Abstract
Tumor microenvironment provides a specialized niche in which a population of stem-like cells is enriched and contributes to cancer progression. Moreover, cancer stem cell (CSC) phenotype has been associated with epithelial-mesenchymal transition (EMT). Here we investigated the effect of tumor microenvironment on the phenotypic characteristics of head and neck cancer cells and expression of CSC markers using a three-dimensional (3D), spheroid, culture system of CAL33 cell line from human tongue squamous cell carcinoma. CAL33 cells derived from 2D monolayer cultures were grown in spheroid cultures containing serum-free medium (epidermal growth factor [EGF], fibroblast growth factor [FGF], and insulin). Adherent CAL33 cells from spheroids or standard control cultures were grown in the presence/absence of serum in combination with hypoxia/normoxia. Markers of EMT, CSC, and hypoxia were analyzed either by Western blotting, immunofluorescence, or reverse transcription quantitative PCR. Spheroid cultures showed hypoxic microenvironment (high carbonic anhydrase IX [CAIX] expression), mesenchymal-like characteristics (reduced E-cadherin and increased vimentin and N-cadherin expression, presence of larger colonies comprised of larger, spread cells with lower density), and increased expression of the CSC marker glioma-associated oncogene homolog 1 (Gli1). These effects were recapitulated in serum-free adherent CAL33 cells maintained for prolonged periods in hypoxia (1% O2) but, in contrast, were completely abolished by the presence of serum. Overall, we found that a combination of hypoxia, EGF and FGF was essential to induce the EMT in adherent CAL33 cell cultures. The addition of serum rapidly reverts the EMT of cells, affects CSC phenotype and, thus, prevents the detection of such cells in tumor cell lines.
Keywords: Head and neck cell carcinoma, cancer stem cells, CSCs, epithelial-mesenchymal transition, EMT, tumor microenvironment, hypoxia, Gli1
INTRODUCTION
Tumor heterogeneity is a term used to describe a phenomenon in which cancer cells within the same tumor or between different tumor subtypes have distinct morphological and functional characteristics as well as proliferation and differentiation potentials. Two main and not mutually exclusive models are used to explain tumor heterogeneity, the cancer stem cell (CSC) and clonal evolution models [1,2]. CSCs are a subpopulation of cells within a tumor which, similar to normal stem cells, have the ability to self-renew and differentiate into other cell types. CSCs have been identified in many cancer types, including leukemia, breast cancer, colorectal cancer and brain cancer [2,3]. According to the standard CSC model of tumor heterogeneity, tumors are hierarchically organized into subpopulations of rare tumorigenic (stem-like) and more numerous non-tumorigenic cells, with CSCs being at the top of the cell hierarchy. In addition, more recent research suggests that CSCs do not necessarily have to be rare or dormant within a tumor tissue and that CSC hierarchical organization may be more plastic than previously thought, depending on the environmental stimuli [1].
Head and neck cancer is a heterogeneous group of cancers originating in most cases from squamous cells that line the mucosal surfaces inside the organs of the head and neck (e.g., the mouth, nose, and throat) and as such are called head and neck squamous cell carcinoma (HNSCC). Additionally, head and neck cancers may occur in the salivary glands. The presence of CSCs has been demonstrated in HNSCCs and among the putative markers used for the characterization and isolation of these cells from HNSCCs are a cell-surface glycoprotein cluster of differentiation (CD)44, transmembrane glycoprotein CD133, the enzyme aldehyde dehydrogenase (ALDH) which is involved in the conversion of retinol to retinoic acid, and ATP-binding cassette (ABC) transporters [4].
Glioma-associated oncogene homolog 1 (Gli1) is a transcription factor that acts downstream of the Sonic hedgehog (Shh) pathway and upregulates genes involved in cell proliferation, tissue development, epithelial-mesenchymal transition (EMT), and stem cell maintenance. In HNSCC, Gli1 was frequently activated and was associated with lymph node metastases and tumor progression after radiotherapy. In lung squamous cell carcinoma (LSCC), Gli1 was an indicator of poor prognosis; moreover, its role as a marker of cancer stem-like cells in LSCC was suggested [5,6].
Three-dimensional (3D) cell culture systems have a number of advantageous features for culturing cancer stem cells in vitro compared to 2D models, such as they can better mimic tumor microenvironments, facilitate the formation of the extracellular matrix (ECM), and provide more accurate proliferation rates with characteristic cellular morphology [7]. In essence, 3D cell cultures have tumor conditions similar to those that exist in vivo. For example, they exhibit regions of hypoxia, zones of quiescent (stem-like) and proliferating cells, and electrochemical gradients together with cell-cell and cell-ECM interactions, all of which are reminiscent of the solid tumor microenvironment [8]. Moreover, 3D cell models such as spheroids or sphere-forming cells have been demonstrated to be useful for the enrichment of CSC populations, especially in cases when specific CSC markers are not well understood [9,10].
The EMT is a multi-stage process in which epithelial cells are transformed into mesenchymal cells through remodeling of epithelial cell architecture and function, including the loss of E-cadherin expression, cell-cell junctions and apical-basal cell polarity, and the acquisition of mesenchymal-like characteristics as well as migratory and invasive capability [11,12]. The EMT is crucial in normal physiological processes such as embryonic development and tissue repair, but is also involved in pathological processes, for example in cancer and fibrosis. An aberrant activation of EMT has been linked to tumor cell invasion and metastasis. Moreover, it has been demonstrated that tumor cells that undergo EMT acquire CSC-like properties [13]; thus targeting CSCs with EMT markers represents a potentially promising therapeutic approach.
Here we investigated the effect of tumor microenvironment on the phenotypic characteristics of head and neck cancer cells and expression of CSC markers using a 3D, spheroid, cell culture system of CAL33 cell line. CAL33 cells expressed high levels of Gli1 in spheroid cultures suggesting its use as a CSC marker in head and neck cancer cell lines. We also showed that spheroid CAL33 cells underwent EMT and the essential factors triggering the EMT were the combination of hypoxia, epidermal growth factor (EGF), and fibroblast growth factor (FGF). In contrast, the presence of serum strongly repressed the transition of CAL33 cells in adherent cultures.
MATERIALS AND METHODS
CAL33 standard and spheroid cell culture in normoxia
Human tongue squamous cell carcinoma line CAL33 was obtained from Centre Antoine-Lacassagne (Nice, France) [14]. CAL33 cells were grown in 2D monolayer culture with Dulbecco’s Modified Eagle’s medium (DMEM, Gibco, CA, USA). The medium contained 10% fetal bovine serum (FBS, Gibco), 50 U/ml penicillin and 50 µg/ml streptomycin. Spheroid cells were derived by plating CAL33 cells at a density of 30,000/ml on non-adherent cell culture plates treated with polyHEMA (Sigma, MO, USA). Spheroid medium, also called serum-free medium (SFM), consisted of DMEM/Nutrient Mixture F-12 (F-12) medium (1:1) supplemented with non-essential amino acid mix (1X, Invitrogen, CA, USA), 1 mM sodium pyruvate (Invitrogen), 5 mM Hepes (Invitrogen), 20 ng/ml EGF (Peprotech, UK), 10 ng/ml FGF (Peprotech), 5 µg/ml insulin (Invitrogen), 50 U/ml penicillin and 50 µg/ml streptomycin. After 10 days of in vitro culture in SFM at 37°C with 5% carbon dioxide, CAL33 spheres were collected by centrifugation (1000 g for 10 minutes), dissociated with trypsin-ethylenediaminetetraacetic acid [EDTA] (0.25%, Thermo Fisher Scientific, MA, USA), suspended in SFM containing 0.1 U/ml soybean trypsin inhibitor (Sigma, MO, USA), and then replated at a density of 30,000/ml on non-adherent 100-mm plates in 10 ml SFM, to form secondary spheres.
CAL33 adherent cell culture in hypoxia
CAL33 cells were plated in standard culture plates at a density of 20,000/ml in EFI medium (EGF, FGF and insulin) in a BugBox anaerobic workstation (Ruskinn Technology Ltd, UK) set at 1% oxygen, 94% nitrogen, and 5% carbon dioxide. The cells were replated every 10 days at the same density, at 37°C inside the BugBox. Each medium change and replating were done with media equilibrated at 1% oxygen to avoid reoxygenation and rinsed with trypsin inhibitor to stop trypsin action. After 30 days, the cells were used for the isolation of RNA and protein or determination of colony-forming ability, as described below. For the reversion of EMT, the cells maintained for 30 days in EFI and hypoxia were maintained in hypoxia or transferred to normoxic conditions for 3, 6 or 10 days.
Colony-forming ability of adherent spheroid or standard CAL33 cells
The colony-forming ability of CAL33 cells was determined in triplicates on adherent 6-well plates. Spheroid or adherent CAL33 cells (control) were dissociated into a single cell suspension; 3 ml of the suspension was subsequently plated at a density of 1,000 cells per dish in DMEM with 10% FBS and incubated at 37°C for 1 week. Colonies were then fixed and stained with Giemsa (Sigma).
Immunoblotting analysis
CAL33 cells were lysed in 3% sodium dodecyl sulfate (SDS) and the protein concentration was determined using the bicinchoninic acid assay (BCA, Pierce, MA, USA). Forty micrograms of each whole-cell extract was resolved by electrophoresis on SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride membrane (Millipore, MA, USA). The membranes were blocked in 5% fat-free milk in TN buffer (50mM Tris-HCl, pH 7.4, 150mM NaCl) and incubated 1 hour to overnight in the presence of the primary antibodies vimentin (Santa Cruz, USA 1/1000) and carbonic anhydrase IX [CAIX] (M75 Bayer Germany 1/10000). The membranes were washed in TN and incubated 1 hour with horseradish peroxidase (HRP)-conjugated secondary antibodies (Promega, WI, USA) in 5% fat-free milk and TN buffer. After washing in TN buffer containing 1% Triton X-100 and then in TN buffer alone, the bands were visualized with the enhanced chemiluminescence (ECL) system (Millipore).
Immunofluorescence staining
CAL33 cells grown on glass coverslips were fixed with 3% paraformaldehyde (PFA) at room temperature and permeabilized with phosphate-buffered saline (PBS) containing 1% Triton X-100, for 20 minutes. After washing two times with PBS for 5 minutes, cells were blocked with PBS containing 3% serum for 30 minutes at room temperature. Cells were then incubated with anti-vimentin (1:2000, Santa Cruz), anti-E-cadherin (1:1000, Abcam, UK) or anti-fibronectin (1:400, Millipore) primary antibodies overnight at room temperature. After washing extensively, they were incubated in PBS containing 3% serum in dark, in the presence of anti-mouse secondary antibody conjugated to Alexa 594 (1:400, Invitrogen) and anti-rabbit secondary antibody conjugated to Alexa 488 (1:400, Invitrogen), for 1 hour at room temperature. Nuclear staining (blue fluorescence) was performed by treating cells with 4’6-diamino-2-phenylindole (DAPI, 20 ng/ml) for 5 minutes at room temperature. After being washed, coverslips were mounted with Citifluor (Amersham Biosciences, UK), slides were observed under a fluorescence microscope (Leica, Leitz DMRB, Germany) and digitized using a Hamamatsu C5810 cooled 3CCD camera (Hamamatsu Photonics Japan), and images were recorded using RS Image software (Photometrics-Roper Scientific Inc UK). No signals were observed when the primary antibodies were omitted.
RNA extraction, reverse transcription (RT)-PCR and real-time quantitative PCR
Total RNA was extracted from spheroid and adherent CAL33 cells using Trizol reagent (Thermo Fisher Scientific USA), according to the manufacturer’s instructions. Complementary DNA (cDNA) synthesis was performed using Omniscript RT kit (Qiagen, Germany). The relative mRNA expression of matrix metalloproteinases (MMPs) and basigin (BSG) was determined by real-time quantitative polymerase chain reaction (qPCR) using TaqMan primer probes (Life Technologies, USA, references can be provided upon request) and qPCR master mix (Eurogentec, Belgium). The gene expression was normalized with RPLP0 housekeeping gene expression. The mRNA expression of markers of EMT and CSCs (zinc finger protein SNAI1 or Snail [SNAI1], GLI1, octamer-binding transcription factor 4 [Oct-4, POU5F1 gene], Nanog transcription factor [NANOG], ATP-binding cassette sub-family G member 2 [ABCG2], c-Myc transcription factor [c-myc], fibroblast growth factor 2 [FGF2], polycomb complex protein BMI-1 [BMI1], Krueppel-like factor 5 [KLF5], vimentin [VIM], epithelial cadherin [E-cadherin, CDH1], neural cadherin [N-cadherin, CDH2], CAIX [CA9], CD133 [PROM1], CD44 [CD44], and CD24 [CD24]) was analyzed with duplex real-time qPCR on a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific, USA). All PCR primers used in the study are provided in Table S1.
TABLE S1.
Primers used in quantitative reverse transcription polymerase chain reaction

Statistical analysis
The colony size was measured using Image J software (imageJ.nih.gov) with the particle analyzer plugin, on 12 images from 3 independent experiments. Student’s t-test was performed using Excel function T.TEST with univariate (one-sample) and two-sample test (control culture vs. spheroid condition). To determine the number of cells in colonies, live cells were labelled with 1µg/ml Hoechst 33342 (Invitrogen) for 10 minutes and imaged under UV illumination with a blue light filter. The number of cells in colonies was determined by counting the nuclei with Image J software on high magnification images (200×). Results are expressed as the mean and standard error of the mean (SEM) of counts from 10 independent images.
RESULTS
Formation of CAL33 spheroids
CAL33 is an established head and neck cancer cell line frequently used as a model system for tumorigenic assays. Our results showed that CAL33 cells spontaneously aggregated into spheroids when cultivated in SFM containing EFI (EGF, FGF and insulin) on polyHEMA coated non-adherent plates. From an inoculum of 105 cells, this cell line produced 20–30 floating spheres with a diameter of 100 µm within 10 days. These spheres (called sph1) were dissociated with trypsin and gave rise to secondary (sph2) and tertiary spheres (sph3) with the same efficiency, which is characteristic of tumor-initiating cells.
Spheroid CAL33 cells form colonies with a mesenchymal-like morphology
When CAL33 cells were dissociated from spheroids and plated at clonal density (1000 cells/60 mm dish) on adherent tissue culture dishes in the presence of serum, their cloning efficiency (1:3) was not different from standard CAL33 cell cultures (control), however, a significantly higher proportion of the colonies from spheroid cultures were comprised of spread cells. Consequently, these colonies were much larger and with a marked increase in colony size compared to control colonies (Figure 1A). The average size of control colonies was 0.09 mm2 (98.6%), while the colonies from sph3 were 1.4 mm2 in size. Mesenchymal-like phenotype and cell morphology were observed in the colonies from spheres, i.e., they had a lower cell density (nuclei/mm2) and larger cells (Figure 1B). The ratio of larger colonies (>0.3 mm2) in the population increased with the sphere formation, indicating an enrichment of CSCs with time (Figure 1C). A similar colony phenotype was observed upon treatment of CAL33 cells with transforming growth factor beta (TGF-β), a cytokine known to induce the EMT (data not shown).
FIGURE 1.

CAL33 cells were dissociated from spheroids and p lated at clonal density (1000 cells/60 mm dish) on adherent culture dishes in the presence of serum for 10 days. The cloning efficiency (1:3) of CAL33 cells from spheroids was not different from standard CAL33 cell cultures (control), but a significantly higher proportion of the colonies from spheroids were comprised of spread cells. The colonies from spheroids were much larger and with a marked increase in colony size compared to the colonies from control (p = 0.02, Student’s t-test), as indicated in (A) Giemsa- and (B) Hoechst 33342 DNA-stained colonies [×50]. The average size of control colonies was 0.09 mm2, while the colonies from sphere 3 were 1.4 mm2 in size. Colonies from spheres had a lower cell density (nuclei/mm2) and larger cells (p = 0.0001) [B right]. The ratio of larger colonies (>0.3 mm2) in the population increased with the sphere formation (a p = 0.02; b p = 0.0001; c p = 0.004) [C]. CAL33 cells: Human tongue squamous cell carcinoma line.
Expression of EMT- and CSC-related genes in CAL33 spheroid cells
To confirm the EMT phenotype in CAL33 cells issued from sphere cultures we measured the expression of EMT markers by immunofluorescence microscopy (Figure 2A) and RT-qPCR (Figure 2B). We observed a strong reduction in E-cadherin protein expression in the colonies issued from spheres, while vimentin expression was progressively increasing with the formation of each new sphere (Figure 2A). Consistently, RT-qPCR analysis showed that the mRNA expression of mesenchymal markers vimentin and N-cadherin was highly increased in spheroids (Figure 2B). There was also a modest increase in E-cadherin mRNA expression in sph1 and sph2, however, this could not compensate for the overall loss of E-cadherin protein expression in CAL33 spheres, which is known to be post-transcriptionally regulated [15].
FIGURE 2.
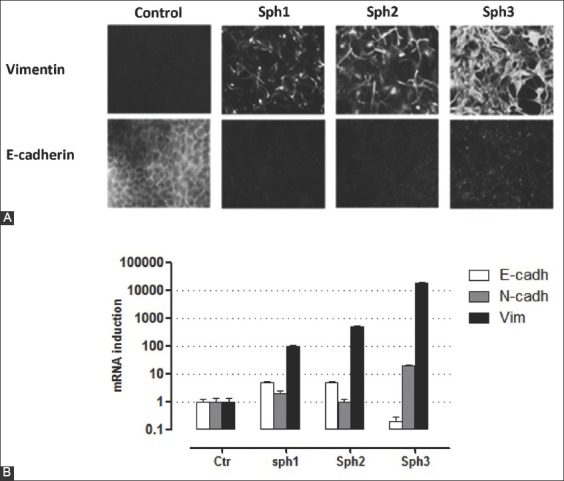
Analysis of EMT markers by immunofluorescence microscopy (A) and RT-qPCR (B) in CAL33 adherent cultures from spheroids and in control cells. Cells from 3 generations (10 days) of spheroids (Sph1-3) or from control adherent CAL33 cultures were spread on a glass slide for 24 hours before fixation and (A) labeled for E-cadherin (epithelial marker) and vimentin (mesenchymal marker). We observed a strong reduction in E-cadherin protein expression in the colonies isolated from spheres, while vimentin expression increased with the formation of each new sphere. (B) Quantification of EMT markers (E-cadherin, N-cadherin, and vimentin) by real-time qPCR from total RNA isolated from the same sphere cultures as described in (A) and relative to control adherent CAL33 cultures (Ctr). The mRNA expression of mesenchymal markers vimentin and N-cadherin was highly increased in spheres. There was also a modest increase in E-cadherin mRNA expression in sph1 and sph2, however, this does not compensate for the overall loss of E-cadherin protein expression, which is known to be regulated post-transcriptionally. CAL33 cells: Human tongue squamous cell carcinoma line; EMT: Epithelial-mesenchymal transition; RT-qPCR: Quantitative reverse transcription polymerase chain reaction; E-cadherin: Epithelial cadherin; N-cadherin: Neural cadherin.
As shown in Figure 3, sphere culture conditions caused a significant decrease in mRNA levels of classical stem cell markers in CAL33 cells. The mRNA expression of ABCG2, CD133, BMI1, Nanog and Oct-4 and of embryonic stem cells markers c-Myc and FGF2 was decreased in spheres compared to controls (adherent CAL33 cell cultures grown in the presence of serum). This decrease in mRNA expression was maintained and enhanced in the successive, secondary and tertiary spheres. On the other hand, we found increased mRNA expression of Gli-1 in the early CAL33 spheroids, and this was maintained during prolonged sphere cultures.
FIGURE 3.
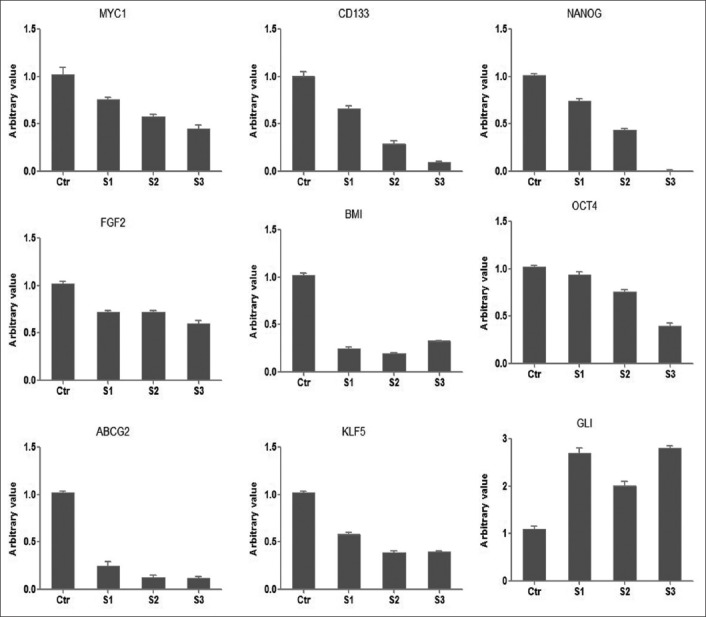
mRNA expression analysis of CSC markers in CAL33 cells from 3 generations of spheres and in control adherent CAL33 cells (Ctr) by RT-qPCR. The mRNA expression of ABCG2, CD133, BMI1, Nanog and Oct-4 and of embryonic stem cells markers c-Myc and FGF2 was decreased in spheroids compared to controls. This decrease in mRNA expression was maintained and enhanced in successive, secondary and tertiary spheres. On the other hand, we found increased mRNA expression of Gli-1 in the early spheroids of CAL33 cells, and this was maintained during prolonged sphere cultures. S1: 10 days spheroid; S2: 20 days spheroid; S3: 30 days spheroid; CAL33 cells: Human tongue squamous cell carcinoma line; CSCs: Cancer stem cells; Gli1: Glioma-associated oncogene homolog 1; Oct-4: Octamer-binding transcription factor 4; ABCG2: ATP-binding cassette sub-family G member 2; FGF2: Fibroblast growth factor 2; BMI1: Polycomb complex protein BMI-1; KLF5: Krueppel-like factor 5; CD133: Cluster of differentiation 133 or prominin-1.
EMT is induced by hypoxia in serum-free medium
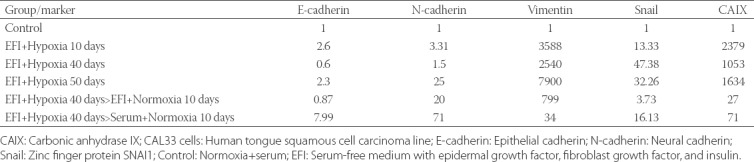
Due to limited oxygen diffusion spheroids have a hypoxic microenvironment even under normoxic culture conditions. The expression of CAIX, a gene that is strongly induced in hypoxia [16], increased 50 folds in spheroid CAL33 cultures, reaching a 65-fold increase in the tertiary spheres (Figure 4). Apparently, the microenvironment inside spheres was hypoxic and a high CAIX mRNA expression was maintained even during the serial passage of spheres in normoxic conditions. To determine the effect of hypoxic microenvironment on EMT induction, adherent CAL33 cells were grown in hypoxic chamber (1% O2) for 30 days (1 passage every 10 days) in serum-containing medium. Under these conditions, CAL33 cells acquired the EMT morphology partially. The cells started to lose cell-cell contacts, they were in scattered cell clusters, and were spindle-shaped (data not shown). After a prolonged exposure of CAL33 cells to hypoxia there was a slight upregulation of N-cadherin and Snail mRNA expression, while the expression of E-cadherin did not decrease (Table 1). Although the hypoxic conditions increased CAIX mRNA expression in CAL33 cells (Table 1), they did not cause EMT in the presence of serum.
FIGURE 4.
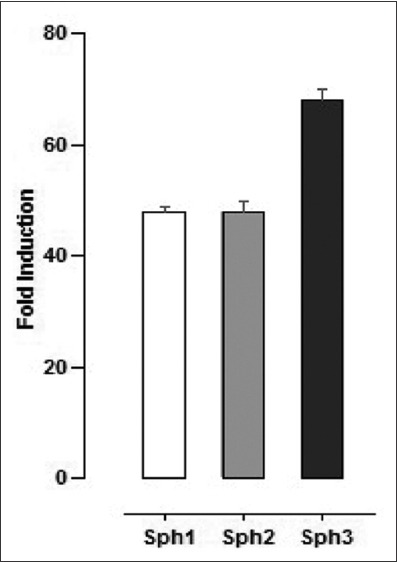
mRNA expression of CAIX, a marker of hypoxia, in CAL33 cells upon prolonged sphere cultures. CAIX mRNA expression increased 50 folds in sphere cultures, reaching a 65-fold increase in tertiary spheres. Sph1: 10 days spheroid; Sph2: 20 days spheroid; Sph3: 30 days spheroid. CAIX mRNA expression was determined by RT-qPCR in the indicated sphere culture relative to CAIX mRNA expression in the corresponding standard two-dimensional cultures. CAL33 cells: Human tongue squamous cell carcinoma line; CAIX: Carbonic anhydrase IX; RT-qPCR: Quantitative reverse transcription polymerase chain reaction.
TABLE 1.
mRNA expression of markers of mesenchymal phenotype and CAIX, a marker of hypoxia, in adherent CAL33 cells grown in the presence of hypoxia and serum

Since a specific growth medium is required for the induction of EMT in spheroid cultures, i.e., a medium with EFI (EGF, FGF and insulin) and without serum, we investigated whether the combination of hypoxia and EFI medium induces a mesenchymal phenotype in adherent CAL33 cells. After a 30-day pre-treatment of CAL33 cultures in different conditions, i.e., serum and normoxia (control), serum and hypoxia or EFI and hypoxia, single cell colonies were grown in standard conditions for 10 days (Figure 5). The cells grown in serum and hypoxia gave slightly larger colonies than control cells, and they did not acquire the spread morphology characteristic of cells from spheroids (Figure 1). However, the combination of hypoxia and EFI resulted in a dramatic change in the cell phenotype and these cells formed much larger colonies. The number of cells in colonies was similar between hypoxia+EFI and control group, however, the colonies in the former group were spread on a much larger surface area. The hypoxia-conditioned cell populations had the same growth rate in the presence of serum or EFI and a similar saturation density (data not shown). When grown to confluence, EFI-hypoxia pre-treated cells did not form a monolayer with epithelial, cobblestone-like morphology, but they rather displayed a spindle-shaped mesenchymal phenotype. The mesenchymal transformation of cells was confirmed by the progressive invasion of vimentin-positive cells in the successive cultures treated with hypoxia and EFI medium (Figure 6A).
FIGURE 5.
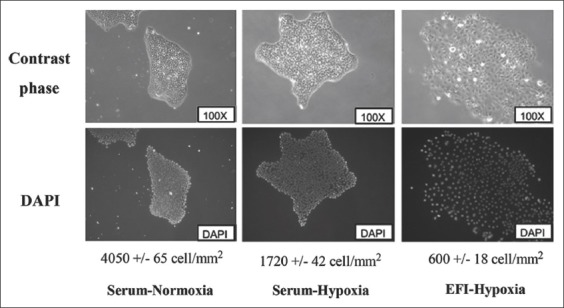
Effect of hypoxia and EFI medium in adherent CAL33 cells on colony formation. After a 30-day pre-treatment of CAL33 cultures in different conditions (serum and normoxia [control], serum and hypoxia, or EFI and hypoxia) single cell colonies were grown in standard conditions for 10 days. The cells grown in serum and hypoxia gave slightly larger colonies than control cells, and they did not acquire the spread morphology characteristic of spheroids. The combination of hypoxia and EFI resulted in much larger colonies. The number of cells in colonies was similar between hypoxia+EFI and control group, however, the colonies in the former group were spread on a much larger surface area. CAL33 cells: Human tongue squamous cell carcinoma line; EFI: Serum-free medium with EGF, FGF and insulin; EGF: Epidermal growth factor; FGF Fibroblast growth factor.
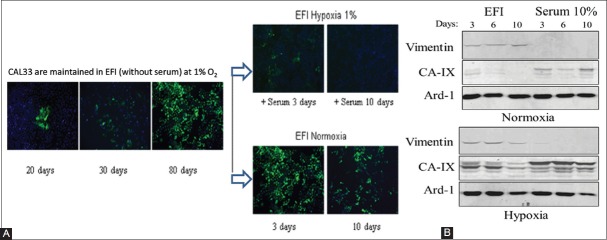
FIGURE 6.
Reversion of EMT by serum in EFI-hypoxia pre-treated CAL33 cells. (A) Immunofluorescence analysis of vimentin protein expression after 20, 30, and 80 days of culture in EFI-hypoxia (right panel). Pre-treated cells did not form a monolayer with epithelial, cobblestone-like morphology, but they rather displayed a spindle-shaped mesenchymal phenotype. The mesenchymal transformation of cells was confirmed by the progressive invasion of vimentin-positive cells in the successive cultures treated with hypoxia and EFI medium. After 80 days in EFI-hypoxia the cells were transferred to normoxic conditions for 3 or 10 days in the same medium (middle lower panel) or maintained in hypoxia with the addition of 10% serum for 3 or 10 days (middle upper panel). (B) Western blot analysis of vimentin expression in total cell extracts from EFI-hypoxia pre-treated CAL33 cells (80 days) and then cultured for 3, 6, 10 days in the indicated conditions, in normoxia (upper panel) or hypoxia (lower panel). After 10 days in normoxia the protein levels of vimentin were still detectable in CAL33 cells. CAL33 cells: Human tongue squamous cell carcinoma line; EFI: Serum-free medium with EGF, FGF and insulin; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; CAIX: Carbonic anhydrase 9; Ard-1: ADP-ribosylation factor domain protein 1.
Overall, a significant increase in the expression of mesenchymal cell markers was observed (Table 2). The CAIX expression was used as an indicator of cellular response to hypoxia; the maximum CAIX expression was observed at 10 days, but it decreased to low levels in normoxic conditions, independently of the presence of serum. Importantly, we observed the upregulation of Snail gene, which is involved in the maintenance of the mesenchymal phenotype, in parallel with vimentin expression. Vimentin and N-cadherin mRNA expression was significantly upregulated after 10 days of treatment and with a progressive increase thereafter. These observations indicate that a prolonged incubation of CAL33 cells in EFI and hypoxia results in a progressive EMT. In contrast, the mRNA expression of E-cadherin, a marker of epithelial phenotype, was not affected by EFI-hypoxia but was highly responsive to serum.
TABLE 2.
mRNA expression of markers of mesenchymal phenotype and CAIX, a marker of hypoxia, in adherent CAL33 cells grown in hypoxia and in the presence or absence of serum, and effects of return to normoxia
Serum severely affects CSC phenotype and reverses EMT in CAL33 cells
To determine the stability of the observed EMT phenotype adherent CAL33 cultures that were conditioned for 30 days in EFI-hypoxia were incubated in the presence of serum and/or normoxia (Figure 6B).
After 10 days in normoxia, the mRNA levels of vimentin and Snail were reduced (Table 2) while the protein levels of vimentin were still detectable (Figure 6B). However, the addition of 10% serum even in hypoxic cultures immediately reduced vimentin expression. The levels of E-cadherin were increased immediately upon the addition of serum (Table 2) indicating that serum not only maintained the epithelial phenotype but also reversed the mesenchymal phenotype. Similarly, in spheroid CAL33 cultures the EMT phenotype was prevented by serum, since spheroids produced in the presence of 10% FBS did not select for cells that form spread colonies (Figure S1). Because the induction of EMT and the expression of EMT markers were suppressed by serum in CAL33 adherent cultures, we also analyzed the effect of hypoxia and serum on the expression of stem cell markers (Figure 7). CAL33 cells exhibited a slightly increased vimentin and CD133 mRNA expression and the CD24high/CD44low ratio in the presence of serum and hypoxia. The 30-day incubation in hypoxia and EFI medium resulted in low expression of CD133 and a CD44high/CD24low phenotype (Figure 6) together with a strong induction of vimentin expression. These results indicate that CD133 expression is controlled by serum, while the absence of serum leads to CSC-like phenotype, i.e., higher vimentin and CD44 expression and lower expression of CD24 in CAL33 cells.
FIGURE S1.
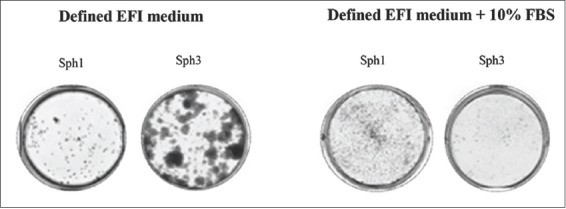
Serum inhibits sphere-induced EMT. Colony-forming assay from single cells issued from 10-day (sph1) or 30-day (sph3) CAL33 sphere cultures in EFI medium in the absence (left) or presence (right) of 10% serum. CAL33 cells: Human tongue squamous cell carcinoma line; EMT: Epithelial-mesenchymal transition; EFI: Serum-free medium with EGF, FGF and insulin; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; FBS: Fetal bovine serum.
FIGURE 7.

The effect of hypoxia and serum on mRNA expression of stem cell markers in CAL33 cells, analyzed by RT-qPCR. The expression of CD133, CD44, CD24 and vimentin in serum and normoxia (control conditions) is compared to a 30-day incubation in EFI-hypoxia (1% oxygen) or the same condition followed by addition of serum for 10 days. In the presence of serum and hypoxia, CAL33 cells exhibited a slightly increased vimentin and CD133 mRNA expression and the CD24high/CD44low ratio. Hypoxia was defined as 1% O2. CAL33 cells: Human tongue squamous cell carcinoma line; EFI: Serum-free medium with EGF, FGF and insulin; EGF: Epidermal growth factor; FGF: Fibroblast growth factor; RT-qPCR: Quantitative reverse transcription polymerase chain reaction; CD133: Cluster of differentiation 133 or prominin-1; CD24: Cluster of differentiation 24 or heat stable antigen CD24; CD44: Cluster of differentiation 44 or homing cell adhesion molecule.
DISCUSSION
CSCs have been identified in many cancer types including HNSCC, and a range of biomarkers have been used for their characterization and isolation from cancer cell lines. In our study, most of well-known stem cell markers, e.g., ABCG2, CD133, BMI1, Nanog, Oct-4, c-Myc and FGF2, were downregulated in CAL33 spheroids, indicating that CAL33 spheroid cultures in serum-free conditions may select cells with a less differentiated phenotype but, which nevertheless do not express the classical stem cell markers. The exception was Gli1 for which increased mRNA expression was observed in early CAL33 spheroids as well as in the prolonged sphere cultures. Gli1, a transcription factor specifically enriched in high-grade gliomas, acts downstream of the Shh pathway and upregulates genes involved in cell proliferation, tissue development, EMT, and stem cell maintenance. The activation of Gli1 was previously reported in HNSCC where it was associated with metastasis and poor survival [6]. Comparably, our results suggest that Gli1 may be a suitable stem cell marker for head and neck cancer cell lines with CSC-like properties.
Furthermore, we found that CAL33 cells isolated from spheroid cultures gave rise to phenotypically different colonies after cloning of cells on adherent culture plates. The colonies issued from spheres were composed of spread cells and were much larger compared to the colonies issued from attached cell cultures, a phenomenon resembling the EMT [17]. Gene expression analyses revealed a marked increase in vimentin mRNA expression (up to 5000 fold), a strong increase in N-cadherin expression and a modest induction of Snail in CAL33 cells from spheroids, suggesting that EMT phenotype was induced in spheroid cultures. We also observed that the protein expression of E-cadherin was lost at the surface of cells grown as spheres (Figure 2), while its mRNA expression was slightly increased during the formation of initial spheres and not affected by the successive sphere passages. This is consistent with the fact that E-cadherin expression is downregulated at the protein level during EMT and cancer metastasis [18]. The downregulation of E-cadherin has been related to tumor hypoxia [19] and this is also in agreement with our results, i.e., we observed a high expression of the hypoxia marker CAIX in the spheroid microenvironment.
The results of our study suggest that it is the particular microenvironment of spheroids rather than cell suspension that favors the ETM. For example, here we showed that CAL33 cells grown as a monolayer in 1% O2 and defined EFI media without serum, for prolonged periods of time, also express the mesenchymal marker vimentin. Comparably, we have recently found that primary breast cancer cells undergo EMT due to the upregulation of SNAI1 and SNAI2 in hypoxic conditions [20]. In the current study, we determined that the essential factors triggering EMT in CAL33 adherent cultures were the combination of hypoxia, EGF and FGF, while the presence of serum strongly repressed the transition of cells. Interestingly, the observation that serum inhibits the EMT in vitro has not been reported previously. In this study, we could detect vimentin expression by immunofluorescence in dispersed cell clusters after 20 days of culture in hypoxia and following that, vimentin was detectable after 40 days of culture in all cells by Western blotting. Upon return to normoxic conditions in the absence of serum the phenotype was maintained for 10 to 15 days. On the other hand, the addition of serum, even in hypoxia, completely inhibited vimentin expression in 1 day. We can conclude that serum contains elements that prevent the loss of epithelial characteristics in cells; however, this feature is distinct from a mitogenic effect because the growth rate obtained in CAL33 cells with purified growth factors was similar to the growth rate obtained with serum. This finding indicates that caution must be taken when assessing the epithelial properties of cell lines that are cultivated in the presence of serum.
With regard to the previously observed association between EMT and CSC properties, we found that the CD44high/CD24low ratio, which is typical of stem cells, was clearly reversed by the addition of serum to CAL33 cells. However, this finding may not apply to every CSC marker or tumor cell model since, for example, some glioblastoma spheres express more markers in the presence of serum [21]. Altogether, our results indicate that the presence of serum in cell cultures severely affects CSC phenotype and prevents the detection of stem-like cells in tumor cell lines.
ACKNOWLEDGMENTS
This research was supported by the grants from the Institut National du Cancer (INCA) in France. The laboratory is funded by the Centre A. Lacassagne, the University of Nice, the CNRS, and INSERM.
In Tunisia, this research was supported by a grant from Tunis el Manar University.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Batlle E, Clevers H. Cancer stem cells revisited. Nat Med. 2017;23(10):1124–34. doi: 10.1038/nm.4409. https://doi.org/10.1038/nm.4409. [DOI] [PubMed] [Google Scholar]
- [2].Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer. 2008;8(10):755–68. doi: 10.1038/nrc2499. https://doi.org/10.1038/nrc2499. [DOI] [PubMed] [Google Scholar]
- [3].Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–8. [PubMed] [Google Scholar]
- [4].Dionne LK, Driver ER, Wang XJ. Head and neck cancer stem cells: From identification to tumor immune network. J Dent Res. 2015;94(11):1524–31. doi: 10.1177/0022034515599766. https://doi.org/10.1177/0022034515599766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cui Y, Cui CA, Yang ZT, Ni WD, Jin Y, Xuan YH. Gli1 expression in cancer stem-like cells predicts poor prognosis in patients with lung squamous cell carcinoma. Exp Mol Pathol. 2017;102(2):347–53. doi: 10.1016/j.yexmp.2017.03.004. https://doi.org/10.1016/j.yexmp.2017.03.004. [DOI] [PubMed] [Google Scholar]
- [6].Chung CH, Dignam JJ, Hammond ME, Klimowicz AC, Petrillo SK, Magliocco A, et al. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003) J Clin Oncol. 2011;29(10):1326–34. doi: 10.1200/JCO.2010.32.3295. https://doi.org/10.1200/JCO.2010.32.3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, Szczylik C, Czarnecka AM. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev Camb Philos Soc. 2017;92(3):1505–20. doi: 10.1111/brv.12293. https://doi.org/10.1111/brv.12293. [DOI] [PubMed] [Google Scholar]
- [8].Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: An update. Mol Carcinog. 2013;52(3):167–82. doi: 10.1002/mc.21844. https://doi.org/10.1002/mc.21844. [DOI] [PubMed] [Google Scholar]
- [9].Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol. 2009;34(5):1381–6. https://doi.org/10.3892/ijo_00000265. [PubMed] [Google Scholar]
- [10].Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–11. doi: 10.1158/0008-5472.CAN-05-0626. https://doi.org/10.1158/0008-5472.CAN-05-0626.11. [DOI] [PubMed] [Google Scholar]
- [11].Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr Opin Cell Biol. 2016;43:7–13. doi: 10.1016/j.ceb.2016.06.002. https://doi.org/10.1016/j.ceb.2016.06.002. [DOI] [PubMed] [Google Scholar]
- [12].Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139(5):871–90. doi: 10.1016/j.cell.2009.11.007. https://doi.org/10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- [13].Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci USA. 2017;114(12):E2337–46. doi: 10.1073/pnas.1618298114. https://doi.org/10.1073/pnas.1618298114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gioanni J, Fischel JL, Lambert JC, Demard F, Mazeau C, Zanghellini E, et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: Establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol. 1988;24(9):1445–55. doi: 10.1016/0277-5379(88)90335-5. https://doi.org/10.1016/0277-5379(88)90335-5. [DOI] [PubMed] [Google Scholar]
- [15].Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65(5):849–57. doi: 10.1016/0092-8674(91)90392-c. https://doi.org/10.1016/0092-8674(91)90392-C. [DOI] [PubMed] [Google Scholar]
- [16].Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol. 2014;4:400. doi: 10.3389/fphys.2013.00400. https://doi.org/10.3389/fphys.2013.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. doi: 10.1016/j.cell.2008.03.027. https://doi.org/10.1016/j.cell.2008.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA. 2002;99(1):365–70. doi: 10.1073/pnas.012425299. https://doi.org/10.1073/pnas.012425299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Beavon IR. Regulation of E-cadherin: Does hypoxia initiate the metastatic cascade? Mol Pathol. 1999;52(4):179–88. doi: 10.1136/mp.52.4.179. https://doi.org/10.1136/mp.52.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Shiraishi A, Tachi K, Essid N, Tsuboi I, Nagano M, Kato T, et al. Hypoxia promotes the phenotypic change of aldehyde dehydrogenase activity of breast cancer stem cells. Cancer Sci. 2017;108(3):362–72. doi: 10.1111/cas.13147. https://doi.org/10.1111/cas.13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hong X, Chedid K, Kalkanis SN. Glioblastoma cell line-derived spheres in serum containing medium versus serum-free medium: A comparison of cancer stem cell properties. Int J Oncol. 2012;41(5):1693–700. doi: 10.3892/ijo.2012.1592. https://doi.org/10.3892/ijo.2012.1592. [DOI] [PubMed] [Google Scholar]