Abstract
Acute kidney injury (AKI) is common in critically ill patients and associated with increased morbidity and mortality. Dysfunction of other organs is an important cause of poor outcomes from AKI. Ample clinical and epidemiological data show that AKI is associated with distant organ dysfunction in lung, heart, brain, and liver. Recent advancements in basic and clinical research have demonstrated physiologic and molecular mechanisms of distant organ interactions in AKI including leukocyte activation and infiltration, generation of soluble factors like inflammatory cytokines/chemokines, and endothelial injury. Oxidative stress and production of reactive oxygen species (ROS) as well as dysregulation of cell death in distant organs are also important mechanism of AKI-induced distant organ dysfunction. This review will update recent clinical and experimental findings on organ crosstalk in AKI and highlight potential molecular mechanisms and therapeutic targets to improve clinical outcomes during AKI.
Keywords: acute kidney injury (AKI), multi-organ dysfunction, organ crosstalk, lung, cardiorenal syndrome (CRS), reno-cerebral reflex, hepatic dysfunction, microbiota, gut-kidney axis, review
INTRODUCTION
Acute kidney injury (AKI) is a serious medical condition that is associated with significantly increased risks for mortality, hospital length of stay, and healthcare-associated costs.1 Based on the KDIGO (Kidney Disease Improving Global Outcomes) definition, AKI complicates 18% of all hospitalized patients, with an associated inhospital mortality of 11%.2 The incidence of AKI increases to 57% in critically ill patients, with 27% in-hospital mortality.3 The mortality rate soars to 45–60% when AKI is complicated by other organ dysfunctions, like pneumonia, acute heart failure, or sepsis.4
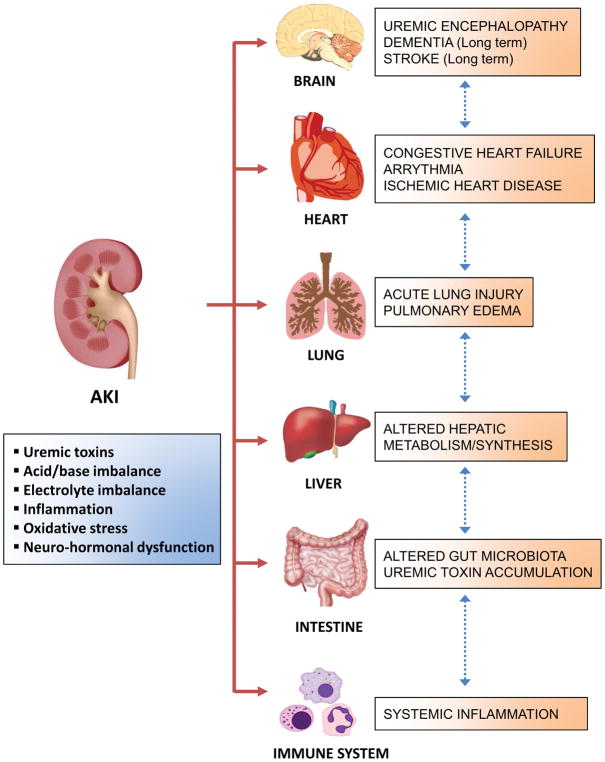
Uremic toxin accumulation, metabolic acidosis, electrolyte imbalances, and fluid overload are the traditionally well-known consequences of AKI that contribute to the high mortality.5 However, a significant proportion of the AKI-associated mortality cannot be simply explained by loss of kidney functions or by complications occurring during AKI treatment. Instead, AKI-induced multi-organ dysfunction is of particular importance in outcomes of critically ill patients with AKI. ‘AKI-induced distant organ crosstalk’ describes the phenomenon when AKI leads to dysfunction of other organs including lung, heart, brain, liver, and intestine via aberrant organ-organ communication.6,7 (Fig 1) Accumulating evidence indicates that interruption of normal immunological balance and generation of inflammatory mediators are important in AKI-induced distant organ crosstalk.8 Additional mechanisms include increased endothelial injury, cellular apoptosis, and oxidative stress.9–11
Figure 1. The impact of acute kidney injury (AKI) on distant organs.
Acute kidney injury (AKI) causes hemodynamic-, humoral-, and immunologic changes, which leads to dysfunction of distant organs including lung, heart, brain, liver, intestine and immune system. Abbreviations: AKI, acute kidney injury.
Organ crosstalk can happen following various types of AKI, but there is no clear data yet whether the etiology of AKI affects the extent of distant organ dysfunction. There is higher chance of distant organ dysfunction with more severe AKI, but even patients with mild to moderate AKI that is not severe enough to require RRT can also experience multi-organ dysfunction.12 Animal models are useful for discovery and mechanistic studies, but do not fully mimic the complexities of human AKI. In addition, inbred animal strains with relatively limited genetic diversity cannot fully represent human populations with polymorphic genetic backgrounds.13 Therefore, researchers need caution in extrapolating animal data to humans. In this review, we will briefly update clinical and experimental findings on distant organ effects of AKI and discuss potential molecular and therapeutic targets.
KIDNEY-LUNG INTERACTIONS
Clinical impact
Acute lung injury is one of the most common extra-renal problems in AKI patients. Accumulation of uremic toxins negatively affects lung mechanics and pulmonary gas exchange.7 Fluid overload can lead to alveolar edema and metabolic acidosis that results in hyperventilation in AKI patients.7 In clinical settings, respiratory complications from AKI include pulmonary edema, respiratory failure needing mechanical ventilation, longer duration of mechanical ventilation, and slower weaning from mechanical ventilation.14 Epidemiological studies demonstrate that respiratory failure requiring mechanical ventilation is an important independent risk factor for mortality in AKI patients.15–17
Clinical evidence
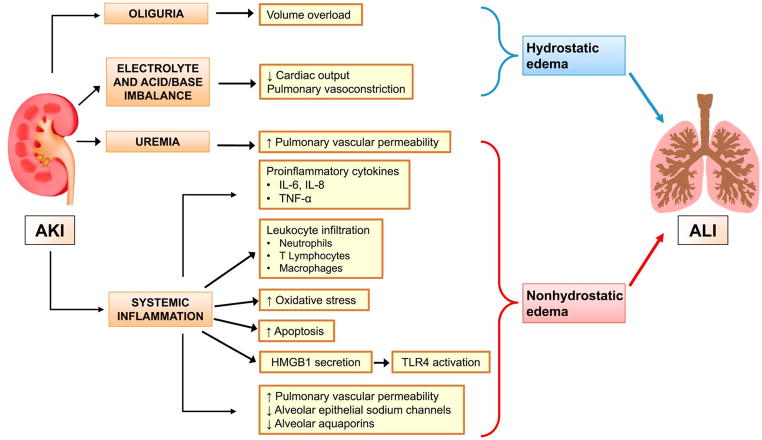
The underlying pathogenesis of respiratory failure in AKI patients is incompletely understood, but several potential mechanisms have been widely studied. (Fig 2) The effects of AKI on lungs are largely due to pulmonary edema, which can be divided into hydrostatic or non-hydrostatic forms.18 Fluid overload, the major cause of hydrostatic edema, occurs because of decreased urine output and dampened cardiac output, and is traditionally recognized as a major cause of lung dysfunction in AKI patients. Fluid overload has been strongly associated with increased mortality in AKI patients accompanying acute lung injury (ALI), and restrictive fluid management improved the lung function and shortened the time that mechanical ventilation is needed in these patients.19
Figure 2. Mechanisms of acute lung injury (ALI) during acute kidney injury (AKI).
Acute kidney injury (AKI) can facilitate development of acute lung injury (ALI) through different mechanisms - volume overload, impairment of cardiac function, systemic inflammation, oxidative stress, increased pulmonary vascular permeability and impaired alveolar fluid clearance. Abbreviations: AKI, acute kidney injury; ALI, acute lung injury; IL, interleukin; TNF-α, tumor necrosis factor alpha; HMGB1, high-mobility group box protein B1; TLR-4, toll-like receptor 4.
Non-hydrostatic pulmonary edema occurs without overt fluid overload. During AKI, the integrity of alveolar-capillary barrier is impaired by uremia, systemic inflammation, and increased oxidative stress, causing fluid accumulation in the lung.7 Basu et al. described this phenomenon as ‘nephrogenic pulmonary edema’ and this condition cannot be improved simply by resolving uremia and controlling fluid balance with dialysis.20 Several clinical studies have suggested the role of inflammation in the initiation and progression of lung injury after AKI. Increased inflammatory cytokines, such as interleukin (IL)-6 and/or IL-8 have been associated with prolonged ventilator weaning times21 and increased mortality22 in AKI patients with ALI. Non-hydrostatic pulmonary edema has been actively studied in the experimental AKI rather than in the clinical setting which will be further described in the next section.
Laboratory evidence
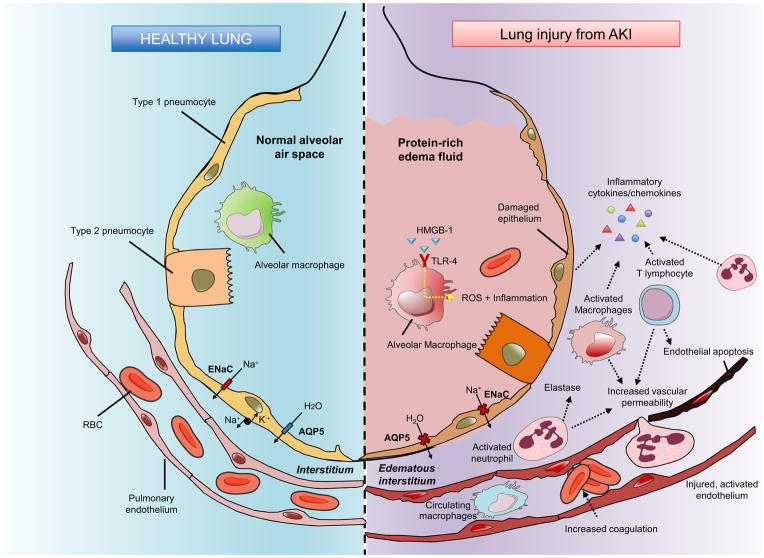
Extending from these clinical observations, a number of experimental animal studies elucidated the underlying mechanisms of AKI-lung crosstalk, demonstrating increased leukocyte infiltration, increased inflammatory cytokines/chemokine expression, and dysregulation of salt/water transporters with abnormal vascular permeability.9,23,24 (Fig 3)
Figure 3. Cascade of lung changes after acute kidney injury (AKI).
Following acute kidney injury (AKI), various inflammatory events occur in the alveolar and pulmonary interstitial spaces. Activated endothelium and increased vascular permeability allow leukocytes to transmigrate into pulmonary interstitium. Infiltrated leukocytes aggravate lung injury through inflammatory cytokine/chemokine secretion, increased oxidative stress and cell damage/apoptosis. Epithelial sodium channel and aquaporin 5 expressions are also downregulated following AKI. Protein-rich fluid accumulation in the alveolar space, alveolar hemorrhage and edematous pulmonary interstitial space are observed in acute lung injury (ALI) following AKI. Abbreviations: AKI, acute kidney injury; Na, sodium, H2O, dihydrogen oxide; AQP, aquaporin; ENaC, epithelial sodium channel; RBC, red blood cell; HMGB-1, high-mobility group box protein B1; TLR-4 - Toll-like receptor 4; ROS, reactive oxygen species. Based on information in Ware & Matthay97
Several immune cells are involved in AKI-associated lung injury. Neutrophil infiltration into lung has been found in two different AKI models [bilateral nephrectomy (BNx) and bilateral ischemia-reperfusion injury (IRI) models]23,25 and the release of neutrophil elastase plays a crucial role in the pathogenesis of ALI following AKI.26 T lymphocyte infiltration into lung also increases during renal IRI and T cell-deficient mice show decreased pulmonary caspase 3 activation, demonstrating the role of T cells in ischemic AKI-induced pulmonary apoptosis.27 Macrophage infiltration into lung increases pulmonary vascular permeability and interstitial edema in an ischemic AKI model,28,29 which is attenuated by administration of the vagus nerve-dependent macrophage deactivator CNI-1493.29
Inflammatory cytokines/chemokines serve important roles in lung dysfunction during AKI. Based on the clinical finding of increased IL-6 level in acute respiratory distress syndrome (ARDS) patients complicated by AKI,30 several experimental studies have demonstrated that IL-6 contribute to AKI-induced lung injury.23,31 Circulating IL-6 induces lung chemokine (C-X-C motif) ligand 1 (CXCL1); in addition, blocking CXCL1 as well as IL-6 can reduce AKI-induced lung injury.31 A recent mouse study showed that high-dose peritoneal dialysis after AKI significantly lowers serum IL-6 concentration as well as lung inflammation.32 However, the counter-inflammatory role of IL-6 was demonstrated in another study showing that systemic IL-6 could upregulate splenic IL-10 production as a compensatory anti-inflammatory mechanism in AKI-induced lung injury.33 Administration of the anti-inflammatory cytokine IL-10 also attenuate pulmonary inflammation.9 Increased tumor necrosis factor α (TNF-α) levels and TNF receptor 1 (TNFR1)-dependent pulmonary apoptosis have been observed in a mouse IRI model.34
Dysfunctional pulmonary water and salt transporters have been studied as potential mediator of non-hydrostatic pulmonary edema after AKI. In normal conditions, apical sodium channels and basolateral adenosine triphosphatase (ATPase) on pneumocytes actively transport sodium from alveolar space into the pulmonary interstitium.35 This active transfer of sodium creates an osmotic gradient with consequent water movement in the same direction.36 In both the ischemic and non-ischemic AKI models, mice show pulmonary septal edema and alveolar hemorrhage in spite of decreased body weight.9 This finding suggests that dysfunctional pulmonary water transport rather than total body water accumulation is a major culprit for AKI-induced pulmonary edema. Another study found significant decreases in lung mRNA expression of epithelial sodium channel and aquaporin 5, consistent with a decreased level of aquaporin 5 protein, suggesting that the decreased expression of alveolar salt and water transporter cause non-hydrostatic pulmonary edema after AKI.24 Recently, Yabuuchi et al. showed that indoxyl sulfate, a typical oxidative stress-inducing uremic toxin, could play a role in the dysregulation of pulmonary aquaporin 5 in AKI using a rat bilateral nephrectomy model.37 This study also showed that administration of oral spherical adsorptive carbon beads, AST-120, lowers the level of indoxyl sulfate in serum and lung, restoring pulmonary aquaporin 5 protein expression.
Potential therapeutic targets
Supporting the clinical and experimental findings above, delivery of IL-10 and blocking IL-6, CXCL1, or TNF-α have efficacy on AKI-induced lung injury in animal models, showing their potential as future therapeutic targets.23,31,34 Administration of atrial natriuretic peptide (ANP), which has anti-inflammatory and natriuretic properties, shows protective effects on the ischemic injured kidney as well as the contralateral kidney, lungs, and heart in a rat IRI model.38 However, clinical trials on ANP have not shown benefit in AKI.39,40
Oxidative stress caused by leukocyte infiltration into lung can lead to tissue damage through systemic inflammatory reaction and active free radical generation.41,42 A recent study demonstrated the protective potential of prostaglandin E1 (PGE1) on modulation of oxidative stress during renal IRI-induced lung injury in rats.42 Administration of PGE1 has been found to decrease both IRI-induced kidney injury and IRI-induced remote lung damage through increased antioxidant enzyme activities and decreased leukocyte activities.
Uremic toxins as well as multiple cytokines and toll-like receptor 4 (TLR-4)/high-mobility group box protein B1 (HMGB1) likely participate in distant organ effects of AKI.43 In the BNx-induced lung injury model, the interaction of TLR-4 with HMGB1 contributes to lung injury induced by AKI and these damages are alleviated in TLR-4-mutant mice or with the use of HMGB1 blockade.44 An in vitro study showed that hemofiltration using surface-treated polyacrylonitrile (AN69ST) membrane could adsorb HMGB145 and another ex vivo study in human cells showed that continuous hemofiltration using a cellulose triacetate membrane could remove various cytokines as well as HMGB1.46 Removal of cytokines and uremic toxins with CRRT using a novel membrane is a promising approach to treat distant organ injury in AKI.
KIDNEY-HEART INTERACTIONS
Clinical impact
The interaction between kidney and heart is often found in patients and primary disorder of one of these organs often results in secondary dysfunction of the other organ. The term, “cardiorenal syndrome (CRS)”, has been increasingly used to explain this relationship, and a working definition/classification of CRS was established in 2008 by Ronco et al..47 (Box 1) These complex interactions between kidney and heart contribute to the worse outcomes from dual organ dysfunction compared to single organ dysfunction. Although the hemodynamic mechanisms in acute cardiac decompensation that leads to AKI are well described, less is known about the effect of AKI on heart.
Box 1. Classification of cardiorenal syndromes.
CRS General Definition
A complex pathophysiological disorder of the heart and the kidneys whereby acute or chronic dysfunction in one organ may induce acute or chronic dysfunction in the other organ47
Types of CRS
-
CRS Type 1 (Acute cardiorenal syndrome)
Description: Abrupt worsening of cardiac function leading to AKI
Examples: Acute coronary syndrome, acute decompensated heart failure
-
CRS Type 2 (Chronic cardiorenal syndrome)
Description: Chronic abnormalities in cardiac function causing progressive and permanent CKD
Examples: chronic heart failure, ischemic heart disease, hypertension
-
CRS Type 3 (Acute renocardiac syndrome)
Description: Abrupt worsening of renal function causing acute cardiac disorder
Examples: Postsurgery AKI, acute glomerulonephritis, rhabdomyolysis
-
CRS Type 4 (Chronic renocardiac syndrome)
Description: CKD contributing to decreased cardiac function, cardiac hypertrophy, fibrosis, and/or increased risk of adverse cardiovascular events
Examples: Cardiac hypertrophy/fibrosis in CKD
-
CRS Type 5 (Secondary cardiorenal syndrome)
Description: Systemic condition causing both acute cardiac and kidney injury and dysfunction
Examples: sepsis, diabetes mellitus
Note: Based on the information presented in Ronco et al..47; Abbreviations: CRS, Cardiorenal Syndrome; AKI, acute kidney injury; CKD, chronic kidney injury
Clinical evidence
CRS can occur following any forms of AKI, but only a limited number of studies have studied cardiovascular outcomes of AKI patients, making the exact estimation for the incidence and outcome of AKI-induced CRS unclear. In one multicenter AKI cohort study, cardiovascular failure was found in 60% of AKI patients in ICU.48 Another study showed that cardiovascular-related deaths were the second most common cause of death after sepsis in AKI patients.15 Long-term cardiovascular effects of AKI have also been observed: postoperative AKI was found to be associated with increased 5-year risk of myocardial infarction and heart failure as well as increased all-cause mortality.49 In another study, patients who recovered from dialysis-requiring AKI had higher long-term risks of coronary events and all-cause mortality regardless of subsequent progression to chronic kidney disease (CKD), showing the independent association of AKI with long-term cardiovascular risk.50 More detailed clinical studies are required to elucidate the exact risk and impact of AKI-induced CRS.
Laboratory evidence
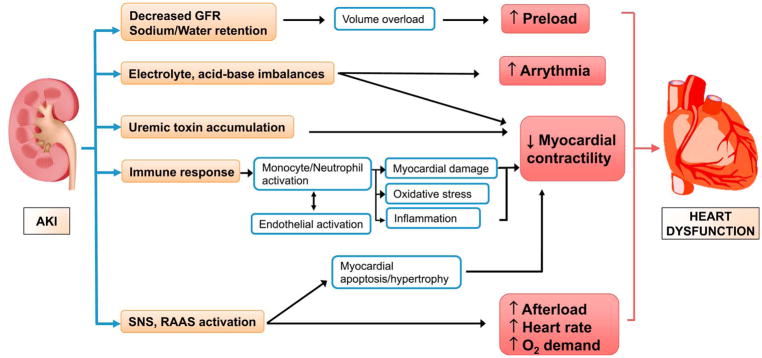
AKI causes cardiac dysfunction through many possible mechanisms. (Fig 4) Fluid overload attenuates myocardial performance and induces maladaptive myocardial remodeling.49 Accumulation of uremic toxins leads to cardiovascular toxicity and can increase risk for myocardial ischemia by compromising coronary vasoreactivity.51 Protein-bound uremic toxins (PBUTs) have deleterious effect on many vital organs including kidney, heart and blood vessels and are poorly removed by current dialysis approaches. Indoxyl sulfate and p-cresyl sulfate, the most well-known PBUTs, have been shown to induce vascular inflammation, endothelial dysfunction, and vascular calcification.52 Metabolic acidosis also affect the myocardial contractility and electrolyte imbalances like hyper/hypokalemia trigger life-threatening arrhythmia.53
Figure 4. Pathophysiology of acute kidney injury (AKI)-induced cardiorenal syndrome.
Acute kidney injury (AKI) triggers cardiac dysfunction through various pathophysiological mechanisms, including volume overload, electrolyte and acid-based imbalances, accumulation of uremic toxins, enhanced immune response and activation of sympathetic nervous system (SNS) and renin–angiotensin–aldosterone system (RAAS). Abbreviations: AKI, acute kidney injury; GFR, glomerular filtration rate; SNS, sympathetic nervous system; RAAS, renin–angiotensin–aldosterone system.
Beside these classically-known mechanisms, there are other important factors with direct and immediate impact on heart, including systemic immune response, activation of the sympathetic nervous system (SNS) and the renin-angiotensin-aldosterone system (RAAS), and increased oxidative stress.54,55 A rat AKI study showed that renal IRI, but not bilateral nephrectomy results in a significant increase in myocardial apoptosis, indicating that systemic inflammation after IRI as opposed to uremia itself has more important role in the myocardial injury following AKI.56 This study also showed the association of ischemic AKI with cardiac injury based on increased myeloperoxidase activity and abnormal echocardiographic findings - increased left ventricular end diastolic/end systolic diameter, relaxation time, and decreased fractional shortening. This was observed to be accompanied by increased systemic and cardiac tissue levels of TNF-α and IL-1 and increased cardiac intercellular adhesion molecule 1 (ICAM-1) expression. In the same study, TNF-α blocker significantly decreased cardiomyocyte apoptosis, implying the role of TNF-α in AKI-induced cardiac damage. Based on the myocardial depressant effect of TNF-α, there have been large multicenter trials about the effect of anti-TNF-α therapies (Etanercept) on congestive heart failure (CHF), but there is still no clear evidence of clinical benefit and some studies even showed worsening CHF.57
Neuroendocrine activation also underlies the pathophysiology of AKI-induced CRS. During AKI, SNS is activated and leads to norepinephrine overflow.53 Activation of SNS alters myocardial function by disrupting myocardial calcium homeostasis, increasing myocardial oxygen demand, and inducing apoptosis/hypertrophy of cardiac myocytes through adrenergic receptor stimulation.51 Activated SNS stimulates β1-adrenergic receptors in the juxtaglomerular apparatus of the kidney, which leads to decreased renal blood flow and activation of RAAS.58 Dysfunctional RAAS activation induces angiotensin II release, increasing systemic vascular resistance. Angiotensin II itself contributes to cardiac myocyte hypertrophy and apoptosis.59,60
Potential therapeutic targets
The immunomodulatory role of heme oxygenase 1 (HO-1) in AKI has been widely studied.61 Lack of HO-1 increases the susceptibility to renal IRI and renal IRI in HO-1-knockout mice increases both IL-6 and its downstream signaling effector pSTAT3 in injured kidney as well as in heart and lung.62 Based on its cyto-protective effects in AKI animal models, HO-1 induction is considered a promising therapeutic target in AKI.
A recent study found that dysfunction of cardiac mitochondria could play an important role in the pathogenesis of cardiac damage induced by AKI.63 This study showed that renal IRI induces fragmentation of mitochondria in a fission-dominant manner through dynamin-related protein 1 (Drp1) activation, which is followed by cardiomyocyte apoptosis in the heart. The use of a Drp1 inhibitor, Mdivi-1, induces a significant decrease in mitochondrial fragmentation and cardiomyocyte apoptosis as well as improvement in cardiac function. These data suggest that preservation of normal mitochondrial dynamics could be a potential therapeutic target in AKI-induced heart dysfunction.
KIDNEY-BRAIN INTERACTIONS
Clinical impact
AKI-induced uremic toxins are well known to cause neurological complications including irritability, attention deficit, hyperreflexia, decreased mental status, seizures, and even death.54 The severity of neurologic impairment is more closely associated with the rate at which kidney function decreases than the level of azotemia itself.64 Therefore, uremic symptoms are usually more severe and advance more rapidly in AKI patients than in patients with CKD.65
Several clinical studies have identified long-term neurological effects of AKI.66,67 One nationwide population study showed that patients who survive dialysis-requiring AKI have higher risk and higher severity of stroke events compared to the control group after the adjustment of progression to consequent CKD or end stage renal disease (ESRD), and the impact is almost similar to that of diabetes.66 Another cohort study showed that among elderly patients who require ICU care during hospitalization, patients with dialysis-requiring AKI has a long-term risk of dementia even after the adjustment of other dementia-related risk factors (Hazard Ratio, 1.70).67
Laboratory evidence
The pathophysiology of neurologic complications after AKI is associated with the accumulation of neurotoxic metabolites that cause disturbances to the blood-brain barrier and an imbalance in cellular water transport.11,68 The central nervous system is generally considered to be an immune-privileged site due to blood-brain barrier, but AKI can lead to disruption of the blood-brain barrier, resulting in cerebral edema as well as infiltration of neuro-toxic metabolites into brain. The short-term effects of AKI on mouse brain include increases of soluble inflammatory proteins (such as keratinocyte-derived chemokine and granulocyte-colony stimulating factor) and increased cellular inflammation, with glial fibrillary acidic protein in astrocytes.69 Post-AKI brain histology shows increased microglial cells and pyknotic neuronal cells in the hippocampus. Another study showed the significant increase of TLR-4 in the hippocampus and striatum following renal IRI, suggesting a role for TLR-4 in AKI-induced neuronal injury.70 In an animal behavioral study, uremic mice showed decreased central dopamine turnover in the striatum, mesencephalon, and hypothalamus, which was correlated with the impairment of motor activity.71
Potential therapeutic targets
A novel reflex pathway between injured kidney and the brain has been recently described, called ‘reno-cerebral reflex’.72 In this study, Cao et al showed that ischemic AKI could co-activate the intrarenal and intracerebral RAAS, oxidative stress and sympathetic activity, promoting ongoing inflammation in both organs. The authors also found that blocking of renal afferent sympathetic activity, central blockade of angiotensin II, or prevention of sympathetic activation could lower the AKI-induced systemic oxidative stress and inflammation. Intervention with RAAS inhibitors, sympatholytic drugs, or renal nerve ablation could be new therapeutic approaches targeting reno-cerebral crosstalk.
KIDNEY-LIVER INTERACTIONS
Clinical impact/evidence
Traditionally, hepatorenal syndrome denotes decrease in kidney function secondary to liver disease. For example, hepatic veno-occlusive disease often leads to reduced kidney function. The efficacy of defibrotide for the treatment of sinusoidal obstruction syndrome, a life-threatening complication following stem cell transplantation shows that treating liver dysfunction can directly lead to improvement of renal function.73 However, the clinical significance of AKI on liver is less well defined.8 Liver dysfunction can be observed in critically-ill AKI patients and hepatic failure increases the in-hospital mortality among these patient groups.74 Several clinical studies have found development of hepatic dysfunction in AKI patients who had alterations in protein synthesis, inflammatory responses, and metabolism of lipid, protein, and drugs.8,75,76 During AKI, there are changes not only in renal drug metabolism, but also in non-renal metabolism, which can have considerable influence on clinical outcomes due to under- or over-dosing and related toxicity problems. The mechanisms by which AKI impacts liver drug metabolism remains unclear, but the interaction is felt to be related to uremic toxins, inflammatory cytokines, activated leucocytes, and other neuro-humoral factors.8 Drug dosing needs to be carefully monitored in the AKI patients with hepatic dysfunction.
Laboratory evidence
An experimental study showed that AKI promotes oxidative stress, inflammation, apoptosis, and tissue damage in hepatocytes while increasing vascular permeability and leukocyte infiltration into liver.77 Recently, Lee and colleagues demonstrated that renal IRI induces peptidyl arginine deiminase 4 (PAD-4) in the liver. Hepatic injury and inflammation in PAD-4 deficient mice were found to be significantly attenuated after renal IRI compared to wild-type (WT) mice, implying that the extra-renal PAD-4 contributes to the distant organ damage after AKI.78 Lee et al also suggested that systemic increase of TNF-α, IL-17A, and IL-6 predispose to liver and small intestine injury after ischemic or non-ischemic AKI.79 Furthermore, AKI-induced damaging changes in the liver and intestine are partly induced by direct activation of small intestine Paneth cells, which release granules containing IL-17A into the intestinal lumen.80
Potential therapeutic targets
Several groups have studied the therapeutic effect of antioxidants in AKI-induced liver damage, targeting activated oxidative stress. Administration of glutathione significantly ameliorates the structural and enzymatic changes in the liver after AKI.77 Another study investigated the effects of vitamin E on AKI-induced liver damage, showing that vitamin E administration alleviates the increase of plasma aspartate transaminase (AST) and alanine transaminase (ALT) level while preserving glutathione activity.81 Administration of thymoquinone, a main constituent of Nigella sativa that has anti-oxidant and anti-inflammatory effect, before renal IRI improves renal and hepatic function and increases antioxidant enzyme activity in both organs.82 This was observed to be accompanied by a decrease of CYP3A1 and spermidine/spermine-N1-acetyl-transferase gene expression in liver, implying less oxidative stress following thymoquinone treatment.
Isoflurane, a well-known volatile anesthetic, shows protective effects on ischemic and non-ischemic AKI-induced hepatic and intestinal injury via induction of the sphingosine kinase 1 (SK-1)/sphingosine-1-phosphate pathway.83,84 Through induction of SK-1 in small intestine, isoflurane ameliorates hepatic and intestinal pro-inflammatory cytokine production and vascular permeability as well as intestinal apoptosis. Given the high incidence of postoperative-AKI, it is possible that using isoflurane may improve the outcome of postoperative AKI-induced distant organ damage by its protective action on liver and intestine as well as less effects on systemic blood pressure/renal blood flow.
KIDNEY-INTESTINAL MICROBIOTA INTERACTIONS
Clinical impact/evidence
Recent clinical and experimental studies have shown the bidirectional relationship between intestinal microbiota and kidney diseases, but most of them have been limited to the interaction of intestinal microbiota with development and progression of CKD/ESRD.85,86 Despite the limited data available on AKI-gut microbiome interactions, accumulating clinical data about the immunomodulatory role of gut microbiome in CKD patients implicates the possible role of intestinal microbiota in the patients with AKI-induced distant organ dysfunction.87
Laboratory evidence/Potential therapeutic targets
The significance of normal gut microbiota on experimental AKI was initially demonstrated by using germ-free mice that lacked any exposure to symbiotic microorganisms and parasites.88 In ischemic AKI, these mice showed worse functional/structural renal damage and enhanced inflammation compared to the control mice. Unexpectedly, they had higher NKT cells in the kidney. Reconstitution of germ-free mice using WT gut microbiota (conventionalization) was found to alleviate their increased susceptibility to AKI. In the analysis of baseline levels of kidney cytokines, the germ-free mice had higher interferon-γ (IFN-γ) and lower IL-4 level compared to the WT mice, which suggests that germ-free mice are more prone to a TH1 type response, similar to that seen in autoimmune disease. This finding implicates a potential immunomodulatory role of gut microbiota in the development of kidney diseases.
During fermentation, gut microbiota generate various metabolites, including multiple uremic retention molecules and short chain fatty acids (SCFAs). SCFAs, the fermentation end products from complex polysaccharides, are mostly comprised of acetate, propionate, and butyrate.89 Accumulating evidences suggest that SCFAs regulate inflammation, energy metabolism, and blood pressure, which affects kidney function through the gut-kidney axis.89 Uremic toxins (e.g. indoles, ammonia, and trimethylamine N-oxide) generated by the gut microbiota, are felt to have a detrimental effect on kidney function.90
Restoring the balance of the gut microbiome has received a lot of attention as a potential therapeutic target for kidney disorders. These interventions include (1) reducing harmful uremic toxins by restricting the intake of uremic toxin precursors (lowering protein intake)91 or by enhancing the disposal of the toxins (adsorbent therapy)92 and (2) supplementing a more balanced gut microbiome using pre-/pro-/syn-biotics.93,94.
Several studies have examined the effects of SCFAs on AKI using ischemia- and gentamicin-induced AKI models.95,96 Administration of the three main SCFAs had protective effect in ischemic AKI by decreasing local and systemic inflammation, oxidative stress, inflammatory cell infiltration, and apoptosis in injured kidney. Administration of acetate, one of the main SCFAs, could also modulate epigenetic modifications in ischemic-injured kidney tissue, by inhibiting the activity of histone deacetylase and reversing the decreased global DNA methylation status.95 Given the therapeutic potential of SCFAs on AKI outcome, more studies are necessary to elucidate the detailed mechanisms and effects of SCFAs on AKI.
CONCLUSION
AKI induces systemic and organ-specific hemodynamic-, humoral-, and immunologic imbalances, which helps explain why patients are not just dying with AKI, but from AKI.18 Based on the current findings about different pathways linking kidney injury with distant organs, therapeutic strategies that targeting just a single molecule are less likely to succeed in reducing the AKI-induced distant organ dysfunctions. (Table 1) Understanding the complex interactions between kidney and distant organs should lead to new diagnostics and therapies to improve outcomes in patients with AKI.
Table 1.
Possible therapeutic targets against AKI-induced distant organ injury
| Organ | Intervention | Subject | Effects | Reference |
|---|---|---|---|---|
| Lung | Blocking of CXCL1 | Mouse | ↓ Lung neutrophil | 31 |
| Blocking of TNF-α | Mouse | ↓ Pulmonary apoptosis | 34 | |
| Administration of IL-10 | Mouse | Improved lung architecture, ↑ Lung neutrophil | 9 | |
| Blocking neutrophil elastase | Mouse | ↓ Lung inflammation, Survival | 26 | |
| Administration of CNI-1493 | Mouse | ↓ Pulmonary vascular permeability | 29 | |
| Administration of ANP | Rat | ↓ Pulmonary edema, Inflammatory cytokine in the lung | 38 | |
| Human | No significant benefit | 39,40 | ||
| Administration of PGE1 | Rat | ↓ Antioxidant stress in lung, Improved lung architecture | 42 | |
| Hemofiltration using novel membrane | Human (Ex vivo) | Efficient removal of systemic cytokines and HMGB1 | 46 | |
| Heart | Blocking of TNF-α | Rat | ↓ Cardiomyocyte apoptosis | 56 |
| Human | No significant benefit | 57 | ||
| Mitochondrial fission protein (Drp1) inhibition | Mouse | ↓ Cardiomyocyte apoptosis, ↑ Cardiac function | 63 | |
| Brain | Inhibition of reno-cerebral reflex using RAAS inhibitors, sympatholytic drugs, or renal nerve ablation | Mouse | ↓ Cerebral inflammation, Down-regulation of cerebral/renal RAAS | 72 |
| Liver | Administration of glutathione | Rat | Improved liver architecture, ALT | 77 |
| Administration of vitamin E | Mouse | ↓ AST and ALT, Improved hepatic oxidant/antioxidant balance | 81 | |
| Administration of thymoquinone | Rat | ↓ Hepatic oxidative stress | 82 | |
| Use of Isoflurane | Mouse | ↓ ALT, Improved hepatic architecture | 83,84 | |
| Intestine | Use of Isoflurane | Mouse | ↓ Intestinal apoptosis, Improved intestinal villi architecture | 83,84 |
| Supplementation with SCFA | Mouse | Modulation of inflammatory process, oxidative stress, & apoptosis in kidney after IRI | 95 | |
| Rat | ↑ Renal antioxidant enzyme activity, ↑ Renal prohibitin protein expression | 96 |
Abbreviations: AKI, Acute kidney injury; CXCL1, Chemokine (C-X-C motif) ligand 1; TNF-α, Tumor necrosis factor α; IL, Interleukin; ANP, Atrial natriuretic peptide; PGE1, Prostaglandin E1; HMGB1, High-mobility group box protein B1; Drp1, Dynamin-related protein 1; RAAS, Renin-angiotensin-aldosterone system; ALT, Alanine transaminase; AST, Aspartate transaminase; SCFA, Short chain fatty acids; IRI, Ischemia-reperfusion injury
Acknowledgments
Support: This work was supported by National Institutes of Health Grants R01-DK111209 and R01-DK104662.
Acronym
- AKI
Acute kidney injury
- ROS
Reactive oxygen species
- KDIGO
Kidney Disease Improving Global Outcomes
- CRRT
Continuous renal replacement therapy
- ICU
Intensive care unit
- ALI
Acute lung injury
- IL
Interleukin
- BNx
Bilateral nephrectomy
- IRI
Ischemia-reperfusion injury
- ARDS
Acute respiratory distress syndrome
- CXCL1
Chemokine (C-X-C motif) ligand 1
- TNF-α
Tumor necrosis factor alpha
- TNFR1
Tumor necrosis factor receptor 1
- ANP
Atrial natriuretic peptide
- PGE1
Prostaglandin E1
- TLR-4
Toll-like receptor 4
- HMGB1
High-mobility group box protein B1
- CRS
Cardiorenal syndrome
- CKD
Chronic kidney disease
- PBUT
Protein-bound uremic toxin
- SNS
Sympathetic nervous system
- RAAS
Renin-angiotensin-aldosterone system
- ICAM-1
Intercellular adhesion molecule-1
- CHF
Congestive heart failure
- ANG II
Angiotensin II
- HO-1
Heme oxygenase-1
- Drp1
Dynamin-related protein 1
- ESRD
End stage renal disease
- PAD-4
Peptidyl arginine deiminase-4
- AST
Aspartate transaminase
- ALT
Alanine transaminase
- SK-1
Sphingosine kinase-1
- WT
Wild-type
- SCFA
Short chain fatty acids
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nature reviews Nephrology. 2014;10(4):193–207. doi: 10.1038/nrneph.2013.282. [DOI] [PubMed] [Google Scholar]
- 2.Zeng X, McMahon GM, Brunelli SM, Bates DW, Waikar SS. Incidence, outcomes, and comparisons across definitions of AKI in hospitalized individuals. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(1):12–20. doi: 10.2215/CJN.02730313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoste EAJ, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Medicine. 2015;41(8):1411–1423. doi: 10.1007/s00134-015-3934-7. [DOI] [PubMed] [Google Scholar]
- 4.Mehta RL, Bouchard J, Soroko SB, et al. Sepsis as a cause and consequence of acute kidney injury: Program to Improve Care in Acute Renal Disease. Intensive Care Med. 2011;37(2):241–248. doi: 10.1007/s00134-010-2089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singbartl K, Joannidis M. Short-term Effects of Acute Kidney Injury. Critical care clinics. 2015;31(4):751–762. doi: 10.1016/j.ccc.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 6.Doi K, Rabb H. Impact of acute kidney injury on distant organ function: recent findings and potential therapeutic targets. Kidney International. 89(3):555–564. doi: 10.1016/j.kint.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 7.Basu RK, Wheeler DS. Kidney–lung cross-talk and acute kidney injury. Pediatric Nephrology. 2013;28(12):2239–2248. doi: 10.1007/s00467-012-2386-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lane K, Dixon JJ, MacPhee IA, Philips BJ. Renohepatic crosstalk: does acute kidney injury cause liver dysfunction? Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2013;28(7):1634–1647. doi: 10.1093/ndt/gft091. [DOI] [PubMed] [Google Scholar]
- 9.Hoke TS, Douglas IS, Klein CL, et al. Acute renal failure after bilateral nephrectomy is associated with cytokine-mediated pulmonary injury. Journal of the American Society of Nephrology : JASN. 2007;18(1):155–164. doi: 10.1681/ASN.2006050494. [DOI] [PubMed] [Google Scholar]
- 10.Yap SC, Lee HT. Acute Kidney Injury and Extrarenal Organ DysfunctionNew Concepts and Experimental Evidence. The Journal of the American Society of Anesthesiologists. 2012;116(5):1139–1148. doi: 10.1097/ALN.0b013e31824f951b. [DOI] [PubMed] [Google Scholar]
- 11.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nature reviews Nephrology. 2015;11(12):707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Murugan R. Effects of non-severe acute kidney injury on clinical outcomes in critically ill patients. Critical Care. 2016;20(1):159. doi: 10.1186/s13054-016-1295-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Justice MJ, Dhillon P. Using the mouse to model human disease: increasing validity and reproducibility. Disease Models and Mechanisms. 2016;9(2):101–103. doi: 10.1242/dmm.024547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faubel S, Edelstein CL. Mechanisms and mediators of lung injury after acute kidney injury. Nature reviews Nephrology. 2016;12(1):48–60. doi: 10.1038/nrneph.2015.158. [DOI] [PubMed] [Google Scholar]
- 15.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S. Acute renal failure in critically ill patients: a multinational, multicenter study. Jama. 2005:294. doi: 10.1001/jama.294.7.813. [DOI] [PubMed] [Google Scholar]
- 16.Waikar SS, Liu KD, Chertow GM. The incidence and prognostic significance of acute kidney injury. Current Opinion in Nephrology and Hypertension. 2007;16(3):227–236. doi: 10.1097/MNH.0b013e3280dd8c35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vieira JM, Jr, Castro I, Curvello-Neto A, et al. Effect of acute kidney injury on weaning from mechanical ventilation in critically ill patients. Crit Care Med. 2007;35(1):184–191. doi: 10.1097/01.CCM.0000249828.81705.65. [DOI] [PubMed] [Google Scholar]
- 18.Basu RK. Pediatric Critical Care Medicine. Springer; 2014. Acute Kidney Injury; pp. 191–212. [Google Scholar]
- 19.Wiedemann HP, Wheeler AP, Bernard GR, et al. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 20.Basu RK, Wheeler D. Effects of ischemic acute kidney injury on lung water balance: nephrogenic pulmonary edema? Pulm Med. 2011;2011:414253. doi: 10.1155/2011/414253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu KD, Altmann C, Smits G, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Critical care (London, England) 2009;13(4):R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simmons EM, Himmelfarb J, Sezer MT, et al. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney International. 2004;65(4):1357–1365. doi: 10.1111/j.1523-1755.2004.00512.x. [DOI] [PubMed] [Google Scholar]
- 23.Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int. 2008;74(7):901–909. doi: 10.1038/ki.2008.314. [DOI] [PubMed] [Google Scholar]
- 24.Rabb H, Wang Z, Nemoto T, Hotchkiss J, Yokota N, Soleimani M. Acute renal failure leads to dysregulation of lung salt and water channels. Kidney Int. 2003;63(2):600–606. doi: 10.1046/j.1523-1755.2003.00753.x. [DOI] [PubMed] [Google Scholar]
- 25.Awad AS, Rouse M, Huang L, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney International. 2009;75(7):689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishii T, Doi K, Okamoto K, et al. Neutrophil elastase contributes to acute lung injury induced by bilateral nephrectomy. The American journal of pathology. 2010;177(4):1665–1673. doi: 10.2353/ajpath.2010.090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie ML, White LE, Santora RJ, Park JM, Rabb H, Hassoun HT. Lung T lymphocyte trafficking and activation during ischemic acute kidney injury. Journal of immunology (Baltimore, Md : 1950) 2012;189(6):2843–2851. doi: 10.4049/jimmunol.1103254. [DOI] [PubMed] [Google Scholar]
- 28.Altmann C, Andres-Hernando A, McMahan RH, et al. Macrophages mediate lung inflammation in a mouse model of ischemic acute kidney injury. American Journal of Physiology - Renal Physiology. 2012;302(4):F421–F432. doi: 10.1152/ajprenal.00559.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer AA, Postler G, Salhab KF, Mendez C, Carey LC, Rabb H. Renal ischemia/reperfusion leads to macrophage-mediated increase in pulmonary vascular permeability. Kidney Int. 1999;55(6):2362–2367. doi: 10.1046/j.1523-1755.1999.00460.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu KD, Glidden DV, Eisner MD, et al. Predictive and pathogenetic value of plasma biomarkers for acute kidney injury in patients with acute lung injury. Crit Care Med. 2007;35(12):2755–2761. [PMC free article] [PubMed] [Google Scholar]
- 31.Ahuja N, Andres-Hernando A, Altmann C, et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. American journal of physiology Renal physiology. 2012;303(6):F864–872. doi: 10.1152/ajprenal.00025.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Altmann C, Ahuja N, Kiekhaefer CM, et al. Early peritoneal dialysis reduces lung inflammation in mice with ischemic acute kidney injury. Kidney International. 2017;92(2):365–376. doi: 10.1016/j.kint.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Andres-Hernando A, Okamura K, Bhargava R, et al. Circulating IL-6 upregulates IL-10 production in splenic CD4+ T cells and limits acute kidney injury–induced lung inflammation. Kidney International. 2017;91(5):1057–1069. doi: 10.1016/j.kint.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 34.White LE, Santora RJ, Cui Y, Moore FA, Hassoun HT. TNFR1-dependent pulmonary apoptosis during ischemic acute kidney injury. American Journal of Physiology - Lung Cellular and Molecular Physiology. 2012;303(5):L449–L459. doi: 10.1152/ajplung.00301.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verkman AS. Role of aquaporins in lung liquid physiology. Respiratory physiology & neurobiology. 2007;159(3):324–330. doi: 10.1016/j.resp.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma T, Fukuda N, Song Y, Matthay MA, Verkman AS. Lung fluid transport in aquaporin-5 knockout mice. The Journal of clinical investigation. 2000;105(1):93–100. doi: 10.1172/JCI8258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yabuuchi N, Sagata M, Saigo C, et al. Indoxyl Sulfate as a Mediator Involved in Dysregulation of Pulmonary Aquaporin-5 in Acute Lung Injury Caused by Acute Kidney Injury. International journal of molecular sciences. 2017;18(1):11. doi: 10.3390/ijms18010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mitaka C, Si MK, Tulafu M, et al. Effects of atrial natriuretic peptide on inter-organ crosstalk among the kidney, lung, and heart in a rat model of renal ischemia-reperfusion injury. Intensive care medicine experimental. 2014;2(1):28. doi: 10.1186/s40635-014-0028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mori Y, Kamada T, Ochiai R. Reduction in the incidence of acute kidney injury after aortic arch surgery with low-dose atrial natriuretic peptide: a randomised controlled trial. European journal of anaesthesiology. 2014;31(7):381–387. doi: 10.1097/EJA.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 40.Nigwekar SU, Navaneethan SD, Parikh CR, Hix JK. Atrial natriuretic peptide for preventing and treating acute kidney injury. The Cochrane database of systematic reviews. 2009;(4):Cd006028. doi: 10.1002/14651858.CD006028.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Carden DL, Granger DN. Pathophysiology of ischaemia–reperfusion injury. The Journal of Pathology. 2000;190(3):255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 42.Oztay F, Kara-Kisla B, Orhan N, Yanardag R, Bolkent S. The protective effects of prostaglandin E1 on lung injury following renal ischemia-reperfusion in rats. Toxicology and industrial health. 2016;32(9):1684–1692. doi: 10.1177/0748233715576615. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Bloom O, Zhang M, et al. HMG-1 as a Late Mediator of Endotoxin Lethality in Mice. Science. 1999;285(5425):248–251. doi: 10.1126/science.285.5425.248. [DOI] [PubMed] [Google Scholar]
- 44.Doi K, Ishizu T, Tsukamoto-Sumida M, et al. The high-mobility group protein B1–Toll-like receptor 4 pathway contributes to the acute lung injury induced by bilateral nephrectomy. Kidney International. 2014;86(2):316–326. doi: 10.1038/ki.2014.62. [DOI] [PubMed] [Google Scholar]
- 45.Yumoto M, Nishida O, Moriyama K, et al. In vitro evaluation of high mobility group box 1 protein removal with various membranes for continuous hemofiltration. Therapeutic apheresis and dialysis : official peer-reviewed journal of the International Society for Apheresis, the Japanese Society for Apheresis, the Japanese Society for Dialysis Therapy. 2011;15(4):385–393. doi: 10.1111/j.1744-9987.2011.00971.x. [DOI] [PubMed] [Google Scholar]
- 46.Yasuda N, Goto K, Yamamoto S, Hidaka S, Hagiwara S, Noguchi T. Removal of 17 Cytokines, HMGB1, and Albumin by Continuous Hemofiltration Using a Cellulose Triacetate Membrane: An Ex Vivo Study. Journal of Surgical Research. 2012;176(1):226–231. doi: 10.1016/j.jss.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 47.Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal Syndrome. Journal of the American College of Cardiology. 2008;52(19):1527–1539. doi: 10.1016/j.jacc.2008.07.051. [DOI] [PubMed] [Google Scholar]
- 48.Liano F, Junco E, Pascual J, Madero R, Verde E. The spectrum of acute renal failure in the intensive care unit compared with that seen in other settings. The Madrid Acute Renal Failure Study Group. Kidney international Supplement. 1998;66:S16–24. [PubMed] [Google Scholar]
- 49.Hansen MK, Gammelager H, Jacobsen C-J, et al. Acute Kidney Injury and Long-term Risk of Cardiovascular Events After Cardiac Surgery: A Population-Based Cohort Study. Journal of Cardiothoracic and Vascular Anesthesia. 2015;29(3):617–625. doi: 10.1053/j.jvca.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 50.Wu V-C, Wu C-H, Huang T-M, et al. Long-term risk of coronary events after AKI. Journal of the American Society of Nephrology. 2014;25(3):595–605. doi: 10.1681/ASN.2013060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Singh K, Xiao L, Remondino A, Sawyer DB, Colucci WS. Adrenergic regulation of cardiac myocyte apoptosis. Journal of Cellular Physiology. 2001;189(3):257–265. doi: 10.1002/jcp.10024. [DOI] [PubMed] [Google Scholar]
- 52.Lekawanvijit S, Kompa AR, Krum H. Protein-bound uremic toxins: a long overlooked culprit in cardiorenal syndrome. American journal of physiology Renal physiology. 2016;311(1):F52–62. doi: 10.1152/ajprenal.00348.2015. [DOI] [PubMed] [Google Scholar]
- 53.Bagshaw SM, Hoste E, Braam B, et al. Cardiorenal syndrome type 3: pathophysiologic and epidemiologic considerations. 2013 doi: 10.1159/000349971. [DOI] [PubMed] [Google Scholar]
- 54.Grams ME, Rabb H. The distant organ effects of acute kidney injury. Kidney Int. 2012;81(10):942–948. doi: 10.1038/ki.2011.241. [DOI] [PubMed] [Google Scholar]
- 55.Cruz DN, Bagshaw SM. Heart-kidney interaction: epidemiology of cardiorenal syndromes. Int J Nephrol. 2011;2011:351291. doi: 10.4061/2011/351291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kelly K. Distant effects of experimental renal ischemia/reperfusion injury. Journal of the American Society of Nephrology. 2003;14(6):1549–1558. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- 57.Anker SD, Coats AJS. How to RECOVER from RENAISSANCE? The significance of the results of RECOVER, RENAISSANCE, RENEWAL and ATTACH. International Journal of Cardiology. 2002;86(2):123–130. doi: 10.1016/s0167-5273(02)00470-9. [DOI] [PubMed] [Google Scholar]
- 58.Saxena PR. Interaction Between the Renin-Angiotensin-Aldosterone and Sympathetic Nervous Systems. Journal of Cardiovascular Pharmacology. 1992;19:S80–S88. doi: 10.1097/00005344-199219006-00013. [DOI] [PubMed] [Google Scholar]
- 59.Kim S, Iwao H. Molecular and cellular mechanisms of angiotensin II-mediated cardiovascular and renal diseases. Pharmacological reviews. 2000;52(1):11–34. [PubMed] [Google Scholar]
- 60.Kajstura J, Cigola E, Malhotra A, et al. Angiotensin II induces apoptosis of adult ventricular myocytesin vitro. Journal of molecular and cellular cardiology. 1997;29(3):859–870. doi: 10.1006/jmcc.1996.0333. [DOI] [PubMed] [Google Scholar]
- 61.Bolisetty S, Zarjou A, Agarwal A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2017;69(4):531–545. doi: 10.1053/j.ajkd.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tracz MJ, Juncos JP, Croatt AJ, et al. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int. 2007;72(9):1073–1080. doi: 10.1038/sj.ki.5002471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sumida M, Doi K, Ogasawara E, et al. Regulation of Mitochondrial Dynamics by Dynamin-Related Protein-1 in Acute Cardiorenal Syndrome. Journal of the American Society of Nephrology : JASN. 2015;26(10):2378–2387. doi: 10.1681/ASN.2014080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fraser CL, Arieff AI. Nervous system complications in uremia. Annals of internal medicine. 1988;109(2):143–153. doi: 10.7326/0003-4819-109-2-143. [DOI] [PubMed] [Google Scholar]
- 65.Burn DJ, Bates D. Neurology and the kidney. Journal of neurology, neurosurgery, and psychiatry. 1998;65(6):810–821. doi: 10.1136/jnnp.65.6.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wu V-C, Wu P-C, Wu C-H, et al. The impact of acute kidney injury on the long-term risk of stroke. Journal of the American Heart Association. 2014;3(4):e000933. doi: 10.1161/JAHA.114.000933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Guerra C, Linde-Zwirble WT, Wunsch H. Risk factors for dementia after critical illness in elderly Medicare beneficiaries. Critical Care. 2012;16(6):1–12. doi: 10.1186/cc11901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clinical Neurology and Neurosurgery. 2004;107(1):1–16. doi: 10.1016/j.clineuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 69.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in the brain. Journal of the American Society of Nephrology. 2008;19(7):1360–1370. doi: 10.1681/ASN.2007080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Salama M, Mohamed Farrag S, Mohamed Amin M, et al. Up-regulation of TLR-4 in the brain after ischemic kidney-induced encephalopathy in the rat. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 2013;12(5):583–586. doi: 10.2174/1871527311312050006. [DOI] [PubMed] [Google Scholar]
- 71.Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive care medicine. 2001;27(10):1655–1660. doi: 10.1007/s001340101067. [DOI] [PubMed] [Google Scholar]
- 72.Cao W, Li A, Li J, et al. Reno-Cerebral Reflex Activates the Renin-Angiotensin System, Promoting Oxidative Stress and Renal Damage After Ischemia-Reperfusion Injury. Antioxidants & redox signaling. 2017;27(7):415–432. doi: 10.1089/ars.2016.6827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carreras E. How I manage sinusoidal obstruction syndrome after haematopoietic cell transplantation. British journal of haematology. 2015;168(4):481–491. doi: 10.1111/bjh.13215. [DOI] [PubMed] [Google Scholar]
- 74.Chertow GM, Christiansen CL, Cleary PD, Munro C, Lazarus J. PRognostic stratification in critically ill patients with acute renal failure requiring dialysis. Archives of internal medicine. 1995;155(14):1505–1511. [PubMed] [Google Scholar]
- 75.Vilay AM, Churchwell MD, Mueller BA. Clinical review: drug metabolism and nonrenal clearance in acute kidney injury. Critical care (London, England) 2008;12(6):235. doi: 10.1186/cc7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun J, Shannon M, Ando Y, et al. Serum metabolomic profiles from patients with acute kidney injury: A pilot study. Journal of Chromatography B. 2012;893–894:107–113. doi: 10.1016/j.jchromb.2012.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Golab F, Kadkhodaee M, Zahmatkesh M, et al. Ischemic and non-ischemic acute kidney injury cause hepatic damage. Kidney International. 2009;75(8):783–792. doi: 10.1038/ki.2008.683. [DOI] [PubMed] [Google Scholar]
- 78.Rabadi M, Kim M, D'Agati V, Lee HT. Peptidyl arginine deiminase-4-deficient mice are protected against kidney and liver injury after renal ischemia and reperfusion. American journal of physiology Renal physiology. 2016;311(2):F437–449. doi: 10.1152/ajprenal.00254.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Park SW, Chen SW, Kim M, et al. Cytokines induce small intestine and liver injury after renal ischemia or nephrectomy. Laboratory investigation; a journal of technical methods and pathology. 2011;91(1):63–84. doi: 10.1038/labinvest.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Park SW, Kim M, Kim JY, et al. Paneth Cell–Mediated Multiorgan Dysfunction after Acute Kidney Injury. The Journal of Immunology. 2012;189(11):5421–5433. doi: 10.4049/jimmunol.1200581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khastar H. Protective effects of vitamin E against liver damage caused by renal ischemia reperfusion. Renal failure. 2015;37(3):494–496. doi: 10.3109/0886022X.2015.1006084. [DOI] [PubMed] [Google Scholar]
- 82.Awad AS, Kamel R, Sherief MA. Effect of thymoquinone on hepatorenal dysfunction and alteration of CYP3A1 and spermidine/spermine N-1-acetyl-transferase gene expression induced by renal ischaemia-reperfusion in rats. The Journal of pharmacy and pharmacology. 2011;63(8):1037–1042. doi: 10.1111/j.2042-7158.2011.01303.x. [DOI] [PubMed] [Google Scholar]
- 83.Kim M, Park SW, Kim M, D'Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against renal ischemia-reperfusion-induced liver and intestine injury. Anesthesiology. 2011;114(2):363–373. doi: 10.1097/ALN.0b013e3182070c3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim M, Park SW, Kim M, D'Agati VD, Lee HT. Isoflurane activates intestinal sphingosine kinase to protect against bilateral nephrectomy-induced liver and intestine dysfunction. American journal of physiology Renal physiology. 2011;300(1):F167–176. doi: 10.1152/ajprenal.00467.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anders H-J, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney International. 2013;83(6):1010–1016. doi: 10.1038/ki.2012.440. [DOI] [PubMed] [Google Scholar]
- 86.Felizardo RJ, Castoldi A, Andrade-Oliveira V, Camara NO. The microbiota and chronic kidney diseases: a double-edged sword. Clinical & translational immunology. 2016;5(6):e86. doi: 10.1038/cti.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ramezani A, Massy ZA, Meijers B, Evenepoel P, Vanholder R, Raj DS. Role of the Gut Microbiome in Uremia: A Potential Therapeutic Target. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2016;67(3):483–498. doi: 10.1053/j.ajkd.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jang HR, Gandolfo MT, Ko GJ, Satpute S, Racusen L, Rabb H. Early exposure to germs modifies kidney damage and inflammation after experimental ischemia-reperfusion injury. American journal of physiology Renal physiology. 2009;297(5):F1457–1465. doi: 10.1152/ajprenal.90769.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li L, Ma L, Fu P. Gut microbiota-derived short-chain fatty acids and kidney diseases. Drug design, development and therapy. 2017;11:3531–3542. doi: 10.2147/DDDT.S150825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mahmoodpoor F, Rahbar Saadat Y, Barzegari A, Ardalan M, Zununi Vahed S. The impact of gut microbiota on kidney function and pathogenesis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2017;93:412–419. doi: 10.1016/j.biopha.2017.06.066. [DOI] [PubMed] [Google Scholar]
- 91.Black AP, Anjos JS, Cardozo L, et al. Does Low-Protein Diet Influence the Uremic Toxin Serum Levels From the Gut Microbiota in Nondialysis Chronic Kidney Disease Patients? Journal of renal nutrition : the official journal of the Council on Renal Nutrition of the National Kidney Foundation. 2018 doi: 10.1053/j.jrn.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 92.Schulman G, Berl T, Beck GJ, et al. Randomized Placebo-Controlled EPPIC Trials of AST-120 in CKD. Journal of the American Society of Nephrology : JASN. 2015;26(7):1732–1746. doi: 10.1681/ASN.2014010042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(9):1603–1610. doi: 10.2215/CJN.00490114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang IK, Wu YY, Yang YF, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: a randomised, double-blind, placebo-controlled trial. Beneficial microbes. 2015;6(4):423–430. doi: 10.3920/BM2014.0088. [DOI] [PubMed] [Google Scholar]
- 95.Andrade-Oliveira V, Amano MT, Correa-Costa M, et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. Journal of the American Society of Nephrology : JASN. 2015;26(8):1877–1888. doi: 10.1681/ASN.2014030288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun X, Zhang B, Hong X, Zhang X, Kong X. Histone deacetylase inhibitor, sodium butyrate, attenuates gentamicin-induced nephrotoxicity by increasing prohibitin protein expression in rats. European journal of pharmacology. 2013;707(1–3):147–154. doi: 10.1016/j.ejphar.2013.03.018. [DOI] [PubMed] [Google Scholar]
- 97.Ware LB, Matthay MA. The acute respiratory distress syndrome. The New England journal of medicine. 2000;342(18):1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]