Abstract
Evaluation of antioxidant and anticancer activities were screened by various Saururus chinensis root extracts. Four solvents (ethyl acetate, methanol, ethanol, and water) extracts were investigated for their total flavonoids, phenol contents and their antioxidant activity of DPPH (2,2-diphenyl-1-picrylhydrazyl), NO (nitric oxide), H2O2 (hydrogen peroxide), ABTS 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid)diammonium assays, FRAP (ferric reducing ability of plasma) assays and anticancer activity. The total phenolic and flavonoid content of extracts were determined by using FC (Folin–Ciocalteu) and AlCl3 colorimetric assay method. Total flavonoid content in these plants ranged from 24.7 to 72.1 mg g−1 and amount of free phenolic compounds was between 11.2 and 67.1 mg g−1 extract. The all extracts have significant levels of phenolics and flavonoids content. Anticancer activity was screened for MCF-7 breast cancer cell line. Ethanol extract shows significant of antioxidant activity and water extract shows significant of anticancer activity compared with standard (BHT) butylated hydroxy toluene. These ethanol and water extracts could be considered as a natural source for using antioxidant, and anticancer agents compared to commercial available synthetic drugs.
Keywords: Saururus chinensis root, Solvent extracts, Antioxidant activity, Anticancer activity
1. Introduction
Saururus chinensis has been used as a diuretic and a detoxifying agent for edema, jaundice, gonorrhoea and several inflammatory diseases treatment in China and India (Rao and Rao, 1990). More than 4000 different naturally occurring flavonoids were identified from other plant materials (Middleton et al., 1986) but very important pharmacological studies have demonstrated that major flavonoids (Kang et al., 2005, Sung et al., 1997) and other ligands (Hwang et al., 2003, Sung and Kim, 2000) from S. chinensis (Fig. 1), which possess a wide array of pharmacological and biochemical activities, such as antidiabetic (Moon et al., 2008), anti-carcinogenic (Omer et al., 2005, Hester et al., 2003), anti-inflammatory (Hwang et al., 2003b), antioxidant (Lee et al., 2003) and hepato-protective (Kim et al., 2004), neuro-protective activity (Nortier and Vanherweghem, 2002), nephrotoxic (Cheng et al., 2006), carcinogenic (Kohara et al., 2002), and mutagenic (Balachandran et al., 2005). Importantly S. chinensis baill shows strong antioxidant activity (Lee et al., 2004, Choi et al., 2002), and its extract has been shown to reduce lipid peroxide levels in rats fed a high-fat diet (Yu et al., 2008). Current cancer chemotherapy is primarily dependent on repeated administrations of synthetic anticancer agents. Although effective, their repeated use has often resulted in the development of resistance, plants have been suggested as an alternative source of materials for cancer therapy because they constitute a potential source of bioactive chemicals. Previous pharmacological studies on S. chinensis have reported that its extracts have cytotoxic activities and EtOH extract from the aerial parts of S. chinensis showed a potent anti-proliferative activity against C33a human cervical cancer cells (Kim et al., 2011).
Figure 1.
Structure of reference compounds from Saururus chinensis leaves extract.
Therefore, studies on herbs have become the top issue at present time for their great potential of biological activity, present investigation the estimated total phenolics and flavonoids in dried root of S. chinensis in various solvent extracts (methanol, ethanol, ethyl acetate, and water) and screened for antioxidant and anticancer activities.
2. Materials and methods
2.1. Preparation of solvent extract
S. chinensis root powder (100 g) was immersed in 500 mL of double distil water. The filtrate was constant stirring with maximum 72 h. The extract was then concentrated under reduced pressure at 40 °C using vacuum rotary evaporator, the yield of water extract was 15.25 g. The aqueous layer further fractionated with ethyl acetate, methanol, and ethanol fractions were concentrated with vacuum rotary evaporator, yield of each fractions 16.1%, 24.6% and 18.0% respectively.
2.2. Determination of total phenolic and flavonoid content
The total phenolic and total flavonoid were identified by using the FC (Folin–Ciocalteu) and aluminium chloride colorimetric assay methods, the method was followed by previously report method (Marinova et al., 2005). Total phenolic and flavonoid content rang are represented in Table 1.
Table 1.
The total phenolic and flavonoid content of Saururus chinensis root extract.
| Extracts | Total phenolica (mg GAE/100 g of dry mass) | Total flavonoidsa (mg CE/100 g of dry mass) |
|---|---|---|
| Ethyl acetate | 11.2 ± 0.2 | 24.7 ± 0.2 |
| Methanol | 22.0 ± 0.6 | 54.0 ± 0.7 |
| Ethanol | 35.2 ± 1.2 | 58.4 ± 0.4 |
| Water | 67.1 ± 0.9 | 72.1 ± 0.1 |
GAE: Gallic Acid Equivalents; CE: Catechin Equivalents.
Values expressed as mean ± standard deviation (n = 3).
2.3. Antioxidant activity of DPPH method
DPPH (2,2-diphenyl-1-picrylhydrazyl) antioxidant activity was screened for S. chinensis root extracts and the method was followed by previously report method (Cefarelli et al., 2006). Accordingly, extracts were examined for their ability to prevent the bleaching of the purple colored methanol solution DPPH 1.6 mL of test sample (25, 50, and 100 μg/mL) and 1.6 mL of 0.004% (w/v) methanol solution of DPPH were reacted at room temperature, absorbance of reaction mixture was measured at 517 nm.
Percentage inhibition was calculated by.
2.4. H2O2 scavenging activity
Hydrogen peroxide scavenging activity was screened for various solvent of S. chinensis root extracts, the method was followed by previously reported literature method (Ruch et al., 1989). A solution of H2O2 (0.6 mL, 40 mM) with phosphate buffer (pH 7.4) and root extract (3.4 mL) with phosphate buffer were reacted, the reaction mixture was measured by absorbance at 230 nm.
Percentage inhibition of H2O2 was calculated by.
2.5. NO scavenging activity
Nitric oxide scavenging activity was calculated using by slight modified version of the method devised by Marcocci et al. (1994). Sodium nitroprusside (1 mL, 10 mM) and 1.5 mL of phosphate buffer saline (0.2 M, pH 7.4) were added to different concentrations of test sample incubated for 150 min at 25 °C. Aliquots (1 mL) of reaction mixtures were then react with 1 mL of Griess reagent (1% sulfanilamide, 2% H3PO4 and 0.1% naphthylethylene diamine dihydrochloride).
Percentage inhibition of nitric oxide scavenging was calculated by.
2.6. ABTS radical cation decolourisation assay
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonicacid)diammonium) assay can be used to determine the antioxidant activity of biological fluids, cells, tissues, natural and other synthetic therapeutic compounds. The assay measures ABTS radical cation formation induced by metmyoglobin and hydrogen peroxide. A water soluble trolox is used as a positive control for inhibiting the formation of the radical cation in the assay (Adedapo et al., 2009).
Preparation: The ABTS reagent was prepared by 5 mL of 14 mM ABTS and 5 mL of 4.9 mM potassium persulfate (K2S2O8), the reaction mixture was kept in dark room at room temperature for 16 h. The reagent absorbance was adjusted to 0.700 ± 0.02 at 734 nm with distilled water and used for the assay purposes.
1 mL of ABTS reagent is added to different concentrations of test sample measured by 734 nm at 3 min interval.
A0 – absorbance of control, A1 – absorbance of the tested sample.
2.7. Ferric reducing antioxidant power (FRAP) assay
The FRAP (ferric reducing ability of plasma) assay was performed according to the previous reported method (Dehghan and Khoshkam, 2012). FRAP reagent was prepared by mixing of 2.5 mL of solutions TPTZ (10 mM, (40 mM) HCl, and FeCl3 (20 mM) in 25 mL of acetate buffer (300 mM, pH 3.6), the light blue reagent contains Fe3+-TPTZ that changes to Fe2+-TPTZ as dark blue. These changes were due to the absorbance increase as monitored at a wavelength of 593 nm for different concentrations of S. chinensis root extracts in FRAP reagent .
2.8. Anticancer activity
Anticancer assays were performed according to the US NCI protocol, which was described elsewhere (Boyd and Paull, 1995).
Cell viability test: The viability of cells was assessed by MTT assay using MCF-7 cell lines.
Cytotoxic assay (MTT method): The anticancer activity was carried out according to the method described in previous literature (Mostafa et al., 2016).
MCF-7 cell lines was treated with these compounds at one primary cytotoxic assay dose of 100 μM for 48 h (MTT anticancer assay). Doxorubicin was used as a standard. In the current protocol, all cell lines were pre-incubated on a microtiter plate. The results of each test were reported as the growth percentage of treated cells compared to untreated control cells. A 0.1 mL aliquot of the cell suspension (5 × 106 cells/100 μL) and 0.1 mL of the test solution (6.25–100 μg) were added to the wells, with the plates kept in an incubator (5% CO2) at 37 °C for 72 h. After 72 h, 20 μL of MTT was added, and the plates were kept in the CO2 incubator for 2 h, followed by the addition of propanol (100 μL). The plates were covered with aluminum foil to protect them from light and subsequently agitated in a rotary shaker for 10–20 min, afterwards the 27-well plates were processed on an ELISA reader to obtain absorption data at 562 nm.
2.9. Statistical analysis
All experiments were performed in triplicate and all data were expressed at least 3 independent evaluations and the standard deviations (SD) were also calculated using Microsoft Excel 2007 software (Microsoft, Redmond, WA, USA).
3. Result and discussion
3.1. Antioxidant activity of S. chinensis root extracts
The S. chinensis root extracts were screened for antioxidant property of 2,2-diphenyl-1-picrylhydrazyl (DPPH), hydrogen peroxide (H2O2), nitric oxide (NO), ABTS and FRAP assays method.
The antioxidant activity of extracts was measured by radical scavenging ability through UV–visible spectrophotometer. Methanolic solutions of DPPH shows a strong purple color, with strong absorption at 517 nm, and when reduced are a yellow color. Free radical scavenging activity of DPPH radical was found to increase with increase in concentration, showing a maximum of 88.71 at 100 μg/mL. The ethanol extract showed a good capacity of scavenging the DPPH free radical activity and highly active (IC50:11.04 μg/mL) compared with standard BHT at concentration 100 μg/mL. Results of DPPH reduction is shown in Table 2.
Table 2.
DPPH scavenging activity (%) of Saururus chinensis root extracts.
| Extracts | Concentration (μg/mL)a |
IC50 (μg/mL)a | ||
|---|---|---|---|---|
| 25 μg/mL | 50 μg/mL | 100 μg/mL | ||
| Ethyl acetate | 10.31 ± 0.17 | 23.23 ± 0.14 | 43.01 ± 0.32 | >100 |
| Methanol | 60.62 ± 0.23 | 71.01 ± 0.16 | 88.71 ± 0.12 | 15.13 ± 0.23 |
| Ethanol | 69.22 ± 0.19 | 74.12 ± 0.22 | 91.02 ± 0.21 | 11.04 ± 0.31 |
| Water | 06.10 ± 0.07 | 12.03 ± 0.21 | 21.21 ± 0.11 | >100 |
| BHT | 54.21 ± 0.22 | 70.30 ± 0.12 | 82.31 ± 0.30 | 18.86 ± 0.21 |
Value expressed are means ± SD.
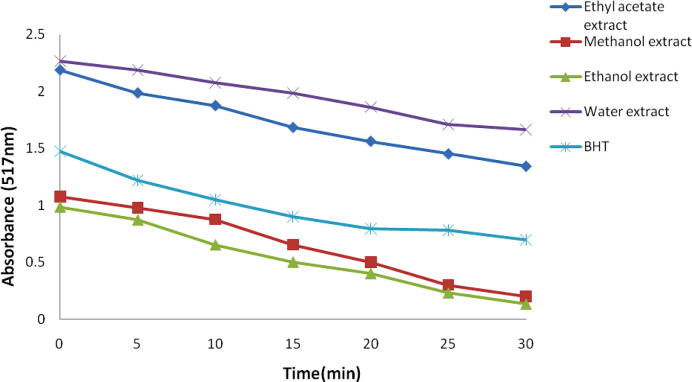
The Fig. 2 shows that kinetic activities of different extracts and estimated the scavenging activity of maximum reaction time. The higher antioxidant activity of the extract may be assumed that –OH groups are primarily involved in H-atom transfer reactions to DPPH, which attributed to the highly significant contribution of 3′,4′-hydroxyl groups on the ring.
Figure 2.
Measurement of absorbance between Saururus chinensis root extracts and DPPH solution in kinetic behavior at concentration 100 μg/mL.
Hydrogen peroxide (H2O2) scavenging activity showed that ethanol extract (12.00 μg/mL) very effective scavenging activity compared with standard BHT (20.35 μg/mL). Results of H2O2 scavenging reduction is shown in Table 3.
Table 3.
Hydrogen peroxide (H2O2) scavenging activity of Saururus chinensis root extracts.
| Extracts | Concentration (μg/mL)a |
IC50 (μg/mL)a | ||
|---|---|---|---|---|
| 25 | 50 | 100 | ||
| Ethyl acetate | 12.16 ± 0.14 | 20.21 ± 0.02 | 43.13 ± 0.04 | >100 |
| Methanol | 55.28 ± 0.11 | 72.00 ± 0.11 | 84.21 ± 0.16 | 18.13 ± 0.02 |
| Ethanol | 68.47 ± 0.04 | 84.17 ± 0.09 | 92.12 ± 0.41 | 12.00 ± 0.15 |
| Water | 16.29 ± 0.35 | 23.12 ± 0.11 | 36.12 ± 0.18 | >100 |
| BHT | 59.01 ± 0.04 | 68.51 ± 0.09 | 82.17 ± 0.53 | 20.35 ± 0.13 |
Value expressed are means ± SD.
The extract showed nitric oxide scavenging activity between 25 and 100 μg/mL concentration. Ethanol extract shows highly active (89.11%) compared with standard BHT (83.32 %) at concentration 100 μg/mL. The nitric oxide (NO•) radical reacts with Griess reagent, to form formazon which was measured spectrophotometrically. The IC50 value of ethanol extract was found to be 14.58 μg/mL. Results of NO scavenging reduction is shown in Table 4. Extracts were screened for ABTS radical-scavenging activity, the EtOH extract (IC50:17.54 μg/mL) was significant of activity compared with standard BHT. Results of ABTS scavenging reduction is shown in Table 5.
Table 4.
NO scavenging activity of Saururus chinensis root extracts.
| Extracts | Concentration (μg/mL)a |
IC50 (μg/mL)a | ||
|---|---|---|---|---|
| 25 | 50 | 100 | ||
| Ethyl acetate | – | 09.14 ± 0.24 | 14.32 ± 0.41 | >100 |
| Methanol | 52.51 ± 0.21 | 69.16 ± 0.10 | 82.12 ± 0.11 | 20.19 ± 0.19 |
| Ethanol | 60.01 ± 0.03 | 71.12 ± 0.64 | 89.11 ± 0.12 | 14.58 ± 0.11 |
| Water | 0.30 ± 0.05 | 17.01 ± 0.11 | 30.10 ± 0.10 | >100 |
| BHT | 53.16 ± 0.02 | 67.65 ± 0.63 | 83.32 ± 0.07 | 22.13 ± 0.23 |
Value expressed are means ± SD.
Table 5.
ABTS radical-scavenging activity of Saururus chinensis root extracts.
| Extracts | Concentration (μg/ml)a |
IC50 (μg/ml)a | ||
|---|---|---|---|---|
| 25 | 50 | 100 | ||
| Ethyl acetate | 21.30 ± 0.04 | 35.72 ± 0.11 | 40.31 ± 0.41 | >100 |
| Methanol | 55.23 ± 0.43 | 62.12 ± 0.10 | 73.20 ± 0.34 | 21.32 ± 0.18 |
| Ethanol | 59.34 ± 0.24 | 69.71 ± 0.08 | 83.23 ± 0.91 | 17.54 ± 0.32 |
| Water | 06.85 ± 0.14 | 11.32 ± 0.14 | 19.07 ± 0.12 | >100 |
| Trolox | 62.02 ± 0.02 | 74.09 ± 0.21 | 92.11 ± 0.56 | 14.21 ± 0.24 |
Value expressed are means ± SD.
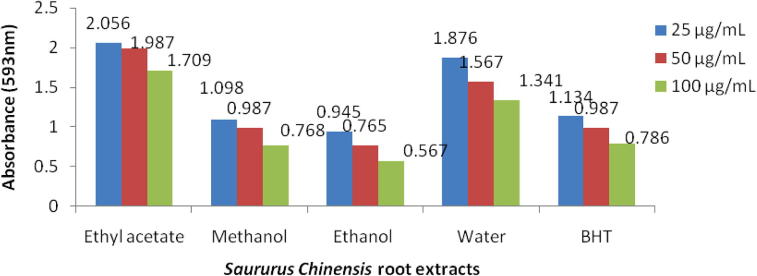
The FRAP assay was measured by ability to reduce the ferric 2,4,6-tripyridyl-s-triazine complex [Fe3+ − (TPTZ)2]3+ to provided blue colored ferrous complex [Fe2+ − (TPTZ)2]2+ in acidic medium at 593 nm absorbance . The ethanol extract was absorbed very low reducing abilities at 25–100 μg/mL compared with standard BHT, activity variation shows in Fig. 3.
Figure 3.
FRAP activity of Saururus chinensis root extracts at concentration 100 μg/mL.
3.2. Anticancer activity of S. chinensis root extracts
The compounds were for primary anticancer assay towards MCF-7 cell lines (human breast cancer). Results were reported as the percent growth of the treated cells when compared to the untreated control cells.
All the synthesized compounds methanol, ethanol, ethyl acetate, and water were evaluated for their anticancer activity against a human cancer cell line such as MCF-7 (Human breast cancer), by employing MTT assay. The results of cell viability data are summarized in Table 6.
Table 6.
Anticancer activity of Saururus chinensis root extracts against MCF-7 cancer cell line.
| Extracts | % Inhibition of cell viability |
LD50 μg/mL | ||
|---|---|---|---|---|
| 25 μg/mL | 50 μg/mL | 100 μg/mL | ||
| Ethyl acetate | – | – | 07.5 | >100 |
| Methanol | – | 04.6 | 18.1 | >100 |
| Ethanol | – | 09.4 | 20.9 | >100 |
| Water | 21.6 | 32.5 | 54.3 | 91.2 |
| Standard | 52.2 | 65.8 | 84.2 | 24.2 |
1,3-Bis(4-chlorobenzenesulfonyl)imidazolidine-2-one used as a standard.
The inhibitory concentration (LD50) value was calculated by the test sample of concentration at which 50% of cells viable, and calculated from the logarithmic trend line of the cytotoxicity graphs. The in vitro screening results revealed that the compound water exhibited promising anticancer activity compared in MCF7 breast cancer cells (54.2% inhibition) were observed at concentration (100 μg/mL). This inhibition at the mentioned concentration indicates a greater potency of compound water with a strong lethal effect over the breast cancer (MCF-7) cell line computed with other solvent extract.
Compounds methanol, ethanol, and ethyl acetate have low inhibitory activity against MCF-7 cell line.
4. Conclusion
In conclusion, the result of this study demonstrates that four different S. chinensis root extracts in methanol, ethanol, ethyl acetate, and water were screened for antioxidant, anticancer activates. An important finding in this study, the S. chinensis root ethanol extract was most important in antioxidant activity and water extract is highly active against anticancer activity against MCF-7 cancer cell line. Current investigation of antioxidant, and anticancer activates were evaluated using in vitro experiments only and further investigation for mechanism of action and in vivo experiments will lead to a more complete assessment of S. chinensis root extracts.
Acknowledgments
The project was supported by king Saud University, Deanship of Scientific Research, Research Chair. We are very grateful to Prince Sultan Research Chair for Environment and Wildlife & Saudi Biological Society. Department of Botany & Microbiology, College of Sciences, King Saud University (KSU), Riyadh, Saudi Arabia for encouragement and support for funding this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adedapo A.A., Jimoh F.O., Koduru S., Masika P.J., Afolayan A.J. Assessment of the medicinal potentials of the methanol extracts of the leaves and stems of Buddleja saligna. BMC Complement Altern. Med. 2009;9(21):1–8. doi: 10.1186/1472-6882-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran P., Wei F., Lin R.C., Khan I.A., Pasco D.S. Structure activity relationships of aristolochic acid analogues: toxicity in cultured renal epithelial cells. Kidney Int. 2005;67:1797–1805. doi: 10.1111/j.1523-1755.2005.00277.x. [DOI] [PubMed] [Google Scholar]
- Boyd M.R., Paull K.D. Some practical considerations and applications of the national cancer institute in vitro anticancer drug discovery screen. Drug Dev. Res. Drug. 1995;34:91–109. [Google Scholar]
- Cheng C.L., Chen K.J., Shih P.H., Lu L.Y., Hung C.F., Lin W.C., Gu J.Y. Chronic renal failure rats are highly sensitive to aristolochic acids, which are nephrotoxic and carcinogenic agents. Cancer Lett. 2006;232:236–240. doi: 10.1016/j.canlet.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Choi C.W., Kim S.C., Hwang S.S., Choi B.K., Ahn H.J., Lee M.Y., Park S.H., Kim S.K. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay-guided comparison. Plant Sci. 2002;163:1161–1168. [Google Scholar]
- Cefarelli G., D’Abrosca B., Fiorentino A., Izzo A., Mastellone C., Pacifico S., Piscopo V. Free-radical-scavenging and antioxidant activities of secondary metabolites from reddened cv. Annurca apple fruits. J. Agric. Food Chem. 2006;54:803–809. doi: 10.1021/jf052632g. [DOI] [PubMed] [Google Scholar]
- Dehghan G., Khoshkam Z. Tin(II)-quercetin complex: synthesis, spectral characterization and antioxidant activity. Food Chem. 2012;131:422–426. [Google Scholar]
- Hwang B.Y., Lee J.H., Nam J.B., Hong Y.S., Lee J.J. Lignans from Saururus chinensis inhibiting the transcription factor NF-κB. Photochemistry. 2003;64:765–771. doi: 10.1016/s0031-9422(03)00391-1. [DOI] [PubMed] [Google Scholar]
- Hwang B.Y., Lee J.H., Jung H.S., Kim K.S., Nam J.B., Hong Y.S., Paik S.G., Lee J.J. Sauchinone, a lignan from Saururus chinensis, suppresses iNOS expression through the inhibition of transactivation activity of RelA of NF-kappaB. Planta Med. 2003;69:1096–1101. doi: 10.1055/s-2003-45189. [DOI] [PubMed] [Google Scholar]
- Hester V.D.W., Anna G.S., Karin S. Biphasic modulation of cell proliferation by quercetin at concentrations physiologically relevant in humans. Cancer Lett. 2003;1:41–47. doi: 10.1016/s0304-3835(03)00412-9. [DOI] [PubMed] [Google Scholar]
- Kang T.H., Cho H., Oh H., Sohn D.H., Kim Y.C. Flavonol glycosides with free radical-scavenging activity of Saururus chinensis. Fitoterapia. 2005;76:115–117. doi: 10.1016/j.fitote.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Kim S.R., Sung S.H., Kang S.Y., Koo K.A., Kim S.H., Ma C.J., Lee H.S., Park M.J., Kim Y.C. Aristolactam BII of Saururus chinensis attenuates glutamate-induced neurotoxicity in rat cortical cultures probably by inhibiting nitric oxide production. Planta Med. 2004;70:391–396. doi: 10.1055/s-2004-818964. [DOI] [PubMed] [Google Scholar]
- Kim H.Y., Choi T.W., Kim H.J., Kim S.M., Park K.R., Jang H.J., Lee E.H., Kim C.Y., Jung S.H., Shim B.S., Ahn K.S. A methylene chloride fraction of Saururus chinensis induces apoptosis through the activation of caspase-3 in prostate and breast cancer cells. Phytomedicine. 2011;18:567–574. doi: 10.1016/j.phymed.2010.10.013. [DOI] [PubMed] [Google Scholar]
- Kohara A., Suzuki T., Honma M., Ohwada T., Hayashi M. Mutagenicity of aristolochic acid in the lambda/lacZ transgenic mouse (MutaMouse) Mutat. Res. 2002;515:63–67. doi: 10.1016/s1383-5718(01)00350-3. [DOI] [PubMed] [Google Scholar]
- Lee A.K., Sung S.H., Kim Y.C. Inhibition of lipopolysaccharide-inducible nitric oxide synthase, NF-alpha and COX-2 expression by sauchinone effects on I-kappaBalpha phosphorylation, C/EBP and AP-1 activation. Br. J. Pharmacol. 2003;139:11–20. doi: 10.1038/sj.bjp.0705231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W.S., Baek Y.I., Kim J.R., Cho K.H., Sok D.E., Jeong T.S. Antioxidant activities of a new lignan and a neolignan from Saururus chinensis. Bioorg. Med. Chem. Lett. 2004;14:5623–5628. doi: 10.1016/j.bmcl.2004.08.054. [DOI] [PubMed] [Google Scholar]
- Marinova D., Ribarova F., Atanassova M. Total phenolic and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005;40:255–260. [Google Scholar]
- Marcocci L., Maguire J.J., Droy-Lefaix M.T., Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem. Biophys. Res. Commun. 1994;201:748–755. doi: 10.1006/bbrc.1994.1764. [DOI] [PubMed] [Google Scholar]
- Middleton E., Kandaswami C., Harborne J.B. Chapman and Hall; London, UK: 1986. The Flavonoids: Advances Research Since. 1993. [Google Scholar]
- Mostafa A.A., Al-Rahmah A.N., Surendra Kumar R., Manilal A., Idhayadhulla A. Biological evaluation of some imidazolidine-2,4-dione and 2-thioxoimidazolidin-4-one derivatives as anticoagulant agents and inhibition of MCF-7 breast cancer cell line. Int. J. Pharm. 2016;12:290–303. [Google Scholar]
- Moon T.C., Seo C.S., Haa K., Kim J.C., Hwang N.K., Hong T.G., Kim J.H., Kim D.H., Son J.K., Chang H.W. Meso-dihydroguaiaretic acid isolated from Saururuschinensis inhibits cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2008;31:606–610. doi: 10.1007/s12272-001-1200-y. [DOI] [PubMed] [Google Scholar]
- Nortier J.L., Vanherweghem J.L. Renal interstitial fibrosis and urothelial carcinoma associated with the use of a Chinese herb (Aristolochiafangchi) Toxicology. 2002;577:181–182. doi: 10.1016/s0300-483x(02)00486-9. [DOI] [PubMed] [Google Scholar]
- Omer C., Mehmet K., Ahmet Sukru O. Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta cell damage in rat pancreas. Pharmacol. Res. 2005;51:117–123. doi: 10.1016/j.phrs.2004.06.002. [DOI] [PubMed] [Google Scholar]
- Rao K.V., Rao N.S. Chemistry of Saururus cernuus, VI: three new neolignans. J. Nat. Prod. 1990;53:212–215. doi: 10.1021/np50067a036. [DOI] [PubMed] [Google Scholar]
- Ruch R.J., Cheng S., Klaunig J.E. Prevention of cytotoxicity and inhibition of intercellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003–1008. doi: 10.1093/carcin/10.6.1003. [DOI] [PubMed] [Google Scholar]
- Sung S.H., Kim Y.C. Hepatoprotective diastereomeric lignans from Saururus chinensis herbs. J. Nat. Prod. 2000;63:1019–1021. doi: 10.1021/np990499e. [DOI] [PubMed] [Google Scholar]
- Sung S.H., Kwon S.H., Cho N.J., Kim Y.C. Hepatoprotective flavonol glycosides of Saururus chinensis herbs. Phytother. Res. 1997;11:500–503. [Google Scholar]
- Yu M.H., Im H.G., Lee J.W., Bo M.H., Kim H.J., Kim S.K., Chung S.K., Lee I.S. Effects of ethanol extract from Saururus chinensis (Bour.) Baill on lipid and antioxidant metabolisms in rats fed a high-fat diet. Nat. Prod. Res. 2008;22:275–283. doi: 10.1080/14786410701590657. [DOI] [PubMed] [Google Scholar]