Abstract
Background
Although several complicated models have been built to evaluate the prognosis of NSCLC patients receiving chemotherapy, simple economic models are still needed to give a preliminary survival assessment of these patients.
Material/Methods
This study retrospectively assessed the clinical and biological parameters of 223 patients with advanced NSCLC. Univariate and multivariate analyses of overall survival (OS) and progression-free survival (PFS) for the parameters and the prognostic score were assessed.
Results
Performance status (PS) score=1, smoking history, fibrinogenemia, thrombocytosis, increased lactate dehydrogenase (LDH) level, and anemia were independent predictors of poor prognosis in the univariate analysis of OS and were assessed in multivariate analysis. There was a significant difference in PS=1 (HR=2.134, p<0.0001), increased LDH level (HR=1.508, p=0.014), thrombocytosis (HR=1.547, p=0.012), and smoking history (HR=1.491, p=0.008), based on which the patients were classified into 3 risk groups: low risk (0–1 points), moderate risk (2 points), and high risk (3–5 points). At p values of <0.0001, the median OS was 565, 340, and 273 days and the median progression-free survival was 250, 209, and 135 days, respectively in these 3 risk groups.
Conclusions
We established a new prognostic score model using PS, LDH level, PLT count, and smoking history to predict the survival of patients receiving first-line chemotherapy for advanced NSCLC, which might be useful in clinical practice.
MeSH Keywords: Lung Neoplasms, Overall, Prognosis, Survival
Background
The rate at which the lung cancer occurs is soaring globally. It has become the malignant cancer with the highest morbidity rates and morality rates [1]. Non-small cell lung cancer (NSCLC) accounts for around four-fifths of overall incidents of lung cancer [2]. Although researchers have endeavored to develop unique “target agents” for treating lung cancer, the rate at which EFGR mutated was only 17% in white patients with non-squamous NSCLC and is 3 times more common in Asians [3]. Chemotherapy remains the first choice for patients with advanced diseases, especially in Asian countries, where half of patients cannot afford target agents. However, even when receiving the same chemotherapy, overall survival (OSs) ranges from several months to more than 1 year [4,5], suggesting that NSCLC is a heterogeneous disease. Therefore, a straightforward and dependable method is required to evaluate the prognosis of patients and to help make the best treatment choice.
In recent years, some survival prognostic factors and prediction models for European NSCLC patients have been reported, indicating that stage IV (versus prior stage), lower performance status (PS), elevated white blood cells (WBCs), distant metastasis, abnormal absolute neutrophil count, age >60, male sex, anemia, abnormal lactate dehydrogenase (LDH) level, and increased tumor markers may be independent negative prognostic factors for survival [4,6,7]. In recent years, coagulation parameters represented by fibrinogen [8], platelets [9], and prevalent inflammatory prognostic indexes such as Glasgow prognostic score 10 (composed of albumin and CRP) have also been verified to predict poor survival. Based on these results, we performed this retrospective analysis using clinical characteristics, blood routine tests, and coagulation parameters, which were easily identified clinical factors influencing the outcomes of patients who received standard first-line chemotherapy, aiming to build a simple model that could be utilized as a part of everyday clinical practice for prediction of long-term survival.
Material and Methods
Patients
In this retrospective analysis, we enrolled 223 patients diagnosed with advanced NSCLC at the First Hospital of China Medical University (Liaoning Province, China) from December 2006 to May 2013. Inclusion criteria were: 1) histologically or cytologically diagnosed as NSCLC; 2) unresectable, relapse, or metastatic NSCLC; 3) without past chemotherapy, or metastatic NSCLC patients without receiving adjuvant chemotherapy in the last 6 months; 4) with an Eastern Cooperative Oncology Group Performance Score of 0 or 1; and 5) with sufficient hepatic, renal, hematologic, and cardiac capacities. All of them received chemotherapy as first-line treatment according to the NCCN guideline using single-agent or combined platinum-based chemotherapy. This study was approved by the Ethics Committee of the First Hospital of China Medical University and we obtained informed consent before enrollment.
Methods
The related information was from daily assessments of patients with cancer in our clinical facility. Pre-chemotherapy blood tests were acquired within 1 week preceding chemotherapy, and citrated blood samples were taken before breakfast and measured within 1 h. The factors utilized to develop the prognostic scores were patient attributes (age, sex, PS, and smoking history), disease (TNM stage and histologic type), routine blood tests (white blood cells [WBC], platelets [PLT], and hemoglobin [Hb]), and blood biochemical tests (albumin, LDH, and fibrinogen [Fg]). The cutoff values of these variables were the limits of normal.
Statistical analysis
The OS was figured from the date of accepting treatment to the day of death or last follow-up visit, which was 16 May 2013. Progression-free survival (PFS) was figured from the date of accepting treatment to the date of definite progression or death. The chi-square test was used to describe the relationship between sex or smoking history and pathology. To assess the correlation between OS and baseline characteristics, all clinical and biological factors were incorporated into a univariate Cox proportional hazard regression model. The parameters with p<0.05 in the univariate Cox model were entered in the multivariate Cox model. The log-hazard rates obtained from the Cox model were utilized to infer weighting elements of a prognostic score to distinguish differential dangers of death, aiming to distinguish differential dangers of dying. Coefficient gauges were ‘normalized’ by dividing and isolating the smallest one and adjusting the subsequent proportions to the closest whole number [11]. The score was based on OS results, but also calculated for PFS. The survival of different groups was analyzed by the Kaplan-Meier method and log-rank tests. A p value of 0.05 for two-tailed analysis was considered to be statistically significant. All statistical analyses were performed using SPSS version 22.0 software (SPSS, Chicago, IL, USA).
Results
Patient characteristics
From December 2006 to May 2013, 223 stage III (not suitable for surgery) or IV NSCLC patients received treatment with first-line single-agent (22/223) or combined platinum-based chemotherapy (201/223), of which 188 patients received cisplatin and 13 patients used carboplatin. The detailed treatment regimens are shown in Table 1. The baseline features of the 223 identified patients are illustrated in Table 2. The median age was 58 years (range 28–77 years). Most patients were males (149/223, 66.8%) and had a good PS score (PS=0) (148/223, 66.3%). The results of chi-square testing showed that sex and pathology were significantly correlated and female patients were more prone to adenocarcinoma (63/74 vs. 86/149, p<0.001, p<0.001). There was also a strong relationship between smoking history and pathology and most of the patients without smoking history had adenocarcinoma (81/107 vs. 68/116, p=0.007).
Table 1.
Therapeutic regimens of patients.
| Chemotherapy | Number (percentage) |
|---|---|
| Total | 223 |
| Single agent | 22 (9.8) |
| Platinum-based combined drugs | 201 (90.2) |
| Docetaxel or Paclitaxel | 58 (26.0) |
| Vinorelbine | 55 (24.7) |
| Gemcitabine | 57 (25.6) |
| Pemetrexed | 31 (13.9) |
Table 2.
Pretreatment clinical and laboratory characteristics of patients.
| Patient characteristics | Number (percentage) |
|---|---|
| Total | 223 (100) |
| Age (year) | |
| <60 | 123 (55) |
| ≥60 | 100 (45) |
| Gender | |
| Male | 149 (67) |
| Female | 74 (33) |
| Smoking history | |
| No | 107 (48) |
| Yes | 116 (52) |
| Histological type | |
| Adenocarcinoma | 149 (67) |
| Squamous carcinoma + others | 74 (33) |
| TNM stage | |
| IIIA+IIIB | 55 (33) |
| IV | 168 (67) |
| PS score | |
| 0 | 148 (66) |
| 1 | 75 (34) |
Clinical outcomes
by May 2014, 194 of the 223 patients had died, with 205 PFS events. The median OS and PFS were 431 days and 219 days, respectively. After 2 cycles of chemotherapy, 53 patients achieved partial remission and 139 achieved stable disease. Only 31 patients progressed and the total disease control rate (DCR) was 86%.
Pretreatment prognostic factors
The relationship between OS and 12 pretreatment parameters, including age, sex, TNM stage, smoking history, pathology, PS score, platelet (PLT), leukocyte (WBC), Hb, fibrinogen (Fg) albumin (ALB), and LDH, were analyzed. The univariate Cox analysis (p<0.05) showed 6 factors predicted poor prognosis: PS=1, history of smoking, fibrinogenemia (Fg >4 g/L), elevated LDH level (LDH >265 U/L), thrombocytosis (PLT >350×109/L), and anemia (Hb <110 g/L for females, Hb <115 g/L for males) (Table 3). Of the positive variables, only 4 elements remained statistically different after Cox multivariate analysis (p<0.05) (Table 4): PS=1 (HR=2.134, p<0.0001), increased LDH level (HR=1.508, p=0.014), thrombocytosis (HR=1.547, p=0.012), and smoking history (HR=1.491, p=0.008).
Table 3.
Results of univariate analysis with regard to OS.
| OS (days) | P* | HR | 95% CI | ||
|---|---|---|---|---|---|
| Gender | Male | Female | 0.250 | ||
| 484 | 407 | ||||
| Smoking history | Smokers | Non-smokers | 0.045 | 1.355 | 1.029–1.784 |
| 351 | 496 | ||||
| Age (year) | ≥60 | <60 | 0.518 | ||
| 418 | 434 | ||||
| PS score | 0 | 1 | <0.001 | 1.98 | 1.482–2.646 |
| 484 | 303 | ||||
| Pathology | Adenocarcinoma | Others | 0.084 | ||
| 446 | 351 | ||||
| Stage | III | IV | 0.242 | ||
| 406 | 434 | ||||
| Fg | Normal | Elevated | 0.015 | 1.423 | 1.068–1.895 |
| 461 | 351 | ||||
| PLT | Normal | Elevated | 0.007 | 1.438 | 1.04–1.988 |
| 446 | 334 | ||||
| WBC | Normal | Elevated | 0.086 | ||
| 438 | 286 | ||||
| Hb | Normal | Anemia | 0.046 | 1.382 | 1.032–1.868 |
| 446 | 345 | ||||
| LDH | Normal | Elevated | 0.003 | 1.594 | 1.182–2.152 |
| 459 | 334 | ||||
| Albumin | Normal | Hypoproteinemia | 0.557 | ||
| 436 | 397 | ||||
WBC – white blood cell; Fg – fibrinogen; Hb – hemoglobin; PLT – platelet; LDH – lactate dehydrogenase.
Performed using the univariate Cox proportional hazard regression model.
Table 4.
Results of multivariate analysis.
| Multivariate analysis | |||
|---|---|---|---|
| HR | 95% CI | P* | |
| PS score | 2.134 | 1.567–2.906 | <0.001 |
| 0 | |||
| 1 | |||
| LDH | 1.508 | 1.088–2.091 | 0.014 |
| Normal | |||
| Abnormal | |||
| PLT | 1.547 | 1.099–2.178 | 0.012 |
| Normal | |||
| Abnormal | |||
| Smoking history | 1.491 | 1.110–2.002 | 0.008 |
| Smokers | |||
| Non-smokers | |||
Fg – fibrinogen; PLT – platelet; LDH – lactate dehydrogenase.
Performed using Cox proportional hazards models with the forward likelihood method.
Prognostic score
The 4 factors from multivariate analysis were assigned points on the basis of their HRs in the Cox analysis (Table 5). The patients were then classified by the total scores, ranging from 0 to 5, into 3 groups: low risk (0–1 points, 115 patients), moderate risk (2 points, 44 patients), and high risk (3–5 points, 64 patients). The median OS of these 3 groups was 565 days, 340 days, and 273 days, respectively (p<0.0001), and this score was also used to estimate their PFSs as 250 days, 209 days, and 135 days, respectively (p<0.0001). The Kaplan-Meier curves of OS and PFS are shown in Figures 1 and 2, respectively.
Table 5.
Definition of the scoring system.
| Point* | |||
|---|---|---|---|
| 0 | 1 | 2 | |
| History of smoking | Non-smokers | Smokers | |
| PS score | 0 | 1 | |
| PLT | Normal | Elevated | |
| LDH | Normal | Elevated | |
Fg – fibrinogen; PLT – platelet; LDH – lactate dehydrogenase.
Coefficient estimates (i.e. logarithm of HR) were ‘normalized’ by dividing by the smallest one and rounding the resulting ratios to the nearest integer value.
Figure 1.
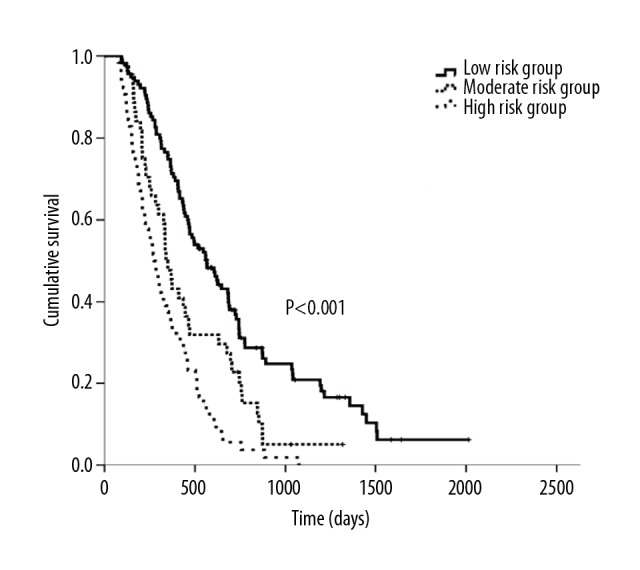
Kaplan-Meier curves of OS according to different prognostic scores.
Figure 2.
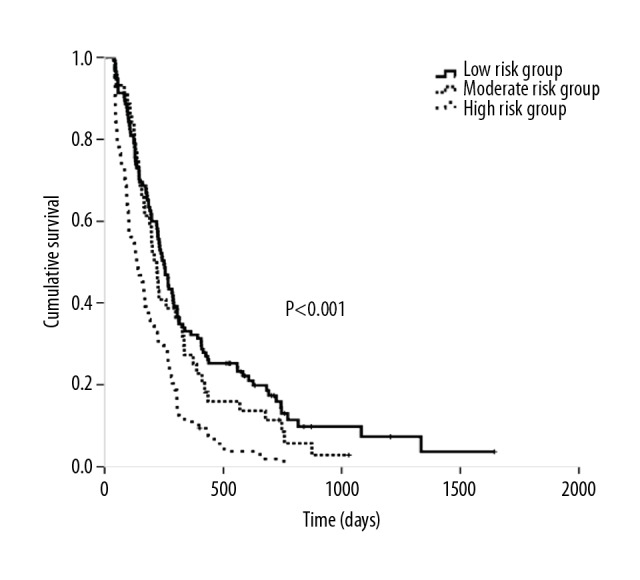
Kaplan-Meier curves of PFS according to different prognostic scores.
Discussion
To the best of our knowledge, this is the first scoring system that combines clinical features and blood test results to give a preliminary and rapid economic judgment of the prognosis of NSCLC patients who received chemotherapies. Prognostic scores help physicians predict which patients will significantly benefit from by the treatment, which is especially important for treatments with clinically related or extreme toxicity, such as chemotherapy. In recent years, several models of NSCLC have been established to predict prognosis. From the recursively partitioned analysis (RPA) [12–14] to nomogram [4,7] prognostic model, the complicated analytic system demanded a profound and intensive assessment of patients (e.g., locus of metastasis), which is time-consuming and has high health insurance costs. Furthermore, few Chinese patients were enrolled and some data were not easily acquired. Recently, new models focusing on the systemic inflammatory response [15] represented by Glasgow prognostic score (GPS) [10,16] and based on a combination of CRP and albumin have been developed to predict the survival of patients with advanced cancers, which were prognostic indexes lacking clinical parameters. Therefore, we propose a prognostic score that is comprehensive, easily acquired, and can be incorporated in daily clinical practice, based on laboratory tests and clinical features.
In our univariate analysis, lower PS, smoking history, high Fg level, anemia, and increased LDH level and PLT count all contributed to worse survival. In addition to poor PS, we found that abnormal LDH level [17, 18] and anemia [19], which are widely accepted poor prognostic factors, increased Fg level, and thrombocytosis and smoking history were proved to be independent prognostic factors that might be useful in prognostic scoring. Fg is a vital component in the connection between malignancy and coagulation, the level of which has prognostic significance in lung cancer, especially in Asian countries [20–22]. Zhao et al. [8] reported that chemotherapy-induced reduction in plasma Fg level may be a promising biomarker, and a higher level of Fg (≥4.4 g/L) was positively correlated with shorter OS. Similarly, in the present study we found that abnormal Fg level was a poor prognostic factor, but in multivariate analysis, only lower PS, smoking history, increased LDH level, and PLT count were used in the new prognostic scoring system.
Thrombocytosis was another key poor prognostic factor in both univariate and multivariate analyses. The reported frequencies of thrombocytosis varied between 4.5% and 41% [21] in lung cancer patients, with 8.1% reported in China [23], and it was also more common in advanced disease [24]; our study found the rate was 21%. Recent research [25] showed that platelet count was associated with metastases to bone, soft tissue, and lymph nodes, in addition to malignant pleural effusion. However, the present study found no association between worse stage and increased PLT count, and the incidence of thrombocytosis in stage III was 23.6% and 19% in IV stage (p=0.462). This result means that the prognostic value did not depend on stage, but rather on some other distinct characteristics of the individual tumor. The mechanism by which increased PLT shortens survival may be as follows: Firstly, platelets interact with fibrin and tumor cells, leading to the formation of platelet-fibrin tumor cells that can protect tumor cells by shielding them from the host immune system and have been report to reduce platelet attachment and diminish the metastatic ability of cancer cells [26]. Secondly, the various cytokines stored in or released by platelets can contribute to tumor growth and invasiveness of cancer cells, including endothelial vascular growth factor (VEGF) and platelet-derived growth factor (PDGF), which play a critical role in directing angiogenesis [27]. Furthermore, platelets promote the development of capillary-like structures in endothelial cells by means of integrin intervening in cell–cell adherence [28]. The Swedish Lung Cancer Radiation Study Group [29] reported that for the patients with PLT >350×109/L and <350×109/L, the mean survival times were 11.2 and 14.9 months, respectively (p<0.0001), which was similar to our results. A meta-analysis from China [30] also defined the prognostic value of PLT count. Nevertheless, Cakar et al. [31] reported that the thrombocytosis group did not have a significantly shorter OS. This difference may be the result of genotype in different human populations.
Another important negative prognostic factor in our analysis in both univariate and multivariate analysis was smoking history. Smoking is not just a risk factor for lung cancer, but also is a strong prognostic factor of survival in advanced NSCLC according to the National Comprehensive Cancer Network (NCCN) [32] and the National Hospital Study Group for Lung Cancer (NHSGLC) [33]. A study from Turkey [34] found that smokers had a higher proportion of stage III or IV disease than non-smokers, but this trend was not found in our analysis because stage III patients were more likely to be smokers (stage III vs. IV, 67.3% vs. 47%, p=0.009), showing that the negative impact of smoking on OS is regardless of stage. In further analysis we found that in patients with adenocarcinoma, the OS of smokers was shorter than in non-smokers (365 days vs. 565 days, p=0.044). We found that in patients with non-adenocarcinomas, the OS difference was no significant (345 days vs. 369days, p=0.957), which was similar to the result of Japanese research [35]. The mechanisms underlying the prognostic effect of smoking are not very clear and may be as follows: Firstly, molecular investigations of lung tumor tests have shown that specific „driver mutations” are more common in never-smoking patients who have lung malignancy as opposed to patients who have a substantial smoking history. These molecularly portrayed subtypes assume a vital part in restorative choices for advanced NSCLC patients. For instance, patients who have epidermal development factor receptor (EGFR) mutations may live longer than patients without EGFR mutations [36]. Unfortunately, data on EGFR mutation was not included in the present study because it was not a routine examination at the time. However, EGFR gene detection is expensive, time-consuming, and complicated, imposing a burden on hospital laboratory personnel and equipment, as well as increasing patient suffering and economic costs, so it may be not suitable for a simple and quick evaluation of prognosis. Secondly, smoking can produce inflammatory mediators [37] and immune depression [38], which can eventually cause malignant cells to spread.
Another finding suggested that this score could also be applied to all patients receiving platinum-based chemotherapy. In the present study, 90% of patients were treated with platinum-based chemotherapy and they were analyzed separately. The median OS of these 3 groups was 565 days, 350 days, and 285 days, respectively (p<0.0001), and this score was also used to estimate their PFSs as 250 days, 225 days, and 141 days, respectively (p<0.0001). ERCC1 is one of the more in-depth prognostic indicators of platinum-based chemotherapy, but its role in the prognosis of lung cancer remains controversial [39]. Arife Ulas et al. showed that ECOG PS >2, a high LDH level, and some other laboratory tests were negative prognostic factors for lung cancer [40]. Because the present study is only a retrospective study in which 10% of monotherapy patients were mixed, we still need prospective clinical trials to verify the efficacy of this prognostic model.
To the best of our knowledge, this is the first prognostic model which covers the clinical features, blood routine tests, and biochemical tests targeting Chinese patients who receive first-line chemotherapy. Furthermore, all data could be readily acquired, without requiring extra time or expense. This score may be employed to maximize the therapeutic gain and to minimize potential morbidity and mortality rates, as well as reducing the health care system costs. Nevertheless, this study did not analyze the expressions of endogenous targeted genes affecting the prognosis of lung cancer chemotherapy, such as ERCC1, EGFR, and HER2. Further prospective trials are needed to confirm of utility of this scoring system.
Conclusions
We established a new prognostic scoring system using PS, LDH level, PLT count, and smoking history to rapidly and economically predict the survival of NSCLC patients, which could be applied in everyday medical treatments
Footnotes
Source of support: This research was supported by the National Science and Technology Major Project of the Ministry of Science and Technology of China (No. 2017ZX09304025), the Science and Technology Plan Project of Liaoning Province (No. 2014226033), the Science and Technology Plan Project of Liaoning Province (N0. 2016007010), the Key Research and Development Program of Shenyang (No. 17-230-9-01), and the National Natural Science Foundation of China (No. 81472193)
Conflict of interest
None.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. Cancer J Clin. 2012;62(1):10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 3.Juan O, Popat S. Treatment choice in epidermal growth factor receptor mutation-positive non-small cell lung carcinoma: Latest evidence and clinical implications. Ther Adv Med Oncol. 2017;9(3):201–16. doi: 10.1177/1758834016687262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoang T, Dahlberg SE, Sandler AB, et al. Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol. 2012;7(9):1361–68. doi: 10.1097/JTO.0b013e318260e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pu X, Li W, Lu B, et al. Single pemetrexed is noninferior to platinum-based pemetrexed doublet as first-line treatment on elderly Chinese patients with advanced nonsquamous nonsmall cell lung cancer. Medicine (Baltimore) 2017;96(11):e6002. doi: 10.1097/MD.0000000000006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trape J, Montesinos J, Catot S, et al. A prognostic score based on clinical factors and biomarkers for advanced non-small cell lung cancer. Int J Biol Markers. 2012;27(3):e257–62. doi: 10.5301/JBM.2012.9314. [DOI] [PubMed] [Google Scholar]
- 7.Hoang T, Xu R, Schiller JH, et al. Clinical model to predict survival in chemonaive patients with advanced non-small-cell lung cancer treated with third-generation chemotherapy regimens based on eastern cooperative oncology group data. J Clin Oncol. 2005;23(1):175–83. doi: 10.1200/JCO.2005.04.177. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Zhao M, Jin B, et al. Tumor response and survival in patients with advanced non-small-cell lung cancer: The predictive value of chemotherapy-induced changes in fibrinogen. BMC Cancer. 2012;12:330. doi: 10.1186/1471-2407-12-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du G, Yang Y, Zhang Y, et al. Thrombocytosis and immunohistochemical expression of connexin 43 at diagnosis predict survival in advanced non-small-cell lung cancer treated with cisplatin-based chemotherapy. Cancer Chemother Pharmacol. 2013;71(4):893–904. doi: 10.1007/s00280-013-2080-6. [DOI] [PubMed] [Google Scholar]
- 10.Zhu L, Chen S, Ma S, Zhang S. Glasgow prognostic score predicts prognosis of non-small cell lung cancer: A meta-analysis. Springerplus. 2016;5:439. doi: 10.1186/s40064-016-2093-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Maio M, Lama N, Morabito A, et al. Clinical assessment of patients with advanced non-small-cell lung cancer eligible for second-line chemotherapy: A prognostic score from individual data of nine randomised trials. Eur J Cancer. 2010;46(4):735–43. doi: 10.1016/j.ejca.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Finkelstein DM, Ettinger DS, Ruckdeschel JC. Long-term survivors in metastatic non-small-cell lung cancer: An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1986;4(5):702–9. doi: 10.1200/JCO.1986.4.5.702. [DOI] [PubMed] [Google Scholar]
- 13.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: The Southwest Oncology Group experience. J Clin Oncol. 1991;9(9):1618–26. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 14.Paesmans M, Sculier JP, Libert P, et al. Prognostic factors for survival in advanced non-small-cell lung cancer: Univariate and multivariate analyses including recursive partitioning and amalgamation algorithms in 1,052 patients. The European Lung Cancer Working Party. J Clin Oncol. 1995;13(5):1221–30. doi: 10.1200/JCO.1995.13.5.1221. [DOI] [PubMed] [Google Scholar]
- 15.Dirican N, Dirican A, Anar C, et al. A new inflammatory prognostic index, based on C-reactive protein, the neutrophil to lymphocyte ratio and serum albumin is useful for predicting prognosis in non-small cell lung cancer cases. Asian Pac J Cancer Prev. 2016;17(12):5101–6. doi: 10.22034/APJCP.2016.17.12.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang AG, Lu HY. The Glasgow prognostic score as a prognostic factor in patients with advanced non-small cell lung cancer treated with cisplatin-based first-line chemotherapy. J Chemother. 2015;27(1):35–39. doi: 10.1179/1973947814Y.0000000188. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Park KR, Kim SJ, et al. Serum lactate dehydrogenase levels at presentation in stage IV non-small cell lung cancer: Predictive value of metastases and relation to survival outcomes. Tumour Biol. 2016;37(1):619–25. doi: 10.1007/s13277-015-3776-5. [DOI] [PubMed] [Google Scholar]
- 18.Inomata M, Hayashi R, Tanaka H, et al. Elevated levels of plasma lactate dehydrogenase is an unfavorable prognostic factor in patients with epidermal growth factor receptor mutation-positive non-small cell lung cancer, receiving treatment with gefitinib or erlotinib. Mol Clin Oncol. 2016;4(5):774–78. doi: 10.3892/mco.2016.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minami S, Ogata Y, Ihara S, et al. Outcomes and prognostic factors of chemotherapy for patients with locally advanced or metastatic pulmonary squamous cell carcinoma. Lung Cancer (Auckl) 2016;7:99–110. doi: 10.2147/LCTT.S107560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang HG, Li J, Shi SB, et al. Value of fibrinogen and D-dimer in predicting recurrence and metastasis after radical surgery for non-small cell lung cancer. Med Oncol. 2014;31(7):22. doi: 10.1007/s12032-014-0022-8. [DOI] [PubMed] [Google Scholar]
- 21.Kim KH, Park TY, Lee JY, et al. Prognostic significance of initial platelet counts and fibrinogen level in advanced non-small cell lung cancer. J Korean Med Sci. 2014;29(4):507–11. doi: 10.3346/jkms.2014.29.4.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng L, Luo M, Sun X, et al. Serum fibrinogen is an independent prognostic factor in operable nonsmall cell lung cancer. Int J Cancer. 2013;133(11):2720–25. doi: 10.1002/ijc.28284. [DOI] [PubMed] [Google Scholar]
- 23.Qiu MZ, Xu RH, Ruan DY, et al. Incidence of anemia, leukocytosis, and thrombocytosis in patients with solid tumors in China. Tumour Biol. 2010;31(6):633–41. doi: 10.1007/s13277-010-0079-8. [DOI] [PubMed] [Google Scholar]
- 24.Maraz A, Furak J, Varga Z, et al. Thrombocytosis has a negative prognostic value in lung cancer. Anticancer Res. 2013;33(4):1725–29. [PubMed] [Google Scholar]
- 25.Ohuchi M, Inoue S, Ozaki Y, Ueda K. Platelet count and mean platelet volume are associated with not only bone, soft tissue, and lymph node metastases but also with malignant pleural effusion in lung cancer patients. Neoplasma. 2017;64(1):140–47. doi: 10.4149/neo_2017_118. [DOI] [PubMed] [Google Scholar]
- 26.Gupta GP, Massague J. Platelets and metastasis revisited: A novel fatty link. J Clin Invest. 2004;114(12):1691–93. doi: 10.1172/JCI23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–42. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 28.Mohr R, Goor DA, Yellin A, et al. Fresh blood units contain large potent platelets that improve hemostasis after open heart operations. Ann Thorac Surg. 1992;53(4):650–54. doi: 10.1016/0003-4975(92)90327-z. [DOI] [PubMed] [Google Scholar]
- 29.Holgersson G, Sandelin M, Hoye E, et al. Swedish lung cancer radiation study group: The prognostic value of anaemia, thrombocytosis and leukocytosis at time of diagnosis in patients with non-small cell lung cancer. Med Oncol. 2012;29(5):3176–82. doi: 10.1007/s12032-012-0247-3. [DOI] [PubMed] [Google Scholar]
- 30.Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: A systematic review and meta-analysis. Int J Clin Exp Med. 2015;8(4):5379–87. [PMC free article] [PubMed] [Google Scholar]
- 31.Cakar B, Karaoglanoglu M, Sayici Y, et al. The prognostic value of thrombocytosis in newly diagnosed lung cancer patients: A retrospective analysis. J BUON. 2011;16(4):677–81. [PubMed] [Google Scholar]
- 32.Ferketich AK, Niland JC, Mamet R, et al. Smoking status and survival in the national comprehensive cancer network non-small cell lung cancer cohort. Cancer. 2013;119(4):847–53. doi: 10.1002/cncr.27824. [DOI] [PubMed] [Google Scholar]
- 33.Kawaguchi T, Takada M, Kubo A, et al. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: A comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5(5):620–30. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- 34.Avci N, Hayar M, Altmisdortoglu O, et al. Smoking habits are an independent prognostic factor in patients with lung cancer. Clin Respir J. 2017;11(5):579–84. doi: 10.1111/crj.12386. [DOI] [PubMed] [Google Scholar]
- 35.Okamoto T, Suzuki Y, Fujishita T, et al. The prognostic impact of the amount of tobacco smoking in non-small cell lung cancer – differences between adenocarcinoma and squamous cell carcinoma. Lung Cancer. 2014;85(2):125–30. doi: 10.1016/j.lungcan.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 36.Sonobe M, Kobayashi M, Ishikawa M, et al. Impact of KRAS and EGFR gene mutations on recurrence and survival in patients with surgically resected lung adenocarcinomas. Ann Surg Oncol. 2012;19(Suppl 3):S347–54. doi: 10.1245/s10434-011-1799-8. [DOI] [PubMed] [Google Scholar]
- 37.Malerba M, Montuschi P. Non-invasive biomarkers of lung inflammation in smoking subjects. Curr Med Chem. 2012;19(2):187–96. doi: 10.2174/092986712803414204. [DOI] [PubMed] [Google Scholar]
- 38.Inamura K, Yokouchi Y, Kobayashi M, et al. Tumor B7-H3 (CD276) expression and smoking history in relation to lung adenocarcinoma prognosis. Lung Cancer. 2017;103:44–51. doi: 10.1016/j.lungcan.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 39.Wang X, Zhu X, Zhang H, et al. ERCC1_202 is a prognostic biomarker in advanced stage non-small cell lung cancer patients treated with platinum-based chemotherapy. J Cancer. 2017;8(14):2846–53. doi: 10.7150/jca.19897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ulas A, Turkoz FP, Silay K, et al. A laboratory prognostic index model for patients with advanced non-small cell lung cancer. PLoS One. 2014;9(12):e114471. doi: 10.1371/journal.pone.0114471. [DOI] [PMC free article] [PubMed] [Google Scholar]


