Abstract
Angiogenic effects of epidermal growth factor (EGF), a potent mitogen, have been demonstrated previously. Moreover, different in vitro studies showed that EGF affects processes associated with bone healing, such as osteoblast differentiation and bone resorption. The aim of this study was to investigate the effect of combined core decompression (CD) and recombinant human EGF (rhEGF) treatment on early-stage osteonecrosis of the femoral head (ONFH) surgically induced in rats. ONFH was induced by dissecting the cervical periosteum and placing a ligature tightly around the femoral neck. Thirty rats were assigned to one of the following groups (n = 10 each group): sham-operated control, CD, and CD+rhEGF group. rhEGF was injected intraosseously into infarcted areas 2 weeks after the surgery. Preservation of femoral head architecture was assessed at 8 weeks post treatment by radiographic and histomorphological analyses. Osteopontin (OPN) and cluster of differentiation 31 (CD31) were detected by immunochemistry, as indicators of bone remodeling and vascular density, respectively. Inter- and intra-group (non-operated left and operated right femur) differences in radiographic and histomorphological results were analyzed. The femoral head area and sphericity were more preserved in CD+rhEGF compared to CD and sham-control group. CD31 levels were significantly different between the three groups, and were higher in CD+rhEGF compared to CD group. OPN levels were increased in CD and CD+rhEGF groups compared to sham control, but with no significant difference between CD and CD+rhEGF groups. Overall, our results indicate that EGF promotes bone formation and microvascularization in ONFH and thus positively affects the preservation of femoral head during healing.
Keywords: Osteonecrosis, epidermal growth factor, EGF, osteopontin, OPN, angiogenic agents, core decompression, CD31, cluster of differentiation 31
INTRODUCTION
Osteonecrosis of the femoral head (ONFH) is a chronic disease with multifactorial etiology, associated with decreased blood flow to the femoral head that eventually leads to cellular death. Due to the complex etiology, the exact mechanisms of ONFH pathogenesis are not completely clear and effective treatment with good long-term outcomes is yet to be developed. At the early (precollapsed) stage of ONFH the treatment modalities may include non-operative (e.g. restricted weight-bearing, pharmacological agents and biophysical modalities) or operative approach, such as osteotomy and core decompression (CD) with or without the addition of bone marrow or bone graft, with the ultimate goal to prevent the onset of subchondral fracture or collapse. The main treatment option for the postcollapsed stage is total hip arthroplasty (THA) [1]. Recent efforts have been focused on the specific role of angiogenesis in bone healing, and the use of osteoinductive and angiogenic agents (in combination with CD) to enhance bone formation and repair, thus preserving the femoral head and avoiding hip replacement surgery.
Angiogenic effects of mitogens such as fibroblast and epidermal growth factor (FGF and EGF) have been demonstrated in various in vitro and in vivo studies [2-4]. Moreover, EGF was shown to suppress osteoblastic differentiation in cultured rat bone marrow stromal cells by increasing the expression of EGF receptor (EGFR) [3], to affect the osteogenic potential of rat calvaria (RC) cells (i.e. increase or decrease depending on the duration of exposure) [5], and to stimulate bone resorption in cultured fetal rat long bone shafts [6]. In addition, EGF treatment in human periodontal ligament cells (HPDLCs) significantly induced secretion of bone morphogenetic protein 2 (BMP-2) and vascular endothelial growth factor (VEGF), as well as gene expression of interleukin-8 (IL-8) and early growth response-1 and -2 (EGR-1/2) indicating that, under inflammatory conditions, upregulated EGF has a role in the healing of injured PDL tissue [7].
The aim of this study was to investigate the effect of combined CD and recombinant human EGF (rhEGF) treatment on early-stage ONFH. We hypothesized that the combination of CD with local administration of rhEGF will enhance bone formation, repair and angiogenesis following the early stage of ONFH. After the induction of ONFH in rats and administration of treatment 2 weeks later (CD or CD+rhEGF) the preservation of femoral head was assessed in sham-operated and treatment groups at 8 weeks post treatment, by radiographic and histomorphological analyses. We also analyzed the levels of osteopontin (OPN) and cluster of differentiation 31 [CD31] (also called platelet endothelial cell adhesion molecule [PECAM-1]) by immunochemistry, as indicators of bone remodeling and vascular density, respectively.
MATERIALS AND METHODS
Ethics statement
All experiments were conducted in accordance with the Ministry of Health of Turkey, the Declaration of Helsinki, and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (NIH) of the United States. The Experimental Animal Center and Ethics Committee of Suleyman Demirel University approved all experimental procedures in this study (Ethics number: 21438139-245).
Animals and surgical procedures
Male Sprague–Dawley rats (20 to 24 weeks of age, weighing 280–370 g) were purchased from the Experimental Animal Center, Isparta, Turkey. Animals were handled regularly for at least one week before surgery and were housed in individual cages at 22 °C and 50% humidity placed in controlled rooms with 12 hours light/dark cycle. A prophylactic antibiotic (4 mg/kg gentamicin via intramuscular injection [i.m.]) was administered to all rats within 60 minutes before surgery. Thirty minutes anesthesia was induced with 100 mg/kg alfamine 10% (Alfasan, Woerden, Netherlands) and 10 mg/kg xylazine 2% (Bioveta, Ivanovice na Hané, Czech Republic) administered intraperitoneally (i.p.). Ischemic osteonecrosis was induced in the right femoral head by the application of a tight ligature around the femoral neck, as previously described [8]. After the induction of osteonecrosis, rats (n = 30) were assigned to three groups as follows: surgical model as sham-operated control (Group 1, n = 10), CD (Group 2, n = 10), and rhEGF+CD group (Group 3, n = 10). Two weeks after the induction of ischemia, a repeated arthrotomy was performed to visualize the femoral head, and the ligatures were tied. At the end of the second week, capsulotomy was carried out in control group and the node (ligature) was removed. After 8 weeks, rats were euthanized by exsanguination under anesthesia.
Core decompression and intraosseous administration of rhEGF
At the end of the second week, arthrotomy was performed after which the femoral head and trochanteric area were visualized (Figure 1). CD was performed in Group 2 (n = 10) and Group 3 (n = 10). The entry point of the drill hole was just below the intertrochanteric crest. A decompression tunnel was created between the lateral femoral cortex and the center of the femoral head through the femoral neck with a 0.4 mm drill. The image intensifier is not necessary during the procedure, but a drill bit with a depth mark should be used to avoid penetrating the femoral head. The intraosseous (i.o.) injection of 75 µg rhEGF (Heberprot-P, CIGB, Havana, Cuba) was dissolved in 5 ml of 0.9% NaCl (saline). A 28-G needle was inserted into the decompression tunnel through the center of the femoral head and 0.2 ml rhEGF (3 µg for each rat, n = 10) was injected i.o. Drill holes were closed with bone wax after the i.o. application of rhEGF. The dose of rhEGF was determined as 10 µg/kg according to previous studies [9,10].
FIGURE 1.
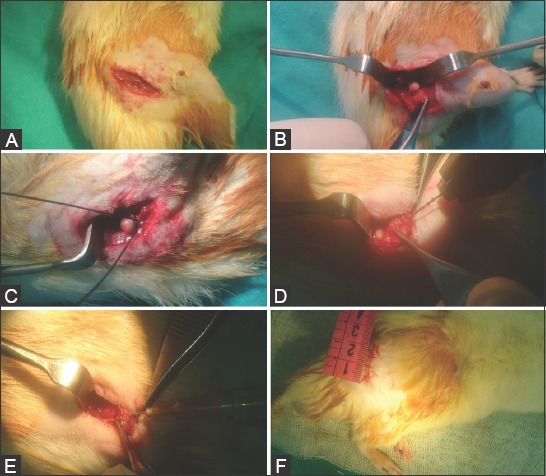
Intraoperative images of rat right femoral head. (A) Skin incision and exposure; (B) Arthrotomy and dislocation; (C) Placing a ligature tightly around the femoral neck; (D) Creating a decompression tunnel with a 0.4-mm drill; (E) Intraosseous injection of rhEGF with a 28 G needle; (F) Skin closure.
Radiographic analysis
After rats were euthanized, the right and left proximal femora were harvested and fixed in 10% neutral buffered formalin (NBF). The bilateral anterior-posterior and lateral x-ray images were obtained for each rat using a Philips Optimus X-Ray Generator (Philips Medical Systems, Hamburg, Germany). The femurs were oriented in standard anterior-posterior and lateral projections in pairs. All images were obtained using the exposure settings of 34 kVp and 3.20 mAs from a 1-meter distance. The femurs were sorted according to the group number and a single image was also taken to avoid incorrect measurements. The bone architecture was not evaluated, as we did not use micro computed tomography (micro-CT).
Digital images were magnified 150% and measured in ImageJ (Java-based image processing program developed by the NIH). Radiographic measurements of the femoral head were sphericity, area, the widest diameter, and height [a vertical distance from the diameter line to the tip] (Figure 2). To determine sphericity, Mose circle [11] measurements were taken by a picture archiving communication system (PACS) at 40% magnification. Circle measurements were classified as spherical, ovoid, or flattened. Sphericity was measured by a concentric circle that best fitted the femoral head. The femoral head diameter, height, and area were measured in the anterior-posterior x-ray images.
FIGURE 2.
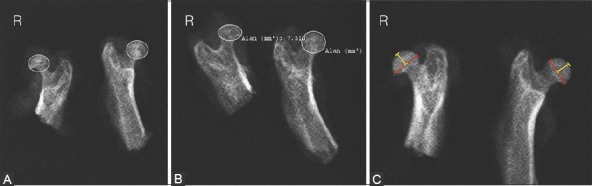
Radiographic measurements of infarcted rat right femoral head. (A) Sphericity; (B) Femoral head area; (C) Femoral head diameter (red dimension line) and height (yellow dimension line).
Histological evaluation
Histological and immunohistochemical analyses were performed blinded with respect to each other and read independently by single investigators. The resected femurs of rats were fixed in 10% NBF, then decalcified in 10% ethylenediaminetetraacetic acid (EDTA) for 10 days or in rapid decalcifying solution for 12 hours, and finally embedded in paraffin blocks. The tissues were longitudinally sectioned at 5 µm and the histological sections were obtained at the midline of the femoral head. The coronal sections of the right (operated) and left (non-operated control) femoral heads were examined histomorphologically. The histological sections were stained with hematoxylin and eosin (H&E) and analyzed under a light microscope. All light microscope images were imported into ImageJ software and measured at 150% magnification.
Analysis of vascular density and bone formation by immunohistochemistry
Tissues were longitudinally sectioned at 5 µm and placed on poly-L-lysine coated slides. Paraffin sections were deparaffinized before quenching endogenous peroxidase activity. After deparaffinization, the sections were treated with sodium citrate buffer in a pressure cooker (LabVision Corporation, Fremont, CA, USA) at 98 °C for 20 minutes for antigen retrieval. Following this procedure, they were cooled at room temperature for 20 minutes in citrate buffer and treated with distilled water. The sections were treated with H2O2 (hydrogen peroxide), incubated for 20 minutes, and then washed with phosphate-buffered saline (PBS, pH 7.6). After being blocked with Ultra V Block (Thermo Fisher Scientific, Fremont, CA, USA), the slides were incubated with OPN rabbit polyclonal (1:50; cat.no: RB-9097-P0 Labvision, Thermo Fisher Scientific Fremont, CA, USA) and with CD31 rabbit polyclonal (1:100; bs-0468R Woburn, Massachusetts, USA) antibody dilution for 60 minutes at room temperature. The sections were treated with PBS (pH 7.6). The secondary antibody was conjugated with biotinylated goat anti-polyvalent (TP-125-BN Labvision, Fremont, CA, USA). After being washed with PBS, the sections were treated with streptavidin peroxidase (TS-125-HR, Labvision, Fremont, CA, USA) and then with 3,3’-diaminobenzidine (DAB) chromogen for 30 seconds. Then, the sections were counterstained with Mayer’s hematoxylin for 15–60 seconds and carefully washed with distilled water. After being treated with ethanol, the sections were dried, placed in xylene, and the slides were covered with a cover slip using Entellan (Merck, Darmstadt, Germany).
Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0. (IBM Corp., Armonk, NY, USA). All data are expressed as the mean ± standard deviation (SD). Nonparametric data were analyzed by the Mann–Whitney U and Wilcoxon tests. For multiple groups, the differences were first analyzed with the Kruskal–Wallis H test followed by Bonferroni’s multiple comparison test. Differences in mineral densities and remodeling phases were compared using Pearson’s chi-squared test. The values of p < 0.05 were considered statistically significant. The femoral head diameter, height, area, trabecular thickness, subchondral distance, and epiphyseal thickness were compared with the Wilcoxon test between the right operated and left non-operated hips in each group. A power calculation was performed with a confidence level of 95% (a = 0.05) and a power (1-b) of 90%, resulting in a minimum requirement of 5 rats per group.
RESULTS
The body weights of rats were measured in week 0, week 2, and week 8 before euthanization. There were no significant differences in the rat weight between the groups. The mean body weight was 329 ± 24.20 g (n = 30). Two rats from Group 2 and two rats from Group 3 were excluded from the study due to femoral neck fracture. One rat from Group 1 was also excluded because of infection in intracapsular fracture of the femoral neck.
Radiographic findings
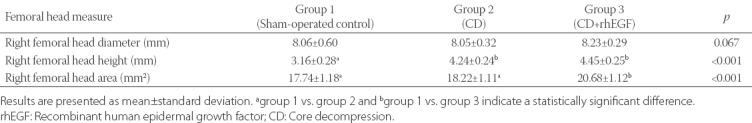
The architecture of the femoral head was partially preserved in the group treated with CD+rhEGF (Group 3). Representative radiographs are shown in Figure 3. The resorption of the femoral neck due to tight ligature was observed in all groups. According to the Mose circle analysis [11], the femoral head was spherical in 11.1% sham-operated control rats (1 out of 9), 37.5% (3 out of 8) rats from Group 2 (CD), and 75% (6 out of 8) rats from Group 3 [CD+rhEGF] (Figure 3). There were no significant differences in the femoral head diameter between the groups. The femoral height was significantly decreased in Group 1 when compared to Group 2 and 3. There was no significant difference in the femoral height between Group 2 and 3. The mean femoral head area values were significantly lower in Group 1 and 2 compared to Group 3 [p < 0.01] (Table 1).
FIGURE 3.
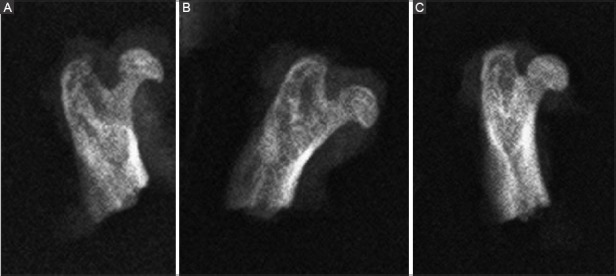
Representative radiographs of rat right femoral head after the induction of osteonecrosis and application of treatments. (A) Sham-operated control (Group 1); (B) CD (Group 2); (C) CD+rhEGF group (Group 3). The sphericity was markedly preserved in Group 3. rhEGF: Recombinant human epidermal growth factor; CD: Core decompression.
TABLE 1.
Effects of CD and CD+rhEGF treatment on infarcted right rat femoral head as assessed by radiographic analysis
Histological and immunohistochemical assessments
Histopathological examination revealed patchy areas characterized by empty osteocyte lacunae and trabecular thinning which led to the diagnosis of osteonecrosis. Histological results were compared between contralateral left non-operated and right operated femoral heads for all three groups (Figure 4). Osteonecrosis was not observed in the left femoral heads, while it was observed in all surgically induced femurs (100%). Trabecular thickness, subchondral distance, and epiphysis thickness were averaged from three different measurement points in each rat. There was no significant difference in the trabecular thickness between the three groups. The subchondral distance was significantly reduced in Group 1 and 2, but largely preserved in Group 3 (Table 2) [p = 0.004].
FIGURE 4.
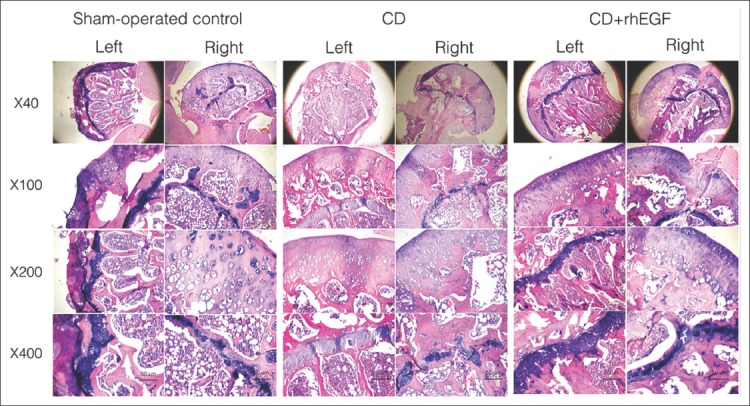
Photomicrographs (hematoxylin and eosin, 40-400×) of rat femoral heads 8 weeks after the induction of osteonecrosis and application of treatments. Right - operated femoral head; Left - non-operated contralateral femoral head. rhEGF: Recombinant human epidermal growth factor; CD: Core decompression. Group 1: Sham-operated control; Group 2: CD; Group 3: CD+rhEGF.
TABLE 2.
Effects of CD and CD+rhEGF treatment on infarcted right rat femoral head as assessed by histomorphological analysis

Immunohistochemical results are summarized in Table 3. OPN levels were similar between Group 2 and 3 (p = 0.435), but were lower in Group 1 compared to the other two groups (p = 0.001). These findings suggest that in the groups treated with CD (with or without rhEGF) the level of mineralization was higher in the femoral head compared to sham-operated control group. Mineral densities and remodeling phases were determined based on OPN staining for each group and statistically analyzed. Pearson’s chi-squared test was conducted to test the differences between groups. CD31 staining was performed to evaluate neovascularization in Group 1-3 (Figure 5). The immunohistochemistry results showed that CD31 levels were significantly different between the three groups (p = 0.001), and were higher in Group 3 compared to Group 2 (p = 0.036). These findings indicate that neovascularization was increased in the group treated with CD+rhEGF (Group 3).
TABLE 3.
Effects of CD and CD+rhEGF treatment on infarcted right rat femoral head as assessed by immunohistochemical analysis of CD31 and OPN
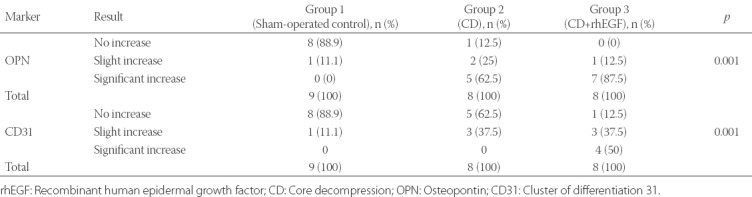
FIGURE 5.
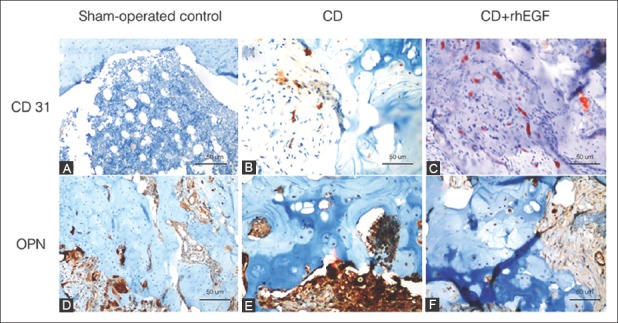
Immunohistochemical staining of CD31 (A-C) and OPN (D-F) in infarcted rat right femoral heads after treatments. (A and D) Group1: Sham-operated control group; (B and E) Group 2: CD group; (C and F) Group 3: CD+rhEGF group. CD31: Cluster of differentiation 31; OPN: Osteopontin; rhEGF: Recombinant human epidermal growth factor; CD: Core decompression.
Finally, we compared the radiographic findings between contralateral left non-operated and right operated femoral heads for all three groups, and these results are presented in Table 4. The height loss of the right femoral heads compared with the left non-operated heads was 25% in Group 1, 22% in Group 2, and 2% in Group 3. The differences between operated and non-operated femurs in Group 1 and 2 were significant for femoral head area and subchondral distance, and in all three groups for epiphyseal thickness. In Group 2, trabecular thickness was also significantly different between the left and right femurs. In Group 3, differences were not significant between the left and right hips except for femoral head height and epiphyseal thickness, indicating that the femoral heads were well preserved after CD+rhEGF treatment in Group 3 (Figure 6).
TABLE 4.
Comparison of radiological and histomorphological findings between operated right and non-operated left rat hips in each group
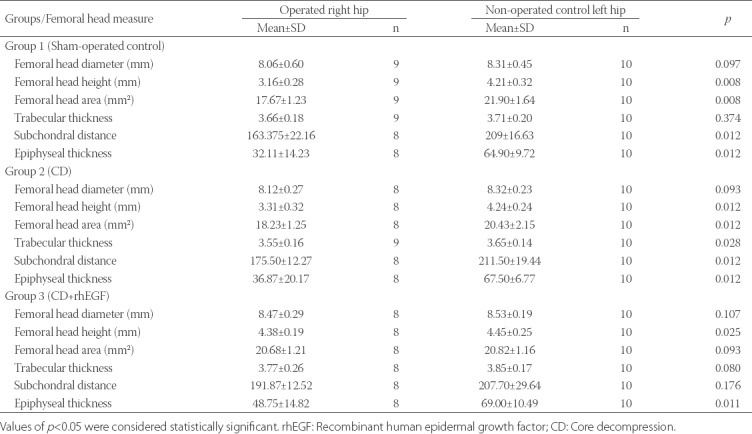
FIGURE 6.
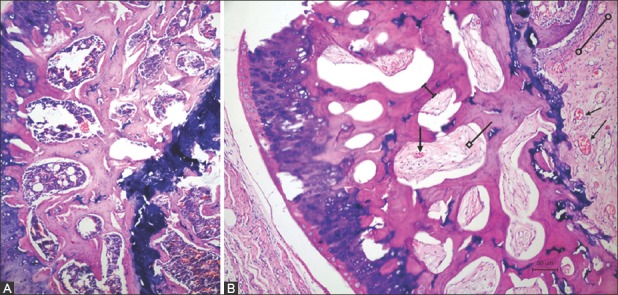
Coronal sections of (A) non-operated left rat femoral head without signs of necrosis and (B) infarcted right rat femoral head in group treated with CD+rhEGF (Group 3). As compared with the left side, osteoblastic activity and angiogenesis (lines with arrows) could be observed in the bone marrow of femoral head in CD+rhEGF-treated group. Trabecular thickness (dimension line), secondary ossification centers, and bone marrow (line with square) were also well preserved. Metaphyseal area was partly replaced with fibrous tissue (line with empty circles). rhEGF: Recombinant human epidermal growth factor; CD: Core decompression.
DISCUSSION
ONFH is a pathological process that occurs in the femoral head under specific conditions and, at the advanced stage od disease, it progresses to the fracture of subchondral bone, collapse and permanent deformity of the femoral head [12,13]. Although many etiological factors (traumatic and nontraumatic) have been associated with the onset of ONFH, the pathological mechanism is still not well understood. Nevertheless, a decreased vascular supply to the subchondral bone of the femoral head has been recognized as the underlying cause of osteocyte death in ONFH. The treatment may include surgical (CD, bone grafting, osteotomy, arthroplasty) and/or pharmaceutical (bisphosphonates, iloprost, statins) approach [12,14-16]. CD was first described by Hopson and Siverhus in 1988 [12], and since then, it has been commonly used in the treatment of early-stage ONFH (stage 1-2). Recently, Zhao and Yu [17] showed that in ONFH resulting only from venous stasis, CD led to better clinical outcomes compared to patients who had venous stasis combined with artery blood supply insufficiency for which long-term efficacy was not successfully achieved after CD. The authors emphasized the importance of evaluating hemodynamics changes in ONFH patients prior to treatment selection [17]. THA is a common treatment for end-stage osteonecrosis with articular collapse. However, total hip replacement is an unsuitable option for young and active population, and thus, the ultimate goal of ON treatment is to prevent the development of femoral head deformity at early stages [13,18,19]. With this regard, recent studies have been focused on the role of angiogenesis in bone healing and the use osteoinductive or angiogenic agents to enhance bone formation and repair and thus preserve the femoral head [8,20-23]. However, in our opinion, a more comprehensive approach is needed for the treatment of ONFH, which would target all aspects of bone healing process including the specific microenvironment, osteogenic differentiation of mesenchymal stem cells (MSCs), balance between bone resorption and formation, and related signaling pathways.
Angiogenic factors produced by osteoclasts, osteoblasts and osteocytes in the bone remodeling compartment (BRC) can regulate local endothelial cells and pericytes, and thus have a role in directional angiogenesis and maintenance of blood supply above BRC canopies during the bone remodeling cycle. These factors, among others, include VEGF, basic fibroblast growth factor (bFGF), BMP7, receptor activator of NF-kB ligand (RANKL) and EGF-like family members, where EGF-like members possibly have a crucial role in the communication between osteoblasts, osteoclasts and endothelial cells during the remodeling cycle [2,24].
Moreover, EGF-like ligands, among other types of molecules, are involved in the regulation of proliferation, differentiation, and survival of osteoprogenitor cells from which mature osteoblast arise. These molecules are well-known potent mitogens for MSCs and osteoprogenitors, and they exert their activity through EGFR [25]. In transgenic (deletion of the EGFR genomic locus) and pharmacologic (inhibition of EGFR in wild-type phenotype) mouse models, Zhang et al. [4] observed that the modified EGFR activity led to a decrease in trabecular bone mass/volume, decrease in osteoblast number and mineralization activity, increase in osteoclast number, and fewer bone marrow MSC and progenitor cells; the authors indicated an anabolic role of EGFR signaling in bone metabolism [4].
In our study, the comparison of radiographic findings between contralateral left non-operated and right operated hips showed that the femoral heads were better preserved in CD+rhEGF treated group compared to the sham and CD groups. For example, the height loss of the right femoral heads was the lowest in CD+rhEGF and there was no significant difference between operated and non-operated hips for the femoral head area and subchondral distance in this group. We also observed increased OPN levels in CD and CD+rhEGF groups compared to sham control, but with no significant difference between CD and CD+rhEGF groups. Although these data indicate that EGF administered i.o. does not cause a significant increase in OPN levels, repetitive applications or sustained release of EGF may cause significant changes in OPN levels.
Other molecules such as cartilage oligomeric matrix protein angiopoietin-1 (COMP-Ang1), hyperbaric oxygen, zoledronate, alendronate, low molecular weight heparin (enoxaparin), VEGF, and CD34+ cells administered intravenously were demonstrated to be effective in the treatment of experimentally induced ONFH [8,18,20,26-30]. However, these findings still need validation in clinical practice. Moreover, Wang et al. [31] showed that osteoblast differentiation and mineralization were accelerated, and consistently osteoblastic marker gene expressions (e.g., OPN and osteocalcin) were increased in MC3T3-E1 cells cultured on mesoporous bioactive glass (MBG)-NH2/EGF, apparently through the extracellular signal-regulated kinase (ERK)-activated Runx2 pathway [31].
Park et al. [8] showed that in surgically induced ischemic necrosis of the femoral head in rats, the angiogenic factor COMP-Ang1, injected i.o., preserved the trabecular framework of the osseous epiphysis, promoted angiogenesis (i.e., higher levels of vascularity were observed in the secondary ossification center of the femoral heads based on the detection of factor VIII on endothelial cells and measurement of vascular densities), and stimulated bone remodeling [8]. Similarly, we observed significantly different CD31 levels between the three groups; moreover CD31 levels were higher in CD+rhEGF compared to CD group, indicating that increased neovascularization in the femoral head was associated with EGF. Which compound (COMP-Ang1 or EGF) might be superior in the treatment of ONFH needs further investigation. However, we also observed no significant difference in the epiphyseal thickness between the three groups and this may be explained by the small sample size used in our study.
CONCLUSION
The femoral head area and sphericity were more preserved in CD+rhEGF compared to CD and sham-control group. Although there was a significant decrease in the femoral head height in CD+rhEGF group, the subchondral distance appeared to be largely protected. No statistical difference was observed in the femoral head area, subchondral distance and trabecular thickness between left non-operated and right operated hip in CD+rhEGF group. The CD31 levels were significantly different between the three groups, and were higher in CD compared to CD+rhEGF group, suggesting that a significant increase in neovascularization may not be achieved with CD alone. Overall, our results indicate that EGF promotes bone formation and microvascularization in ONFH. The positive effect of recombinant EGF has been demonstrated in non-healing wounds of diabetic patients and the results of our study suggest that EGF may also be applied in osteonecrotic lesions. In ongoing research on therapeutic application of stem cells and growth factors in tissue repair and healing, special attention should be given to the role of EGF and EGF-like factors.
ACKNOWLEDGMENTS
This work was grant funded by Scientific Research Projects, Suleyman Demirel University, Turkey (No. 4136-TU2-14). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- [1].Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015;49(1):28–45. doi: 10.4103/0019-5413.143911. https://doi.org/10.4103/0019-5413.143911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chim SM, Tickner J, Chow ST, Kuek V, Guo B, Zhang G, et al. Angiogenic factors in bone local environment. Cytokine Growth Factor Rev. 2013;24(3):297–310. doi: 10.1016/j.cytogfr.2013.03.008. https://doi.org/10.1016/j.cytogfr.2013.03.008. [DOI] [PubMed] [Google Scholar]
- [3].Matsuda N, Kimar NM, Ramakrishnan PR, Cho M-I. Role of epidermal growth factor receptor in osteoblastic differentiation of rat bone marrow stromal cells. J Bone Miner Metab. 1996;14(3):137–45. https://doi.org/10.1007/BF02239481. [Google Scholar]
- [4].Zhang X, Tamasi J, Lu X, Zhu J, Chen H, Tian X, et al. Epidermal growth factor receptor plays an anabolic role in bone metabolism in vivo. J Bone Miner Res. 2011;26(5):1022–34. doi: 10.1002/jbmr.295. https://doi.org/10.1002/jbmr.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Antosz ME, Bellows CG, Aubin JE. Biphasic effects of epidermal growth factor on bone nodule formation by isolated rat calvaria cells in vitro. J Bone Miner Res. 1987;2(5):385–93. doi: 10.1002/jbmr.5650020505. https://doi.org/10.1002/jbmr.5650020505. [DOI] [PubMed] [Google Scholar]
- [6].Raisz LG, Simmons HA, Sandberg AL, Canalis E. Direct stimulation of bone resorption by epidermal growth factor. Endocrinology. 1980;107(1):270–3. doi: 10.1210/endo-107-1-270. https://doi.org/10.1210/endo-107-1-270. [DOI] [PubMed] [Google Scholar]
- [7].Teramatsu Y, Maeda H, Sugii H, Tomokiyo A, Hamano S, Wada N, et al. Expression and effects of epidermal growth factor on human periodontal ligament cells. Cell Tissue Res. 2014;357(3):633–43. doi: 10.1007/s00441-014-1877-x. https://doi.org/10.1007/s00441-014-1877-x. [DOI] [PubMed] [Google Scholar]
- [8].Park BH, Jang KY, Kim KH, Song KH, Lee SY, Yoon SJ, et al. COMP-Angiopoietin-1 ameliorates surgery-induced ischemic necrosis of the femoral head in rats. Bone. 2009;44(5):886–92. doi: 10.1016/j.bone.2009.01.366. https://doi.org/10.1016/j.bone.2009.01.366. [DOI] [PubMed] [Google Scholar]
- [9].Konturek S, Dembinski A, Warzecha Z, Brzozowski T, Gregory H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology. 1988;94(6):1300–7. doi: 10.1016/0016-5085(88)90667-1. https://doi.org/10.1016/0016-5085(88)90667-1. [DOI] [PubMed] [Google Scholar]
- [10].Sarıkaya B, Yumuşak N, Yigin A, Sipahioğlu S, Yavuz Ü, Altay MA. Comparison of the effects of human recombinant epidermal growth factor and platelet-rich plasma on healing of rabbit patellar tendon. Eklem Hastalik Cerrahisi. 2017;28(2):92–9. doi: 10.5606/ehc.2017.55396. https://doi.org/10.5606/ehc.2017.55396. [DOI] [PubMed] [Google Scholar]
- [11].Mose K. Methods of measuring in Legg-Calve-Perthes disease with special regard to the prognosis. Clin Orthop Relat Res. 1980;150:103–9. [PubMed] [Google Scholar]
- [12].Hopson CN, Siverhus SW. Ischemic necrosis of the femoral head. Treatment by core decompression. J Bone Joint Surg Am. 1988;70(7):1048–51. [PubMed] [Google Scholar]
- [13].Lieberman JR, Berry DJ, Mont MA, Aaron RK, Callaghan JJ, Rajadhyaksha AD, et al. Osteonecrosis of the hip: Management in the 21stcentury. Instr Course Lect. 2003;52:337–55. [PubMed] [Google Scholar]
- [14].Classen T, Becker A, Landgraeber S, Haversath M, Li X, Zilkens C, et al. Long-term clinical results after iloprost treatment for bone marrow edema and avascular necrosis. Orthop Rev (Pavia) 2016;8(1):6150. doi: 10.4081/or.2016.6150. https://doi.org/10.4081/or.2016.6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nozaki Y, Kumagai K, Miyata N, Niwa M. Pravastatin reduces steroid-induced osteonecrosis of the femoral head in SHRSP rats. Acta Orthop. 2012;83(1):87–92. doi: 10.3109/17453674.2011.641103. https://doi.org/10.3109/17453674.2011.641103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Peled E, Bejar J, Zinman C, Reis DN, Boss JH, Ben-Noon H, et al. Alendronate preserves femoral head shape and height/length ratios in an experimental rat model: A computer-assisted analysis. Indian J Orthop. 2009;43(1):22–6. doi: 10.4103/0019-5413.44630. https://doi.org/10.4103/0019-5413.44630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhao DW, Yu XB. Core decompression treatment of early-stage osteonecrosis of femoral head resulted from venous stasis or artery blood supply insufficiency. J Surg Res. 2015;194(2):614–21. doi: 10.1016/j.jss.2014.12.007. https://doi.org/10.1016/j.jss.2014.12.007. [DOI] [PubMed] [Google Scholar]
- [18].Norman D, Miller Y, Sabo E, Misselevich I, Peskin B, Zinman C, et al. The effects of enoxaparin on the reparative processes in experimental osteonecrosis of the femoral head of the rat. APMIS. 2002;110(3):221–8. doi: 10.1034/j.1600-0463.2002.100304.x. https://doi.org/10.1034/j.1600-0463.2002.100304.x. [DOI] [PubMed] [Google Scholar]
- [19].Ding S, Peng H, Fang HS, Zhou JL, Wang Z. Pulsed electromagnetic fields stimulation prevents steroid-induced osteonecrosis in rats. BMC Musculoskelet Disord. 2011;12:215. doi: 10.1186/1471-2474-12-215. https://doi.org/10.1186/1471-2474-12-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Zhou L, Yoon SJ, Jang KY, Moon YJ, Wagle S, Lee KB, et al. COMP-angiopoietin1 potentiates the effects of bone morphogenic protein-2 on ischemic necrosis of the femoral head in rats. PLoS One. 2014;9(10):e110593. doi: 10.1371/journal.pone.0110593. https://doi.org/10.1371/journal.pone.0110593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Okazaki S, Nagoya S, Matsumoto H, Mizuo K, Shimizu J, Watanabe S, et al. TLR4 stimulation and corticosteroid interactively induce osteonecrosis of the femoral head in rat. J Orthop Res. 2016;34(2):342–5. doi: 10.1002/jor.23008. https://doi.org/10.1002/jor.23008. [DOI] [PubMed] [Google Scholar]
- [22].Yu Z, Fan L, Li J, Ge Z, Dang X, Wang K. Lithium prevents rat steroid-related osteonecrosis of the femoral head by beta-catenin activation. Endocrine. 2016;52(2):380–90. doi: 10.1007/s12020-015-0747-y. https://doi.org/10.1007/s12020-015-0747-y. [DOI] [PubMed] [Google Scholar]
- [23].Zhang YL, Yin JH, Ding H, Zhang W, Zhang CQ, Gao YS. Vitamin K2 prevents glucocorticoid-induced osteonecrosis of the femoral head in rats. Int J Biol Sci. 2016;12(4):347–58. doi: 10.7150/ijbs.13269. https://doi.org/10.7150/ijbs.13269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Tong PJ, Xu L, Hu BS, Jin HT, Li TY, Fang X. In vitro induction studies of YouGui drink on culture of steroid induced necrosis of femoral head rat osteoblast. [Article in Chinese] Zhongguo Gu Shang. 2010;23(1):23–7. [PubMed] [Google Scholar]
- [25].Chandra A, Lan S, Zhu J, Siclari VA, Qin L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J Biol Chem. 2013;288(28):20488–98. doi: 10.1074/jbc.M112.447250. https://doi.org/10.1074/jbc.M112.447250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bejar J, Peled E, Boss JH. Vasculature deprivation-induced osteonecrosis of the rat femoral head as a model for therapeutic trials. Theor Biol Med Model. 2005;2:24. doi: 10.1186/1742-4682-2-24. https://doi.org/10.1186/1742-4682-2-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Peled E, Bejar J, Barak M, Orion E, Norman D. Core decompression and alendronate treatment of the osteonecrotic rat femoral head: Computer-assisted analysis. Int J Exp Pathol. 2013;94(3):212–6. doi: 10.1111/iep.12022. https://doi.org/10.1111/iep.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Peled E, Davis M, Axelman E, Norman D, Nadir Y. Heparanase role in the treatment of avascular necrosis of femur head. Thromb Res. 2013;131(1):94–8. doi: 10.1016/j.thromres.2012.09.018. https://doi.org/10.1016/j.thromres.2012.09.018. [DOI] [PubMed] [Google Scholar]
- [29].Terayama H, Ishikawa M, Yasunaga Y, Yamasaki T, Hamaki T, Asahara T, et al. Prevention of osteonecrosis by intravenous administration of human peripheral blood-derived CD34-positive cells in a rat osteonecrosis model. J Tissue Eng Regen Med. 2011;5(1):32–40. doi: 10.1002/term.285. https://doi.org/10.1002/term.285. [DOI] [PubMed] [Google Scholar]
- [30].Vadasz Z, Misselevich I, Norman D, Peled E, Boss JH. Localization of vascular endothelial growth factor during the early reparative phase of the rats’ vessels deprivation-induced osteonecrosis of the femoral heads. Exp Mol Pathol. 2004;77(2):145–8. doi: 10.1016/j.yexmp.2004.06.002. https://doi.org/10.1016/j.yexmp.2004.06.002. [DOI] [PubMed] [Google Scholar]
- [31].Wang X, Chen W, Liu Q, Gao K, Wang G, Gao L, et al. Function and mechanism of mesoporous bioactive glass adsorbed epidermal growth factor for accelerating bone tissue regeneration. Biomed Mater. 2017;12(2):025020. doi: 10.1088/1748-605X/aa65d8. https://doi.org/10.1088/1748-605X/aa65d8. [DOI] [PubMed] [Google Scholar]


