Abstract
Risk for Alzheimer’s disease (AD) is affected by multiple factors, including aging, obesity, and low testosterone. We previously showed that obesity and low testosterone independently and interactively exacerbate AD-related outcomes in young adult rodents. The goals of the current study are two-fold: to examine whether the effects of obesogenic diet differ with increasing age, and to determine if testosterone treatment in middle-aged and aged animals mitigates negative effects of diet. Male brown Norway rats were maintained on control or high-fat diets for 12 weeks beginning in young adulthood, middle-age, or advanced age. Separate cohorts of middle-aged and aged animals were treated with testosterone during dietary manipulations. Endpoints included metabolic indices, inflammation, cognitive performance, and neural health outcomes. Aging was associated with poorer outcomes that were generally exacerbated by high fat diet, especially at middle-age. Testosterone treatment was largely without benefit, exerting only subtle effects on a select number of measures. Understanding how the deleterious effects of obesity are affected by advancing age and the ability of protective strategies such as testosterone to reduce these effects may provide significant insight into both the development and prevention of age-related cognitive decline and AD.
Keywords: Aging, Diet-induced obesity, High fat diet, Neuroinflammation, Testosterone
1. Introduction
Obesity is a growing public health concern that increases risks for death and several diseases, including type 2 diabetes, cardiovascular disease, and various cancers (Zheng et al., 2017). Obesity is also associated with numerous adverse neural outcomes (Asih et al., 2017; Jayaraman and Pike, 2014; Lee and Mattson, 2014). For example, obesity is linked with decreases in hippocampal volume and white matter integrity (Ho et al., 2010; Jagust et al., 2005; Stanek et al., 2011) as well as accelerated cognitive decline (Cournot et al., 2006; Elias et al., 2005). Importantly, obesity also increases up to three-fold the risk of dementias (Whitmer et al., 2008), including Alzheimer’s disease (AD) (Profenno et al., 2010) and vascular dementia (Hayden et al., 2006).
Though advanced age is the single greatest risk factor for cognitive decline and dementia (Niccoli and Partridge, 2012), whether and how aging affects neural outcomes of obesity is not well understood. Weight gain increases during adulthood (Sheehan et al., 2003) leading to rates of obesity that peak at late middle-age (Mizuno et al., 2004) and are associated with a wide range of serious health outcomes (Zheng et al., 2017). Several studies indicate that obesity during middle-age is especially harmful, increasing rates of cognitive decline (Bischof and Park, 2015; Cournot et al., 2006; Dahl et al., 2013) and risk of dementia (Emmerzaal et al., 2015; Fitzpatrick et al., 2009) later in life. In animal studies, diet-induced obesity (DIO) is associated with reduced hippocampal neurogenesis (Lindqvist et al., 2006; Park et al., 2010), cognitive impairment (Kanoski et al., 2010; Knight et al., 2014; Stranahan et al., 2008), and increased AD-related pathology (Barron et al., 2013; Ho et al., 2004; Julien et al., 2010; Kohjima et al., 2010). The role of aging in the neural effects of DIO has only been addressed by a limited number of studies (Boitard et al., 2012; Erdos et al., 2011; Larkin et al., 2001; Spencer et al., 2017).
One normal physiological change that occurs in middle-aged men and is linked with the development of both obesity (Zitzmann, 2009) and dementia (Pike et al., 2009) is a decrease in estosterone levels. Importantly, there appears to be a bidirectional relationship between testosterone loss and obesity, in that loss of testosterone can increase adiposity and risk for obesity, and obesity, in turn, can decrease testosterone levels (De Maddalena et al., 2012; Fui et al., 2014). Because testosterone exerts a number of neural benefits, including increased hippocampal neurogenesis, neuroprotection, improved performance in select cognitive tasks, and reduction of AD-related neuropathology (Galea, 2008; Rosario and Pike, 2008), age-related testosterone depletion also may be expected to affect several neural consequences related to obesity. Consistent with this possibility, our prior work in male rodents identified interactions between testosterone and obesity in terms of both metabolic and neural outcomes (Jayaraman et al., 2014). Such findings suggest that testosterone therapy may provide a valuable approach to reduce negative effects of obesity during aging. Indeed, testosterone therapy has been reported to improve body composition (Isidori et al., 2005) by reducing body weight, body mass index, symptoms of metabolic syndrome (Traish et al., 2014; Yassin et al., 2014) and visceral adiposity, while increasing skeletal muscle (Allan et al., 2008). However, a recent meta-analysis of randomized clinical trials reported only limited net benefits of testosterone (Ponce et al., 2018). Further, new findings from the Testosterone Trials show no significant cognitive improvement (Resnick et al., 2017) and only modest metabolic benefits (Snyder et al., 2018) in aging, hypogonadal men treated with testosterone. The mixed evidence of testosterone treatment underscores the need for greater understanding of the contexts and outcomes for which testosterone therapy may have significant efficacy.
The available literature suggests that advancing age and obesity may act not only individually, but also cooperatively to drive a number of adverse outcomes. The extent to which protective actions of testosterone against obesity may offer a therapeutic approach to minimize the deleterious consequences of obesity during aging are unclear. To investigate these interactions, we conducted two parallel studies in male brown Norway rats, which have been demonstrated to model age-related testosterone loss in men (Gruenewald et al., 2008; Wang et al., 2002, 1993). Our goal was to examine the effects of high fat diet (HFD) across the lifespan, as well as the role of testosterone in this relationship. Thus, we compared, in two separate experiments, the effects of both age and testosterone supplementation on metabolic, cognitive, inflammatory, and neural health outcomes associated with HFD. First, we examined the effects of aging by comparing HFD outcomes in male rats at young adulthood (3 mo), middle-age (13 mo), and advanced age (23 mo). In the second experiment, we evaluated whether testosteronesupplementation alters the effects of HFD in middle-aged and aged animals that are vulnerable to normal, age-related testosterone depletion.
2. Methods
2.1. Animal procedures
Experiment 1: Aging and diet.
Male brown Norway rats at 3 (young), 13 (middle-aged), and 23 (aged) months of age were provided by the Aged Rodent Colony at the National Institute on Aging and subsequently maintained at vivarium facilities at University of Southern California. All animals were sexually naïve. Throughout the experimental period, animals were housedunder a 12-hour light/dark cycle with lights on at 6 AM and had ad libitum access to food and water. After a 1-week gap to allow animals to acclimate to the room in which they were housed, they were randomized to one of two dietary treatment groups: control diet (10% fat; #D12450J, Research Diets, Inc., New Brunswick, NJ, USA; CTL) or high fat diet (60% fat; #D12492, Research Diets, Inc.; HFD). The animals were kept on experimental diets for 12 weeks duringwhich body weight (expressed as both actual body weight and % change in body weight) andfood consumption (expressed as mean kcal consumed per day) were recorded weekly.
At the end of the treatment period, rats were euthanized with inhalant carbon dioxide and the brains were rapidly removed. One hemi-brain was immersion fixed for 48 h in 4% paraformaldehyde/0.067 M Sorensen’s phosphate buffer, then stored at 4°C in 0.1 M PBS/0.03% NaN3 until processed for immunohistochemistry. The remaining hemi-brain was snap frozen, then stored at −80°C prior to processing for RNA extraction. Blood was collected in EDTA-coated tubes via cardiac puncture and centrifuged to separate plasma, which was aliquoted and stored at −80°C. The liver was dissected and snap frozen for RNA extraction. Gonadal and retroperitoneal fat were dissected and weighed as a measure of adiposity.
Experiment 2: Testosterone and diet.
In order to examine the effects of testosterone supplementation on diet-associated outcomes during aging, we enrolled additional groups of 13 (middle-aged) and 23 (aged) month-old brown Norway rats that were treated with testosterone and randomly assigned to either CTL or HFD. Rats were housed under the same conditions as described above. Testosterone treatment was initiated simultaneously with dietary treatments. Animals were anesthetized with inhalant isoflurane, and subcutaneously implanted between the shoulder blades with a silastic capsule (1.57 mm ID x 3.18 mm OD; Dow Corning, Midland, MI, USA) that was a total length of 2 cm with the inner 1 cm packed with testosterone (Steraloids, Newport, RI, USA). Capsules were removed and replaced with fresh testosterone-containing capsules at the 6-week time point. Dosing was based on previous studies showing that this treatment results in physiological testosterone levels (Edinger and Frye, 2004; Frye et al., 2010; Pinilla et al., 1999). The highly androgen-responsive tissues levator ani muscle, seminal vesicles, and prostrate were collected and weighed as established bioassays to confirm efficacy of testosterone treatment (Owens et al., 2006). All animal procedures were carried out under aprotocol approved by the University of Southern California Institutional Animal Care and Use Committee and in accordance with the National Institute of Health standards.
2.2. Glucose homeostasis
A glucose tolerance test (GTT) was performed at week 11. After a 16 h overnight fast, baseline glucose levels were recorded and rats were administered a glucose bolus (2 g/kg body weight) via intraperitoneal injection. Blood glucose levels were recorded 15, 30, 60, and 120 min after administration of the glucose bolus. Blood was collected from the lateral tail vein and immediately assessed for glucose levels using the Precision Xtra Blood Glucose and Ketone Monitoring System (Abbott Diabetes Care, Alameda, CA, USA). The extent to which animals returned to their baseline glucose reading after 120 min, and the total area under the curve across 120 min were calculated.
2.3. Leptin and testosterone ELISAs
Plasma leptin levels were determined by ELISA using a commercially available kit (Rat Leptin ELISA, #EZRL-83K, Millipore, Burlington, MA, USA). All samples were run in duplicate according to manufacturer’s instructions. Plasma samples were also assayed for testosterone using an adaptation of the ImmuChem Double Antibody testosterone RIA kit from MP Biomedicals (#07–189102, Costa Mesa, CA, USA) with [125I] testosterone as the tracer and all reagent volumes halved. The testosterone antibody (solid phase) cross-reacts slightly with 5α-DHT (3.4%), 5 α-andros-tane-3β, 17β-diol (2.2%) and 11-oxotestosterone (2%) but does not cross-react with progesterone, estradiol, or the glucocorticoids (all <0.01%). The minimum detectable testosterone concentration was 0.1 ng/ml and the intra-assay coefficient of variation was 6.0%.
2.4. Immunohistochemistry and quantification
Fixed hemi-brains were completely sectioned across the horizontal plane at 40 μm using a vibratome (Leica Biosystems, Buffalo Grove, IL, USA). Immunohistochemistry was performed using a standard avidin/biotin peroxidase approach with ABC Vector Elite kits (Vector Laboratories, Burlingame, CA, USA). Every thirteenth section was processed for glial fibrillary acidic protein (GFAP), ionized calcium binding adaptor molecule 1 (IBA-1), and doublecortin (DCX). Briefly, for GFAP staining, sections were rinsed in TBS before being treated with an endogenous peroxidase blocking solution for 10 min. After three 10 min washes in 0.1% Triton-X/TBS, sections were incubated for 30 min in a blocking solution consisting of 2% bovine serum albumin in TBS. Blocked sections were incubated overnight at 4°C in primary antibody directed against GFAP (#Z0334, 1:10,000 dilution, Dako, Carpinteria, CA, USA) that was diluted in blocking solution. Sections were then rinsed and incubated in biotinylated secondary antibody diluted in blocking solution. Immunoreactivity was visualized using 3,3′-diaminobenzidine (Vector Laboratories).
Immediately adjacent tissue sections were similarly immunostained for IBA-1 with the following slight modifications. First, a 0.2% Triton-X/TBS solution was used for rinses on Day 1, and both the blocking solution and primary antibody solution contained 0.2% Triton-X. Second, sections were blocked for 60 min before being incubated overnight in IBA-1 (#019–19741, 1:500 dilution, Wako Chemicals, Richmond, VA, USA).
In addition, sections were stained for doublecortin (DCX) as previously described (Brummelte and Galea, 2010). Briefly, sections were treated with 0.6% hydrogen peroxidase for 30 min, and then incubated for 24 h in primary antibody at 4°C (#sc-8066, 1:1000 dilution, goat anti-doulecortin, Santa Cruz Biotechnology, Santa Cruz, CA, USA), that was diluted in PBS with 0.4% Triton-X and 3% Normal Rabbit Serum. Sections were then incubated with secondary antibody (#BA-5000, 1:500 dilution, biotinylated rabbit anti-goat, Vector Laboratories) for 24 h at 4°C and with Avidin-Biotin complex (1:1000 avidin and 1:1000 biotin, Vector Laboratories) for 4 h at room temperature, before being visualized using 3,3′-diaminobenzidine with 1.6% nickel (Vector Laboratories). Tissue was rinsed 3 times in PBS for 10 min each between each step. The tissue was mounted onto microscope slides, dehydrated, cleared with xylene, and cover-slipped with Permount (Fisher Scientific).
Microglia density and activation were quantified using live imaging (Olympus, BX50, CASTGrid software, Olympus, Tokyo, Japan) and a 40x objective. IBA-1 immunoreactive cells were scored as exhibiting either resting or reactive phenotypes based on morphological criteria, as described in previous studies (Ayoub and Salm, 2003; Moser and Pike, 2017). In brief, IBA-1 labeled cells were scored as resting (type 1) microglia if their cell bodies were spherical with numerous thin and highly ramified processes. Cells were scored as having an activated phenotype if they had either a) enlarged, rod-shaped cell bodies with fewer and thicker processes (type 2), or b) very few, short processes, or no processes with or without filopodia (type 3). The hippocampus was quantified starting in CA1 where the pyramidal cell layer begins. Five alternating fields were scored in each of 3 brain sections for a total of 15 fields and an average of ~170 cells per brain.
Astrocytes were visualized by GFAP immunostaining and scored based upon morphological phenotype. Digital images were captured using a 40X objective with an Olympus BX50 microscope equipped with a DP74 camera and CellSens software. Images of four alternating fields were taken starting in CA1 of the hippocampus where the pyramidal cell layer begins, and repeated across 3 brain sections for a total of 12 images per brain. This approach yielded an average of ~450 cells scored per brain. GFAP-immunoreactive cells were scored using NIH Image J 1.50i (National Institutes of Health, Rockville, MD, USA) with the cell counter plugin to mark cells as having either a resting or a reactive morphological phenotype, as previously described (Moser and Pike, 2017; Wilhelmsson et al., 2006). In brief, cells were scored as having a resting phenotype if they displayed normal-sized somas with typical,generally short projections. Conversely, GFAP-immunoreactive cells were scored as having a reactive phenotype when they exhibited enlarged cell bodies and large, thick processes.
DCX staining was quantified using a Nikon E600 light microscope (Nikon, Tokyo, Japan) with a 40X objective lens. Cells were counted throughout the granule cell layer and area measurements were made of the granule cell layer using NIH Image J. A total of 10 sections per brain were quantified. To obtain cell density, the estimated total number of DCX-positive cells was divided by the total area counted per brain.
2.5. RNA isolation and quantitative PCR
RNA extractions and PCR were performed as previously described (Jayaraman et al., 2014; Moser & Pike, 2017). For RNA extractions, the liver, hippocampus, and hypothalamus were homogenized using TRIzol reagent (Invitrogen Corporation, Carlsbad, CA, USA) following the manufacturer’s protocol. The RNA pellet was then treated with RNase-free DNase I (Epicentre, Madison, WI, USA) for 30 min at 37°C to remove any remaining DNA contamination, and a phenol-chloroform extraction was performed to isolate RNA. Purified RNA (1 µg) was used to reverse transcribe cDNA using the iScript cDNA synthesis system (Bio-Rad, Hercules, CA, USA). Real-time quantitative PCR was then run on the resulting cDNA using SsoAdvanced Universal SYBR Green Supermix (Bio-Rad) and a Bio-Rad CFX Connect Thermocycler. All samples were run in duplicates. PCR products were quantified by normalizincorresponding β-actin expression levels using the ΔΔ-CT method to acquire relative Mrna levels. Hippocampus and hypothalamus were probed for levels of interleukin-1 beta (IL-1β), cluster of differentiation 68 (CD68), and glial fibrillary acidic protein (GFAP). Liver was probed for levels of CD68, sterol regulatory element binding protein-1 (SREBP1), and stearoyl-CoA desaturase (SCD-1). Primer pair sequences are shown in Table 1.
Table 1.
Primer sequences. Gene targets for the PCR analyses are listed with their corresponding oligonucleotide sequences for the forward and reverse primers.
| Target Gene | Sequence |
|---|---|
| Interleukin-1β (IL-1β) | Forward: 5′-GACTTCACCATGGAACCCGT-3′ Reverse: 5′-GGAGACTGCCCATTCTCGAC-3′ |
| Cluster of differentiation 68 (CD68) | Forward: 5′-AAGCAGCACAGTGGACATT-3′ Reverse: 5′-TTCCGCAACAGAAGCTTTG-3′ |
| Glial fibrillary acidic protein (GFAP) | Forward: 5′-TCAATGCCGGCTTCAAAGA-3′ Reverse: 5′-AGCGCCTTGTTTTGCTGTTC-3′ |
| Sterol regulatory element binding protein-1(SREBP1) |
Forward: 5′-GCTCACAAAAGCAAATCACT-3′ Reverse: 5′-GCGTTTCTACCACTTCAGG-3′ |
| Stearoyl-CoA desaturase (SCD-1) | Forward: 5′-GGGAAAGTGAAGCGAGCAA-3′ Reverse: 5′-GTGGTCGTGTAGGAACTGGAGA-3′ |
| β-actin | Forward: 5′-AGCCATGTACGTAGCCATCC-3′ Reverse: 5′-CTCTCAGCTGTGGTGGTGAA-3′ |
2.6. Behavioral testing: Barnes maze
Spatial memory was assessed during week 10 of the treatment period, using a modified Barnes maze protocol (McLay et al., 1999). The maze was composed of a circular platform with 20 holes located around the border with an escape box located beneath one hole. The maze was housed inside an area isolated with black curtains on which visual cues were placed on each of the four walls. Rats were habituated to the behavioral testing room and to the maze 24 h before the first training day. Specifically, after habituating to the room in their home cage for 30 min, they were placed on the maze in a vertical cylinder that they could not see out of for 3 min. The cylinder was then moved to guide the animal into the escape box for 1 min before they were returned to their home cages. Animals were trained to locate the escape box over the following 4 days, with 3 trials per day, and a 15 min inter-trial interval during which they were returned to their home cage. During training trials, a bright light was directed above the maze, and static was played from a speaker below the maze. On each training day, rats were again habituated to the testing room for 30 min, before being placed in the cylinder on the maze for 10 s. The rats were then given 3 min to move about the maze freely and locate the escape box. The animal was given 1 min in the box once it located it, before being returned to its home cage. If animals did not locate the box within 3 min, they were gently guided into it and allowed to remain inside for 1 min. The maze was cleaned with 70% ethanol between each animal. Forty-eight h after the last training trial, rats were tested on the probe trial, in which the escape box was removed and rats were given 3 min to freely explore the maze.
The training and probe trials were recorded using Noldus Ethovision XT software (Leesburg, VA, USA). For training trials, the latency to locate the escape box was recorded, while for the probe trial, the number of correct hole investigations (defined as the hole where the escape box was previously located, as well as 1 hole on either side), errors, and the distance traveled to reach the correct hole for the first time were recorded.
2.7. Statistical analyses
All data are reported as the mean ± the standard error of the mean (SEM). Raw data were analyzed using Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA). For Experiment 1, data were analyzed using a 2×3 analysis of variance (ANOVA) that contained the variables of diet (CTL, HFD) and age (young, middle-aged, aged). For Experiment 2, data were analyzed using a 2×2 ANOVA that contained the variables of diet (CTL, HFD) and age (middle-aged, aged). Experiments 1 and 2 were analyzed separately; groups from the two experiments were not directly compared. For the analyses of body weight and glucose tolerance, which are measured across time, repeated measures ANOVAs were conducted. In the case of significant main effects, planned comparisons between groups of interest were made using the Bonferroni correction. Significance was set at a threshold of p<0.05. Additionally, correlations between metabolic, inflammatory, and behavioral outcomes were performed using the Spearman correlation coefficient.
3. Results
3.1. Body weight and adiposity
3.1.1. Body weight and adiposity: Diet and aging
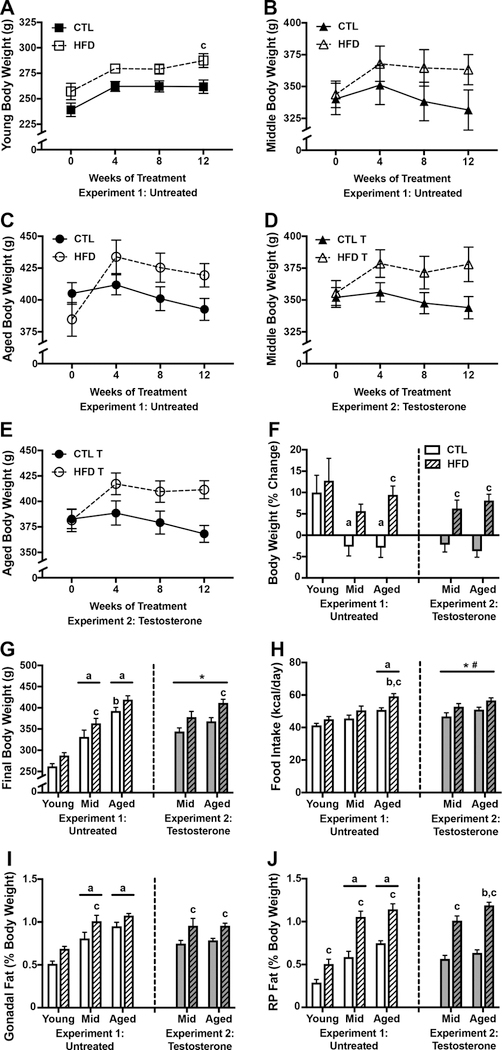
In order to examine the independent and interactive effects of aging and HFD, we first analyzed changes in body weight across the 12-week treatment period (Fig. 1A-C). There was a significant effect of age (F=4.85, p=0.003) in which both middle-aged and aged animals weighed more than young animals, and aged animals weighed more than middle-aged at every time point (p<0.05). There was also a significant effect of diet in the young animals (F=10.04, p=0.007; Fig. 1A). However, this effect reached statistical significance only at the 12-week time point (p=0.017). There was no significant effect of diet on body weight at any time point in either middle-aged (Fig. 1B) or aged animals (Fig. 1C).
Figure 1.
Body weight and adiposity outcomes associated with high fat diet in young, middle aged, and aged brown Norway rats. A-C) Body weights in A) young, B) middle-aged, and C) aged male brown Norway rats maintained on control and high fat diet taken at baseline (week 0) and 4-week intervals across the 12-week diet treatment. D-E) Body weights in testosterone treated D) middle-aged and E) aged rats fed control or high fat diet, across 12 weeks. F) Percent change in body weight from baseline to the end of the 12-week dietary treatment and G) final body weight, in young, middle-aged, and aged male brown Norway rats after 12 weeks of high fat diet-feeding. H) Food consumption as measured by the average daily kilocalories consumed across the 12-week dietary exposure. Adiposity is measured as weight of the I) gonadal fat pads and J) retroperitoneal fat pads, relative to total body weight. Data are presented as mean (±SEM) values; n=7–8/group. For figures A-E, young animals are shown as squares, middle-aged animals as triangles, and aged animals as circles; control diet are open symbols, high fat diet are closed symbols. For figures F-H, animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p < 0.05 relative to young rats in same diet condition. b p < 0.05 relative to middle-aged animal in same diet condition. c p < 0.05 relative to control diet-fed of the same age. * p < 0.05 for main effect of age that does not reach statistical significance in post hoc tests. # p < 0.05 for main effect of diet that does not reach statistical significance in post hoc tests.
When examining the percent change in body weight from baseline to the end of the 12-week dietary treatment, we found significant effects of both age (F=5.65, p=0.007; Fig. 1F) and diet (F=9.34, p=0.004). Between group comparisons revealed that, on control diet, young animals had a greater percent increase in body weight than both middle-aged (p=0.024) and aged animals (p=0.021), and that the effect of diet was significant only in aged animals (p=0.022). Though comparisons between CTL and HFD failed to reach statistical significance when examined across time, we found a significant interaction effect between age and diet when comparing final body weights at 12 weeks (F=6.84, p=0.003; Fig. 1G), such that HFD only significantly increased body weight in middle-aged animals (p<0.001). Moreover, there was a significant main effect of age (F=94.92, p<0.001), even in the absence of diet. Between group comparisons revealed that middle-aged and aged animals weighed more than young rats on both CTL and HFD (p<0.01), but aged animals weighed more than middle-aged animals only on a CTL diet (p<0.001).
As differences in body weight can reflect, in part, differential food intake across age, we examined food intake, expressed as the average kilocalories consumed per day. We found a significant main effect of age on food intake (F=20.07, p<0.0001; Fig. 1H), with aged animals consuming more food than young animals on both control (p=0.003) and HFD (p<0.0001) diets, and more than middle-aged animals on HFD (p=0.006). Moreover, there was a significant main effect of diet (F=14.06, p=0.0005), which was significant only in aged animals (p=0.008).
In addition to increasing body weight, HFD is also associated with increased adiposity. Thus, we analyzed weights of the gonadal and retroperitoneal (RP) fat pads. Aging was associated with increases in weights of the gonadal (F=36.56, p<0.001; Fig. 1I) and RP (F=51.98, p<0.001; Fig. 1J) fat pads, across both diets (p<0.01). HFD increased weight of the gonadal fat pads (F=16.44, p<0.001), though this effect reached statistical significance only in middle-aged animals (p<0.05). HFD also increased weight of the RP fat pads (F=61.29, p<0.0001), across all age groups (p<0.05). There were no significant interactions between age and diet on fat pad weights.
3.1.2. Body weight and adiposity: Diet and testosterone treatment
We also examined the effects of CTL versus HFD on metabolic outcomes in middle-aged and aged rats that had received testosterone treatment throughout the experimental period (Fig. 1D-E). When examining plasma testosterone levels, we found that the majority of animals that had not been treated with testosterone had levels below the detection threshold. Of those that did have detectable readings, young animals had an average of 0.256 ± 0.124 ng/mL; middle-aged had 0.298 ± 0.129 ng/mL, and aged animals had 0.166 ± 0.004 ng/mL. All testosterone-treated animals had detectable hormone levels, with middle-aged animals having an average of 0.382 ± 0.030 ng/mL, and aged animals having 0.294 ± 0.024 ng/mL. To confirm efficacy of testosterone treatment, we compared weights of levator ani muscle, an established in vivo bioassay of androgens. Middle-aged rats on control diet treated with testosterone had significantly heavier levator ani than untreated middle-agedrats (0.920±0.025g vs. 0.630±0.033g, p<0.01), a finding also observed in aged rats (0.916±0.018g vs. 0.730±0.032g, p<0.01). Similar relationships were found for the androgen-responsive tissues prostate and seminal vesicles in both middle-aged and aged rats (data not shown). Thus, testosterone treatment increased plasma testosterone levels and weights of androgen–responsive tissues in both middle-aged and aged rats.
Although rats maintained on HFD showed higher mean body weights, there was no statistically significant main effect of diet on body weight across the 12 weeks in middle-aged animals (Fig. 1D) or in aged animals (Fig. 1E). However, there was a main effect of diet on the percent of weight gained across the treatment period (F=37.5, p<0.0001; Fig. 1F), and this effect was significant in both middle-aged (p=0.002) and aged animals (p<0.0001). Similarly, we found a significant main effect of diet on final body weight (F=14.38, p<0.001; Fig. 1F), which was significant only in aged animals (p=0.011). Additionally, there was a main effect of age on final body weight (F=7.98, p=0.009; Fig. 1G), which failed to reach statistical significance when examined across diets.
Levels of food intake showed significant main effects of both age (F=4.77, p=0.038; Fig. 1H) and diet (F=10.1, p=0.004). The main effect of age on food intake failed to reach statistical significance by post hoc test, and there were nonsignificant trends towards an effect of diet in middle-aged (p=0.058) and aged animals (p=0.072).
For measures of adiposity, we found significant main effects of diet on weights of both the gonadal (F=14.31, p<0.001; Fig. 1I) and RP (F=137.4, p<0.001; Fig. 1J) fat pads, with post-hoc tests revealing that HFD significantly increased fat pad weights across both ages (p<0.05). Additionally, there was a significant effect of age on RP fat pad weight (F=8.37, p=0.007) with aged animals on HFD having greater fat pad weight than middle-aged animals (p=0.013).
3.2. Glucose homeostasis
3.2.1. Glucose homeostasis: Diet and aging
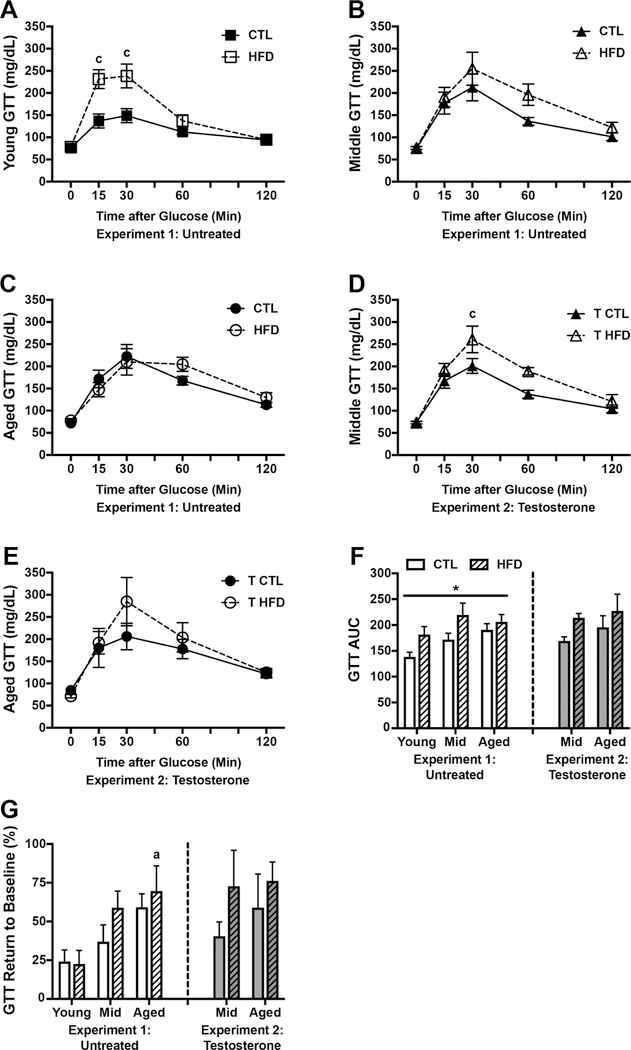
One of the major systems disrupted by diet-induced obesity is glucose homeostasis. To examine the effects of diet and aging on this system we performed glucose tolerance tests (GTT). We found a significant main effect of diet on glucose clearance in young animals (F=9.67, p=0.008; Fig. 2A) such that HFD-fed rats had higher glucose readings, though this effect reached statistical significance only at 15 min (p<0.001) and 30 min (p<0.001) post glucose bolus. Diet did not significantly affect rates of glucose clearance in middle-aged (Fig. 2B) and aged (Fig. 2C) animals. There were significant effects of both diet (F=8.27, p=0.006) and age (F=4.02, p=0.026) on GTT as measured by area under the curve (AUC; Fig. 2F), though both of these effects failed to reach statistical significance when examined across ages and diets, respectively. Finally, we assessed the extent to which animals were able to restore glucose homeostasis by calculating percent return to baseline glucose value (Fig. 2G). We found a significant main effect of age (F=7.09, p=0.002), but not of diet, on GTT return to baseline. Between group comparisons showed that aged animals on HFD were significantly impaired at returning to baseline glucose levels compared with young animals on HFD (p=0.011).
Figure 2.
Glucose homeostasis as assessed by glucose tolerance testing in young, middle aged, and aged brown Norway rats on control or high fat diet. A-C) Blood glucose levels measured in mg/dL at baseline (0 minutes) and 15, 30, 60, and 120 minutes after administration of a glucose bolus in A) young, B) middle-aged, and C) aged rats on control or high fat diet. D-E) Glucose levels in testosterone-treated D) middle-aged, and E) aged rats fed control or high fat diet and administered a glucose bolus. F) Area under the curve (AUC) for the glucose tolerance test. G) The extent to which animals returned to their baseline glucose levels after 120 min, calculated as a percent change from their baseline level. For figures A-E, young animals are shown as squares, middle-aged animals as triangles, and aged animals as circles; control diet are open symbols, high fat diet are closed symbols. For figures F-G, animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p< 0.05 relative to young rats in same diet condition. c p < 0.05 relative to control diet-fed rats of the same age. * p < 0.05 for main effect of age that does not reach statistical significance in post hoc tests.
3.2.2. Glucose homeostasis: Diet and testosterone treatment
In testosterone-treated animals, we found a significant main effect of diet on GTT in middle-aged animals (F=7.97, p=0.014; Fig. 2D) in which glucose levels were higher in HFD-fed animals at 30 min (p=0.025). Diet did not significantly increase glucose levels in aged animals at any time point (F=0.48, p=0.50; Fig. 2E). GTT AUC did not differ by age, though there was a trend towards an increase in HFD groups that failed to reach statistical significance (F=3.54, p=0.07; Fig. 2F). Finally, neither age nor diet had a significant effect on the extent to which glucose returned to baseline levels (Fig. 2G).
3.3. Peripheral effects of HFD
3.3.1. Peripheral effects of HFD: Diet and Aging
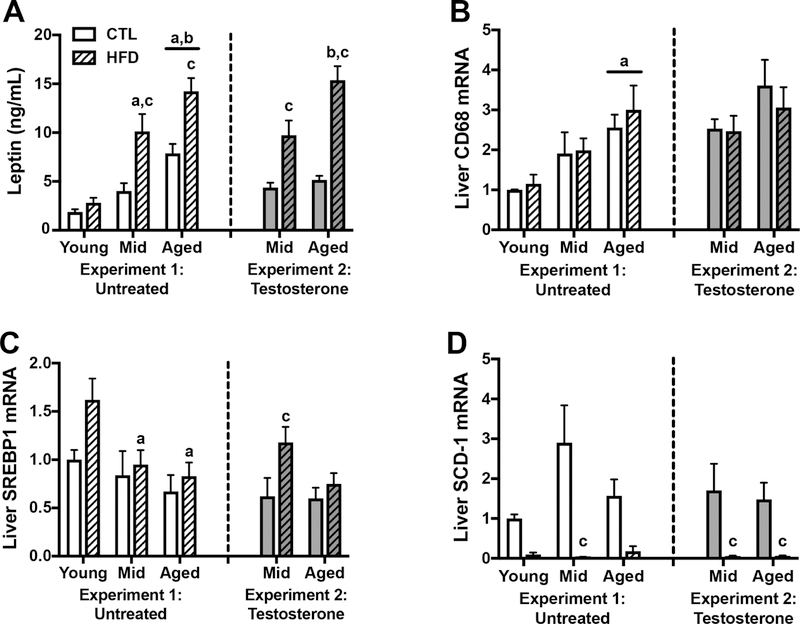
In addition to alterations in body weight and glucose homeostasis, HFD has numerous other effects on a variety of peripheral organs and systems. One change is increased plasma levels of the hormone leptin, which is released by adipose tissue. We observed a significant interaction between age and diet on plasma leptin levels (F=3.87, p=0.029; Fig. 3A) such that HFD was associated with increased leptin in middle-aged and aged rats (p<0.001), but not in young rats. Additionally, there was a main effect of age (F=31.6, p<0.0001) in which middle-aged animals on HFD and aged animals on either diet exhibited higher leptin than young adult rats (p<0.05). There was a further significant increase in leptin levels in aged as compared to middle-aged animals (p<0.05).
Figure 3.
Peripheral effects of control or high fat diet in young, middle-aged, and aged brown Norway rats. A) Plasma leptin levels measured in ng/mL in young, middle-aged, and aged rats on control or high fat diet, at the end of the experimental period. Relative mRNA expression in the liver of B) CD68, a macrophage marker; C) SREBP1, a transcription factor regulating lipogenesis and glycolysis; and D) SCD-1, a fatty acid metabolism enzyme, as determined by qPCR. Data show fold differences relative to the young rats on control diet. Animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p< 0.05 relative to young rats in same diet condition. b p < 0.05 relative to middle-aged animal in same diet condition. c p < 0.05 relative to control diet-fed rats of the same age.
The liver is also vulnerable to the effects of HFD. We examined the gene expression of CD68 as a measure of hepatic inflammation, and of SREBP-1 and SCD-1, as measures of liver fatty acid metabolism and lipogenesis. We found a significant effect of age on CD68 mRNA levels (F=9.26, p=0.0005; Fig. 3B) in which aged animals had higher levels than young animals across both diets (p<0.05). There was no significant effect of diet, nor was there an interaction between age and diet on CD68 levels. Levels of the transcription factor SREBP-1 were significantly affected by age (F=5.10, p=0.010; Fig. 3C) with HFD-fed middle-aged and aged animals having decreased expression relative to young animals. Additionally, there was a non-significant trend toward an effect of diet on SREBP-1 (F=3.99, p=0.053), but no significant interaction between age and diet. Finally, we found a significant effect of diet on mRNA levels of SCD1 (F=23.54, p<0.0001; Fig. 3D), which was significant only in middle-aged animals (p<0.0001). Though there was a trend towards an interaction between age and diet on SCD1 levels, this failed to reach statistical significance (F=2.80, p=0.073), and there was no significant effect of age.
3.3.2. Peripheral effects of HFD: Diet and testosterone treatment
Examination of leptin levels in testosterone-treated animals showed a significant interaction between age and diet (F=5.01, p=0.03; Fig. 3A). Post-hoc tests revealed that the effect of age was only significant in HFD-fed rats (p=0.002), whereas diet increased leptin levels at both ages (p<0.01).
Assessment of gene expression in liver revealed a non-significant trend toward increased CD68 levels with age (F=3.38, p=0.077; Fig. 3B) and no significant main effect of diet or interaction between diet and age. However, there were significant effects of diet on levels of both SREBP-1 (F=5.87, p=0.02; Fig. 3C), and SCD1 (F=15.57, p=0.0005; Fig. 3D). Between group comparisons showed that for SREBP-1, HFD increased expression significantly in middle-aged animals only (p=0.02), and for SCD1 HFD was associated with significantly decreased expression in both middle aged and aged animals (p<0.05).
3.4. Gliosis
3.4.1. Gliosis: Diet and aging
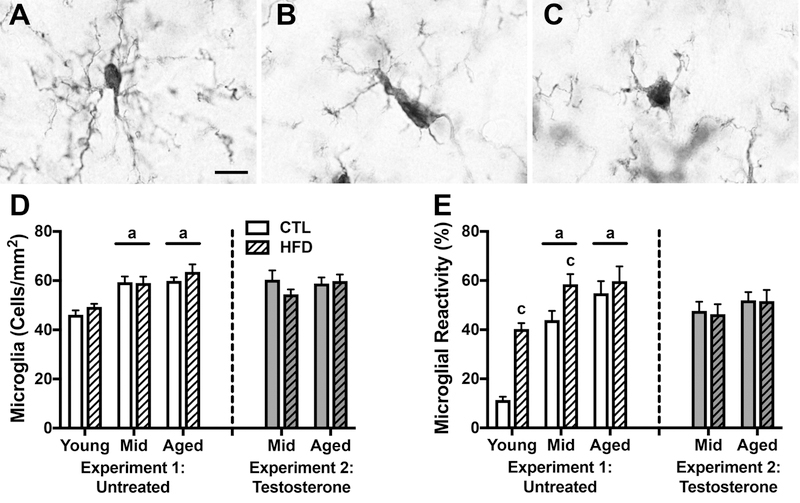
Both HFD and advanced age increase activation states of microglia and astrocytes, the two neural cell types most responsible for regulating neuroinflammation. We examined the independent and interactive effects of diet and age on glial number and activation state. Microglial reactivity was assessed by morphology. Figure 4A shows a resting microglial cell, characterized by numerous thin, branching processes (type 1). Reactive cells typically have enlarged rod-shaped cell bodies with fewer, thicker processes (type 2; Fig. 4B), or are amoeboid (type 3; Fig. 4C). We first looked at microglial density in hippocampus, and observed a significant main effect of age (F=22.95, p<0.001; Fig. 4D) in which middle-aged and aged animals across both diets had greater numbers of cells than young animals (p<0.01). There was no significant main effect of diet on the density of microglia. We then examined the proportion of microglia with a reactive phenotype and found a significant interaction between age and diet (F=4.25, p=0.021; Fig. 4E) such that HFD was associated with increased microglial reactivity in young (p<0.001) and middle-aged (p<0.05), but not aged animals. Additionally, there was a significant main effect of age (F=32.35, p<0.001), with middle-aged and aged animals having higher proportions of reactive microglia than young animals across both diets (p<0.01).
Figure 4.
Microglia number and morphological status as assessed by IBA-1immunohistochemistry in young, middle-aged, and aged brown Norway rats across dietary and testosterone treatments. A-C) Representative images of microglial morphology. Scale bar =10μm. A) A type 1 or resting cell characterized by a small cell body with numerous, ramified projections. Reactive cells are either B) type 2 cells with rod shaped cell bodies and fewer, thicker processes, or C) amoeboid cells with no branches or filopodia. D) Densities of IBA-1immunoreactive microglia in young, middle-aged, and aged rats on control or high fat diet were quantified in hippocampus. E) Percentages of reactive microglia (types 2 and 3) were quantified in hippocampus. Animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p < 0.05 relative to young rats in same diet condition. c p < 0.05 relative to control diet-fed rats of the same age.
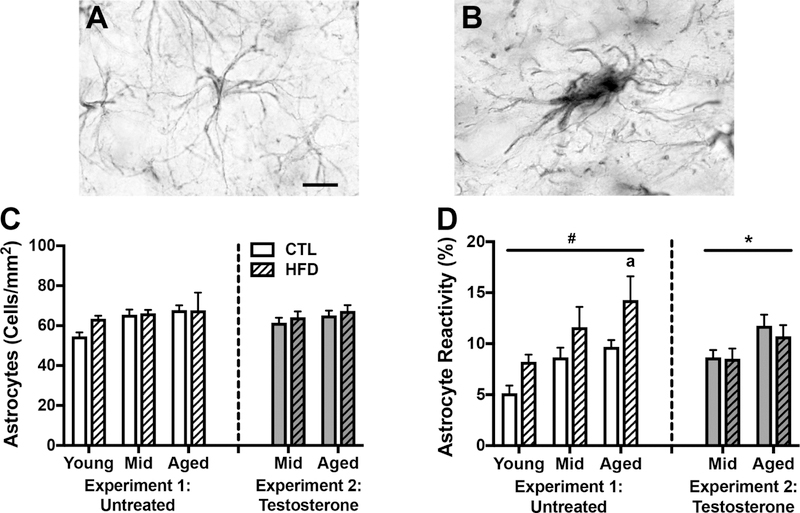
We next performed parallel density and reactivity analyses with astrocytes. As shown in Figure 5, nonreactive astrocytes have normally sized somas with several long, thin branches (Fig. 5A), whereas reactive astrocytes have enlarged somas and projections (Fig. 5B). We found no significant effect of either diet or age on astrocyte density (Fig. 5C). However, there were significant main effects of both age (F=7.41, p<0.002) and diet (F=9.28, p<0.004) on astrocyte reactivity (Fig. 5D). Between group analyses revealed that aged animals had a higher percentage of reactive astrocytes than young animals (p=0.012), but the effect of diet did not reach statistical significance when separated across ages.
Figure 5.
Astrocyte number and morphological status as assessed by GFAP immunohistochemistry in young, middle-aged, and aged brown Norway rats across diet and testosterone treatments. A-B) Representative images of astrocyte morphology. Scale bar =10μm. A) Non-reactive astrocytes have normally sized cell bodies and thin projections. B) Reactive astrocytes have enlarged somas and thicker processes. C) Densities of GFAP immune reactive astrocytes and D) percentages of reactive astrocytes were quantified in hippocampus of young, middle-aged, and aged rats on control or high fat diet and untreated or treated with testosterone. Animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p < 0.05 relative to young rats in same diet condition. * p < 0.05 for main effect of age that does not reach statistical significance in post hoc tests. # p < 0.05 for main effect of diet that does not reach statistical significance in post hoc tests.
3.4.2. Gliosis: Diet and testosterone treatment
Next, we examined the relationship between aging and diet on inflammation outcomes in middle-aged and aged animals that received testosterone supplementation. We found no significant main effects or interactions on measures of microglial density (Fig. 4D) or the proportion of reactive microglia (Fig. 4E). Similarly, there were no significant effects of diet or age on astrocyte density (Fig. 5C). We found a significant main effect of age on the proportion of reactive astrocytes (F=7.24, p=0.012; Fig. 5D), however, this effect was not significant when examined separately across diets.
3.5. Inflammatory gene expression
3.5.1. Inflammatory gene expression: Diet and aging
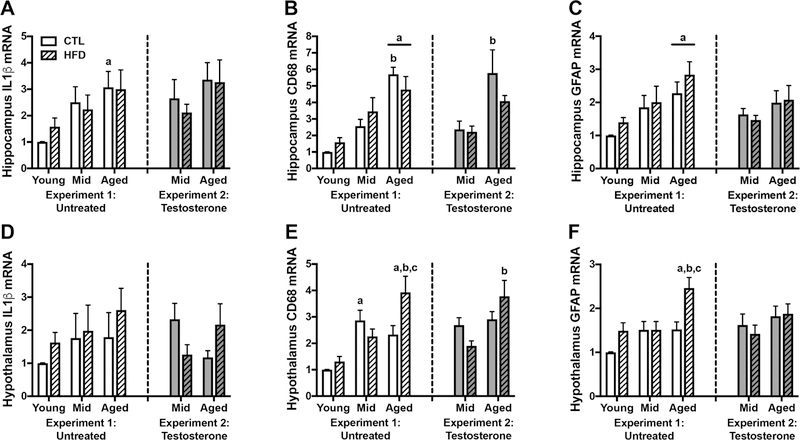
Another aspect of neuroinflammation that can be affected by HFD and aging is geneexpression of pro-inflammatory and glial factors. We used qPCR to examine gene expression of several such genes in both hippocampus and hypothalamus. We found significant main effects of age on hippocampal gene expression of IL-1β (F=5.41, p=0.002; Fig. 6A), CD68 (F=25.85, p<0.001; Fig. 6B), and GFAP (F=8.19, p=0.001; Fig. 6C). Between group analyses revealed that (1) on CTL diet, aged animals had greater levels of IL-1β than young animals (p=0.029); (2) aged animals had higher expression of CD68 than both young and middle-aged animals on CTL diet, and had higher levels than young animals on HFD (p<0.001), and (3) aged animals had higher levels of GFAP than young animals on either diet (p<0.05). Neither diet nor the interaction between age and diet significantly affected expression levels of these genes in hippocampus.
Figure 6.
Inflammatory gene expression in young, middle-aged, and aged brown Norway rats across diet and testosterone treatments. A-C) Relative mRNA levels in hippocampus were determined by qPCR for A) IL1b, a pro-inflammatory cytokine; B) CD68, a microglia/macrophage marker; and C) GFAP, an astrocyte marker. D-F) Relative mRNA levels for D) IL1b; E) CD68; and F) GFAP, were also determined in hypothalamus. Data show fold differences relative to the young rats on control diet. Animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p < 0.05 relative to young rats in same diet condition. b p < 0.05 relative to middle-aged animal in same diet condition. c p < 0.05 relative to control diet-fed rats of the same age.
Hypothalamic gene expression of IL-1β was not significantly affected by either age or diet (Fig. 6D). There was a significant interaction between age and diet on levels of CD68 (F=3.68, p=0.034; Fig. 6E) and a trend towards an interaction effect on GFAP levels (F=2.98, p=0.062; Fig. 6F), with diet increasing gene expression only in aged animals (p<0.01). Additionally, there was a significant main effect of age on levels of CD68 (F=12.35, p<0.001) and GFAP (F=7.69, p=0.002). Post-hoc tests revealed that on CTL diet middle-aged animals had higher levels of CD68 than young animals (p<0.01), and on HFD aged animals had higher levels of CD68 and GFAP than both young and middle-aged animals (p<0.05).
3.5.2. Inflammatory gene expression: Diet and testosterone treatment
Among testosterone-treated rats, we found no significant effects of age, diet, or their interaction on expression of IL-1β (Fig. 6A) and GFAP (Fig. 6C) in hippocampus. However, there was an effect of age on levels of hippocampal CD68 (F=13.21, p=0.001; Fig. 6B), with post-hoc tests showing that aged animals on CTL diet had higher CD68 than matched middle-aged animals (p=0.006). Hypothalamic gene expression showed a significant interaction between age and diet on levels of IL-1β (F=6.29, p=0.018; Fig. 6D), though this interaction failed to achieve statistical significance in post-hoc analysis. Likewise, we found a significant interaction between age and diet on CD68 expression in hypothalamus (F=5.55, p=0.026; Fig.6E), in which aged animals had higher levels than middle-aged animals, but only on HFD(p=0.002). Finally, levels of hypothalamic GFAP did not differ by age, diet, or their interaction (Fig. 6F).
3.6. Behavioral and neurogenesis outcomes
3.6.1. Behavioral and neurogenesis outcomes: Diet and aging
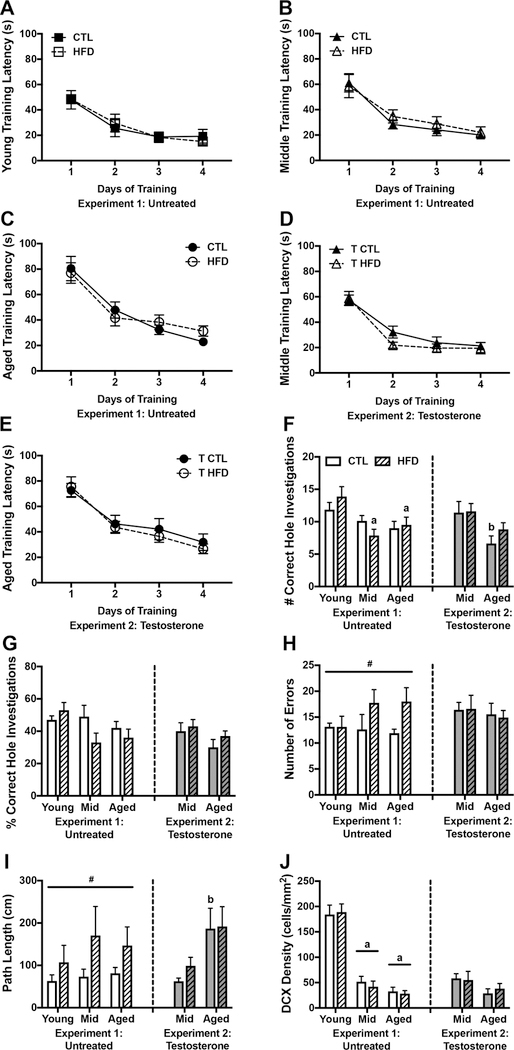
In order to determine whether age and diet modulate cognitive performance, we examined behavior on the Barnes maze, a test of spatial reference learning and memory. We first assessed learning behavior by examining the latency to reach the escape box on each trial during training. Figures 7A-C show the latency averaged across the 3 trials per day. For all age and diet groups the animals showed significant learning, as indicated by shorter latencies to reach the escape box on days 2–4, than on day 1 of training (p<0.05). However, rates of learning differed across groups. There was a significant main effect of age across CTL diet animals (F=7.02, p=0.004), with aged animals being slower at locating the escape platform than both young (p=0.005), and middle-aged animals (p=0.045). However, this effect is likely due to slower locomotion in aged animals, rather than to a learning disability, as all animals were able to locate the escape box within 30 s by the last training day. There were no significant effects of diet in any age group, suggesting that diet did not impair ability to learn the location of the escape box.
Figure 7.
Effects of diet and testosterone on behavioral performance in the Barnes maze and on neurogenesis in young, middle-aged, and aged brown Norway rats. A-C) Average latency to located the escape box over the course of 4 days of training in A) young, B) middle-aged, and C) aged male brown Norway rats maintained on control and high fat diet. D-E) Average latency to locate the escape box in testosterone-treated D) middle-aged and E) aged rats fed control or high fat diet. F) The number and G) percentage of correct hole visits during the Barnes maze probe trial, 48 h after the last training trial. H) The number of errors made during the probe trial and I) the distance traveled to locate the escape box for the first time during the probe trial. J) Neurogenesis as measured by the density of double cortin-expressing cells in the dentate gyrus. Animals not treated with testosterone are shown in white (Experiment 1), and animals treated with testosterone in gray (Experiment 2); control diets are solid white or gray bars, high fat diets are striped bars. a p < 0.05 relative to young rats in same diet condition. b p < 0.05 relative to middle-aged animal in same diet condition. # p < 0.05 for main effect of diet that does not reach statistical significance in post hoc tests.
When examining performance on the probe trial, we found a significant main effect of age (F=6.79, p=0.003; Fig. 7F) such that on HFD, young animals approached the correct hole more frequently than either middle aged (p=0.002) or aged animals (p=0.021). Likewise, when looking at the percent of correct hole approaches (Fig. 7G), we found a trend towards a main effect of age with younger age exhibiting more correct hole approaches (F=2.54, p=0.089),though this failed to reach statistical significance. Neither diet, nor the interaction between age and diet had significant effects on number or percent correct hole approaches. There was a significant main effect of diet on errors, where rats fed HFD approached more incorrect holes (F=4.42, p=0.041; Fig. 7H), though this was not statistically significant when examined separately across the ages. Finally, we found a significant main effect of diet on path length, with greater distance travelled in HFD-fed rats (F=4.54, p=0.039; Fig. 7I), that did not reach statistical significance when examined across the age groups. However, our data show a greater percent increase in path length associated with HFD in middle-aged (~132%) rats than in young (~70%) and aged (~80%) rats, suggesting that middle-aged animals were most susceptible to the effects of HFD. Neither age, nor the interaction between age and diet, had significant effects on errors or path length.
To examine neurogenesis, we analyzed the density of DCX-expressing cells in the dentate gyrus. As shown in Figure 7J, there was a main effect of age (F=98.65, p<0.0001) in which young animals had a higher density of DCX-expressing cells than middle-aged and aged animals across both diets (p<0.0001). There was no significant effect of diet, nor of an interaction between diet and age, on the density of DCX-expression.
3.6.2. Behavioral and neurogenesis outcomes: Diet and testosterone treatment
Behavior in the Barnes maze was also assessed in testosterone-treated animals. Both middle-aged (Fig. 7D) and aged (Fig. 7E) rats located the escape box more quickly on day 2 and all subsequent days of training than on day one (p<0.05). There was a main effect of age for both CTL diet (F=5.61, p=0.029) and HFD animals (F=14.6, p=0.001) with aged animals taking longer to find the escape box. There was no significant effect of diet, nor was there an interaction between age and diet on training latency.
Examination of probe trial performance yielded results similar to those observed in animals not treated with testosterone. That is, we found a significant main effect of age (F=8.68, p=0.005; Fig. 7F) with aged animals on CTL diet having less correct hole approaches than middle-aged animals (p=0.026). Further, there again was a trend towards reduced correct hole investigations with age (F=3.34, p=0.076; Fig. 7G). However, neither diet nor the interaction between age and diet had significant effects on number or percent of correct hole investigations. There were no significant main or interactive effects of aging and diet on the number of probe trial errors (Fig. 7H). We found a significant main effect of age on path length (F=9.14, p=0.005; Fig. 7I) such that in CTL diet animals, aged rats traveled further to locate the correct hole than did middle-aged (p=0.04). There was no main effect of diet, nor was there an interaction between age and diet on path length.
When examining DCX-labeled cell density, we found a non-significant trend towards an effect of age (F=3.73, p=0.064; Fig. 7J), but neither diet, nor the interaction between diet and age, had a significant effect on DCX density.
3.7. Correlations
We examined correlations between various metabolic and inflammatory markers and performance in the Barnes maze, specifically in animals from Experiment 1, results of which are shown in Table 2. As expected, metabolic outcome measures of adiposity, leptin, and GTT AUC, are highly correlated with each other, and with food intake. The percent change in body weight correlates with food intake, but not with any other metabolic outcome measure, nor with any glial or behavioral outcome. There is also high correlation between the glial measures ofmicroglia and astrocyte activation, and the behavioral outcome measures of correct hole entries, errors, and path length.
Table 2.
Correlations among metabolic, inflammatory, and behavioral outcomes. Data are presented as Spearman r values
| Measure | kCal | Body weight (% change |
Adiposity (RP fat) |
Leptin | GTT AUC | Microglia activation |
Astrocyte activation |
Correct hole entries |
Errors | Path length |
|---|---|---|---|---|---|---|---|---|---|---|
| kCal | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ |
| Body weight (% change |
.35* | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ |
| Adiposity (RP fat) |
.72**** | .11 | _____ | _____ | _____ | _____ | _____ | _____ | _____ | _____ |
| Leptin | .77**** | .07 | .91**** | _____ | _____ | _____ | _____ | _____ | _____ | _____ |
| GTT AUC | .17 | −.01 | .40** | .37* | _____ | _____ | _____ | _____ | _____ | _____ |
| Microglia Activation |
.49*** | −.08 | .63**** | .59**** | .48*** | _____ | _____ | _____ | _____ | _____ |
| Astrocyte Activation |
.49*** | .03 | .53**** | .39** | .12 | .39** | _____ | _____ | _____ | _____ |
| Correct entries |
−.13 | .23 | −.28T | −.28T | −.24 | −.38** | −.21 | _____ | _____ | _____ |
| Errors | .11 | .18 | .21 | .22 | .17 | .23 | .14 | .14 | _____ | _____ |
| Path length |
.10 | .09 | .09 | .17 | .21 | .33* | .14 | −.28T | .66**** | _____ |
p < 0.05
p < 0.01
p < 0.001
p < 0.0001
p < 0.07.
Metabolic measures are highly correlated with both measures of glial activation, but show non-significant correlations with behavioral outcomes, except for a trend towards significance in adiposity and leptin on correct hole entries. However, microglial activation is significantly correlated with both correct hole entries and path length, highlighting the role of microglia in this context. Thus, metabolic outcomes were correlated with glial activation, which was then correlated with behavioral outcomes.
3.8. Effects of testosterone treatment
The main goals of this study were to examine (1) interactions between age and diet, and (2) the potential of testosterone to modulate diet outcomes during aging. Although not a planned goal of the study, the dataset does provide an opportunity to assess the effects of testosterone treatment in middle-aged and aged rats across diets. Because different sets of animals were used in Experiments 1 and 2, we were unable to directly compare by statistical analyses the effects of testosterone treatment. Nonetheless, some descriptive examination of the data is possible.
We observed only a few positive effects of testosterone treatment upon comparison of Experiment 1 middle-aged and aged rats without testosterone treatment versus age- and diet-matched rats with testosterone treatment. Specifically, testosterone was associated with a ~20% reduction in gonadal fat mass (Fig. 1I) and a ~50% reduction in levels of plasma leptin (Fig. 3A) in aged rats. Testosterone had negligible effects in CTL-fed middle-aged animals, with only an ~8% decrease in gonadal fat mass and no appreciable change in leptin levels, suggesting that any testosterone protection against age-related metabolic impairments may be limited to advanced ages.
Testosterone treatment did not alter inflammatory outcomes in CTL-fed rats, but appears to have yielded subtle reductions in gliosis. In untreated middle-aged animals HFD was associated with ~25% increases in both microglial (Fig. 4E) and astroglial (Fig. 5D) activated morphology. In testosterone-treated middle-aged animals, HFD was not associated with increases in microglial and astroglial reactivity, suggesting that testosterone treatment diminished the effect of HFD in middle-aged animals. Similar effects of were observed in aged animals.
In the Barnes maze, testosterone treatment in CTL-fed animals had opposite effects at middle-age and old age. That is, during the probe trial, testosterone was associated with a~17% decrease in path length in middle-aged animals, but a ~56% increase in aged animals 600 (Fig. 7I), suggesting that it negatively affected cognitive performance in 26-month old rats. 601 When examining HFD-associated increases in path length in middle-aged animals, we found a 602 ~130% increase in untreated rats, that was reduced to a ~60% increase in testosterone treated 603 rats, suggesting testosterone treatment is associated with modest protection from the effects of 604 HFD on behavioral performance at middle-age.
4. Discussion
A primary goal of this study was to determine whether HFD differentially affected various metabolic, inflammatory, and cognitive outcomes across advancing age. We found that, regardless of diet, aging had the strongest adverse effects on a number of measures, corroborating the idea that aging is the central driving force for negative health consequences and disease. For example, aging was associated with increased body weight, adiposity, and leptin levels, indicating a general shift towards worse metabolic outcomes with age. Consistent with our findings, previous studies in rodent models also showed increased adiposity with age (Larkin et al., 2001; Wolden-Hanson et al., 1999) as well as greater weight gain in response to HFD in older animals (Erdos et al., 2011). Moreover, we found that both middle-aged and aged animals had increased microglial reactivity, and aged animals showed higher expression of several pro-inflammatory factors. These findings of increased inflammation with age are well documented in in humans (Freund et al., 2010) and rodent models (Sierra et al., 2007; Wu et al., 2007).
In addition to aging, we found generally modest effects of HFD on metabolic, inflammatory, and behavioral changes. As expected, HFD was associated with a number of adverse metabolic results, including increases in body weight, adiposity, and leptin levels as well as reduced glucose clearance in young animals. Moreover, HFD increased microgliosis without significantly affecting astrocyte reactivity or cytokine expression. However, because animals were not perfused, it is possible that cytokine expression is affected by blood and leukocyte infiltration, although the changes associated with this would be expected to be rather small (York et al., 2012). Though some prior studies have shown HFD-associated increases in both glial reactivity and cytokine levels (Pistell et al., 2010), others show changes in only a subset of neuroinflammatory markers (Setti et al., 2015) or only in select brain regions (Guillemot-Legris et al., 2016). We also found that overall HFD was associated with worsened spatial memory performance, which is consistent with other reports (Molteni et al., 2002). Thus, age and diet were both independently associated with a range of adverse metabolic, inflammatory, and behavioral changes.
It is important to note that we observed only moderate increases in body weight in response to HFD. Comparison of mean final body weights showed that HFD was associated with ~12% increase in body weight in young animals, and a ~7% increase in middle-aged and aged animals, over the three-month period. When evaluated as percent change in individual animals over the course of the study, HFD in middle-aged and aged animals yielded net increases in body weight of ~7 and ~12%, respectively. For comparison, for an average height man with normal BMI, an increase of 9% body weight would correspond to an elevation in BMI of ~2 units. Thus, the relatively modest effects of HFD may reflect the mild increases in body weight and adiposity yielded by this HFD. By this perspective, the HFD-induced effects may reflect long-term exposure to an obesogenic diet rather than obesity. It is of interest that recent observations show that short-term HFD exposure is also associated with deleterious neural effects that are exacerbated in aged animals (Spencer et al., 2017). Perhaps unexpectedly, we observed that increasing age exerted a much stronger effect on body weight than HFD. In rats on control diet, mean body weight progressively increased from young adulthood to middle-age and old age, such that there was an overall ~33% weight increase with aging. In line with the age-related increase in body weight, aging was also associated with greater food consumption, with aged rats consuming a greater amount of kcal per day across both diets. The observed age-related increases in body weight and adiposity may reasonably have contributed to the effects of age on metabolic, inflammation, and neurogenesis measures.
In addition to their independent effects, age and diet interactively affected several outcomes. For example, we observed significant effects of aging on measures of ability to return to baseline in GTT, astrocyte reactivity, expression of hypothalamic CD68 and GFAP, and number of correct hole visits in the Barnes maze probe, specifically in HFD-fed animals. Similar findings of age and diet interactions were recently demonstrated in the context of short-term HFD exposure in which only aged animals on HFD showed cognitive impairments (Spencer et al., 2017). Conversely, it has also been shown that obesity impairs cognitive performance in mice exposed to HFD during adolescence, but not in adulthood (Boitard et al., 2012), suggesting that the brain may be especially vulnerable to effects of diet during both early and late life. Collectively, these findings support the “two hit hypothesis” that has been proposed for a number of diseases (Hawkes et al., 2007; Zhu et al., 2004) and consequences of age-related inflammation (Franceschi et al., 2000). According to this position, aging may act as the first hit that increases vulnerability of several systems to the adverse effects associated with a second challenge, like HFD. Such interactive effects between various risk factors may have important implications for prevention and/or treatment of diseases.
Interestingly, we found that middle-aged animals were more vulnerable to some effects of HFD. More specifically, HFD induced relatively more robust changes in body weight, adiposity, leptin levels, liver SCD1 expression, and microglial activation in middle-aged animals than in both young and aged animals, which exhibited HFD-induced effects in some but not all of these measures. Moreover, although not statistically significant, the effects of HFD on cognitive performance in the Barnes maze appeared strongest in middle-aged animals. A number of studies in the human literature have demonstrated increased risk for dementia (Emmerzaal et al., 2015; Fitzpatrick et al., 2009) and accelerated brain aging (Ronan et al., 2016) in response to midlife obesity. Effects of obesity at midlife have thus far not been thoroughly investigated in experimental models. One study found that middle-aged rats showed memory impairments, loss of dendrites, and microglial activation in response to a diet high in cholesterol and saturated fatty acids (Granholm et al., 2008). However, young adult and aged animals were not included in this study to compare whether dietary effects were particularly harmful in middle-age. Our study is unique in its focus on comparing effects of HFD across animals in young adulthood, middle-aged, and advanced aged. Though we did not directly examine how HFD in middle-aged rats affected outcomes in advanced age, our data suggest that the pronounced effect of HFD at middle-age may serve as a catalyst that pushes the aging trajectory to poorer outcomes later in life.
A second goal of this study was to evaluate the ability of testosterone treatment to attenuate consequences of HFD in middle-aged and aged rats. Overall, we found very limited effects of testosterone treatment on the range of metabolic, inflammatory, and neural outcomes assessed in this study. Comparisons across the two experiments showed that some consequences of HFD observed in the absence of testosterone (Experiment 1) were less robust in the presence of testosterone (Experiment 2). Specifically, in Experiment 2, HFD was not associated with increased in microglial activation in middle-aged rats, nor elevated astrocyte activation and GFAP expression in aged animals. These observations are consistent with a prior report showing testosterone decreases age-related increases in GFAP expression (Day et al., 1998).
In terms of cognitive function, we found only non-significant trends of improved function with testosterone in middle-aged rats. However, testosterone treatment was associated with reduced spatial memory in aged rats maintained on control diet, suggesting an unfavorable effect of testosterone in these animals. Though some studies have shown improved cognition with testosterone treatment in men (Cherrier et al., 2005; Janowsky et al., 1994) and rodents (Frye and Seliga, 2001), many studies have failed to show an association (Emmelot-Vonk et al., 2008; Haren et al., 2005; Lu et al., 2006; Puts et al., 2010; Resnick et al., 2017), or have found deleterious effects of testosterone under conditions of oxidative stress (Cunningham et al., 2014). Moreover, though levels of neurogenesis in adult animals have been shown to be decreased with obesity (Lindqvist et al., 2006; Park et al., 2010) and increased with testosterone (Spritzer and Galea, 2007), we observed a significant reduction in doublecortin-expressing cells in middle-aged and aged animals that did not differ with diet or testosterone treatments. Within the limits of our assessments, our observations in male rats are consistent with much of the human literature in suggesting that testosterone does not have significant neural benefits during aging. Indeed, despite numerous potential advantages of testosterone therapy (Bassil et al., 2009), our overall findings align well with emerging evidence of limited positive outcomes of testosterone in aging men (Snyder et al., 2018).
Though the exact pathways linking obesity to cognitive decline and dementia are unknown, one hypothesis is that metabolic impairments drive neural impairment. It is well established that there are changes in metabolic factors in the AD brain (de la Monte and Wands, 2008). There is evidence that insulin deficiency and resistance in the brain are associated with cognitive impairment and AD (Craft, 2005), and reductions in insulin signaling are exacerbated as AD progresses (Rivera et al., 2005). Moreover, high plasma insulin can increase levels of Aβ42 in cerebrospinal fluid of healthy older adults (Watson et al., 2003), and there is evidence that improving insulin sensitivity in AD patients is associated with better cognitive outcomes (Reger et al., 2006; Watson et al., 2005). However, we found that neither impairments in GTT nor adiposity significantly correlated with behavioral performance, though there was a non-significant trend toward a correlation between leptin levels and the number of correct hole entries. Interestingly, a high density of leptin receptors is found in hippocampus and leptin resistance is tied to deficits in synaptic plasticity and hippocampal-dependent behaviors (Harvey et al., 2006; Irving and Harvey, 2014; Münzberg and Morrison, 2015). Interestingly, leptin receptors are found on astrocytes, and leptin can affect astrocyte morphology and function (García-Cáceres et al., 2011; Kim et al., 2014), suggesting that leptin may contribute to relationships between HFD and gliosis. While our findings suggest that metabolic impairments largely did not predict behavioral outcomes, we did not specifically examine metabolic pathways in brain and thus, cannot rule out their potential effects.
Although there is a role for metabolic factors in regulation of cognitive function, there also is increasing evidence for a role of glial cells. For example, gliosis is hallmark of both aging and AD and has been proposed as a possible mechanism underlying cognitive impairment and AD pathogenesis (Blasko et al., 2004; Glass et al., 2010). In fact, several studies clearly point to a role for inflammation rather than metabolic changes, in the effects of obesity on the brain. For instance, a prior study showed that 8 weeks of HFD resulted in cognitive deficits, decreased synaptic spines, and microglial activation, but no changes in insulin or glucose levels (Bocarsly et al., 2015). The finding that just 3 days of HFD exposure leads to cognitive impairments in aged rats (Spencer et al., 2017) also suggests that these changes are likely independent of adiposity and metabolic outcomes. Inflammatory and glial changes, however, can be observed after just 1 day of HFD-feeding and before metabolic changes occur (Thaler et al., 2012). In fact, inflammation may be driving the metabolic changes seen with HFD-feeding and obesity (Maldonado-Ruiz et al., 2017), as central administration of pro-inflammatory cytokines has been shown to impair peripheral insulin signaling (Arruda et al., 2011), and exacerbate the effects of HFD-feeding (Oh-I et al., 2010). Our findings of significant correlations between microglial reactivity and behavioral impairments support the role of gliosis as a central factor in mediating the effects of obesity on the brain. Interestingly, we found that astrocyte activation did not significantly correlate with behavioral outcomes, nor were they significantly increased in response to HFD, which may suggest that microglia have a greater role in this relationship. This is supported by other studies showing a central role for microglia in obesity (De Luca et al., 2016; Tucsek et al., 2014), including the finding that HFD causes synaptic stripping by microglia (Hao et al., 2016). Taken together, these findings suggest that inflammation is an early consequence of HFD and may drive many of the adverse changes associated with obesity.
This study has limitations that should be addressed in future research. First, the design of this study limits our interpretations of testosterone actions. Experiments 1 and 2 were designed to assess the effects of aging and testosterone treatment during aging, respectively, on the neural and metabolic outcomes induced by HFD. The independent design of the two experiments precludes direct statistical comparison of groups between Experiments 1 and 2. Thus, the data limit the ability to analyze how testosterone may have affected age-related changes in the absence of HFD beyond qualitative comparisons. Second, due to limited sensitivity of the testosterone assay, we were unable to determine whether age and/or HFD affected endogenous plasma testosterone levels, although we did confirm efficacy of testosterone treatment. The choice of brown Norway rat may also have impacted the magnitude of the observed HFD-induced effects. Although an excellent rodent model of male reproductive aging (Gruenewald et al., 2008; Wang et al., 2002, 1993), brown Norway rats are a relatively lean strain in comparison to Sprague-Dawley rats (Gordon et al., 2016) and show more modest weight gain in response to HFD than strains such as Sprague-Dawley or Wistar rats (Buettner et al., 2007). Thus, the interaction between HFD and aging may be more pronounced in other animal models. Finally, although groups in Experiment 2 were controlled for surgeries, anesthesia is known to effect neural outcomes (Zhu et al., 2010) and may have affected findings differently across Experiments 1 and 2.
Collectively, our results show that the effects of HFD vary across increasing ages, and that middle-age may present a particularly vulnerable period. Importantly, the rate of obesity peaks at middle-age, with ~43% of US adults between the ages of 40 and 59 classified as obese (Hales et al., 2017). This represents a large population whose risk for a number of age-related diseases, including AD, could be mitigated by lifestyle changes. Midlife likely presents an especially important time-point for protective interventions including testosterone treatment in men, as interventions at midlife are well positioned to alter the trajectory of age-related diseases. However, consistent with an increasing literature on effects of testosterone in aging men (Emmelot-Vonk et al., 2008; Snyder et al., 2018; Storer et al., 2017), our findings in aging rats do not indicate robust benefits of testosterone supplementation. Determining the underlying pathological processes that are driving adverse effects of obesity is critical. Findings in our study suggest an important role for gliosis. In fact, it may be the case that factors like obesity are especially harmful at middle-age because of their role in driving inflammatory processes, as it was recently shown that inflammation in middle-age is associated with greater loss of brain volume later in life (Walker et al., 2017). Thus, it will be important to explore various risk factors 788 that drive adverse aging trajectories in midlife, as well as the mechanisms underlying their effects, with the ultimate goal of identifying strategies and interventions to alter these trajectories.
Aging and high-fat diet regulate metabolic, inflammatory, and neural outcomes.
Effects of high-fat diet are generally exacerbated at middle-age.
Gliosis is correlated with metabolic and behavioral effects of high-fat diet.
Testosterone treatment has negligible effects on consequences of high-fat diet.
Acknowledgements
This study was supported by NIH grants AG034103 (to CJP), AG051521 (to CE Finch/CJP), AG058068 (to CJP), Alzheimer’s Association grant (AARF-17-505302 to CRB), and a Canadian Institutes of Health Research operating grant (MOP102568 to LAMG). CRB was supported in part by NIH grant AG000037. The authors thank Dr. Wayne Yu (University of British Columbia) for the plasma testosterone assay, Dr. Camille Sample for contributions to behavioral analyses, and Ms. Christina Sisliyan for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan CA, Strauss BJG, Burger HG, Forbes EA, McLachlan RI, 2008. Testosteronetherapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobeseaging men. J. Clin. Endocrinol. Metab 93, 139–146. 10.1210/jc.2007-1291 [DOI] [PubMed] [Google Scholar]
- Arruda AP, Milanski M, Coope A, Torsoni AS, Ropelle E, Carvalho DP, Carvalheira JB, Velloso LA, 2011. Low-grade hypothalamic inflammation leads to defective thermogenesis, insulin resistance, and impaired insulin secretion. Endocrinology 1521314–1326. 10.1210/en.2010-0659 [DOI] [PubMed]
- Asih PR, Tegg ML, Sohrabi H, Carruthers M, Gandy SE, Saad F, Verdile G, Ittner LM, Martins RN, 2017. Multiple mechanisms linking type 2 diabetes and alzheimer’s disease: testosterone as a modifier. J. Alzheimers Dis 59, 445–466. 10.3233/JAD-161259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoub AE, Salm AK, 2003. Increased morphological diversity of microglia in the activatedhypothalamic supraoptic nucleus. J. Neurosci 23, 7759–7766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron AM, Rosario ER, Elteriefi R, Pike CJ, 2013. Sex-specific effects of high fat diet onindices of metabolic syndrome in 3xTg-AD mice: implications for Alzheimer’s disease. PLoS One 8, e78554 10.1371/journal.pone.0078554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil N, Alkaade S, Morley JE, 2009. The benefits and risks of testosterone replacementtherapy: a review. Ther Clin Risk Manag 5, 427–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof GN, Park DC, 2015. Obesity and aging: consequences for cognition, brain structure, and brain function. Psychosom. Med 77, 697–709. 10.1097/PSY.0000000000000212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasko I, Stampfer-Kountchev M, Robatscher P, Veerhuis R, Eikelenboom P, Grubeck-Loebenstein B, 2004. How chronic inflammation can affect the brain and support the development of Alzheimer’s disease in old age: the role of microglia and astrocytes. Aging Cell 3, 169–176. 10.1111/j.1474-9728.2004.00101.x [DOI] [PubMed] [Google Scholar]
- Bocarsly ME, Fasolino M, Kane GA, LaMarca EA, Kirschen GW, Karatsoreos IN, McEwen BS, Gould E, 2015. Obesity diminishes synaptic markers, alters microglialmorphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 112, 15731–15736. 10.1073/pnas.1511593112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boitard C, Etchamendy N, Sauvant J, Aubert A, Tronel S, Marighetto A, Layé S, Ferreira G, 2012. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 22, 2095–2100. 10.1002/hipo.22032 [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LAM, 2010. Chronic corticosterone during pregnancy and postpartumaffects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58, 769–779. 10.1016/j.yhbeh.2010.07.012 [DOI] [PubMed] [Google Scholar]
- Buettner R, Schölmerich J, Bollheimer LC, 2007. High-fat diets: modeling the metabolicdisorders of human obesity in rodents. Obesity (Silver Spring) 15, 798–808. 10.1038/oby.2007.608 [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S, 2005. Testosterone improves spatial memory in men withAlzheimer disease and mild cognitive impairment. Neurology 64, 2063–2068. 10.1212/01.WNL.0000165995.98986.F15 [DOI] [PubMed] [Google Scholar]
- Cournot M, Marquié JC, Ansiau D, Martinaud C, Fonds H, Ferrières J, Ruidavets JB, 2006. Relation between body mass index and cognitive function in healthy middle-agedmen and women. Neurology 67, 1208–1214. 10.1212/01.wnl.0000238082.13860.50 [DOI] [PubMed] [Google Scholar]
- Craft S, 2005. Insulin resistance syndrome and Alzheimer’s disease: age- and obesity-related effects on memory, amyloid, and inflammation. Neurobiol. Aging 26 Suppl 1, 65–69. 10.1016/j.neurobiolaging.2005.08.021 [DOI] [PubMed] [Google Scholar]
- Cunningham RL, Singh M, O’Bryant SE, Hall JR, Barber RC, 2014. Oxidative stress, testosterone, and cognition among Caucasian and Mexican-American men with andwithout Alzheimer’s disease. J. Alzheimers Dis 40, 563–573. 10.3233/JAD-131994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl AK, Hassing LB, Fransson EI, Gatz M, Reynolds CA, Pedersen NL, 2013. Bodymass index across midlife and cognitive change in late life. Int. J. Obes 37, 296–302. 10.1038/ijo.2012.37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day JR, Frank AT, O’Callaghan JP, Jones BC, Anderson JE, 1998. The effect of ageand testosterone on the expression of glial fibrillary acidic protein in the rat cerebellum. Exp. Neurol 151, 343–346. 10.1006/exnr.1998.6801 [DOI] [PubMed] [Google Scholar]
- de la Monte SM, Wands JR, 2008. Alzheimer’s disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol 2, 1101–1113. 10.1177/193229680800200619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Luca SN, Ziko I, Sominsky L, Nguyen JCD, Dinan T, Miller AA, Jenkins TA, Spencer SJ, 2016. Early life overfeeding impairs spatial memory performance by reducing microglial sensitivity to learning. J. Neuro inflammation 13, 112 10.1186/s12974-016-0578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Maddalena C, Vodo S, Petroni A, Aloisi AM, 2012. Impact of testosterone on body fatcomposition. J. Cell Physiol 227, 3744–3748. 10.1002/jcp.24096 [DOI] [PubMed] [Google Scholar]
- Edinger KL, Frye CA, 2004. Testosterone’s analgesic, anxiolytic, and cognitive-enhancing effects may be due in part to actions of its 5alpha-reduced metabolites in the hippocampus. Behav. Neurosci 118, 1352–1364. 10.1037/0735-7044.118.6.1352 [DOI] [PubMed] [Google Scholar]
- Elias MF, Elias PK, Sullivan LM, Wolf PA, D’Agostino RB, 2005. Obesity, diabetes and cognitive deficit: The Framingham Heart Study. Neurobiol. Aging 26 Suppl 1, 11–16. 10.1016/j.neurobiolaging.2005.08.019 [DOI] [PubMed] [Google Scholar]
- Emmelot-Vonk MH, Verhaar HJJ, Nakhai Pour HR, Aleman A, Lock TMTW, Bosch JLHR, Grobbee DE, van der Schouw YT, 2008. Effect of testosterone supplementation on functional mobility, cognition, and other parameters in older men: a randomized controlled trial. JAMA 299, 39–52. 10.1001/jama.2007.51 [DOI] [PubMed] [Google Scholar]
- Emmerzaal TL, Kiliaan AJ, Gustafson DR, 2015. 2003–2013: a decade of body mass index, Alzheimer’s disease, and dementia. J. Alzheimers Dis 43, 739–755. 10.3233/JAD-141086 [DOI] [PubMed] [Google Scholar]
- Erdos B, Kirichenko N, Whidden M, Basgut B, Woods M, Cudykier I, Tawil R, Scarpace PJ, Tumer N, 2011. Effect of age on high-fat diet-induced hypertension. Am. J. Physiol. Heart Circ. Physiol 301, H164–72. 10.1152/ajpheart.01289.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick AL, Kuller LH, Lopez OL, Diehr P, O’Meara ES, Longstreth WT, Luchsinger JA, 2009. Midlife and late-life obesity and the risk of dementia: cardiovascular health study. Arch. Neurol 66, 336–342. 10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, 2000. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci 908, 244–254. 10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- Freund A, Orjalo AV, Desprez P-Y, Campisi J, 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med 16, 238–246. 10.1016/j.molmed.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Edinger KL, Lephart ED, Walf AA, 2010. 3alpha-androstanediol, but not testosterone, attenuates age-related decrements in cognitive, anxiety, and depressive behavior of male rats. Front. Aging Neurosci 2, 15 10.3389/fnagi.2010.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Seliga AM, 2001. Testosterone increases analgesia, anxiolysis, and cognitive performance of male rats. Cogn Affect Behav Neurosci 1, 371–381. [DOI] [PubMed] [Google Scholar]
- Fui MNT, Dupuis P, Grossmann M, 2014. Lowered testosterone in male obesity: mechanisms, morbidity and management. Asian J Androl 16, 223–231. 10.4103/1008-682X.122365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LAM, 2008. Gonadal hormone modulation of neurogenesis in the dentate gyrus ofadult male and female rodents. Brain Res. Rev 57, 332–341. 10.1016/j.brainresrev.2007.05.008 [DOI] [PubMed] [Google Scholar]
- García-Cáceres C, Fuente-Martín E, Burgos-Ramos E, Granado M, Frago LM, Barrios V, Horvath T, Argente J, Chowen JA, 2011. Differential acute and chronic effects ofleptin on hypothalamic astrocyte morphology and synaptic protein levels. Endocrinology 152, 1809–1818. 10.1210/en.2010-1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH, 2010. Mechanisms underlying inflammation in neurodegeneration. Cell 140, 918–934. 10.1016/j.cell.2010.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Jarema K, Johnstone AFM, Phillips PM, 2016. Effect of genetic strain and gender on age-related changes in body composition of the laboratory rat. J Toxicol Environ Health Part A 79, 376–392. 10.1080/15287394.2016.1169237 [DOI] [PubMed] [Google Scholar]
- Granholm A-C, Bimonte-Nelson HA, Moore AB, Nelson ME, Freeman LR, Sambamurti K, 2008. Effects of a saturated fat and high cholesterol diet on memory and hippocampal morphology in the middle-aged rat. J. Alzheimers Dis 14, 133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald DA, Naai MA, Hess DL, Matsumoto AM, 2008. The Brown Norway Rat as a Model of Male Reproductive Aging: Evidence for Both Primary and Secondary Testicular Failure 1–9 [DOI] [PubMed]
- Guillemot-Legris O, Masquelier J, Everard A, Cani PD, Alhouayek M, Muccioli GG, 2016. High-fat diet feeding differentially affects the development of inflammation in the central nervous system. J. Neuroinflammation 13, 206 10.1186/s12974-016-0666-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales CM, Carroll MD, Fryar CD, Ogden CL, 2017. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS data brief, no 288 Hyattsville, MD: National Center for Health Statistics. [PubMed] [Google Scholar]
- Hao S, Dey A, Yu X, Stranahan AM, 2016. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav. Immun 51, 230–239. 10.1016/j.bbi.2015.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haren MT, Wittert GA, Chapman IM, Coates P, Morley JE, 2005. Effect of oral testosterone undecanoate on visuospatial cognition, mood and quality of life in elderly men with low-normal gonadal status. Maturitas 50, 124–133. 10.1016/j.maturitas.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Harvey J, Solovyova N, Irving A, 2006. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res 45, 369–378. 10.1016/j.plipres.2006.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CH, Del Tredici K, Braak H, 2007. Parkinson’s disease: a dual-hit hypothesis. Neuropathol Appl Neurobiol 33, 599–614. 10.1111/j.1365-2990.2007.00874.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden KM, Zandi PP, Lyketsos CG, Khachaturian AS, Bastian LA, Charoonruk G, Tschanz JT, Norton MC, Pieper CF, Munger RG, Breitner JCS, Welsh-Bohmer KA, Cache County Investigators, 2006. Vascular risk factors for incident Alzheimer disease and vascular dementia: the Cache County study. Alzheimer Dis Assoc Disord 20, 93–100. 10.1097/01.wad.0000213814.43047.86 [DOI] [PubMed] [Google Scholar]
- Ho AJ, Raji CA, Becker JT, Lopez OL, Kuller LH, Hua X, Lee S, Hibar D, Dinov ID, Stein JL, Jack CR, Weiner MW, Toga AW, Thompson PM, Cardiovascular Health Study ADNI, 2010. Obesity is linked with lower brain volume in 700 AD and MCI patients. Neurobiol. Aging 31, 1326–1339. 10.1016/j.neurobiolaging.2010.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L, Qin W, Pompl PN, Xiang Z, Wang J, Zhao Z, Peng Y, Cambareri G, Rocher A, Mobbs CV, Hof PR, Pasinetti GM, 2004. Diet-induced insulin resistance promotes amyloidosis in a transgenic mouse model of Alzheimer’s disease. FASEB J 18, 902–904. 10.1096/fj.03-0978fje [DOI] [PubMed] [Google Scholar]
- Irving AJ, Harvey J, 2014. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. Lond. B, Biol. Sci 369, 20130155 10.1098/rstb.2013.0155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isidori AM, Giannetta E, Greco EA, Gianfrilli D, Bonifacio V, Isidori A, Lenzi A, Fabbri A, 2005. Effects of testosterone on body composition, bone metabolism and serum lipid profile in middle-aged men: a meta-analysis. Clin. Endocrinol. (Oxf.) 63, 280–293. 10.1111/j.1365-2265.2005.02339.x [DOI] [PubMed] [Google Scholar]
- Jagust W, Harvey D, Mungas D, Haan M, 2005. Central obesity and the aging brain. Arch. Neurol 62, 1545–1548. 10.1001/archneur.62.10.1545 [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Oviatt SK, Orwoll ES, 1994. Testosterone influences spatial cognition in older men. Behav. Neurosci 108, 325–332. [DOI] [PubMed] [Google Scholar]
- Jayaraman A, Lent-Schochet D, Pike CJ, 2014. Diet-induced obesity and low testosteroneincrease neuro inflammation and impair neural function. J. Neuro inflammation 11, 162 10.1186/s12974-014-0162-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Pike CJ, 2014. Alzheimer’s disease and type 2 diabetes: multiple mechanisms contribute to interactions. Curr Diab Rep 14, 476 10.1007/s11892-014-0476-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien C, Tremblay C, Phivilay A, Berthiaume L, Emond V, Julien P, Calon F, 2010. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol. Aging 31, 1516–1531. 10.1016/j.neurobiolaging.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Zhang Y, Zheng W, Davidson TL, 2010. The effects of a high-energy diet onhippocampal function and blood-brain barrier integrity in the rat. J. Alzheimers Dis 21, 207–219. 10.3233/JAD-2010-091414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JG, Suyama S, Koch M, Jin S, Argente-Arizon P, Argente J, Liu Z-W, Zimmer MR, Jeong JK, Szigeti-Buck K, Gao Y, Garcia-Caceres C, Yi C-X, Salmaso N, Vaccarino FM, Chowen J, Diano S, Dietrich MO, Tschöp MH, Horvath TL, 2014. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci 17, 908–910. 10.1038/nn.3725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight EM, Martins IVA, Gümüsgöz S, Allan SM, Lawrence CB, 2014. High-fat diet induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol. Aging 35, 1821–1832. 10.1016/j.neurobiolaging.2014.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohjima M, Sun Y, Chan L, 2010. Increased food intake leads to obesity and insulin resistance in the tg2576 Alzheimer’s disease mouse model. Endocrinology 151, 1532–1540. 10.1210/en.2009-1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin LM, Reynolds TH, Supiano MA, Kahn BB, Halter JB, 2001. Effect of aging and obesity on insulin responsiveness and glut-4 glucose transporter content in skeletal muscle of Fischer 344 x Brown Norway rats. J. Gerontol. A, Biol. Sci. Med. Sci 56, B486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EB, Mattson MP, 2014. The neuropathology of obesity: insights from human disease. Acta Neuropathol 127, 3–28. 10.1007/s00401-013-1190-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C, 2006. High-fat diet impairs hippocampal neurogenesis in male rats. Eur. J. Neurol 13, 1385–1388. 10.1111/j.1468-1331.2006.01500.x [DOI] [PubMed] [Google Scholar]
- Lu PH, Masterman DA, Mulnard R, Cotman C, Miller B, Yaffe K, Reback E, Porter V, Swerdloff R, Cummings JL, 2006. Effects of testosterone on cognition and mood in male patients with mild Alzheimer disease and healthy elderly men. Arch. Neurol 63, 177–185. 10.1001/archneur.63.2.nct50002 [DOI] [PubMed] [Google Scholar]
- Maldonado-Ruiz R, Montalvo-Martínez L, Fuentes-Mera L, Camacho A, 2017. Microgliaactivation due to obesity programs metabolic failure leading to type two diabetes. Nutr Diabetes 7, e254 10.1038/nutd.2017.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLay RN, Freeman SM, Harlan RE, Kastin AJ, Zadina JE, 1999. Tests used to assess the cognitive abilities of aged rats: their relation to each other and to hippocampal morphology and neurotrophin expression. Gerontology 45, 143–155. 10.1159/000022077 [DOI] [PubMed] [Google Scholar]
- Mizuno T, Shu I-W, Makimura H, Mobbs C, 2004. Obesity over the life course. Sci. AgingKnowledge Environ. 2004, 10.1126/sageke.2004.24.re4 [DOI] [PubMed]
- Molteni R, Barnard RJ, Ying Z, Roberts CK, Gómez-Pinilla F, 2002. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. NSC 112, 803–814. [DOI] [PubMed] [Google Scholar]
- Moser VA, Pike CJ, 2017. Obesity Accelerates Alzheimer-Related Pathology in APOE4 butnot APOE3 Mice. Eneuro 4 10.1523/ENEURO.0077-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münzberg H, Morrison CD, 2015. Structure, production and signaling of leptin. Metab. Clin. Exp 64, 13–23. 10.1016/j.metabol.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L, 2012. Ageing as a risk factor for disease. Curr. Biol 22, R741–52. 10.1016/j.cub.2012.07.024 [DOI] [PubMed] [Google Scholar]
- Oh-I S, Thaler JP, Ogimoto K, Wisse BE, Morton GJ, Schwartz MW, 2010. Central administration of interleukin-4 exacerbates hypothalamic inflammation and weight gain during high-fat feeding. Am. J. Physiol. Endocrinol. Metab 299, E47–53. 10.1152/ajpendo.00026.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens W, Zeiger E, Walker M, Ashby J, Onyon L, Gray LE, 2006. The OECD program to validate the rat Hershberger bioassay to screen compounds for in vivo androgen and antiandrogen responses. Phase 1: use of a potent agonist and a potent antagonist to test the standardized protocol. Environ. Health Perspect 114, 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park K-Y, Chung HY, Lee J, 2010. A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor Neurosci. Lett 482, 235–239. 10.1016/j.neulet.2010.07.046 [DOI] [PubMed] [Google Scholar]
- Pike CJ, Carroll JC, Rosario ER, Barron AM, 2009. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol 30, 239–258. 10.1016/j.yfrne.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinilla L, Seoane LM, Gonzalez L, Carro E, Aguilar E, Casanueva FF, Dieguez C, 1999. Regulation of serum leptin levels by gonadal function in rats. Eur. J. Endocrinol 140, 468–473. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ, 2010. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol 219, 25–32. 10.1016/j.jneuroim.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponce OJ, Spencer-Bonilla G, Alvarez-Villalobos N, Serrano V, Singh-Ospina N, Rodriguez-Gutierrez R, Salcido-Montenegro A, Benkhadra R, Prokop LJ, Bhasin S, Brito JP, 2018. The efficacy and adverse events of testosterone replacement therapy in hypogonadal men: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Endocrinol. Metab 10.1210/jc.2018-00404 [DOI] [PubMed]
- Profenno LA, Porsteinsson AP, Faraone SV, 2010. Meta-analysis of Alzheimer’s disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 67, 505–512 10.1016/j.biopsych.2009.02.013 [DOI] [PubMed] [Google Scholar]
- Puts DA, Cárdenas RA, Bailey DH, Burriss RP, Jordan CL, Breedlove SM, 2010. Salivary testosterone does not predict mental rotation performance in men or women. Horm. Behav 58, 282–289. 10.1016/j.yhbeh.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Reger MA, Watson GS, Frey WH, Baker LD, Cholerton B, Keeling ML, Belongia DA, Fishel MA, Plymate SR, Schellenberg GD, Cherrier MM, Craft S, 2006. Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol. Aging 27, 451–458. 10.1016/j.neurobiolaging.2005.03.016 [DOI] [PubMed] [Google Scholar]
- Resnick SM, Matsumoto AM, Stephens-Shields AJ, Ellenberg SS, Gill TM, Shumaker SA, Pleasants DD, Barrett-Connor E, Bhasin S, Cauley JA, Cella D, Crandall JP, Cunningham GR, Ensrud KE, Farrar JT, Lewis CE, Molitch ME, Pahor M, Swerdloff RS, Cifelli D, Anton S, Basaria S, Diem SJ, Wang C, Hou X, Snyder PJ, 2017. Testosterone Treatment and Cognitive Function in Older Men With Low Testosterone and Age-Associated Memory Impairment. JAMA 317, 717–727. 10.1001/jama.2016.21044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera EJ, Goldin A, Fulmer N, Tavares R, Wands JR, de la Monte SM, 2005. Insulin and insulin-like growth factor expression and function deteriorate with progression of Alzheimer’s disease: link to brain reductions in acetylcholine. J. Alzheimers Dis 8, 247–268. [DOI] [PubMed] [Google Scholar]
- Ronan L, Alexander-Bloch AF, Wagstyl K, Farooqi S, Brayne C, Tyler LK, Cam-CAN, Fletcher PC, 2016. Obesity associated with increased brain age from midlife. Neurobiol. Aging 47, 63–70. 10.1016/j.neurobiolaging.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Pike CJ, 2008. Androgen regulation of beta-amyloid protein and the risk of Alzheimer’s disease. Brain Res. Rev 57, 444–453. 10.1016/j.brainresrev.2007.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setti SE, Littlefield AM, Johnson SW, Kohman RA, 2015. Diet-induced obesity attenuates endotoxin-induced cognitive deficits. Physiol. Behav 141, 1–8. 10.1016/j.physbeh.2014.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan TJ, DuBrava S, DeChello LM, Fang Z, 2003. Rates of weight change for blackand white Americans over a twenty year period. Int J Obes Relat Metab Disord 27, 498–504. 10.1038/sj.ijo.0802263 [DOI] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K, 2007. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424. 10.1002/glia.20468 [DOI] [PubMed] [Google Scholar]
- Snyder PJ, Bhasin S, Cunningham GR, Matsumoto AM, Stephens-Shields AJ, Cauley JA, Gill TM, Barrett-Connor E, Swerdloff RS, Wang C, Ensrud KE, Lewis CE, Farrar JT, Cella D, Rosen RC, Pahor M, Crandall JP, Molitch ME, Resnick SM, Budoff M, Mohler ER, Wenger NK, Cohen HJ, Schrier S, Keaveny TM, Kopperdahl D, Lee D, Cifelli D, Ellenberg SS, 2018. Lessons from the testosterone trials. Endocr. Rev 39, 369–386. 10.1210/er.2017-00234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, D’Angelo H, Soch A, Watkins LR, Maier SF, Barrientos RM, 2017. High-fat diet and aging interact to produce neuroinflammation and impair hippocampal- and amygdalar-dependent memory. Neurobiol. Aging 58, 88–101. 10.1016/j.neurobiolaging.2017.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spritzer MD, Galea LAM, 2007. Testosterone and dihydrotestosterone, but not estradiol, enhance survival of new hippocampal neurons in adult male rats. Dev. Neurobiol 67, 1321–1333. 10.1002/dneu.20457 [DOI] [PubMed] [Google Scholar]
- Stanek KM, Grieve SM, Brickman AM, Korgaonkar MS, Paul RH, Cohen RA, Gunstad JJ, 2011. Obesity is associated with reduced white matter integrity in otherwise healthy adults. Obesity (Silver Spring) 19, 500–504. 10.1038/oby.2010.312 [DOI] [PubMed] [Google Scholar]
- Storer TW, Basaria S, Traustadottir T, Harman SM, Pencina K, Li Z, Travison TG, Miciek R, Tsitouras P, Hally K, Huang G, Bhasin S, 2017. Effects of testosterone supplementation for 3 years on muscle performance and physical function in older men. J. Clin. Endocrinol. Metab 102, 583–593. 10.1210/jc.2016-2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Norman ED, Lee K, Cutler RG, Telljohann RS, Egan JM, Mattson MP, 2008. Diet-induced insulin resistance impairs hippocampal synaptic plasticity and cognition in middle-aged rats. Hippocampus 18, 1085–1088. 10.1002/hipo.20470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaler JP, Yi C-X, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW, 2012. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest 122, 153–162. 10.1172/JCI59660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traish AM, Haider A, Doros G, Saad F, 2014. Long-term testosterone therapy in hypogonadal men ameliorates elements of the metabolic syndrome: an observation allong-term registry study. Int. J. Clin. Pract 68, 314–329. 10.1111/ijcp.12319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucsek Z, Toth P, Sosnowska D, Gautam T, Mitschelen M, Koller A, Szalai G, Sonntag WE, Ungvari Z, Csiszar A, 2014. Obesity in aging exacerbates blood-brain barrier disruption, neuroinflammation, and oxidative stress in the mouse hippocampuseffects on expression of genes involved in beta-amyloid generation and Alzheimer’sdisease. J. Gerontol. A, Biol. Sci. Med. Sci 69, 1212–1226. 10.1093/gerona/glt177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker KA, Hoogeveen RC, Folsom AR, Ballantyne CM, Knopman DS, Windham BG, Jack CR, Gottesman RF, 2017. Midlife systemic inflammatory markers are associated with late-life brain volume: The ARIC study. Neurology 10.1212/WNL.0000000000004688 [DOI] [PMC free article] [PubMed]
- Wang C, Hikim AS, Ferrini M, Bonavera JJ, Vernet D, Leung A, Lue Y-H, Gonzalez-Cadavid NF, Swerdloff RS, 2002. Male reproductive ageing: using the brown norway rat as a model for man, in: Chadwick DJ, Goode JA (Eds.), Endocrine Facets of Ageing, Novartis Foundation Symposia John Wiley & Sons, Ltd, Chichester, UK, pp. 82–97. 10.1002/0470846542.ch6 [PubMed] [Google Scholar]
- Wang C, Leung A, Sinha-Hikim AP, 1993. Reproductive aging in the male brown-Norwayrat: a model for the human. Endocrinology 133, 2773–2781. 10.1210/endo.133.6.8243304 [DOI] [PubMed] [Google Scholar]
- Watson GS, Cholerton BA, Reger MA, Baker LD, Plymate SR, Asthana S, Fishel MA, Kulstad JJ, Green PS, Cook DG, Kahn SE, Keeling ML, Craft S, 2005. Preserved Cognition in Patients With Early Alzheimer Disease and … Am. J. Geriatr. Psychiatry 13, 950–958. [DOI] [PubMed] [Google Scholar]
- Watson GS, Peskind ER, Asthana S, Purganan K, Wait C, Chapman D, Schwartz MW, Plymate S, Craft S, 2003. Insulin increases CSF Abeta42 levels in normal older adults. Neurology 60, 1899–1903. [DOI] [PubMed] [Google Scholar]
- Whitmer RA, Gustafson DR, Barrett-Connor E, Haan MN, Gunderson EP, Yaffe K, 2008. Central obesity and increased risk of dementia more than three decades later. Neurology 71, 1057–1064. 10.1212/01.wnl.0000306313.89165.ef [DOI] [PubMed] [Google Scholar]
- Wilhelmsson U, Bushong EA, Price DL, Smarr BL, Phung V, Terada M, Ellisman MH, Pekny M, 2006. Redefining the concept of reactive astrocytes as cells that remain within their unique domains upon reaction to injury. Proc. Natl. Acad. Sci. USA 103, 17513–17518. 10.1073/pnas.0602841103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolden-Hanson T, Marck BT, Smith L, Matsumoto AM, 1999. Cross-sectional and longitudinal analysis of age-associated changes in body composition of male Brown Norway rats: association of serum leptin levels with peripheral adiposity. J. Gerontol. A, Biol. Sci. Med. Sci 54, B99–107. [DOI] [PubMed] [Google Scholar]
- Wu D, Ren Z, Pae M, Guo W, Cui X, Merrill AH, Meydani SN, 2007. Aging up regulates expression of inflammatory mediators in mouse adipose tissue. J. Immunol 179, 4829–4839. 10.4049/jimmunol.179.7.4829 [DOI] [PubMed] [Google Scholar]
- Yassin D-J, Doros G, Hammerer PG, Yassin AA, 2014. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J. Sex. Med 11, 1567–1576. 10.1111/jsm.12523 [DOI] [PubMed] [Google Scholar]
- York JM, Blevins NA, Meling DD, Peterlin MB, Gridley DS, Cengel KA, Freund GG, 2012. The biobehavioral and neuroimmune impact of low-dose ionizing radiation. Brain Behav. Immun 26, 218–227. 10.1016/j.bbi.2011.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Manson JE, Yuan C, Liang MH, Grodstein F, Stampfer MJ, Willett WC, Hu FB, 2017. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA 318, 255–269. 10.1001/jama.2017.7092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K, 2010. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J. Cereb. Blood Flow Metab 30, 1017–1030. 10.1038/jcbfm.2009.274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Perry G, Smith MA, 2004. Alzheimer’s disease: the two-hit hypothesis. Lancet Neurol 3, 219–226. 10.1016/S1474-4422(04)00707-0 [DOI] [PubMed] [Google Scholar]
- Zitzmann M, 2009. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat. Rev. Endocrinol 5, 673–681. 10.1038/nrendo.2009.212 [DOI] [PubMed] [Google Scholar]