Abstract
Mitochondria are organelles involved in the production of cellular energy, regulation of Ca2+ and redox signaling, and are critical for normal functioning of eukaryotic cells. The dysfunction of mitochondria has been implicated in a wide range of diseases, including metabolic and neurodegenerative disorders and different types of cancers. To better understand the role of mitochondria in healthy and disease states, the development of efficient and reliable tools for the assessment of mitochondrial function is particularly important. Janus green B (JG-B) is a supravital lipophilic cationic dye which, in its oxidized form, has a green-blue color. As JG-B is taken up and reduced by metabolically active mitochondria, the dye has been used for assessing the purity, integrity and metabolic activity of mitochondria with microscopy-based methods. Here we present a simple, time- and cost-efficient JG-B-based colorimetric assay for assessing mitochondrial function, activity and toxicity. The method is based upon reduction of JG-B by mitochondrial dehydrogenases to diethylsafranine, which is pink colored and has a maximum absorption at 550 nm. In this proof of principle study, using in vitro mitochondrial preparations isolated from rat brain, we provide evidence that monitoring JG-B conversion to diethylsafranine can be used as a reliable and robust indicator of mitochondrial activity and toxicity. Because of its simplicity and efficiency in terms of costs and time, this assay has a wide potential in analytical as well as therapeutic areas of biomedical research.
Keywords: Janus green B, diethylsafranine, dehydrogenases, mitochondrial integrity, mitochondrial energization
INTRODUCTION
Mitochondria are organelles responsible for the production of cellular energy in the form of adenosine triphosphate (ATP), and the regulation of Ca2+ and redox signaling among other processes. In neurons, the normal functioning of mitochondria is particularly important for the energy consuming processes including the formation of electrochemical gradients, cell excitability and synaptic activity. Consequently, mitochondrial dysfunction and associated cell death can lead to the development of different pathological conditions, including neurodegenerative disease. Thus, analyzing mitochondrial function in healthy and disease states is essential for understanding the mechanisms underlying such conditions as well as for developing effective therapeutic strategies.
Janus green B (JG-B) or 8-[[4-(dimethylamino)phenyl]diazenyl]-N,N-diethyl-10-phenylphenazin-10-ium-2-amine is a supravital lipophilic cationic dye. Like other lipophilic cations, JG-B is taken up only by metabolically active mitochondria in which the inside-negative membrane potential is intact [1,2]. Due to this property, JG-B has been extensively used for assessing the purity, integrity and metabolic activity of mitochondria with microscopy-based methods [3-14]. In its oxidized form JG-B has a green-blue color and absorbs maximally at 595 nm. Upon reduction by mitochondrial dehydrogenases, JG-B is converted to diethylsafranine which is pink colored and has an absorption maxima at 550 nm [15-18]. Altered mitochondrial dehydrogenase activity is often associated with mitochondrial damage and dysfunction [19], thus, the capacity of enzymes to convert JG-B to diethylsafranine can be used as an indicator of mitochondrial function.
Spectrophotometric analysis of mitochondrial function with JG-B has been reported previously [20,21], however, those colorimetric assays were based on the detection of changes in JG-B concentration (at 595-610 nm) rather than formation of diethylsafranine, which is the actual end product of mitochondrial reduction of JG-B. JG-B can also be reduced by dehydrogenases from cytosolic fractions, but predominantly to a colorless end product leucosafranine. Thus, measuring JG-B levels at 595-610 nm does not differentiate between mitochondrial (formation of diethylsafranine) and non-mitochondrial reduction of JG-B (formation of leucosafranine). This may lead to erroneous results, i.e. the overestimation of JG-B reduction by mitochondrial dehydrogenases. On the other hand, the formation of diethylsafranine is specific to mitochondrial reduction of JG-B, and measuring the absorbance at 550 nm can provide a better estimation of mitochondrial activity.
In this study, we presented a modified and improved colorimetric assay for assessing mitochondrial function, which is based on the detection of diethylsafranine as the product of JG-B reduction by mitochondrial dehydrogenases. The ratio of the absorbance at 550 (absorption maximum of diethylsafranine) and 595 nm (absorption maximum of JG-B) [22] was used to assess the concentration of diethylsafranine. Our results indicate that this colorimetric assay is a robust, reliable and economical tool for analyzing mitochondrial function and toxicity, and it can be used independently or in a combination with the established fluorescence-based assays [23,24].
MATERIALS AND METHODS
Chemicals and reagents
All chemicals used in this study were of analytical grade and obtained from Merck (Darmstadt, Germany) or Sigma-Aldrich (Steinheim, Germany). Janus green B was obtained from Merck.
Animals
All experiments involving animals were carried out in accordance with the institutional guidelines for animal care and use in scientific research and the Helsinki Declaration (1975), and conducted after the approval from the Institutional Review Board of the Imam Abdulrahman Bin Faisal University. Adult Wistar rats were housed in cages under a 12/12 light/dark cycle in rooms with controlled temperature of 25 °C, and with free access to food and water.
Mitochondrial isolation
Rats were decapitated under anesthesia and brain cortices were dissected out immediately. Mitochondria from cerebral cortices were isolated using discontinuous sucrose gradient ultracentrifugation protocol, as described previously and with some modifications [25]. Briefly, cerebral cortices were homogenized in 10 times volume of isolation buffer [10 mM Tris, 1 mM EDTA(K), 320 mM sucrose, pH 7.4] using a Potter-Elvehjem pestle and glass tube on ice. The homogenate was then centrifuged at 1300 g for 10 minutes at 4 °C and the resultant supernatant (post-nuclear supernatant) was further centrifuged at 17,000 g for 10 minutes at 4 °C to obtain crude mitochondrial pellet (CMP). CMP was resuspended in isolation buffer and loaded onto a discontinuous 1.2-1.0-0.8 M sucrose gradient and centrifuged at 100,000 g for 60 minutes at 4 °C. This separated CMP into myelin (at first interface), synaptosomes (at second interface), and mitochondria as a pellet. The enriched mitochondrial pellet was then washed twice with isolation buffer at 10,000 g for 10 minutes at 4 °C. Mitochondria were resuspended in isolation buffer, aliquoted and stored at -80°C. The purity of mitochondrial fraction was assessed by the enrichment of succinate dehydrogenase (SDH) activity, a well-known mitochondrial marker.
Activity assay of Complex II (succinate dehydrogenase)
SDH activity was measured in homogenate, post-nuclear supernatant, post-mitochondrial supernatant, and crude and enriched mitochondrial fractions to verify mitochondrial enrichment at each stage of purification. Assay for SDH activity was performed as described previously [26]. Briefly, purified and crude mitochondrial or subcellular fractions (0.1 mg of protein each) were suspended in 50 mM (K) phosphate buffer (pH 7.4) containing 3 mM potassium ferricyanide (III) acting as an exogenous electron acceptor. A decrease in the absorption (420 nm) upon the addition of 50 mM succinate was followed to measure the rate of potassium ferrocyanide (II) formation at 30 °C for 2 minutes. The reaction rate was calculated as nmol ferrocyanide formed per minute per mg protein and represented with respect to the rate of homogenate (ε420 for potassium ferricyanide = 1040 M-1 cm-1). Pure mitochondria pretreated with 500 mM malonate, a competitive inhibitor of SDH, was used as a negative control.
Mitochondrial integrity assay
The integrity of mitochondria isolated from rat cerebral cortices was evaluated by measuring fumarase activity (a marker of mitochondrial matrix), as reported previously [27]. The assay is based on the formation of fumarate upon the addition of L-malate to mitochondrial samples; fumarase catalyzes reversible conversion of malate to fumarate which is observed as an increase in the absorbance at 240 nm. Briefly, an increase in the absorbance (240 nm) was followed after the addition of 50 mM malate to mitochondria (0.1 mg protein) suspended in isolation buffer in the presence or absence of 0.2 % Triton X-100 detergent. The linear portion of the kinetic curve was used to calculate the slope of fumarase activity, expressed as nmol fumarate formed/min/mg protein (ε240 for fumarate = 2.44 mM-1 cm-1). The integrity of mitochondria was calculated as 1-(ν-TX/ν+TX), where ν-TX and ν+TX represent fumarase reaction rate in the absence and presence of Triton X-100 respectively, and expressed as a percentage.
Mitochondrial reduction of JG-B
To evaluate mitochondrial reduction of JG-B, 10 µM JG-B was incubated with different amounts of mitochondrial preparations resuspended in isolation buffer. The absorbance was recorded at 550 and 595 nm before and 10 minutes after the addition of mitochondrial preparations. For the experiments involving mitochondrial uptake of JG-B and its subsequent reduction, mitochondria were incubated with JG-B and then pelleted down at 17,000 g for 5 minutes to remove the excess unbound JG-B. Mitochondria bound with JG-B were then resuspended in isolation buffer and the absorbance was recorded. The reduction of JG-B by energized mitochondria was carried out by incubation of 10 µM JG-B with mitochondria (500 µg protein) in presence of varying concentrations of mitochondrial substrates glutamate and malate. In the experiments involving blockade of dehydrogenases of the electron transport chain (ETC), mitochondria were pretreated with 10 µM rotenone, 100 mM malonate and 10 mM sodium azide for 10 minutes to block the activity of complex I, II and IV, respectively, before incubation with the substrates malate and glutamate (25 mM each). The integrity of mitochondria in some experiments was compromised by their preincubation with 0.2% Triton X-100 for 10 minutes at room temperature.
Spectrophotometric measurements
All spectroscopic measurements were carried out using a Tecan Infinite 200 PRO microplate reader (Tecan, Mannedorf, Switzerland).
Statistics
Statistical differences between two groups were determined using unpaired two-tailed Student’s t-test. All graphical representation of data and statistical analyses including the linear and non-linear (semi-log) analysis were performed with GraphPad Prism software (CA, USA).
RESULTS
Absorption spectra of JG-B and its derivative diethylsafranine
Absorption spectra of JG-B and diethylsafranine, the derivative obtained after reductive cleavage of JG-B, were recorded to determine their respective absorption maxima (Figure 1). Reductive cleavage of JG-B carried out using sodium dithionite as described elsewhere [22], resulted in the red shift of the absorption maxima from 595 to 550 nm, corresponding to that of pink-colored diethylsafranine.
FIGURE 1.
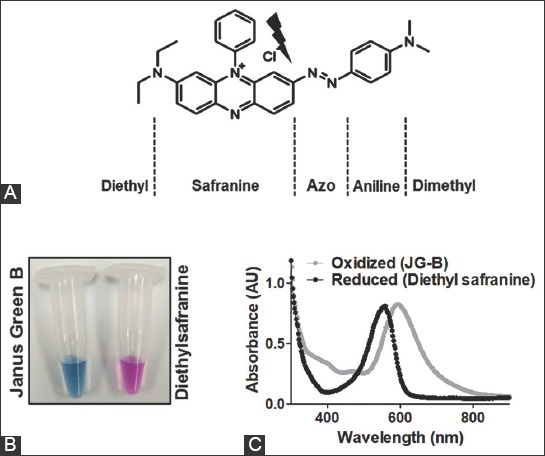
Janus green B (JG-B) and its reductive cleavage product diethylsafranine have distinct absorption spectra. (A) The chemical structure of JG-B is shown along with the chemical groups. The thunderbolt sign represents the site of reductive cleavage of JG-B leading to the formation of diethylsafranine. (B) Reductive cleavage of green-blue JG-B upon its treatment with reducing agent sodium dithionite leads to the formation of pink-colored diethylsafranine. (C) Absorption spectra of JG-B and diethylsafranine are shown; reduction of JG-B to diethylsafranine shifts the absorption maximum from 595 nm to 550 nm.
Analysis of enrichment and integrity of mitochondrial preparations
Enrichment/purity of mitochondria prepared from rat cerebral cortices was confirmed by analyzing the relative levels of SDH activity (Figure 2). There was a ~6.5 fold increase in the activity of SDH in the purified mitochondrial fraction compared to the initial homogenate, indicating a high enrichment of mitochondria. In addition, the integrity of mitochondria was confirmed using fumarase assay and found to be 93.57 ± 3.20% (mean ± SD).
FIGURE 2.
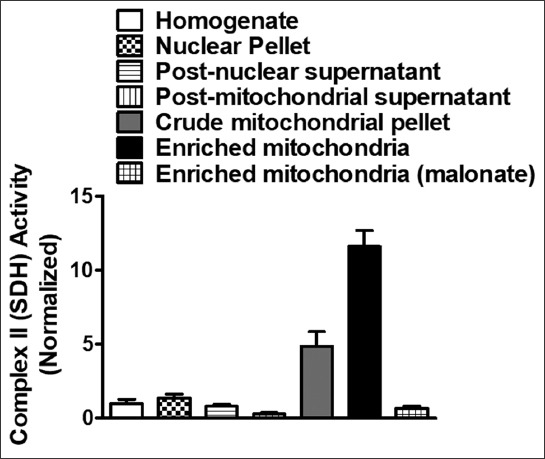
Density gradient subcellular fractionation results in highly pure rat brain cortical mitochondria. Enrichment of complex II (succinate dehydrogenase [SDH]) activity was spectrophotometrically measured for cortical mitochondria isolated using discontinuous sucrose gradient ultracentrifugation method. SDH activity levels were enriched by 6.5 ± 0.7 (mean ± SD) times compared to homogenate. Data is presented as mean ± standard error of the mean [SEM] (n = 4).
Reductive cleavage of JG-B to diethylsafranine occurs specifically in the presence of mitochondria and increases linearly with increasing amount of mitochondria
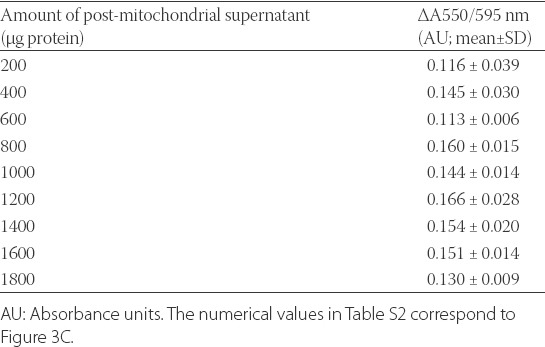
To confirm the reduction of JG-B to diethylsafranine, the absorption spectrum of JG-B incubated with mitochondria was recorded and compared with free-aqueous JG-B. As seen in Figure 3A, an absorption peak at 550 nm (corresponding to diethylsafranine) was observed only when JG-B was incubated with mitochondria, confirming that mitochondria can reduce JG-B to diethylsafranine. To determine whether mitochondria reduces JG-B to diethylsafranine in a dose dependent manner, JG-B was incubated with increasing amounts of mitochondria. The increase in the absorbance ratio at 550/595 nm with the increasing amounts of mitochondria fractions followed a linear trend (Figure 3B). For the highest amount of mitochondria analyzed (2000 µg protein), the ratio of absorbance at 550/595 nm was 0.359 ± 0.029 absorbance units [AU] (mean ± SD), approximately 3 folds higher compared to the ratio obtained with 200 µg of mitochondrial protein (Table S1). Moreover, the incubation of JG-B with increasing amounts of post-mitochondrial supernatant, which is essentially deprived of all mitochondria, did not increase the absorbance ratio (550/595 nm; Table S2; Figure 3C), suggesting that the conversion of JG-B to diethylsafranine is specific for mitochondria.
FIGURE 3.
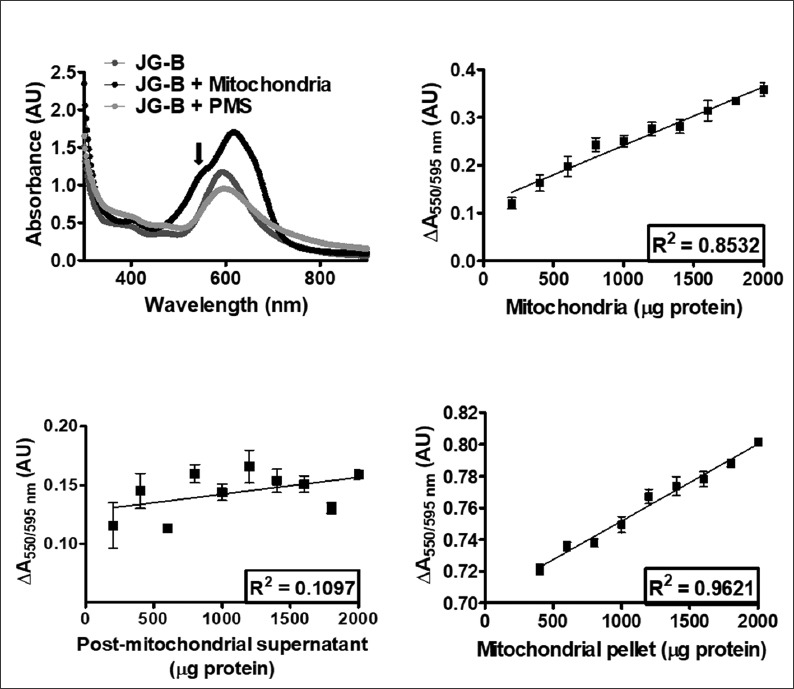
Reductive conversion of Janus green B (JG-B) to diethylsafranine is specifically observed only in the presence of mitochondria (and not in the presence of post-mitochondrial supernatant), and the amount of formed diethylsafranine correlates significantly with the increase in the amount of mitochondria. (A) Absorption spectra of JG-B alone, incubated with mitochondria, or with post-mitochondrial supernatant are shown. Conversion of JG-B to diethylsafranine occurs only in the presence of mitochondria, observed as a peak at 550 nm (marked with the arrow). (B) Reductive cleavage of JG-B to diethylsafranine, measured as the absorbance ratio 550 nm/595 nm increases with the increase in the amount of mitochondria and follows a linear trend. (C) No formation of diethylsafranine was observed when JG-B was incubated with mitochondria-depleted post-mitochondrial supernatant, even up to 2000 µg of protein. (D) Binding and conversion of JG-B to diethylsafranine, assessed after mitochondrial pelleting and removal of free (unbound) JG-B, follows a linear trend with increasing amounts of mitochondria. Data is presented as mean ± standard error of the mean [SEM] (n = 4).
TABLE S1.
Numerical values of the absorbance ratio 550/595 nm for the reduction of Janus green B (JG-B) to diethylsafranine in the presence of increasing amounts of mitochondria
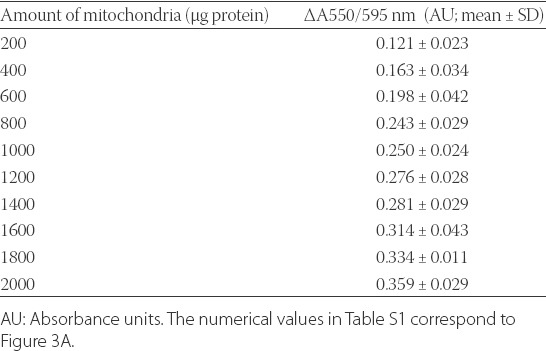
TABLE S2.
Numerical values of the absorbance ratio 550/595 nm for the reduction of Janus green B (JG-B) to diethylsafranine in the presence of increasing amounts of post-mitochondrial supernatant
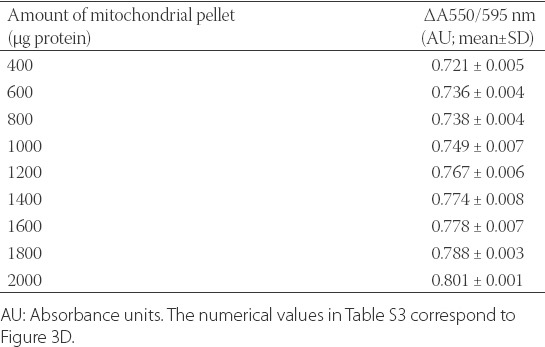
To further confirm that JG-B binds to and is converted to diethylsafranine by mitochondria, JG-B was incubated with increasing amounts of mitochondria for 15 minutes, followed by pelleting of mitochondria and removal of unbound free JG-B. The absorbance ratio (550/595 nm) increased linearly with the increasing amounts of resuspended mitochondrial pellet, indicating that JG-B enters mitochondria where it is reduced to diethylsafranine by mitochondrial enzymes. For the highest amount of resuspended mitochondrial pellet (2000 µg protein) the absorbance ratio (550/595 nm) was 0.801 ± 0.001 (mean ± SD), which was significantly higher than the absorbance ratio of 0.721 ± 0.005 (mean ± SD) obtained for 400 µg of mitochondrial pellet (Table S3). Moreover, similar to the previous experiment, the amount of reduced JG-B followed a linear relationship with increasing amounts of mitochondria (Figure 3D).
TABLE S3.
Numerical values of the absorbance ratio 550/595 nm for the reduction of Janus green B (JG-B) to diethylsafranine in the presence of increasing amounts of mitochondrial pellet
Energization of mitochondria by substrates increases mitochondrial entry of JG-B and its subsequent reduction to diethylsafranine in a dose-dependent manner
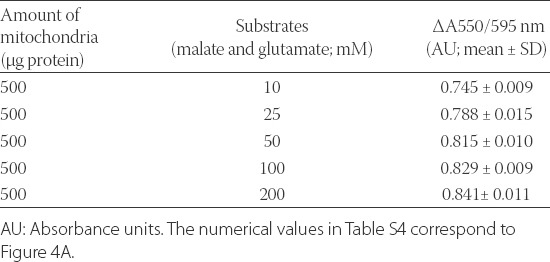
Because JG-B is a lipophilic cation, energization of mitochondria in the presence of substrates increases the uptake of JG-B [2]. Moreover, substrates increase mitochondrial reduction of JG-B due to the activation of oxidoreductases in the ETC [28,29]. Therefore, we energized mitochondria with varying concentrations of glutamate and malate and observed their effects on JG-B reduction. The addition of increasing amounts of mitochondrial substrates increased the reduction of JG-B to diethylsafranine in a logarithmic manner, following a linear trend up to 50 mM glutamate and malate (Figure 4A; Table S4). The pretreatment of mitochondria with the inhibitors of the ETC (rotenone, malonate, and azide) reduced the conversion of JG-B to diethylsafranine, from 0.905 ± 0.053 AU (mean ± SD) in the presence of 50 mM malate and glutamate to 0.243 ± 0.077 AU (mean ± SD) in the presence of both the ETC substrates and inhibitors (Figure 4B), as assessed by the absorbance ratio (550/595 nm). These results indicate that the proposed colorimetric JG-B-based assay is a robust and reliable method to analyze energy status and function of mitochondria.
FIGURE 4.
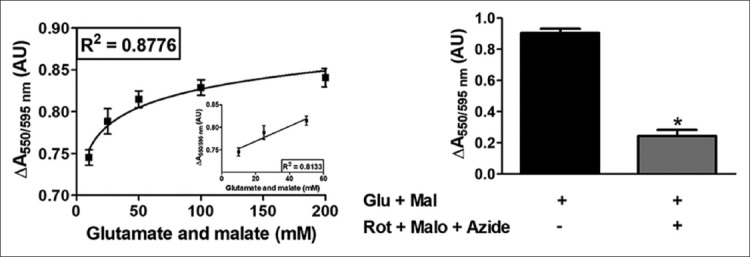
Mitochondrial reduction of Janus green B (JG-B) to diethylsafranine is increased in the presence of substrates malate and glutamate in a dose-dependent manner. (A) Mitochondria (500 µg protein) were incubated with varying concentrations of malate and glutamate and 10 µM JG-B. Availability of substrates increases the bio-energetic activity of mitochondria leading to increased reduction of JG-B to diethylsafranine, measured as the absorbance ratio 550 nm/595 nm. The increased formation of diethylsafranine in the presence of energized mitochondria followed a logarithmic trend. The increase in formation of diethylsafranine was, however, linear up to 50 mM of malate and glutamate (A inset). (B) Pretreatment of mitochondria with inhibitors of the complex I, II and IV (rotenone, malonate and sodium azide; respectively) inhibited the formation of diethylsafranine. Mal and glu refer to malate and glutamate and Rot, Malo and Azide refer to rotenone, malonate and sodium azide; respectively. Data is represented as mean ± standard error of the mean [SEM] (n = 5) and *denotes statistical significance (p < 0.0001).
TABLE S4.
Numerical values of the absorbance ratio 550/595 nm for the reduction of Janus green B (JG-B) to diethylsafranine in the presence of increasing amounts of mitochondria and substrates malate and glutamate
Triton X-100 compromises mitochondrial integrity and lowers its ability to reduce JG-B to diethylsafranine
JG-B can only bind to and is reduced by intact mitochondria, and loss of mitochondrial integrity reduces both the binding and reduction of JG-B [6,30]. To determine whether the proposed assay could detect changes in mitochondrial integrity, mitochondria were incubated with and without 0.2% Triton X-100 for 10 minutes. The conversion of JG-B to diethylsafranine (measured by the absorption ratio at 550/595 nm) was reduced in mitochondria pretreated with Triton X-100 (0.407 ± 0.060; mean ± SD) compared to untreated intact mitochondria (0.821 ± 0.197; mean ± SD; Figure 5). This provides evidence that the described colorimetric assay is suitable for assessing alterations in mitochondrial integrity.
FIGURE 5.
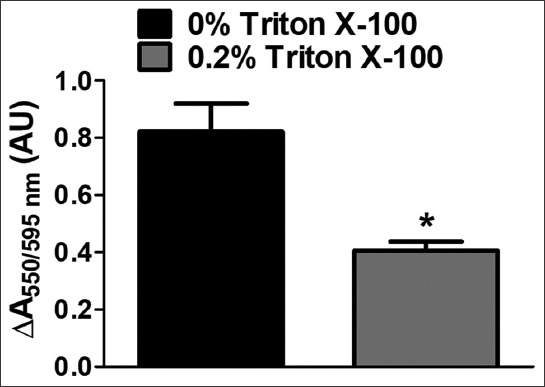
Compromised integrity of mitochondria in the presence of Triton X-100 lowers its ability to reduce Janus green B (JG-B) to diethylsafranine. Mitochondria (500 µg protein) were exposed to 0.2% Triton X-100 for 10 minutes and subsequently allowed to reduce JG-B (10 µM). Mitochondrial reduction of JG-B to diethylsafranine was restricted when the integrity of mitochondria was compromised by Triton X-100. Data is represented as mean ± standard error of the mean [SEM] (n = 5) and *denotes statistical significance (p = 0.0069).
DISCUSSION
In the earlier published studies, the reduction of JG-B was considered to be specific only to mitochondria due to the absence of the Krebs cycle and ETC dehydrogenases in non-mitochondrial fraction [15], which suggested that JG-B could be used as a mitochondria-specific dye. However, other studies showed that, in addition to the mitochondrial reduction of JG-B to pink-colored diethylsafranine [16,17], dehydrogenases in non-mitochondrial fraction could also reduce JG-B, but to a colorless leucosafranine instead of diethylsafranine as the end product [16,28]. Our results confirm that the conversion of JG-B to pink-colored diethylsafranine is specifically carried out by mitochondria, as we did not observe the reduction of JG-B to diethylsafranine after incubation with post-mitochondrial supernatant (Figure 3C). Based on this JG-B property, we have developed a reliable, time- and cost-efficient colorimetric assay for assessing mitochondrial function, which uses diethylsafranine (measured at 550 nm) as an indicator of mitochondrial activity, integrity and toxicity.
Two important factors should be considered in JG-B-based assessment of mitochondria. First, studies on supravital staining of mitochondria showed that, when JG-B is exposed to mitochondrial enzymes for an extended period of time, all diethylsafranine is eventually converted to colorless leucosafranine [16,17,28]. However, this does not impair the quality of our assay as it requires that JG-B is exposed to mitochondrial dehydrogenases for 10-15 minutes, while the conversion of diethylsafranine to leucosafranine proceeds slowly and decolorization is observed only after several hours of exposure. Second, cytotoxic effects of JG-B were observed when the dye was used in very high concentrations or for extended periods of time [1,31]. This property of JG-B also does not affect the functionality of the proposed assay, which requires low concentrations of JG-B (10 µM) and short exposure (15 minutes); however, it makes JG-B unsuitable for longer-term monitoring of mitochondrial function in living cells [1,32,33]. Moreover, the specificity of JG-B to mitochondria is lost when the dye is used in high concentrations [3].
Other lipophilic cations, such as fluorescent rhodamine and cyanine dyes are widely used for functional characterization of mitochondria [23,24]. With this regard, our modified JG-B-based assay may be used independently or in combination with the established tools for assessing mitochondrial function.
CONCLUSION
Overall, this study provides a proof of principle for an improved assay of mitochondrial function which is based on reductive cleavage of JG-B to diethylsafranine. The described assay represents a reliable, time- and cost-efficient tool for assessing mitochondrial activity and toxicity in both basic and applied biomedical research.
ACKNOWLEDGMENTS
The study was partly funded by Deanship of Scientific Research, Imam Abdulrahman Bin Faisal University (Project No. 2016-087-IRMC).
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Chen LB. Potential use of mitochondria as a reservoir for photosensitive lipophilic cations. New Dir Photodyn Ther. 1987;847:46–50. https://doi.org/10.1117/12.942688. [Google Scholar]
- [2].Ross MF, Kelso GF, Blaikie FH, James AM, Cocheme HM, Filipovska A, et al. Lipophilic triphenylphosphonium cations as tools in mitochondrial bioenergetics and free radical biology. Biochemistry (Mosc) 2005;70(2):222–30. doi: 10.1007/s10541-005-0104-5. https://doi.org/10.1007/s10541-005-0104-5. [DOI] [PubMed] [Google Scholar]
- [3].Todd AS, Barnetson WK. Use of dark ground microscopy in haematology. J Clin Pathol. 1988;41(7):786–92. doi: 10.1136/jcp.41.7.786. https://doi.org/10.1136/jcp.41.7.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chaiyarit S, Thongboonkerd V. Changes in mitochondrial proteome of renal tubular cells induced by calcium oxalate monohydrate crystal adhesion and internalization are related to mitochondrial dysfunction. J Proteome Res. 2012;11(6):3269–80. doi: 10.1021/pr300018c. https://doi.org/10.1021/pr300018c. [DOI] [PubMed] [Google Scholar]
- [5].Chen R, Liu W, Zhang G, Ye J. Mitochondrial proteomic analysis of cytoplasmic male sterility line and its maintainer in wheat (Triticum aestivum L.) Agric Sci China. 2010;9(6):771–82. https://doi.org/10.1016/S1671-2927(09)60154-1. [Google Scholar]
- [6].Dutta M, Ghosh AK, Mohan V, Mishra P, Rangari V, Chattopadhyay A, et al. Antioxidant mechanism(s) of protective effects of Fenugreek 4-hydroxyisoleucine and trigonelline enriched fraction (TF4H (28%)) Sugaheal against copper-ascorbate induced injury to goat cardiac mitochondria in vitro. J Pharm Res. 2014;8(6):798–811. [Google Scholar]
- [7].Onyia GOC, Gahan PB, Norman H. The use of new probes for protoplast integrity following isolation and purification of photoplasts from tubers of white yam (Discorea rotundata, poir) Plant Sci Lett. 1984;33(2):231–8. https://doi.org/10.1016/0304-4211(84)90013-0. [Google Scholar]
- [8].Yu G, Xiang H, Tian J, Yin J, Pinkert CA, Li Q, et al. Mitochondrial haplotypes influence metabolic traits in porcine transmitochondrial cybrids. Sci Rep. 2015;5:13118. doi: 10.1038/srep13118. https://doi.org/10.1038/srep13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Xiong H, Du S, Ni J, Zhou J, Yao J. Mitochondria and nuclei dual-targeted heterogeneous hydroxyapatite nanoparticles for enhancing therapeutic efficacy of doxorubicin. Biomaterials. 2016;94:70–83. doi: 10.1016/j.biomaterials.2016.04.004. https://doi.org/10.1016/j.biomaterials.2016.04.004. [DOI] [PubMed] [Google Scholar]
- [10].Xue T, Luo P, Zhu H, Zhao Y, Wu H, Gai R, et al. Oxidative stress is involved in Dasatinib-induced apoptosis in rat primary hepatocytes. Toxicol Appl Pharmacol. 2012;261(3):280–91. doi: 10.1016/j.taap.2012.04.010. https://doi.org/10.1016/j.taap.2012.04.010. [DOI] [PubMed] [Google Scholar]
- [11].Ye Y, Huang A, Huang C, Liu J, Wang B, Lin K, et al. Comparative mitochondrial proteomic analysis of hepatocellular carcinoma from patients. Proteomics Clin Appl. 2013;7(5-6):403–15. doi: 10.1002/prca.201100103. https://doi.org/10.1002/prca.201100103. [DOI] [PubMed] [Google Scholar]
- [12].Yao X, Li M, He J, Zhang G, Wang M, Ma J, et al. Effect of early acute high concentrations of iodide exposure on mitochondrial superoxide production in FRTL cells. Free Radic Biol Med. 2012;52(8):1343–52. doi: 10.1016/j.freeradbiomed.2012.02.002. https://doi.org/10.1016/j.freeradbiomed.2012.02.002. [DOI] [PubMed] [Google Scholar]
- [13].Ma WW, Hou CC, Zhou X, Yu HL, Xi YD, Ding J, et al. Genistein alleviates the mitochondria-targeted DNA damage induced by beta-amyloid peptides 25-35 in C6 glioma cells. Neurochem Res. 2013;38(7):1315–23. doi: 10.1007/s11064-013-1019-y. https://doi.org/10.1007/s11064-013-1019-y. [DOI] [PubMed] [Google Scholar]
- [14].Stephanova E, Topouzova-Hristova T, Konakchieva R. Mitochondria are involved in stress response of A549 alveolar cells to halothane toxicity. Toxicol Vitr. 2008;22(3):688–94. doi: 10.1016/j.tiv.2007.12.012. https://doi.org/10.1016/j.tiv.2007.12.012. [DOI] [PubMed] [Google Scholar]
- [15].Du Buy HG, Showacre J. Enzymes catalyzing sequential reactions in mouse brain and liver supernatant fractions: I. Differential use of Janus green B and phenazine methosulfate. J Histochem Cytochem. 1959;7(6):361–9. doi: 10.1177/7.6.361. https://doi.org/10.1177/7.6.361. [DOI] [PubMed] [Google Scholar]
- [16].Lazarow A, Cooperstein S. Studies on the enzymatic basis for the Janus green B staining reaction. J Histochem Cytochem. 1953;1(4):234–41. doi: 10.1177/1.4.234. https://doi.org/10.1177/1.4.234. [DOI] [PubMed] [Google Scholar]
- [17].Tanaka Y. Deposition of Janus green B and pinocyanol in mitochondria of supravitally stained KB cells. Exp Cell Res. 1968;52(2):338–48. doi: 10.1016/0014-4827(68)90475-8. https://doi.org/10.1016/0014-4827(68)90475-8. [DOI] [PubMed] [Google Scholar]
- [18].Showacre JL. A critical study of Janus Green B coloration as a tool for characterizing mitochondria. J Natl Cancer Inst. 1953;13(4):829–45. [PubMed] [Google Scholar]
- [19].Udhayabanu T, Manole A, Rajeshwari M, Varalakshmi P, Houlden H, Ashokkumar B. Riboflavin responsive mitochondrial dysfunction in neurodegenerative diseases. J Clin Med. 2017;6(5):E52. doi: 10.3390/jcm6050052. https://doi.org/10.3390/jcm6050052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ghazi-Khansari M, Mohammadi-Bardbori A, Hosseini MJ. Using Janus green B to study paraquat toxicity in rat liver mitochondria: Role of ACE inhibitors (thiol and nonthiol ACEi) Ann N Y Acad Sci. 2006;1090:98–107. doi: 10.1196/annals.1378.010. https://doi.org/10.1196/annals.1378.010. [DOI] [PubMed] [Google Scholar]
- [21].Mohammadi-Bardbori A, Ghazi-Khansari M. Comparative measurement of cyanide and paraquat mitochondrial toxicity using two different mitochondrial toxicity assays. Toxicol Mech Methods. 2007;17(2):87–91. doi: 10.1080/15376510600822664. https://doi.org/10.1080/15376510600822664. [DOI] [PubMed] [Google Scholar]
- [22].Cooperstein SJ, Lazarow A, Patterson JW. II. Reactions and properties of Janus green B and its derivatives. Exp Cell Res. 1953;5(1):69–82. doi: 10.1016/0014-4827(53)90095-0. https://doi.org/10.1016/0014-4827(53)90095-0. [DOI] [PubMed] [Google Scholar]
- [23].Perry SW, Norman JP, Barbieri J, Brown EB, Gelbard HA. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques. 2011;50(2):98–115. doi: 10.2144/000113610. https://doi.org/10.2144/000113610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cottet-Rousselle C, Ronot X, Leverve X, Mayol JF. Cytometric assessment of mitochondria using fluorescent probes. Cytometry A. 2011;79(6):405–25. doi: 10.1002/cyto.a.21061. https://doi.org/10.1002/cyto.a.21061. [DOI] [PubMed] [Google Scholar]
- [25].Hamberger A, Blomstrand C, Lehninger AL. Comparative studies on mitochondria isolated from neuron enriched and glia enriched fractions of rabbit and beef brain. J Cell Biol. 1970;45(2):221–34. doi: 10.1083/jcb.45.2.221. https://doi.org/10.1083/jcb.45.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Dua R, Gill KD. Effect of aluminium phosphide exposure on kinetic properties of cytochrome oxidase and mitochondrial energy metabolism in rat brain. Biochim Biophys Acta. 2004;1674(1):4–11. doi: 10.1016/j.bbagen.2004.05.003. https://doi.org/10.1016/j.bbagen.2004.05.003. [DOI] [PubMed] [Google Scholar]
- [27].Valenti D, Vacca RA, de Pinto MC, De Gara L, Marra E, Passarella S. In the early phase of programmed cell death in Tobacco Bright Yellow 2 cells the mitochondrial adenine nucleotide translocator, adenylate kinase and nucleoside diphosphate kinase are impaired in a reactive oxygen species-dependent manner. Biochim Biophys Acta. 2007;1767(1):66–78. doi: 10.1016/j.bbabio.2006.11.004. https://doi.org/10.1016/j.bbabio.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [28].Cooperstein SJ, Dixit PK, Lazarow A. Studies on the mechanism of Janus green B staining of mitochondria. IV. Reduction of Janus green B by isolated cell fractions. Anat Rec. 1960;138(1):49–66. doi: 10.1002/ar.1091380107. https://doi.org/10.1002/ar.1091380107. [DOI] [PubMed] [Google Scholar]
- [29].Lazarow A, Cooperstein SJ. Studies on the enzymatic basis for the Janus Green B staining reaction. J Histochem Cytochem. 1953;1(4):234–41. doi: 10.1177/1.4.234. https://doi.org/10.1177/1.4.234. [DOI] [PubMed] [Google Scholar]
- [30].Braun S, Erdelyi M, Udvardy A. Janus Green B and the biologic “oxygen effect’’. Cancer Res. 1967;27(4):660–7. [PubMed] [Google Scholar]
- [31].Koide T, Baba K, Wa K, Keda M. Mitochondrial injury produced by Janus Green B - Enzyme-morphological and ultrastructural observation. Acta Histochem Cytochem. 1971;4(3):137–52. https://doi.org/10.1267/ahc.4.137. [Google Scholar]
- [32].Johnson LV, Walsh ML, Chen B, Buchanan JM. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sc USA Cell Biol. 1980;77(2):990–4. doi: 10.1073/pnas.77.2.990. https://doi.org/10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chen Y, Qiao L, Yu B, Li G, Liu C, Ji L, et al. Mitochondria-specific phosphorescent imaging and tracking in living cells with an AIPE-active iridium(III) complex. Chem Commun (Camb) 2013;49(94):11095–7. doi: 10.1039/c3cc46957c. https://doi.org/10.1039/c3cc46957c. [DOI] [PubMed] [Google Scholar]


