Microbiol. 2001;41:185–227. doi: 10.1002/1521-4028(200107)41:3/4<185::aid-jobm185>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Lowry O.H., Rosenbrough N.J., Farr A.L., Randal R.J. Protein measurement with Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mansur M., Suárez T., Fernández-Larrea J.B., Brizuela M.A., González A.E. Identification of a laccase gene family in the new lignin-degrading basidiomycetes CECT 20197. Appl. Environ. Microbiol. 1997;63:2637–2646. doi: 10.1128/aem.63.7.2637-2646.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongkolthanaruk W., Tongbopit S., Bhoonontong A. Independent behaviour of bacterial laccases to inducers and metal ions during production and activity. Afr. J. Biotechnol. 2012;11:9391–9398. [Google Scholar]
- Nakamura Y., Sangusis M.G., Sawada T., Kuwahara M. Lignin degradind enzyme production by Bjerkendera adusta immobilized on polyurethane foam. J. Biosci. Bioeng. 1999;88:41–47. doi: 10.1016/s1389-1723(99)80173-x. [DOI] [PubMed] [Google Scholar]
- Nozaki K., Beh H.C., Mizuno M., Isobe T., Shiroishi M. Screening and investigation of dye decolorization activities of basidiomycetes. J. Biosci. Bioeng. 2008;105:69–72. doi: 10.1263/jbb.105.69. [DOI] [PubMed] [Google Scholar]
- Palanisami S., Uma L. Laccase and polyphenol oxidase activities of marine cyanobacteria: a study with Poly R 478 decolourization. World J. Microbiol. Biotechnol. 2010;26:63–69. [Google Scholar]
- Palmieri G., Giardiana P., Bianco C., Fontanella B., Sannia G. Copper induction of laccase isoenzymes in the ligninolytic fungus Pleurotus ostreatus. Appl. Environ. Microbiol. 2000;66:920–924. doi: 10.1128/aem.66.3.920-924.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parik A., Madamwar D. Textile dyes decolorization using cyanobacteria. Biotechnol. Lett. 2005;27:323–326. doi: 10.1007/s10529-005-0691-7. [DOI] [PubMed] [Google Scholar]
- Patel H., Gupte S., Gahlout M., Gupte A. A purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. 3 Biotech. 2014;4:77–84. doi: 10.1007/s13205-013-0129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pointing S.B., Jones E.B.G., Vrijmoed L.L.P. Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia. 2000;92:139–144. [Google Scholar]
- Revankar M., Desai S., Lele K.M. Solid-state fermentation for enhanced production of laccase using indigenously isolated Ganoderma sp. Appl. Biochem. Biotechnol. 2007;143:16–26. doi: 10.1007/s12010-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Rindle E., Troll W.J. Metabolic reduction of benzidine azo dyes to benzidine in the rhesus monkey. J. Natl. Cancer Inst. 1975;55:181–185. doi: 10.1093/jnci/55.1.181. [DOI] [PubMed] [Google Scholar]
- Robinson T., McMullan G., Marchant R., Nigam P. Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001;77:247–255. doi: 10.1016/s0960-8524(00)00080-8. [DOI] [PubMed] [Google Scholar]
- Saito T., Hong P., Kato K., Okazaki M., Inagaki H., Maeda S., Yokogawa Y. Purification and characterization of an extracellular laccase of a fungus (family Chaetomiaceae) isolated from soil. Enzyme Microb. Technol. 2003;33:520–526. [Google Scholar]
- Scheel T., Hofer M., Ludwig S., Holker U. Differential expression of manganese peroxidase and laccase in white-rot fungi in the presence of manganese or aromatic compounds. Appl. Microbiol. Biotechnol. 2000;54:686–691. doi: 10.1007/s002530000427. [DOI] [PubMed] [Google Scholar]
- Shraddha R.S., Sehgal S., Kamthania M., Kumar A. Laccase: microbial sources, production, purification and potential biotechnological applications. Enzyme Res. 2011 doi: 10.4061/2011/217861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares G.M.B., Pessoa-de Amorim M.T., Costa-Ferreira M. Use of laccase together with redox mediators to decolorize Remazol Brilliant Blue R. J. Biotechnol. 2001;89:123–129. doi: 10.1016/s0168-1656(01)00302-9. [DOI] [PubMed] [Google Scholar]
- Stajic M., Persky L., Friesem D., Hadar Y., Wasser S.P., Nevo E. Effect of different carbon and nitrogen sources on laccase and peroxidases production by selected Pleurotus species. Enyzme Microbiol. Technol. 2006;38:65–73. [Google Scholar]
- Tavares A.P.M., Cristóvão R.O., Loureiro J.M., Boaventura R.A.R., Macedo E.A. Optimisation of reactive textile dyes degradation by laccase–mediator system. J. Chem. Technol. Biotechnol. 2008;83:1609–1615. [Google Scholar]
- Thurston C.F. The structure and function of fungal laccases. Microbiology. 1994;140:19–26. [Google Scholar]
- Yaropolov A.I., Skorobogatko O.V., Vartanov S.S., Varfolomeyev S.D. Laccase: properties, catalytic mechanism, and applicability. Appl. Biochem. Biotechnol. 1994;49:257–280. [Google Scholar]
- Yemendzhiev H., Alexieva Z., Krastanov A. Decolorization of synthetic dye reactive blue 4 by mycelia culture of white-rot fungi Trametes versicolor. Biotechnol. Biotechnol. 2009;23:1337–1339. [Google Scholar]
- Zadrazil, F., Gonser, A., Lang, E., 1999. Influence of incubation temperature on the secretion of extracellular ligninolytic enzymes of Pleurotus sp. and Dichomitus squalens into oil. In: Proc Conf Enz Env. Granada, Spain Ecology and Applications, pp. 12–16.
- Zille A., Tzanov T., Gübitz G.M., Cavaco-Paulo M. Immobilized laccase for decolourization of reactive black dyeing effluent. Biotechnol. Lett. 2003;25:1473–1477. doi: 10.1023/a:1025032323517. [DOI] [PubMed] [Google Scholar]