Abstract
Artemisinin is the first-line drugs for the treatment of malaria. In recent years, a large number of reports showed that artemisinin exhibit anti-tumor activity. In this study, we used C6 glioma cells and rat C6 brain-glioma model to study anti-tumor activity of artemisinin in vivo and in vitro. We found that artemisinin inhibited the proliferation in C6 cells and induced cell cycle arrest and a caspase-3-dependent cell apoptosis. It also inhibited the growth of C6 brain-glioma in vivo and enhanced living state of rat brain-glioma model. These results suggested that artemisinin had significant anti-tumor activities on C6 cells both in vitro and in vivo. Artemisinin might be exploited as a promising clinical anti-cancer drug in future.
Keywords: Artemisinin, Glioma, Anti-tumor, C6, Apoptosis
1. Introduction
Gliomas have been the most severe brain tumors in the world (Paw et al., 2015). The incidence of gliomas is relative to 80% of malignant brain tumors (Goodenberger and Jenkins, 2012). Surgical resection is the key procedure of glioma treatment. However, despite development in surgical techniques, the 5-year survival rate of patients with gliomas remains low (Ater et al., 2016). With the improvements after resection, tumor recurrence and metastasis are virtually inevitable and are a major cause of death in glioma patients. Therefore, inhibition of residual glioma cells after surgery is highly relied on adjuvant radiotherapy or chemotherapy (Huang et al., 2016).
Artemisia annua L., a Chinese medicinal herb, has significant efficacy against malaria with low toxicity. Artemisinin, along with its derivatives such as dihydroartemisinin, artemether, arteether and artesunate are the safe, predominant compounds in anti-malarial treatment (Klayman, 1985). In recent years, many studies reported that artemisinin and its derivatives inhibit tumor growth both in vitro and in vivo. Some compounds with novel structures were synthesized and showed powerful anti-tumor activity that even better than the chemotherapy drugs currently in use. For example, Lu et al. reported that artemisinin and its derivatives significantly inhibited lung tumorigenesis and tumor metastasis (Tong et al., 2016). Artemisinin and its derivative dihydroartemisinin induced radiosensitivity in cervical cancer cells (Luo et al., 2014). Artemisinin also showed anti-tumor activity against a variety of cancer cells, such as acute myeloid leukemia (Drenberg et al., 2016), gallbladder cancer (Jia et al., 2016), colon cancer and breast cancer (Chen et al., 2016).
Dihydroartemisinin has been reported that it could penetrate into cerebrospinal fluid (Davis et al., 2003). Furthermore, Chinese researchers suggested that digydroartemisinin inhibited proliferation and induced apoptosis of rat glioma C6 cells in vitro (Ma et al., 2007). To date, whereas whether artemisinin could suppress rat C6 glioma cells in vitro or in vivo, as well as the potential molecular mechanism remains unclear. Therefore, in the current study, by utilizing C6 glioma cells and rat brain-glioma model, the anti-tumor activities of artemisinin were addressed both in vitro and in vivo. This paper has provided some information that artemisinin may become promising hopeful clinical anticancer compounds.
2. Materials and methods
2.1. Reagents
Artemisinin was purchased from Shanghai Yuanye Biological Technology Co., Ltd. (63968649). RPMI1640 and fetal bovine serum were purchased from Gibco. Propidium iodide (PI) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchase from Sigma. Oligo-dT primers and rTaq premix were purchased from Takara, Otsu, Japan. Reverse transcriptase (M-MLV), RNase inhibitor, Taq polymerase and nuclease-free water were all purchased from Promega, Madison, WI, USA. Anti-bcl-2 (0032R, Bioss), anti-bax (0127R, Bioss), anti-caspase-3 (0081R, Bioss), and anti-β-actin (RG00120, Solarbio life sciences) polyclonal or monoclonal antibodies were purchased from Bioss and Solarbio life sciences. HRP-labeled goat anti-rabbit/mouse IgG was purchased from ZSGB-BIO (Beijing, China). All the plates used in present study were purchased from Nunc. All chemicals used were of analytical grade.
2.2. Cell culture and animals
C6 brain glioma cells lines were purchased from Shanghai Institute of Cellular Biology of Chinese Academy of Sciences. Male Wistar rats (4–6 weeks old, weighing 200–250 g) were provided by the Animal Experimental Center, Basic Medical College of Jilin University. They were housed at 20 ± 2 °C, with humidity at 40–50%, and natural illumination. All procedures were in strict accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals of Jilin University and with the guidelines of P.R. China legislation.
2.3. Cell proliferation assay
The cells were plated at 1 × 104 cells/well into 96-well plates and incubated for 24 h. Then the cells were treated with different concentrations (2.5, 5, 10, 20, 40 μg/ml) of artemisinin for 48 h. Control cells were treated similarly without the addition of artemisinin. Subsequently, the media were removed and MTT solution (0.5 mg/ml) was added to each well. The plate was incubated for 4 h in a humidified atmosphere at 37 °C, and the media were carefully aspirated. Dimethyl sulfoxide (100 μl) was added, and the absorbance was measured at 570 nm by a microplate reader (Bio-Rad). The experiments were performed in triplicate at least.
2.4. Flow cytometry for cell cycle analysis
C6 cells were treated with different concentration of artemisinin as described above. Then the cells were collected and fixed in cold 70% ethanol for 30 min. After centrifugation, cell pellets were re-suspended in PBS containing 20 μg/ml PI and 200 μg/ml DNase-free RNase, 30 min prior to the examination on a FACScan follow cytometer (BD).
2.5. RT-PCR assay
C6 cells were plated in 6-well plates at 2.5 × 105 cells/well for 24 h, followed by treatment with increasing concentrations of artemisinin for 48 h. Total RNA of the treated cells were extracted using Trizol reagent (Invitrogen) according to the manufacturer’s instruction. Each reverse transcription (RT) reaction and polymerase chain reaction (PCR) reaction were carried out to detect mRNA expression using RT Reagent Kit and Ex Taq according to the manufacturer’s instructions.
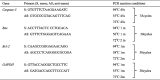
PCR reaction conditions and primers are shown in Table 1. The amplified products were visualized and analyzed by a UVP gel imaging system (BioPro, Farmingdale, NY, USA).
Table 1.
Primers and PCR reaction conditions for gene expression analysis.
 |
2.6. Western blotting assay
C6 cells were plated at a seeding density of 3.5 × 105 cells/well in 6-well plates for 24 h. The cells were treated with artemisinin at 2.5, 5, 10, 20 and 40 μg/ml for 48 h. Then the cells were collected and homogenized in lysis buffer for 30 min on ice. The cell lysates were separated by 12% sodium dodecyl sulfate (SDS)-polyacrylamide gel. After blocking in 5% non-fat milk, the membrane was incubated with the following primary antibodies: Bcl-2 (1:1000), Bax (1:1000), Caspase-3 (1:1000), β-actin (1:1000). The proteins were detected by ECL Plus Western Blotting Detection Kit (GE Healthcare).
2.7. Establishment rat brain-glioma model
The rats were placed on a stereotaxic head holder and C6 cells of glioma were stereotaxically implanted into the right cerebral cortex region of Wistar rats. Model rat were randomly divided into groups of 10 mice each. Another 10 non-model rat mice used as control group. After 3 days, treated group were intraperitoneal injected artemisinin (10 mg/kg); model rat and control groups were given normal saline. After 10 days of consecutive administration, we observed the living state of rats, tumor volumes, tumor histopathology and the expressions of bax, bcl-2 and caspase-3 in tumors by pathology and immunohistochemistry.
2.8. Hematoxylin and eosin (H&E) staining
The tissues were fixed in formalin overnight, followed by serial dehydration in 70% ethanol (3 h), 80% ethanol (3 h), 95% ethanol (2 h), 100% ethanol (1.5 h, twice) and xylene (0.5 h, twice). After immersion in paraffin (1 h at 55 °C and 2 h at 60 °C), tissues were embedded and sectioned in to 3-μm slices. Using xylene and gradient ethanol for dewaxing, slices were sequentially stained in hematoxylin (1 min) and eosin (10 s), and were mounted on glass slides. The pathological changes were evaluated by three independent observers in a blinded manner.
2.9. Immunohistochemical analysis
Bax, Bcl-2 and caspase-3 expressions were determined by IHC assay of the tissue sections. All specimens were fixed with 4% formaldehyde, dehydrated, embedded, and cut into 4-μm histologic sections. Intrinsic peroxidase activity was blocked by 3% hydrogen peroxide for 10 min. For high-pressure antigen retrieval, the slides were washed and incubated in 0.01 M sodium citrate buffer (pH 6.0) for 3 min. Slides were then incubated with the primary antibody at 4 °C overnight. Bax, bcl-2 and caspase-3 antibody concentrations were determined by performing a series of serial antibody dilutions. After 3 washes in PBS, the slides were incubated with the corresponding secondary antibody for 10 min at room temperature. The antigen-antibody complexes were examined by the streptavidin-biotin-peroxidase method using diaminobenzidine (DAB) as the chromogenic substrate (Maxim, Fuzhou, China). Finally, the slides were counterstained with 10% hematoxylin, dehydrated, mounted, and detected by light microscopy. In all cases, negative controls were performed by omitting the primary antibody.
2.10. Statistical analysis
The results were presented as mean ± standard deviation (SD). All the data were analyzed by SPSS 17.0 software. Statistical significance compared between the treatment and the control groups was checked by one-way ANOVA or analyzed by Student’s t test. The differences were considered significant when *P < 0.05; **P < 0.01. All experiments were carried out in triplicate.
3. Results
3.1. Effect of artemisinin on C6 cell proliferation
C6 cells were treated with artemisinin at concentrations of 2.5–40 μg/ml, and cell viability was determined by MTT assay. As shown in Fig. 1, artemisinin had a dose-dependent anti-proliferative activity on C6 cells. The anti-proliferative activity of artemisinin at low concentration (2.5 μg/ml) was 13.9%. With the doses increased, the inhibitory effect of artemisinin increased significantly. At the high dose of 40 μg/ml, the inhibitory rate of artemisinin was 51.2%.
Fig. 1.
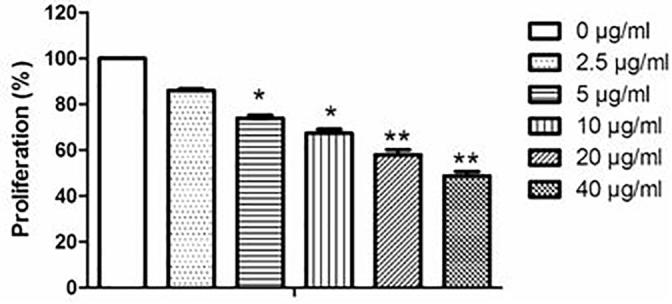
Effects of artemisinin on C6 cell proliferation. Cells were treated with artemisinin in different doses (0, 2.5, 5, 10, 20, 40 μg/ml). The effects of artemisinin were determined by the MTT assay. The data are shown as means ± SD. *P < 0.05, **P < 0.01 vs. control.
3.2. Effect of artemisinin on C6 cell cycle
To investigate the potential mechanism by which artemisinin inhibited cell proliferation, cells treated with different concentrations of artemisinin were subjected to cell cycle assay (Fig. 2 and Table 2). The results showed that artemisinin increased the population of S phase cells at lower concentrations. At high concentrations of artemisinin, G0/G1 phase population increased significantly, indicating that artemisinin induced G0/G1 phase arrest. The S phase cell population reached maximum at 5 μg/ml. At higher concentrations, the proportion of S phase cells decreased. We also found that sub-G1 phase cells population increased with artemisinin treatment. The cells at sub-G1 phase accounted for 2.23% of total cells at 40 μg/ml. These results are in line with an inhibition of cell proliferation by MTT assay, and suggested that artemisinin inhibited cell proliferation might induce S phase arrest at lower concentrations, and induce apoptosis and G0/G1 phase arrest at higher concentrations.
Fig. 2.
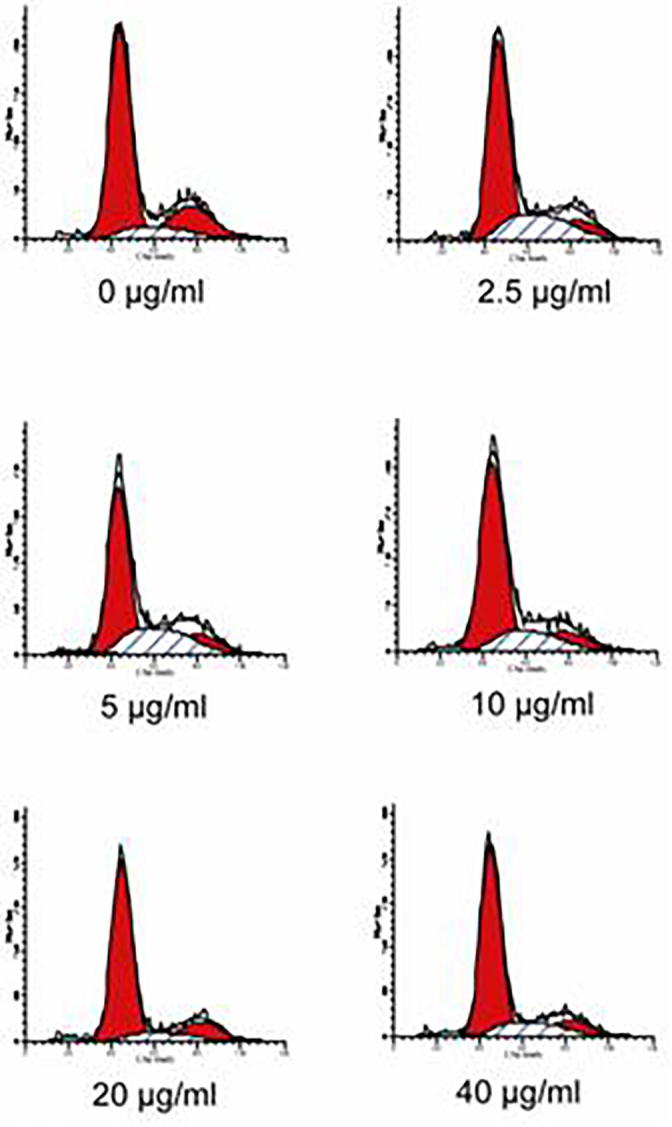
Effects of artemisinin on C6 cell cycle. Cells treated with 0, 2.5, 5, 10, 20, 40 μg/ml of artemisinin for 48 h were stained with PI, and then analyzed by flow cytometry. The percentages of cells in G0/G1, S, G2/M and sG1 phase are presented. Data are representatives of three independent experiments with similar results.
Table 2.
Effect of artemisinin on C6 cell cycle.
| Artemisinin (μg/ml) | G0/G1 (%) | S (%) | G2/M (%) | sG1 (%) |
|---|---|---|---|---|
| 0 | 68.31 | 10.50 | 21.20 | 0.57 |
| 2.5 | 62.86 | 24.98 | 12.15 | 0.68 |
| 5 | 58.24 | 27.72 | 14.04 | 1.42 |
| 10 | 67.86 | 18.36 | 13.78 | 1.77 |
| 20 | 73.82 | 10.47 | 15.71 | 1.82 |
| 40 | 71.39 | 16.35 | 12.25 | 2.23 |
3.3. Effect of artemisinin on bax, bcl-2 and caspase-3 mRNA expressions of C6 cells
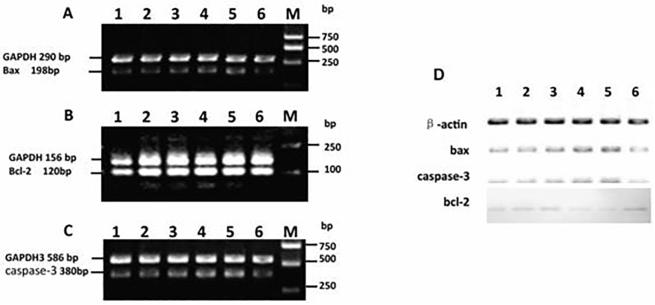
To elucidate the mechanisms involved in the observed anti-proliferative activity, we measured the levels of several cell cycle-transcripts in C6-cells treated with various concentrations of artemisinin. As shown in Fig. 3, the cells treated with artemisinin showed a significant increment in the mRNA levels of bax (Fig. 3A) as well as caspase-3 (Fig. 3C). In contrast, artemisinin treatment decreased the mRNA expression of bcl-2 (Fig. 3B) in a slight dose-dependent manner. These data indicated that artemisinin induced C6 glioma cells apoptosis.
Fig. 3.
Effects of artemisinin on expressions of bax, bcl-2 and caspase-3 in C6 cells. Cells were treated with artemisinin at 0 (lane 6), 2.5, 5, 10, 20 and 40 (lane 1–5) μg/ml for 48 h and then harvested. The total RNA was extracted, and then cDNA was synthesized from total RNA was amplified using the sets of primers for examining the expression of bax (A), bcl-2 (B) and caspase-3 (C). GAPDH mRNA was used as the standard for PCR. Western blot analysis of protein extracts of C6 cells treated with artemisinin using antibodies to Bax, Bcl-2, Caspase-3 and β-actin. C6 cells were treated with 0 (lane 6), 2.5, 5, 10, 20 and 40 (lane 1–5) μg/ml of artemisinin for 48 h. The expression of Bax, Bcl-2, cleaved Caspase-3 and actin were detected by western blotting (D).
3.4. Effect of artemisinin on C6 cell apoptosis
We used Western Blotting assay to investigate whether artemisinin induced apoptosis in C6 cells or not. As shown in Fig. 3D, artemisinin treatment increased both Bax and cleaved Caspase-3 level in a dose-dependent manner. In contrast, artemisinin had a dose-dependent inhibitory effect on Bcl-2 expression. These results are in accordance with the data of both MTT assay and cell cycle analyses, indicating that artemisinin inhibited C6 cell proliferation possibly by induction of cell cycle arrest and apoptosis.
3.5. Effect of artemisinin on rat brain-glioma model
Rat brain implanted with C6 glioma cells were used to study the anti-tumor effects of artemisinin in vivo. After 3 days inoculation, the mice were administered intraperitoneally each day with either normal saline (control and model) or 10 mg/kg of artemisinin for 10 consecutive days. The anti-tumor activities were evaluated by comparing the tumor weights of artemisinin-treated group with the control group and model rat group. We found that the formation rate of brain tumor was 100% in Wistar rats, none of them had distant metastasis. After 3 days inoculation of the cells, rats of both model rat and control group were observed left side hemiparalysis, doldrums, lassitude, lose weight, and death one after another. On the contrary, rats of treated group, the rats gained weight after 10 day treatment. As shown in Fig. 4, artemisinin inhibited the tumor growth compared with both control and model rat group, indicating that artemisinin had anti-tumor activity in vivo, in accordance with its anti-proliferative effect in vitro.
Fig. 4.

Effect of artemisinin on C6 glioma growth in rat. TheC6 cells bearing rats were administered intraperitoneally with artemisinin for 10 consecutive days and then sacrificed. Their tumor weights were measured. Comparison of artemisinin (C) with model rat group (B) and control group (A).
3.6. Effect of artemisinin on morphology
To further evaluate morphological effect of artemisinin, the tumor was confirmed with H&E staining. As shown in Fig. 5, artemisinin treated group and model rat group were both observed typical colloid tumor-like change and vessel lumen. We also found some necrosis area, while in Fig. 5A, control group showed normal brain tissue with clear boundary.
Fig. 5.
H&E staining of rat glioma C6 model tissue morphology. The rats implanted C6 cells were administered intraperitoneally with artemisinin for 10 consecutive days and then sacrificed. Tumor was confirmed with hematoxylin and eosin staining. Control group (A), model rat group (B) and artemisinin treated group (C).
3.7. Effects of artemisinin on Bax, Bcl-2 and Caspase-3 expressions of rat model
To further investigate the role of artemisinin in rat model, we evaluated expressions of Bax, Bcl-2 and Caspase-3 by immunohistochemical analysis in rat model. As shown in Fig. 6, we observed that with treatment of artemisinin, expression of Bcl-2 was decreased, while expression of Bax and Caspase-3 were both increased. These results were in line with the data of RT-PCR analyses in C6 glioma cells, indicating that artemisinin had anti-tumor activities in vivo, in accordance with its anti-proliferative and pro-apoptotic effect in vitro.
Fig. 6.
Effects of artemisinin on Bax, Bcl-2 and Caspase-3 expression of rat model. The rats implanted C6 cells were administered intraperitoneally with artemisinin for 10 consecutive days and then sacrificed. Representative IHC showing bax, bcl-2 and caspase-3-stained images of xenografts from rat model.
4. Discussion
Artemisinin has been well known for its anti-malaria for years. In recent years, artemisinin reveals remarkable activity against many malignancies, while its mechanism was remain unclear. The anti-tumor activity of artemisinin is associated with multiple mechanisms, including reactive oxygen species (ROS), DNA damage, sustained DNA double-strand breaks and apoptosis (Luo et al., 2014, Zhou et al., 2012, Berdelle et al., 2011, Jiang et al., 2012). In our paper, we found that artemisinin inhibited the proliferation and induce cell cycle arrest at S phase at low concentration and G0/G1 phase at high concentration and a caspase-3-dependent cell apoptosis in C6 glioma cells. It also inhibited the growth of C6 brain-glioma in vivo. It indicates that artemisinin may become promising hopeful clinical anti-cancer compounds, and deserve further investigation.
Caspases (cysteinyl aspartate-specific protease) are enzymes from cysteine protease family which play important role in cell apoptosis. They cleave peptide bonds specifically after an aspartic acid residue. Caspases are indispensable for the execution of apoptosis, following the cleavage of critical cellular proteins. These enzymes are participants in a proteolytic cascade leading to cell death via apoptosis (Hasanbasic et al., 2016). Caspase-3 is one of the major executioners in its family. Caspase-3 is a frequently activated death protease, catalyzing the specific cleavage of many key cellular proteins (Porter and Janicke, 1999).
Bcl-2 is a blocker of programmed cell death and apoptosis. It has been suggested that overexpression of the Bcl-2 protein inhibits cell apoptosis induced by nearly all cytotoxic anti-tumor drugs (Reed et al., 1996, Gao et al., 2017). Bax, a bcl-2-like protein 4, is found incytosol. However, with the initiation of apoptotic signaling, Bax undergoes a conformational shift, leading to release of cytochrome c and other pro-apoptotic factors and induce activation of caspases (Weng et al., 2005, Zhang and Saghatelian, 2013).
In present study, we analyzed mRNA and protein expression of bax, bcl-2 and caspase-3 by RT-PCR and western blotting. The results suggested that artemisinin treatment increased both bax and caspase-3 level in a dose-dependent manner in C6 cells. On the contrary, bcl-2 expression decreased with artemisinin treatment. We conjectured that Bcl-2 may bind to Bax and become Bcl-2-Bax heterodimer which terminate apoptosis. The distinct mechanisms are not yet elucidated and deserve further investigations.
To date, surgical resection and radiotherapy or chemotherapy are suggested treatment for glioma. However, the 5-year survival rate of patients with gliomas remains low. Therefore, it is urgent to exploit drug candidates for application of human glioma in pharmaceutical industry. With the development of study for artemisinin, structural modification of artemisinin may be obtained better anti-tumor effect and lower toxicity. Artemisinin might be exploited for the prevention and therapy of human glioma in future.
5. Conclusions
In the present study, we investigated effects of artemisinin on cell proliferation and apoptosis-related protein expression in vivo and in vitro. The results suggested that artemisinin inhibited the proliferation in C6 cells and induced cell cycle arrest and a caspase-3-dependent cell apoptosis. It also inhibited the growth of C6 brain-glioma in vivo and enhanced living state of rat brain-glioma model. Our results indicate that artemisinin may serve as a potential clinical anti-cancer drug in future.
Acknowledgments
Funding/Support: This work was supported by the National Natural Science Foundation of China (Grant No. 81401966), the research project of Jilin province development and reform commission (No. 20110706) and the research project of Bethune plan of Jilin University (No. 2015332).
Footnotes
Peer review under responsibility of King Saud University.
References
- Ater J.L., Xia C., Mazewski C.M., Booth T.N. Nonrandomized comparison of neurofibromatosis type 1 and non-neurofibromatosis type 1 children who received carboplatin and vincristine for progressive low-grade glioma: a report from the Children's Oncology Group. Cancer. 2016;122:1928–1936. doi: 10.1002/cncr.29987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berdelle N., Nikolova T., Quiros S. Artesunate induces oxidative DNA damage, sustained DNA double-strand breaks, and the ATM/ATR damage response in cancer cells. Mol. Cancer Ther. 2011;10:2224–2233. doi: 10.1158/1535-7163.MCT-11-0534. [DOI] [PubMed] [Google Scholar]
- Chen G., Gong R., Shi X. Halofuginone and artemisinin synergistically arrest cancer cells at the G1/G0 phase by upregulating p21Cip1 and p27Kip1. Oncotarget. 2016 doi: 10.18632/oncotarget.10367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis T.M., Tq Binh, Kf Ilett. Penetration of dihydroartemisinin into cerebrospinal fluid after administration of intravenous artesunate in severe falciparum malaria. Antimicrob. Agents Chemother. 2003;47:368–370. doi: 10.1128/AAC.47.1.368-370.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drenberg C.D., Buaboonnam J., Orwick S.J. Evaluation of artemisinins for the treatment of acute myeloid leukemia. Cancer Chemother. Pharmacol. 2016;77:1231–1243. doi: 10.1007/s00280-016-3038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Wang Y., Wang W., Shi L. The first multiplication atom-bond connectivity index of molecular structures in drugs. Saudi Pharm. J. 2017;25(4):548–555. doi: 10.1016/j.jsps.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenberger M.L., Jenkins R.B. Genetics of adult glioma. Cancer Genet. 2012;205:613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Huang D., Lin C., Wen X. A potential nanofiber membrane device for filling surgical residual cavity to prevent glioma recurrence and improve local neural tissue reconstruction. PLoS One. 2016;11:e0161435. doi: 10.1371/journal.pone.0161435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasanbasic S., Jahic A., Karahmet E. The role of cysteine protease in Alzheimer disease. Mater. Sociomed. 2016;28:235–238. doi: 10.5455/msm.2016.28.235-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Z., Chai J., Chuang H. Artesunate induces G0/G1 cell cycle arrest and iron-mediated mitochondrial apoptosis in A431 human epidermoid carcinoma cells. Anticancer Drugs. 2012;23:606–613. doi: 10.1097/CAD.0b013e328350e8ac. [DOI] [PubMed] [Google Scholar]
- Jia J., Qin Y., Zhang L. Artemisinin inhibits gallbladder cancer cell lines through triggering cell cycle arrest and apoptosis. Mol. Med. Rep. 2016;13:4461–4468. doi: 10.3892/mmr.2016.5073. [DOI] [PubMed] [Google Scholar]
- Klayman D.L. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- Luo J., Zhu W., Tang Y. Artemisinin derivative artesunate induces radiosensitivity in cervical cancer cells in vitro and in vivo. Radiat. Oncol. 2014;9:1–12. doi: 10.1186/1748-717X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z.Q., Huang X.J., Zhang W.P. Dihydroartemisinin inhibits proliferation and induces apoptosis of rat glioma C6 cells. Zhejiang Da Xue Xue Bao Yi Xue Bao. 2007;36:267–272. doi: 10.3785/j.issn.1008-9292.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Different. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Paw I., Carpenter R.C., Watabe K. Mechanisms regulating glioma invasion. Cancer Lett. 2015;362:1–7. doi: 10.1016/j.canlet.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J.C., Miyashita T., Takayama S. BCL-2 family proteins: regulators of cell death involved in the pathogenesis of cancer and resistance to therapy. J. Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Tong, Y., Liu, Y., Zheng, H., et al., 2016. Artemisinin and its derivatives can significantly inhibit lung tumorigenesis and tumor metastasis through Wnt/beta-catenin signaling. LID. Oncotarget. 10.18632/oncotarget.8920. [DOI] [PMC free article] [PubMed]
- Weng C., Li Y., Xu D. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J. Biol. Chem. 2005;280:10491–10500. doi: 10.1074/jbc.M412819200. [DOI] [PubMed] [Google Scholar]
- Zhou C., Pan W., Wang X.P. Artesunate induces apoptosis via a Bak-mediated caspase-independent intrinsic pathway in human lung adenocarcinoma cells. J. Cell Physiol. 2012;227:3778–3786. doi: 10.1002/jcp.24086. [DOI] [PubMed] [Google Scholar]
- Zhang T., Saghatelian A. Emerging roles of lipids in BCL-2 family-regulated apoptosis. Biochim. Biophys. Acta. 2013;1831:1542–1554. doi: 10.1016/j.bbalip.2013.03.001. [DOI] [PubMed] [Google Scholar]