Abstract
Antibody–drug conjugates are an emerging class of biopharmaceuticals changing the landscape of targeted chemotherapy. These conjugates combine the target specificity of monoclonal antibodies with the anti-cancer activity of small-molecule therapeutics. Several antibody–drug conjugates have received approval for the treatment of various types of cancer including gemtuzumab ozogamicin (Mylotarg®), brentuximab vedotin (Adcetris®), trastuzumab emtansine (Kadcyla®), and inotuzumab ozogamicin, which recently received approval (Besponsa®). In addition to these approved therapies, there are many antibody–drug conjugates in the drug development pipeline and in clinical trials, although these fall outside the scope of this article. Understanding the pharmacokinetics and pharmacodynamics of antibody–drug conjugates and the development of pharmacokinetic/pharmacodynamic models is indispensable, albeit challenging as there are many parameters to incorporate including the disposition of the intact antibody–drug conjugate complex, the antibody, and the drug agents following their dissociation in the body. In this review, we discuss how antibody–drug conjugates progressed over time, the challenges in their development, and how our understanding of their pharmacokinetics/pharmacodynamics led to greater strides towards successful targeted therapy programs.
1. Introduction
Despite significant improvements in therapeutic agents and surgical techniques, cancer remains the second leading cause of death in USA [1]. Chemical-based treatment of cancer gained significant interest in the early 1900s. Paul Ehrlich, the esteemed German chemist, first sought to treat infectious diseases with chemical agents, coining the term “chemotherapy”. Ehrlich was also interested in using chemical drugs to treat cancers, though his success was limited [2]. In his career, Ehrlich described his vision of a “magic bullet” therapy, which would be used to target and kill diseased tissues while leaving healthy tissues intact [3]. Until the 1960s, conventional treatment of cancer employed surgical and radiotherapeutic approaches, until it was realized that the addition of drugs to these therapies could allow practitioners to optimize tumor treatment while limiting unwanted toxicities [2, 4]. Since then, countless chemotherapeutic agents have been designed, tested, and marketed for many diseases. However, curative rates of treatment leave room for improvement for a number of reasons including acquired multidrug resistance, insufficient target specificity, and intolerable toxicities [5]. There remains an unmet need to develop new therapeutic modalities that specifically target cancer cells and exhibit relatively minimal side effects. As a result, immunotherapy was explored as a new modality that carries a great potential for the treatment of cancer mainly owing to its target specificity [6].
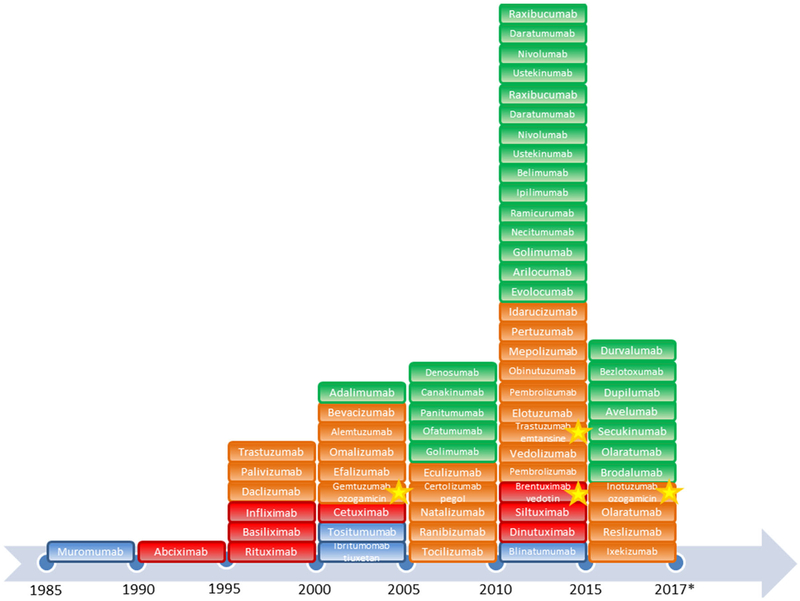
Immunotherapy dates back to the 1970s and the development of hybridoma technology, allowing for the reliable production of antibodies first by Kohler and Milstein [7]. In 1980, the first patient with relapsed lymphoma was treated with therapeutic antibodies after in vitro screening showed promising anti-tumor activity. While this initial trial proved unsuccessful because of a lack of prolonged efficacy in patients, the development of these biological agents continued as they were generally well tolerated. Currently, over 60 monoclonal antibodies have been approved for the treatment of various health conditions (Fig. 1), most prevalently in the field of oncology [8, 9].
Fig. 1.
Timeline of the US Food and Drug Administration approval of monoclonal antibody therapeutics and antibody–drug conjugates. Antibody–drug conjugates discussed in this review are highlighted with a yellow star. The color of each block denotes the type of antibody: blue, murine; red, chimeric; orange, humanized; green, human. *This timeline only includes therapeutics approved at the time of writing this review (2017). The number of approvals between 2015 and 2017 is in line with the increasing trend in approved biologic therapeutics
Monoclonal antibodies are highly specific and can bind to the same antigenic epitope because they are secreted from identical immune cells that are all clones of a unique parent cell. This makes monoclonal antibodies attractive therapeutic tools for targeted therapeutic approaches. In addition to treating cancer, monoclonal antibodies can be used to treat certain forms of arthritis, systemic lupus erythematosus, multiple sclerosis, inflammatory bowel disease, and other autoimmune diseases [10]. Four major antibody types have been developed: murine, chimeric, humanized, and human. The guiding principle was to develop antibodies that can escape immunological rejection by the host while still maintaining their bioactive properties [11]. Murine antibodies, denoted with the suffix ‘-omab’, were the first to be developed into therapeutics. The major drawback to these therapeutics was the recognition by the host as foreign proteins and the development of vigorous immune responses resulting in adverse events, increased drug clearance (Cl), and reduced efficacy [12]. This led to the idea of the development of chimeric antibodies (suffix, ‘-ximab’) made by fusing varying ratios of murine antigen-binding domains with human effector domains. The human sequences usually represent about 70% of the whole protein. As a result of the incorporation of more human proteins, these antibodies were not as foreign to the immune system as murine antibodies and thus exhibited decreased immunogenicity and increased serum half-lives [13]. The most successful chimeric monoclonal antibody to date is rituximab, an anti-CD20 antibody used in the treatment of B-cell lymphomas [14]. It has also proven effective in the treatment of autoimmune diseases such as rheumatoid arthritis and multiple sclerosis [14, 15]. Most antibodies being developed today are either humanized (suffix, ‘-zumab’), generated by combining mouse hypervariable regions with human constant domains, or fully human (suffix, ‘-umab’), produced in transgenic mice or by using phage display technology. The humanized antibodies boast over 90% human sequences and are thus less foreign to the immune system than chimeric antibodies. The main difference between humanized and human antibodies is that humanized antibodies have non-human origins. Trastuzumab and adalimumab represent two of the most successful humanized and human antibodies on the market today, respectively [16, 17].
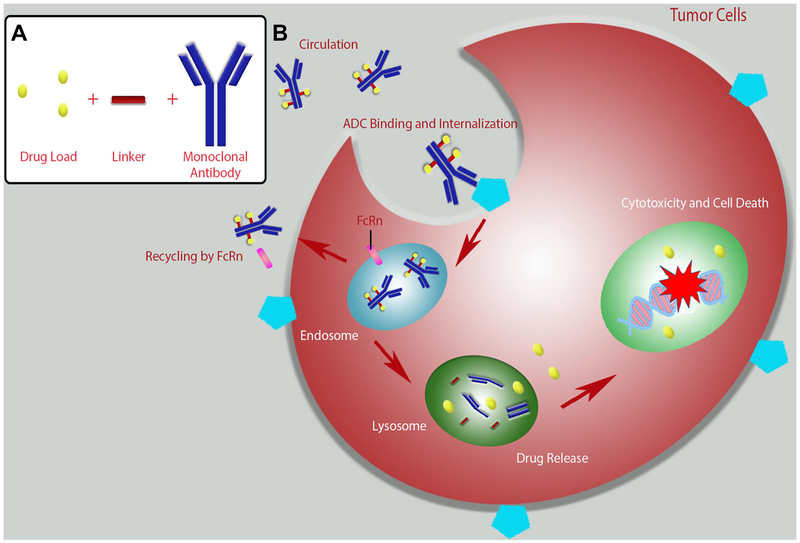
Antibody–drug conjugates (ADCs) are an emerging class of biopharmaceutical agents designed for targeted treatment primarily for patients with cancer. These conjugate drugs closely resemble the “magic bullet” vision of Paul Ehrlich. By conjugating active drug moieties to targeted antibodies, ADCs are designed to attack only cancerous cells while remaining non-toxic to healthy tissue [18–20]. This targeted delivery of antineoplastic agents is designed to enhance the potency of antibody therapeutics while widening the often narrow therapeutic index of chemotherapeutic drugs [21]. The process of ADC binding, internalization, cleavage, and cytotoxicity is described in Fig. 2. Briefly, the monoclonal antibody moiety of the ADC will bind to the target antigen on the surface of cells. The entire ADC complex is then internalized, the conjugated drug is released, and the cell is killed by the cytotoxic effect of the conjugated drug. This strategy has also been employed for overcoming multidrug resistance in target cells [22].
Fig. 2.
Antibody–drug conjugate (ADC) assembly and interaction with target cells. a Assembly of an ADC. b Typical mechanism of action of an ADC. The administered ADC binds to antigens expressed on the surface of target tumor cells. Following binding, the ADC is internalized. Some of the ADC is recycled back to circulation by the neonatal Fc receptor (FcRn). The remainder of the ADC is trafficked from the late endosome to the lysosome where the antibody is degraded and the linked drug is released. The free drug enters the nucleus of the cell and damages DNA leading to cell death
To date, only four ADCs have received US Food and Drug Administration (FDA) approval. The first of which, gemtuzumab ozogamicin (Mylotarg®), was approved in 2001 for the treatment of acute myelogenous leukemia (AML). It was withdrawn from the market in June 2010 as it was linked to a serious and potentially fatal liver condition known as veno-occlusive disease. Gemtuzumab ozogamicin was resubmitted for approval with a fractionated dosing regimen and was recently (September 2017) approved by the FDA for the treatment of adults with newly diagnosed CD33+ AML and adults and children aged 2 years and older with relapsed or refractory CD33+ AML. Three other FDA-approved ADCs remain on the market including brentuximab vedotin (Adcetris®) for the treatment of CD30+ Hodgkin lymphoma and systemic anaplastic large cell lymphoma, trastuzumab emtansine (Kadcyla®) for treating human epidermal growth factor 2 (HER2)+ metastatic breast cancer, and inotuzumab ozogamicin (Besponsa®), which targets CD22+ non-Hodgkin lymphoma (NHL) and was recently approved for use. The structure and targets of these ADCs are listed in Table 1 [23, 24].
Table 1.
Structure and targets of antibody–drug conjugates (ADCs)
| ADC name | Antibody origin | Target | Linker | Payload |
|---|---|---|---|---|
| Gemtuzumab ozogamicin | Humanized | CD33 | 4-(4-Acetylphenoxy)butanoic acid | NAC |
| Brentuximab vedotin | Chimeric | CD30 | Valine-citrulline | MMAE |
| Trastuzumab emtansine | Humanized | HER2 | MCC | DM1 |
| Inotuzumab ozogamicin | Humanized | CD22 | 4-(4-Acetylphenoxy)butanoic acid | NAC |
CD20/22/30 B-cell receptor CD20/22/30, DM1 emtansine, HER2 human epidermal growth factor receptor 2, MCC maleimidomethyl cyclohexane-1-carboxylate, MMAE monomethyl aurostatin E, NAC N-acetyl-γ-calicheamicin
There are several factors contributing to the overall efficacy of ADC therapies including tumor penetration and accumulation, target binding and cellular uptake, release of active catabolic products within the target cells, and the potency of these products, as well as the pharmacokinetic (PK) profile of the ADC. The significant majority (~ 98%) of the total ADC is comprised by the antibody component and the pharmacokinetics of the ADCs are influenced greatly by the properties of the antibody backbone. Antibody properties governing ADC pharmacokinetics include target-specific binding, neonatal Fc receptor-dependent recycling, and Fc (fragment, crystallizable) effector functions, and ADCs exhibit the same absorption, distribution, metabolism, and excretion properties associated with unconjugated antibodies including a low volume of distribution, slow Cl, a long half-life, and proteolysis-mediated catabolism [25, 26]. Applications and recommended PK considerations of various ADCs have been the subject of numerous review articles [27–29].
In addition to PK challenges, ADCs come with risks of toxicity and immunogenicity, which can be mediated by any of the components of the ADC complex. Expression of a target antigen on normal cells can lead to toxicity in healthy tissue and early cleavage of the linker leading to drug release can result in more systemic toxicities. The majority of the reported toxicity from ADCs, including those discussed in this article, arises from the payload drug. Monomethyl aurostatin E (MMAE) has been associated with peripheral neuropathy and neutropenia while emtan-sine (DM1) is known to cause thrombocytopenia and elevated liver enzymes [30]. In the cases of brentuximab vedotin and gemtuzumab ozogamicin, ADC immunogenicity has been reported to manifest as infusion reactions and transient shortness of breath, respectively [31, 32]. Immunological rejection is another caveat and is often associated with negative impacts on the PK properties of the drug such as increased drug Cl. Impacts of these risks on the efficacy and safety of the drug formulations need to be carefully assessed.
Until recently, the development of ADCs has been carried out empirically, without significant understanding of the correlation between in vitro, preclinical, in vivo, and clinical results. Here, we explore the progression of ADCs, challenges in their development, and some early mechanistic and quantitative pharmacology models, which have been developed from previously conducted preclinical studies and clinical trials, with the intention of creating better predictive models for accelerating ADC candidate selection and development. We also discuss the results of clinical studies carried out with clinically advanced ADCs. The overall results of these clinical studies are summarized in Table 2.
Table 2.
Clinical studies performed with antibody–drug conjugates (ADCs)
| ADC | Year | Study design | Major findings | References |
|---|---|---|---|---|
| Gemtuzumab ozogamicin | 2001 | PK study in 59 patients with AML in the first relapse given a single agent (9 mg/m2), two doses 2 weeks apart | Determined Cmax, t1/2, AUC, Cl Observed elevated plasma concentrations following second administration |
[39] |
| 2001 | 29 male and 29 female patients of mean age 53 ± 16 years given a 2-h IV infusion (9 mg/m2), single 2-h infusion | Age has no impact (i.e., non-significant covariate) on the pharmacokinetics of gemtuzumab ozogamicin | [40] | |
| 2004 | 29 pediatric patients with AML given 6, 7.5, or 9 mg/m2 of gemtuzumab ozogamicin, two doses, 14–28 days apart | Mean PK values in pediatric patients similar to those reported in adult populations Adult and pediatric patients exhibited large interindividual PK variability |
[41] | |
| 2009 | PK study in 40 Japanese patients with relapsed/refractory AML, two doses (9 mg/m2), 2 weeks apart | Japanese patients demonstrated a similar toxicity profile to other ethnicities Japanese patients remained in remission longer than previous reports in other ethnic groups |
[42] | |
| Brentuximab vedotin | 2011 | Dose-escalation (0.4–1.4 mg/kg on days 1, 8, and 15) study to determine the MTD in patients with relapsed/refractory CD30+ hematologic malignancies, dosed weekly | Determined the MTD as 1.2 mg/kg in this population | [46] |
| 2014 | Combination of brentuximab vedotin with rituximab, two cycles of brentuximab vedotin (1.8 mg/kg) followed by six cycles of CHOP or brentuximab plus CHOP once every 3 weeks | 88% objective response rate when brentuximab vedotin was administered simultaneously with rituximab, while 85% objective response rate when brentuximab vedotin was administered in succession with rituximab | [90] | |
| Trastuzumab emtansine | 2010 | Dose-escalation study in 24 patients. Dose range 0.3–4.8 mg/kg every 3 weeks | Determined the MTD to be 4.8 mg/kg t1/2 = 3.5 days Cl greater at doses less than 1.2 mg/kg |
[68] |
| 2011 | Administered at 3.6 mg/kg in 112 patients once every 3 weeks as a single agent | Strong anticancer activity and well tolerated as a single agent at this dose | [72] | |
| 2012 | PK study in patients with HER2+ breast cancer who were given 3.6 mg/kg as a single agent once every 3 weeks | Pharmacokinetics of single-agent trastuzumab emtansine was well characterized and consistent with previous reports Exposure to trastuzumab emtansine does not correlate with clinical response or adverse events |
[64] | |
| 2012 | Dose escalation (1.2–2.4 mg/kg) with weekly dosing in 28 patients to determine the MTD, safety, tolerability, and pharmacokinetics at this dosing frequency | Determined the MTD to be 2.4 mg/kg when administered weekly Exposure to the drug in patients is dose proportional at doses less than 1.2 mg/kg |
[71] | |
| 2015 | Pharmacokinetics, toxicity, and the MTD examined in ten Japanese patients with HER2+ breast cancer. Dose escalation study from 1.8–3.6 mg/kg every 3 weeks | THe MTD in Japanese patients matched other ethnic groups, 3.6 mg/kg | [70] | |
| 2016 | 73 Japanese patients with previously treated HER2+ breast cancer given 3.6 mg/kg of trastuzumab emtansine every 3 weeks | Safety and efficacy profile of trastuzumab emtansine in Japanese populations closely matches other investigated ethnic populations | [91] | |
| Inotuzumab ozogamicin | 2006 | Dose-escalation study (0.4–2.4 mg/m2) at 14 European and US sites with 36 patients followed by an expanded trial of clinical activity at MTD (1.8 mg/m2 every 4 weeks) | Determined the MTD of inotuzumab ozogamicin to be 1.8 mg/m2 every 4 weeks with a manageable overall safety profile | [82, 92] |
| 2008 | Fixed dose of rituximab (375 mg/m2) on day 1 followed by inotuzumab ozogamicin (0.8–1.8 mg/m2) on day 2 in patients with CD22+ B-cell NHL | This combination provides efficacy in this patient population with a manageable safety profile similar to that of inotuzumab ozogamicin monotherapy | [83] | |
| 2010 | 13 Japanese patients with relapsed/refractory CD22+ NHL previously treated with R-CHOP, dose escalation 1.3—1.8 mg/m2 administered once every 28 days | Confirmed the previously reported MTD of inotuzumab ozogamicin of 1.8 mg/kg in Japanese patients | [86] | |
| 2012 | Inotuzumab ozogamicin administered in combination with rituximab in patients with relapsed/refractory B-cell NHL, administered once every 28 days for up to eight cycles (1.8 mg/m2) | PK profile of inotuzumab ozogamicin closely resembled that of single-agent inotuzumab ozogamicin | [87] | |
| 2013 | Dose-escalation study in patients with relapsed/refractory ALL. First 49 patients received 1.3—1.8 mg/m2 of single-agent inotuzumab ozogamicin once every 3–4 weeks, the next 41 patients received 0.8 mg/m2 on day 1, 0.5 mg/m2 on days 8 and 15, and every 3–4 weeks thereafter | Single-agent inotuzumab ozogamicin is highly active and safe in this patient population | [85] | |
| 2013 | Escalating doses of inotuzumab ozogamicin (0.8–1.8 mg/m2) with a fixed dose of rituximab (375 mg/m2) to determine the MTD Combination of inotuzumab ozogamicin and rituximab administered at the MTD (375 mg/m2 of rituximab on day 1, 1.8 mg/m2 of inotuzumab ozogamicin every 4 weeks for up to eight cycles) in patients with CD20/22+ NHL |
This combination has strong response rates and long-term progression-free survival with a manageable toxicity profile | [88] |
ALL acute lymphoblastic leukemia, AML acute myelogenous leukemia, AUC area under the curve, CD20/22/30 B-cell receptor CD20/22/30, Cl clearance, Cmax maximum observed concentration, HER2 human epidermal growth factor receptor 2, IV intravenous, MTD maximum tolerated dose, NHL non-Hodgkin lymphoma, PK pharmacokinetic, R-CHOP rituximab, cyclophosphamide, doxorubicin, vincristine, prednisone, t1/2 half-life
2. Gemtuzumab Ozogamicin
Gemtuzumab ozogamicin (Mylotarg®) received accelerated approval from the FDA in 2000 for the treatment of AML. Gemtuzumab ozogamicin is a humanized anti-CD33 monoclonal antibody covalently linked to the antitumor antibiotic N-acetyl-γ-calicheamicin by a bifunctional linker, 4-(4-acetylphenoxy)butanoic acid. It is indicated for the treatment of patients with CD33+ AML in the first relapse who are 60 years of age or older and who are not candidates for other cytotoxic chemotherapeutic interventions. Gemtuzumab ozogamicin binds to the CD33 antigen expressed on the surface of leukemic blasts, normal myeloid cells, and leukemic clonogenic precursors [33]. The fact that CD33 is not expressed on pluripotent hematopoietic stem cells is a big advantage as it allows for gemtuzumab ozogamicin-induced myelosuppression reversal [34]. The binding of gemtuzumab ozogamicin to CD33 results in the formation of a complex that is internalized followed by the release of the anti-tumor antibiotic inside the lysosome of the cell. The antibiotic binds to DNA, which leads to DNA double-strand breaks and cell death [33].
In accordance with the FDA Accelerated Approval Program, a randomized phase III control trial (SWOG S0106) was initiated in 2004. This trial was terminated early because of observed fatal toxicities in the gemtuzumab ozogamicin treatment group as compared with the control group that received a standard chemotherapeutic treatment [35, 36]. In the ADC-treated group, the overall mortality rate was 5.7% (16/283 patients) as compared with1.4% (4/281 patients) in the standard-of-care therapy group. This toxicity was attributed to veno-occlusive disease, a condition in which blood flow within small blood vessels of the liver is obstructed [37]. After 10 years, per the request of the FDA, this ADC was withdrawn from the US market in 2010, as it showed no improvement in patient survival in addition to an increased risk of mortality [35]. Although this drug was absent from the US market, it remained available in Japan and was recently approved by the FDA with a modified dosing regimen [38]. Despite the limited success off gemtuzumab ozogamicin to date, valuable lessons were learned from its development process, clinical studies, and PK/pharmacodynamic (PD) data.
2.1. Clinical Studies with Gemtuzumab Ozogamicin
In 2001, Dowell et al. examined the pharmacokinetics of gemtuzumab ozogamicin in patients with AML in their first relapse. In this study, 59 patients received a single dose (9 mg/m2, intravenous infusion) of gemtuzumab ozogamicin and plasma samples were collected at specified time points. The PK parameters of gemtuzumab ozogamicin in this population were estimated to be: maximum observed concentration (Cmax), 2.86 ± 1.35 mg/L; area under the curve (AUC), 123 ± 105 mg h/L; half-life, 72.4 ± 42.0 h; and Cl, 0.265 ± 0.229 L/h. The authors also observed elevated plasma concentrations following a second dose of the ADC and speculated that this increase may have arisen from a decrease in Cl by CD33+ blast cells resulting from decreased tumor burden [39].
2.2. Impact of Age, Ethnicity, and Sex on Gemtuzumab Ozogamicin Pharmacokinetic/Pharmacodynamics
In 2001, Korth-Bradley et al. examined the impact of age and sex of individuals on the pharmacokinetics of gemtuzumab ozogamicin. In a 58-patient sex-balanced study with a mean age of 53 ± 16 years, the authors determined that no differences in the pharmacokinetics of the antibody or drug component of gemtuzumab ozogamicin were based on age or sex(i.e., age and sex were not significant covariates) [40].
As gemtuzumab ozogamicin was initially approved for the treatment of patients over 60 years of age, there was a great interest in expanding its usage to other populations. As such, in 2004, Buckwalter et al. sought to characterize the pharmacokinetics of gemtuzumab ozogamicin in pediatrics. Twenty-nine pediatric patients with refractory or relapsed AML received dosages of 6, 7.5, and 9 mg/m2. The mean PK parameters of gemtuzumab ozogamicin were similar to those in adult populations with both populations demonstrating large inter-individual variability. The authors concluded that treatment with gemtuzumab ozogamicin could be equally efficacious in pediatric patients as in adults at a 9-mg/m2 dose [41].
In 2009, Kobayashi et al. sought to examine the pharmacokinetics of gemtuzumab ozogamicin in patients with relapsed or refractory CD33+ AML. The dose-limiting toxicities associated with gemtuzumab ozogamicin were determined in a phase I study. Consistent with previous reports, the major toxicities associated with this ADC were hepatotoxicities and the optimum dose was determined to be 9 mg/m2. In a phase II study, five patients achieved complete remission and another achieved remission without platelet recovery. Interestingly, the authors reported that the Japanese patients included in the study remained in remission longer than non-Japanese patients from previous studies [42].
2.3. Lessons Learned from Gemtuzumab Ozogamicin
Although the commercial life of gemtuzumab ozogamicin was limited to only 10 years (2000–2010), it provided very useful information regarding this developing class of therapeutics. Importantly, it demonstrated the need for controlled clinical trials to confirm the benefits of ADC therapy over traditional therapy as well as post-marketing surveillance to monitor toxicity. It is noteworthy that this ADC remained available for 10 years while providing minimal clinical benefit over conventional chemotherapy and causing untoward hepatotoxicity. This liver toxicity may have arisen from the ADC binding to healthy sinusoidal cells in the liver expressing CD33 on their surface, demonstrating the need to better understand the target expression, an issue that was addressed while developing the ADCs that followed [43].
Another important lesson learned from the development and clinical lifetime of gemtuzumab ozogamicin was the investigation of fractionated doses of the ADC allowing for the safe administration of greater cumulative doses. This dosing strategy was investigated in the Acute Leukemia French Association trial ALFA-0701 in which patients received 3 mg/m2 of gemtuzumab ozogamicin on days 1, 4, and 7 in conjunction with standard front-line chemotherapy, which yielded significantly improved patient outcomes [44, 45]. These efforts indicate the importance of investigating various dosing regimens of ADCs early in the clinical development process. In fact, gemtuzumab ozogamicin was recently re-approved using an altered dosing regimen.
3. Brentuximab Vedotin
Brentuximab vedotin (Adcetris®) was approved for the treatment of CD30+ Hodgkin lymphoma and systemic anaplastic large cell lymphoma. Brentuximab vedotin is composed of brentuximab, a chimeric monoclonal antibody that targets CD30 conjugated to the antimitotic chemotherapeutic agent, MMAE, via a cathepsin cleavable linker (valine–citrulline). Currently, several phase III trials involving brentuximab vedotin are underway assessing its utility in patients with lymphoma compared with other biological therapies and combination chemotherapies.
3.1. Early Clinical Studies with Brentuximab Vedotin
In 2011, a dose-escalation study was conducted by Fanale et al. to examine the safety, maximum tolerated dose (MTD), and activity of brentuximab vedotin dosed weekly in patients with relapsed or refractory CD30+ hematologic malignancies. Brentuximab vedotin was given intravenously on days 1, 8, and 15 of each 28-day cycle at doses that ranged from 0.4 to 1.4 mg/kg. Doses were increased by 0.2 mg/kg weekly until dose-limiting toxicity was observed. The results of this study indicated that the MTD of brentuximab vedotin was 1.2 mg/kg administered weekly with the most common side effects presenting as peripheral sensory neuropathy, fatigue, nausea, diarrhea, arthralgia, and pyrexia. Tumor regression was achieved in 85% of patients and the overall objective response rate was 59% with 34% of patients achieving complete remission. This trial demonstrated that weekly administration of brentuximab vedotin results in tumor regression and lengthy remissions in patients with CD30+ malignancies with manageable toxicities [46].
3.2. Pharmacokinetic/Pharmacodynamic Modeling of Brentuximab Vedotin
Shah et al. sought to develop a PK/PD model of ADCs using brentuximab vedotin as an example to better understand and predict pre-clinical to clinical translation of ADC efficacy [47, 48]. The authors used data from various reports to develop and validate the model based on in vitro and in vivo PK data for ADC and unconjugated drugs [49–51], drug concentrations in tumors [52–54], preclinical tumor growth inhibition data [50, 55], ADC and drug pharmacokinetics in patients [56], and prediction of clinical responses using the developed PK/PD model [46, 47, 56, 57]. The authors were able to successfully predict xenograft tumor and plasma drug concentrations, and predicted complete response and progression-free survival rates that closely matched results from clinical studies [47]. The success of this model may be owed to notable submodels included within the overall PK/PD model. These submodels included an in vivo intracellular ADC kinetic model, which allowed for extrapolation to humans using species-specific target densities as well as a tumor disposition model based on drug molecular weight and tumor size, which provided a valuable platform to extrapolate this model to other drugs [47, 58].
In 2014, Chen et al. developed the first physiologically based pharmacokinetic model to predict potential enzyme-mediated drug–drug interactions (DDIs) resulting from MMAE release from an ADC complex, acknowledging this important aspect of developing ADCs as therapeutics. In this study, a minimal physiologically based pharmacokinetic model was developed to link antibody-conjugated MMAE to unconjugated MMAE using valine-citrulline-MMAE ADCs and validated using clinical PK data from brentuximab vedotin. This model sought to overcome the challenge of determining the pharmacokinetics of unconjugated MMAE formed via cleavage of the linker of ADCs, as the mechanisms and kinetics of this process are yet to be fully characterized [27, 59]. The constructed model by Chen et al. used both ‘bottom-up’ and ‘top-down’ approaches combining existing preclinical and clinical data in addition to a physiologically-based pharmacokinetics (PBPK) distribution model. The authors estimated total Cl of MMAE to be 8 L/h scaled from a metabolic Cl of ~ 4 L/h using in vitro in vivo extrapolation. Using this model, the authors were able to successfully demonstrate that these MMAE conjugates have a limited risk of enzyme-mediated DDIs [60].
In 2016, Flerlage et al. examined the pharmacokinetics and safety of brentuximab vedotin dosed weekly in pediatric patients with Hodgkin lymphoma. The observed AUC and maximum observed concentration were lower in pediatric patients than previously reported in adult studies by 25 and 11%, respectively while other factors, including toxicity, remained consistent. The authors concluded that patient body weight was a significant covariate explaining the intersubject variability in Cl of brentuximab vedotin in pediatric patients and that weekly dosing of brentuximab vedotin is safe in these patients [61].
Further, in 2016, Zhao et al. investigated the impact of renal and hepatic impairment on exposure to brentuximab vedotin. Exposure to MMAE was increased by 2.3-fold in patients with severe hepatic impairment and 1.9-fold in those with severe renal impairment. Furthermore, exposure to the intact ADC decreased in both of these patient groups. The authors proposed that poor outcomes and adverse events following treatment with brentuximab vedotin were attributable to poor baseline attributes resulting from co-morbid conditions [62].
3.3. Lessons Learned from Brentuximab Vedotin
The results from these clinical studies have indicated that weekly administration of brentuximab vedotin at a relatively low dose leads to tumor regression and manageable toxicities, indicating the benefit of optimizing dose and frequency as mentioned above regarding gemtuzumab ozogamicin. This is the first example of a minimal PBPK model used to predict potential DDIs resulting from drug release from an ADC. This model demonstrated that there is little risk of enzyme-mediated DDIs for this ADC. Several groups reported that impaired hepatic and renal function can significantly impact exposure to both the intact ADC and the released drug. While the authors of these studies did not report additional adverse events in patients with altered renal and hepatic function, increased exposure to the drug could lead to unwanted toxicity. Further development of predictive models of the disposition of ADCs in patients with co-morbid conditions is warranted as it may influence patient treatment.
4. Trastuzumab Emtansine
Trastuzumab emtansine (Kadcyla®) was approved for the treatment of HER2+ breast cancers in 2009. It consists of a humanized HER2-targeted monoclonal antibody, trastuzumab, linked to a cytotoxic agent, DM1, a maytansinoid conjugate, via a stable thioether linker, MCC. Trastuzumab emtansine exhibits favorable pharmacokinetics and minimal to no systemic accumulation of the antibody or drug following multiple doses administered once every 3 weeks [63, 64]. These PK findings have been used to develop mechanistic models of antibody–maytansinoid conjugates to predict the disposition of the conjugated entity as well as the catabolites, and the resulting antitumor activity.
4.1. Preclinical Characterization and Modeling of Trastuzumab Emtansine
Several groups have been interested in the mechanisms of internalization of trastuzumab maytansinoid conjugates and their translocation in cells. In 2015, Hamblett et al. established solute carrier family 46 member A3 (SLC46A3) as a direct transporter of maytansine-based catabolites from the lysosome to the cytoplasm of cells; silencing the expression of SLC46A3 resulted in increased concentrations of the catabolites within the lysosomes [65].
Moving beyond in vitro cell-based assays, many groups have investigated the pharmacokinetics and PD of trastuzumab emtansine in mouse models. In 2010, Jumbe et al. developed a PK/PD model of trastuzumab emtansine in mice [66]. The pharmacokinetics of trastuzumab emtansine fits a two-compartment model based on data obtained from single- and multiple-dose studies, as well as time-dose-fractionation studies in animal models and HER2-expressing cells. Subsequently, a population-based PK/PD model was developed to examine the antineoplastic activity of trastuzumab emtansine. Specifically, the authors were able to develop a cell-cycle-phase, non-specific, tumor cell kill model, which included transit compartments of the ADC and offered an accurate representation of the tumor growth inhibition achieved by trastuzumab emtansine [66].
Beyond this initial PK investigation in mice, Cilliers et al. sought to examine the tissue and cellular distribution of trastuzumab emtansine and thus developed a multi-scale PBPK model coupling the systemic and organ-level distributions of the drug with the tissue-level detail of a tumor penetration model. Using this model, the authors were able to examine the impact of the drug-antibody ratio on tumor penetration, the net result of drug deconjugation, and the impact of using an unconjugated antibody to assist the ADC in further penetrating the tumor tissue. Overall, this model, which is based on in vivo mouse tumor xenograft studies, offers quantitative mechanistic support to experimental studies working to elucidate the complex mechanisms of action of these drug conjugate therapies [67].
4.2. Clinical Studies of Trastuzumab Emtansine
Many phase I studies with trastuzumab emtansine have been conducted. In 2010, a clinical study enrolled 24 patients who had received, on average, four prior chemotherapeutic treatments. They received increasing doses of trastuzumab emtansine from 0.3 to 4.8 mg/kg on an every-3-weeks treatment schedule. The MTD of trastuzumab emtansine was determined to be 4.8 mg/kg as a result of transient thrombocytopenia. The half-life of this ADC was estimated to be 3.5 days with peak DM1 levels below 10 ng/mL. The Cl of the drug was found to be greater at lower doses (less than 1.2 mg/kg), perhaps owing to saturation of the HER2-binding sites at increased doses [68]. Similar dosage-based variability in trastuzumab Cl estimates has been previously reported [69].
In early 2012, Girish et al. characterized the pharmacokinetics of trastuzumab emtansine in patients with HER2+ metastatic breast cancer by assessing the data from four studies in which patients received trastuzumab emtansine as a single agent every 3 weeks at a 3.6-mg/kg dose. In this report, the PK parameters for the conjugated ADC trastuzumab emtansine, the drug alone, and the antibody alone, remained consistent across all studies. Trastuzumab emtansine pharmacokinetics was not altered by residual trastuzumab in circulation from prior therapy or by the circulating extracellular domain of HER2. The authors concluded that the pharmacokinetics of single-agent trastuzumab emtansine (3.6 mg/kg dosed once every 3 weeks) is well characterized and that the exposure to trastuzumab emtansine is not altered by liver or kidney function (e.g., aspartate aminotransferase, alanine amino-transferase, total bilirubin, and albumin) and does not correlate with adverse events including thrombocytopenia or increased levels of transaminases [64].
Later, in 2012, a clinical study investigating weekly dosing of trastuzumab emtansine in patients with advanced HER2+ breast cancer was conducted by Beeram et al. The aim of this multi-center, open-label, dose-escalation study was to examine the safety, tolerability, and pharmacokinetics of trastuzumab emtansine administered weekly in patients with breast cancer. In this trial, 28 patients received weekly doses of trastuzumab emtansine. The treatment was well tolerated and the MTD was determined to be 2.4 mg/kg administered weekly and the exposure was dose proportional. This is in contrast with other clinical studies reporting an MTD of 3.6 mg/kg administered once every 3 weeks [68, 70–72]. In 13 patients, partial tumor growth inhibitory responses were reported with a median tumor inhibition duration of 18.6 months. A weekly dose of trastuzumab emtansine at 2.4 mg/kg had effective anti-tumor activity and was well tolerated in patients with HER2+ breast cancers [71].
More recently, Yamamoto et al. examined the pharmacokinetics, toxicity, and MTD of trastuzumab emtansine in Japanese patients. Patients with HER2+ metastatic breast cancer received trastuzumab emtansine intravenously at doses of 1.8, 2.4, or 3.6 mg/kg every 3 weeks for a median of seven cycles. The dose-limiting toxicity was reported as a grade-3 elevation of aspartate aminotransferase/alanine aminotransferase at the 2.4-mg/kg dose. Trastuzumab emtansine administration of up to 3.6 mg/kg was generally well tolerated by Japanese patients with breast cancer with toxicities that tended to be more severe than was previously reported [68, 70, 71].
Moving beyond these phase I trials, several phase II investigations have been performed to further examine the safety and efficacy of trastuzumab emtansine in patients with HER2+ breast cancer. In 2011, Burris et al. conducted a phase II study in this patient population with individuals who had already received some form of HER2-directed therapy but had subsequent tumor progression. In this study, 112 patients received 3.6 mg/kg of trastuzumab emtansine once every 3 weeks. At this dose, the ADC showed strong anticancer activity when administered as a single agent and was well tolerated at the recommended dose [72].
In a phase III study (MARIANNE), patients with advanced HER2+ breast cancer with no previous therapy for advanced disease received trastuzumab plus taxane, trastuzumab emtansine plus placebo, or trastuzumab emtansine plus pertuzumab at standard doses. The primary endpoint of the study was progression-free survival of patients. In this study, none of the groups receiving trastuzumab emtansine showed superior progression-free survival compared with trastuzumab plus taxane, though fewer patients discontinued treatment because of adverse events in the trastuzumab emtansine arms. Overall, it was concluded that trastuzumab showed noninferior, but not superior, efficacy and better tolerability than taxane plus trastuzumab for the first-line treatment of advanced HER2+ breast cancer [73].
4.3. Pharmacokinetic/Pharmacodynamic Modeling of Trastuzumab Emtansine
Li et al. compiled the results of eight clinical studies to assess the ethnic sensitivity of trastuzumab emtansine to assess whether the clinically recommended dose (3.6 mg/kg) is sufficient and appropriate across ethnicities. The authors used four approaches to analyze the data including: non-compartmental analysis, population-PK analysis, comparative pharmacokinetics of trastuzumab emtansine in Japanese patients compared with the global population, and exposure-response analyses to examine the impact of ethnicity on pharmacokinetics. The non-compartmental analysis parameters reported were consistent across different ethnic groups; the reported AUCs were 475, 442, and 518 day lg/mL for white individuals (n = 461), Asian individuals (n = 68), and others (n = 57), respectively. The population-PK analysis of these three groups indicated that ethnicity was not a significant covariate that can affect the pharmacokinetics of trastuzumab emtansine. The exposure-response analyses indicated that ethnicity played no role in efficacy or hepatotoxicity risk, but individuals from Asian populations demonstrated a trend toward greater thrombocytopenia risk. Most Asians exhibiting thrombocytopenia were able to continue receiving treatment following a dose adjustment consistent with the recommendations made for the global population [74].
Thrombocytopenia is a frequently noted side effect of treatment with trastuzumab emtansine in patients with breast cancer [68, 70, 71]. As such, several investigators have found it pertinent to develop PK/PD models to characterize the effects of this treatment on patient platelet counts. In 2012, Bender et al. reported a semi-mechanistic population PK/PD model with transit compartments to mimic platelet development and circulation, which was fit to platelet concentration–time course data from two trastuzumab emtansine single-agent studies [71, 72]. This model predicted that with trastuzumab emtansine administration of 3.6 mg/kg once every 3 weeks, the lowest platelet nadir would be observed following the first administered dose. It was also able to predict a subgroup of patients with variable downward-drifting platelet concentration–time profiles, predicted to stabilize by the eighth treatment cycle. However, the authors note that baseline characteristics were not significant covariates in this model [75].
Chudasama et al. described a semi-mechanistic population-PK model of multivalent trastuzumab emtansine. The authors used preclinical data of trastuzumab emtansine to develop a PK model of the intact ADC and the trastuzumab monoclonal antibody alone. In this model, a series of transit compartments with the same disposition parameters was used to represent the deconjugation process from greater to lesser drug antibody ratios. The authors postulated that this model could be used to examine inter-individual variability in ADC pharmacokinetics and that these variabilities could further be correlated to clinical outcomes [76].
In 2014, Wada et al. sought to employ PK/PD modeling to gain insight into the complex behavior and disposition of antibody–maytansinoid conjugates. To this end, the authors applied mechanistic PK/PD modeling to simulate the processes of ADC tumor uptake, catabolism, and response. Much like the models used by Shah et al. describing brentuximab vedotin, the models described by Wada et al. used a comprehensive, multi-scale, mechanism-based PK/PD approach to translate the ADC PK/PD data from pre-clinical to clinical, which may provide a better understanding of ADC disposition and improved ADC design [47, 58].
In their studies, Wada and colleagues postulated that tumor catabolite concentrations would more closely correlate to efficacy than to other measured concentrations such as plasma ADC concentration and tumor total maytansinoid concentration. As such, the driver of tumor response in their developed model was the catabolite concentration at the tumor site. Using their model, they were able to demonstrate that for trastuzumab emtansine, the catabolite concentrations achieved in tumor cells were highly sensitive to catabolite efflux rate, but less sensitive to the rate of catabolism, which takes place outside of tumor cells. Further, they were able to demonstrate that changing the catabolism rate of the ADC, for example, by changing a more or less stable linker, may have a lesser impact on efficacy than altering the ability of the ADC to exit the tumor [58].
In 2014, Lu et al. described the population pharmacokinetics of trastuzumab emtansine with a linear two-compartment model with first-order elimination from the central compartment. The Cl of trastuzumab emtansine was0.7 L/day, the volume of distribution was 3.1 L, and the terminal half-life was 3.9 days. The authors examined the impact of age, race, geographic region, and renal function and concluded that these covariates are not significant in describing the pharmacokinetics of trastuzumab emtansine [63]. Further, they state that refinements in dose based on baseline covariates other than body weight would not explain the inter-individual variability in trastuzumab emtansine pharmacokinetics in this population [63].
In a 2016 publication, Singh et al. described a cellular disposition model that built upon previously published models by including greater intracellular detail including ADC degradation and passive diffusion of an unconju-gated drug across tumor cells. Further, different biological and chemotherapy measures for trastuzumab emtansine were incorporated into the model to characterize the pharmacokinetics of this ADC in vitro in three HER2+ cell lines. Upon combining this cellular disposition model with the tumor disposition model, the authors were able to a priori predict tumor DM1 concentrations in xenograft mice. Their analysis indicated that non-specific deconjugation of the drug and its passive diffusion across the tumor cell membrane were key parameters for cellular drug exposure [77].
In 2017, these authors validated this modeling and simulation-based strategy for ADC disposition using trastuzumab emtansine as a case study. Using their model, they developed a PK/PD model able to characterize in vivo efficacy of trastuzumab emtansine in preclinical tumor models. Parameter estimates for trastuzumab emtansine were taken from preclinical data while the human pharmacokinetics of the ADC was predicted a priori using allometric scaling from PK parameters in monkeys. The predicted human pharmacokinetics, estimated efficacy data from preclinical results, and clinically observed breast tumor volume and growth parameters were combined to develop the full PK/PD model for trastuzumab emtansine. The authors state that this model suggested that a fractionated dosing regimen may provide improved efficacy with trastuzumab emtansine. It was concluded that this modeling and simulation strategy for ADC pharmacokinetics/pharmacodynamics was capable of predicting the clinical efficacy of ADCs a priori and the authors were able to retrospectively validate this strategy for all clinically approved ADCs [78].
4.4. Lessons Learned from Trastuzumab Emtansine
In contrast to previous attempts to correlate preclinical PK/PD data to clinical application, the predictive model from Singh et al. represents the first generalized PK/PD modeling and simulation-based strategy for the bench-to-bedside translation of ADCs and is an exciting new tool in ADC development. Using preclinical efficacy data, predicted PK data, and estimated PK parameters from monkeys, the authors were able to accurately evaluate the efficacy of various dosing regimens with trastuzumab emtansine and also apply this strategy to the other clinically approved ADCs. This model provides a key mechanism for evaluating ADCs that will be useful both in the development process as well as clinical study design.
5. Inotuzumab Ozogamicin
Inotuzumab ozogamicin is an ADC recently approved in the UK and USA for the treatment of relapsed or refractory B-cell precursor acute lymphocytic leukemia (ALL). This conjugate is made up of inotuzumab, a humanized anti-CD22 monoclonal antibody linked to an anticancer agent from the calicheamicin class, N-acetyl-γ-calicheamicin via an acid-labile 4-(4-acetylphenoxy)butanoic acid linker. [79]. This ADC has been the subject of many clinical trials including two phase II trials for the treatment of NHL. A recent phase III study concluded that treatment with single-agent inotuzumab was associated with significantly increased remission rates than standard chemotherapy approaches in adults with relapsed or refractory B-cell ALL [80].
5.1. Preclinical Characterization of Inotuzumab Ozogamicin
In 2007, Dijoseph et al. employed an ALL xenograft tumor study to investigate the anti-tumor activity of inotuzumab ozogamicin in mice. Administration of inotuzumab ozogamicin resulted in the dose-dependent inhibition of tumor growth of the xenografted leukemia cells, producing complete tumor regression at the greatest administered dose (160 μg/kg). At the conclusion of the study, significantly fewer ALL cells were isolated from the bone marrow of mice treated with inotuzumab ozogamicin compared with the placebo group. These results provided a solid foundation for the treatment of CD22+ leukemias with inotuzumab ozogamicin [81].
5.2. Clinical Studies with Inotuzumab Ozogamicin
In 2006, Fayad et al. reported a dose-escalation study performed across 14 European and US sites to determine the MTD of inotuzumab ozogamicin in patients with CD22+ B-cell NHL. In this study, the MTD of inotuzuomab ozogamicin was observed to be 1.8 mg/m2 administered once every 4 weeks. This dosing scheme was carried forward in a further clinical study examining the safety of inotuzumab ozogamicin in this patient population, which concluded that the toxicity was clinically manageable with the most prominent side effect being thrombocytopenia [82].
This study was advanced further by this group in 2008 by exploring the combination of inotuzumab ozogamicin with rituximab. In this study, a fixed dose of rituximab (375 mg/m2) was administered on day 1 followed by inotuzumab ozogamicin (0.8–1.8 mg/m2) on day 2 of each 28-day cycle for a maximum of eight cycles. Anti-tumor responses were seen in all patients in the study and the safety profile of this combination closely resembled that of inotuzumab ozogamicin monotherapy [83].
Another early study characterizing the safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin was performed in 2010 by Advani et al. In this clinical study on patients with relapsed or refractory CD22+ B-cell NHL, the authors sought to determine the MTD, safety, and efficacy of inotuzumab ozogamicin. Patients were administered inotuzumab ozogamicin intravenously as a single agent at a dose ranging from 0.4 to 2.4 mg/m2 once every 3 or 4 weeks. The MTD was determined to be 1.8 mg/m2. Frequently reported adverse events at this dose included thrombocytopenia (90%), asthenia (67%), nausea (51%), and neutropenia (51%). The objective response rate among patients at the cessation of treatment was 39% for all 79 enrolled patients, 68% for patients with follicular NHL treated with the MTD, and 15% for all patients with diffuse large B-cell lymphoma (DLBCL) treated with the MTD. In the enrolled patients, the median progression-free survival was 317 days for patients with follicular NHL and 49 days for patients with DLBCL. Inotuzumab ozogamicin exhibited potent antitumor activity against CD22+ B-cell lymphoma with reversible thrombocytopenia as the most frequently observed toxicity [84].
In 2013, Kantarjian et al. reported a clinical study in which patients with refractory/relapsed ALL received either single-dose inotuzumab ozogamicin intravenously at increasing doses from 1.3 to 1.8 mg/m2 every 3–4 weeks, or a lower 0.8-mg/m2 dose on day 1, followed by 0.5 mg/m2 on days 8 and 15, repeating every 3–4 weeks [85]. Response rates between these two treatment groups were similar (57 and 59%, respectively), while the median survival for the first group was reported to be 5 months, vs. 7.1 months in the latter group. Side effects, including reversible bilirubin elevation, fever, and hypotension, were observed less frequently in the weekly treated group as well. The authors concluded that inotuzumab ozogamicin single-agent treatment was highly active and safe in patients with refractory-relapsed ALL and that frequent administration at lower doses appeared to be equally effective and less toxic than elevated single-dose therapy [85].
In a clinical study in 2010, Ogura et al. examined the safety, efficacy, tolerability, and pharmacokinetics of inotuzumab ozogamicin in Japanese patients with relapsed or refractory CD22+ B-cell-derived NHL. All 13 patients included in this trial had follicular lymphoma, were previously treated with rituximab alone or a rituximab-containing chemotherapy-regimen (e.g., R-CHOP), and were enrolled into two dose cohorts (1.3 mg/m2, three patients; 1.8 mg/m2, ten patients). None of the 13 patients had dose-limiting toxicities, and the previously reported MTD of 1.8 mg/m2 in Japanese patients was confirmed. Adverse events reported in this trial included thrombocytopenia (100%), leukopenia (92%), lymphopenia (85%), neutropenia (85%), elevated aspartate aminotransferase (85%), anorexia (85%), and nausea (77%). There were several reported cases of grade 3/4 adverse events in these patients as well, including thrombocytopenia (54%), lymphopenia (31%), neutropenia (31%), and leukopenia (15%). The AUC and maximum observed concentration estimates of inotuzumab ozogamicin increased linearly in a dose-dependent manner. Moreover, the PK parameters estimates in Japanese patients were comparable to those in non-Japanese patients. Within this particular study, seven patients achieved complete response (54%), four patients had partial response (31%), and two patients had stable disease (15%), yielding an overall response rate of 85%. This ADC was well tolerated in these patients at doses up to the MTD of 1.8 mg/m2 and showed efficacy in relapsed or refractory follicular lymphomas following treatment with a rituximab-containing regimen [86].
In 2012, this group investigated the combination of inotuzumab ozogamicin and rituximab in patients with relapsed or refractory B-cell NHL. This clinical study examined the tolerability, efficacy, safety, and pharmacokinetics of intravenously administered inotuzumab ozogamicin alongside rituximab in Japanese patients. Ten patients received a 375-mg/m2 dose of rituximab followed by inotuzumab ozogamicin administered at the previously determined MTD of 1.8 mg/m2. These doses were repeated every 28 days for up to eight cycles or until disease progression or intolerable toxicity. The safety profile of this combination was similar to that of singly administered inotuzumab ozogamicin; the most common grade 3 or greater adverse events were thrombocytopenia (70%), neutropenia (50%), leukopenia (30%), and lymphopenia (30%). The reported overall response rate with this combination was 80% (eight of ten patients). Exposure to the conjugated drug increased with successive doses, similar to the observed PK profiles observed in preliminary studies with inotuzumab ozogamicin monotherapy [86, 87].
In 2013, Fayad et al. performed a clinical trial of inotuzumab ozogamicin plus rituximab (R-INO) for the treatment of CD20/CD22+ B-cell NHL. The first phase of this study was a dose-escalation phase to determine the MTD of the combination with inotuzumab ozogamicin doses ranging from 0.8 to 1.8 mg/m2 combined with a fixed 375-mg/m2 dose of rituximab. In phase II, an expanded cohort of patients was treated to further examine the safety and efficacy of R-INO at the previously determined MTD. Patients with relapsed follicular lymphoma, relapsed DLBCL, or refractory aggressive NHL received R-INO at the MTD every 4 weeks for up to eight cycles. Between the two phases of this study, 118 patients received at least one cycle of R-INO (median, four cycles). Similarly, the most commonly reported grade 3/4 adverse events were thrombocytopenia (31%) and neutropenia (22%). Other common toxicities resulting from this combination included hyperbilirubinemia (25%) and increased aspartate aminotransferase (36%). As reported previously by Ogura et al. in Japanese patients, the MTD of inotuzumab ozogamicin with co-administered rituximab at 375 mg/m2 was consistent with the MTD of single-agent inotuzumab ozogamicin at 1.8 mg/m2 [86, 87]. Treatment at this dose achieved overall response rates of 87, 74, and 20% for follicular lymphoma, DLBCL, and NHL, respectively. The 2-year progression-free survival rates in these patient groups were 68% for follicular lymphoma and 42% for DLBCL, indicating that R-INO had strong response rates and long-term progression-free survival in these patients with a manageable toxicity profile [88].
5.3. Pharmacokinetic/Pharmacodynamic Modeling of Inotuzumab Ozogamicin
In 2016, Betts et al. performed a retrospective analysis of inotuzumab ozogamicin to develop a model correlating preclinical with clinical PK/PD data. The authors integrated preclinical data into a mechanistic PK/PD model, which included a plasma PK model describing the disposition and Cl of inotuzumab ozogamicin and its released drug (N-acetyl-γ-calicheamicin), a tumor disposition model describing diffusion of the ADC into the extracellular environment of target tumors, a cellular model describing binding of the ADC to CD22 and its subsequent internalization, release of the drug, and drug binding to DNA and/or efflux from the cell, and tumor growth and inhibition in mouse xenograft models. The authors were able to correlate preclinical data with applicable clinical situations by incorporating human PK data for inotuzumab ozogamicin and clinically relevant tumor volumes, growth rates, and values for CD22 expression in patient populations. The authors were able to predict progression-free survival rates for treatment with inotuzumab ozogamicin in patients with B-cell malignancies, which were comparable to those observed in the clinic. Moreover, they demonstrate that a fractionated dosing regimen was more effective in patients being treated for ALL but not in those receiving treatment for NHL. Furthermore, simulations using this model indicated that the growth of tumors is a highly sensitive parameter and correlates well with predictive outcomes of treatment [89].
5.4. Lessons Learned from Inotuzumab Ozogamicin
The story of inotuzumab ozogamicin stresses the need to pay particular attention to the dose and dose frequency of ADCs. In the clinic, patients who received lower doses more frequently achieved comparable tumor regression while reporting fewer untoward effects of their treatment. Through modeling, one group has been able to correlate preclinical efficacy data with clinical PK/PD data to effectively predict progression-free survival, and to demonstrate that a fractionated dosing regimen is advantageous.
6. Conclusions
The development and approval of ADCs have changed the landscape of targeted therapy, in particular, cancer therapy. Indeed, there is a staggering number of ADC development programs supported by industry, academia, and regulatory agencies and certainly new ADCs are expected to be introduced as new therapeutic modalities in the next few years. Pharmacokinetic/pharmacodynamic models are indispensable for successful and efficient development programs of ADCs. However, PK/PD modeling of these conjugates represents a unique challenge because of the myriad of dynamic and complex processes that follow their administration. These processes include disposition and Cl of the ADC and the deconjugated moieties, site-specific binding, and small-molecule translocation inside cancer cells. All these processes must be taken into account for the better development of mechanistic PK/PD models.
In this article, we discussed several cases where advanced unique mechanistic models of ADCs and their constituents were reported. Many of these models employed state-of-the art quantitative pharmacological approaches and were able to combine the disposition of the ADC, antibody, and drug while factoring in biological parameters such as tumor volume as well as drug tumor and plasma concentration data for predicting exposure and efficacy. Improved models combined with improved therapeutic and post-marketing surveillance can prevent ineffective or toxic agents from entering or remaining in the market as was seen with gemtuzumab ozogamicin. These approaches improved our understanding of ADCs and their utilization in targeted therapy, and undoubtedly they will aid in the discovery and development of Paul Ehrlich’s famed “magic bullet” chemotherapy.
Key Points.
Pharmacokinetic/pharmacodynamic models are essential for the successful and efficient development of antibody–drug conjugates.
Unique mechanistic models of antibody–drug conjugates and their constituents have been developed and used to predict drug release, exposure, and efficacy following administration.
Improved pharmacokinetic/pharmacodynamic models combined with therapeutic and post-marketing surveillance can prevent toxic or ineffective antibody–drug conjugates from entering or remaining on the market.
Acknowledgments
Funding
This work was supported in part by intramural funding awarded to Hazem E. Hassan. William D. Hedrich is supported by a pre-doctoral fellowship from the PhRMA Foundation as well as a training grant (T32 GM066706) from the National Institutes of Health.
Footnotes
Conflict of interest
William D. Hedrich, Tamer E. Fandy, HossamM. Ashour, Hongbing Wang, and Hazem E. Hassan have no conflicts of interest directly relevant to the content of this article.
References
- 1.Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, et al. The global burden of cancer 2013. JAMA Oncol. 2015;1(4):505–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeVita VT Jr, Chu E. A history of cancer chemotherapy. Cancer Res. 2008;68(21):8643–53. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz RS. Paul Ehrlich’s magic bullets. N Engl J Med. 2004;350(11):1079–80. [DOI] [PubMed] [Google Scholar]
- 4.DeVita VT Jr. The evolution of therapeutic research in cancer. N Engl J Med. 1978;298(16):907–10. [DOI] [PubMed] [Google Scholar]
- 5.Plenderleith IH. Treating the treatment: toxicity of cancer chemotherapy. Can Fam Physician. 1990;36:1827–30. [PMC free article] [PubMed] [Google Scholar]
- 6.Schietinger A, Philip M, Schreiber H. Specificity in cancer immunotherapy. Semin Immunol. 2008;20(5):276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256(5517):495–7. [DOI] [PubMed] [Google Scholar]
- 8.Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40(9):3147–54. [PubMed] [Google Scholar]
- 9.Ritz J, Schlossman SF. Utilization of monoclonal antibodies in the treatment of leukemia and lymphoma. Blood. 1982;59(1):1–11. [PubMed] [Google Scholar]
- 10.Berger M, Shankar V, Vafai A. Therapeutic applications of monoclonal antibodies. Am J Med Sci. 2002;324(1):14–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu JK. The history of monoclonal antibody development: progress, remaining challenges and future innovations. Ann Med Surg (Lond). 2014;3(4):113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Courtenay-Luck NS, Epenetos AA, Moore R, Larche M, Pecta-sides D, Dhokia B, et al. Development of primary and secondary immune responses to mouse monoclonal antibodies used in the diagnosis and therapy of malignant neoplasms. Cancer Res. 1986;46(12 Pt 1):6489–93. [PubMed] [Google Scholar]
- 13.Zuckier LS, Chang CJ, Scharff MD, Morrison SL. Chimeric human-mouse IgG antibodies with shuffled constant region exons demonstrate that multiple domains contribute to in vivo half-life. Cancer Res. 1998;58(17):3905–8. [PubMed] [Google Scholar]
- 14.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–81. [DOI] [PubMed] [Google Scholar]
- 15.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–88. [DOI] [PubMed] [Google Scholar]
- 16.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–72. [DOI] [PubMed] [Google Scholar]
- 17.Furst DE, Schiff MH, Fleischmann RM, Strand V, Birbara CA, Compagnone D, et al. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis). J Rheumatol. 2003;30(12):2563–71. [PubMed] [Google Scholar]
- 18.Ducry L, Stump B. Antibody–drug conjugates: linking cytotoxic payloads to monoclonal antibodies. Bioconjug Chem. 2010;21(1):5–13. [DOI] [PubMed] [Google Scholar]
- 19.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, et al. Antibody–drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–21. [DOI] [PubMed] [Google Scholar]
- 20.Kovtun YV, Goldmacher VS. Cell killing by antibody–drug conjugates. Cancer Lett. 2007;255(2):232–40. [DOI] [PubMed] [Google Scholar]
- 21.Kovtun YV, Audette CA, Mayo MF, Jones GE, Doherty H, Maloney EK, et al. Antibody–maytansinoid conjugates designed to bypass multidrug resistance. Cancer Res. 2010;70(6):2528–37. [DOI] [PubMed] [Google Scholar]
- 22.Loganzo F, Sung M, Gerber HP. Mechanisms of resistance to antibody–drug conjugates. Mol Cancer Ther. 2016;15(12):2825–34. [DOI] [PubMed] [Google Scholar]
- 23.Diamantis N, Banerji U. Antibody–drug conjugates: an emerging class of cancer treatment. Br J Cancer. 2016;114(4):362–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FDA news release. FDA approves new treatment for adults with relapsed or refractory acute lymphoblastic leukemia. 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm572131.htm. Accessed 30 Aug 2017.
- 25.Lobo ED, Hansen RJ, Balthasar JP. Antibody pharmacokinetics and pharmacodynamics. J Pharm Sci. 2004;93(11):2645–68. [DOI] [PubMed] [Google Scholar]
- 26.Lin K, Tibbitts J, Shen BQ. Pharmacokinetics and ADME characterizations of antibody–drug conjugates. Methods Mol Biol. 2013;1045:117–31. [DOI] [PubMed] [Google Scholar]
- 27.Lin K, Tibbitts J. Pharmacokinetic considerations for antibody drug conjugates. Pharm Res. 2012;29(9):2354–66. [DOI] [PubMed] [Google Scholar]
- 28.Bornstein GG. Antibody drug conjugates: preclinical considerations. AAPS J. 2015;17(3):525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamath AV, Iyer S. Preclinical pharmacokinetic considerations for the development of antibody drug conjugates. Pharm Res. 2015;32(11):3470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donaghy H Effects of antibody, drug and linker on the preclinical and clinical toxicities of antibody–drug conjugates. MAbs. 2016;8(4):659–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baxley AA, Kumm DE, Bishop CB, Medina PJ, Holter-Chakrabarty J. Severe infusion reactions to brentuximab vedotin in two patients with Hodgkin lymphoma previously treated with allogeneic stem cell transplantation. J Oncol Pharm Pract. 2013;19(3):279–83. [DOI] [PubMed] [Google Scholar]
- 32.Hanbali A, Wollner I, Neme K, Ulreich C. Fatal hypersensitivity reaction to gemtuzumab ozogamicin associated with platelet transfusion. Am J Health Syst Pharm. 2007;64(13):1401–2. [DOI] [PubMed] [Google Scholar]
- 33.Bross PF, Beitz J, Chen G, Chen XH, Duffy E, Kieffer L, et al. Approval summary: gemtuzumab ozogamicin in relapsed acute myeloid leukemia. Clin Cancer Res. 2001;7(6):1490–6. [PubMed] [Google Scholar]
- 34.Walter RB, Appelbaum FR, Estey EH, Bernstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119(26):6198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121(24):4854–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petersdorf S, Kopecky K, Stuart RK, Larson RA, Nevill TJ, Stenke L, et al. Preliminary results of Southwest Oncology Group Study S0106: an international intergroup phase 3 randomized trial comparing the addition of gemtuzumab ozogamicin to standard induction therapy versus standard induction therapy followed by a second randomization to post-consolidation gemtuzumab ozogamicin versus no additional therapy for previously untreated acute myeloid leukemia. Blood. 2009;114(22):790. [Google Scholar]
- 37.Tack DK, Letendre L, Kamath PS, Tefferi A. Development of hepatic veno-occlusive disease after Mylotarg infusion for relapsed acute myeloid leukemia. Bone Marrow Transplant. 2001;28(9):895–7. [DOI] [PubMed] [Google Scholar]
- 38.Kell J The addition of gemtuzumab ozogamicin to chemotherapy in adult patients with acute myeloid leukemia. Expert Rev Anticancer Ther. 2016;16(4):377–82. [DOI] [PubMed] [Google Scholar]
- 39.Dowell JA, Korth-Bradley J, Liu H, King SP, Berger MS. Pharmacokinetics of gemtuzumab ozogamicin, an antibody-targeted chemotherapy agent for the treatment of patients with acute myeloid leukemia in first relapse. J Clin Pharmacol. 2001;41(11):1206–14. [DOI] [PubMed] [Google Scholar]
- 40.Korth-Bradley JM, Dowell JA, King SP, Liu H, Berger MS, Mylotarg Study Group. Impact of age and gender on the pharmacokinetics of gemtuzumab ozogamicin. Pharmacotherapy. 2001;21(10):1175–80. [DOI] [PubMed] [Google Scholar]
- 41.Buckwalter M, Dowell JA, Korth-Bradley J, Gorovits B, Mayer PR. Pharmacokinetics of gemtuzumab ozogamicin as a single-agent treatment of pediatric patients with refractory or relapsed acute myeloid leukemia. J Clin Pharmacol. 2004;44(8):873–80. [DOI] [PubMed] [Google Scholar]
- 42.Kobayashi Y, Tobinai K, Takeshita A, Naito K, Asai O, Dobashi N, et al. Phase I/II study of humanized anti-CD33 antibody conjugated with calicheamicin, gemtuzumab ozogamicin, in relapsed or refractory acute myeloid leukemia: final results of Japanese multicenter cooperative study. Int J Hematol. 2009;89(4):460–9. [DOI] [PubMed] [Google Scholar]
- 43.Rajvanshi P, Shulman HM, Sievers EL, McDonald GB. Hepatic sinusoidal obstruction after gemtuzumab ozogamicin (Mylotarg) therapy. Blood. 2002;99(7):2310–4. [DOI] [PubMed] [Google Scholar]
- 44.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf S, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncol. 2014;15(9):986–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castaigne S, Pautas C, Terre C, Raffoux E, Bordessoule D, Bastie JN, et al. Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379(9825):1508–16. [DOI] [PubMed] [Google Scholar]
- 46.Fanale MA, Forero-Torres A, Rosenblatt JD, Advani RH, Franklin AR, Kennedy DA, et al. A phase I weekly dosing study of brentuximab vedotin in patients with relapsed/refractory CD30-positive hematologic malignancies. Clin Cancer Res. 2012;18(1):248–55. [DOI] [PubMed] [Google Scholar]
- 47.Shah DK, Haddish-Berhane N, Betts A. Bench to bedside translation of antibody drug conjugates using a multiscale mechanistic PK/PD model: a case study with brentuximab-vedotin. J Pharmacokinet Pharmacodyn. 2012;39(6):643–59. [DOI] [PubMed] [Google Scholar]
- 48.Shah DK, Betts AM. Towards a platform PBPK model to characterize the plasma and tissue disposition of monoclonal antibodies in preclinical species and human. J Pharmacokinet Pharmacodyn. 2012;39(1):67–86. [DOI] [PubMed] [Google Scholar]
- 49.Sanderson RJ, Hering MA, James SF, Sun MM, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res. 2005;11(2 Pt 1):843–52. [PubMed] [Google Scholar]
- 50.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, et al. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–70. [DOI] [PubMed] [Google Scholar]
- 51.Okeley NM, Miyamoto JB, Zhang X, Sanderson RJ, Benjamin DR, Sievers EL, et al. Intracellular activation of SGN-35, a potent anti-CD30 antibody–drug conjugate. Clin Cancer Res. 2010;16(3):888–97. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt MM, Wittrup KD. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol Cancer Ther. 2009;8(10):2861–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60(12):1421–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thurber GM, Schmidt MM, Wittrup KD. Factors determining antibody distribution in tumors. Trends Pharmacol Sci. 2008;29(2):57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francisco JA, Cerveny CG, Meyer DL, Mixan BJ, Klussman K, Chace DF, et al. cAC10-vcMMAE, an anti-CD30-monomethyl auristatin E conjugate with potent and selective antitumor activity. Blood. 2003;102(4):1458–65. [DOI] [PubMed] [Google Scholar]
- 56.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010;363(19):1812–21. [DOI] [PubMed] [Google Scholar]
- 57.Zhao Y, Kosorok MR, Zeng D. Reinforcement learning design for cancer clinical trials. Stat Med. 2009;28(26):3294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wada R, Erickson HK, Lewis Phillips GD, Provenzano CA, Leipold DD, Mai E, et al. Mechanistic pharmacokinetic/pharmacodynamic modeling of in vivo tumor uptake, catabolism, and tumor response of trastuzumab maytansinoid conjugates. Cancer Chemother Pharmacol. 2014;74(5):969–80. [DOI] [PubMed] [Google Scholar]
- 59.Lu D, Sahasranaman S, Zhang Y, Girish S. Strategies to address drug interaction potential for antibody–drug conjugates in clinical development. Bioanalysis. 2013;5(9):1115–30. [DOI] [PubMed] [Google Scholar]
- 60.Chen Y, Samineni D, Mukadam S, Wong H, Shen BQ, Lu D, et al. Physiologically based pharmacokinetic modeling as a tool to predict drug interactions for antibody–drug conjugates. Clin Pharmacokinet. 2015;54(1):81–93. [DOI] [PubMed] [Google Scholar]
- 61.Flerlage JE, Metzger ML, Wu J, Panetta JC. Pharmacokinetics, immunogenicity, and safety of weekly dosing of brentuximab vedotin in pediatric patients with Hodgkin lymphoma. Cancer Chemother Pharmacol. 2016;78(6):1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Chen R, O’Connor OA, Gopal AK, Ramchandren R, Goy A, et al. Brentuximab vedotin, an antibody–drug conjugate, in patients with CD30-positive haematologic malignancies and hepatic or renal impairment. Br J Clin Pharmacol. 2016;82(3):696–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lu D, Girish S, Gao Y, Wang B, Yi JH, Guardino E, et al. Population pharmacokinetics of trastuzumab emtansine (T-DM1), a HER2-targeted antibody–drug conjugate, in patients with HER2-positive metastatic breast cancer: clinical implications of the effect of covariates. Cancer Chemother Pharmacol. 2014;74(2):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Girish S, Gupta M, Wang B, Lu D, Krop IE, Vogel CL, et al. Clinical pharmacology of trastuzumab emtansine (T-DM1): an antibody–drug conjugate in development for the treatment of HER2-positive cancer. Cancer Chemother Pharmacol. 2012;69(5):1229–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hamblett KJ, Jacob AP, Gurgel JL, Tometsko ME, Rock BM, Patel SK, et al. SLC46A3 is required to transport catabolites of noncleavable antibody maytansine conjugates from the lysosome to the cytoplasm. Cancer Res. 2015;75(24):5329–40. [DOI] [PubMed] [Google Scholar]
- 66.Jumbe NL, Xin Y, Leipold DD, Crocker L, Dugger D, Mai E, et al. Modeling the efficacy of trastuzumab-DM1, an antibody drug conjugate, in mice. J Pharmacokinet Pharmacodyn. 2010;37(3):221–42. [DOI] [PubMed] [Google Scholar]
- 67.Cilliers C, Guo H, Liao J, Christodolu N, Thurber GM. Multiscale modeling of antibody–drug conjugates: connecting tissue and cellular distribution to whole animal pharmacokinetics and potential implications for efficacy. AAPS J. 2016;18(5):1117–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Krop IE, Beeram M, Modi S, Jones SF, Holden SN, Yu W, et al. Phase I study of trastuzumab-DM1, an HER2 antibody–drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol. 2010;28(16):2698–704. [DOI] [PubMed] [Google Scholar]
- 69.Bruno R, Washington CB, Lu JF, Lieberman G, Banken L, KleinP. Population pharmacokinetics of trastuzumab in patients with HER2+ metastatic breast cancer. Cancer Chemother Pharmacol. 2005;56(4):361–9. [DOI] [PubMed] [Google Scholar]
- 70.Yamamoto H, Ando M, Aogi K, Iwata H, Tamura K, Yonemori K, et al. Phase I and pharmacokinetic study of trastuzumab emtansine in Japanese patients with HER2-positive metastatic breast cancer. Jpn J Clin Oncol. 2015;45(1):12–8. [DOI] [PubMed] [Google Scholar]
- 71.Beeram M, Krop IE, Burris HA, Girish SR, Yu W, Lu MW, et al. A phase 1 study of weekly dosing of trastuzumab emtansine (TDM1) in patients with advanced human epidermal growth factor 2-positive breast cancer. Cancer. 2012;118(23):5733–40. [DOI] [PubMed] [Google Scholar]
- 72.Burris HA 3rd, Rugo HS, Vukelja SJ, Vogel CL, Borson RA, Limentani S, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol. 2011;29(4):398–405. [DOI] [PubMed] [Google Scholar]
- 73.Perez EA, Barrios C, Eiermann W, Toi M, Im YH, Conte P, et al. Trastuzumab emtansine with or without pertuzumab versus trastuzumab plus taxane for human epidermal growth factor receptor 2-positive, advanced breast cancer: primary results from the phase III MARIANNE Study. J Clin Oncol. 2017;35(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li C, Wang B, Lu D, Jin JY, Gao Y, Matsunaga K, et al. Ethnic sensitivity assessment of the antibody–drug conjugate trastuzumab emtansine (T-DM1) in patients with HER2-positive locally advanced or metastatic breast cancer. Cancer Chemother Pharmacol. 2016;78(3):547–58. [DOI] [PubMed] [Google Scholar]
- 75.Bender BC, Schaedeli-Stark F, Koch R, Joshi A, Chu YW, Rugo H, et al. A population pharmacokinetic/pharmacodynamic model of thrombocytopenia characterizing the effect of trastuzumab emtansine (T-DM1) on platelet counts in patients with HER2-positive metastatic breast cancer. Cancer Chemother Pharmacol. 2012;70(4):591–601. [DOI] [PubMed] [Google Scholar]
- 76.Chudasama VL, Schaedeli Stark F, Harrold JM, Tibbitts J, Girish SR, Gupta M, et al. Semi-mechanistic population pharmacokinetic model of multivalent trastuzumab emtansine in patients with metastatic breast cancer. Clin Pharmacol Ther. 2012;92(4):520–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singh AP, Maass KF, Betts AM, Wittrup KD, Kulkarni C, King LE, et al. Evolution of antibody–drug conjugate tumor disposition model to predict preclinical tumor pharmacokinetics of trastuzumab-emtansine (T-DM1). AAPS J. 2016;18(4):861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singh AP, Shah DK. Application of a PK-PD modeling and simulation-based strategy for clinical translation of antibody–drug conjugates: a case study with trastuzumab emtansine (TDM1). AAPS J. 2017;19(4):1054–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yilmaz M, Richard S, Jabbour E. The clinical potential of inotuzumab ozogamicin in relapsed and refractory acute lymphocytic leukemia. Ther Adv Hematol. 2015;6(5):253–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab ozogamicin versus standard therapy for acute lymphoblastic leukemia. N Engl J Med. 2016;375(8):740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–5. [DOI] [PubMed] [Google Scholar]
- 82.Fayad L, Patel H, Verhoef G, Czuczman M, Foran J, Gine E, et al. Clinical activity of the immunoconjugate CMC-544 in B-cell malignancies: preliminary report of the expanded maximum tolerated dose (MTD) cohort of a phase 1 study. Blood. 2006;108(11):2711. [Google Scholar]
- 83.Fayad L, Patel H, Verhoef G, Smith MR, Johnson PWM, Czuczman MS, et al. Safety and clinical activity of the anti-CD22 immunoconjugate inotuzumab ozogamicin (CMC-544) in combination with rituximab in follicular lymphoma or diffuse large B-cell lymphoma: preliminary report of a phase 1/2 study. Blood. 2008;112(11):266. [Google Scholar]
- 84.Advani A, Coiffier B, Czuczman MS, Dreyling M, Foran J, Gine E, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin’s lymphoma: results of a phase I study. J Clin Oncol. 2010;28(12):2085–93. [DOI] [PubMed] [Google Scholar]
- 85.Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M, et al. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ogura M, Tobinai K, Hatake K, Uchida T, Kasai M, Oyama T, et al. Phase I study of inotuzumab ozogamicin (CMC-544) in Japanese patients with follicular lymphoma pretreated with rituximab-based therapy. Cancer Sci. 2010;101(8):1840–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogura M, Hatake K, Ando K, Tobinai K, Tokushige K, Ono C, et al. Phase I study of anti-CD22 immunoconjugate inotuzumab ozogamicin plus rituximab in relapsed/refractory B-cell non-Hodgkin lymphoma. Cancer Sci. 2012;103(5):933–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fayad L, Offner F, Smith MR, Verhoef G, Johnson P, Kaufman JL, et al. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J Clin Oncol. 2013;31(5):573–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Betts AM, Haddish-Berhane N, Tolsma J, Jasper P, King LE, Sun Y, et al. Preclinical to clinical translation of antibody–drug conjugates using PK/PD modeling: a retrospective analysis of inotuzumab ozogamicin. AAPS J. 2016;18(5):1101–16. [DOI] [PubMed] [Google Scholar]
- 90.Fanale MA, Horwitz SM, Forero-Torres A, Bartlett NL, Advani RH, Pro B, et al. Brentuximab vedotin in the front-line treatment of patients with CD30+ peripheral T-cell lymphomas: results of a phase I study. J Clin Oncol. 2014;32(28):3137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kashiwaba M, Ito Y, Takao S, Doihara H, Rai Y, Kanatani K, et al. A multicenter phase II study evaluating the efficacy, safety and pharmacokinetics of trastuzumab emtansine in Japanese patients with heavily pretreated HER2-positive locally recurrent or metastatic breast cancer. Jpn J Clin Oncol. 2016;46(5):407–14. [DOI] [PubMed] [Google Scholar]
- 92.Advani A, Giné E, Gisselbrecht C, Rohatiner A, Rosen S, Smith M, et al. Preliminary report of a phase 1 study of CMC-544, an antibody-targeted chemotherapy agent, in patients with B-cell non-Hodgkin’s lymphoma (NHL). Blood. 2005;106(11):230. [Google Scholar]