Abstract
Germination bioassay was carried out to test the biological activity of Achillea santolina L. (ASAE), Artemisia monosperma Del. (AMAE), Pituranthus tortuosus L. (PTAE) and Thymus capitatus L. (TCAE) aqueous extracts (collected from Taif region, KSA) on germination percentage (GP), plumule (PL) and radicle (RL) lengths (mm) besides seedling dry weight (SDW) (mg/seedlings) of Medicago polymorpha L. The inhibitory effect of P. tortuosus was insignificant compared to the other three donor species which attained the strongest allelopathic potential in the following order: A. santolina > A. monosperma > T. capitatus. Growth experiment using crude powder of the four donor species was conducted to examine their effects on leaf area index (LAI), photosynthetic pigments, total available carbohydrates (TAC) and total protein (TP). It is worth mentioning that each of the four donor species crude powders mixed with clay loam soil appeared to have a great inhibitory allelopathic effect on LAI, total photosynthetic pigment and chlorophyll a (Chl a) while carotenoids exhibited a slight increase with the application of the four donor species crude powders. TAC and TP were significantly decreased with increasing the crude powder concentrations while a slight decrease was recorded for carbon/nitrogen (C/N) ratio. There is possibility of using these allelochemicals directly or as structural leads for the discovery and development of environmentally friendly herbicides to control weeds. The study recommended that these species must be phytochemically examined in future for their allelochemicals in order to provide information on the possibilities of using one or more of these species as bioherbicides.
Keywords: Achillea santolina, Artemisia monosperma, Pituranthus tortuosus, Thymus capitatus, Bioherbicides
1. Introduction
The loss of crop yields due to weeds is enormous (Appleby et al., 2000). Potential yield reductions caused by uncontrolled weed growth throughout a crop season have an estimated range of 45–95%, depending on ecological and climatic conditions (Moody, 1991) whereas; it is even greater in the developing and underdeveloped countries. Lacey (1985) stated that sometimes weeds cause even 100% of crop loss. The overuse of synthetic herbicides may affect the environment, human health and food (Khanh et al., 2005). Furthermore, the increasing use of the herbicides has resulted in a dramatic increase in the herbicide resistance among weeds, and over 307 weed resistant biotype belonging to 183 species (110 dicots and 73 monocots) have been identified world over (Heap, 2006, Batish et al., 2007, Gianessi and Reigner, 2007). The use of crops having allelopathic potential can reduce the dependency on synthetic herbicides and increase crop yields (Khanh et al., 2005). The phenomenon of allelopathy has been suggested to be one of the possible alternatives for achieving sustainable weed management (Abu-Romman et al., 2010, Salhi et al., 2011, Salhi et al., 2012). At present, there is a trend toward searching for novel natural plant products to develop bioherbicides. Numerous plants are reported to possess allelopathic potential, and efforts have been made to apply them for weed control (Salhi et al., 2014). Although most common allelopathic plants have potential for weed suppression, (Xuan et al., 2004a). The use of allelopathy for controlling weeds could be either through the direct utilization of natural allelopathic interactions or by using allelochemicals as natural herbicides (Singh et al., 2009, Batish et al., 2004). The need for finding stronger allelopathic plants for weed control is indispensable (Xuan et al., 2003).
The use of allelopathic and medicinal plants has been suggested as a viable option for alternative weed management under sustainable agriculture (Fujii et al., 2003, Hong et al., 2003, Batish et al., 2007, Zeng et al., 2009, Salhi et al., 2011, Salhi et al., 2012, El-Kenany and El-Darier, 2013, Salhi et al., 2013, Salhi et al., 2014). Medicago polymorpha (bur clover) is an annual broad leaf plant which inhabits agricultural lands especially wheat fields, road sides and other disturbed areas (Malik et al., 2012). The species can be considered as an invasive weed and can sometimes be toxic to livestock, and the seed pods can be a serious contaminant of wool (Cal-IPC, 2005). M. polymorpha can cause photosensitisation in horses, occasionally red gut in sheep, and bloat in cattle. Phytoestrogens/coumestrols can potentially have negative effects on the reproduction of grazing livestock (Cabi Database, 2014). The allelopathic potential of Medicago species has been previously reported against edible plants (El-Darier et al., 2014). The main objective of the present study was to explore the possible allelopathic effects of Achillea santolina L., Artemisia monosperma Del., Pituranthus tortuosus L. and Thymus capitatus L. (donor species) aqueous extracts and crude powder on germination efficiency, some growth parameters as well as some metabolic changes of the weedy species; M. polymorpha L. under laboratory conditions.
2. Materials and methods
2.1. Plant materials and soil
Samples of fresh aerial shoots of four medicinal plants as donor/allelopathic species (A. santolina L., A. monosperma Del., P. tortuosus L. and T. capitatus L.) were collected from different locations at Taif region Saudi Arabia during spring 2015. The seeds of the weedy species; M. polymorpha L., were collected locally from the same locations and surface cleaned then stored in paper bags at room temperature (20 ± 2 °C) until further use. Additionally, soil samples were excavated from some agricultural fields in the same locations and applied in a pot experiment.
2.2. Phytochemical screening
Test for sterols, tannins, flavonoids, coumarins and alkaloids was carried out according to the methods described by Harborne, 1973, Harborne, 1998, Harborne, 1999 respectively. Additionally, glycosides, saponins, were detected by the procedures of Farnsworth, 1966, Lewis and Smith, 1967 respectively. On the other hand, the content of total phenols and flavonoids was determined according to Meda et al. (2005) while extraction and estimation of essential oils followed the method described by Odalo et al. (2005).
2.3. Preparation of donor species aqueous extracts
The collected aerial shoot samples were air-dried, then, cut into 0.5–1 cm pieces. Stock aqueous extract was obtained by soaking 150 g of the air-dried donor plant materials in one liter of distilled water at room temperature (20 ± 2 °C) for 24 h. The mixture was filtered through four layers of cheesecloth to remove the fiber debris and kept at 5 °C in the dark until further use. Different concentrations (5%, 10%, 15% and 20%) were prepared from the stock solution, in addition to the control (distilled water).
2.4. Germination bioassay
Petri-dish experiment was carried out to investigate the potential allelopathic effects of donor species aqueous extracts on germination percentage (GP), plumule (PL) and radicle (RL) lengths, as well as seedlings dry weight (SDW) of the recipient species. Consequently, twenty-five seeds of the recipient species were arranged in 9-cm diameter Petri-dishes on two disks of Whatman No. 1 filter paper under normal laboratory conditions with day temperature ranging from 20 °C to 23 °C and night temperature from 14 °C to 16 °C. Ten cm3 of each level of the donor species extracts were added to four replicates. Before sowing, the seeds were treated with H2SO4 (96%) for 2 min then washed several times with distilled water. Thereafter, the seeds were surface sterilized by soaking for two minutes in 4% sodium hypochlorite, then, rinsed four times with distilled water. Treatments were arranged in a complete randomized block design with four replications. Measurements of GP, PL and RL were recorded after 9 days at the end of the experiment. Inhibition percentage (IP) was calculated according to the following equation (Khanh et al., 2005):
while the reduction in Pl and RL was calculated according to the routine equation:
Seedlings were dried at 105 °C till constant weight for the determination of seedling dry weight (SDW).
2.5. Growth experiment
Pot experiment was performed to validate the effect of different levels of each of the four donor species (A. santolina, A. monosperma, P. tortuosus and T. capitatus) crude powder mixed (w/w) with clay loam soil (collected from control locations) on leaf area index (LAI), photosynthetic pigments in addition to carbohydrate and protein content of M. polymorpha (recipient species). Total plant leaf area was measured using Digital Planimeter (Placom KP-90) to the nearest cm2. Leaf area index (LAI) was calculated by dividing the total plant leaf area by its specific ground area. The photosynthetic pigments chlorophyll a, b (Chl a, Chl b) and carotenoids (Carot) were extracted and determined using the spectrophotometric method described by Metzner et al. (1965). Total available carbohydrates (TAC) were estimated by the procedure described by Murata et al. (1968). Total proteins were extracted according to Rausch (1981) and estimated as described by Hartree (1972).
2.6. Statistical analysis
All the data of the present study were subjected, where appropriate, to standard one-way analysis of variance (ANOVA) and t-test (Zar, 1984) using the COSTAT 2.00 statistical analysis software manufactured by CoHort Software Company. Pairwise comparisons of means were performed using Least Significant Differences (LSD) at 0.05 probability level.
3. Results
3.1. Soil analysis
The routine analyses for soil applied in the current study are presented in Table 1. The soil texture was sandy loam with pH value of about 7.4., the CaCO3 and OM percentage attained values of about 23.8 and 2.3 respectively. Additionally, cations attained considerable values.
Table 1.
Determination of some physical and chemical properties ± SD of soil collected from at Taif region (KSA) during the year of 2015.
| Character | Mean ± SD |
|---|---|
| pH | 7.4 ± 1.5 |
| EC (mmhos cm−1) | 3.5 ± 0.6 |
| OM (%) | 2.3 ± 0.4 |
| OC (%) | 1.7 ± 0.1 |
| CaCO3 (%) | 23.8 ± 3.2 |
| Soluble cations and anions (mg/100 g soil) | |
| Total N | 65 ± 5.6 |
| P+++ | 1.2 ± 0.2 |
| K+ | 67 ± 6.1 |
| Ca++ | 125 ± 7.2 |
| Mg++ | 23.4 ± 2.5 |
| Na+ | 90.8 ± 5.3 |
| Cl− | 100.7 ± 6.3 |
| HCO3− | 9.20 ± 2.1 |
| Soil texture (particle size distribution) | |
| Sand % | 53 ± 5 |
| Silt % | 30 ± 3 |
| Clay % | 17 ± 2 |
Data are means of 5 replicates ± SD.
EC: electrical conductivity, OM: organic matter, OC: organic carbon.
3.2. Phytochemical screening of the donor species
The phytochemical screening of the donor species is presented in Table 2. The data showed that all the donor species were found to contain essential oils, flavonoids, glycosides, total phenols, sterols and tannins with different incidence. However, saponins were not detected in P. tortuosus, which is the only plant species that contains coumarins. Alkaloids were detected only in A. santolina and A. monosperma. In addition, data in Table 3 clearly demonstrated that the highest value of total flavonoids (937 mg/100 g dw), total phenols (6935 mg/100 g dw), and essential oil (2%) were detected in A. monosperma while the lowest values of the three phytochemical groups (246, 1057 mg/100 g dw and 0.12% respectively) were detected in P. tortuosus compared to the other plant species.
Table 2.
Qualitative phytochemical screening of the four donor species collected from different locations at Taif region (KSA) during the year of 2015.
| Chemical group | Plant species |
|||
|---|---|---|---|---|
| Achillea santolina | Artemisia monosperma | Pituranthus tortuosus | Thymus capitatus | |
| Alkaloids | + | + | − | − |
| Coumarins | − | − | ++ | − |
| Essential oil | ++ | +++ | ++ | +++ |
| Flavonoids | ++ + | +++ | + | ++ |
| Glycosides | ++ | ++ | + | ++ |
| Total phenols | ++ | ++++ | + | +++ |
| Saponins | ++ | + | − | + |
| Sterols | ++ | ++ | ++ | ++ |
| Tannins | + | + | + | + |
+: detected, −: not detected.
The notations, ++++, +++, ++, + and − refer to highly appreciable amounts (positive within <2 min); appreciable amounts (positive within 5 min), moderate amounts (positive after 5 min but within 10 min); trace amounts (positive after 10 min but within 15 min) and completely absent, respectively.
Table 3.
Variation in the content of total flavonoids and total phenolics (mg/100 g dw) as well as essential oils (%) detected in the four donor species collected from different locations at Taif region (KSA) during the year of 2015.
| Chemical group | Plant species |
|||
|---|---|---|---|---|
| Achillea santolina | Artemisia monosperma | Pituranthus tortuosus | Thymus capitatus | |
| Total flavonoids | 824 ± 24 | 937 ± 32 | 246 ± 12 | 500 ± 18 |
| Total phenolics | 2672 ± 56 | 6935 ± 87 | 1057 ± 29 | 3842 ± 64 |
| Essential oil | 0.65 ± 0.01 | 2.00 ± 0.12 | 0.12 ± 0.02 | 1.00 ± 0.07 |
Data are means of three replicates ± SD.
Total flavonoids were determined as rutin and total phenolics as chlorogenic acid.
A quantitative analysis of each essential oil component was carried out by peak area normalization measurement.
3.3. Germination and growth parameters
The germination percentage (GP), plumule (PL) and radicle (RL) lengths as well as SDW of M. polymorpha were significantly affected by applying different concentrations of A. santolina (ASAE), A. monosperma (AMAE), P. tortuosus (PTAE) and T. capitatus (TCAE) aqueous extracts (Table 4, Table 5, and Fig. 1). The inhibitory effect of the four donor species was in the following order: A. santolina > A. monosperma > T. capitatus > P. tortuosus. Noticeably, GP exhibited a gradual decrease with the increase of all donor species aqueous extracts and the germination was completely inhibited (100% inhibition) at 20% for ASAE and AMAE (Table 4). On the other hand, PTAE and TCAE exercised germination inhibition percentages (IP) of about 46.34 and 77.78 respectively compared to control at the same concentration.
Table 4.
Suppression effect of aqueous extracts from different medicinal plants on germination percentage (GP) of Medicago polymorpha.
| Treatment (%) | Plant species |
|||
|---|---|---|---|---|
| Achillea santolina | Artemisia monosperma | Pituranthus tortuosus | Thymus capitatus | |
| 0 | 84a ± 2 | 89a ± 2 | 82a ± 4 | 90a ± 2 |
| 5 | 49b ± 3 | 55b ± 2 | 67b ± 3 | 50b ± 1 |
| 10 | 35c ± 2 | 38c ± 3 | 64c ± 1 | 40c ± 1 |
| 15 | 13d ± 2 | 17d ± 1 | 57d ± 4 | 25d ± 3 |
| 20 | 0.0e | 0.0e | 44e ± 2 | 20e ± 2 |
Data are means of 5 replicates ± SD.
Different letters within each column indicate a significant difference at P < 0.05 level of probability as evaluated by one-way ANOVA test.
Table 5.
Suppression effect of aqueous extracts from different medicinal plants on plumule (PL) and radicle (RL) lengths (mm) of Medicago polymorpha.
| Treatment(%) | Plant species |
|||||||
|---|---|---|---|---|---|---|---|---|
|
Achillea santolina |
Artemisia monosperma |
Pituranthus tortuosus |
Thymus capitatus |
|||||
| PL | RL | PL | RL | PL | RL | PL | RL | |
| 0 | 41a ± 4 | 70a ± 6 | 46a ± 4 | 75a ± 6 | 49a ± 4 | 78a ± 6 | 41a ± 4 | 70a ± 6 |
| 5 | 26b ± 2 | 35b ± 3 | 29b ± 3 | 38b ± 3 | 29b ± 3 | 39b ± 4 | 32b ± 2 | 51b ± 4 |
| 10 | 14c ± 2 | 24c ± 3 | 18c ± 2 | 28c ± 2 | 23c ± 3 | 32c ± 3 | 26c ± 3 | 38c ± 5 |
| 15 | 9d ± 1 | 10d ± 1 | 12d ± 2 | 18d ± 2 | 16d ± 2 | 22d ± 3 | 20d ± 3 | 31d ± 4 |
| 20 | 0.0e | 0.0e | 0.0e | 0.0e | 9e ± 2 | 10e ± 2 | 10e ± 1 | 9e ± 1 |
Data are means of 5 replicates ± SD.
Different letters within each column indicate a significant difference at P < 0.05 level of probability as evaluated by one-way ANOVA test.
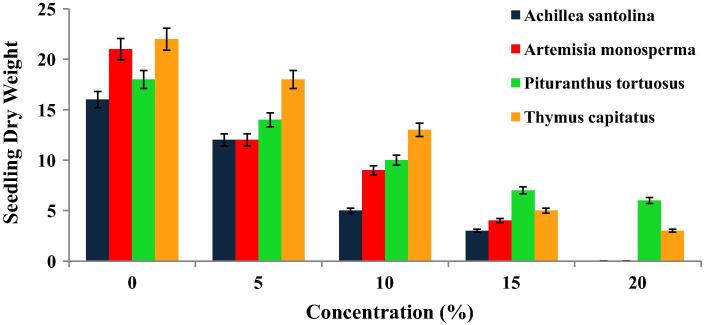
Figure 1.
Suppression effect of aqueous extracts from different medicinal plants on seedling dry weight (SDW) (mg/seedling) of Medicago polymorpha. Vertical bar above each column represents SD.
Markedly, the plumule and radicle growth followed the same trend exhibited by the GP (Table 5). The reduction exerted as a result of application of PTAE and TCAE on the recipient species for PL was 81.36% and 75.61% and for RL was 87.18% and 87.14% respectively compared to control at the maximum concentration (20%). The other two extracts attained their maximum reduction percentages of about 78.05% for PL and 85.71% for RL as well as 37.91% for PL and 76% for RL with regard to ASAE and AMAE respectively at 15% concentration level.
Consequently, the maximum reduction percentages in SDW (mg/seedling) of M. polymorpha (81.25% and 80.95%) were achieved respectively at the highest ASAE and AMAE concentration level (15%) compared to control (Fig. 1). The equivalent values were 66.66% and 86.36% respectively for PTAE and TCAE at the highest extract concentration level (20%).
3.4. Physiological parameters
3.4.1. Leaf area index (LAI)
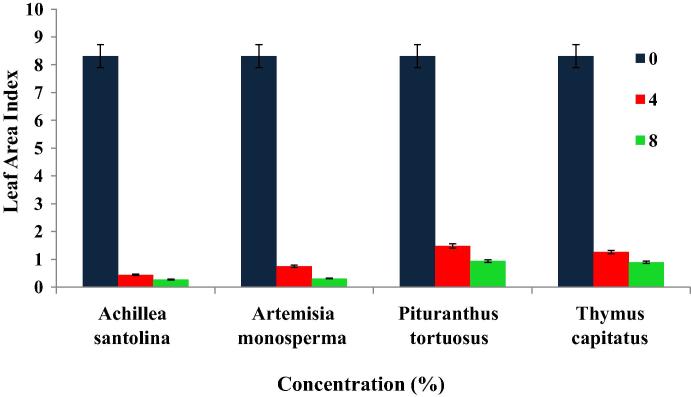
Leaf area index (LAI) (cm2/cm2) achieved a significant reduction due to the ascending of the four donor species crude powders (ASCP, AMCP, PTCP and TCCP) (Fig. 2). The control value was about 8.31 (mm2/mm2) while at 8%, LAI exhibited reduced values of about 0.27, 0.31, 0.94 and 0.89 (mm2/mm2) for the four crude powders respectively.
Figure 2.
Suppression effect of crude powders from different medicinal plants on leaf area index (LAI) (cm2/cm2) of Medicago polymorpha. Vertical bar above each column represents SD.
3.4.2. Pigment content
A significant decrease was attained in the total photosynthetic pigment contents of the recipient species as affected by application of A. santolina (ASCP), A. monosperma (AMCP), P. tortuosus (PTCP) and T. capitatus (TCCP) donor species crude powders (Table 6). The reduction percentages were about 51.8, 45.7, 31.02 and 40.36 respectively which are compatible with 60.15%, 56.19%, 37.1% and 55.04% reduction percentages for Chl a respectively. On the other hand, carotenoids exhibited a slight increase with the application of the four donor species crude powders.
Table 6.
Suppression effect of crude powders from different medicinal plants on pigment content ± SD of Medicago polymorpha.
| Donor species | Treatment (%) | Chlorophyll fractions |
|||
|---|---|---|---|---|---|
| Chl a | Chl b | Carot | Total | ||
| Achillea santolina | 0 | 15.66a ± 2.1 | 5.89a ± 0.5 | 0.92b ± 0.01 | 22.47a ± 1.3 |
| 4 | 7.23b ± 1.4 | 2.81b ± 0.2 | 2.15a ± 0.03 | 12.19b ± 0.9 | |
| 8 | 6.24c ± 1.2 | 1.93c ± 0.1 | 2.66a ± 0.02 | 10.83c ± 1.3 | |
| Artemisia monosperma | 0 | 15.66a ± 2 | 5.89a ± 0.4 | 0.92c ± 0.01 | 22.47a ± 1.7 |
| 4 | 9.21b ± 1.5 | 3.32b ± 0.6 | 1.98b ± 0.1 | 14.51b ± 1.7 | |
| 8 | 6.86c ± 1.2 | 2.00c ± 0.2 | 3.34a ± 0.3 | 12.20c ± 2 | |
| Pituranthus tortuosus | 0 | 15.66a ± 1.6 | 6.62a ± 1 | 0.92b ± 0.04 | 22.47a ± 2.6 |
| 4 | 14.85b ± 1.4 | 5.89b ± 1.1 | 1.14ab ± 0.01 | 21.88b ± 1.5 | |
| 8 | 9.85c ± 2 | 3.87c ± 0.9 | 1.78a ± 0.2 | 15.50c ± 2 | |
| Thymus capitatus | 0 | 15.66a ± 2.1 | 5.89a ± 0.6 | 0.92b ± 0.02 | 22.47a ± 1.3 |
| 4 | 8.95b ± 2 | 4.42b ± 0.5 | 2.20a ± 0.02 | 15.57b ± 1.4 | |
| 8 | 7.01c ± 1.6 | 3.56c ± 0.8 | 2.83a ± 0.1 | 13.40c ± 1.2 | |
Data are means of 5 replicates ± SD.
Chl a: chlorophyll a.
Chl b: chlorophyll b.
Carot: carotenoids.
Different letters within each column and receptor species indicate a significant difference at P < 0.05 level of probability as evaluated by one-way ANOVA test.
3.4.3. Total available carbohydrates, total proteins and C/N ratio
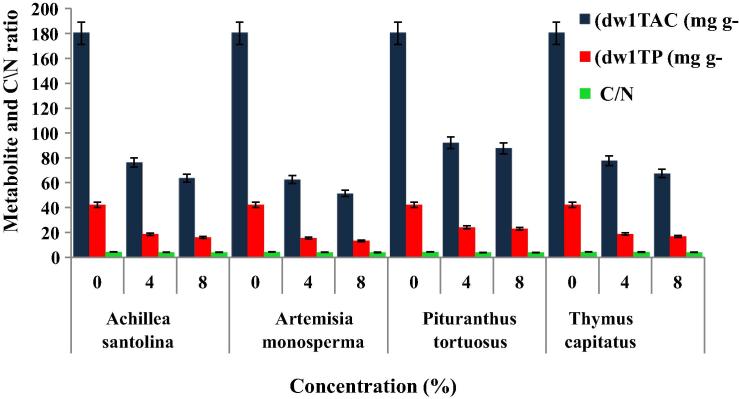
Generally, total available carbohydrates (TAC) and total protein (TP) (mg g−1 dw) of the recipient species were significantly decreased with increasing crude powder concentrations (Fig. 3). Application of ASCP with 8% crude powder concentration created a decrease of about 64% and 62% while the decrease due to AMCP attained values of about 71% and 68% compared to control respectively. Similarly, decrease percentages of about 51% and 45% as well as 62% and 62% were achieved for TAC and TP content as affected by PTCP and TCCP respectively. On the other hand, carbon/nitrogen ratio exhibited a slight decrease upon applying donor species crude powders.
Figure 3.
Suppression effect of crude powders from different medicinal plants on total available carbohydrates (TAC), total proteins (TP) as well as C/N ratio of Medicago polymorpha. Vertical bar above each column represents SD.
4. Discussion
Medicinal plants have inhibitory effects on selected weeds and its allelochemicals inhibiting weed growth (Lin et al., 2003, Lin et al., 2004, Hemada and El-Darier, 2015). Therefore, it was easier to screen allelopathic plants from medicinal ones than other plants possibly because they have the ability to accumulate certain metabolic compounds curing many diseases of mankind (Qasem and Hassan, 2003, Salhi et al., 2014). In the present study, the aqueous extract of all the donor species (A. santolina, A. monosperma, P. tortuosus and T. capitatus) suppressed seed germination of M. polymorpha under different concentrations. The degree of inhibition was enhanced by increasing the concentration. The allelopathic effect of the donor species was ranked as follows: A. monosperma > T. capitatus > A. santolina > P. tortuosus. Noticeably, this alternative effect of the allelopathic substance could be related to the species specific growth regulatory effect of allelochemicals and could be concentration dependent (Einhellig, 1996, Salhi et al., 2012) expressed in the phytochemical screening of the four donor species. These results are in agreement with those previously reported by Kato-Noguchi (2001) who found that Melissa officinalis shoots aqueous extract inhibited the seed germination of the weed species; Amaranthus caudatus, Digitaria sanguinalis, Phleum pratense and Lolium multiflorum. Additionally, such an inhibition was supported by data provided by other studies (Batish et al., 2004, Xuan et al., 2004, Khanh et al., 2005, Khanh et al., 2006). Moreover, Ashrafi et al. (2008) demonstrated that aqueous extract of Azadirachta indica (Neem) shoots reduced the seed germination of Amaranthus rotundus, Cirsium arvense, D. sanguinalis, Sinapis arvensis and L. multiflorum as weed species.
Nearly, all reports on allelopathy described some types of bioassay method used to demonstrate allelopathic activity (Macias et al., 2000). The most widely used bioassay is the technique of seed germination. However, several studies had revealed that this was not the most sensitive parameter (Leather and Einhellig, 1986, Fuentes et al., 2004) and growth bioassays were often more sensitive than germination bioassays (Bhowmik and Doll, 1984) especially for the evaluation of phytotoxicity. Additionally, Hall and Henderlong (1989) and Chung and Miller (1995) had also observed that the seedling growth was more sensitive to allelochemicals than the associated seed germination.
Results of the current study suggested that aqueous extracts of all the donor species exhibited inhibitory effect on plumule (PL) and radicle (RL) lengths of the recipient species under all concentration levels, which support the previously documented GP implications. Both PL and RL of the recipient species were affected negatively due to the addition of different plant extracts and this effect was directly proportional to the concentration and more significant in the case of the radicle compared to the plumule. These results are in agreement with the declaration that water extracts of allelopathic plants have generally more pronounced effects on radicle length rather than plumule length (Turk and Tawaha, 2002, Ashrafi et al., 2007). Additionally, Ashrafi et al. (2008) stated that radicle length was more sensitive to allelochemicals than hypocotyls. Wakjira et al. (2005) reported that lettuce roots were more sensitive to the allelochemicals than shoots on applying Parthenium hysterophorus extracts. Similar findings were also reported by Batish et al., 2004, Modallal and Al-Charchafchi, 2006, Vasilakoglou et al., 2007 and Mekky (2008). In a parallel work, aqueous extracts of Lantana camara reduced root growth of Phalaris minor and Sorghum bicolor (El-Kenany and El-Darier, 2013). This may be attributable to the fact that radicle had direct contact with soil or extract and can absorb many allelochemicals. The most probable explanation for the reductions in seedling root and shoot is the reduced rate of cell division and cell elongation due to the presence of allelochemicals in the aqueous extracts (Javaid and Anjum, 2006). Furthermore, allelochemicals could inhibit the elongation, expansion and division of cells which were a prerequisite for seedling growth (Einhellig, 1995). Several studies had shown that compounds of plant origin, such as allelochemicals, affect mitotic activity of growing roots (Rizvi et al., 1992, Einhellig, 1996). Such an inhibitory effect on mitotic may directly decrease plant growth, and so mitotic activity can be used to evaluate root growth resulting from cell division of meristematic cells and cell expansion in the elongation zone of roots (Dayan et al., 2000).
It was reported that measuring the seedling length might result in complicated curling and other morphological alterations such as the seedling dry weight (SDW) which may be a better selection criteria for allelopathic potential than the seedling length (Ahn and Chung, 2000). These findings agreed with that obtained by Terzi et al. (2003). The authors emphasized that the dry weights of root and the stem of cucumber seedlings were negatively influenced by the decomposed walnut leaves and juglone, depending on the concentration. Under the present study, the aqueous extract of all the donor species reduced SDW of the recipient species at different concentration levels. Reduction increased as the concentration of the donor plant increased; however the reduction degree was varied and was species dependent. A. monosperma extract had the greatest allelopathic potential at all concentrations, while P. tortuosus had the lowest ones. This reduction may be attributed to the presence of allelochemicals in the aqueous extracts. Baziramakenga et al. (1994) found that allelochemicals reduced the number of lateral roots, root and shoot dry biomass of soybean. Similar findings were also reported by Batish et al. (2004) who found that eucalypt oil could significantly reduce the seedling growth, as well as the chlorophyll content and the cellular respiration of the tested plants. Additionally, coumarin derivatives inhibited the dry matter production, the leaf area expansion, and the photosynthesis in the seedling of tobacco, sunflower and pigweed (Einhellig et al., 1970). An analogous work was conducted to determine the allelopathic effect of Passiflora edulis aqueous extracts on growth and seedling dry weight of two major paddy weeds Echinochloa crusgalli and Monochoria vaginalis. It was reported that the aqueous extracts strongly suppressed seedling growth.
Data also revealed that all the donor species crude powder concentrations suppressed LAI of the recipient species. This reduction may be attributed to the presence of allelochemicals in the aqueous extracts and may be related to the inhibition of cell division and/or cell expansion (El-Darier, 2002, Javaid and Anjum, 2006). Currently, total photosynthetic pigments were significantly decreased in the recipient species with the increasing of donor species crude powder levels which may be attributed to the decrease of chlorophyll a content. In general, the decrease in photosynthetic pigment may be related to the inhibitory effect of the released allelochemical substances from the donor species on the synthesis of porphyrin precursors of chlorophyll biosynthesis (Rice, 1984), inhibition of specific enzymes responsible for the synthesis of chlorophyll and/or the activation of chlorophyllase which catalyzed the catabolism of chlorophyll (Yang et al., 2004) or the modification in the integrity of chloroplast and thylakoid membranes in response to allelochemicals (Batish et al., 2004).
Throughout the present study, the total available carbohydrates (TAC) in the recipient species were significantly decreased with the increasing of donor species crude powder levels. Furthermore, the crude powder of A. monosperma showed the greatest allelopathic potential, while P. tortuosus had the lowest effect. Such decrease may be attributed to the inhibitory effect of the released allelochemical substances on the synthesis of photosynthetic pigment, and hence, on photosynthesis. Bernat et al. (2004) reported that the decrease of TAC of Sinapis albaunder allelochemical stress may be related to the reduction of the photosynthetic leaf area and the stomatal frequency, as well as, the inhibitory effect on the photosynthetic apparatus which may result in a decrease of CO2 diffusion and assimilation. In addition, the reduction of photosynthetic allocation may be due to the destructive effect of allelochemical stress on plasma membrane and phloem elements (Singh and Rao, 2003). Also, Gniazdowska and Bogatek (2005) concluded that the consumption of most photosynthates in respiration for diverting energy to maintenance of the physiological processes, under allelochemicals stress, resulted in a decrease of TAC in Zea mays seedlings. Therefore, under prevailing experimental conditions, insufficient non-structural saccharides were converted to structural carbohydrates which were incorporated as carbon skeleton for the growth of recipient species. Thus, this suggestion, in line with the present study, may explain the decrease in dry mass and growth of the tested crop and weedy species.
It had been demonstrated that allelopathic substance stress generally induced a marked decrease in total protein content. Under the present study, total protein content in the recipient species was significantly decreased with the increasing of donor species crude powder levels. Inhibition of photosynthesis and other impaired metabolic activities, under this study, may have resulted in a decrease of protein synthesis and/or the stimulation of the protein degradation. Mersie and Singh (1993) and Hoque et al. (2007) reported that the degradation of the protein to amino acids, such as proline, might be an adaptation mechanism against the allelochemical stress and/or a means of osmolytes to prevent water loss. In addition, allelochemicals are known to generate reactive oxygen species (ROS) which caused oxidative modification/degradation of proteins (Lara-Nunez et al., 2006, Venkateshwarlu et al., 2001). Therefore, the decline in total protein content, under this study, may be related to the enhancement of protein degradation, and/or alteration in the incorporation of amino acids into protein, hence, reduce the growth of target species.
5. Conclusion
The present study evaluated the allelopathic potential of ASAE, AMAE, PTAE and TCAE on GP, PL, RL, SDW, LAI, pigments, TAC and TP of M. polymorpha. All these parameters were significantly decreased at different concentration levels. Based on these results, the recipient species showed distinct poor growth and metabolism under the effect of the different donor species. The species with the strongest allelopathic potential (A. monosperma, T. capitatus and A. santolina) must be examined for their selective action on other specific plants including crops and weeds under field conditions, and their allelopathic activity should be further studied in detail.
Acknowledgements
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (G-1436-130-487). The authors, therefore, acknowledge with thanks DSR technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abu-Romman S., Shatnawi M., Shibli R. Allelopathic effects of spurge (Euphorbia hierosolymitana) on wheat (Triticum durum) American-Eurasian J. Agric. Environ. Sci. 2010;7:298–302. [Google Scholar]
- Ahn J.K., Chung I.M. Allelopathic potential of rice hulls on germination and seedling growth of Barnyard grass. Agron. J. 2000;92:1162–1167. [Google Scholar]
- Appleby A.P., Muller F., Carpy S. Weed control. In: Muller F., editor. Agrochemicals. Willy VCH; New York: 2000. pp. 687–709. [Google Scholar]
- Ashrafi Z.Y., Mashhadi H.R., Sadeghi S. Allelopathic effects of barley (Hordeum vulgare) on germination and growth of wild barley (Hordeum spontaneum) Pak. J. Weed Sci. Res. 2007;13:99–112. [Google Scholar]
- Ashrafi Z.Y., Sadeghi S., Mashhadi H.R., Hassan M.A. Allelopathic effects of sunflower (Helianthus annuus) on germination and growth of wild barley (Hordeum spontaneum) J. Agric. Technol. 2008;4:219–229. [Google Scholar]
- Batish D.R., Setia N., Singh H.P., Kohli R.K. Phytotoxicity of lemon-scented eucalypt oil and its potential use as a bioherbicides. Crop Prot. 2004;23:1209–1214. [Google Scholar]
- Batish D.R., Arora K., Singh H.P., Kohli R.K. Potential utilization of dried powder of Tagetes minuta as a natural herbicide for managing rice weeds. Crop Prot. 2007;26:566–571. [Google Scholar]
- Baziramakenga R., Simard R.R., Lerroux G.D. Effects of benzoic and cinnamic acids on growth, mineral composition and chlorophyll content of soybean. J. Chem. Ecol. 1994;20:2821–2833. doi: 10.1007/BF02098391. [DOI] [PubMed] [Google Scholar]
- Bernat W., Gawronska H., Gawronski S.W. Physiological effects of allelopathic activity of sunflower on mustard. Zesz. Probl. Post. Nauk Roln. 2004;496:275–287. [Google Scholar]
- Bhowmik P.C., Doll J.D. Allelopathic effects of annual weed residues on growth and nutrient uptake of corn and soyabeans. Agron. J. 1984;76:383–388. [Google Scholar]
- Cabi Database, 2014. <http://www.cabi.org/isc/datasheet/33031/> (accessed 28.01.2016).
- Cal-IPC, 2005. California Invasive Plant Council reports on plant assessment form for Medicago polymorpha, Berkeley, California, USA. <http://www.cal-ipc.org/paf/site/paf/380/> (accessed 17.01.2016).
- Chung I.M., Miller D.A. Natural herbicide potential of alfalfa residues on selected weed species. Agron. J. 1995;87:920–925. [Google Scholar]
- Dayan F., Romagni J.G., Duke S.O. Investigating the mode of action of natural phytotoxins. J. Chem. Ecol. 2000;26:2079–2094. [Google Scholar]
- Einhellig F.A. Allelopathy: current status and future goals. In: Inderjit Dakshini K.M.M., Einhellig F.A., editors. Allelopathy: Organisms, Processes, and Applications. American Chemical Society; Washington, DC: 1995. pp. 1–24. [Google Scholar]
- Einhellig F.A. Interactions involving allelopathy in cropping systems. Agron. J. 1996;88:886–893. [Google Scholar]
- Einhellig F.A., Rice E.L., Risser P.G., Wender S.H. Effects of scopoletin on growth, CO2 exchange rates and concentration of scopoletin, scopolin and chlorogenic acids in tobacco, sunflower and pigweed. Bull. Torrey Bot. Club. 1970;97:22–23. [Google Scholar]
- El-Darier S.M. Allelopathic effects of Eucalyptus rostrata on growth, nutrient uptake and metabolite accumulation of Vicia faba L. and Zea mays L. Pak. J. Biol. Sci. 2002;5:6–11. [Google Scholar]
- El-Darier S.M., Abdelaziz H.A., Zein El-Dien M.H. Effect of soil type on the allelotoxic activity of Medicago sativa L. residues in Vicia faba L. agroecosystems. J. Taibah Univ. Sci. 2014;8:84–89. [Google Scholar]
- El-Kenany E.T., El-Darier S.M. Suppression effects of Lantana camara L. aqueous extracts on germination efficiency of Phalaris minor Retz. and Sorghum bicolor L. (Moench) J. Taibah Univ. Sci. 2013;7:67–71. [Google Scholar]
- Farnsworth N.R. Biological and phytochemical screening of plants. J. Pharm. Sci. 1966;55:225–276. doi: 10.1002/jps.2600550302. [DOI] [PubMed] [Google Scholar]
- Fuentes A., Liorens M., Saez J., Aguilar M.I., Ortuno J.F., Meseguer V.F. Phytotoxicity and heavy metals speciation of stabilized sewage sludges. J. Hazard. Mater. 2004;108:161–169. doi: 10.1016/j.jhazmat.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Fujii Y., Parvez S.S., Parvez M.M., Ohmae Y., Iida O. Screening of 239 medicinal plant species for allelopathic activity using the sandwich method. Weed Biol. Manage. 2003;3:233–241. [Google Scholar]
- Gianessi L.P., Reigner N.P. The value of herbicides in US crop production. Weed Technol. 2007;21:559–566. [Google Scholar]
- Gniazdowska A., Bogatek R. Allelopathic interactions between plants. Multisite action of allelochemicals. Acta Physiol. Plant. 2005;27:395–407. [Google Scholar]
- Hall M.H., Henderlong P.R. Alfalfa autotoxic fraction characterization and initial separation. Crop Sci. 1989;29:425–428. [Google Scholar]
- Harborne J.B. Chapman and Hall; London: 1973. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; p. 279. [Google Scholar]
- Harborne J.B. second ed. Chapman and Hall; London: 1998. Phytochemical Methods: A Guide to Modern Techniques of Plants Analysis; pp. 54–84. [Google Scholar]
- Harborne J.B. Classes and functions of secondary products from plants. In: Walton N.J., Brown D.E., editors. Chemicals from Plants-Perspectives on Plant Secondary Products. Imperial College Press; London: 1999. pp. 1–25. [Google Scholar]
- Hartree E.F. Determination of protein: a modification of the lowry method that gives a linear photometric response. Anal. Biochem. 1972;48:422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Heap, I., 2006. International survey of herbicide resistant weeds. Available online at URL: <www.weedscience.com>.
- Hemada M.M., El-Darier S.M. Management of a noxious weed; Melilotus indicus L.via allelopathy of Cotula cinerea Del. Int. J. Adv. Res. 2015;3:553–561. [Google Scholar]
- Hong N.H., Xuan T.D., Tsuzuki E., Terao H., Matsuo M., Khanh T.D. Screening for allelopathic potential of higher plants from Southeast Asia. Crop Prot. 2003;22:829–836. [Google Scholar]
- Hoque M.A., Banu M.N., Okuma E., Amako K., Nakamura Y., Shimoishi Y., Murata Y. Exogenous proline and glycine betaine increase NaCl-induced ascorbate-glutathione cycle enzyme activities and proline improves salt tolerance more than glycine betaine in tobacco bright yellow-2 suspension cultured cells. J. Plant Physiol. 2007;164:1457–1468. doi: 10.1016/j.jplph.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Javaid A., Anjum T. Control of Parthenium hysterophorus L., by aqueous extracts of allelopathic grasses. Pak. J. Bot. 2006;38:139–145. [Google Scholar]
- Kato-Noguchi H. Effects of lemon balm, Melissa officinalis extract on germination and seedling growth of six plants. Acta Physiol. Plant. 2001;23:49–53. [Google Scholar]
- Khanh T.D., Chung I.M., Xuan T.D., Tawata S. The exploitation of allelopathy in sustainable agricultural production. J. Agron. Crop Sci. 2005;191:172–184. [Google Scholar]
- Khanh T.D., Chung I.M., Tawata S., Xuan T.D. Weed suppression by Passiflora edulis and its potential allelochemicals. Weed Res. 2006;46:296–303. [Google Scholar]
- Lacey A.J. Weed control. In: Haskell P.T., editor. Pesticide Applications: Principles and Practices. Oxford University Press; Oxford: 1985. pp. 456–485. [Google Scholar]
- Lara-Nunez A., Romero-Romero T., Ventura J.L., Blancas V., Anaya A.L., Cruz-Ortega R. Allelochemical stress causes inhibition of growth and oxidative damage in Lycopersicon esculentum Mill. Plant Cell Environ. 2006;29:2009–2016. doi: 10.1111/j.1365-3040.2006.01575.x. [DOI] [PubMed] [Google Scholar]
- Leather G.R., Einhellig F.A. Bioassays in the study of allelopathy. In: Putnan A.R., Tang C.-S., editors. The Science of Allelopathy. Wiley-Interscience; New Jersey: 1986. pp. 133–145. [Google Scholar]
- Lewis H., Smith C. Sugars alcohol in fungi and green plants. Methods of detection and estimation. New Phytol. 1967;66:185–204. [Google Scholar]
- Lin D., Tsuzuki E., Sugimoto Y., Dong Y., Matsuo M., Terao H. Assessment of dwarf lilyturf (Ophiopogon japonicus K.) dried powders for weed control in transplanted rice. Crop Prot. 2003;22:431–435. [Google Scholar]
- Lin D., Tsuzuki E., Sugimoto Y., Dong Y., Matsuo M., Terao H. Elementary identification and biological activities of phenolic allelochemicals from dwarf lilyturf plant (Ophiopogon japonicus K.) against two weeds of paddy rice field. Plant Prod. Sci. 2004;7:260–265. [Google Scholar]
- Macias F.A., Castelland D., Molinillo M.J. Search for a standard phytotoxic bioassay for allelochemicals. Selection of standard target species. J. Agric. Food Chem. 2000;48:2512–2521. doi: 10.1021/jf9903051. [DOI] [PubMed] [Google Scholar]
- Malik M.A., Khan Z., Khan A. Weed diversity in wheat fields of upper Indus plains in Punjab. Pak. J. Weed Sci. Res. 2012;18:413–421. [Google Scholar]
- Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. [Google Scholar]
- Mekky M.S. Allelopathic effects of blue gum (Eucalyptus globules), sweet basil (Ocimum basilicum), wormwood (Artemisia annua) and sweet potato (Ipomoea batatas) extracts on seeds germination and seedling development of some weed species. Egypt. J. Appl. Sci. 2008;23:95–106. [Google Scholar]
- Mersie W., Singh M. Phenolic acids affect photosynthesis and protein synthesis by isolated, leaf cells of velvet-leaf. J. Chem. Ecol. 1993;19:1293–1301. doi: 10.1007/BF00984876. [DOI] [PubMed] [Google Scholar]
- Metzner H., Rau H., Senger H. Untersuchungen Zur Synchronisier barkeep ein Zelner pigment Mango I Mutanten Von chlorella. Planta. 1965;65:186. [Google Scholar]
- Modallal N.M., Al-Charchafchi F.M. Allelopathic effect of Artemisia herbaalba on germination and seedling growth of Anabasis setifera. Pak. J. Biol. Sci. 2006;9:1795–1798. [Google Scholar]
- Moody K. Weed management in rice. In: Pimentel D., editor. Handbook of Pest Management in Agriculture. CRC Press; Boca Raton, Florida: 1991. pp. 301–328. [Google Scholar]
- Murata K., Miyamoto T., Tauchi M. Biosynthesis of B vitamins with Rhizopus oligosporus. J. Vitaminol. 1968;14:191–197. doi: 10.5925/jnsv1954.14.191. [DOI] [PubMed] [Google Scholar]
- Odalo J.O., Omolo M.O., Malebo H., Angira J., Njeru P.M., Ndiege I.O., Hassan A.A. Repellency of essential oils of some plants from the Kenyan coast against Anopheles gambiae. Acta Trop. 2005;95:210–218. doi: 10.1016/j.actatropica.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Qasem R., Hassan A.A. Herbicidal properties of some medicinal plants against Malva sylvestris and Portulaca oleracea. Dirasat Agric. Sci. 2003;30:84–100. [Google Scholar]
- Rausch T. The estimation of micro-algal protein content and its meaning to the evaluation of algal biomass I. Comparison of methods for extracting protein. Hydrobiologia. 1981;78:237–251. [Google Scholar]
- Rice E.L. second ed. Academic Press; Florida: 1984. Allelopathy; p. 422. [Google Scholar]
- Rizvi S.J.H., Haque H., Singh H.K., Rizvi V. A discipline called allelopathy. In: Rizvi S.J.H., Rizvi V., editors. Allelopathy: Basic and Applied Aspects. Chapman and Hall Press; New York: 1992. pp. 1–10. [Google Scholar]
- Salhi N., El-Darier S.M., Halilat M.T. Allelopathic effect of some medicinal plants on germination of two dominant weeds in Algeria. Adv. Environ. Biol. 2011;5:443–446. [Google Scholar]
- Salhi N., El-Darier S.M., Halilat M.T. The allelochemicals effect of Zygophyllum album on control of Bromus tectorum. J. Life Sci. 2012;6:182–186. [Google Scholar]
- Salhi N., El-Darier S.M., Halilat M.T. Allelopathic effect of Euphorbia guyoniana aqueous extract and their potential uses as natural herbicides. Sains Malays. 2013;42:1501–1504. [Google Scholar]
- Salhi N., El-Darier S.M., Halilat M.T. Allelotoxicity of Oudneya africana R. Br. aqueous leachate on germination efficiency of Bromus tectorum L. and Triticum aestivum L. Afr. J. Biotechnol. 2014;13:1194–1197. [Google Scholar]
- Singh D., Rao Y.B. Allelopathic evaluation of Andrographis paniculata aqueous leachates on rice (Oryza sativa L.) Allelopathy J. 2003;11:71–76. [Google Scholar]
- Singh A., Singh D., Singh N.B. Allelochemical stress produced by aqueous leachate of Nicotiana plumbaginifolia Viv. Plant Growth Regul. 2009;58:163–171. [Google Scholar]
- Terzi I., Kocaçalişkan I., Benliglu O., Solak K. Effects of juglone on growth of cucumber seedlings with respect to physiological and anatomical parameters. Acta Physiol. Plant. 2003;25:353–356. [Google Scholar]
- Turk M.A., Tawaha A.M. Inhibitory effects of aqueous extracts of barley on germination and growth of lentil. Pak. J. Agron. 2002;1:28–30. [Google Scholar]
- Vasilakoglou I., Dhima K., Wogiatzi E., Elefherohorinos I., Lithourgidis A. Herbicidal potential of essential oils of oregano or marjoram (Origanum spp.) and basil (Ocimum basilicum) on Echinochloa crus-galli (L.) P. Beauv. and Chenopodium album L. weeds. Allelopathy J. 2007;20:297–306. [Google Scholar]
- Venkateshwarlu G., Ravindra V., Prabha C. Mangiferin: an allelopathin from mango (Mangifera indica L.) leaves. Allelopathy J. 2001;8:221–224. [Google Scholar]
- Wakjira M., Berecha G., Bulti B. Allelopathic effects of Parthenium hysterophorus extracts on seed germination and seedling growth of lettuce. Trop. Sci. 2005;45:159–162. [Google Scholar]
- Xuan T.D., Tsuzuki E., Matsuo M., Khanh T.D. Paddy weed control by alfalfa, rice by-products, and their incorporation. Weed Biol. Manage. 2003;3:137–144. [Google Scholar]
- Xuan T.D., Tsuzuki E., Terao H., Matsuo M., Khanh T.D., Chung I.M. Evaluation on phytotoxicity of neem (Azadirachta indica. A. Juss) to crops and weeds. Crop Prot. 2004;23:335–345. [Google Scholar]
- Xuan T.D., Tawata S., Hong N.H., Khanh T.D., Chung I.M. Assessment of phytotoxic action of Ageratum conyzoides L. (billy goat weed) on weeds. Crop Prot. 2004;23:915–922. [Google Scholar]
- Yang C.M., Chang I.F., Lin S.J., Chou C.H. Effects of three allelopathic phenolics on chlorophyll accumulation of rice (Oryza sativa) seedlings: II. Stimulation of consumption–orientation. Bot. Bull. Acad. Sin. 2004;45:119–125. [Google Scholar]
- Zar J.H. Prentice-Hall:Inc.; New Jersey: 1984. Biostatistical Analysis; p. 718. [Google Scholar]
- Zeng H.Y., Alan A.R., Saxena P.K. Evaluation of in vitro shoots of Artemisia judaica for allelopathic potential. Acta Physiol. Plant. 2009;31:1237–1248. [Google Scholar]