Abstract
Background:
The prevalence of isolated cleft mitral valve (MV; no concomitant congenital heart disease or degenerative MV disease) with significant mitral regurgitation (MR) diagnosed using two-dimensional echocardiography (2DE) has been reported to be very low. Three-dimensional echocardiography (3DE) has enabled a more comprehensive visualization of the MV and detailed understanding of the mechanisms of MR and can potentially reveal isolated cleft MV that is not recognized with 2DE. The aim of this study was to determine, using 3DE, the prevalence, location, and associated MV annular and left ventricular characteristics of isolated cleft MV, in the absence of associated congenital heart disease, in patients with significant MR.
Methods:
A total of 1,092 patients with unexplained moderate or greater MR on two-dimensional transthoracic echocardiography who were referred for three-dimensional transesophageal echocardiography between 2005 and 2017 (n = 626) were retrospectively studied. Left ventricular dimensions and function were determined, and quantitative MR assessment and three-dimensional analysis of the MV annulus was performed.
Results:
Twenty-one patients (prevalence 3.3%) were diagnosed with isolated cleft MV using three-dimensional transesophageal echocardiography but not 2DE. The majority of these patients (n = 16) were noted to have anterior cleft MVs, with most located in the mid-A1 (n = 10) or mid-A3 (n = 5) scallops. Posterior clefts were less common (n = 5) and occurred at the site of the natural scallop indentations (three between P1 and P2 and two between P2 and P3). Among patients with either anterior or posterior MV cleft, there were no differences in left ventricular ejection fraction or three-dimensional MV geometry (annular distance, height, circumference, and area). There was a trend toward worse MR severity in patients with anterior cleft MV.
Conclusions:
In patients with otherwise unexplained significant MR referred for transesophageal echocardiography, 3DE uncovered a considerably higher prevalence of isolated cleft MV than previously reported by 2DE, with the majority located in the anterior MV. Although the annular geometry was similar between patients with anterior and posterior cleft MVs, a trend toward more severe MR in anterior clefts may reflect underlying abnormalities in the embryologic development of the anterior MV leaflet. Evaluation of MV pathology is improved by 3DE, which should be used routinely in the setting significant MR. (J Am Soc Echocardiogr 2018;31:1161-7.)
Keywords: Transthoracic echocardiography, Transesophageal echocardiography, Mitral valve, Mitral regurgitation
Cleft mitral valve (MV) is rare and is usually associated with congenital heart disease, most notably ostium primum atrial septal defect or other conditions, including MV prolapse, myxomatous MV disease, Marfan syndrome, and malrotation of the papillary muscles.1,2 Isolated cleft MV in either the anterior or posterior leaflet, in the absence of congenital heart disease, is diagnosed infrequently. One study using two-dimensional (2D) transthoracic echocardiography (TTE) reported a prevalence of isolated posterior cleft MV with moderate or severe mitral regurgitation (MR) of 0.07% (13 of 19,320 patients),3 while the prevalence of isolated anterior cleft MV is unknown. The diagnosis of cleft MV using 2D TTE is particularly challenging. High-quality short-axis views of the MV that simultaneously visualize the entire anterior or posterior leaflet without artifacts or dropout are generally required.
The development of three-dimensional echocardiography (3DE) has allowed unparalleled anatomic visualization of the MV in the “surgeon’s view.” This has led, in turn, to the identification of the mechanisms of MR in many patients in whom the diagnosis was otherwise unclear on the basis of 2D echocardiography (2DE).4–6 The recently updated valvular regurgitation guidelines recommend comprehensive imaging with routine incorporation of 3DE when assessing etiologies of MR.7 As such, the increasing use of three-dimensional (3D) transesophageal echocardiography (TEE) has resulted in better understanding of the mechanisms of MR (including cleft MV) before surgical or percutaneous intervention. When patients with significant MR due to unrecognized cleft MV are referred for surgical intervention, the likelihood of its diagnosis in a nonbeating, flaccid heart at the time of surgery is lower. If a cleft MV is left unaddressed during surgical intervention, persistent regurgitation after repair is possible, or in other cases, repair may be converted to valve replacement.
Whereas the posterior MV leaflet has indentations separating the respective scallops (P1, P2, and P3), the anterior leaflet lacks indentations (Figure 1). When the indentation depth of a posterior scallop exceeds 50% of the depth of the adjacent scallop or reaches the annulus, the diagnosis of cleft has been proposed.8 Because of the absence of indentations in the anterior leaflet, the diagnosis of cleft is made solely when an indentation reaches the MV annulus (Figure 1). Visualizing this level of anatomic detail is generally very difficult with 2DE but can be readily made with 3D TEE. Accordingly, it is possible that the true prevalence of isolated cleft MV would be higher when consistently using 3D TEE of the MV.
Figure 1.
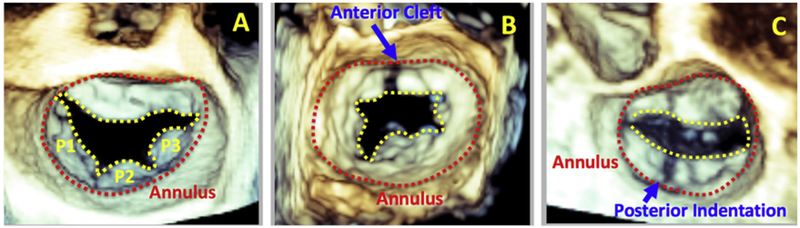
Three-dimensional transesophageal echocardiographic view of a normal MV (A) with the annular boundary noted in red and the division between anterior and posterior leaflets shown in yellow. Anterior (B) and posterior (C) MV cleft that extends to the annular boundary is shown. The scallops are labeled P1-P3.
The aim of this study was to determine, using 3DE, the prevalence, location, and echocardiographic morphology (including MV annular geometry) of isolated cleft MV, in the absence of associated congenital heart disease, in patients with moderate or more MR.
METHODS
Patient Population
We retrospectively evaluated consecutive adult patients with moderate or greater MR on 2D TTE without an apparent mechanism who were referred for clinically indicated TEE between 2005 and 2017 at our medical center. Both 2D and 3D TEE were performed using commercial equipment (iE33 or EPIQ Philips Healthcare, Andover, MA). Patients with congenital heart disease or prior MV surgery or interventions were excluded from the study.
Nomenclature
Because the nomenclature of clefts is confusing and inconsistent in the literature, we used the following definitions in this study. Whereas the anterior leaflet has no indentations, there are naturally occurring scallops in the posterior leaflet (without a clear embryologic explanation). We defined an indentation in the anterior leaflet that reaches the annulus as a cleft. In the posterior leaflet, indentations can range from <50% of the posterior leaflet depth to >50% (profound indentations). Indentations that reach the posterior annulus boundary are inconsistently labeled as fissures, clefts, and profound indentations. Because of the presence of naturally occurring scallops on the posterior leaflet, we defined indentations that reached the annulus boundary as profound indentations, rather than clefts.
Two-Dimensional TTE
Comprehensive TTE was performed according to standard protocol. Systolic and diastolic left ventricular (LV) internal dimensions were measured in the parasternal long-axis view. LV ejection fraction was calculated using the biplane method of disks (modified Simpson rule). The severity of MR was determined quantitatively by measuring vena contracta (VC) width in the parasternal long-axis view and proximal isovelocity surface area (PISA), to determine effective regurgitant orifice area and regurgitant volume.7 Qualitative grading of MR severity used the following scale: 0 = none, 1 = trivial, 2 = mild, 3 = moderate, and 4 = severe.
Two-Dimensional and 3D TEE
Patients who underwent TEE for at least moderate MR noted on TTE were included in the study. Comprehensive TEE was performed according to a standard protocol. Frame rates were maximized to optimize spatial and temporal resolution by imaging at minimal depth and sector angle. Single- or multibeat zoom or, in some cases, multibeat full-volume acquisitions of the MV were obtained. The severity of MR on TEE was similarly assessed quantitatively (VC and PISA). Three-dimensional echocardiographic acquisitions were analyzed off-line using QLAB version 10.8 (Philips Healthcare). The MV was carefully inspected from both the atrial and ventricular perspectives for diagnosis of isolated cleft. MV annular dimensions, annulus height, 3D annular circumference and area, and MV annular ellipticity were measured at end-systole using MV Navigator (QLAB).
Statistical Analysis
Categorical variables are expressed as absolute counts and percentages and continuous variables as mean ± SD. For all continuous variables, unpaired two-tailed Student’s t tests were used to determine the significance of the differences between anterior and posterior MV clefts. These analyses were performed using Excel (Microsoft, Redmond, WA).
RESULTS
Between 2005 and 2017, 1,092 patients with moderate or severe MR of unclear etiology on TTE were referred for TEE. Six hundred twenty-six of these patients had 3D echocardiographic studies for analysis. From this cohort, a total of 21 patients were identified as having isolated cleft MVs on 3D TEE (prevalence 3.3%; Figure 2). Three-dimensional color Doppler imaging (when available) confirmed that the regurgitant jet originated at the site of the cleft MV. The demographic characteristics are detailed in Table 1. Notably, patients with anterior MV clefts were younger than those with posterior MV clefts (49 ± 19 vs 61 ± 20 years), but this did not reach statistical significance (P = .14). LV dimension, volumes, and function and assessment of MR on TTE are described in Table 2. There were no notable differences in hemodynamic parameters, LV internal dimension in diastole, LV volumes, or LV ejection fraction between patients with anterior or posterior cleft MVs. Although the qualitative (color Doppler) and quantitative (PISA) severity of MR was higher in patients with anterior cleft MV, this did not reach statistical significance. Table 3 shows the transesophageal echocardiographic assessment of MR. Similar to the transthoracic assessment, the quantitative (VC and PISA) severity of MR was higher in patients with anterior cleft MVs. However, this did not reach statistical significance either.
Figure 2.
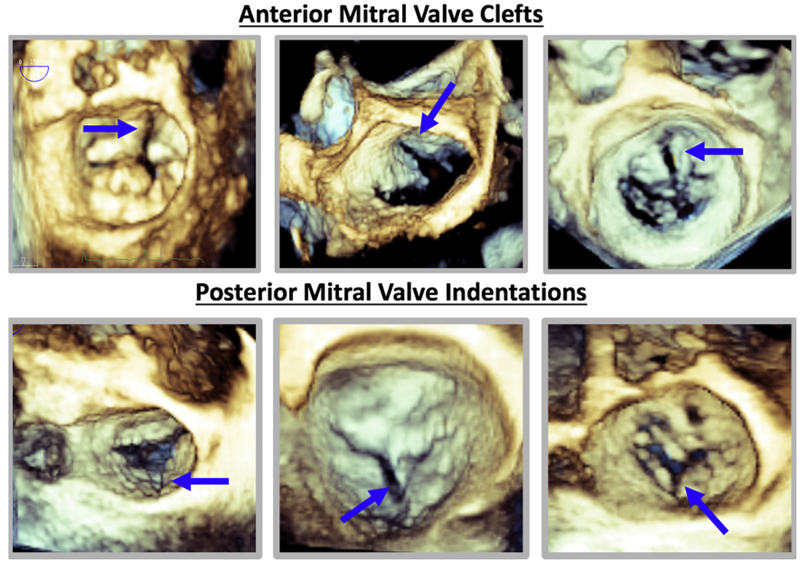
Examples of anterior and posterior cleft MV acquired using 3D TEE (blue arrows).
Table 1.
Baseline demographics and medical histories of patients with isolated cleft MVs
| Variable | Total |
|---|---|
| Mean age (y) | 50 ± 20 |
| Women | 8 (38) |
| Hypertension | 12 (57) |
| Hyperlipidemia | 4 (19) |
| Diabetes | 3 (14) |
| Smoking history | 4 (19) |
| Stroke | 2 (10) |
| Coronary artery disease | 3 (14) |
| Heart failure | 9 (43) |
| Atrial fibrillation | 2 (10) |
| Chronic kidney disease (GFR <60 mL/min/1.73 m2) | 4 (19) |
GFR, Glomerular filtration rate.
Data are expressed as mean ± SD or as number (percentage).
Table 2.
Two-dimensional transthoracic echocardiographic characteristics: comparing isolated anterior and posterior cleft MV
| Variable | Total | Anterior MV cleft | Posterior MV indentation | P* |
|---|---|---|---|---|
| Systolic BP (mm Hg) | 122 ± 16 | 121 ± 16 | 122 ± 21 | .90 |
| Diastolic BP (mm Hg) | 71 ± 12 | 72 ± 12 | 69 ± 20 | .67 |
| Heart rate (beats/min) | 74 ± 14 | 74 ± 14 | 75 ± 18 | .89 |
| LV internal dimension in diastole (mm) | 5.1 ± 1.0 | 5.1 ± 1.1 | 5.3 ± 0.7 | .68 |
| LV ejection fraction (%) | 54 ± 17 | 52 ± 13 | 60 ± 24 | .33 |
| MR VC (cm) | 0.78 ± 0.15 | 0.80 ± 0.16 | 0.70 ± 0.04 | .40 |
| Effective regurgitant orifice area (mm2) | 35 ± 15 | 37 ± 16 | 26 ± 3 | .15 |
| Regurgitant volume (mL) | 52 ± 21 | 55 ± 22 | 39 ± 1 | .13 |
| Qualitative MR (0–4) | 3.3 ± 0.5 | 3.3 ± 0.5 | 3.2 ± 0.5 | .64 |
BP, Blood pressure.
Data are expressed as mean ± SD.
Comparison between anterior and posterior MV cleft.
Table 3.
Transesophageal echocardiographic parameters of qualitative and quantitative assessment of MR
| Variable | Total | Anterior MV cleft | Posterior MV indentation | P* |
|---|---|---|---|---|
| Systolic BP (mm Hg) | 126 ± 17 | 116 ± 16 | 137 ± 10 | .01 |
| Diastolic BP (mm Hg) | 67 ± 14 | 66 ± 17 | 68 ± 13 | .90 |
| Heart rate (beats/min) | 86 ± 19 | 86 ± 21 | 87 ± 17 | .84 |
| MR VC (cm) | 0.67 ± 0.09 | 0.68 ± 0.09 | 0.64 ± 0.10 | .38 |
| Effective regurgitant orifice area (mm2) | 33 ± 20 | 41 ± 22 | 30 ± 3 | .05 |
| Regurgitant volume (mL) | 54 ± 31 | 66 ± 35 | 35 ± 5 | .07 |
BP, Blood pressure.
Data are expressed as mean ± SD.
Comparison between anterior and posterior MV cleft.
The locations of the isolated cleft MVs identified in the study are depicted in Figure 3. The majority of patients (n = 16) had a single anterior cleft MV, with the majority of clefts located at mid-A1 (n = 10) or mid-A3 (n = 5). Posterior clefts were less common and occurred at the site of the natural scallop indentations, either between P1 and P2 (n = 3) or between P2 and P3 (n = 2). All of the clefts reached the annular boundary.
Figure 3.
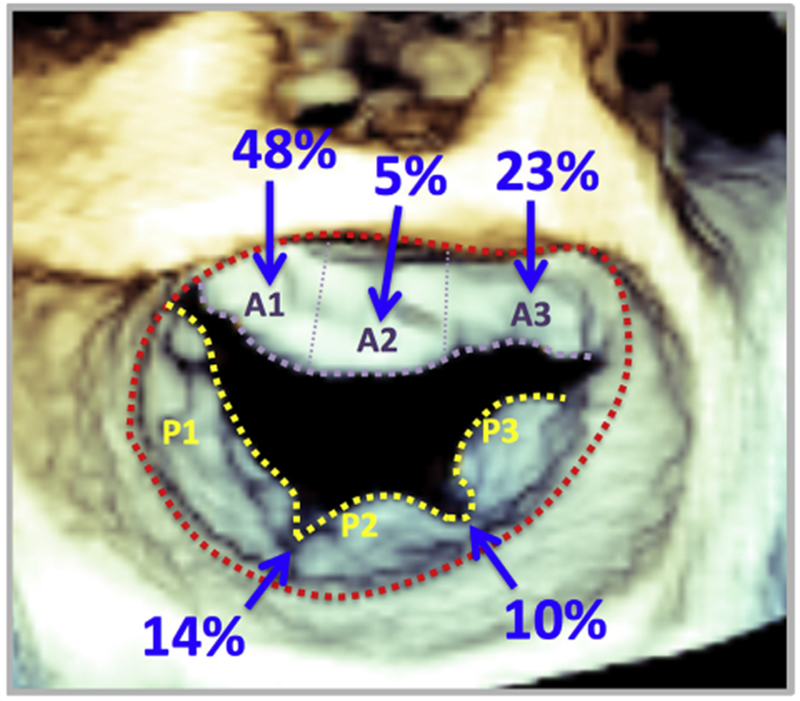
Location of isolated cleft MV identified by 3D TEE. The majority of the clefts identified were located on the anterior MV leaflet. The scallops are labeled A1-A3 and P1-P3.
Analysis of geometry of the MV from the transesophageal echocardiographic images was compared between patients with anterior and posterior MV clefts (Table 4). Overall, the annular geometry was comparable in both groups of patients. The dimensions of the MV annulus (anterolateral-posteromedial or anterior-posterior distance) were similar between the two groups of patients. Similarly there was no difference in MV annular height in patients who had anterior versus posterior MV clefts. Three-dimensional annular circumference and area were greater in patients who had anterior MV clefts, but this did not reach statistical significance.
Table 4.
Assessment of MV annular geometry using 3D TEE
| Variable | Total | Anterior MV cleft | Posterior MV indentation | P* |
|---|---|---|---|---|
| AL-PM distance (mm) | 50 ± 11 | 51 ± 10 | 48 ± 8 | .54 |
| Anterior-posterior distance (mm) | 44 ± 8 | 44 ± 8 | 43 ± 7 | .89 |
| Annular height (mm) | 11 ± 3 | 10 ± 3 | 12 ± 4 | .36 |
| 3D annular circumference (mm) | 160 ± 31 | 162 ± 31 | 155 ± 27 | .69 |
| 3D annular area (mm2) | 1,843 ± 692 | 1,910 ± 709 | 1,681 ± 498 | .54 |
| Annulus ellipticity (%) | 114 ± 12 | 116 ± 11 | 111 ± 11 | .44 |
AL-PM, Anterolateral-posteromedial.
Data are expressed as mean ± SD.
Comparison between anterior and posterior MV cleft.
DISCUSSION
This is the first study in which 3DE was used to describe the prevalence of isolated cleft MV and the clinical and echocardiographic characteristics of patients with this condition. The main findings of our study were as follows: (1) the prevalence (3.3%) of isolated cleft MV in patients with significant MR (on 2D TTE) referred for 3D TEE was considerably higher compared with what has been previously reported on the basis of 2D echocardiographic studies in patients with otherwise unexplained significant MR; (2) the majority of isolated cleft MVs with significant MR were located on the anterior leaflet (mostly between A1 and A2); (3) cleft MV in the posterior MV leaflet occurred at the site of natural scallop edges, presenting as profound indentations; (4) there was a trend toward more severe MR in patients with isolated anterior cleft; and (5) there were no differences between anterior and posterior cleft MV in the morphology of the MV annulus.
MR is a growing problem worldwide. At least mild MR was detected using 2D TTE in nearly 20% of subjects in the Framingham Heart Study, and moderate or severe MR was noted on 2D TTE in 2.1% of patients in the Strong Heart Study.9,10 The predominant etiologies of significant MR in the developed world include degenerative or ischemic heart disease, while rheumatic MV disease is a leading cause in the developing world. When the etiology of significant MR is not elucidated by 2D TTE, TEE is often the next diagnostic test performed because of its higher resolution, ability to image in a variety of planes, and 3D capability.7 A previous investigation revealed that up to 21% of patients with at least moderate MR (diagnosed by 2D TTE or TEE) had no clearly defined etiology.11 The development of 3DE has allowed improved understanding of the mechanisms of MR that could potentially be easily missed by 2D imaging, such as isolated cleft MV.
Previous 2D echocardiographic investigations have reported a low prevalence of isolated cleft MV,3 likely because of inadequate anatomic visualization of the MV, in part because of incomplete imaging of the leaflets in relation to the annulus, echo dropout, and artifacts. Using 3D TEE, we found a considerably higher prevalence of isolated cleft MV. A recent investigation has similarly shown that a substantial proportion of cleft MV cases were accurately identified by 3D TEE and not by 2DE.8 The advantage of using 3DE to visualize the MV is the ability to crop and rotate the valve at multiple levels and planes while simultaneously visualizing the anterior and posterior leaflets in relation to the MV annulus. The anterior leaflet can be carefully inspected for abnormal clefts, while on the posterior leaflet, one can readily identify the natural scallop indentations and understand whether pathologic indentations are present. With the use of 3D color Doppler, the MR jet can also be confirmed to originate from within the cleft.
We noted that nearly three quarters of isolated clefts were located in either the mid-A1 or mid-A3 position. This confirms what was previously observed in a postmortem study of patients with isolated clefts.12 Despite naturally occurring scallops in the posterior leaflet, a minority of clefts were identified in the posterior leaflet in our study. This is also consistent with the observation that isolated posterior MV clefts are less common than isolated anterior MV clefts.
The embryologic explanation for isolated MV cleft development is not well elucidated. The normal anterior MV leaflet is formed when the superior and inferior atrioventricular (endocardial) cushions fuse together. Failure of this fusion results in cleft anterior MV, which is typically associated with ostium primum atrial septal defect. Many of these patients are diagnosed during childhood because of the presence of murmurs on physical examination. When anterior MV clefts are diagnosed in the absence of associated congenital heart defects, the mechanism responsible for cleft development remains unclear. In our study, no additional pathologies of the anterior MV leaflet were observed other than the cleft. Additionally, the MV annulus was not significantly dilated to explain the degree of MR. It is possible that incomplete fusion of the endocardial cushions may be the primary reason for cleft formation in the anterior leaflet. The majority of anterior clefts were located off the “midline” of A2, suggesting that endocardial fusion maybe potentially asymmetric.
Although the posterior MV leaflet has naturally occurring indentations, the embryologic explanation for scallops is unknown. The indentation depth can vary considerably among scallops. It remains unclear if profound indentations are truly pathologic or represent a normal spectrum. Similar to anterior MV clefts, the mechanisms governing these profound posterior indentations are unknown. In our study, besides the profound indentations, there were no obvious pathologies of the posterior leaflet or the MV annulus that could explain the degree of MR.
Similar to our findings, in which we did not observe significant morphologic abnormalities of the MV annular geometry, a previous investigation of 17 posterior MV clefts also found no association between excess annular enlargement (resulting in MV stretching) or excess leaflet prolapse (causing scallop separation) compared with cases of myxomatous MV degeneration.8
The degree of MR noted on TTE was quantitatively greater in patients with anterior cleft MVs compared with profound posterior leaflet indentations, although the difference did not reach statistical significance, likely because of the small sample size. Nonetheless, the observation that anterior clefts have more severe MR can potentially be explained by the fact that the anterior MV leaflet has no naturally occurring indentations, and hence any disruption of the anterior leaflet is more likely to cause regurgitation than a prominent indentation in the posterior leaflet. Additionally, the anterior leaflet typically has a larger area compared with the posterior leaflet. As such, a cleft in the anterior leaflet is more likely to result in more severe MR compared with a profound indentation in the posterior MV leaflet. The difference in MR severity between patients with anterior cleft MV and profound posterior indentation cannot be explained on the basis of differences in annular geometry.
Three-dimensional TEE is invaluable in determining the mechanisms of unexplained severe MR, particularly when due to isolated cleft MV. Recognizing cleft MV, when present, as the mechanism of severe MR is paramount for optimal surgical or percutaneous procedural planning. The shape of MV clefts is heterogeneous, ranging from V-shaped to C- or S-shaped clefts.13 Three-dimensional echocardiography allows the determination of the exact shape and location of the cleft and may thus help decide whether surgical or percutaneous correction would be beneficial.
The treatment options for MV cleft have traditionally been surgical repair (direct suture of the cleft or insertion of an autologous pericardial patch with or without an annuloplasty ring) or valve replacement.14–18 Novel options include percutaneous repair using the MitraClip system (Abbott Vascular, Santa Clara, CA) to suture-close the cleft. The percutaneous repair option is particularly attractive for patients with multiple comorbidities who are at high surgical risk (Figure 4). The goal of both surgical and percutaneous repair is to minimize MR by closing the cleft. Real-time 3D TEE is necessary to evaluate success of repair.
Figure 4.
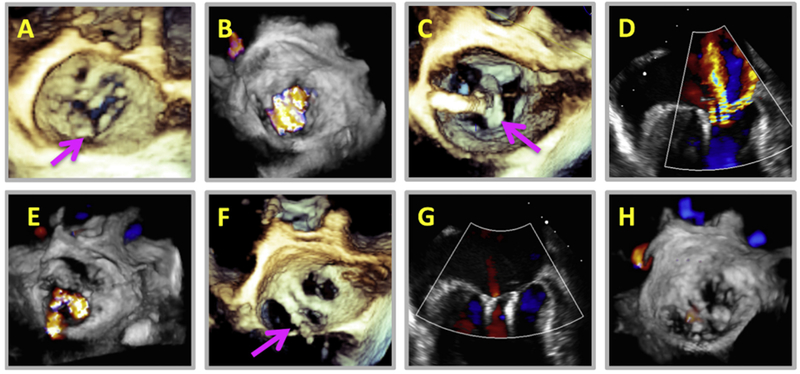
An 81-year-old female patient with a history of severe MR due to isolated posterior indentation MV (A, arrow) and multiple heart failure admissions was referred for percutaneous clip after being deemed too high risk for surgical intervention. Three-dimensional color Doppler revealed significant MR (B) arising for the indentation. A MitraClip was placed in the A2-P2 position lateral to the indentation (C, arrow), but color Doppler showed minimal reduction in severity of MR (D, E). As a result, a second MitraClip was placed slightly medial to the first (F, arrow). The resultant MR was trace (G, H). The patient had no further heart failure admissions at 1-year follow-up.
Limitations
There were several limitations to this study. Although this is the first study to comprehensively assess isolated cleft MV using 3DE, the overall sample size was still small, even though it was larger than the majority of previous studies and perhaps the largest 3DE study. Additionally, the true prevalence of isolated cleft MV with any degree of MR is not reflected in this study, because (1) we evaluated only patients with significant MR who were referred for 3DE, and thus patients with trivial or mild MR were not included, and (2) only 57% of patients (n = 626) with unclear etiologies of MR underwent 3D TEE in our study; although 3DE was routinely performed during the later period of the study, this was not the case with patients enrolled earlier. Similarly, this study was conducted at a large tertiary care academic medical center with a referral bias for complex cases. This too may have affected the true prevalence. The measurement of MR severity in patients with cleft MVs may not be accurate using the geometric assumptions of a hemisphere used in the 2D PISA calculations. It would have been ideal to use a 3D PISA to assess MR. However, many of our studies are >10 years old, and this methodology was unavailable at the time. Accordingly, we measured VC, which was found to be higher (though not statistically significantly) in patients with anterior cleft MV versus posterior cleft MV on TTE and TEE (Tables 2 and 3). Ideally, future studies should examine MR using 3D PISA, as this would take into account noncircular orifices. Finally, our sample size was too small for reliable analysis of the relationship between cleft shape and severity of MR.
CONCLUSION
Evaluation of MV pathology has been aided significantly by 3DE. In patients with unexplained moderate or severe MR referred for TEE, 3DE uncovered a higher prevalence of isolated cleft MV than previously reported by 2DE. Additionally, although the annular geometry did not differ between patients with anterior and posterior cleft MVs, patients with anterior cleft MV tended to have more severe regurgitation. Evaluation of MV pathology is improved by 3DE, which should be used routinely in the setting significant MR.
HIGHLIGHTS.
Using 3DE, isolated cleft MV is more prevalent than previously reported.
The majority of isolated cleft MV are located on the anterior MV leaflet.
Patients with isolated cleft MV on the anterior leaflet tend to have more MR.
3DE should be utilized when evaluating patients with MR of unclear etiology.
Acknowledgments
This study was funded by a T32 Cardiovascular Sciences Training Grant (5T32HL7381) to Dr. Narang. Dr. Lang has received research grants from Philips Healthcare for other unrelated studies. Bijoy K. Khandheria, MD, FASE, served as guest editor for this report.
Abbreviations
- 2D
Two-dimensional
- 2DE
Two-dimensional echocardiography
- 3D
Three-dimensional
- 3DE
Three-dimensional echocardiography
- LV
Left ventricular
- MR
Mitral regurgitation
- MV
Mitral valve
- PISA
Proximal isovelocity surface area
- TEE
Transesophageal echocardiography
- TTE
Transthoracic echocardiography
- VC
Vena contracta
REFERENCES
- 1.Izgi C, Feray H, Saltan Y, Kahraman R. Isolated cleft of the posterior mitral valve leaflet in a patient with Marfan syndrome. Int J Cardiol 2010;145: e102–4. [DOI] [PubMed] [Google Scholar]
- 2.Kent SM, Markwood TT, Vernalis MN, Tighe JF. Cleft posterior mitral valve leaflet associated with counterclockwise papillary muscle malrotation. J Am Soc Echocardiogr 2001;14:303–4. [DOI] [PubMed] [Google Scholar]
- 3.Wyss CA, Enseleit F, van der Loo B, Gr€unenfelder J, Oechslin EN, Jenni R. Isolated cleft in the posterior mitral valve leaflet: a congenital form of mitral regurgitation. Clin Cardiol 2009;32:553–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muraru D, Cattarina M, Boccalini F, Dal Lin C, Peluso D, Zoppellaro G, et al. Mitral valve anatomy and function: new insights from three-dimensional echocardiography. J Cardiovasc Med (Hagerstown) 2013; 14:91–9. [DOI] [PubMed] [Google Scholar]
- 5.Lang RM, Badano LP, Tsang W, Adams DH, Agricola E, Buck T, et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. J Am Soc Echocardiogr 2012;25:3–46. [DOI] [PubMed] [Google Scholar]
- 6.Sugeng L, Coon P,Weinert L, Jolly N, Lammertin G, Bednarz JE, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr 2006;19:413–21. [DOI] [PubMed] [Google Scholar]
- 7.Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for Noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–71. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani F, Clavel MA, Vatury O, Suri RM, Mankad SV, Malouf J, et al. Cleft-like indentations in myxomatous mitral valves by three-dimensional echocardiographic imaging. Heart 2015;101:1111–7. [DOI] [PubMed] [Google Scholar]
- 9.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, et al. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897–902. [DOI] [PubMed] [Google Scholar]
- 10.Jones EC, Devereux RB, Roman MJ, Liu JE, Fishman D, Lee ET, et al. Prevalence and correlates of mitral regurgitation in a population-based sample (the Strong Heart Study). Am J Cardiol 2001;87: 298–304. [DOI] [PubMed] [Google Scholar]
- 11.Martínez-Sellés M, García-Fernández MA, Larios E, Moreno M, Pinto A, García-Robles JA, et al. Etiology and short-term prognosis of severe mitral regurgitation. Int J Cardiovasc Imaging 2009;25:121–6. [DOI] [PubMed] [Google Scholar]
- 12.Van Praagh S, Porras D, Oppido G, Geva T, Van Praagh R. Cleft mitral valve without ostium primum defect: anatomic data and surgical considerations based on 41 cases. Ann Thorac Surg 2003;75: 1752–62. [DOI] [PubMed] [Google Scholar]
- 13.Yuan X, Zhou A, Chen L, Zhang C, Zhang Y, Xu P. Diagnosis of mitral valve cleft using real-time 3-dimensional echocardiography. J Thorac Dis 2017;9:159–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinonez LG, Del Nido PJ. Valve reconstruction for congenital mitral valve disease. Multimed Man Cardiothorac Surg 2015;2015. [DOI] [PubMed] [Google Scholar]
- 15.Abadir S, Fouilloux V, Metras D, Ghez O, Kreitmann B, Fraisse A. Isolated cleft of the mitral valve: distinctive features and surgical management. Ann Thorac Surg 2009;88:839–43. [DOI] [PubMed] [Google Scholar]
- 16.Artrip JH, Rumball EM, Finucane K. Repair of left atrioventricular valve cleft defects with patch augmentation. Ann Thorac Surg 2012;93: 2081–3. [DOI] [PubMed] [Google Scholar]
- 17.Perier P, Clausnizer B. Isolated cleft mitral valve: valve reconstruction techniques. Ann Thorac Surg 1995;59:56–9. [DOI] [PubMed] [Google Scholar]
- 18.Miglioranza MH, Muraru D, Mihaila S, Haertel JC, Iliceto S, Badano LP. Isolated anterior mitral valve leaflet cleft: 3D transthoracic echocardiography-guided surgical strategy. Arq Bras Cardiol 2015;104:e49–52. [DOI] [PMC free article] [PubMed] [Google Scholar]


