Abstract
We report a severe defect of neuromuscular transmission in a consanguineous patient with a homozygous variant in the laminin α5 subunit gene (LAMA5). The variant c.8046C>T (p.Arg2659Trp) is rare and has a predicted deleterious effect. The affected individual, who also carries a rare homozygous sequence variant in LAMA1, had normal cognitive function, but magnetic resonance brain imaging showed mild volume loss and periventricular T2 prolongation. Repetitive nerve stimulation at 2 Hz showed 50% decrement of compound muscle action potential amplitudes but 250% facilitation immediately after exercise, similar to that seen in Lambert-Eaton myasthenic syndrome. Endplate studies demonstrated a profound reduction of the endplate potential quantal content but normal amplitudes of miniature endplate potentials. Electron microscopy showed endplates with increased postsynaptic folding that were denuded or only partially occupied by small nerve terminals. Expression studies revealed that p.Arg2659Trp caused decreased binding of laminin α5 to SV2A and impaired laminin-521 cell adhesion and cell projection support in primary neuronal cultures. In summary, this report describing severe neuromuscular transmission failure in a patient with a LAMA5 mutation expands the list of phenotypes associated with defects in genes encoding α-laminins.
Keywords: congenital myasthenic syndrome (CMS), presynaptic, LAMA5, laminin α5
Introduction
Congenital myasthenic syndromes (CMSs) are a complex group of neurologic disorders of neuromuscular transmission that are characterized by muscle weakness and fatigability. Most types of CMS result from defects in genes involved in the synthesis of proteins located in the post- and presynaptic compartments.1 However, in recent years, reports have described CMS variants resulting from defects in genes encoding proteins of the synaptic extracellular compartment, including agrin,2 laminin β2,3 and collagen XIII α1.4 In addition, one of the first-described forms of CMS was congenital endplate acetylcholinesterase deficiency, which is caused by genetic defects of the extracellular acetylcholinesterase collagenic tail (ColQ).5
Laminins are the most abundant non-collagenous proteins of the basal lamina (BL), which is a thin layer of fibrous tissue that covers the muscle fiber, including the primary and secondary postsynaptic clefts of the neuromuscular junction (NMJ).6 They are large extracellular matrix (ECM) heterotrimeric glycoproteins composed of one of five α subunits, one of three β subunits, and one of three γ subunits.7
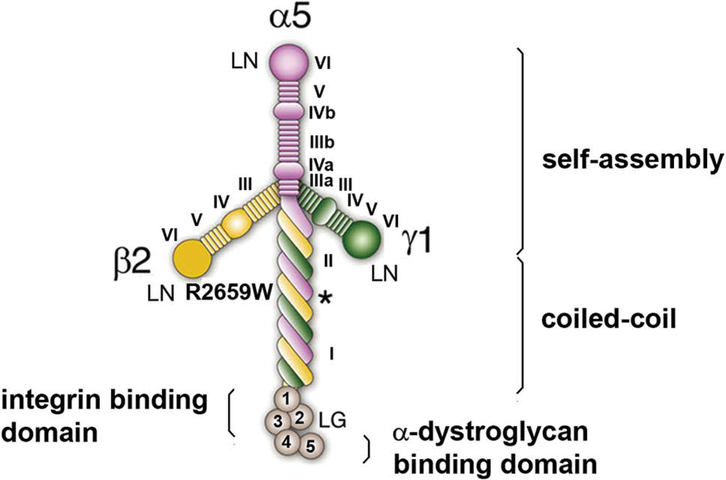
Laminins present a characteristic cross-shaped structure in rotary shadowing electron micrographs.8,9 The long arm of the cross is composed of a ~80-nm α-helical coiled-coil structure formed by the three subunits, while each of the ~35–50 nm short arms of the cross is formed by one of the three subunits (Fig. 1). The distal part of the α subunit comprises five globular-like (LG) domains that serve as attachment sites for integrin receptors and α-dystroglycan.
Figure 1.
Laminin domains. Self-assembly is accomplished by the N-terminal domain (VI) of each of the laminin short arms that join at ternary nodes with the LN of contiguous laminins. The distal laminin globular (LG) domain contains binding sites for integrin receptors (LG 1–3) and for α-dystroglycan (LG4 and LG5).10 The coiled-coil region comprises domains I and II and contains a binding site for agrin to the γ-subunit (asterisk).11 Modified from Ref. 12.
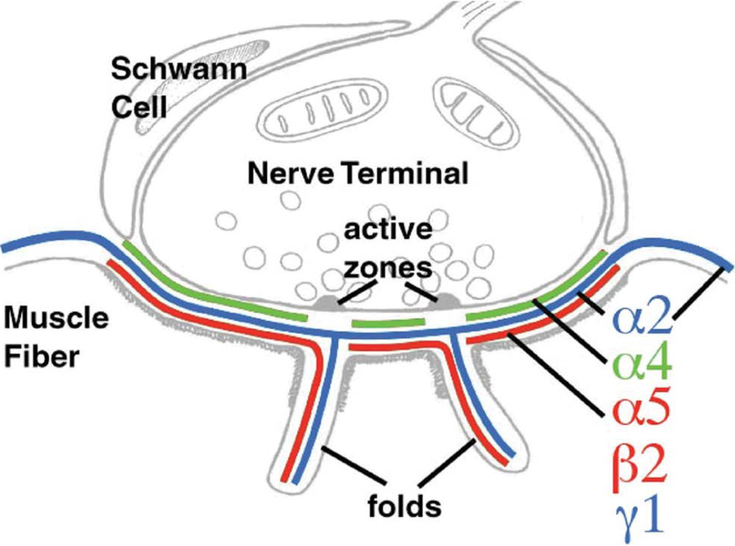
The distal laminin N-terminal (LN) domain of each of the subunits is important for the self-assembly and polymerization of laminins that confer mechanical support to the BL. Laminins are also involved in signaling and in important cellular processes, such as the development and maintenance of the NMJ and regeneration of the nerve terminal.6 The arrangement of the laminin isoforms α2, α4, α5, β2 and γ1 in laminins α2β2γ1, α4β2γ1, and α5β2γ1 is fundamental for the NMJ, because these are the only laminin variants expressed at the BL that covers the adult motor endplate (Fig. 2).13
Figure 2.
Laminin subunits of the adult synaptic BL. While laminin α2 (blue) is present in the synaptic and extrasynaptic BL, laminins α5 and β2 are present only in the synaptic BL. By contrast, laminin α4 localizes only to the BL of the primary synaptic cleft and is absent at the segments of the BL apposing the active zones. Modified with permission from Ref. 18.
Laminin β2 is essential for the organization of the NMJ. It has been shown to bind directly to one of the extracellular loops of the α-subunit of the P/Q- and N-types of the voltage-gated calcium channel (VGCC).14 Mice lacking laminin β2 are normal at birth but later develop generalized weakness and massive proteinuria and die before the end of the first month of life.15 The NMJs of laminin β2 gene knockout mice show a number of pre- and postsynaptic abnormalities, including lack of active zones, dispersion of synaptic vesicles (SVs), invasion of the synaptic cleft by processes of the Schwann cell, and underdeveloped postsynaptic folding. Similar structural defects to those described in the laminin β2 gene knockout mice were observed in a rare human case of CMS.3 The patient was a long-term survivor of a biallelic truncating mutation in LAMB2 (Pierson syndrome) as the result of a highly compatible renal transplant. This syndrome is very rare, because most patients with severe forms of Pierson syndrome die of renal failure. However, it was observed in another survivor of Pierson syndrome following a successful renal transplant (A.G. Engel, personal communication).
The role of laminin α4 at the NMJ has been inferred largely from mice lacking laminin α4. This model shows a peculiar loss of accurate apposition of presynaptic active zones to secondary synaptic clefts, which suggests that laminin α4 plays a role in the correct alignment of pre- and postsynaptic specialized structures.16
The precise function of laminin α5 at the NMJ has been difficult to ascertain, because mice lacking laminin α5 die at late embryonic stages due to multiple developmental abnormalities and placental insufficiency.17 To assess the role of the α5 subunit in later stages of neuromuscular synaptogenesis, a conditional laminin α5 allele has been developed.18 Even though, in this conditional laminin α5 gene knockout, no obvious phenotypic manifestations are present, presynaptic differentiation appears to be primarily affected, since motor nerves only partially cover the postsynaptic membrane during the initial period of postnatal development. Furthermore, in double mutants lacking laminins α4 and α5, the normal synapse elimination is not followed by axonal regeneration into acetylcholine receptor (AChR)-rich areas previously innervated by retracting axons.18 These findings suggest an important role for laminin α4 and α5 in presynaptic maturation.
Another important laminin α-chain isoform that actively participates in the process of nerve outgrowth is laminin α1.19,20 Although laminin α1 is not expressed at the adult NMJ, it plays an important role in dendritic growth and axonal formation similar to that of laminins α4 and α5. In humans, homozygous or compound heterozygous mutations in the gene encoding laminin α1 (LAMA1) has been linked to Poretti-Boltshauser syndromePoretti-Boltshauser syndrome (PBS), which comprises cerebellar ataxia due to cerebellar cysts/dysplasia, intellectual disability, and frequent myopia or retinopathy.21,22 Importantly, PBS does not include muscle weakness.
We recently reported an individual with a severe CMS attributed to a homozygous mutation in the gene encoding the laminin α5 subunit (LAMA5) (MIM 601033), which showed selective presynaptic ultrastructural changes reminiscent of those found in the conditional Lama5 mutant mouse.23 In addition, the patient had physiologic changes similar to those seen in Lambert-Eaton myasthenic syndrome (LEMS).
Here, we amplify the description of the phenotype of the patient with CMS attributed to a homozygous LAMA5 mutation.
Clinical report
The patient is a 25-year-old woman who was born weak and hypotonic but without arthrogryposis. She underwent tracheostomy after birth and currently requires mechanical ventilation for most of the day and a gastric tube for feeding. Her respiratory function worsens with intercurrent infections, but she has no episodes of apnea. Her parents, who are second cousins, and three siblings are healthy, but an older brother died of muscle weakness and respiratory failure. Except for restrictive lung disease, her pulmonary, cardiac, and kidney functions are normal. However, she has chronic inflammatory bowel disease and myopia without retinal abnormalities, and she is unable to ambulate, in part due to bilateral knee flexion contractures. In addition, she has occasional facial muscle twitches and tics. Her most recent physical examination showed minor dysmorphic features that included elongated face, ptosis, high arched palate, and mild body hirsutism. Her mental function was normal, but the cranial nerve examination showed ptosis and weakness of extraocular, facial, tongue, and soft palate muscles. She had moderate weakness of neck flexors and proximal limb muscles, and her deep tendon reflexes were depressed. The rest of the neurologic examination was unremarkable.
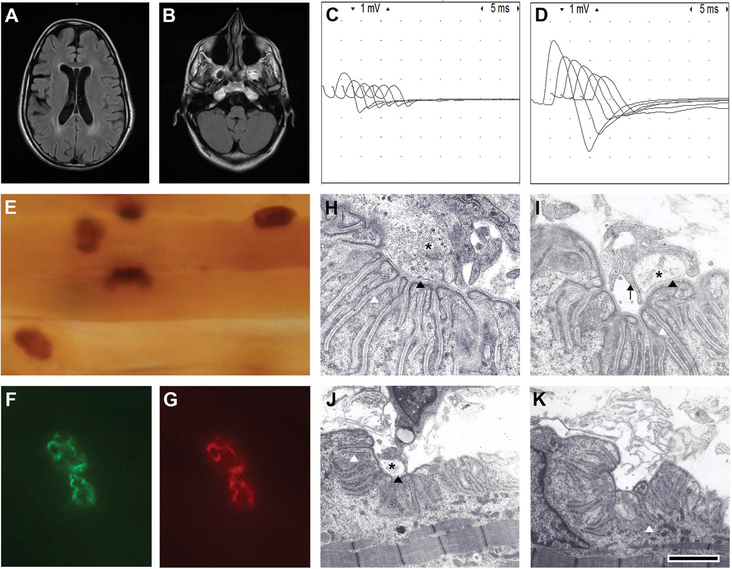
A recent brain magnetic resonance imaging showed mild volume loss for age and periventricular T2 prolongation (Fig. 3A and 3B). Repetitive stimulation of the right spinal accessory nerve at 2 Hz revealed severe reduction of compound muscle action potential (CMAP) amplitudes and 55% decrement, but there was more than 250% increment of CMAP amplitudes immediately after 30 s of maximal muscle activation (Fig. 3C and 3D). An anconeus muscle biopsy performed at age 7 revealed muscle fiber type I predominance and normal expression of acetylcholinesterase (AChE), laminin α5, and AChRs (Fig. 3E–3G). Electron microscopy of the NMJ showed the following distinctive features: (1) endplates with normal or increased postsynaptic folding that were denuded or innervated by small nerve terminals; (2) encasement of nerve endings by projections of Schwann cells that did not intrude into the synaptic space; and (3) moderate reduction of the density of SVs (Fig. 3I–3K). Endplate intracellular microelectrode recordings showed severe reduction of the average endplate potential quantal content, which markedly increased after exposure of the muscle to 3,4 diaminopyridine (3,4 DAP). The amplitudes and frequencies of miniature endplate potentials were normal.
Figure 3.
Clinical and laboratory findings. (A) Fluid-attenuated inversion recovery (FLAIR) brain MRI demonstrates periventricular T2 prolongation and mild volume loss. (B) There are no cerebellar cysts or cerebellar dysplasia. Repetitive stimulation shows marked decrement (C) before and (D) after exercise. Notice the distinct increment of CMAP amplitudes after exercise. (E) Normal configuration of endplates and AChE expression. Normal expression of laminin α5 and AChRs at an endplate exposed to (F) a primary antibody against laminin α5 and (G) counterstained with rhodamine-αBGT. (H) A normal NMJ from a control individual showing normal nerve terminal (asterisk), normal postsynaptic folds, and normal width of the primary and secondary synaptic clefts (black and white arrowheads, respectively). (I and J) Examples of NMJs of the patient showing small nerve terminals encased by Schwann cell projections (black arrow) that do not invade the synaptic space. Note the normal width of the primary synaptic clefts (black arrowheads). (K) An example of a NMJ from the patient denuded of its nerve terminal. The calibration mark = 100 μM in E, 10 μM in F and G, 1 μm in H and I, and 2 μm in J and K. Modified with permission from Ref. 23.
Genetic analysis performed with whole-exome sequencing revealed only two homozygous variants that were potentially relevant to the phenotype of the patient. One was LAMA1 (rs532162048), p.Asp210Asn; the other was LAMA5 (rs201012962), p.Arg2659Trp. They were present in the parents in the heterozygous state and absent in the non-affected sister (DNA from the deceased sibling and two brothers was not available). Both variants are rare and have potential damaging effects, according to software predicting the effects of sequence variants on protein function. However, LAMA1 is unlikely to have caused the CMS, because laminin α1 is not expressed at the adult NMJ and because biallelic mutations in LAMA1 are linked to PBS, which does not involve muscular weakness.21 Thus, we concluded that the CMS phenotype was caused primarily by the LAMA5 variant.
To further characterize the LAMA5 variant, we introduced it into a clone of human wild-type (WT) laminin α5 and tested its effects on signaling, cell adhesion, and neurite outgrowth.
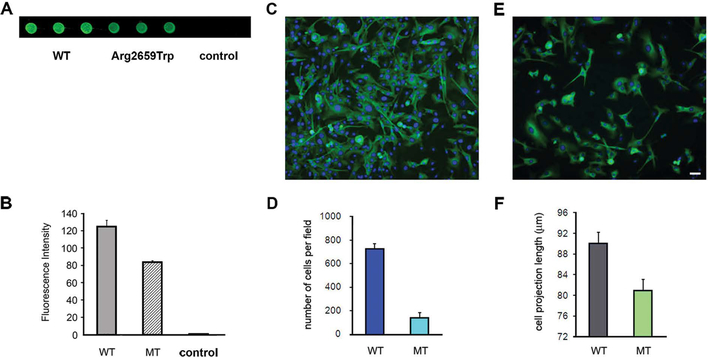
Since α5-containing laminins form a strong complex with the SV protein SV2, which may play an important role in SV trafficking, we tested the binding of LAMA5 p.Arg2659Trp to SV2A using the dot-blotting assay.24 The assay showed that, compared with WT, the binding of p.Arg2659Trp to SV2A was significantly reduced (Fig. 4A and 4B).
Figure 4.
Laminin α5 interactions with SV2A and support of cell adhesion and cell outgrowth. (A and B) Decreased immunoreactivity against SV2A in immobilized lysates from HEK cells transfected with mutant LAMA5 cDNA (wells 4–6) in comparison with WT (wells 1–3). The controls are lysates of untransfected cells (wells 7–9). (C) Normal cell density and neurite outgrowth in a slide coated with WT laminin. (D) A bar graph summarizing four transfection experiments. The cell adhesion to slides coated with the mutant laminin-521 was only about 25% of that of slides coated with WT laminin-521. (E) Low cell density and reduced neurite outgrowth length in a slide coated with mutant laminin. (F) Bar graph showing shorter cell projections in slides coated with mutant laminin in comparison with those coated with WT laminin. The calibration mark = 25 μm in C and E. Reprinted with permission from Ref. 23.
In order to assess the effect of p.Arg2659Trp on cell adhesion and neurite outgrowth, we first sequentially transfected HEK cells with the three laminin subunits to obtain either WT or mutant laminin-521 and then coated slides with lysates from transfected cells. We found that the adherence of primary cultured neurons from newborn spinal cords to slides coated with lysates from cells transfected with the mutant laminin-521 was only about 25% of that of cells that attached to slides coated with lysates from cells transfected with WT laminin-521. In addition, the average length of cell projections in slides coated with mutant laminin was shorter than that in slides coated with WT laminin (80.94 ± 1.67 μm (n = 774) vs. 90.02 ± 2.13 μm (n = 478), P < 0.001) (Fig. 4C–4F).
Discussion
We describe a complex phenotype that includes severe failure of neuromuscular transmission and central nervous system (CNS) manifestations in a patient with a homozygous sequence variant in LAMA5. Although the patient carried another rare homozygous sequence variant in LAMA1 that may partially explain the CNS symptoms, this possibility is unlikely, since our patient did not have the classical manifestations of PBS, such as cerebellar cysts, cerebellar dysplasia, or retinal abnormalities.21,22
It is also surprising that, given the high expression of laminin α5 in lungs, skin, and kidneys, our patient did not have any primary manifestations in these organs. However, since the LAMA5 variant does not preclude the expression of laminin α5 at the NMJ, it is likely that it disrupts neuromuscular transmission by affecting a fundamental signaling pathway that operates selectively at the NMJ but not in other tissues. Moreover, a defective laminin α5 may be sufficient to provide support to tissues like lung, skin and kidneys, but may be insufficient to maintain function at more active metabolic sites, such as the NMJ.
Another unexpected finding was the association of an ECM protein with a presynaptic failure resulting from at least two distinctive mechanisms. The first was a reduction of the number of SVs readily available for release, predicted by the small size of the nerve terminals and reduced number of active zones. This can be directly explained by the deficient support of cell adhesion and neurite outgrowth by the mutant laminin α5. The second mechanism involved a reduction of the probability of SV release, similar to that seen in LEMS. This was also surprising given the localization of the laminin α5 to the ECM. Nevertheless, a LEMS-like defect has been found by electrophysiological studies in patients with mutations in the N-terminal domain of agrin, which also localizes to the ECM.25 Since laminin α5 Arg2659Trp may disturb the laminin coiled-coil conformation, which is required for agrin attachment to the ECM,11 Arg2659Trp may have similar consequences to mutations in the N-terminal domain of agrin (Fig. 5).
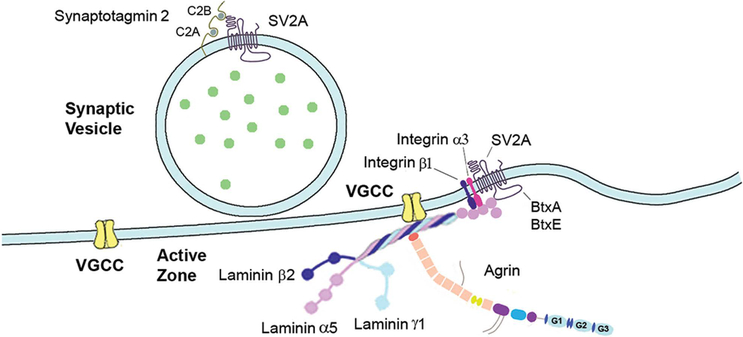
Figure 5.
Schematic drawing showing laminin 521 interactions. Laminin β2 binds to one of the extracellular loops of the P/Q- and N-type VGCC and the N-terminal of agrin binds to the laminin γ subunit at the coiled-coil domain adjacent to laminin α5 Arg2659Trp. The LG domain interacts with integrin receptors and with the large intraluminal loop between the seventh and eighth transmembrane domains of SV2A, which is also the receptor for botulinum toxins A and E (BtxA and BtxE). The N-terminal of SV2A interacts with the C2B domain of synaptotagmin 2.
Alternatively, the LEMS-like defect may be explained by an impaired interaction of laminin α5 with SV2A, as all SV2 isoforms regulate presynaptic Ca2+ dynamics,26,27 which has direct impact on the probability of SV release. In addition, the cytoplasmic N-terminal of the three SV2 isoforms binds to synaptotagmin, which is the putative Ca2+ sensor involved in SV exocytosis.28 In support of this argument is the recent report of two different mutations in the C2B domain of synaptotagmin 2 that cause autosomal-dominant CMS with LEMS-like features.29
Finally, the LEMS-like deficiency caused by Arg2659Trp may be due in part to an impaired coiled-coil formation at the C-terminal region of laminin β2 causing disruption of the interaction of laminin with the α-subunit of P/Q- and N-type VGCCs.13
In summary, a presynaptic deficit of neuromuscular transmission was found in a patient with a CMS attributed to a homozygous LAMA5 variant. The presynaptic failure involved both reduction of the pool of SVs readily available for release and a diminished probability of SV release, similar to that seen in LEMS. Identification of similar cases is important, as our patient had an excellent response to a combined treatment with pyridostigmine sulfate and 3,4 DAP.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Engel AG, Shen XM, Selcen D, et al. 2015. Congenital myasthenic syndromes: pathogenesis, diagnosis, and treatment. Lancet Neurol 4:420–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huzé C, Bauché S, Richard P, et al. 2009. Identification of an agrin mutation that causes congenital myasthenia and affects synapse function. Am J Hum Genet. 85:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maselli RA, Ng JJ, Anderson JA, Cagney O, et al. 2009. Mutations in LAMB2 causing a severe form of synaptic congenital myasthenic syndrome. J Med Genet. 2009. 46:203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Logan CV,Cossins J, Rodríguez Cruz PM, et al. 2015. Congenital Myasthenic Syndrome Type 19 Is Caused by Mutations in COL13A1, Encoding the Atypical Non-fibrillar Collagen Type XIII α1 Chain. Am J Hum Genet. 97:878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engel AG, Lambert EH, Gomez MR 1977. A new myasthenic syndrome with end-plate acetylcholinesterase deficiency, small nerve terminals, and reduced acetylcholine release. Ann Neurol. 1:315–330. [DOI] [PubMed] [Google Scholar]
- 6.Sanes JR 2003. The basement membrane/basal lamina of skeletal muscle. J Biol Chem. 278:12601–12604. [DOI] [PubMed] [Google Scholar]
- 7.Aumailley M, Bruckner-Tuderman L, Carter WG, et al. 2005. A simplified laminin nomenclature. Matrix Biol. 5:326–332. [DOI] [PubMed] [Google Scholar]
- 8.Engel J, Odermatt E, Engel A, et al. 1981. Shapes, domain organizations and flexibility of laminin and fibronectin, two multifunctional proteins of the extracellular matrix. J Mol Biol. 150:97–120. [DOI] [PubMed] [Google Scholar]
- 9.Beck K, Hunter I, Engel J 1990. Structure and function of laminin: anatomy of a multidomain glycoprotein. FASEB J. 4:148–160. [DOI] [PubMed] [Google Scholar]
- 10.Hohenester E, Yurchenco PD 2013. Laminins in basement membrane assembly. Cell Adh Migr. 7:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kammerer RA, Schulthess T, Landwehr R, et al. 1999. Interaction of agrin with laminin requires a coiled-coil conformation of the agrin-binding site within the laminin gamma1 chain. EMBO J. 18:6762–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suleiman H, Zhang L, Roth R, et al. 2013. Nanoscale protein architecture of the kidney glomerular basement membrane. eLife. 2:e01149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patton BL, Miner JH, Chiu AY, et al. 1997. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol 139:1507–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishimune H, Sanes JR & Carlson SS 2004. A synaptic laminin-calcium channel interaction organizes active zones in motor nerve terminals. Nature 432:580–587. [DOI] [PubMed] [Google Scholar]
- 15.Noakes PG, Gautam M, Mudd J, et al. 1995. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/laminin β2. Nature 374:258–262. [DOI] [PubMed] [Google Scholar]
- 16.Patton BL, Cunningham JM, Thyboll J, et al. 2001. Properly formed but improperly localized synaptic specializations in the absence of laminin alpha4. Nat. Neurosci 6:597–604. [DOI] [PubMed] [Google Scholar]
- 17.Miner JH, Cunningham J & Sanes JR 1998. Roles for laminin in embryogenesis exencephaly, syndactyly, and placentopathy in mice lacking the laminin 5 chain. J. Cell Biol 143:1713–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimune H, Valdez G, Jarad G, et al. 2008. Laminins promote postsynaptic maturation by an autocrine mechanism at the neuromuscular junction. J. Cell Biol 182:1201–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mruthyunjaya S, Parveen D, Shah RD, et al. 2015. Gene expression analysis of laminin-1-induced neurite outgrowth in human mesenchymal stem cells derived from bone marrow. J. Biomed. Mater. Res. A 103:746–761. [DOI] [PubMed] [Google Scholar]
- 20.Mruthyunjaya S, Manchanda R, Godbole R, et al. 2010. Laminin-1 induces neurite outgrowth in human mesenchymal stem cells in serum/differentiation factors-free conditions through activation of FAK-MEK/ERK signaling pathways. Biochem. Biophys. Res. Commun 391:43–48. [DOI] [PubMed] [Google Scholar]
- 21.Aldinger KA, Mosca SJ, Tétreault M, et al. 2014. Mutations in LAMA1 cause cerebellar dysplasia and cysts with and without retinal dystrophy. Am. J. Hum. Genet 95:227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vilboux T, Malicdan MC, Chang YM, et al. 2016. Cystic cerebellar dysplasia and biallelic LAMA1 mutations: a lamininopathy associated with tics, obsessive compulsive traits and myopia due to cell adhesion and migration defects. J. Med. Genet 53:318–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maselli RA, Arredondo J, Vázquez J, et al. 2017. Presynaptic congenital myasthenic syndrome with a homozygous sequence variant in LAMA5 combines myopia, facial tics, and failure of neuromuscular transmission. Am J Med Genet A. 173:2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arredondo J, Lara M, Ng F, et al. 2014. COOH-terminal collagen Q (COLQ) mutants causing human deficiency of endplate acetylcholinesterase impair the interaction of ColQ with proteins of the basal lamina. Hum. Genet 133:599–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicole S, Chaouch A, Torbergsen T, et al. 2014. Agrin mutations lead to a congenital myasthenic syndrome with distal muscle weakness and atrophy. Brain. 137:2429–2443. [DOI] [PubMed] [Google Scholar]
- 26.Janz R, Südhof TC, Hammer RE, et al. 1999. Essential roles in synaptic plasticity for synaptogyrin I and synaptophysin I. Neuron 24:687–700. [DOI] [PubMed] [Google Scholar]
- 27.Chang WP & Südhof TC 2009. SV2 renders primed synaptic vesicles competent for Ca2+ -induced exocytosis. J. Neurosci 29:883–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perin MS, Johnston PA, Ozcelik T, et al. 1991. Structural and functional conservation of synaptotagmin (p65) in Drosophila and humans. J. Biol. Chem 266:615–622. [PubMed] [Google Scholar]
- 29.Herrmann DN, Horvath R, Sowden JE, et al. 2014. Synaptotagmin 2 mutations cause an autosomal-dominant form of lambert-eaton myasthenic syndrome and nonprogressive motor neuropathy. Am J Hum Genet. 95:332–339. [DOI] [PMC free article] [PubMed] [Google Scholar]