Abstract
Objectives:
Human glutaminyl cyclases (QC and isoQC) play an important role in maintaining inflammatory conditions. Meanwhile a glutaminyl cyclase synthesized by Porphyromonas gingivalis (PgQC), a key pathogen in developing periodontitis and a potential link of periodontitis with rheumatoid arthritis (RA), was discovered. This study was aimed to determine the expression of QC, isoQC and PgQC in patients with chronic periodontitis (CP) and RA.
Design:
Thirty volunteers were enrolled in a pilot study and divided into 3 groups (healthy, CP and RA individuals). Blood samples, biofilm and gingival crevicular fluid (GCF) were analysed for mRNA expression of QC, isoQC and P. gingivalis QC. Major bacteria being associated with periodontal disease were quantified in subgingival biofilm and protein levels for monocyte chemoattractant protein (MCP)-1, MCP-3 and interleukin (IL)-1β) were determined in the GCF. Expression of PgQC on the mRNA and protein levels was assessed in two P. gingivalis strains.
Results:
PgQC is expressed in P. gingivalis strains and the protein seems to be located mainly in peri-plasmatic space. mRNA expression of QC was significantly increased in the peripheral blood from RA patients vs. healthy subjects and CP patients (p=0.013 and p=0.003, respectively). In GCF of RA patients, QC mRNA was detected more frequently than in healthy controls (p=0.043). In these samples IL-1β levels were also elevated compared to GCF from periodontally healthy individuals (p=0.003). PgQC was detected in eight out of the 13 P. gingivalis positive biofilm samples.
Conclusion:
Activity of QC may play a supportive role in maintaining chronic periodontal inflammation and destruction in RA. PgQC is expressed in vivo but further research is needed to evaluate biological importance of this enzyme and if it constitutes a potential target in periodontal antimicrobial therapy.
Keywords: Biomarkers, Chronic inflammatory diseases, Gingival crevicular fluid, Peripheral blood
1. Introduction
Recently, glutaminyl cyclases (QC) gained considerable attention. These enzymes catalyze the post-translational formation of pyroglutamic acid (pGlu) from N-terminal glutamine or glutamic residues. In animals two secreted proteins (QC, isoQC) originated from two different genes have been identified (Schilling, Wasternack, & Demuth, 2008). The pGlu at the N-termini protects of peptides and proteins against activity of aminopeptidases and may influence interaction of bioactive peptides with their specific receptors (Schilling et al., 2008). Several lines of evidence support pathophysiological involvement of the human QC in the generation of pGlu at the N-terminus of the Aβ peptide forming amyloid in the brain of Alzheimer’s patients (Schilling et al., 2008).
In vitro monocyte chemoattractant protein 1 (MCP-1=CCL2) and MCP-3 (=CCL-7) are substrates for QC while both QC and isoQC catalyze the conversion of Glu-CCL2 to pGlu-CCL2 (Cynis et al., 2011). Using knockout mice, it was found that in vivo mainly isoQC modifies chemokines. This prevents truncation of Glu-CCL2 by aminopeptidases and preserves this chemokine activity to recruit monocytes thus contributing to inflammatory Process (Cynis et al., 2011). A pharmacological inhibition of isoQC seems to be an interesting approach to suppress monocyte infiltration (Cynis et al., 2011). In a study using peripheral blood-derived monocytes inhibition of both QC and isoQC reduced LPS-stimulated migration of monocytes (Chen et al., 2012). In septic conditions QC/isoQC seem to be also involved in neutrophil infiltration. In a septic arthritis mice model the application of QC/isoQC inhibitors reduced neutrophil infiltration and delayed the onset of clinical signs of arthritis (Hellvard et al., 2013).
Increasing number of studies focus on the relationship between periodontitis and rheumatoid arthritis (RA). In patients with RA, periodontitis was found to be more prevalent than in those without (Joseph, Rajappan, Nath, & Paul, 2013; Pischon et al., 2008; Torkzaban, Hjiabadi, Basiri, & Poorolajal, 2012). Opposite, an increased prevalence of RA was reported in periodontitis patients (Nesse et al., 2010). A few studies including a small number of patients suggested that an efficient RA treatment resulted in improvement of periodontal parameters (Kobayashi, Okada, et al., 2014) whereas disease activity of RA decreased after periodontal therapy (Biyikoglu et al., 2013; Erciyas et al., 2013). Both RA and periodontitis are chronic inflammatory diseases with a similar pathobiology characterized by bone and tissue destruction and high levels of inflammatory markers, e.g. interleukin (IL)-1 (Culshaw, McInnes, & Liew, 2011). In RA as an autoimmune disease, IgG antibodies against citrullinated proteins have been characterized as disease-specific (Mewar & Wilson, 2006; Wegner, Lundberg, et al., 2010). Citrullination is a post-translational modification of arginine residues in polypeptides (peptidylarginine) to citrulline residues. The reaction is catalysed by a family of five peptidylarginine deiminase (PAD) enzymes (Darrah & Andrade, 2018).
In developing periodontal disease, Porphyromonas gingivalis, a gram-negative anaerobic bacterium is postulated to be a keystone pathogen ( Hajishengallis, Darveau, & Curtis, 2012) Gingipains (two arginine specific cysteine proteases RgpA and RgpB, and one lysine specific cysteine protease Kgp) are the most important virulence factors (Guo, Nguyen, & Potempa, 2010). They modulate immune response, e.g. by defective clearance of apoptotic macrophages (Castro et al., 2017) and platelet activation (Klarstrom Engstrom, Khalaf, Kalvegren, & Bengtsson, 2015) and play an essential role in synergistic biofilm formation with T. denticola (Zhu et al., 2013). Meanwhile P. gingivalis gathers considerable attention as a link between periodontitis and rheumatoid arthritis. Antibody levels against P. gingivalis are elevated in patients with RA (Bender, Burgin, Sculean, & Eick, 2016; Mikuls et al., 2009; Okada et al., 2011). A unique among bacteria P. gingivalis peptidylarginine deiminase is able to citrullinate fibrinogen and α-enolase (Wegner, Wait, et al., 2010). In a recent study (Laugisch et al., 2016) we have confirmed an activity of this enzyme in the gingival fluid in RA and periodontitis patients. Furthermore, the analysis of proteins expressed in P. gingivalis in planktonic stage and in biofilm suggested that QC is produced by this bacterium (Ang, Veith, Dashper, & Reynolds, 2008)
To our best knowledge no report has been published focusing on expression or activity of human glutaminyl cyclases related to periodontitis. Also data about a potential role of QC in RA are scarce. This is perplexing in the context of a study showing that the mRNA expression profile of peripheral blood mononuclear cells, the QC expression level was a factor most significantly differentiating patients with active rheumatoid arthritis from healthy controls (Batliwalla et al., 2005).
Because nearly nothing was known about the expression of the QC enzymes in periodontium and their importance in periodontitis, preliminary experiments should confirm the expression of P. gingivalis QC per se first. Next a pilot study was aimed to determine the expression of human and P. gingivalis QCs in relation to periodontitis and RA. In other words, we addressed the question if and in which quantity human and bacterial QCs are expressed in periodontal area and if there is any association of local and systemic expression of human QC with chronic periodontitis (CP) and/or RA.
2. Material and Methods
2.1. Expression of P. gingivalis QC
The database (accession # AE 015924.1) was searched for glutamine cyclotransferase-related protein. After identifying the respective region (2263083…2264084) primers were designed (5’- TGC ACA CAG AGA CCT TCG AC-3’, 5’- TGA TCA TCT GTC AGA GCG CC-3’). sod as a highly expressed enzyme was used as a comparator gene (primers: 5′-AAT TCC ACC ACG GTA AGC AC-3′, 5′- TTC TCG ATG GAC AGT TTG CC-3′) as described recently (Frohlich et al., 2013).
In preliminary experiments P. gingivalis ATCC 33277 (the reference strain) and P. gingivalis J430–1 (clinical isolate) were included. Strains were precultivated on Schaedler agar plates (Oxoid, Basingstoke, UK) with 5% sheep blood and vitamin K addition, in an anaerobic atmosphere for 24 h. Thereafter, bacterial suspension in 0.9% w/v NaCl equal to McFarland 4 was prepared and mixed with Wilkins-Chalgren broth (Oxoid) supplemented with 5 mg/l β-NAD in a ratio 1:50. Tubes were incubated in an anaerobic atmosphere for 1 h, 2 h, 4 h and 24 h at 37°C. After washing cultures ones with 0.9% w/v NaCl, total RNA was purified using an innuPREP RNA mini kit (Analytic Jena AG, Jena, Germany). Then cDNA was synthesized from 100 ng total RNA using an RevertAid RT kit (Thermo Fisher Scientific Inc., Waltham, MA, USA). Finally, real-time polymerase chain reaction was carried out using GoTaq qPCR Master Mix (Promega Corporation, Madison, WI, USA) and the 7500 Real-time PCR System (Applied Biosystems™, Foster City, USA). All the procedures were performed according to the manufacturer’s instructions.
In a second series of preliminary experiments, QC protein expression was identified by using Western blot technique. Therefore, first a polyclonal antibody (MWT-Ab-00001) was generated by a 5-step immunization procedure of rabbits using the former expressed (Escherichia coli) and purified full protein without His-Tag and followed by affinity purification of the antiserum. Theoretical molecular weight of purified HisPgQC or PgQC without His-Tag of 37 KDa or 34 KDa could be approved. Strains were cultured as described before. At the respective times, (1 h, 2 h, 4 h and 24 h) media containing the bacteria were centrifuged at 10’000 g for 5 min. Bacteria were collected by centrifugation and pellets containing approximately 2 × 109 bacteria were suspended with 500 µl of sample buffer (0.125 M Tris– HCl, 20% glycerol, 4% SDS), before being subjected to SDS–PAGE (10% polyacrylamide including 0.1% SDS) under reduced conditions. Next, resolved proteins were transferred (Mini Trans-Blot System; Bio-Rad, Hercules, CA, USA) on to nitrocellulose membranes (GE Healthcare, Buckinghamshire, GB). Non-specific binding sites on the membranes were blocked overnight in 5% skimmed milk (BD, Franklin Lakes, NJ). Blots were then probed with the mentioned polyclonal rabbit antibody MWT-Ab-00001 against P. gingivalis QC followed by goat anti-rabbit IgG horseradish peroxidase-conjugated antibodies (Dako Deutschland GmbH, Hamburg, Germany). The blots were developed using the ECL Plus (GE Healthcare) substrate kit and visualized by using a gel documentation system (VWR® Imager CHEMI premium, VWR International, Radnor, PA, USA).All experiments were run in independent duplicates.
For freeze-fracture replica immunolabelling (FRIL) electron microscopy, the two P. gingivalis strains were cultured for 24 h. Then preparation and immuno-gold labelling was made as described recently (Schlormann et al., 2010). As a primary antibody the polyclonal rabbit antibody MWT-Ab-00001 against P. gingivalis was used again and as secondary gold-conjugated antibody (goat anti rabbit IgG with 10 nm gold, British Biocell International, Cardiff, UK) was used. Primary and secondary antibodies were diluted 1:50.
2.2. Study participants
This study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The Ethical Committee of the Canton Bern approved the study protocol (KEK 326/2014). All included 30 study participants signed an informed consent and their privacy right were always observed. Twenty systemically healthy volunteers (10 periodontally healthy and 10 with CP) were consecutively recruited from the patients referred to the Department of Periodontology, University of Bern, School of Dental Medicine. Another 10 outpatients with rheumatoid arthritis where consecutively selected at the Clinic of Rheumatology, Immunology and Allergology, University Hospital Bern.
According to the group the inclusion criteria were CP, periodontal health and rheumatoid arthritis respectively. Diagnosis of CP was made according to the criteria of Armitage (Armitage, 1999). In periodontitis, at least in four non adjacent sites a probing depth (PD) of ≥5 mm had to be measured. All patients with rheumatoid arthritis had to meet the criteria of the 2010 classification criteria of the American College of Rheumatology (ACR)/European League against Rheumatism (EULAR) (Aletaha et al., 2010). Study participants had to be aged between 25 and 70 years. As ethnic variation in RA should be considered (Yamamoto, Okada, Suzuki, & Kochi, 2015) another criterion for inclusion was the ethnical background; only Caucasians were included.
Exclusion criteria were the intake of antibiotics 3 months prior to the study, any type of/orperiodontal therapy (e.g. scaling and root planning and/or periodontal surgery) within the last 6 months. Also patients with a medical diagnosis of diabetes or other severe systemic disorders affecting the immune system (except for RA in the RA group) were excluded. Further, pregnant and lactating women were not included in the study.
2.3. Data and sample collection
Demographic (gender, age and smoking status as well as the medical history)and periodontal clinical data were recorded. Clinical data included the Periodontal Screening and Recording index (PSR) (Lo Frisco, Cutler, & Bramson, 1993), the number of teeth and the sites with PD ≥ 5 mm. Patients were asked for the reason of tooth loss. In addition, DAS-CRP scores in RA patients were recorded.
At the same visit, about 2 ml peripheral blood were obtained into an EDTA container. Gingival crevicular fluid was collected by applying 25 µl of 0.9% NaCl solution and aspiration for two times per pocket at 4 sites and finally biofilm was sampled by inserting a sterile paper point for 20 s into the same pockets used for sampling the crevicular fluid. The samples per patient were pooled.
2.4. Laboratory analysis of clinical samples
From paper points, DNA and RNA were extracted simultaneously using a DNA/RNA extraction kit (innuPREP DNA/RNA Mini Kit, Analytik Jena, Jena, Germany) according to the manufacturer’s instructions.
Real-time PCR using DNA extracted from periodontal biofilm was carried out for the bacteria highly associated with periodontal disease, i.e., P. gingivalis, T. forsythia, Treponema denticola, Prevotella intermedia, and Aggregatibacter actinomycetemcomitans, by using GoTaq qPCR Master Mix (Promega Corporation, Madison, WI, USA), as described recently (Eick, Straube, Guentsch, Pfister, & Jentsch, 2011). The primers published before (P. gingivalis (Ashimoto, Chen, Bakker, & Slots, 1996), T. denticola (Ashimoto et al., 1996) A. actinomycetemcomitans (Tran & Rudney, 1999) or designed according to database (http://www.oralgen.lanl.gov, T. forsythia, gene TF0367; 5’-GTC TGC GAT CAA GCA ACC T-3’, 5’-TCC ATA TTC TCC TTG AGG TGT C-3’), P. intermedia, gene PI0811; 5’-ACA AGG CTT CTG ATG GCA AG-3’, 5’-AAG TAA TGT TCT TGC CTA CGA GTG-3’) were used in the reaction. The detection level was 103 bacteria per sample.
From RNA, cDNA was generated and real-time PCR for P. gingivalis QC expression (primers 5’-TGC ACA CAG AGA CCT TCG AC-3’, 5’-TGA TCA TCT GTC AGA GCG CC-3’ designed according to #NP_906209.1; glutamine cyclotransferase-like protein) was performed as described above.
From EDTA blood and GCF, total RNA was purified and cDNA was synthesized as described above. Similarly, real-time PCR was applied to quantify QC and isoQC expression. Here the primers were designed as published recently (Cynis et al., 2011). GADPH (Shen et al., 2010) was used as a reference gene.
In addition, GCF levels of IL-1β, MCP-1, and MCP-3 were determined using commercially available enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems Europe Ltd., Abingdon, UK) according to the manufacturer’s instruction. The detection level of each kit was 2 pg/site.
2.5. Statistical analysis
Statistical analysis was performed using the software SPSS® Statistics 24 (IBM Corporation, New York,NY, USA). Clinical data were compared with ANOVA followed by post-hoc Bonferroni testing. Non-parametric tests for intergroup comparisons (Kruskal-Wallis test and Mann Whitney U–test, respectively) were used for continuous variables of laboratory data. Correlations were analysed by using Spearman test. The Chi2-test compared dichotomized variables and McNemar-Bowker test categorized variables.
A level of α ≤0.05 was considered as being significant. The unit of analysis in all statistical tests was the individual.
2.6. Methods to minimize bias
Analysis of samples was made coded at the Laboratory of Microbiology in Bern. The investigators did not know the group to which the samples belonged to.
3. Results
3.1. QC is expressed in P. gingivalis
In both analysed strains, mRNA expression was detected. First expression was low, but after 24 h of incubation it was comparable to sod (Fig. 1A). In culture supernatants a positive signal for PgQC was never found in Western blots (data not shown). Using highly concentrated bacteria, a signal was visible from 1 h of incubation (Fig. 1B). Freeze-fracture replica immunolabelling (FRIL) electron microscopy visualized the location of the PgQC in bacterial cells. The enzyme-indicating gold particles were found mostly at membrane fracture faces directed towards the periplasmic space. An extracellular secretion can be assumed, some enzyme indicating gold particles are placed in groups at the outer fracture face of outer membranes and also outside of the cells (Fig. 2).
Fig. 1.
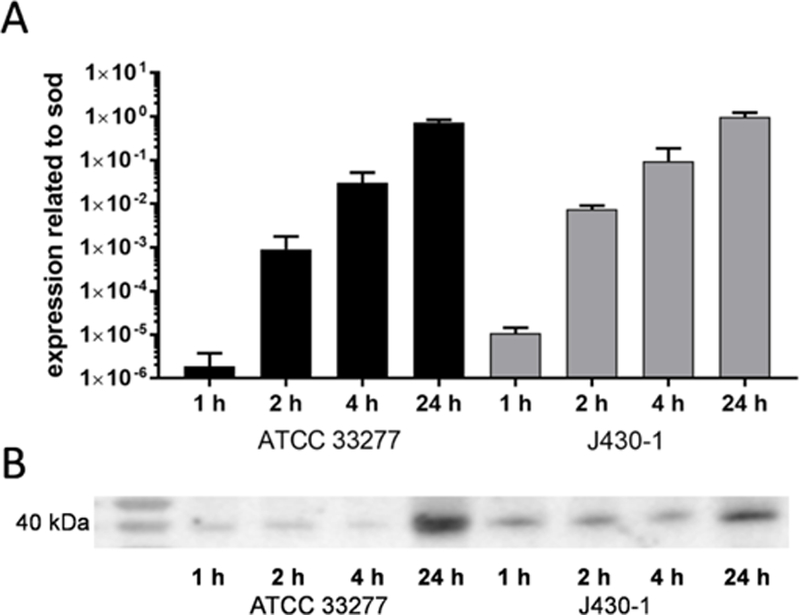
Expression of bacterial glutaminyl cyclase (A: mRNA; B: protein) in P. gingivalis ATCC 33277 and P. gingivalis J430–1 cultures after anaerobic incubation for 1 h, 2 h, 4 h and 24 h
Fig. 2.
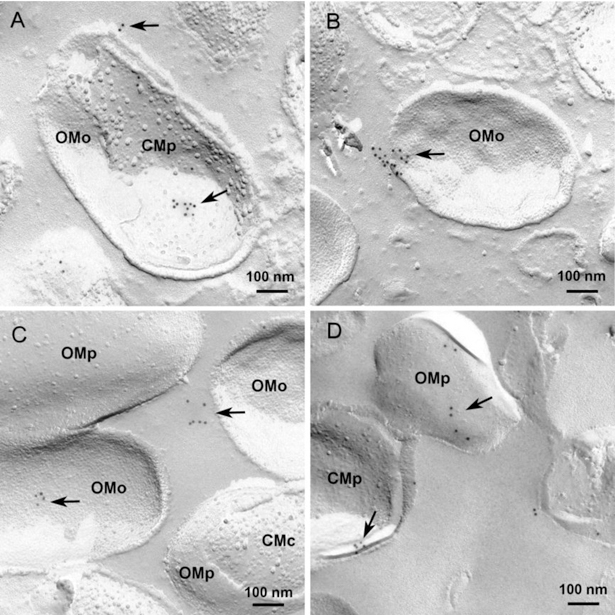
Visualization of bacterial glutaminyl cyclase in P. gingivalis ATCC 33277 (A-C) and in P. gingivalis J430–1 (D) bacterial cells after 24 h of anaerobic incubation and freeze-fracture replica immunolabelling (FRIL) electron microscopy. Glutaminyl cyclase indicating gold particles are mainly located in groups at the membrane fracture faces facing the bacterial periplasm (arrows) some gold particles were also found at the outer membrane outer fracture face and outside of the cells (arrowheads).
OMo: outer membrane outer fracture face (concave, many small intra-membrane particles); OMp: outer membrane periplasmic fracture face (convex few intra-membrane particles); CMp: cytoplasma membrane, periplasmic fracture face (concave, few intra-membrane particles), CMc: cytoplasma membrane, cytoplasmic fracture face (convex many intra-membrane particles). Bars: 100 nm
3.2. Demographics and clinical periodontal data
Thirty patients (each 10 in the RA, CP and healthy groups) were included in the study. Demographic data and periodontal parameters are presented in Table 1. The RA patients had disease duration of 11.2 years (range 2 – 22 years) and a DAS-CRP score of 2.43 (range 1.1 – 3.9) in mean at the time of the study, all of them received an anti-inflammatory therapy. The control and RA groups comprised fewer smokers in comparison to the periodontitis group. The patients with RA (20.6±6.4) had significantly (p=0.001) fewer teeth than the healthy controls (28±0) and periodontitis patients (27.1±3). Reason for tooth loss was mainly periodontitis both in RA and periodontitis patients. The patients with periodontitis had significantly (p<0.001) more sites with PD ≥5 mm (51.2±39.8) and higher PSR scores (3.80±0.23) compared to both other groups (Table 1).
Table 1.
Demographic and clinical periodontal data
| Variable | RA | periodontitis | controls | P |
|---|---|---|---|---|
| (n=10) | (n=10) | (n=10) | ||
| Age (mean±SD) | 62.8±12.4 | 57.6±11.1 | 37.7±8.1 | <0.001 |
| Gender male/female | 2 / 8 | 4 / 6 | 7 / 3 | 0.0610 |
| Smoker (no/yes/former) | 8 / 1 / 1 | 4 / 6 / 0 | 9 / 1 / 0 | 0.0361 |
| #of teeth (mean±SD) | 20.6±6.4 | 27.1±3.0 | 28±0 | 0.001 |
| #of sites with PD≥5 mm (mean±SD) | 0.45±0.93 | 51.2±39.8 | 0±0 | <0.001 |
| Mean PSR (mean±SD) | 2.29±0.40 | 3.80±0.23 | 1.72±0.42 | <0.001 |
RA: rheumatoid arthritis; SD standard deviation; PD probing depth
3.3. Bacterial counts in subgingival biofilm
P. gingivalis and T. denticola were most prevalent in CP. In RA, 5 of the 10 samples were tested positively for A. actinomycetemcomitans (Table 2).
Table 2.
Prevalence of bacteria in subgingival biofilm samples
(total of positive results (samples with counts ≥105) / total of analysed samples)
| RA | periodontitis | Controls | McNemar-Bowker test P |
|
|---|---|---|---|---|
| P. gingivalis | 3 (1) / 10 | 10 (6) / 10 | 0 (0) / 10 | 0.018 |
| T. forsythia | 8 (6) / 10 | 10 (9) / 10 | 2 (1) / 10 | n.s. |
| T. denticola | 4 (2) / 10 | 9 (5) / 10 | 0 (0) / 10 | 0.026 |
| P. intermedia | 7 (1) / 10 | 9 (3) / 10 | 1 (0) / 10 | n.s. |
| A. actinomycetemcomitans | 5 (1) / 10 | 4 (3) / 10 | 1 (0) / 10 | 0.028 |
RA: rheumatoid arthritis; n.s.: not significant
3.4. Levels of selected inflammatory markers in GCF
When the GCF levels of biomarkers for inflammation were analysed there was a significant difference for IL-1β levels between the groups (p=0.003). Specifically, the IL-1β levels were higher in GCF of RA patients than the healthy controls (p=0.003). Although there was a clear tendency for MCP-3 levels being highest in the RA group (vs. healthy controls p=0.157), levels of MCP-1 and MCP-3 did not differ significantly between the groups (Fig. 3). Furthermore, elevated levels of MCP-1 and MCP-3 were found in the QC and isoQC negative samples in comparison to positive samples but the difference was not statistically significant (data not shown).
Fig. 3.
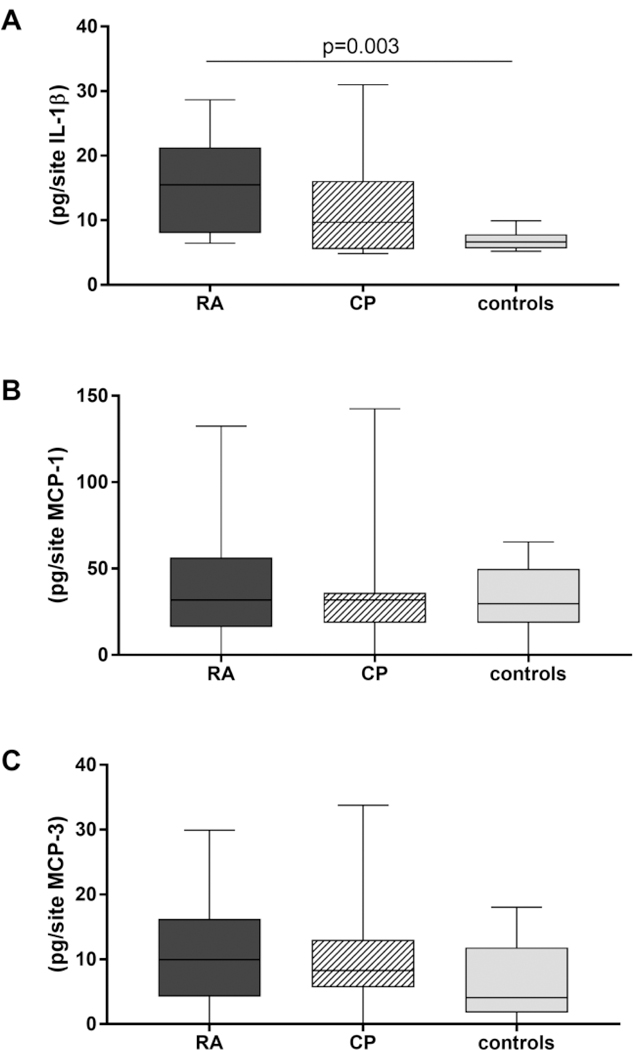
Levels of interleukin-1β (A), chemoattractant protein (CMP)-1 (B) and CMP-3 (C) in gingival crevicular fluid of patients with rheumatoid arthritis (RA, n=10), periodontitis (n=10) and healthy controls (n=10)
3.5. Expression of human QC and isoQC
The qualitative analysis of expression of QC-mRNA in the EDTA-blood (Table 3) showed comparable proportions of blood samples tested positively for QC and isoQC (p=0.371 and p=0.866 respectively). Quantitatively, there were significant differences between the groups (p=0.007). Further analysis revealed an increased mRNA expression of QC in RA patients compared to CP patients (p=0.003) and healthy controls (p=0.013) (Fig. 4A). The expression of isoQC-mRNA in the same samples failed to reach statistical significance, but showed a similar tendency in favor of the RA EDTA-blood samples (Fig. 4B).
Table 3.
Positive results (n/total) of the mRNA expression of human glutaminyl and iso glutaminyl cyclases in EDTA blood and gingival crevicular fluid
| Material | Gene | RA | periodontitis | controls | X2 P |
|---|---|---|---|---|---|
| EDTA blood | QC | 9/9 | 8/9 | 8/10 | 0.371 |
| EDTA blood | isoQC | 6/9 | 7/9 | 7/10 | 0.866 |
| EDTA blood | GAPDH | 9/9 | 9/9 | 10/10 | |
| GCF | QC | 4/10 | 1/10 | 0/10 | 0.044* |
| GCF | isoQC | 2/10 | 1/10 | 0/10 | 0.329 |
| GCF | GAPDH | 10/10 | 6/10 | 7/10 | 0.089 |
RA: rheumatoid arthritis; QC: human glutaminyl cyclase; isoQC: human iso glutaminyl cyclases
Fig. 4.
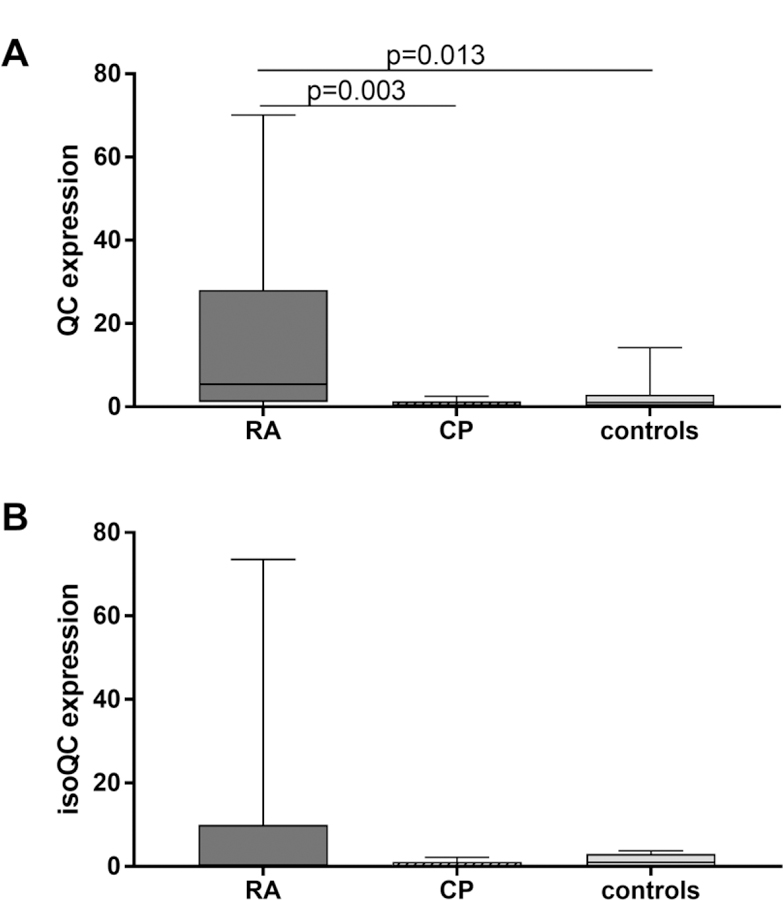
mRNA-expression of human glutaminyl cyclases (QC and isoQC) in EDTA blood obtained from patients with rheumatoid arthritis (RA, n=10), periodontitis (n=10) and healthy controls (controls, n=10) related to healthy controls
The mRNA expression was always related to those of the healthy controls. The median of the values related to GAPDH (a reference gene) of that group was set to 1.
Five of 30 analysed GCF samples were tested positively for QC expression, whereas isoQC expression was detectable only in three samples. Except for one sample all QC-positive samples were obtained from RA patients. he difference between groups was significant for QC (p=0.044). All GCF samples from RA patients but not all from CP and healthy controls were positive for the reference control gene GAPDH.
mRNA QC expression in blood correlated positively with those in GCF (R=0.418; p=0.024); on the other hand, analysing only RA patients the correlation factor was R=0.642 (p=0.045). isoQC was not related due to low number of positive readings in GCF.
3.6. Expression of P. gingivalis QC in subgingival biofilm
In eight of the 13 biofilm samples tested positively for P. gingivalis, PgQC expression was detected. Expression in these samples was in median 0.442, with a maximum of 38.13 related to sod expression as the housekeeping gene. Detection of the protein in GCF by Western blotting failed in any case.
DNA analysis for PgQC was made in the five samples being negatively tested for PgQC mRNA expression. No positive result was obtained.
4. Discussion
The focus in this study was on bacterial and two human glutaminyl cyclases in relation to periodontal disease and rheumatoid arthritis. Although not being the primary focus, it became obvious that study participants with RA had a lower number of teeth with less sites with a PD ≥5 mm than those with CP. The lower number of teeth is in accordance with a few other studies (Ishi Ede, Bertolo, Rossa, Kirkwood, & Onofre, 2008; Schmickler et al., 2017; Yamakawa et al., 2002). But contrary to us, high numbers of sites with pathologically increased PD were reported in RA (Schmickler et al., 2017). atients with RA in our study had been treated already with disease modifying antirheumatic drugs (DMARDs) for several years which may influence clinical periodondontal indices. E.g., RA treatment with tumor necrosis factor α-inhibitor had a beneficial effect on gingival inflammation and periodontal disease (Kobayashi, Yokoyama, et al., 2014). On the other hand, the general oral health awareness was high in these patients, most of them were enrolled in a regular supportive care program.
In this pilot study, the only report about glutaminyl cyclases in RA (Batliwalla et al., 2005) was confirmed: expression of QC was higher in EDTA blood of study participants with RA than in other groups. Also in GCF, mainly samples of RA patients were tested positively for the expression of QC or isoQC. GCF washes obtained by a non-invasive method were analyzed .Using periodontal tissue biopsies cell counts might be higher and would reveal more positive results with the possibility of quantification. Moreover, it might be of interest to develop a method allowing measurement of QC and isoQC protein level in GCF. mRNA expression of QC in the A blood correlated with QC expression in GCF, however the small sample size in general and those with quantified mRNA QC expression in both sets of samples excludes possibility of more elaborative analysis for an association between systemic and local inflammation. Nevertheless, such association can be assumed.
As QC and isoQC stabilize the levels of MCP-1 and MCP-3 in vivo (Chen et al., 2012; Cynis et al., 2011), the levels of these molecules were determined. In samples being tested positively for QC or isoQC, there was a tendency for higher levels of MCP-1 and MCP-3 than in the QC and isoQC negative samples. IL-1β levels were significantly higher (p=0.007) in the positive samples, with tendency visible even within the RA group. It is well-known that IL-1β is a product of stimulated monocytes or macrophages (Krakauer, 1986). Blocking its activity resolves non-infectious inflammation (Dinarello, 2011). In periodontitis, IL-1β is one of the most thoroughly investigated cytokines. Its expression is induced by bacteria associated with periodontal disease and then this cytokine drives secretion of inflammatory mediators and destructive enzymes (Preshaw & Taylor, 2011). Recently, it was reported that stimulation of endothelial cells by IL-1β increases expression of QC together with its substrates MCP-1 and CX3CL1 and the N-terminally modified pGlu-CX3CL1 induced a stronger inflammatory response than the immature glutamate-variant (Kehlen et al., 2017). If further research confirms a potential role of QC or isoQC in pathogenesis of periodontitis in patients with RA, topical blocking of QC and isoQC activity might be an interesting approach in the periodontal therapy. At the moment, the main focus is the development of QC inhibitors for treatment of Alzheimer’s disease (Hoffmann et al., 2017). Inhibitiors are based on the imidazole structure (Buchholz et al ., 2006) but also flavonoids (Li et al., 2016) or natural products of microalgae (Hielscher-Michael et al., 2016) represent potential candidates.
First bacterial QC has been described in Streptococcus bovis as being important in generation of energy (Cook & Russell, 1993). In this study, the focus was on P. gingivalis QC. P. gingivalis was detected in all patients with CP, but only in three patients with RA. This low prevalence is in contrast to other reports (Schmickler et al., 2017) including ours (Laugisch et al., 2016) It might be related to the small number of participants in this pilot study. Sampling of biofilm after GCF collection might have affected the bacterial counts in the biofilm samples, which is a limitation of our methodology. This precluded calculation of association between P. gingivalis QC expression and RA. Five of the 13 positive . gingivalis samples were tested negatively for QC, among them two (one CP, one RA) with counts ≥106 P .gingivalis subgingival with biofilm.Analysing a larger study population is needed to see if there is any association with periodontal disease activity.
Results using two P. gingivalis strains showed expression of QC, which seemed to be increased in the stationary growth phase. The enzyme was detectable in concentrated bacterial sediment but not in culture supernatant and GCF samples. This suggests that the enzyme is mostly located in the bacterial cell and not released into the environment. Very recently more detailed data about . gingivalis QC have been published (Bochtler et al., 2018). Using several fractions of cell extracts, QC activity and presence of QC (determined by Western blotting of fractions) was localized at the inner membrane only (Bochtler et al., 2018). Our FRIL electron microscopy photographs confirm a location of the enzyme in the periplasm.
Very little is known about the importance of the PgQC. Grouped location in periplasm suggest a role in transport or modification of molecules being transported via translocation in periplasm. E.g., gingipains are translocated from periplasm via outer membrane by type IX secretion system (T9SS), where a modification of the enzymes occurs (de Diego et al., 2016). The very recent report mentioned before (Bochtler et al., 2018) found N-terminal pGlu in 27 proteins, and it is speculated that PgQC pyroglutaminates N-terminal of majority of proteins secreted by P. gingivalis. All these proteins with N-terminal pGlu are putative substrates for signal peptidase I, an enzyme catalysing release of proteins from the membrane. However, only for RgpA but not for RgpB and Kgp a role for glutaminyl cyclization in T9SS could be assumed when using different P. gingivalis W83 mutants (Bochtler et al., 2018). The exact function of P. gingivalis QC should be a topic in further research.
In conclusion, human glutaminyl cyclases may play a role in maintaining inflammation and destruction of the periodontium in patients with RA. More investigation is needed to verify if human glutaminyl cyclases might be a target to retard progression of periodontal disease in these patients. As to PgQC, further research should specify the importance of the enzyme as a potential virulence factor in biofilm formation and modulating immune response. Moreover, P. gingivalis QC activity related to clinical parameters of the disease should be analysed in a larger study group. Here, the development of a more sensitive method for detection of the protein seems to be of importance. All these analyses together might help to answer the question if inhibition of P. gingivalis QC might be an alternative adjunctive therapy in the treatment of periodontal disease.
Highlights.
Porphyromonas gingivalis glutaminyl cyclase is expressed in vitro and in vivo.
Porphyromonas gingivalis glutaminyl cyclase is located in bacterial cell wall.
Human glutaminyl cyclases are expressed in periodontium of RA patients.
Expression of human glutaminyl cyclases is correlated with those of interleukin-1β.
Acknowledgements
We are grateful to Fabiola Costanzo, Anna Magdoń and Ekaterina Volkova (University of Bern, Department of Periodontology, Laboratory of Oral Microbiology) for technical assistance.
Funding:
This study was funded by a grant from the European Commission (FP7-HEALTH-F3–2012-306029 “TRIGGER”). JP was supported by a grant DE 022597 from NIH NIDCR.
Glossary
- CP
chronic periodontitis
- GCF
gingival crevicular fluid
- IL
interleukin
- isoQC
human glutaminyl cyclase
- Kgp
Porphyromonas gingivalis lysine specific cysteine protease
- MCP
monocyte chemoattractant protein
- pGlu
pyroglutamic acid
- PgQC
Porphyromonas gingivalis glutaminyl cyclase
- PSR
Periodontal Screening and Recording index
- QC
human glutaminyl cyclase
- RA:
rheumatoid arthritis
- RgpA and RgpB
Porphyromonas gingivalis arginine specific cysteine proteases A and B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, … Hawker G (2010). 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum, 62(9), 2569–2581. [DOI] [PubMed] [Google Scholar]
- Ang CS, Veith PD, Dashper SG, & Reynolds EC (2008). Application of 16O/18O reverse proteolytic labeling to determine the effect of biofilm culture on the cell envelope proteome of Porphyromonas gingivalis W50. Proteomics, 8(8), 1645–1660. [DOI] [PubMed] [Google Scholar]
- Armitage GC (1999). Development of a classification system for periodontal diseases and conditions. Ann Periodontol, 4(1), 1–6. [DOI] [PubMed] [Google Scholar]
- Ashimoto A, Chen C, Bakker I, & Slots J (1996). Polymerase chain reaction detection of 8 putative periodontal pathogens in subgingival plaque of gingivitis and advanced periodontitis lesions. Oral Microbiol Immunol, 11(4), 266–273. [DOI] [PubMed] [Google Scholar]
- Batliwalla FM, Baechler EC, Xiao X, Li W, Balasubramanian S, Khalili H, … Gregersen PK (2005). Peripheral blood gene expression profiling in rheumatoid arthritis. Genes Immun, 6(5), 388–397. [DOI] [PubMed] [Google Scholar]
- Bender P, Burgin WB, Sculean A, & Eick S (2016). Serum antibody levels against Porphyromonas gingivalis in patients with and without rheumatoid arthritis - a systematic review and meta-analysis. Clin Oral Investig [DOI] [PubMed]
- Biyikoglu B, Buduneli N, Aksu K, Nalbantsoy A, Lappin DF, Evrenosoglu E, & Kinane DF (2013). Periodontal therapy in chronic periodontitis lowers gingival crevicular fluid interleukin-1beta and DAS28 in rheumatoid arthritis patients. Rheumatol Int, 33(10), 2607–2616. [DOI] [PubMed] [Google Scholar]
- Bochtler M, Mizgalska D, Veillard F, Nowak ML, Houston J, Veith P, … Potempa J (2018). The Bacteroidetes Q-Rule: Pyroglutamate in Signal Peptidase I Substrates. Front Microbiol, 9, 230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz M, Heiser U, Schilling S, Niestroj AJ, Zunkel K, & Demuth HU (2006). The first potent inhibitors for human glutaminyl cyclase: synthesis and structure-activity relationship. J Med Chem, 49(2), 664–677. [DOI] [PubMed] [Google Scholar]
- Castro SA, Collighan R, Lambert PA, Dias IH, Chauhan P, Bland CE, … Devitt A (2017). Porphyromonas gingivalis gingi pains caused effective macrophage migration towards apoptotic cells and inhibit phagocytosis of primary apoptotic neutrophils. Cell Death Dis, 8(3), e2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Huang KF, Kuo WC, Lo YC, Lee YM, & Wang AH (2012). Inhibition of glutaminy l cyclase attenuates cell migration modulated by monocyte chemoattractant proteins. Biochem J, 442(2), 403–412. [DOI] [PubMed] [Google Scholar]
- Cook GM, & Russell JB (1993). The glutamine cyclotransferase reaction of Streptococcus bovis: a novel mechanism of deriving energy from non-oxidative and non-reductive deamination. FEMS Microbiol Lett, 111(2–3), 263–268. [DOI] [PubMed] [Google Scholar]
- Culshaw S, McInnes IB, & Liew FY (2011). What can the periodontal community learn from the pathophysiology of rheumatoid arthritis? J Clin Periodontol, 38 Suppl 11, 106–113. [DOI] [PubMed] [Google Scholar]
- Cynis H, Hoffmann T, Friedrich D, Kehlen A, Gans K, Kleinschmidt M, … Demuth HU (2011). The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions. EMBO Mol Med, 3(9), 545–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah E, & Andrade F (2018). Rheumatoid arthritis and citrullination. Curr Opin Rheumatol, 30(1), 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Diego I, Ksiazek M, Mizgalska D, Koneru L, Golik P, Szmigielski B, … Potempa J (2016). The outer-membrane export signal of Porphyromonas gingivalis type IX secretion system (T9SS) is a conserved C-terminal beta-sandwich domain. Sci Rep, 6, 23123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA (2011). A clinical perspective of IL-1beta as the gatekeeper of inflammation. Eur J Immunol, 41(5), 1203–1217. [DOI] [PubMed] [Google Scholar]
- Eick S, Straube A, Guentsch A, Pfister W, & Jentsch H (2011). Comparison of real-time polymerase chain reaction and DNA-strip technology in microbiological evaluation of periodontitis treatment. Diagn Microbiol Infect Dis, 69(1), 12–20. [DOI] [PubMed] [Google Scholar]
- Erciyas K, Sezer U, Ustun K, Pehlivan Y, Kisacik B, Senyurt SZ, … Onat AM (2013). Effects of periodontal therapy on disease activity and systemic inflammation in rheumatoid arthritis patients. Oral Dis, 19(4), 394–400. [DOI] [PubMed] [Google Scholar]
- Frohlich E, Kantyka T, Plaza K, Schmidt KH, Pfister W, Potempa J, & Eick S (2013). Benzamidine derivatives inhibit the virulence of Porphyromonas gingivalis. Mol Oral Microbiol, 28(3), 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Nguyen KA, & Potempa J (2010). Dichotomy of gingipains action as virulence factors: from cleaving substrates with the precision of a surgeon’s knife to a meat chopper-like brutal degradation of proteins. Periodontol 2000, 54(1), 15–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajishengallis G, Darveau RP, & Curtis MA (2012). The keystone-pathogen hypothesis. Nat Rev Microbiol, 10(10), 717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellvard A, Maresz K, Schilling S, Graubner S, Heiser U, Jonsson R, … Mydel P (2013). Glutaminyl cyclases as novel targets for the treatment of septic arthritis. J Infect Dis, 207(5), 768–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hielscher-Michael S, Griehl C, Buchholz M, Demuth HU, Arnold N, & Wessjohann LA (2016). Natural Products from Microalgae with Potential against Alzheimer’s Disease: Sulfolipids Are Potent Glutaminyl Cyclase Inhibitors. Mar Drugs, 14(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann T, Meyer A, Heiser U, Kurat S, Bohme L, Kleinschmidt M, … Schilling S (2017). Glutaminyl Cyclase Inhibitor PQ912 improves cognition in mouse models of Alzheimer’s disease - studies on relation to effective target occupancy. J Pharmacol Exp Ther [DOI] [PubMed]
- Ishi Ede P, Bertolo MB, Rossa C Jr., Kirkwood KL, & Onofre MA (2008). Periodontal condition in patients with rheumatoid arthritis. Braz Oral Res, 22(1), 72–77. [DOI] [PubMed] [Google Scholar]
- Joseph R, Rajappan S, Nath SG, & Paul BJ (2013). Association between chronic periodontitis and rheumatoid arthritis: a hospital-based case-control study. Rheumatol Int, 33(1), 103–109. [DOI] [PubMed] [Google Scholar]
- Kehlen A, Haegele M, Bohme L, Cynis H, Hoffmann T, & Demuth HU (2017). N-terminal pyroglutamate formation in CX3CL1 is essential for its full biologic activity. Biosci Rep, 37(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarstrom Engstrom K, Khalaf H, Kalvegren H, & Bengtsson T (2015). The role of Porphyromonas gingivalis gingipains in platelet activation and innate immune modulation. Mol Oral Microbiol, 30(1), 62–73. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Okada M, Ito S, Kobayashi D, Ishida K, Kojima A, … Yoshie, H. (2014). Assessment of interleukin-6 receptor inhibition therapy on periodontal condition in patients with rheumatoid arthritis and chronic periodontitis. J Periodontol, 85(1), 57–67. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Yokoyama T, Ito S, Kobayashi D, Yamagata A, Okada M, … Yoshie H (2014). Periodontal and Serum Protein Profiles in Patients With Rheumatoid Arthritis Treated With Tumor Necrosis Factor Inhibitor Adalimumab. J Periodontol, 1–12. [DOI] [PubMed]
- Krakauer T (1986). Human interleukin 1. Crit Rev Immunol, 6(3), 213–244. [PubMed] [Google Scholar]
- Laugisch O, Wong A, Sroka A, Kantyka T, Koziel J, Neuhaus K, … Eick S (2016). Citrullination in the periodontium--a possible link between periodontitis and rheumatoid arthritis. Clin Oral Investig, 20(4), 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Dong Y, Yu X, Zou Y, Zheng Y, Bu X, … Wu H (2016). Inhibitory effect of flavonoids on human glutaminyl cyclase. Bioorg Med Chem, 24(10), 2280–2286. [DOI] [PubMed] [Google Scholar]
- Lo Frisco C, Cutler R, & Bramson JB (1993). Periodontal screening and recording: perceptions and effects on practice. J Am Dent Assoc, 124(7), 226–229, 231–222. [DOI] [PubMed] [Google Scholar]
- Mewar D, & Wilson AG (2006). Autoantibodies in rheumatoid arthritis: a review. Biomed Pharmacother, 60(10), 648–655. [DOI] [PubMed] [Google Scholar]
- Mikuls TR, Payne JB, Reinhardt RA, Thiele GM, Maziarz E, Cannella AC, … O’Dell JR (2009). Antibody responses to Porphyromonas gingivalis (P. gingivalis) in subjects with rheumatoid arthritis and periodontitis. Int Immunopharmacol, 9(1), 38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesse W, Dijkstra PU, Abbas F, Spijkervet FK, Stijger A, Tromp JA, … Vissink A (2010). Increased prevalence of cardiovascular and autoimmune diseases in periodontitis patients: a cross-sectional study. J Periodontol, 81(11), 1622–1628. [DOI] [PubMed] [Google Scholar]
- Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, … Yoshie H (2011). Antibody responses to periodontopathic bacteria in relation to rheumatoid arthritis in Japanese adults. J Periodontol, 82(10), 1433–1441. [DOI] [PubMed] [Google Scholar]
- Pischon N, Pischon T, Kroger J, Gulmez E, Kleber BM, Bernimoulin JP,…Detert J (2008). Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol, 79(6), 979–986. [DOI] [PubMed] [Google Scholar]
- Preshaw PM, & Taylor JJ (2011). How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol, 38 Suppl 11, 60–84. [DOI] [PubMed] [Google Scholar]
- Schilling S, Wasternack C, & Demuth H . (2008). Glutaminyl cyclases from animals and plants: a case of functionally convergent protein evolution. Biol hem, 389(8), 983–991. [DOI] [PubMed] [Google Scholar]
- Schlormann W, Steiniger F, Richter W, Kaufmann R, Hause G, Lemke C, & Westermann M (2010). The shape of caveolae is omega-like after glutaraldehyde fixation and cup-like after cryofixation. Histochem Cell Biol, 133(2), 223–228. [DOI] [PubMed] [Google Scholar]
- Schmickler J, Rupprecht A, Patschan S, Patschan D, Muller GA, Haak R, … Ziebolz D (2017). Cross-Sectional Evaluation of Periodontal Status and Microbiologic and Rheumatoid Parameters in a Large Cohort of Patients With Rheumatoid Arthritis. J Periodontol, 88(4), 368–379. [DOI] [PubMed] [Google Scholar]
- Shen Y, Li Y, Ye F, Wang F, Lu W, & Xie X (2010). Identification of suitable reference genes for measurement of gene expression in human cervical tissues. Anal Biochem, 405(2), 224–229. [DOI] [PubMed] [Google Scholar]
- Torkzaban P, Hjiabadi T, Basiri Z,& Poorolajal J (2012). Effect of rheumatoid arthritis on periodontitis: a historical cohort study. J Periodontal Implant Sci, 42(3), 67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran SD, & Rudney JD (1999). Improved multiplex PCR using conserved and species-specific 16S rRNA gene primers for simultaneous detection of Actinobacillus actinomycetemcomitans, Bacteroides forsythus, and Porphyromonas gingivalis. J Clin Microbiol, 37(11), 3504–3508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegner N, Lundberg K, Kinloch A, Fisher B, Malmstrom V, Feldmann M, & Venables PJ (2010). Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev, 233(1), 34–54. [DOI] [PubMed] [Google Scholar]
- Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, … Venables PJ (2010). Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and alpha-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum, 62(9), 2662–2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa M, Ansai T, Kasai S, Ohmaru T, Takeuchi H, Kawaguchi T, & Takehara T (2002). Dentition status and temporomandibular joint disorders in patients with rheumatoid arthritis. Cranio, 20(3), 165–171. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okada Y, Suzuki A, & Kochi Y (2015). Genetics of rheumatoid arthritis in Asia--present and future. Nat Rev Rheumatol, 11(6), 375–379. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Dashper SG, Chen YY, Crawford S, Slakeski N, & Reynolds EC (2013). Porphyromonas gingivalis and Treponema denticola synergistic polymicrobial biofilm development. PLoS One, 8(8), e71727. [DOI] [PMC free article] [PubMed] [Google Scholar]


