Abstract
Group B streptococcal infection (Streptococcus agalactiae) is one of the leading causes of life-threatening disease in the early neonatal period, resulting in sepsis, pneumonia, and meningitis. During invasive infections, an excessive release of pro-inflammatory cytokine, such as interleukin-6 (IL-6), thus IL-6 gene is significant, as a diagnostic marker of systemic infection of the newborns. The present study aimed to describe the epidemiology diagnostic of GBS disease in neonatal by phenotypic and genotypic methods. Nine hundred and ninety-six samples were taken at Maternity and Children Hospital, Jeddah, Saudi Arabia for a period of one year (2011–2012). Results indicated that out of 217 infected samples, twenty (9.23.0%) were positive for group B Streptococci bacteria. This study also shows that female infants are more susceptible than males. The level of IL-6 was higher in mothers above 30 years. Twenty positive Streptococci group B isolates showed bands with the cylE gene primers in the border between 228 bp, 267 bp and 50 bp. Molecular detection by Real time polymerase chain reaction was also done to detect the target (Sip gene) encoding the Sip surface immunogenic protein. Specific primers and TaqMan probe were chosen for this purpose. A Real-time PCR method targeting the sip gene of GBS in neonates after delivery has been evaluated.
Keywords: Group B Streptococcus (GBS); Interlukin-6 (IL-6) neonatology; Meningitis; cylE, Sip gene
1. Introduction
Streptococcus agalactiae, (GBS) is a facultative, beta-hemolytic, fastidious, Gram-positive cocci belonging to the phylum Firmicutes. GBS can be found as a part of normal vaginal, rectal, and oral flora. Group B Streptococcus (GBS) is the most common cause of life-threatening disease in the early neonatal period, resulting in sepsis, pneumonia, and meningitis; thus, GBS is the primary focus of any discussion about infections and pregnancy (Broomand et al., 2008). Early onset sepsis usually presents within the first 72 h of life. In severe cases, the neonate may be symptomatic in utero (fetal tachycardia, poor beat to beat variability) or within a few hours after birth. The source of infection is generally the maternal genital tract. Many adults are asymptomatically colonized with GBS in the genital and gastrointestinal tracts but colonized pregnant women are at increased risk of perinatal transmission to their neonates. Clinically, neonates usually present with respiratory distress and pneumonia (Gray et al., 2007). Late onset sepsis usually presents after 72 h of birth. The source of infection is either nosocomial or community-acquired and neonates usually present with septicemia, pneumonia or meningitis (Deborah Money et al., 2013, Jeeva et al., 2008). The identification and classification of Streptococci in microbiology laboratories generally begin with their classification based on their hemolytic reactions on blood agar medium. GBS are differentiated from other beta hemolytic Streptococci by lancefield serological typing. S. agalactiae subgroups are determined by serotyping based on cell wall polysaccharides. In contrast to developed countries, where type III accounts for more than two-thirds of all GBS related neonatal disease cases and is a predominant serotype (González et al., 2002).
Interleukin-6 (IL-6) acts as both a pro-inflammatory and anti-inflammatory cytokine. In humans, it is encoded by the IL6 gene. IL-6 is secreted by T cells and macrophages to stimulate immune response, e.g. during infection and after trauma, especially burns or other tissue damage leading to inflammation (Kishimoto, 2007).
Over the past few years, cytokines have been widely studied as reliable markers of neonatal infection. IL-6 has been associated with maternal chorioamnionitis and is used to provide diagnosis of early-onset of neonatal sepsis, when high levels are detected in cord blood (Dulcimar et al., 2010). Microorganisms most commonly associated with early onset infection include group B Streptococcus (GBS), Escherichia coli, Hemophilus influenzae and Listeria monocytogenes. In case of late onset infection causative organisms are coagulase negative Staphylococci, Staphylococcus aureus, E. coli, Klebsiella, Pseudomonas, Enterobacter, Candida, GBS, Serratia, Acinobacter and anaerobes (Noor et al., 2008).
Serotyping of GBS can be done by immunoprecipitation, latex agglutination, coagglutination, double immunodiffusion, and enzyme immunoassays and recently by molecular methods for genotyping. PCR, PFGE, MLEE and MLST, can be used to characterize bacterial genes and distinguish specific bacterial clones as well as emerge and spread of new clone (Pierre et al., 2014).
2. Material and methods
2.1. Sample collection
This study was conducted at Maternity and Children Hospital, Jeddah, Saudi Arabia for a period of one year (2011–2012). Three hundred thirty-two (332) infants were delivered and admitted in the hospital due to medical problems with an average stay for both mother and child of 2–3 days. Three control groups with five normal children in each group were assigned. Five samples each were taken from throat swabs, skin swabs and blood. All isolates were collected, couple of minutes after infants’ delivery and just after admission. Throughout, various studies took advantage of participants, causing trauma, and in some cases, death. Because of this, our research follows primary and certain research ethics especially when dealing with patient and working with isolates. The present research work does not require the approval and check before starting.
2.2. Blood samples
For serum, venous blood was drawn into sterile tubes and centrifuged at 3600 rpm for 10 min, then freeze-preserved at −70 °C for enzyme-linked immunosorbent assay (ELISA) to calculate the Inerlukin-6 levels. All positive (Streptococci group B) samples were preserved in Tryptic Soy Broth (TSB) with 20% glycerol, at −20 °C for molecular typing. From each neonate with suspected infection, blood sample of 1000–1500 μL was drawn by venipuncture for IL-6 determinants, between 0 and 48 h of life before the first doses of antimicrobials.
2.3. Skin and throat swabs
Swabs taken were immersed in AMIES transport medium with charcoal. Blood agar with 5% sheep blood was used. Swabs were streaked and the agar plates were incubated at 37 °C for 24 h. Blood agar plates were examined after 24 and 48 h. Colonies were examined by Gram stain, tested for catalase production and agglutination test.
2.4. Enzyme-linked immunosorbent assay (ELISA)
The DIA source IL-6-EASIA kit was used (Engvall and Perlmann, 1971). The assay uses monoclonal antibodies (MAbs) directed against distinct epitopes of IL-6. Calibrators and samples react with the capture monoclonal antibody (MAb 1) coated on microtiter well and with a monoclonal antibody (MAb 2) labeled with horseradish peroxidase (HRP). After an incubation period to allow the formation of a sandwich: coated MAb 1 – human IL-6 – MAb 2 – HRP, the microtiter plate is washed to remove unbound enzyme labeled antibody. Bound enzyme labeled antibody is measured through a chromogenic reaction. Chromogenic solution (TMB) is added and incubated. The reaction is stopped with the addition of stop solution and the microtiter plate is then read at the appropriate wavelength. The amount of substrate turnover is determined colorimetrically by measuring the absorbance, which is proportional to the IL-6 concentration. The plates were first read at 450 nm against a reference filter set at 650 nm (or 630 nm) by use of BioTek ELx808 reader. A second reading was performed at 490 nm against the same reference filter. The software automatically drove the reader and integrated both readings into a polychromatic model. Data were statistically analyzed and represented as mean ± sd for IL-6.
2.5. Molecular epidemiological typing
Knowledge of the epidemiology of GBS infections requires typing methods that can identify changes of virulence or emergence of new serotypes of GBS. Serotyping of GBS was done by immunoprecipitation, latex agglutination, coagglutination, double immunodiffusion, enzyme immuno assays and recently by molecular methods. All isolates with a positive culture for Streptococci group B were tested using the molecular test for both multiplex polymerase chain reaction (MPCR) and Real-time PCR following the below methods. For DNA extraction, a (QIAamp DNA Mini and Blood kit 51104-QIAGEN) kit was used (Madzivhandila et al., 2011).
2.6. Multiplex polymerase chain reaction (MPCR)
Primers for the target (cyIE) that coding for b-hemolysin/cytolysin (cylE) were prepared for MPCR reaction. GoTaq® Hot Start Green Master Mix-M5121 Promega kit was used (Wolach, 1997). It includes GoTaq Hot Start Green Master Mix, and Nuclease-Free Water. The stock was prepared by adding 50 μl from GoTaq Master Mix to 10 μl water. The reaction mixture contains the master mix 12.5 μl, upstream primer 0.5 μl, downstream 0.5 μl, DNA template 1 μl, free water 25 μl. One negative control was included in the runs, as negative control sterile water was added instead of DNA template. Aliquots for PCR product were analyzed by gel electrophoresis.
2.7. Real time PCR
The three different primers were used in this analysis to target (Sip gene) that encoding the Sip surface immunogenic protein. Light Cycler Fast Start DNA Master plus Hybprobe Roche kit was used for this reaction. (Light Cycler® Real-Time PCR Systems Roche, USA) It contained Light Cycler Reaction mix Enzyme and Nuclease-Free Water. The stock was prepared by adding 10 μl from the master mix and from 10 μl to 50 μl water. The reaction contains the master mix 8.6 μl, upstream primer 1 μl, downstream 1 μl, 04 μ probe, DNA template 1 μl, free water 4 μl. One negative control was included in the runs, as negative control sterile water was added instead of DNA template. The Real time PCR program (Light Cycler 2.0 Roche) read the result on (530/640) fluorescence (Elbaradie et al., 2009).
3. Results
3.1. Bacterial identification
The swabs were tested for group B Streptococci (GBS) growth by seeding them on 5% sheep blood agar plates. The test showed, bacteria of unknown growth were observed in 217 (32.7%) of the total samples. One hundred and twenty 55.3% of them were of throat while 97 (44.7%) were of skin. Out of two hundred seventeen (217) positive swab samples sixty-six (30.4%) showed growth in both skin and throat swab cultures. Bacterial isolates were identified by catalase test, coagulase test. Group B Streptococci growth shows beta (β) hemolysis on blood sheep agar. The colonies of GBS are gray to whitish-gray surrounded by a weak zone of beta hemolysis of the red blood cells in the blood culture media.
3.2. Demographic data
The relationship between the total numbers of swabs with positive bacterial growth in comparison with total number of swabs positive of GBS Streptococci group B bacteria was observed. Twenty positive samples for group B Streptococci, GBS eleven (55%) samples were from skin swaps, and nine (54%) from throat swaps. The majority (53.0%) of the mothers’ age of infected new born are between 30 and 41 years old and (47.0%) are under 30 years old. In comparison with the mothers’ age of the GBS infected infants (60.0%) they are between 31 and 41 years old and only 40.0% were between 21 and 30 years old. These results showed that either the GBS bacteria or unknown bacterial growth is more prevalent in older women compared to the younger ones.
Regarding the nationality of mothers, majority of the mother total isolates are Saudi (80.1%) compared to (19.9%) non Saudi. While the Saudi mothers of the infected infants were higher (65%) than that of the non Saudi mothers (35%). This result is not statistically significant since the majority of the total samples are Saudi. GBS infection was higher in females: eleven (55%) while 45% were males. 42.9% of males were born full term, while 46.1% preterm. Similarly with females, the percentage of infected babies born full term (57.1%) is more than that of preterm (53.9%). In normal birth weight babies the prevalence of GBS bacteria (70%) is more than the low birth weight babies (30%) (Fig. 1b). Preterm infants have higher positive results than the full term infants. Also this result shows that females are more susceptible to these bacteria than males. Distribution of GBS that isolated from different sources of skin and throat according to mothers (age, nationality) and infants (weigh, gender and duration of pregnancy) was observed.
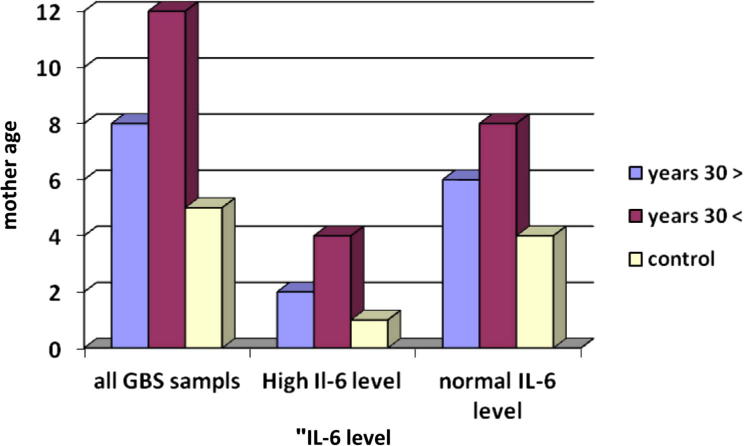
Figure 1b.
Interleukin-6 level according to infant’s mother’s age.
Enzyme-linked immunosorbent assays (ELISA) have been done to calculate IL-6 level in infant’s blood serum. The test was done for all infants with positive GBS bacteria. Fourteen with 70% of total 20 samples were in normal range >60 pg/ml, and other (6) represents 30% of their result were in high range >60 pg/ml. All control samples were with normal result except one sample which was in a high level of IL-6 in serum.
3.3. Evaluation of the relation between interleukin-6 and gestation period
Interleukin-6 (IL-6) level was higher in full term but the difference between the full term and pre term and control was not statistically significant (Fig. 1a and Table 1). F test was used to compare between full term and pre term in relation to control. P value = 0.4 > 0.05 and that is not statistically significant.
Figure 1a.

Evaluation of the IL-6 level with pregnancy duration.
Table 1.
Interlukin-6 (IL-6) levels according to pregnancy duration.
| Duration of pregnancy | No. | IL-6 level X ± SD (range) | Median | F test | P value |
|---|---|---|---|---|---|
| Full term | 8 | 529.9 ± 836 (12.85–1946.86) | 48.07 | 1.8 | 0.4 |
| Pre term | 12 | 185.2 ± 443.7 (0.0–1517.25) | 20 | ||
| Control | 5 | 30.6 ± 16.9 (18.1–60.18) | 25 |
SD = standard division, X = mean of IL-6 value, range = (highest value − lowest value).
3.4. Relation between Interlukin-6 (IL-6) and infant’s mother age
The level of IL-6 was higher in mothers above thirty years but the difference in relation to control was not statically significant (Table 2). F test was used to compare between mothers from twenty to thirty (20–30) year and mothers above 30 years in relation to control.
Table 2.
IL-6 level according to mother age.
| Mother age | No. | IL-6 level X ± SD (range) | Median | F test | P value |
|---|---|---|---|---|---|
| >30 years | 8 | 67.5 ± 116.6 (12.85–352.82) | 22.3 | 0.29 | 0.85 |
| <30 years | 12 | 493.4 ± 778.2 (0.0–1996) | 20.7 | ||
| Control | 5 | 30.6 ± 16.9 (18.1–60.18) | 25 |
SD = standard division, X = mean of IL-6 value, range = (highest value − lowest value).
P value = 0.85 > 0.05 and that is not statistically significant (Fig. 1b).
3.5. Correlation between Inerlikun-6 (IL-6) level and symptoms of infected GBS infants
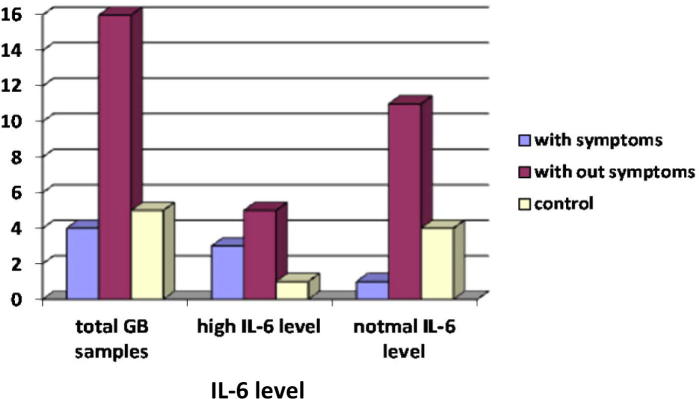
The level of IL-6 in infants with symptoms different such as fever, jaundice, difficult breathing was significantly higher among those without symptoms by using F test P value = 0.036 < 0.05 and that is statistically significant (Table 3 and Fig. 2a).
Table 3.
IL-6 level according to presence of symptoms.
| With/without symptoms | No. | IL-6 level X ± SD (range) | Median | F test | P value |
|---|---|---|---|---|---|
| With symptoms | 5 | 826.8 ± 963.8 (12.85–1946) | 352.8 | 3.76 | 0.036 Sig |
| Without symptoms | 15 | 155.2 ± 398.3 (0.0–151t.25) | 20 | ||
| Control | 5 | 30.6 ± 16.9 (18.1–60.18) | 25 |
SD = standard division, X = mean of IL-6 value, range = (highest value − lowest value).
Figure 2a.
The relation between Interleukin-6 (IL-6) level and presence of symptoms.
3.6. Evaluation the relation between Interlukin-6 level and neonatal weight
Il-6 was not statistically significant when comparing control with the neonate weight, neonatal above or equal to 2.5 kg and neonatal under 2.5 kg (Fig. 2b). P value = 0.5 > 0.05 by using F test (Table 4).
Figure 2b.

The relation between Interleukin-6 (IL-6) and infected infants’ weight.
Table 4.
IL-6 level according to neonatal weight.
| Neonatal weight | No. | IL-6 level X ± SD (range) | Median | F test | P value |
|---|---|---|---|---|---|
| >2.5 kg | 8 | 269.2 ± 533 (17.77–1517.25) | 20 | 0.5 | 0.5 |
| ⩾2.5 kg | 12 | 358.9 ± 713.1 (0.0–1946.86) | 26 | ||
| Control | 5 | 30.6 ± 16.9 (18.1–60.18) | 25 |
SD = standard division, X = mean of IL-6 value, range = (highest value − lowest value).
3.7. Interlukin-6 (IL-6) level according to infant’s nationality
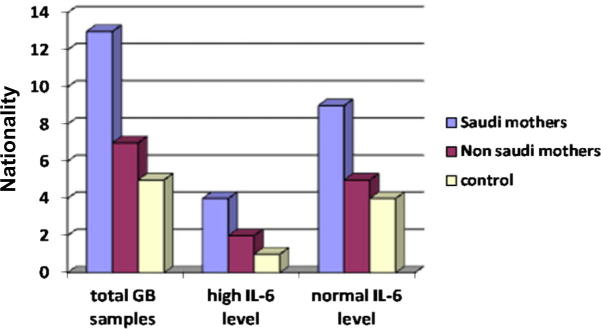
The level of IL-6 was higher among Saudi nationality than non Saudi but the difference between these two groups in relation to control was not statistically significant, P value = 0.5 > 0.05 (Table 5 and Fig. 2c).
Table 5.
IL-6 level according to mother nationality.
| Nationality | No. | IL-6 level X ± SD (range) | Median | F test | P value |
|---|---|---|---|---|---|
| Saudi | 13 | 346.1 + 641.2 (0–1946.86) | 20 | 0.5 | 0.5 |
| Non Saudi | 7 | 280.3 + 667.6 (15.79–1793.85) | 21 | ||
| Control | 5 | 30.6 + 16.9 (18.1–60.18) | 25 |
SD = standard division, X = mean of IL-6 value, range = (highest value − lowest value.
Figure 2c.
Interleukin-6 level in relation to infant’s mother’s nationality.
3.8. Multiplex polymerase chain reaction (MPCR)
Molecular detection study confirmed that all positive Streptococci group B samples showed bands with the cylE gene primers in the border between 228 bp, 267 bp and 50 bp DNA ladder marker have been used (Figure 3a, Figure 3b).
Figure 3a.
Multiplex PCR-typing for the fifteen samples targeting cylE primers gene, 50 bp DNA ladder marker in the border between 228 bp and 267 bp. Lan 1 and 2–5, 6–10, 11–15 was positive for cylE primers gene. (M: marker 50), (−ve: negative control).
Figure 3b.
Multiplex PCR-typing for the five samples targeting cylE gene primers, using 50 bp DNA ladder marker in the border between 228 bp and 267 bp. lan 16–20 was positive for cylE gene primers (M: marker 50), (−ve: negative control).
3.9. Real time polymerase chain reaction (Real time-PCR)
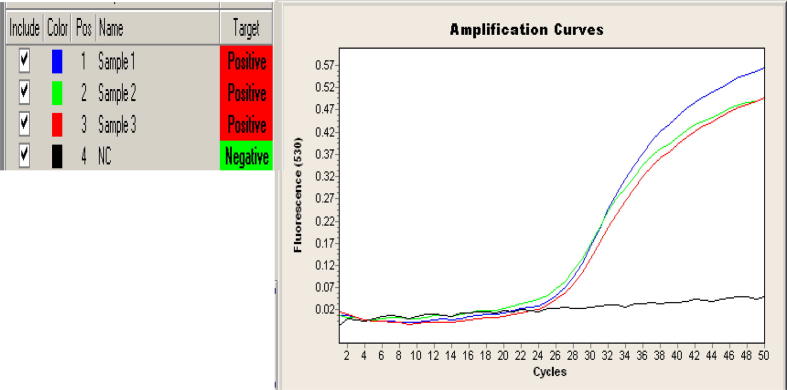
Molecular detection by RT-PCR was done to detect the target (Sip gene) by using Light Cycler 2.0 Roche. All the 20 samples were positive of the reaction. The results were read on (530/640) fluorescence (Figure 4a, Figure 4b).
Figure 4a.
Real time PCR detecting sip gene primer for three sample (1–3) were positive to the target (sip gene) at (530/640) fluorescence wavelength. Negative control (NC).
Figure 4b.
Real time PCR detecting sip gene primer for seventeen samples (4–17) was positive to the target (sip gene) at (530/640) fluorescence wavelength. Negative control (NC).
4. Discussion
4.1. Demographic data group B Streptococci (GBS)
Group B Streptococci (GBS) is a major cause of severe systemic and local infections in newborns. This study was performed to evaluate the presence of GBS in the early neonatal period related to the different sources. To investigate the relation between serotype group B Streptococci (GBS) of patients with infection or colonization according to the infant’s mother age, mother’s nationality, percentage of GBS isolated according to the infants gender, age and clinical manifestation.
Three hundred and thirty-two infants were included in the study with the prevalence of unknown bacterial growth of 32.7%. Results indicated that the group B Streptococcus (GBS) isolates were catalase negative as are all other Streptococci when tested with 3% hydrogen peroxide. Agglutination were employed to determine the GBS bacterial growth that shows twenty (9.23%) out of 217 infected samples. Similarly, in USA positive results of 3.4 of this bacteria upon birth of 900 samples. 2.2% of neonatal were negative at birth but gave positive result for swabs taken on discharge (Onipede et al., 2012, Ferrieri et al., 1977). The relation between positive samples for GBS infection and source of swabs (Table 4) showed 9 (45%), and 11 (55%) from the throat and skin. The results proved the group B Streptococci (GBS) were noticed in the skin with higher percentage than in the throat.
A study investigated the vaginal colonization rate of GBS in population of non-pregnant and pregnant women in 2098 samples in Poland. The prevalence of GBS in women was (91/4.33%), (59/1222) from the Obstetrics Ward (Stupak et al., 2010).
Broomand F., reported similar findings to ours for GBS colonization rate, he noted that past history of preterm labor and neonate hospitalization were more common in PPROM (Preterm Premature Rupture of Membranes) group but without any significant relationship to positive cultures. The study showed a significant difference of GBS colonization rate between PPROM and full term babies (Broomand et al., 2008).
Infants born to women with GBS bacteria during pregnancy are more frequently and more heavily colonized with GBS, and may be at increased risk for invasive GBS disease; however different studies are published (Gray et al., 2007).
In the United States, 5–35% of pregnant women are carriers of GBS. And 29–70% of newborns are colonized by this bacterium, which predisposes the neonates to infections such as septicemia, meningitis, and pneumonia. The higher rates of isolation had been from ear canal (80%) and nose (60%). Of the 17 GBS positive mothers, 5 transmitted S. agalactiae to their newborns (Nahaei et al., 2007). Sixty-five pregnant women from 406 cases were positive for GBS. This result reflects a high prevalence of 16% of pregnant women receiving antenatal care at Thammasat Hospital (Tapisiz et al., 2007). Six to 13% of the newborns colonized with SGB during the first 48 h of life are born to women with negative SGB cultures. SGB colonization during pregnancy increases the risk of spontaneous abortion and influences the pathogenesis of premature rupture of the fetal membranes, of preterm labor and of low neonatal birth weight, though the consequences differ widely (El Beitune et al., 2005). Our findings confirmed that the GBS bacterial growth is more prevalent in babies with mothers >30 years, which is similar to a study conducted by Schuchat et al. (1994) in metropolitan Atlanta, that noted a prevalence of GBS bacteria in infants with mothers >29 years (Al-Kadri et al., 2013, Schuchat et al., 1994).
4.2. Interleukin-6 (enzyme-linked immunosorbent assay (ELISA))
Enzyme-linked immunosorbent assay (ELISA) was done to calculate IL-6 level in infant’s blood serum. The test was done for all infants with positive GBS bacteria. Result for (15) 70% of total 20 samples were in normal range >60 Pg/ml, and (6) 30% of their result were in high range >60 Pg/ml. All control samples were with normal result except one sample which was in a high level of IL-6 in serum. All neonates with positive culture for GBS were examined to calculate the level of IL-6 in blood serum IL-6-EASIA by ELISA. The level of IL-6 was higher in mothers above 30 years. Regarding infants, our results show that the level of IL-6 was higher in infants with symptoms and this is similar to other studies like Nurdan et al., 2010 who noted that the serum IL-6 levels were high in both infection and Transient Tachypnea new born but no significant difference was observed between them. This study showed that IL-6 cannot distinguish between sepsis and Transient Tachypnea (TTN) in newborn infants (Nahaei et al., 2007). Just similarly comes Tabesis results concluded that eighteen septic and 23 control patients were included in the study. IL-6 levels were significantly higher in septic patients, so IL-6 levels were high in septic newborns (Tapisiz et al., 2007). IL-6 was not statistically related to neonate weight, while it was higher among Saudis. IL-6 level was higher in full term but the difference between the full term and pre term and control was not statistically significant.
Arad et al. (2010) studied the association of cord blood levels of IL-6 and NT-proBNP with perinatal variables of premature infants and examined the relationship between the obtained values (Arad et al., 2010). Cord blood IL-6 levels were measured in 89 infants with a birth weight in the range 445–1920 g and gestational age weeks in the range 24–31 weeks. IL-6 was higher in 69% of infants with gestational age <28 weeks. There were no statistically significant associations with pregnancy complications, maternal age or origin, multiple gestation, and being small for gestational age. There was no association between high levels and early hypotension N and no correlation was found with initial blood acidity. There were no statistically significant differences between groups of infants with or without elevated IL-6 levels with regard to respiratory distress syndrome, bronchopulmonary dysplasia, patent ductus arteriosus, intraventricular hemorrhage, periventricular leukomalacia or retinopathy of prematurity (Arad et al., 2010).
The correlation of more infants with raised IL-6 levels with decreasing gestational age found by Arad et al. (2010) was reported previously by Romero et al. (2003) and is in line with the higher prevalence of clinical or subclinical intrauterine infection at an earlier gestation (Nurdan et al., 2010). It has been shown that corticosteroids may alter the ratio of pro inflammatory to anti-inflammatory cytokines, resulting in the reduction of the pro inflammatory effect (Wolach, 1997). These findings suggested that most infants who were exposed to antenatal steroids had low IL-6 cord blood levels and appear to support these data. A similar study by Noor et al. (2008) concluded that interleukin-6 is a very early marker in the diagnosis of neonatal infection. IL-6 level was raised in suspected septic cases. IL-6 was positive within twenty-four hours of the onset of sepsis in comparison with other tests. It may be concluded that the estimation of IL-6 done at the time of onset of signs and symptoms suggestive of infection is useful in the early diagnosis of neonatal sepsis (Noor et al., 2008).
4.3. Molecular epidemiological typing
Group B streptococcus (GBS) is an important etiological agent of serious neonatal infections. Prevention of severe neonatal meningitis, pneumonia and sepsis associated with S. agalactiae requires various molecular gene typing methods, mostly involving culture, which are used for the detection of specific genes to identify Group B Streptococcus (GBS). The polymerase chain reaction (PCR) is a biochemical technology in molecular biology to amplify a single or a few copies of a piece of DNA across several orders of magnitude.
4.4. Multiplex polymerase chain reaction (MPCR)
Molecular detection by multiplex polymerase chain reaction (PCR) targeting the cylE gene, coding for b-hemolysin/cytolysin (cylE) that induces apoptosis, promotes cellular invasion, penetration of epithelial barriers and induction of sepsis syndrome. All the positive Streptococci group B samples showed bands with the cylE gene primers in the border between 228 bp and 267 bp, 50 bp DNA ladder marker has been used.
4.5. Real time polymerase chain reaction (Real time-PCR)
The sip gene encoding Sip-surface immunogenic protein was chosen as a target as this protein is universally expressed in GBS with specific primers and TaqMan probe as previously described (Bergseng et al., 2007). Molecular detection by RT-PCR was done to detect the target (Sip gene) by using Light Cycler 2.0 Roche. All the 20 samples were positive of the reaction. The results were read on (530/640) fluorescence. RT-PCR provides reliable assessment of intrapartum carriage of group B Streptococci. The rapid RT-PCR test was robust and was able to reliably detect the presence of GBS in women in labor within 1 h of specimen submission to the laboratory. They recommend that the rapid PCR test be targeted to women who present in labor with unknown GBS status. In cases where the laboratory does not offer 24-h availability of testing, sample collection followed by PCR testing the next morning is still valuable and provides reliable results 24–48 h faster than culture and will aid appropriate decision-making regarding continuing or stopping antibiotics for neonates of women with unknown GBS status.
A combined rectal/vaginal specimen was collected. GBS culture and PCR were discordant for 10 women at the time of labor. The results demonstrated a sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of 90.5%, 96.1%, 86.4%, and 97.4%, respectively, for rapid PCR. Of the 196 women evaluated, 29 (14.8%) presented with unknown GBS status, 11 (37.9%) of whom received unnecessary intrapartum antibiotics (Siripen et al., 2006). Screening and grouping B Streptococci by SCPB gene based PCR for maternal and neonatal to detect the magnitude of group B streptococcal (GBS) colonization and disease among a sample of pregnant women and their infants in Egypt. The study concluded that the maternal GBS carriage is associated with a significant increase in neonatal infection rate but is not associated with an increase in neonatal intensive care admission. An accurate evaluation of colonization rate (using a larger sample) is desired to evaluate neonatal invasive disease and determine the cost effectiveness of PCR to select an appropriate preventive strategy in Egypt (Elbaradie et al., 2009). Thus the RT-PCR assay is now a common, fast, highly sensitive, specific and often indispensable technique used in medical and biological research for detecting GBS colonization.
Acknowledgements
This research project was supported by a grant from the “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Kadri Hanan M., Bamuhair Samira S., Al Johani Sameera M., Al-Buriki Namsha A., Tamim Hani M. Maternal and neonatal risk factors for early-onset group B streptococcal disease: a case control study. Int. J. Womens Health. 2013;2013(5):729–735. doi: 10.2147/IJWH.S52206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arad I., Bar-Oz B., Ergaz Z., Nir A., Barak V. Interleukin-6 and N-terminal pro-brain natriuretic peptide cord blood levels in premature infants: correlations with perinatal variables. Isr. Med. Assoc. J. 2010;12:419–423. [PubMed] [Google Scholar]
- Bergseng H., Bevanger L., Rygg M., Bergh K. Real-time PCR targeting the sip gene for detection of group B streptococcus colonization in pregnant women at delivery. J. Med. Microbiol. 2007;56:223–228. doi: 10.1099/jmm.0.46731-0. [DOI] [PubMed] [Google Scholar]
- Broomand F., Abbasy F., Rahim N., Yekta Z., Nanbaksh F., Mirfakhraie G. Group B Streptococcus positive culture’s results in pregnants with preterm premature rupture of membranes. J. Family Reprod. Health. 2008;2:139–141. [Google Scholar]
- Deborah Money M.D., Vancouver B.C., Victoria M., Allen M.D., Halifax N.S. The prevention of early-onset neonatal group B streptococcal disease. J. Obstet. Gynaecol. Can. 2013;35(10):1–10. doi: 10.1016/S1701-2163(15)30818-5. [DOI] [PubMed] [Google Scholar]
- Dulcimar P., Marcos V., Juliana R., Lúcio R., Virmondes R., Cristina H. Early-onset neonatal sepsis: cord blood cytokine levels at diagnosis and during treatment. J. Pediatr. 2010;6:86. doi: 10.2223/JPED.2043. [DOI] [PubMed] [Google Scholar]
- El Beitune P., Duarte G., Maffei C. Colonization by Streptococcus agalactiae during pregnancy: maternal and perinatal prognosis. Braz. J. Inf. Dis. 2005;9:276–282. doi: 10.1590/s1413-86702005000400002. [DOI] [PubMed] [Google Scholar]
- Elbaradie S., Mahmoud M., Farid M. Maternal and neonatal screening for group B Streptococci by SCPB gene based PCR: a preliminary study. Indian J. Med. Microbiol. 2009;27:17–21. [PubMed] [Google Scholar]
- Engvall E., Perlmann P. Enzyme linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 1971;8:871–875. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- Ferrierpi P., Cleary P., Seeds A. Epidemiology of group B Streptococcal carriage in pregnant women and newborn infants. J. Med. Microbial. 1977;10:103–114. doi: 10.1099/00222615-10-1-103. [DOI] [PubMed] [Google Scholar]
- González A., Biól P., Zaragoza M., Mota R., Biól V. Serotypes and antimicrobial susceptibility of group B Streptococcus isolated from pregnant women in Mexico. Rev. Latinoam. Microbiol. 2002;44:133–136. [PubMed] [Google Scholar]
- Gray K., Bennett S., French N., Phiri A., Graham S. Invasive group B Streptococcal infection in infants, Malawi. Emerg. Infect. Dis. 2007;13:223–229. doi: 10.3201/eid1302.060680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeeva, M., Ramesh, A., Ashok, K., Vinod, K., 2008. Sepsis in the Newborn. AIIMS-NICU protocols 2008.
- Kishimoto T. The biology of Interleukin-6. J. Am. Soc. Hematol. 2007;74:1–10. [PubMed] [Google Scholar]
- Madzivhandila M., Adrian P., Cutland C., Kuwanda L., Schrag J., Madhi mail S. Serotype distribution and invasive potential of group B streptococcus isolates causing disease in infants and colonizing maternal-newborn dyads. PLoS One. 2011;6:e17861. doi: 10.1371/journal.pone.0017861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahaei M., Ghandchilar N., Bilan N., Ghahramani P. Maternal carriage and neonatal colonization of Streptococcus agalactiae in Tabriz, Northwest Iran. Iran. J. Med. Sci. 2007;32:177–181. [Google Scholar]
- Noor M., Shahidullah M., Rahman H., Mutanabbi M. Interleukin-6: a sensitive parameter for the early detection of neonatal sepsis. BSMMU J. 2008;1:1–5. [Google Scholar]
- Nurdan U., Ahmet K., Alparslan T., Emin M., Semra K., Musemma K., Mustafa M. Serum interleukin-6 levels in differential diagnosis of sepsis and transient tachypnea of newborn. Med. J. Trakya Univ. 2010;27:257–260. [Google Scholar]
- Onipede A., Adefusi O., Adeyemi A., Adejuyigbe E., Oyelese A., Ogunniyi T. Group B Streptococcus carriage during late pregnancy in ile-ife, Nigeria. Afr. J. Clin. Exp. Microbiol. 2012;13(3):135–143. [Google Scholar]
- Pierre E.F., Gregory D., Didier R. Clinical detection and characterization of bacterial pathogens in the genomics era. Genome Med. 2014;6:114. doi: 10.1186/s13073-014-0114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Chaiworapongsa T., Espinoza J. Micronutrients and intrauterine infection, preterm birth and the fetal inflammatory response syndrome. J. Nutr. 2003;133:1668S–1673S. doi: 10.1093/jn/133.5.1668S. [DOI] [PubMed] [Google Scholar]
- Schuchat A., Deaver-Robinson K., Plikaytis B., Zangwill K., MohleBoetani J., Wenger J.D. Multistate case-control study of maternal risk factors for neonatal group B Streptococcal disease. The active surveillance study group. Pediatr. Infect. Dis. 1994;13:623–629. doi: 10.1097/00006454-199407000-00008. [DOI] [PubMed] [Google Scholar]
- Siripen T., Pharuhat T., Wanwarang H. The prevalence of Streptococcus agalactiae (Group B) colonization in pregnant women at thammasat hospital. J. Med. Assoc. Thai. 2006;89:411–414. [PubMed] [Google Scholar]
- Stupak A., Kwasniewska A., Semczuk M., Zdzienicka G., Malm A. The colonization of women genital tract by Streptococcus agalactiae. Arch. Perinatal. Med. 2010;16:48–50. [Google Scholar]
- Tapisiz A., Ergenkon E., Yaldiz A. Interleukin-6 and nitric oxide levels in neonatal sepsis. Turk. J. Med. Sci. 2007;37:261–266. [Google Scholar]
- Wolach B. Neonatal sepsis: pathogenesis and supportive therapy. Semin. Perinatol. 1997;21:28–38. doi: 10.1016/s0146-0005(97)80017-1. [DOI] [PubMed] [Google Scholar]