Abstract
Chlorpyrifos (O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate), is an organophosphate insecticide effective against a broad spectrum of insect pests of economically important crops. The present study investigated the effects of chlorpyrifos application on sulfate assimilation and macro elemental composition in different plant parts at different phenological stages. Field experiments were conducted in the month of April 2008–2009. The individual plot size was 6 m2 (4 m × 1.5 m) having 4 rows with a row-to-row distance of 15 inches and plant to plant distance of 10 inches. The number of plants per m2 was 15. Seedlings were collected at 5 (preflowering), 10 (flowering) and 20 (postflowering) DAT (day after treatment) to analyze the effect of chlorpyrifos on APR activity and elemental composition. At harvest stage, seed from individual treatments were analyzed for sulfur containing amino acids like methionine and cysteine content. Twenty-day-old seedlings of Vigna radiata L. were subjected to chlorpyrifos at different concentrations ranging from 0 to 1.5 mM through foliar spray in the field condition. A significant increase (50% in cysteine content and 50–92% in methionine content) in sulfur containing amino acids at a higher dose rate of 1.5 mM was recorded in seeds, however the increased activity of adenosine 5-phosphosulfate reductase (APR), the key enzyme in sulfate assimilation was recorded in all the three parts of the plant (leaf, stem, root.). Transiently lower nitrogen, sulfur and carbon content in 0.6 and 1.5 mM chlorpyrifos application in V. radiata L. supports the inhibition of metabolic processes. However, reverse trend was exhibited at 0.3 mM for same parameters. These results suggest the stimulatory effects on sulfate assimilation in V. radiata L. while as inhibitory effects were prevalent on elemental composition.
Keywords: Green gram, Insecticide, Sulfur, Nitrogen, Carbon, Assimilation
1. Introduction
Mung bean [Vigna radiata (L.) Wilczek] is an important legume crop widely cultivated in Asia. The crops are utilized in several ways. Seeds, sprouts and young pods are consumed as sources of protein, amino acids, vitamins and minerals, and plant parts are used as fodder and green manure (Somta and Srinives, 2007). In India it is under cultivation on about 3.25 million hectares and an annual production is 1.45 million tons. Major obstacles to the growth and productivity of widely cultivated green gram in arid and semiarid regions are nutrient imbalance, pest infestation and salinity. Right from seed germination to maturity, mung bean is adversely attacked by many serious pests. Chemical control represents major treatment for these pests to avoid economic loss. However, the indiscriminate use of potentially hazardous insecticides possesses a serious threat to the human and environment. Due to the long persistence of organochlorines in the environment, the organophosphorus insecticides are taking the major share of insecticide consumption in India (Adityachaudhury et al., 1997). Chlorpyrifos (O,O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate), is one such organophosphate insecticide effective against a broad spectrum of insect pests of economically important crops. In the study of Parween et al. (2011), foliar application of a lower dose of chlorpyrifos proved stimulant whereas a higher concentration showed detrimental for growth and enzyme activity in V. radiata L. In another study foliar application of chlorpyrifos enhanced lipid peroxidation rate and proline content with 1.5 mM at Day 20, whereas dehydroascorbate, oxidized and total glutathione were increased in 1.5 mM at Day 10. Among the enzymatic antioxidants, activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase enhanced significantly in all the concentrations at Day 10 whereas maximum catalase activity was observed at Day 10 in control and it declined thereafter (Parween et al., 2012). Kashyap and Kumar (2013), Studied the effects of Chlorpyrifos on Growth and Yield in Green Gram (V. radiata L.) at different phenological stages and concluded that the application of chlorpyrifos at a lower concentration (50 ppm) may be a useful tool to increase the seed quality as well as quantity in Green gram plant, apart from their pesticidal properties.
With this background the present study was undertaken to determine the effects of chlorpyrifos on the elemental composition at different phenological stages, variation in sulfur containing amino acids and changes in the associated key enzyme-adenosine 5-phosphosulfate reductase (APR), in different plant parts at different phenological stages of V. radiata L.
2. Materials and methods
2.1. Experimental setup and treatment
The seeds of V. radiata (L.) R. Wilcz. were procured from division of genetics, pulse research centre, Indian Agricultural Research Institute (IARI), New Delhi. Field experiments were conducted in the month of April 2008–2009. The individual plot size was 6 m2 (4 m × 1.5 m) having 4 rows with a row-to-row distance of 15 inches and plant to plant distance of 10 inches. The number of plants per m2 was 15. The field was ploughed and leveled prior to sowing. The plots were made with proper bunds along with necessary irrigation channels. Irrigation was done as and when required. The crop was kept free from weeds by manual weeding operation regularly. 20-day-old seedlings were subjected to foliar application of three different concentrations of chlorpyrifos (20% E.C.) viz., 0 (control), 0.3, 0.6 and 1.5 mM/100 ml prepared by dissolving the required amount of chlorpyrifos in double distilled water. Each experiment was repeated three times with five replicates. Seedlings were collected at 5 (preflowering), 10 (flowering) and 20 (postflowering) DAT (day after treatment) to analyze the effect of chlorpyrifos on APR activity and elemental composition. At harvest stage, seed from individual treatments were analyzed for sulfur containing amino acids like methionine and cysteine content.
2.2. Elemental composition
Plant parts were analyzed at different samplings for assessing nitrogen (N), carbon (C), and sulfur (S) status. Five plants were collected from each treatment and were wiped free of any adhering dust. Samples were first washed in running tap water for 1 min followed by 1 min in distilled water. Each sample was dried for 48 h in a hot air oven at 65 °C. The dried samples were fine powdered and passed through a 72 mm mesh screen. The powder was stored in polyethylene bags labeled and used for analysis later.
Estimation: Nitrogen (N), sulfur (S) and carbon (C) contents (% dry weight) were analyzed by packing the known weight of plant sample-powder in tin boats with the help of Elemantar system (CHNS Analyse, Vario EL.)
2.3. Aminoacid estimation
The extraction procedure of Bieleski and Turner (1966) was modified as follows. First the seeds were homogenized in 100 ml of methanol–chloroform-water (4:5:1, by vol.), using Virtis homogenizer operated at full speed for 2 min. This was followed by centrifugation at 20,000g for 10 min in the SS34 head of the Sorvall RC2 refrigerated centrifuge operated at 2 °C. The pellets were treated by boiling for 3 min in 80% (v/v) ethanol, and then homogenizing in a Virtis homogenizer for 2 min at full speed, and the homogenate was centrifuged in the Sorvall centrifuge under the conditions described above. The supernatant from this step was termed the ethanol extract. The methanol–chloroform-water supernatant was stirred for 15 min with 50 ml of chloroform plus 75 ml of water using a magnetic stirrer. The resulting mixture was centrifuged in the Sorvall centrifuge, under the conditions described above. The aqueous layer was collected and added to the ethanol extract. The chloroform layer was further extracted with 2 × 50 ml of water, the aqueous layers were separated as described above and added to the ethanol extract. The final mixture of the ethanol extract, plus all aqueous layers was dried in vacuo at 50 °C using a Buchler rotary evaporator. The dried material was then extracted with 2 × 25 ml of di-ethyl ether, and the oil-free material dissolved in 10 ml of 0.1 M-HCl-0.07 M-2-mercaptoethanol. This mixture was then fractionated on a column (1 cm × 5 cm) of Dowex 50W ion-exchange resin (X8; 200–400 mesh; H+ form). The column was washed with 50 m1 of deionized water, and then the acidic and neutral amino acids were eluted with 8. 5 ml of 0.2 M-pyridine (Biemann and Deffner, 1961). The pyridine eluate was evaporated to dryness at 60 °C in vacuo and dissolved in 10 ml of the HCl-mercaptoethanol solution.
2.3.1. Identification and determination of amino acids
The sulfur-containing amino acids were identified by co-chromatography with authentic amino acids, followed by detection with ninhydrin spray [0.3 g of ninhydrin in 100 ml of 3% (v/v) acetic acid in butan-l-ol] and iodoplatinate spray (0.2 g of platonic chloride and 4 g of KI in 100 ml of water). Amino acid spots were also subjected to oxidation with hydrogen peroxide as a further confirmatory test. The areas corresponding to methionine, cysteine or homocysteine were cut out from the chromatograms and eluted in 1 M-HCl. They were then oxidized with 0.1 ml of 30% (v/v) hydrogen peroxide. The products of oxidation were then re-chromatographed on TLC plates in the chloroform–methanol-aq. 17% NH3 – mercaptoethanol solvent. Authentic compounds were also oxidized in a similar manner and chromatographed together with the test compounds. The RF values of oxidized methionine, homocysteine and cysteine were 0.377, 0.154 and 0.160 respectively. The oxidation and chromatography of cystathionine was carried out by the procedure of Mudd et al. (1965).
Quantitative measurements of the sulfur-containing amino acids were obtained using the iodoplatinate colorimetric procedure of Awwad and Adelstein (1966), which is specific for sulfur containing amino acids. Standard curves were produced for each of the sulfur-containing amino acids determined.
2.4. APR enzyme measurement
Plants were washed for 1 min with H2O at 4 °C, and extracts were prepared by grinding 1:10v(w/v) in 0.1 m Tris–HCl, pH 8.0, containing 100 mm KCl, 20 mm MgCl2, and 10 mm dithioerythritol in a glass homogenizer. The homogenate was centrifuged for 10 min at 10,000g and the supernatant was used for the enzyme assays. APR activity in extracts was measured as the production of [35S] sulfite, assayed as acid volatile radioactivity, formed from [35S] APS in the presence of dithioerythritol (Brunold and Suter, 1990).
3. Results and discussion
3.1. Nitrogen content
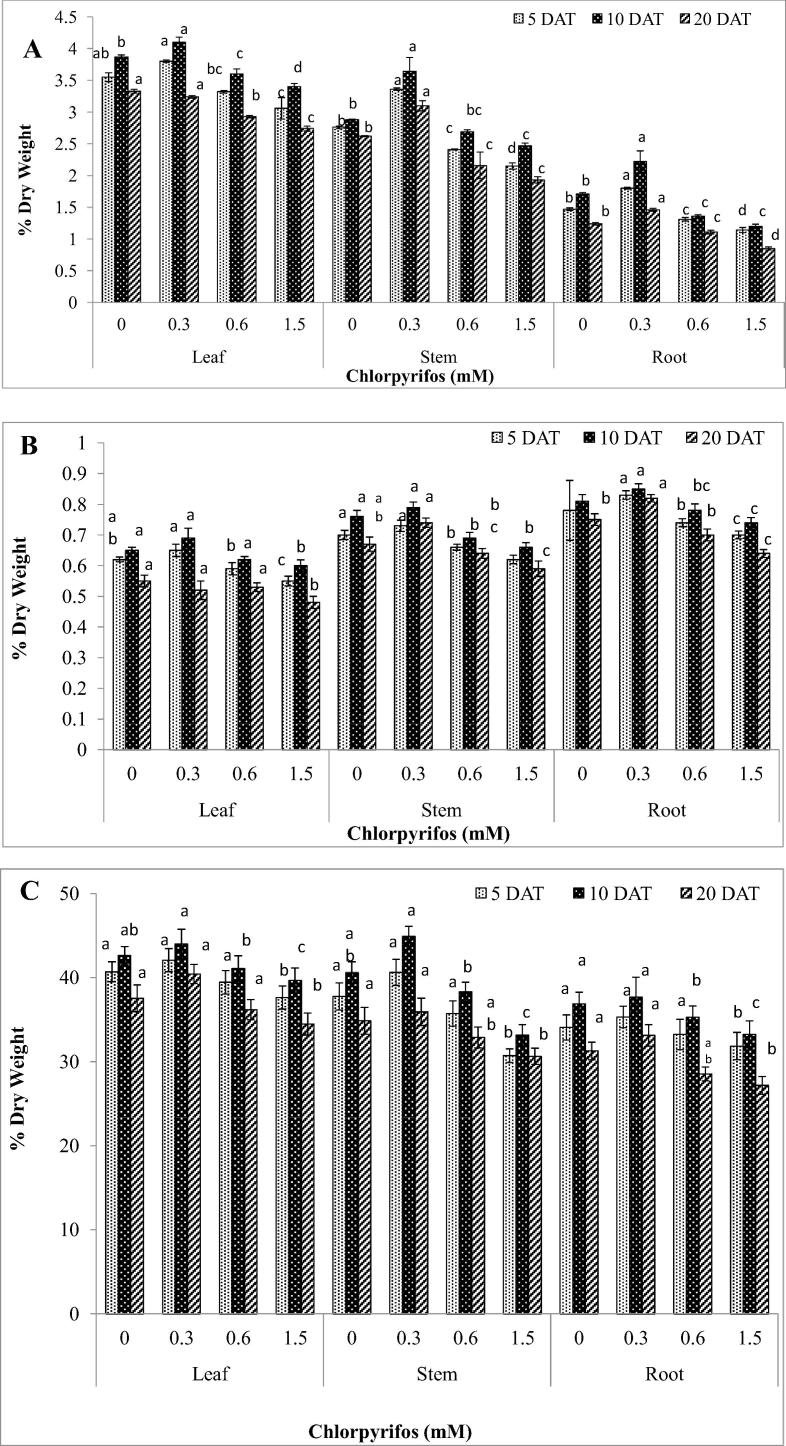
The nitrogen content in the roots, stem and leaves of V. radiata also showed a significant (p < 0.05) enhancement with the age of the plant up to the flowering stage (10 DAT) followed by preflowering (5 DAT) and post-flowering stages (20 DAT) in control as well as treated plants (Fig.1A). The maximum decline in nitrogen content was observed at postflowering stage with 1.5 mM insecticidal treatment. However, nitrogen content showed a significant increase with 0.3 mM insecticidal treatment. Maximum variation in root nitrogen (31.56%) was recorded with 1.5 mM at post-flowering stage followed by flowering (29.96%) and preflowering (24.01%) stages respectively. Similarly, maximum variation in stem nitrogen (26.43%) was recorded with 1.5 mM at post-flowering stage followed by flowering (26.16%) and preflowering (22.02%) stages respectively. Maximum variation in leaf nitrogen (18.05%) was recorded with 1.5 mM at postflowering stage followed by preflowering (13.55%) and flowering (12.75%) stages respectively. Leaf and stem nitrogen content in V. radiata L. plants were highest during flowering stage following 0.3 mM treatment with chlorpyrifos but declined thereafter. This decline coincided with plants treated with higher doses of chlorpyrifos. The content of nitrogen in stem was less than that in the leaves. However, roots show lowest nitrogen at all development stages. Our results are in agreement with findings of Maman et al. (1999) that the content of nitrogen in most tissues of crop plants was high during the early stage of growth and decreased with advancement of plant age. The demand for nitrogen is closely related to plant growth, and nitrogen deficiency is, after water stress, the most frequently reported limitation to growth (Kozlowski, 1971, Kramer and Kozlowski, 1979). The persistence and metabolism of chlorpyrifos in Gangetic alluvial soil of West Bengal was used to evaluate the effect on the availability of the major plant nutrients (N, P and K) in soil following the application of chlorpyrifos. The inhibitory effect on available N was attributable to 3,5,6-trichloro-2 methoxypyridine (TMP) and for 3,5,6-trichloro-2-pyridinol (TCP), it was due to the presence of TCP and TMP rather than chlorpyrifos itself as revealed by the step wise multiple regression technique (Sardar and Kole, 2005). Microorganisms were involved in the degradation of chemical insecticides deriving energy and nutrient for their growth and metabolism (Lal, 1982). The application of chlorpyrifos and quinalphos decreased the nitrogenase activity in Azospirillum (Gadagi et al., 2004). As the concentration of the insecticides increased the inhibition of nitrogenase activity also increased. Similarly, inhibition of nitrogenase activity by the application of organophosphorus, methidathion also occurred (Gomez et al., 1998). The arginine ammonification activity of rhizospheric microbes was inhibited after seed treatment with chlorpyrifos and quinalphos and their principal metabolites, TCP and TMP and hydroxyquinoxaline and quinoxaline-2-thiol, respectively (Menon et al., 2004).
Figure 1.
Variation in elemental composition (%) of Vigna radiata L. in root, stem and leaf at different phenological stages treated with different concentrations of chlorpyrifos (A) Nitrogen content (B) Sulfur content, and (C) Carbon content. Value represents mean ± SE (n = 5). Values with different superscripts are significantly (p < 0.05) different from each other (Duncan’s multiple range test).
3.2. Sulfur content
The sulfur content in the root, leaves and stem of V. radiata L. also showed a significant (p < 0.05) enhancement with the age of the plant up to the flowering stage followed by preflowering and postflowering stages in control as well as treated plants (Fig.1B). The maximum decline was observed at postflowering stage with 1.5 mM insecticidal treatment. However, sulfur content showed a significant increase with 0.3 mM insecticidal treatment. Maximum variation in root (13.06%) was recorded with 1.5 mM at postflowering stage followed by preflowering (10.08%) and flowering (9.29%) stages respectively. Maximum variation in stem (12.59%) was recorded with 1.5 mM at flowering stage followed by preflowering (12.18%) and postflowering (12.13%) stages respectively. Maximum variation in leaves (12.99%) was recorded with 1.5 mM at postflowering stage followed by preflowering (10.28%) and flowering (7.78%) stages respectively. Organophosphates have been reported to inhibit activities of many enzymes involved in plant metabolism. Pesticides can alter the chemical composition of plants. The changes that occur appear to be specific for both the plant and the pesticides involved. For example, certain organo chlorine insecticides have increased the amounts of some macro- and micro-element constituents (Al, B, Ca, Cu, Fe, K, Mg, Mn, N, P, Sr, and Zn) of corn and beans, and decreased the amounts of others (Cole et al., 1968). The four basic biochemical reactions commonly involved in pesticide metabolism in higher plants (oxidation, reduction, hydrolysis and conjugation) are discussed in relation to the enzyme systems capable of catalyzing these reactions. The use of enzymes from these classes to study pesticide metabolism is reviewed. The following enzymes are considered: peroxidases, mixed function oxidases, hydroperoxidases, aryl nitroreductases, aryl acylamidases, esterases, glutathione S-transferases, cysteine S-transferases and various glucose-conjugating enzyme systems (Lamoureux and Frear, 1979).
3.3. Carbon content
The carbon content in the root, stem and leaves of V. radiata L. also showed a significant (p < 0.05) enhancement with the age of the plant up to the flowering stage followed by preflowering and postflowering stages in control as well as treated plants (Fig.1C). The maximum decline was observed at postflowering stage with 1.5 mM insecticidal treatment. However, carbon content showed a significant increase with 0.3 mM insecticidal treatment. Maximum variation (13.37%) in root was recorded with 1.5 mM at post flowering stage followed by flowering (9.60%) and preflowering (6.39%) stages respectively. Maximum variation in stem (18.66%) was recorded with 1.5 mM at preflowering stage followed by flowering (18.35%) and postflowering (11.84%) stages respectively. Maximum variation (8.96%) in leaf was recorded with 1.5 mM at postflowering stage followed by preflowering (7.24%) and flowering (6.04%) stages respectively. Earlier studies suggested that toxicant produced by the pesticides application retarded the carbon metabolism by inducing alteration in cytochrome oxidase activity, blocking alternative respiratory pathways and accumulation of carbon metabolites like succinate (Berger and Cwick, 1990, Siddiqui and Ahmed, 2002). Dimethoate pesticides are electrophilic in nature and they may attack other enzymes necessary for carbohydrate and nitrogenous metabolism. Carbohydrate and nitrogenous metabolism were significantly affected due to the hypoxic conditions occurred by the exposure of pesticide, as total protein, nucleic acids (DNA and RNA), pyruvate levels and cytochrome oxidase activity were significantly decreased, while the total free amino acids and lactic dehydrogenase activity was increased after the sub-lethal exposure to dimethoate (Siddiqui and Ahmed, 2000). Atrazine- treated mung bean had an increase in the number of plastoglobuli and the absence of starch grains. Metsulfuron-treated plants had more starch grains compared to the control, which can be attribute to the reduced ability of leaf tissue to load sucrose into the phloem (Kaushik and Inderjit, 2006). Kim et al. (1997) showed that chlorsulfuron-treated canola (B. napus) leaves at 72 h after treatment had swollen and disorganized chloroplasts and starch granules were present in companion cells and mesophyll cells but not in the untreated control.
3.4. APR activity
A significant increase of APR activity was observed in roots, stem and leaves compared with the non-treated plants at flowering stage (10 DAT) after chlorpyrifos application with 0.6 and 1.5 mM (Table 1). The increase in APR activity ranged from 7% to 94% in roots, from 10% to 97% in stems, and from 6% to 78% in leaves. These ranges depend on chlorpyrifos application rate and stage of application. At a chlorpyrifos application rate of 1.5 mM and 10 days after the chlorpyrifos application, APR showed a significant increase in all plant parts compared with untreated plants. The chlorpyrifos application of 1.5 mM caused a significant increase in APR activity in roots, stem and leaves. The sulfate assimilation pathway provides reduced sulfur for the synthesis of the amino acids cysteine and methionine. These are the essential building blocks of proteins and further sources of reduced sulfur for the synthesis of coenzymes and various secondary compounds. Several recent reports identified the adenosine 5′-phosphosulfate reductase (APR) as the enzyme with the greatest control over the pathway. APR catalyzes a thiol-dependent two-electron reduction of APS to sulfite (Suter et al., 2000). Chlorpyrifos affects sulfur metabolism by exerting its toxicity by irreversibly sulphahydral group of aminoacid. Chlorpyrifos is reported to decrease sulfur containing enzyme glutathione-S-transferase following exposure to 0.4 nM for 48 h.
Table 1.
Variation in adenosine 5-phosphate reductase (APR) activity (nmol mg protein−1 min−1) in leaf, stem, and root at different phenological stages treated with different concentrations of chlorpyrifos application in Vigna radiata L.
| Organ/Stage | Control | 0.3 mM | 0.6 mM | 1.5 mM |
|---|---|---|---|---|
| Leaf | ||||
| Preflowering | 2.20c (0) |
2.34ab (6.36) |
2.64b (20) |
3.93a (78.63) |
| Flowering | 3.36c (0) |
3.85b (14.5) |
4.23ab (25.83) |
5.10a (51.78) |
| Postflowering | 2.28a (0) |
2.65b (16.45) |
3.45bc (51.31) |
3.89b (70.61) |
| Stem | ||||
| Preflowering | 3.55b (0) |
3.76ab (5.91) |
3.80a (7.04) |
3.93a (10.70) |
| Flowering | 3.26c (0) |
4.80a (47.23) |
5.79a (77.60) |
6.45a (97.85) |
| Postflowering | 2.7b (0) |
2.98bc (10.73) |
3.45ab (27.77) |
3.79a (40.37) |
| Root | ||||
| Preflowering | 3.45b (0) |
3.69bc (6.95) |
3.93a (13.91) |
4.23a (22.60) |
| Flowering | 5.09a (0) |
5.65b (11.0) |
6.36a (24.95) |
6.53a (28.29) |
| Post-flowering | 3.19c (0) |
4.3b (34.79) |
5.93a (85.89) |
6.20a (94.35) |
Values represent mean (n = 5). Values in parenthesis indicate percent variation with reference to respective controls. Values with different superscripts are significantly (p < 0.05) different from each other (Duncan’s multiple range test).
Brassinosteroids (BRs) are a class of phytohormones involved in the regulation of plant growth, development and stress response. Jiang (2012) and Xia (2009), found that BRs-induced stress tolerance is associated with changes in cellular redox homeostasis and expression of a wide range of stress-related genes including those encoding P450 and GST involved in metabolism of xenobiotic compounds. Zhou et al. (2015) studied a novel approach for minimizing pesticide residues in crops by exploiting plants’ own detoxification mechanisms. They showed that brassinosteroids (BRs), a class of natural plant hormones, decreased residues of common organophosphorus, organochlorine and carbamate pesticides by 30–70% in tomato, rice, tea, broccoli, cucumber, strawberry, and other plants when treated externally. Genome-wide microarray analysis showed that fungicide chlorothalonil (CHT) and BR co-upregulated 301 genes, including a set of detoxifying genes encoding cytochrome P450, oxidoreductase, hydrolase and transferase in tomato plants. The level of BRs was closely related to the respiratory burst oxidase 1 (RBOH1)-encoded NADPH oxides-dependent H2O2 production, glutathione biosynthesis and the redox homeostasis, and the activity of glutathione S-transferase (GST). Gene silencing treatments showed that BRs decreased pesticide residues in plants likely by promoting their metabolism through a signaling pathway involving BRs-induced H2O2 production and cellular redox change.
3.5. Sulfur containing amino acids
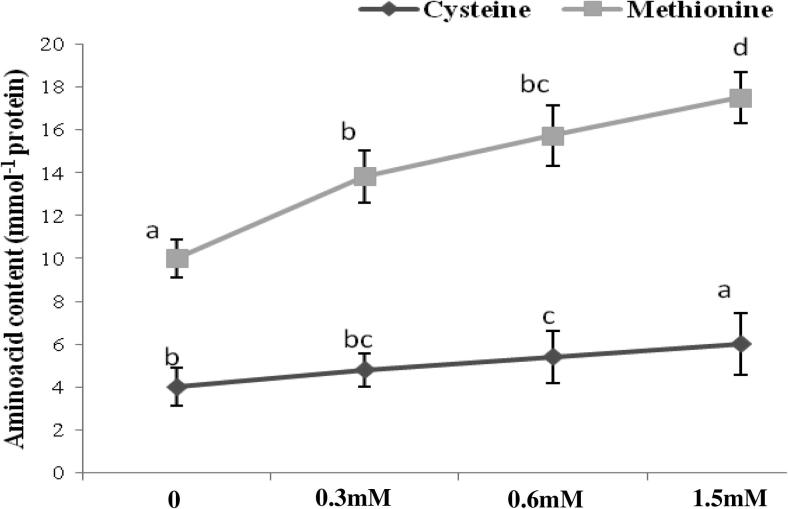
The application of chlorpyrifos at 0.6 and 15 mM dose rate significantly increased sulfur containing amino acids of seed (Fig. 2). There was 50% increment in cysteine content following a higher dose rate of 1.5 mM while as methionine increased from 50% to 92% with same dose rate. In another study, DDT, aldrin, endrin, and lindane were found to stimulate the synthesis of the important amino acids arginine, histidine, leucine, lysine, proline, and tyrosine in corn, but decreased the content of tryptophan (Thakre and Saxena, 1972).
Figure 2.
Variation in sulfur containing amino acid (mmol−1 protein) in seeds of Vigna radiata L. exposed to different concentrations of chlorpyrifos. Values represent mean ± SE (n = 5). Values with different superscripts are significantly (p < 0.05) different from each other (Duncan’s multiple range test).
The pesticide tends to increase essential amino acids in cotton seeds i.e. cysteine and lysine, in considerable proportions while the rest of the amino acids decrease in concentration. The increasing tendency is very significant in seven aminoacids, particularly in cysteine, histidine and lysine, and in appreciable amounts in alanine, glutamic acid, leucine and tyrosine in the samples treated with monocrotophos. The influence of OP pesticides i.e. chlorpyrifos-methyl and pirimiphos-methyl on all the amino acids in wheat grain was found to be quite identical, while another OP pesticide, monocrotophos, produced adverse effects on majority of the amino acids in cotton seed (Afridi et al., 1995). On the contrary, chlorpyrifos was found to have no effect on cysteine content in Aspergillus flavus while as aminoacids like α- aminoadipic acid, α-amino butyric acid, α-amino iso-butyric acid and α-amino n-butyric acid increased (Abdel-Ghany et al., 2009).
4. Conclusion
The current research suggests that the partitioning of nitrogen, sulfur and carbon depends on phenological stage of plant as well as exposure to different doses of insecticide applied. Further studies investigated the increase in sulfur containing aminoacids. However, an in-depth study is required to interpret the relationships between N and S supply and uptake, and seed composition constituent accumulation, especially amino acids following different insecticide exposure. This study showed that chlorpyrifos application affects sulfate assimilation by ameliorating APR activity. Chlorpyrifos application affects nitrogen, sulfur and carbon metabolism resulting in a significant differences in seed composition, suggesting carbon metabolism alteration.
Acknowledgement
First author is highly thankful to University Grants Commission (UGC), Government of India for providing fellowship during this study.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Ghany T.M., Abdel-Mongy M., Afify M.M. Dynamic changes in amino acids and volatile metabolites of soil fungus Aspergillus flavus as a result of chlorpyrifos methyl application. J. Appl. Sci. Res. 2009;5(1):121–128. [Google Scholar]
- Adityachaudhury N., Banerjee H., Kole R.K. An appraisal of pesticide use in Indian agriculture with special reference to their consumption in West Bengal. Sci. Cult. 1997;63:223–228. [Google Scholar]
- Afridi I.A.K., Parveen Z., Masud S.Z. Organochlorine, organophosphorus and synthetic pyrethroid pesticides affecting amino acids in cotton seeds and wheat grain during storage. J. Islamic. Acad. Sci. 1995;8(1):47–52. [Google Scholar]
- Awwad H.K., Adelstein S.J. A quantitative method for the determination of the specific radioactivity of sulfur-containing amino acids separated by paper chromatography. Anal. Biochem. 1966;16:433–437. [Google Scholar]
- Berger S., Cwick K. Selected aspect of adverse nutritional effect of pesticides. Ernahrung. 1990;14:411–415. [Google Scholar]
- Bieleski R.L., Turner N.A. Separation and estimation of amino acids in crude plant extracts by thin-layer electrophoresis and chromatography. Anal. Biochem. 1966;17(2):278–293. doi: 10.1016/0003-2697(66)90206-5. [DOI] [PubMed] [Google Scholar]
- Biemann K., Deffner G.G.J. Determination of n$sup 15$ in amino acid mixtures without separation into individual components. Biochem. Biophys. Res. Commun. 1961;4:283–287. [Google Scholar]
- Brunold C., Suter M. Adenosine 59-phosphosulfate sulfotransferase. In: Lea P., editor. Meth. Plant. Biochem. Academic Press; London: 1990. pp. 339–343. [Google Scholar]
- Cole H., Mackenzie D., Smith C.B., Bergman E.L. Influence of various persistent chlorinated insecticides on the macro and micro element constituents of Zea mays and Phaseolus vulgaris growing in soil containing various amounts of these materials. Bull. Environ. Contam. Toxicol. 1968;3:141–153. doi: 10.1007/BF01684450. [DOI] [PubMed] [Google Scholar]
- Gadagi R.S., Tongmin S.A., Chung J.B. Chemical insecticide effects on growth and nitrogenase activity of Azospirillum sp. OAD-Commun. Soil Sci. Plant Anal. 2004;35(3):495–503. [Google Scholar]
- Gomez F., Salmeron V., Rodelas B., Martinez-Toledo M.V., Gonzalez-lopez J. Response of Azospirillum brasilense to the pesticides bromopropylate and methidathion on chemically defined media and dialysed-soil media. Ecotoxicology. 1998;7:43. [Google Scholar]
- Jiang Y.P. Cellular glutathione redox homeostasis plays an important role in the brassinosteroid-induced increase in CO2 assimilation in Cucumis sativus. New Phytol. 2012;194:932–943. doi: 10.1111/j.1469-8137.2012.04111.x. [DOI] [PubMed] [Google Scholar]
- Kashyap V., Kumar M. Studies on the effects of Chlorpyrifos on Growth and Yield in Green Gram (Vigna radiata L.) at different phenological stages. J. Biol. Chem. Res.2013;30(2):734–740. [Google Scholar]
- Kaushik S., Inderjit Phytotoxicity of selected herbicides to mung bean (Phaseolus aureus Roxb.) Environ. Exp. Bot.2006;55:41–48. [Google Scholar]
- Kim S., Han S., Vanden Born W.H. Effect of chlorsulfuron on assimilate transport: ultra structural implications. Weed Sci. 1997;45:470–473. [Google Scholar]
- Kozlowski, T.T., 1971. Growth and Development of Trees, vol. I, Seed germination, ontogeny, and shoot growth, Academic Press, New York.
- Kramer P.J., Kozlowski T.T. Academic Press; New York: 1979. Physiology of Woody Plants. [Google Scholar]
- Lal R. Accumulation, metabolism and effects of organophosphorus insecticides on microorganisms. Adv. Appl. Microbiol. 1982;28:149–200. doi: 10.1016/s0065-2164(08)70235-1. [DOI] [PubMed] [Google Scholar]
- Lamoureux G.L., Frear D.S. Pesticide metabolism in higher plants: in vitro enzyme studies. Am. Chem. Soc. 1979:77–128. (Chapter 3) [Google Scholar]
- Maman N., Mason S.C., Galusha T., Clegg M.D. Hybrid and nitrogen influence on pearl millet production in Nebraska: yield, growth, and nitrogen uptake, and nitrogen use efficiency. Agro. J. 1999;91:737–743. [Google Scholar]
- Menon P., Gopal M., Prasad R. Influence of two insecticides, chlorpyrifos and quinalphos, on arginine ammonification and mineralizable nitrogen in two tropical soil types. J. Agric. Food Chem. 2004;52(24):7370–7376. doi: 10.1021/jf049502c. [DOI] [PubMed] [Google Scholar]
- Mudd S.H., Finkelstein J.D., Irreverre F., Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J. Biol. Chem. 1965;240:4382–4392. [PubMed] [Google Scholar]
- Parween T., Jan S., Mahmooduzzafar, Fatma T. Alteration in nitrogen metabolism and plant growth during different developmental stages of green gram (Vigna radiata L.) in response to chlorpyrifos. Acta Physiol. Planta. 2011;33:2321–2328. [Google Scholar]
- Parween T., Jan S., Mahmooduzzafar, Fatma T. Evaluation of oxidative stress in Vigna radiata L. in response to chlorpyrifos. Int. J. Environ. Sci. Technol. 2012;9(4):605–612. [Google Scholar]
- Sardar D., Kole R.K. Metabolism of chlorpyrifos in relation to its effect on the availability of some plant nutrients in soil. Chemosphere. 2005;61(9):1273–1280. doi: 10.1016/j.chemosphere.2005.03.078. [DOI] [PubMed] [Google Scholar]
- Siddiqui Z.S., Ahmed S. Effect of systemic fungicide on nutritive composition of diseased and healthy plant of Triticum aestivum L. Pak. J. Biol. Sci. 2000;3:2148–2150. [Google Scholar]
- Siddiqui Z.S., Ahmed S. Effects of systemic fungicides on protein, carbohydrate, aminoacids and phenolics contents of susceptible (Mexipak) and resistant (Poven) varieties of Triticum aestivum L. Turk. J. Bot. 2002;26:127–130. [Google Scholar]
- Somta P., Srinives P. Genome research in Mung bean [Vigna radiata (L.) Wilczek] and Blackgram [V. mungo (L.) Hepper] Sci. Asia. 2007;33(1):69–74. [Google Scholar]
- Suter M., Von Ballmoos P., Kopriva S., Opden Camp R., Schaller J., Kuhlemeier C., Schurmann P., Brunold C. Adenosine 59-phosphosulfate sulfotransferase and adenosine 59-phosphosulfate reductase are identical enzymes. J. Biol. Chem. 2000;275:930–936. doi: 10.1074/jbc.275.2.930. [DOI] [PubMed] [Google Scholar]
- Thakre S.K., Saxena S.N. Effect of soil application of chlorinated insecticides on amino acid composition of maize (Zea mays) Plant Soil. 1972;37:415–418. [Google Scholar]
- Xia X.J. Reactive oxygen species are involved in brassinosteroid-induced stress tolerance in cucumber. Plant Physiol. 2009;150:801–814. doi: 10.1104/pp.109.138230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Xia X., Yu G., Wang J., Wu J., Wang M., Yang Y., Shi K., Yu Y., Chen Z., Gan J., Yu J. Brassinosteroids play a critical role in the regulation of pesticide metabolism in crop plants. Sci. Rep. 2015;5:9018. doi: 10.1038/srep09018. [DOI] [PMC free article] [PubMed] [Google Scholar]