Abstract
The study was aimed to investigate the effect of baicalein, a flavonoid molecule isolated from the plant Oroxylum indicum on bladder cancer cell viability. The results revealed that baicalein treatment of T24 and 253J bladder cancer cells targeted the expression of mRNA and proteins corresponding to the anti-apoptotic genes. RT-PCR assay showed that anti-apoptotic genes were markedly over-expressed in the bladder cancer cells. Exposure of the bladder cancer cells to baicalein at 5 mg/mL doses for 72 h led to reduction in the expression of mRNA levels of antiapoptotic genes. In T24 cells, the levels of BCL2, Bcl-xL, XIAP and surviving was reduced by 65, 69, 58 and 72%, respectively. In T24 and 253J cells exposure to baicalein for 72 h resulted respectively in 39 and 46% reduction in cell viability. Baicalein treatment also induced apoptosis in the bladder cancer cells. In T24 and 253J cells baicalein treatment at 5 mg/mL for 72 h induced apoptosis in 79 and 86% cells respectively. Thus, baicalein mediated reduction in antiapoptotic gene expression inhibits viability and induces apoptosis in bladder cancer cells. Therefore, baicalein is of therapeutic importance for the development of bladder cancer treatment strategy.
Keywords: Survivin, Bladder cancer, Apoptosis, Viability, Reduction
1. Introduction
Invasive bladder carcinoma patients have been treated by using radical cystectomy as the standard treatment method. But this method has the limitation of affecting the quality of life of the patients after treatment. It has been estimated that 40% patients suffering from invasive bladder carcinoma have a survival period of less than 5 years (Patton et al., 2001, Leissner et al., 2003, Hong et al., 2005, Stenzl et al., 2008). The use of newly developed trimodality therapy has been reported to be safe and effective, yielding a complete response (CR) in more than 60% of cases (Kaufman et al., 2000, Weiss et al., 2007, Gogna et al., 2006). However, the survival rate using this treatment method was found to be similar to that for radical cystectomy (Hagan et al., 2003). A highly effective, but non- or only minimally invasive therapy that conserves the bladder is therefore needed. Down-regulation of the genes responsible for survival and proliferation of cancer cells can have a therapeutic effect in bladder cancer. For maintenance of normal tissue homeostasis and selective removal of infected or damaged cells apoptosis is responsible (Burz et al., 2009). However, cancer cells have the ability to escape the process of apoptosis which may be due to the over expression of antiapoptotic genes like BCL2, Bcl-xL, XIAP and surviving (Hanahan and Weinberg, 2011). The members of BCL2 family are reported to inhibit the cytochrome c release from mitochondria and inhibit caspase activation (Brunelle and Letai, 2009). Caspases on activation are known to induce apoptosis resulting in decomposition of the proteins involved in maintaining cell stability and function (Taylor et al., 2008). In more than 80% of the bladder cancer tissues BCL2 protein was found positively and its expression correlated with the stage and grade of tumour (Korkolopoulou et al., 2002).
Metabolites derived from medicinal plants play a crucial role in the treatment of various diseases and disorders (Antonisamy et al., 2015, Balamurugan, 2015, Rathi et al., 2015, Sreeshma et al., 2016). Oroxylum indicum poly herbal formulations have a well known traditional medicinal importance in Indian system of medicine and exhibits anticancer potential. Its ethyl alcohol extract at a concentration of 0.05% showed promising cytotoxic activity against Hep2 cell lines (Narisa et al., 2006). Phytochemical investigation of Oroxylum indicum leads to the isolation of a flavonoid molecule, baicalein which exhibited inhibitory effect on proliferation of HL-60 cell lines at 25–30 μM (Roy et al., 2007, Serasanambati and Chilakapati, 2016). Oroxylum indicum has showed extensive cytotoxicity against cancer cell lines both in form of methanolic and aqueous extracts. Furthermore, the two extracts also prevented oxidative stress of DNA (Kumar et al., 2010). In the present study effect of baicalein on knockdown of antiapoptotic genes including BCL2, Bcl-xL, XIAP and survivin in human cervical carcinoma cell lines was studied.
2. Materials and methods
2.1. Cell culture and chemicals
The human bladder carcinoma cell lines T24, 253J and J82 were purchased from American Type of Culture Collection (ATCC, Manassas, VA, USA). The cells were cultured under standard conditions at 37 °C in humidified atmosphere containing 5% CO2. Baicalein a kind of gift from Professor Rajneesh was dissolved in dimethyl sulfoxide (DMSO) to a concentration of 50 µM as a stock solution. Rabbit antihuman Caspase-3, mouse antihuman Bcl-2, and β-actin were purchased from Cell Signaling (China).
2.2. Cell viability assay
WST-1 colorimetric assay was used to determine the viability of T24, 253J and J82 bladder carcinoma cells. The cells were seeded at a density of 2.5 × 105 cells per well in 96 well tissue culture plates and were treated with baicalein at indicated concentrations. After treatment cells were incubated with WST-1 for 4 h at 37 °C as per the manual protocol. The Victor 3 microplate reader was used to measure the absorbance at 450 nm. All the measurements were performed three times and the mean of three measurements was calculated based on average absorbance of control cells.
2.3. Assessment of apoptosis
The cells were treated with the indicated concentrations of baicalein and subjected to examination of nuclear morphology using fluorescence microscopy employing cell-permeable Hoechst 33342 dye. Five fields with 100 cells per field were randomly selected for calculation of cells containing condensed nuclei. Annexin-V binding assay was used for monitoring the phosphatidyl serine exposure. Cells were treated with the indicated doses of baicalein and then trypsinized. The cells were then washed with PBS and re-suspended in Annexin-V binding buffer (10 mM HEPES/NaOH pH 7.5, 140 mM NaCl, 2.5 mM CaCl2), containing Annexin-V Alexa Fluor 488 conjugate (1:50) for 15 min at 25 °C. The inverted fluorescence microscope (Leica DM IRB, Germany) was used to capture the images.
2.4. Reverse transcriptase PCR
Total cell RNA was isolated from the cells treated with baicalein using RNeasy Kits (Qiagen). The Omniscript RT (Qiagen) was used to reverse transcribe the cDNA using 500 ng RNA sample. After transcription the cDNA was then employed for quantitative real-time PCR (qPCR) using Taq PCR Master Mix Kit (Qiagen) according to the manufacturer’sprocedure.
2.5. Western blot analysis
Baicalein treated cells were lysed mechanically in ice-cold hypotonic buffer containing protease inhibitors (10 mM Tris HCl pH 7.2, 5 mM KCl, 1 mM MgCl2, 1 mM EGTA, 1% Triton X-100; 10 μM Leu-pep and Pep-A, 100 μM PMSF). The samples of proteins were loaded and separated on SDS–PAGE and transferred to nitrocellulose membrane. The membrane was incubated with the primary antibody (1:1000) overnight at 4 °C followed by incubation with HRP-conjugated secondary antibody (1:1000) for 1 h at 25 °C. The enhanced chemi-luminescence reagent was used for the visualization of the proteins. NIH Image J software was used for quantification of the digitized and band intensified images.
2.6. Cell motility assay
T24 and 253J cells were grown into 35-mm cell culture dishes to attain 90% confluence and then pipette tip was used for making a wound through the cell layer. The dishes were then washed with phosphate-buffered saline (PBS) followed by addition of various concentrations of baicalein of dimethyl sulfoxide (DMSO) as control. The cells were incubated for 72 h and the cell migration into wounded area was examined by taking photographs of the plates at 50× magnification under the microscope.
2.7. Statistical analysis
The data expressed is the mean of ±SD. The difference between the baicalein treatment culture and the DMSO (control) cells was analyzed using Student's t-test. The probability of p < 0.05 was taken to indicate statistically significant differences.
3. Results
3.1. Expression of antiapoptotic genes in bladder cancer cells
Analysis of the expression of anti-apoptotic genes in bladder cancer cells was performed by quantitative PCR analysis. The examination revealed a marked increase in the expression of anti-apoptotic genes in the three tested bladder cancer cells, T24, 253J and J82 (Table 1). These findings correlated with the earlier reports showing enhanced expression of the anti-apoptotic genes in bladder cancer cells.
Table 1.
The levels of mRNA in three bladder cancer cell lines.
| Cell line | Bcl-xL | XIAP | Survivin | BCL2 |
|---|---|---|---|---|
| T24 | 28.5 | 5.07 | 6.21 | 0.333 |
| 253J | 26.3 | 4.71 | 5.43 | 0.327 |
| J82 | 22.6 | 4.45 | 4.98 | 0.299 |
The data shown is expressed by taking TBP as the control.
3.2. Baicalein exhibits inhibitory effect on expression of anti-apoptotic gene
The bladder cancer cells, T24 and 253J were exposed to 1, 2, 3, 4 and 5 mg/mL doses of baicalein followed by analysis of anti-apoptotic gene expression. The results showed that anti-apoptotic gene expression in both the bladder cancer cells was markedly reduced on exposure to 5 mg/mL doses of baicalein for 72 h. The rate of inhibition was found to be 20, 21, 19 and 27% for BCL2, Bcl-Xl, XIAP and survivin respectively at 5 mg/mL concentration of baicalein in T24 cells (Fig. 1). The similar results were observed for 253J cells. Therefore, baicalein treatment caused a marked reduction in the expression of anti-apoptotic genes in bladder cancer cells after 72 h.
Fig. 1.
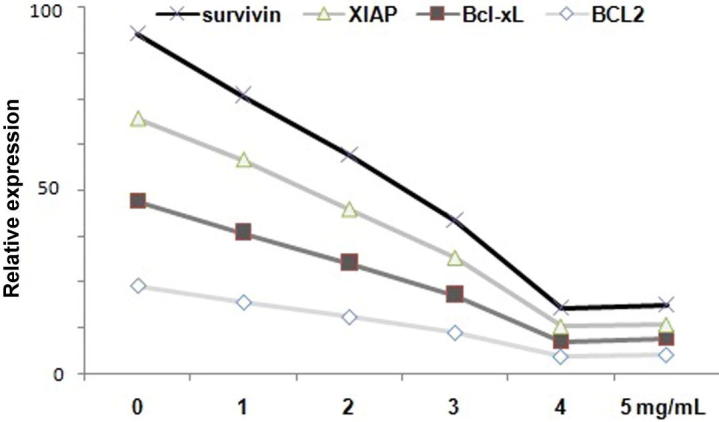
Inhibition of anti-apoptotic gene by baicalein treatment after 72 h in T24 and 253J bladder cells. The cells were incubated with 1, 2, 3, 4 and 5 mg/mL doses of baicalein for 72 h and the expression of BCL2, Bcl-Xl, XIAP and survivin genes was analyzed using RT-PCR assay.
3.3. Baicalein exhibits molecular effects on antiapoptotic gene inhibition
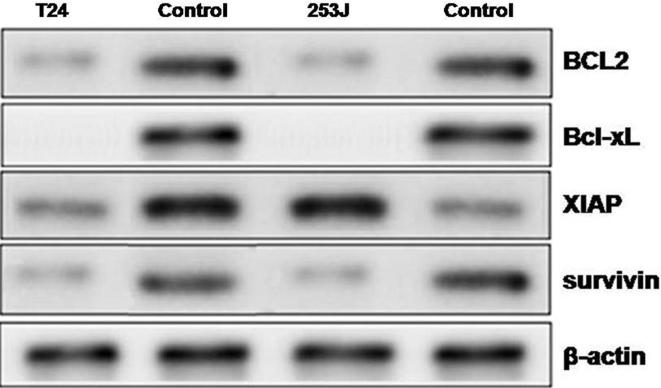
Exposure of the bladder cancer cells to baicalein at 1, 2, 3, 4 and 5 mg/mL doses for 72 h lead to reduction in the expression of mRNA levels of anti-apoptotic genes. In T24 cells, the levels of BCL2, Bcl-xL, XIAP and surviving was reduced by 65, 69, 58 and 72%, respectively after 72 h. Western blot analysis also showed that baicalein treatment at 5 mg/mL doses reduced the expression of proteins in both T24 and 253J bladder cancer cell lines at 72 h (Fig. 2).
Fig. 2.
Baicalein inhibits expression of anti-apoptotic genes. T24 and 253J cells were subjected to incubation with 1, 2, 3, 4 and 5 mg/mL doses of baicalein for 72 h. The expression of mRNA corresponding to BCL2, Bcl-xL, XIAP and survivin protein was examined by western blot assay.
3.4. Cellular effects of baicalein exposure
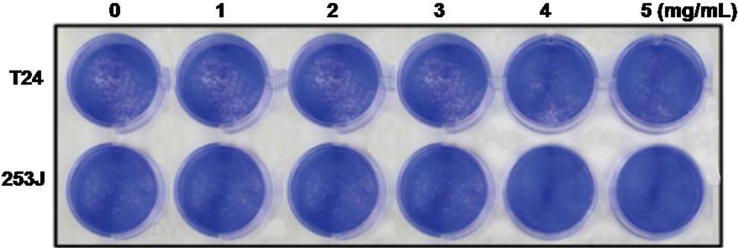
Investigation of the effect of baicalein treatment on viability of bladder cancer cells was performed following 72 h of exposure. Incubation of bladder cancer cells with 1, 2, 3, 4 and 5 mg/mL doses of baicalein for 72 h caused a marked decrease in the viability (Fig. 3). In T24 and 253J cells exposure to baicalein for 72 h resulted respectively in 39 and 46% reduction of cell viability.
Fig. 3.
T24 and 253J bladder carcinoma cell viability is inhibited by baicalein treatment. The cells were subjected incubation with 1, 2, 3, 4 and 5 mg/mL doses of baicalein or with DMSO (control) for 72 h and then cell viability was analyzed using WST-1 colorimetric assay.
3.5. Effects of baicalein on DNA damage and apoptosis in T24 cells
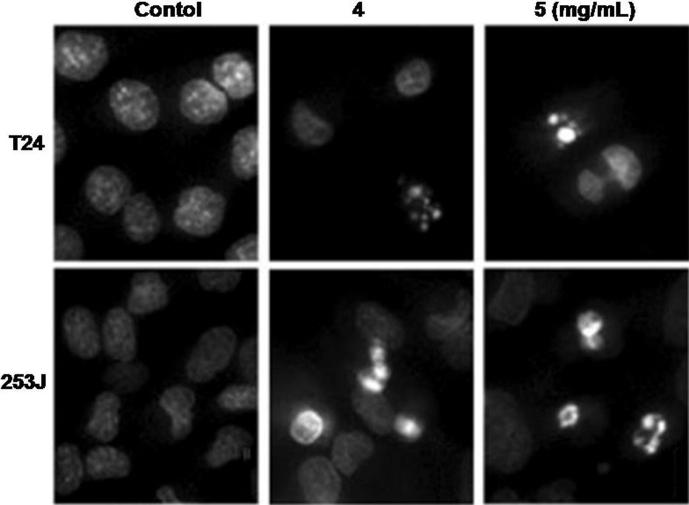
Baicalein treatment for 72 h induced apoptosis in the bladder cancer cells. The apoptosis induction in both the cancer cell lines was significant from 4 mg/L concentration of baicalein. In T24 and 253J cells baicalein treatment at 5 mg/mL for 72 h induced apoptosis in 79 and 86% cells respectively (Fig. 4).
Fig. 4.
Percentage of early and late apoptotic cells 72 h after baicalein treatment. The cells after incubation with 4 and 5 mg/L doses of baicalein or with DMSO (control) were analyzed for apoptosis induction using flow cytometry. Values represented are the mean of three independent experiments.
3.6. Baicalein suppresses migration
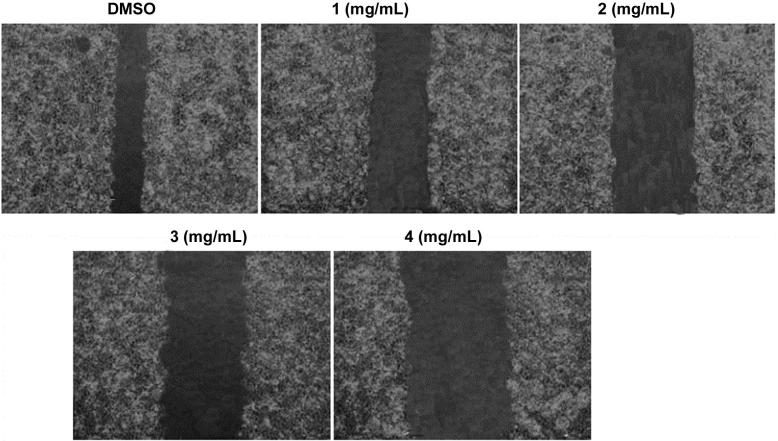
Wound-healing assay revealed that treatment of T24 and 253J cells with various concentration of baicalein caused inhibition of migration in comparison to the control cells. T24 and 253J cells after incubation with 1, 2, 3 and 4 mg/mL concentration of baicalein for 72 h were analyzed for migration potential. The migration potential of both the cells lines was reduced markedly on treatment with 4 mg/mL concentration of baicalein (Fig. 5).
Fig. 5.
Baicalein treatment inhibits migration of bladder cancer cells. T24 and 253J cells cultured in 35 mm cell culture dishes were allowed to grown up to confluence and subsequently pipette tip was used to make a scratch through cell layer. Cells were then washed with PBS followed by incubation with various concentrations of baicalein or DMSO (control) for 72 h. the migration of cells through wounded area was examined by taking the photographs of the culture dishes.
4. Discussion
Bladder cancer patients treated by radical cystectomy show poor quality of life after the treatment. More than 40% invasive bladder cancer patients have a survival period of less than 5 years (Dalbagni et al., 2001). Trimodality therapy despite being safe and effective yields results similar to that for radical cystectomy (Kaufman et al., 2000). The current study demonstrates the role of baicalein in inhibiting the viability and inducing the apoptosis of bladder cancer cells. The results revealed that baicalein treatment inhibits viability and induces apoptosis in bladder cancer cells through targeting anti-apoptotic gene expression. Carcinoma cells are known to undergo proliferation and promote formation of tumor growth at a very high rate. In the present study results revealed that baicalein treatment inhibits the viability of T24 and 253J bladder cancer cells at doses of 5 mg/mL after 72 h. Anti-apoptotic genes play a vital role in overcoming the process of apoptosis and are expressed abundantly in cancer cells and tissues (Li et al., 2007, Hunter et al., 2007). It is believed that suppression of anti-apoptotic gene expression exhibits inhibitory effect on invasive potential of bladder cancer and can be of therapeutic importance for bladder cancer treatment. Our results from the current study showed that baicalein treatment exhibits inhibitory effect on the expression of anti-apoptotic genes, mRNA and the corresponding protein in bladder cancer cells. In addition, baicalein treatment also reduced the viability of the bladder carcinoma cells. Apoptosis constitutes a physiological process that plays an important role in maintaining homeostasis in the living organisms by controlling proliferation of cells and regulating cell number (Trump et al., 1997). Induction of apoptosis in cancer cells is of great therapeutic value for the development of cancer treatment strategy (Thompson, 1995). The anti-apoptotic genes including BCL2, Bcl-xL, XIAP and survivin enable the cancer cells to escape the process of apoptosis. Our data from the present study showed that exposure of bladder cancer cells to baicalein for 72 h enhanced the rate of apoptosis. In T24 and 253J cells baicalein treatment at 5 mg/mL for 72 h induced apoptosis in 79 and 86%, respectively.
5. Conclusion
Baicalein treatment inhibits viability and induces apoptosis in bladder cancer cells through targeting expression of anti-apoptotic gene expression. Therefore, baicalein is of therapeutic significance for the treatment of bladder cancer.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Antonisamy P., Duraipandiyan V., Ignacimuthu S., Kim J.-H. Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha lam. in Wistar rats. South Ind. J. Biol. Sci. 2015;1:34–37. [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci. 2015;1:47–51. [Google Scholar]
- Brunelle J.K., Letai A. Control of mitochondrial apoptosis by the Bcl-2 family. J. Cell Sci. 2009;122:437–441. doi: 10.1242/jcs.031682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burz C., Berindan-Neagoe I., Balacescu O., Irimie A. Apoptosis in cancer: key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48:811–821. doi: 10.1080/02841860902974175. [DOI] [PubMed] [Google Scholar]
- Dalbagni G., Genega E., Hashibe M. Cystectomy for bladder cancer: a contemporary series. J. Urol. 2001;165:1111–1116. [PubMed] [Google Scholar]
- Gogna N.K., Matthews J.H., Turner S.L. Efficacy and tolerability of concurrent weekly low dose cisplatin during radiation treatment of localised muscle invasive bladder transitional cell carcinoma: a report of two sequential phase II studies from the Trans Tasman Radiation Oncology Group. Radiother. Oncol. 2006;81:9–17. doi: 10.1016/j.radonc.2006.09.001. [DOI] [PubMed] [Google Scholar]
- Hagan M.P., Winter K.A., Kaufman D.S. RTOG 97–06: initial report of a phase I-II trial of selective bladder conservation using TURBT, twice-daily accelerated irradiation sensitized with cisplatin, and adjuvant MCV combination chemotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2003;57:665–672. doi: 10.1016/s0360-3016(03)00718-1. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Hong S., Kwak C., Jeon H., Lee E., Lee S. Do vascular, lymphatic, and perineural invasion have prognostic implications for bladder cancer after radical cystectomy. Urol. 2005;65:697–702. doi: 10.1016/j.urology.2004.10.048. [DOI] [PubMed] [Google Scholar]
- Hunter A.M., LaCasse E.C., Korneluk R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis. 2007;12:1543–1568. doi: 10.1007/s10495-007-0087-3. [DOI] [PubMed] [Google Scholar]
- Kaufman D.S., Winter K.A., Shipley W.U. The initial results in muscle-invading bladder cancer of RTOG 95-06: phase I/II trial of transurethral surgery plus radiation therapy with concurrent cisplatin and 5-fluorouracil followed by selective bladder preservation or cystectomy depending on the initial response. Oncologist. 2000;5:471–476. doi: 10.1634/theoncologist.5-6-471. [DOI] [PubMed] [Google Scholar]
- Korkolopoulou P., Lazaris A., Konstantinidou A.E. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur. Urol. 2002;41:274–283. doi: 10.1016/s0302-2838(02)00003-9. [DOI] [PubMed] [Google Scholar]
- Kumar R.A., Rajkumar V., Guha G., Mathe L. Therapeutic potentials of Oroxylum indicum bark extracts. Chinese J. Nat. Med. 2010;8(2):121. [Google Scholar]
- Leissner J., Koeppen C., Wolf H. Prognostic significance of vascular and perineural invasion in urothelial bladder cancer treated with radical cystectomy. J. Urol. 2003;169:955–960. doi: 10.1097/01.ju.0000043639.55877.17. [DOI] [PubMed] [Google Scholar]
- Li M., Song T., Yin Z.F., Na Y.Q. XIAP as a prognostic marker of early recurrence of nonmuscular invasive bladder cancer. Chin. Med. J. (Engl.) 2007;120:469–473. [PubMed] [Google Scholar]
- Narisa K., Jenny M.W., Heather M.A.C. Cytotoxic effect of four Thai edible plants on mammalian cell proliferation. Thai Pharm. Health Sci. J. 2006;1(3):189. [Google Scholar]
- Patton S., Hall M., Ozen H. Bladder cancer. Curr. Opin. Oncol. 2001;14:265–272. doi: 10.1097/00001622-200205000-00003. [DOI] [PubMed] [Google Scholar]
- Rathi M.A., Meenakshi P., Gopalakrishnan V.K. Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci. 2015;1:60–65. [Google Scholar]
- Roy M.K., Nakahara K., Na T.V., Trakoontivakorn G., Takenaka M., Isobe S. Baicalein, a flavonoid extracted from a methanolic extract of Oroxylum indicum inhibits proliferation of a cancer cell line in vitro via induction of apoptosis. A. Pharmazie. 2007;62(2):149. [PubMed] [Google Scholar]
- Serasanambati M., Chilakapati S.R. Function of nuclear factor kappa B (NF-kB) in human diseases – a review. South Ind. J. Biol. Sci. 2016;2:368–387. [Google Scholar]
- Sreeshma P.S., Raphael K.R., Baby A.A. Pharmacognostic studies of leaves of Naravelia zeylanica (Linn) DC. South Ind. J. Biol. Sci. 2016;2:179–182. [Google Scholar]
- Stenzl A., Cowan N.C., De Santis M. Guidelines on bladder cancer muscle-invasive and metastatic. Eur. Assoc. Urol. 2008;3(1–59):2008. [Google Scholar]
- Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Thompson C.B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Trump B.F., Berezeskyi K., Chang S.H., Phelps P.C. The pathways of cell death: oncosis, apoptosis and necrosis. Toxicol. Pathol. 1997;25:82–88. doi: 10.1177/019262339702500116. [DOI] [PubMed] [Google Scholar]
- Weiss C., Engehausen D.G., Krause F.S. Radiochemotherapy with cisplatin and 5-fluorouracil after transurethral surgery in patients with bladder cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007;68:1072–1080. doi: 10.1016/j.ijrobp.2007.01.054. [DOI] [PubMed] [Google Scholar]