Abstract
Aldehyde dehydrogenase 1 (ALDH1) has been identified as a marker of cancer stem cells in breast cancer (BC). Recent studies showed that ALDH1 expression is correlated with poor prognostic parameters and worse clinical outcome in BC. We evaluated ALDH1 expression by immunohistochemistry in a series of 217 invasive BCs and investigated the correlation between ALDH1 expression and clinicopathological parameters, molecular subtypes (luminal A, luminal B, human epidermal growth factor receptor 2 [HER2] type, and triple-negative BC [TNBC]), and patient survival. There was a significant association between ALDH1 expression and tumor grade (p < 0.001), i.e., the expression of ALDH1 was higher in high-grade tumors. ALDH1 expression was significantly associated with estrogen and progesterone receptor (ER and PR) negativity (p < 0.001) and HER2 positivity (p = 0.001). ALDH1 expression ratios were higher in HER2 type and TNBC. There was a statistically significant correlation between ALDH1 negativity and luminal A subtype (p < 0.001). The overall and disease free survival were shorter in ALDH1+ tumors, although without statistical significance. We confirm that ALDH1 is a potentially important, poor prognostic factor in BC, associated with high histological grade, ER/PR negativity and HER2 positivity. For more accurate results, ALDH1 expression should be evaluated in larger case series including various types/subtypes of BC.
Keywords: ALDH1, breast carcinoma, invasive breast carcinoma, cancer stem cells, molecular subtype
INTRODUCTION
Breast cancer (BC) is one of the most common malignant tumors in women worldwide. It shows a high intra and intertumor heterogeneity, and different subtypes of BC differ in morphological and histopathological characteristics, proteomic/genomic/transcriptomic profiles, metastatic potential, and therapeutic response [1,2].
Cancer stem cells (CSCs) are a subpopulation of cells within tumors that have features similar to normal stem cells, i.e., CSCs have the ability to self-renew and differentiate into mature cancer cells through asymmetric cell divisions. These cells are characterized by dysregulated gene expression and altered signaling pathways, and have been implicated in the onset, maintenance, recurrence and distant metastasis of tumors, as well as tumor resistance to therapy. Based on the expression of specific cell surface markers, CSCs have been reported in a number of cancer types, including BC [3-5].
Aldehyde dehydrogenase 1 (ALDH1), specifically its isotype ALDH1A1, has been identified as a marker of BC CSCs [6,7]. Aldehyde dehydrogenases are a group of enzymes involved in the detoxification of endogenous and exogenous aldehyde substrates to carboxylic acids, through nicotinamide adenine dinucleotide phosphate (NADP+) dependent oxidation [6]. ALDH1A1 is a highly conserved cytosolic isozyme, in addition to the other two cytosolic isotypes ALDH1A2 and ALDH1A3, and is able to catalyze the oxidation of retinal (vitamin A aldehyde) to retinoic acid (RA) which regulates gene expression and is important for normal development and maintenance of adult organs and tissues [6].
Many studies investigated the clinicopathological and prognostic value of ALDH1/ALDH1A1 in BC types/subtypes [7-12], most of them showing that higher ALDH1 expression is associated with larger tumor size, higher histological grade, invasive BCs, higher epidermal growth factor receptor 2 (HER2), and/or lower estrogen receptor (ER) and progesterone receptor (PR) expression. Increased likelihood of lymph node metastasis (LNM) was also associated with ALDH1 expression in some studies [7] although not in all [9]. Moreover, in one study, the percentage of ALDH1+ cells was significantly higher in triple-negative BC (TNBC) and HER2 type compared to luminal and luminal-HER2 BC [10]. Shorter disease-free survival (DFS), relapse-free survival (RFS) and/or overall survival (OS) were reported for ALDH1+ compared to ALDH1- BC patients [10-12].
CD133 or prominin-1 is another biomarker that has been utilized to identify specific CSC and progenitor cell subpopulations in many types of neoplasms including BC [13,14]. For example, Ieni et al. [14] showed that CD133 was highly efficient for detecting hematopoietic progenitor cells (HPCs) in non-metastatic lymph nodes obtained from patients that had been surgically treated for invasive BC, and the role of CD133 as a positive predictor of metastasis risk in BC was suggested [14]. Moreover, Kim et al. [3] reported that CD133 or the combination of CD133 and ALDH1 expression were associated to a higher degree with the presence of adverse biomarkers and subtypes of BC compared to ALDH1 expression alone, indicating their potential predictive role in the management of patients with invasive BC [3].
However, despite the extensive research, clinicopathological and prognostic value of ALDH1 in BC remains controversial. To contribute to the ongoing efforts in this field, we investigated the association of ALDH1 expression with clinicopathological parameters and survival in a sample of invasive BCs. Based on immunohistochemistry (IHC), we grouped BC cases into the four molecular subtypes, i.e., luminal A, luminal B, HER2 type and TNBC. We then evaluated the expression of ALDH1 in relation to these groups.
MATERIALS AND METHODS
Patients
This study included 217 invasive BC cases, diagnosed at the Department of Pathology and treated at the Department of Medical Oncology of Cerrahpasa School of Medicine, Istanbul University, Turkey, between 1992 and 2002. The patients treated with neoadjuvant therapies were excluded from the study. Hematoxylin and eosin (H&E) stained slides and pathology reports were retrospectively reviewed. Clinicopathological parameters including age, sex, multifocality/multicentricity (MF/MC), tumor size, histological type, histological grade, lymphovascular invasion (LVI), axillary lymph node status, local/distant metastases, ER, PR, and HER2 status were recorded for each case. Tumor size and lymph node status were classified based on the TNM classification [15]. The histological types were evaluated according to the World Health Organization (WHO) Classification of Breast Tumors, 4th Edition (2012) [16]. The modified Bloom–Richardson grading system was used for histological grading [17]. OS and DFS times were calculated. OS was defined as the time from diagnosis to death from any cause or until the most recent follow-up. DFS was defined as the time from diagnosis to recurrence or death from any cause.
Tissue microarray (TMA) construction
Representative areas of each tumor were selected on H&E-stained slides and then marked on individual paraffin blocks. Three tissue cores (2 mm in diameter) were obtained from each selected specimen and transferred to a recipient paraffin block using a tissue-arraying instrument. Fourteen TMA blocks were constructed. The non-neoplastic kidney, liver and spleen were used as control tissues in each block.
Immunohistochemistry
Sections of 3 µm in thickness were obtained with a microtome, transferred to positively charged slides and dried at 56 °C for 12 hours. Immunohistochemical staining with ALDH1 antibody was assessed on an automated Ventana Benchmark instrument using the ultraView Universal DAB Detection Kit (Ventana Medical Systems, Arizona, USA). The slides were incubated with primary antibody (ALDH1A1; dilution 1:25; Cell Marque, California, USA) for 1 hour and 8 minutes. Then the slides were stained with Mayer’s hematoxylin for 3 minutes.
Interpretation of immunohistochemistry
Cases were considered positive for ER and PR when strong nuclear staining was observed in at least 10% of tested tumor cells. HER2 immunostaining was considered positive when strong membranous staining (score 3+) was observed in at least 30% of tumor cells [18]. Regardless of the extent or intensity, ALDH1 staining was considered positive when the cytoplasm showed a positive reaction [1]. Stromal staining of ALDH1, observed in some tumors, was not considered.
Molecular classification
A total of 217 cases were classified into the 4 molecular subtypes of BC, based on ER/PR and HER2 status. ER and/or PR (hormone receptor: HR) positive but HER2 negative tumors were classified as luminal A; HR and HER2 positive as luminal B; HR negative but HER2 positive as HER2 type; and tumors negative for ER, PR and HER2 were classified as TNBC.
Statistical analysis
Descriptive statistics were used to describe the data. Normal distribution was tested by the Kolmogorov–Smirnov and Shapiro–Wilk tests. Non-parametric data were compared using Chi-square test. The Kaplan-Meier method was used for survival analysis, and the log-rank test (Mantel–Cox) was performed to compare the survival curves between the groups. The confidence intervals were calculated at the 95% confidence level and differences at p < 0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, Version 21.0. (IBM Corp., Armonk, NY).
RESULTS
Clinicopathological characteristics
In total, 217 invasive BC cases were analyzed. All but 5 patients were women. The age range was 21–92 years in the overall sample (mean ± standard deviation [SD]: 53.0 ± 13.12).
The surgeries performed were modified radical mastectomy (MRM) in 172 cases (79.3%), breast-conserving surgery in 43 cases (19.8%), and simple mastectomy in 2 cases (0.9%). Axillary dissections were performed with all breast-conserving surgeries except one case. The tumor sizes varied from 1 to 10 cm. Cases with multiple tumors were interpreted based on the largest tumor size. Thirty-two (14.7%) cases showed MF/MC and 4 (1.8%) cases had bilateral tumors. Positive lymph nodes were detected in 129 (59.4%) and negative in 84 (38.7%) cases. Lymph node status could not be assessed (unknown) in 2 cases with simple mastectomy, 1 case with MRM, and 1 case with breast-conserving surgery.
The 217 BC cases were grouped according to the ER, PR and HER2 expression as follows: 151 luminal A (69.6%), 22 luminal B (10.1%), 24 HER2 type (11.1%), and 20 TNBC cases (9.2%). The clinicopathological characteristics of the patients and tumors are summarized in Table 1.
TABLE 1.
Clinicopathological characteristics of breast cancer patients

The follow-up periods were available for 196/217 patients and ranged from 4 to 178 months. During this period, 52 (26.5%) cases developed recurrence and 19 (9.7%) died. The time of recurrence was unknown in 1 case. The earliest recurrence developed in 3 months. The earliest death occurred in 7 months.
Association between ALDH1 expression and clinicopathological parameters
ALDH1 positivity was observed in 40 (18.4%) of 217 cases (Figure 1), and all ALDH1-positive (ALDH1+) tumors were from female patients. ALDH1 expression was not correlated with the patient age (p = 0.252).
FIGURE 1.
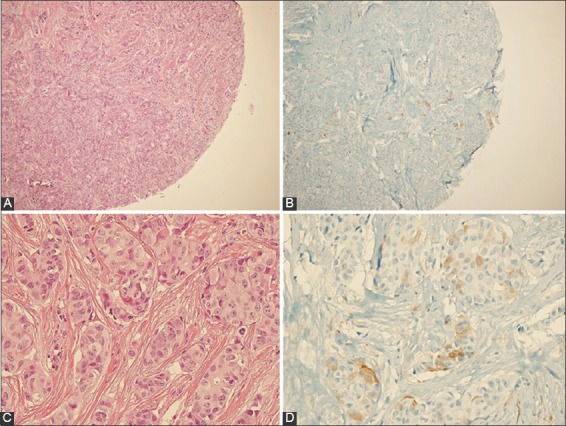
Positive staining of aldehyde dehydrogenase 1 (ALDH1) in a case of invasive breast carcinoma. (A) Hematoxylin and eosin (H&E)×100, (B) immunohistochemistry (IHC)×100, (C) H&E×400, (D) IHC×400.
There was a significant association between ALDH1 expression and tumor grade (p < 0.001). The expression of ALDH1 was increased in relation to the tumor grade; ALDH1 positivity ratios were 4.8%, 11.3% and 38.1% in grade I, II and III tumors, respectively. However, ALDH1 expression was not associated with MF/MC (p = 0.349), tumor size (p = 0.156), and LVI (p = 0.543).
For statistical analysis, 4 cases with unknown lymph node status were ignored and, due to the small number of patients, the remaining 213 cases were divided into groups with positive and negative lymph node status. No significant association was observed between the lymph node status and ALDH1 positivity (p = 0.424).
The histological type of 35/40 ALDH1+ cases was invasive ductal carcinoma (IDC). The remaining cases were 1 invasive lobular carcinoma (ILC), 1 mixed IDC and ILC, 1 cribriform carcinoma, 1 medullary carcinoma, and 1 metaplastic carcinoma [squamous cell carcinoma] (Table 2).
TABLE 2.
Analysis of ALDH1 expression in relation to clinicopathological parameters of breast cancer patients
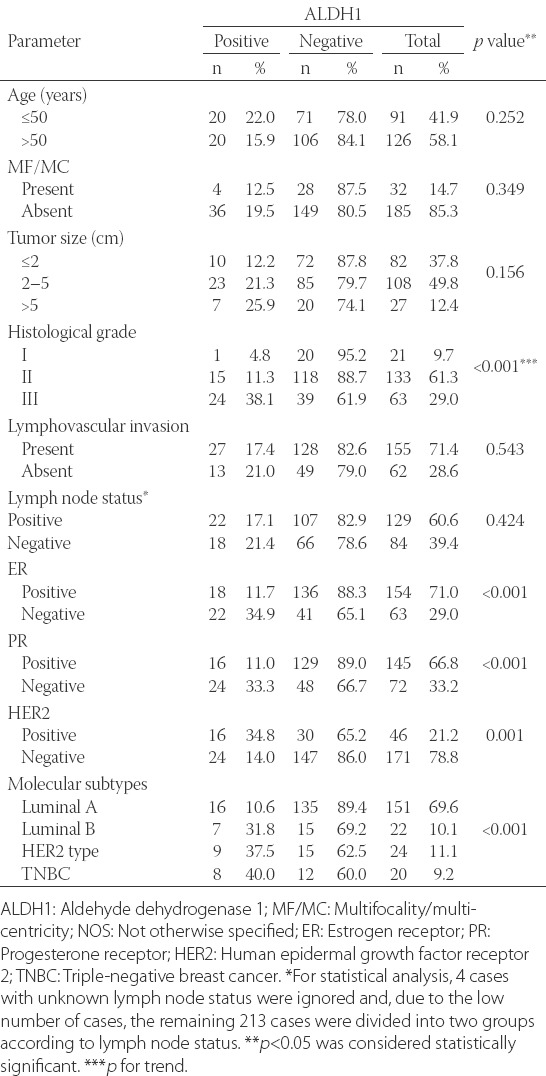
Association of ALDH1 expression with ER, PR, HER2 expression and molecular subtypes of BC
ALDH1 expression was statistically associated with ER negativity (p < 0.001), PR negativity (p < 0.001) and HER2 positivity (p = 0.001). ALDH1 expression ratios were higher in ER- and PR- (34.9% and 33.3%) compared to ER+ and PR+ tumors (11.7% and 11.0%, respectively). In contrast, ALDH1 expression ratios were higher in HER2+ (34.8%) compared to HER2- tumors [14.0%] (Table 2).
ALDH1 expression ratios in each molecular subtype of BC were as follows: 10.6%, 31.8%, 37.5%, 40.0% in luminal A, luminal B, HER2 type and TNBC, respectively. Apparently, ALDH1 expression was higher in HER2 type and TNBC compared to luminal A and luminal B types. The majority of luminal A cases (89.4%) were ALDH1- and there was significantly more ALDH1- cases in luminal A subtype compared to the number of ALDH1- cases in other BC molecular subtypes (p < 0.001).
Association between ALDH1 expression and survival outcomes
The OS rate was 85.3% and 91.4% in ALDH1+ and ALDH1- groups, respectively. However, there was no statically significant difference between the groups (log rank = 1.251, p = 0.263). The DFS rate was 70.6% and 74.1% in ALDH1+ and ALDH1- cases, respectively, with no significant difference between the groups [log rank = 0.437, p = 0.508] (Figure 2).
FIGURE 2.

Overall survival [OS] (A) and disease free survival [DFS] (B) in aldehyde dehydrogenase 1 (ALDH1) positive and negative cases. The OS and DFS were shorter in ALDH1+ tumors, although without statistical significance.
DISCUSSION
Recently, a growing number of studies have been investigating the clinicopathological and prognostic value of CSC marker ALDH1 in BC. Most of those studies showed that ALDH1 expression was associated with poor prognostic parameters and worse clinical outcome in BC patients [3,7-11]. In this study, we immunohistochemically evaluated ALDH1 expression in a series of 217 invasive breast carcinomas and analyzed the correlation between ALDH1 expression and clinicopathological parameters, molecular subtypes of BC, and patient survival.
We grouped 217 cases according to the age (≤50 and >50 years old) and found no significant difference in ALDH1 expression between the two groups. Five out of 217 BC patients were male and all of them were negative for ALDH1 expression. Similarly, in a meta-analysis including 21 studies on the relationship between ALDH1 expression and clinical pathological features of BC patients, no significant correlation between ALDH1 expression and patient age was observed [9].
When we analyzed ALDH1+ cases in terms of histological types of BC, most of ALDH1+ tumors (35/50) were IDC. The remaining cases were ILC, mixed IDC and ILC, cribriform, medullary or metaplastic carcinoma. Only 1 out of 5 metaplastic carcinoma, a type which is known to have poor prognosis, was ALDH1+.
Pan et al. [8] showed a lower rate of ALDH1 expression in cases with DC in situ (DCIS) and a higher in patients with invasive cancer without extensive intraductal component (EIC). Notably, in the same tumor, the rate of ALDH1 expression was higher in the invasive component than in the in situ component. Also, in their study, ALDH1+ invasive BCs were significantly more likely to have large tumor size, high grade, and high Ki67 expression [8].
In a meta-analysis covering 921 ALDH1A1+ BC cases and 2353 controls (ALDH1A1-) [7], in the overall sample, higher ALDH1A1 expression was associated with larger tumor size, higher histological grade, increased likelihood of LNM, higher HER2, and lower ER and PR expression. Also, the prognosis of ALDH1A1+ patients was poorer compared to ALDH1A1- group [7]. Another meta-analysis showed significant association (pooled analysis) of ALDH1 expression with histological grade, ER expression and PR expression in BC, however, not with the tumor size, LNM, LVI, and HER2 expression [9]. Kida et al. indicated that in their group of invasive BC specimens ALDH1 expression significantly correlated with larger tumor size, node metastasis, higher nuclear grade, and with HER2+ and PR/ER- subtypes [10].
Comparably, we showed a significant association between ALDH1 expression and the tumor grade (p < 0.001). The ALDH1 positivity ratios were 4.8%, 11.3% and 38.1% in grade I, II and III tumors, respectively. On the contrary, we did not observe a significant difference in ALDH1 expression in relation to the tumor size, i.e. between the tumors of ≤2 cm, 2–5 cm, and >5 cm in size. There was also no significant association between ALDH1 expression and MF/MC. Nevertheless, consistently with the previous studies [7,10], ALDH1 expression in our sample was significantly associated with ER negativity (p < 0.001), PR negativity (p < 0.001) and HER2 positivity (p = 0.001).
Some authors reported a significant correlation between ALDH1 expression and axillary lymph node metastasis in BC [7,10]. Moreover, in a study that included only ER+/HER2- breast carcinomas, ALDH1 expression was significantly associated with LNM in the group of patients with early recurrences [19]. Due to the small number of ALDH1+ cases in our study, we only compared ALDH1 expression between positive and negative lymph node status, and did not observe significant difference between the two groups. Similarly, we did not find any association between ALDH1 expression and LVI.
Kim et al. reported that ALDH1, as well as CD133, expression correlated significantly with nonluminal subtype and TNBC [3]. In the study of Kida et al. [10] the percentage of ALDH1+ cells was significantly higher in TNBC and HER2 type compared to luminal and luminal-HER2 type. Moreover, they reported that ALDH1 expression significantly affected the prognosis of luminal types, but not that of TNBC and HER2 type [10]. In the study which included only ER+/HER2- BC types, ALDH1 expression was significantly higher in the early recurrence group compared to the group without recurrence [19]. We compared the ratios of ALDH1 expression in each molecular subtype of BC and observed that ALDH1 expression was higher in HER2 type and TNBC (37.5% and 40.0%, respectively) compared to luminal A and luminal B types (10.6% and 31.8%, respectively). The majority of our cases (69.6%) were luminal A and 89.4% of them showed ALDH1 negativity. There was significantly more ALDH1- cases in luminal A subtype compared to the number of ALDH1- cases in other BC molecular subtypes (p < 0.001).
In two studies investigating the clinical significance of ALDH1 expression in TNBC, ALDH1 expression was correlated with shorter RFS [12,20] and OS [12], and in both studies ALDH1 expression was an independent prognostic indicator according to multivariate analysis. In our study 69.6% of cases were luminal A. The OS rate was 85.3% and 91.4%; DFS rate was 70.6% and 74.1%, respectively, in ALDH1+ and ALDH1- groups. However, the difference was not statistically significant (OS: log rank = 1.251, p = 0.263; DFS: log rank = 0.437, p = 0.508), and this might be related to the small sample size and lower occurrence of deaths during the follow-up period.
A limitation of our study is that, due to the nature of TMA technique, immunohistochemical staining was performed only in small (millimetric) areas of tumors. Thus, ALDH1 staining was considered positive based only on a positive cytoplasmic reaction and regardless of the extent or intensity of staining. Furthermore, ALDH1+ tumor areas could have been missed during the sampling and, therefore, some cases may have been mistakenly assessed as ALDH1-. Larger sample size is necessary for more accurate evaluation of ALDH1 expression in different types/subtypes of BC.
CONCLUSION
Overall, our results on 217 invasive BCs indicate that ALDH1 is an important, poor prognostic factor associated with high histological grade, ER/PR negativity and HER2 positivity. We observed a significant correlation between luminal A subtype and ALDH1 negativity. ALDH1 expression also effected the patient survival in our sample, although without statistical significance. For more accurate and comprehensive results, the prognostic value of ALDH1, especially in invasive breast carcinomas, should be further studied in larger case series.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Kim YS, Jung MJ, Ryu DW, Lee CH. Clinicopathologic characteristics of breast cancer stem cells identified on the basis of aldehyde dehydrogenase 1 expression. J Breast Cancer. 2014;17(2):121–8. doi: 10.4048/jbc.2014.17.2.121. Erratum in: J Breast Cancer 2016;19:340. https://doi.org/10.4048/jbc.2014.17.2.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Collina F, Di Bonito M, Li Bergolis V, De Laurentiis M, Vitagliano C, Cerrone M, et al. Prognostic value of cancer stem cells markers in triple-negative breast cancer. Biomed Res Int. 2015;2015:158682. doi: 10.1155/2015/158682. https://doi.org/10.1155/2015/158682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kim SJ, Kim YS, Jang ED, Seo KJ, Kim JS. Prognostic impact and clinicopathological correlation of CD133 and ALDH1 expression in invasive breast cancer. J Breast Cancer. 2015;18(4):347–55. doi: 10.4048/jbc.2015.18.4.347. https://doi.org/10.4048/jbc.2015.18.4.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].LV X, Wang Y, Song Y, Pang X, Li H. Association between ALDH1+/CD133+ stem-like cells and tumor angiogenesis in invasive ductal breast carcinoma. Oncol Lett. 2016;11(3):1750–6. doi: 10.3892/ol.2016.4145. https://doi.org/10.3892/ol.2016.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ginestier C, Hur MH, Charafe-Jauffret E, Monville F, Dutcher J, Brown M, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1(5):555–67. doi: 10.1016/j.stem.2007.08.014. https://doi.org/10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Tomita H, Tanaka K, Tanaka T, Hara A. Aldehyde dehydrogenase 1A1 in stem cells and cancer. Oncotarget. 2016;7(10):11018–32. doi: 10.18632/oncotarget.6920. https://doi.org/10.18632/oncotarget.6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Liu Y, Lv DL, Duan JJ, Xu SL, Zhang JF, Yang XJ, et al. ALDH1A1 expression correlates with clinicopathologic features and poor prognosis of breast cancer patients: A systematic review and meta-analysis. BMC Cancer. 2014;14:444. doi: 10.1186/1471-2407-14-444. https://doi.org/10.1186/1471-2407-14-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pan H, Wu N, Huang Y, Qin Li, Liu C, Liang M, et al. Aldehyde dehydrogenase 1 expression correlates with the invasion of breast cancer. Diagn Pathol. 2015;10:66. doi: 10.1186/s13000-015-0301-5. https://doi.org/10.1186/s13000-015-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liu JF, Xia P, Hu WQ, Wang D, Xu XY. Aldehyde dehydrogenase 1 expression correlates with clinicopathologic features of patients with breast cancer: A meta-analysis. Int J Clin Exp Med. 2015;8(6):8425–32. [PMC free article] [PubMed] [Google Scholar]
- [10].Kida K, Ishikawa T, Yamada Shimada K, Narui K, Sugae S, et al. Effect of ALDH1 on prognosis and chemoresistance by breast cancer subtype. Breast Cancer Res Treat. 2016;156(2):261–9. doi: 10.1007/s10549-016-3738-7. https://doi.org/10.1007/s10549-016-3738-7. [DOI] [PubMed] [Google Scholar]
- [11].Dong Y, Bi LR, Xu N, Yang HM, Zhang HT, Ding Y, et al. The expression of aldehyde dehydrogenase 1 in invasive primary breast tumors and axillary lymph node metastases is associated with poor clinical prognosis. Pathol Res Pract. 2013;209(9):555–61. doi: 10.1016/j.prp.2013.05.007. https://doi.org/10.1016/j.prp.2013.05.007. [DOI] [PubMed] [Google Scholar]
- [12].Ma F, Li H, Li Y, Ding X, Wang H, Fan Y, et al. Aldehyde dehydrogenase 1 (ALDH1) expression is an independent prognostic factor in triple negative breast cancer (TNBC) Medicine (Baltimore) 2017;96(14):e6561. doi: 10.1097/MD.0000000000006561. https://doi.org/10.1097/MD.0000000000006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Giuffrè G, Adamo V, Ieni A, Colonese F, Barresi V, Caristi N, et al. Hematopoietic progenitor cells (HPCs) in node-negative invasive breast carcinomas: Immunohistochemical analysis and clinico-pathological correlations. Pathol Res Pract. 2011;207(8):487–91. doi: 10.1016/j.prp.2011.05.013. https://doi.org/10.1016/j.prp.2011.05.013. [DOI] [PubMed] [Google Scholar]
- [14].Ieni A, Giuffrè G, Adamo V, Tuccari G. Prognostic impact of CD133 immunoexpression in node-negative invasive breast carcinomas. Anticancer Res. 2011;31(4):1315–20. [PubMed] [Google Scholar]
- [15].Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A AJCC cancer staging manual. 7th Edn. New York, NY: Springer; 2010. [Google Scholar]
- [16].Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon, France: IARC Press; 2012. [Google Scholar]
- [17].Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. https://doi.org/10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- [18].Kim MJ, Ro JY, Ahn SH, Kim HH, Kim SB, Gong G. Clinicopathologic significance of the basal-like subtype of breast cancer: A comparison with hormone receptor and Her2/neu-overexpressing phenotypes. Hum Pathol. 2006;37(9):1217–26. doi: 10.1016/j.humpath.2006.04.015. https://doi.org/10.1016/j.humpath.2006.04.015. [DOI] [PubMed] [Google Scholar]
- [19].Miyoshi Y, Shien T, Ogiya A, Ishida N, Yamazaki K, Horii R, et al. Differences in expression of the cancer stem cell marker aldehyde dehydrogenase 1 among estrogen receptor-positive/human epidermal growth factor receptor type 2-negative breast cancer cases with early, late, and no recurrence. Breast Cancer Res. 2016;18:73. doi: 10.1186/s13058-016-0731-3. https://doi.org/10.1186/s13058-016-0731-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ohi Y, Umekita Y, Yoshioka T, Souda M, Rai Y, Sagara Y, et al. Aldehyde dehydrogenase 1 expression predicts poor prognosis in triple-negative breast cancer. Histopathology. 2011;59(4):776–80. doi: 10.1111/j.1365-2559.2011.03884.x. https://doi.org/10.1111/j.1365-2559.2011.03884.x. [DOI] [PubMed] [Google Scholar]


