Abstract
Onions (Allium cepa L.) comprise a valuable vegetable crop in many countries. Modern scientific research has shown that onions possess many biological activities, including antibacterial, anticancer, hypoglycemic, hypolipidemic, antiplatelet aggregation, and antioxidant activities. The goal of this study was to investigate the impact of total onion polyphenols on antioxidant and xanthine oxidase (XO) inhibitory activities. Total onion polyphenols showed significant antioxidant activity in DPPH, FRAP, and OH-assays (IC50 [µg/mL]), 43.24, 560.61, and 12.97, respectively). In a X/XO system, antioxidant properties of these polyphenols significantly inhibited XO activity (IC50 [µg/mL], 17.36). These results indicated that total onion polyphenols showed promising antioxidant and anti-gout properties and might be used as potential, natural drugs against oxidative diseases after successful studies in vivo as well as clinical trials.
Keywords: Total polyphenols, Onion, Antioxidant, Xanthine oxidase, Inhibitory activities
1. Introduction
Onion (Allium cepa L.) is a plant of the Liliaceae family that grows naturally in China and is an important vegetable crop (Shokoohinia et al., 2015, Griffiths et al., 2002, Abdelrahman et al., 2016, Zhang et al., 2016). Modern scientific research has shown that onions have antibacterial, anticancer, hypoglycemic, hypolipidemic, antiplatelet aggregating, and antioxidant activities (Benkeblia et al., 2007, Rodríguez Galdón et al., 2008, Zhu and Li, 2016). It is known to contain many phytochemicals, such as carotenoids, copaenes, flavonoids, minerals, phenolics, phytoestrogens, terpenoids, vitamins, anthocyanins, and amino acids. Polyphenols, as one kind of phenolic hydroxyl compounds, are a class of natural, functional onion constituent.
Abnormal xanthine oxidase (XO) activity and high serum uric acid concentrations are common health problems that occur in many diseases, particularly in gout and metabolic syndromes (Ferreira Antunes et al., 2016, Song et al., 2016). In particular, XO and serum uric acid concentration abnormalities prepare the ground for the precipitation of gout. Therefore, keeping XO and serum uric acid concentrations within normal limits is very important in treating or ameliorating gout. Accordingly, the potential effects of various natural plant active ingredients on XO regulation and serum uric acid concentrations are being studied in gout-disease models (Nile and Park, 2015, Tang et al., 2016, Wan et al., 2016, Gao et al., 2017).
From a literature survey, it was found that total polyphenols from onion contain many bioactive constituents, which in pure form or derivatives might be potentially useful in the treatment of various diseases. Therefore, in this study, the goal was to investigate the impact of total onion polyphenols on antioxidant and XO inhibitory activities.
2. Material and methods
2.1. Material and chemicals
Total polyphenols from onion, according to gallic acid content calibration (polyphenols content of 68%) were isolated in-house. 2-Diphenyl-1-picrylhydrazine radical (DPPH), gallic acid, X, allopurinol (AL), and XO were obtained from Sinopharm Chemical Reagent Co. (Shanghai, China). All other chemicals and solvents used were HPLC grade from Hunan Chemical Reagent Factory (Changsha, China) and used as received or dried by standard procedures, unless stated otherwise.
2.2. DPPH radical-scavenging assay
DPPH is a stable free radical, appearing dark purple in alcoholic solution, with a 515-nm maximum absorption peak. A free-radical scavenger in a reaction system reduces the A515nm by pairing with DPPH’s single electron, such that free-radical scavenging is detected by changes in the A515nm, allowing evaluation of a sample’s antioxidant ability. A total of 0.18 mL of 1 × 10−3 M DPPH and 10 mg of sample were examined by mixing in absolute ethanol. The resulting samples were mixed, incubated for 45 min using ultrasound, and then monitored against blanks using a spectrophotometer (UV-757 Shanghai reached US spectrum Instrument Co. at 515 nm until the reaction reached a steady state (Atta et al., 2017). All measurements were performed in triplicate and free-radical scavenging activity calculated using absorbance. The IC50 values of samples were obtained from a radical-scavenging activity curve (Nile and Park, 2015, Tang et al., 2016, Wan et al., 2016, Nile et al., 2013).
2.3. Ferric reducing antioxidant power radical-scavenging assay
Antioxidants can also reduce Fe3+ into Fe2+ and antioxidation ability measured by spectrophotometry, using a ferric-reducing antioxidant power (FRAP) assay. The Fe3+ reduction ability of samples was determined as described previously with slight modifications. The reaction mixture contained 10 mL of sample (10 mg) in 3 mL of potassium ferricyanide solution (1 mM). The resulting mixture was then mixed in a water bath at 50 °C for 20 min. After incubation, 0.5 mL of trichloroacetic acid (TCA, 10% by vol) was added to terminate the reaction (Daya and Pant, 2017). The solution’s upper phase (1 mL) was mixed with distilled water (1 mL) and 0.1 mL of FeCl3 solution (0.01% by wt). The reaction mixture was then incubated at room temperature for 10 min and the A700 measured using a spectrophotometer against a blank solution; all tests were performed in triplicate. Higher reaction mixture absorbances indicated greater reducing power, with glutathione (2 mM) used as a reference compound. The IC50 values of samples were obtained from a radical scavenging activity curve (Nile et al., 2013, 2014).
2.4. OH• radical scavenging assay
In this experiment, 2-deoxyribose was degraded by condensation with thiobarbituric acid (TBA) and evaluated using a spectrophotometer. The OH• radical was generated using the Fe3+-ascorbate-EDTA-H2O2 system (Fenton reaction). This reaction mixture contained, in a final 1 mL volume, 2-deoxy-2-ribose (2.8 mM), KH2PO4-KOH buffer (20 mM, pH 7.4), FeCl3 (100 µM), EDTA (100 µM), H2O2 (1.0 mM), and ascorbic acid (100 µM). Each test sample, such as a 0.3 mL sample (10 mg) and a 6-paradol (2 mM) solution, was added separately. The reaction mixture with added sample was then stirred at 37 °C for 1 h, at which time 1 mL of 3% TCA and 1 mL of 2% aqueous TBA solution was added. This reaction mixture was incubated at 90 °C for 15 min for color development (Ishaq and Jafri, 2017). After cooling, the A532 was recorded against a blank solution, with tests performed in triplicate and the percent inhibition calculated by comparing test and blank solutions. The IC50 values of the samples were obtained from a radical-scavenging activity curve (Nile et al., 2013, 2014).
2.5. XO inhibition
Bovine milk XO activity was measured at 25 °C using a spectrophotometer at 550 nm. The reaction mixtures in sample wells were incubated with XO (500 µM final concentration) in phosphate buffer (0.01 M, pH 8.75, 30 µL), NBT (50 µL, 100 µM final concentration), PMS (50 µL, 100 µM final concentration), and Triton X-100 (20 µL, 0.5%). A reaction sample (10 mg) was added to the reaction mixture to inhibit XO by formazan formation. The total mixture was then mixed in a water bath at 37 °C for 2 h, and the reaction product’s A550 recorded. For blanks and controls in this assay, X was omitted from the sample, and AL used as a positive control. All values were expressed as the average of triplicate experiments. The samples’ IC50 values were obtained from inhibitor concentration-activity curves (Nile and Park, 2015, Tang et al., 2016, Wan et al., 2016, Nile et al., 2013).
3. Results and discussion
3.1. Antioxidant activity
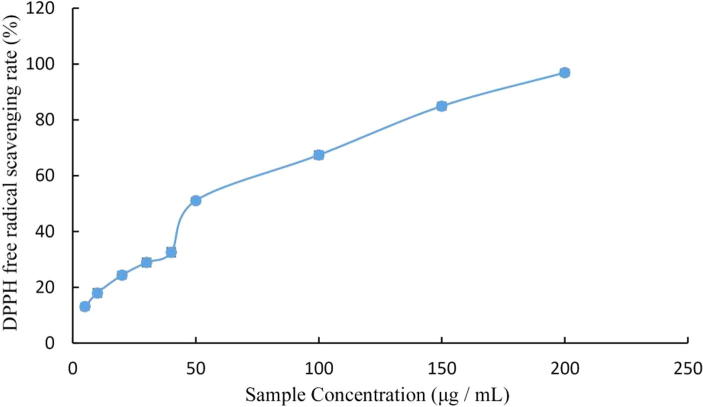
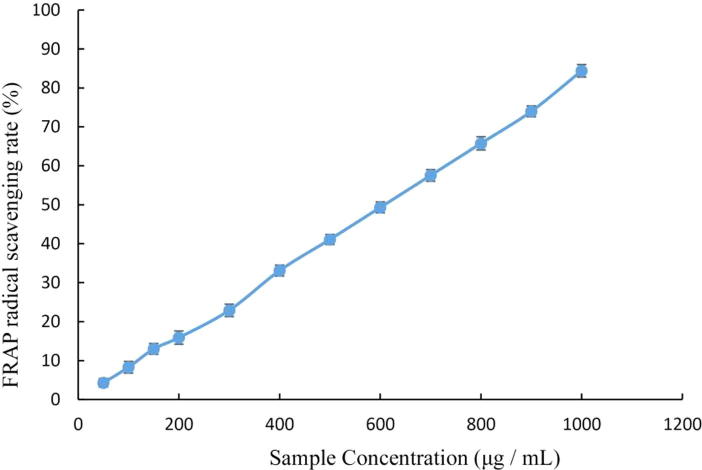
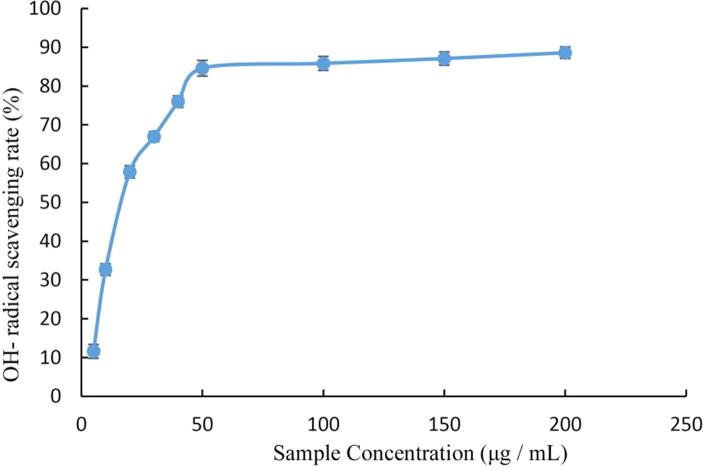
Total onion polyphenols were investigated for antioxidant activity in vitro against DPPH, FRAP, and OH• radicals. Different concentrations of total onion polyphenols were used and the results presented in Fig. 1, Fig. 2, Fig. 3.
Fig. 1.
DPPH free radical-scavenging rate by total onion polyphenols.
Fig. 2.
FRAP radical-scavenging rate by total onion polyphenols.
Fig. 3.
OH• radical-scavenging rate by total onion polyphenols.
Total onion polyphenols showed better DPPH scavenging ability, which increased with increased polyphenol concentrations (Kumruzzaman and Sarker, 2017). When the concentration was 50 and 200 µg/L, the scavenging rate was 51.05 and 96%, respectively (Fig. 1). The IC50 for DPPH radical-scavenging activity was 43.24 µg/mL.
The FRAP radical-scavenging activity of total onion polyphenols also increased with increased polyphenol concentrations, showing a significant dose-effect relationship (Fig. 2). The IC50 for FRAP radical-scavenging activity was 560.61 µg/mL.
With increased of total polyphenol concentrations, OH• radical-scavenging was enhanced (Fig. 3). At concentrations >50 µg/mL, OH• radical-scavenging activity was relatively stable and slow. The IC50 of OH• radical scavenging activity was 12.97 µg/mL (see Table 1).
Table 1.
The antioxidant activity of total onion polyphenols.
| Antioxidant activity | IC50 (µg mL−1) |
|---|---|
| DPPH | 43.24 |
| FRAP | 560.61 |
| OH• | 12.97 |
Therefore, the results of these assays showed that total onion polyphenols possessed good antioxidant activities. It has been reported that free radicals cause oxidative damage to biomolecules and have significant effects on cardiovascular diseases, aging, cancer, and inflammatory diseases (Jimenez et al., 2011, Katsampa et al., 2015). Antioxidants have good prophylactic and therapeutic potential for free radical-related disease disorders (Cao et al., 2015, Hougaard et al., 2016). Many studies have shown that polyphenols have strong antioxidant properties in vitro (Gangopadhyay et al., 2016, Jurinjak Tušek et al., 2016, Zielinska and Michalska, 2016). Studies of the active constituents of onions, such as polyphenols, have shown that they exhibit good antioxidant properties in vivo and in vitro (Jimenez et al., 2011, Katsampa et al., 2015). Some active ingredients in onions also possess good antibacterial activity (Jimenez et al., 2011, Katsampa et al., 2015). The data obtained in the present study showed that total onion polyphenols exerted significant free-radical repression and were the main antioxidants that react with free radicals.
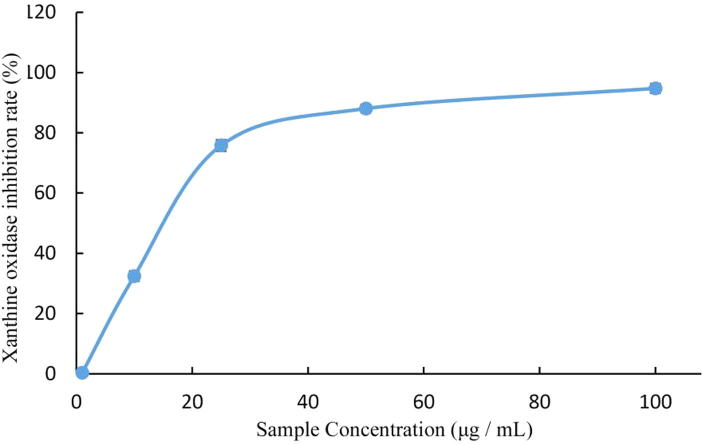
3.2. Xanthine oxidase inhibition
The results showed that total onion polyphenols possessed good XO activity inhibition, from experimental and screening studies using gradient multiplexing at different concentrations. These results showed that there was a dose-effect relationship between XO concentration and XO activity inhibition (Fig. 4). At 100 µg/mL, the XO inhibition rate was >90% and the IC50 of XO activity inhibition was 17.36 µg/mL.
Fig. 4.
XO inhibition rate by total onion polyphenols.
To our knowledge, there have been no studies or reports to date regarding inhibition of XO activity by total onion polyphenols, but a small number of studies of plant, vegetable, and flavor extracts have been reported to possess XO-activity inhibition (Ong et al., 2017). The direct antioxidant activity of dietary polyphenols is significant because of the association between the intake of polyphenolic-rich foods (e.g., fruits and vegetables) and the incidence of oxidative stress-related diseases. However, through general reductions in oxidative stress, indirect antioxidant effects (e.g., inhibition of ROS-producing enzymes) might also be associated with health benefits (Nile and Park, 2015, Nile et al., 2013).
Due to modern diets and lifestyle changes, the incidence of gout and hyperuricemia in the world has increased dramatically. Antihypertensive agents, including XO inhibitors and uric acid excretion agents, are commonly used to treat chronic gouty arthritis. In general, AL is a commonly used drug, but it also has serious side effects. Therefore, new alternatives with better therapeutic effects and fewer side effects need to be identified (Nile et al., 2013, Nile and Park, 2014). Thus, we have tried to identify phytochemicals that can serve as XO inhibitors from a variety of foods and food products, including onions.
4. Conclusion
The present results showed that total onion polyphenols have significant antioxidant and XO inhibitory activities. Although the efficacy of practical applications was inferred here from results in vitro, this study showed that onion polyphenols had an associated pharmacological activity, suggesting that they might have value in human and animal health. The data showed that total onion polyphenols and their biologically active compounds might be potential sources of new antioxidants and anti-gout drugs, providing a preliminary, scientific basis for new drug applications of onion polyphenols. However, further studies in vivo as well as clinical studies are needed to demonstrate that these chemicals are useful as drugs for various diseases associated with XO and free radicals.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (Project No. 31560192; Youth Science Funds, Project No. 31100421), the Natural Science Foundation of Hunan Province (Project No. 13JJB011), and the Science and Technology Project of the Key Laboratory for Ecotourism of Hunan Province (Project No. JSSTLY1516), and the JSPS Invitation Fellowships for Research in Japan of the Japan Society for the Promotion of Science (Long-term, Project No. L15711).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Hui Ouyang, Email: oyhmail@163.com.
Wanxi Peng, Email: pengwanxi@163.com.
References
- Abdelrahman M., Abdel-Motaal F., El-Sayed M. Dissection of Trichoderma longibrachiatum-induced defense in onion (Allium cepa L.) against Fusarium oxysporum f. sp. cepa by target metabolite profiling. Plant Sci. 2016;246:128–138. doi: 10.1016/j.plantsci.2016.02.008. [DOI] [PubMed] [Google Scholar]
- Atta A., Mustafa G., Sheikh M.A., Shahid M., Xiao H. The biochemical significances of the proximate, mineral and phytochemical composition of selected vegetables from Pakistan. Matrix Sci. Pharma. 2017;1(1):06–09. [Google Scholar]
- Benkeblia N., Shiomi N., Osaki M. Kinetics and hydrolysis parameters of total fructooligosaccharides of onion bulbs: effects of temperature regimes and cultivars. J. Food Biochem. 2007;31(1):14–27. [Google Scholar]
- Cao H., Xie Y., Chen X. Type 2 diabetes diminishes the benefits of dietary antioxidants: evidence from the different free radical scavenging potential. Food Chem. 2015;186:106–112. doi: 10.1016/j.foodchem.2014.06.027. [DOI] [PubMed] [Google Scholar]
- Daya B., Pant K. Biomonitoring of wetland using macrophytes and macroinvertebrates. Malaysian J. Sustain. Agricult. 2017;1(1):11–14. [Google Scholar]
- Ferreira Antunes M., Eggimann F.K., Kittelmann M. Human xanthine oxidase recombinant in E. coli: a whole cell catalyst for preparative drug metabolite synthesis. J. Biotechnol. 2016;235:3–10. doi: 10.1016/j.jbiotec.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Gangopadhyay N., Rai D.K., Brunton N.P., Gallagher E., Hossain M.B. Antioxidant-guided isolation and mass spectrometric identification of the major polyphenols in barley (Hordeum vulgare) grain. Food Chem. 2016;210:212–220. doi: 10.1016/j.foodchem.2016.04.098. [DOI] [PubMed] [Google Scholar]
- Gao W., Wang Y., Basavanagoud B., Jamil M.K. Characteristics studies of molecular structures in drugs. Saudi Pharmaceut. J. 2017;25(4):580–586. doi: 10.1016/j.jsps.2017.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G., Trueman L., Crowther T., Thomas B., Smith B. Onions—a global benefit to health. Phytother. Res. 2002;16(7):603–615. doi: 10.1002/ptr.1222. [DOI] [PubMed] [Google Scholar]
- Hougaard A.B., Pindstrup H., Arneborg N., Andersen M.L., Skibsted L.H. Free radical formation by Lactobacillus acidophilus NCFM is enhanced by antioxidants and decreased by catalase. Food Res. Int. 2016;79:81–87. [Google Scholar]
- Ishaq S., Jafri L. Biomedical importance of cocoa (Theobroma cacao): significance and potential for the maintenance of human health. Matrix Sci. Pharma. 2017;1(1):01–05. [Google Scholar]
- Jimenez L., Alarcón E., Trevithick-Sutton C., Gandhi N., Scaiano J.C. Effect of γ-radiation on green onion DNA integrity: role of ascorbic acid and polyphenols against nucleic acid damage. Food Chem. 2011;128(3):735–741. [Google Scholar]
- Jurinjak Tušek A., Benković M., Belščak Cvitanović A., Valinger D., Jurina T., Gajdoš Kljusurić J. Kinetics and thermodynamics of the solid-liquid extraction process of total polyphenols, antioxidants and extraction yield from Asteraceae plants. Indust. Crops Prod. 2016;91:205–214. [Google Scholar]
- Katsampa P., Valsamedou E., Grigorakis S., Makris D.P. A green ultrasound-assisted extraction process for the recovery of antioxidant polyphenols and pigments from onion solid wastes using Box-Behnken experimental design and kinetics. Indust. Crops Prod. 2015;77:535–543. [Google Scholar]
- Kumruzzaman M., Sarker A. Water requirements for various crops and impact of irrigation in barind area. Malaysian J. Sustain. Agricult. 2017;1(1):04–07. [Google Scholar]
- Nile S.H., Kumar B., Park S.W. In vitro evaluation of selected benzimidazole derivatives as an antioxidant and xanthine oxidase inhibitors. Chem. Biol. Drug Des. 2013;82(3):290–295. doi: 10.1111/cbdd.12141. [DOI] [PubMed] [Google Scholar]
- Nile S.H., Park S.W. Antioxidant, α-Glucosidase and Xanthine oxidase inhibitory activity of bioactive compounds from maize (Zea mays L.) Chem. Biol. Drug Des. 2014;83(1):119–125. doi: 10.1111/cbdd.12205. [DOI] [PubMed] [Google Scholar]
- Nile S.H., Park S.W. Chromatographic analysis, antioxidant, anti-inflammatory, and xanthine oxidase inhibitory activities of ginger extracts and its reference compounds. Indust. Crops Prod. 2015;70:238–244. [Google Scholar]
- Ong S.Q., Lee B.B., Tan G.P., Maniam S. Capacity of black soldier fly and house fly larvae in treating the wasted rice in Malaysia. Malaysian J. Sustain. Agricult. 2017;1(1):08–10. [Google Scholar]
- Rodríguez Galdón B., Rodríguez Rodríguez E., Díaz Romero C. Flavonoids in onion cultivars (Allium cepa L.) J. Food Sci. 2008;73(8):C599–C605. doi: 10.1111/j.1750-3841.2008.00903.x. [DOI] [PubMed] [Google Scholar]
- Shokoohinia Y., Rashidi M., Hosseinzadeh L., Jelodarian Z. Quercetin-3-O-β-d-glucopyranoside, a dietary flavonoid, protects PC12 cells from H2O2-induced cytotoxicity through inhibition of reactive oxygen species. Food Chem. 2015;167:162–167. doi: 10.1016/j.foodchem.2014.06.079. [DOI] [PubMed] [Google Scholar]
- Song J.U., Jang J.W., Kim T.H. Structure-based design and biological evaluation of novel 2-(indol-2-yl) thiazole derivatives as xanthine oxidase inhibitors. Bioorg. Med. Chem. Lett. 2016;26(3):950–954. doi: 10.1016/j.bmcl.2015.12.055. [DOI] [PubMed] [Google Scholar]
- Tang H.J., Zhang X.W., Yang L. Synthesis and evaluation of xanthine oxidase inhibitory and antioxidant activities of 2-arylbenzo[b]furan derivatives based on salvianolic acid C. Europ. J. Med. Chem. 2016;124:637–648. doi: 10.1016/j.ejmech.2016.08.019. [DOI] [PubMed] [Google Scholar]
- Wan Y., Zou B., Zeng H., Zhang L., Chen M., Fu G. Inhibitory effect of verbascoside on xanthine oxidase activity. Int. J. Biol. Macromole. 2016;93(Part A):609–614. doi: 10.1016/j.ijbiomac.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Zhang S.L., Deng P., Xu Y.C., Lü S.W., Wang J.J. Quantification and analysis of anthocyanin and flavonoids compositions, and antioxidant activities in onions with three different colors. J. Integrat. Agricult. 2016;15(9):2175–2181. [Google Scholar]
- Zhu Y.B., Li J.S. Analysis on the harm and preventive of computer network viruses. J. Mech. Eng. Res. Develop. 2016;39(2):469–472. [Google Scholar]
- Zielinska M., Michalska A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016;212:671–680. doi: 10.1016/j.foodchem.2016.06.003. [DOI] [PubMed] [Google Scholar]
Further reading
- Liu H.B., Liu Z.L. Recycling utilization patterns of coal mining waste in China. Resources, Reservat. Recycl. 2010;12:1331–1340. [Google Scholar]
- Peng W. Effect of weakly alkaline salt pretreatment on bio-boards for medicine safety. J. Pure Appl. Microbiol. 2015;9(3):1913–1917. [Google Scholar]
- Viju N., Satheesh S., Vincent S. Antibiofilm activity of coconut (Cocos nucifera Linn.) husk fibre extract. Saudi J. Biol. Sci. 2013;20(1):85–91. doi: 10.1016/j.sjbs.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]