Abstract
Objectives:
To prospectively assess rates and detailed predictors of morbidity and mortality among HIV-exposed uninfected children and HIV-unexposed children in Botswana in a more recent era.
Study design:
We enrolled HIV-infected and HIV-uninfected mothers and their children in the prospective observational Tshipidi study at 2 sites (1 city and 1 village) in Botswana from May 2010-July 2012. Live-born children and their mothers were followed for 24 months postpartum. Detailed sociodemographic data, health, and psychosocial characteristics were collected at baseline and prospectively, and health outcomes ascertained. Mothers chose infant feeding method with counselling.
Results:
893 live-born HIV-uninfected children (436 HEU, 457 HIV-unexposed) were followed. HIV-infected mothers had a median CD4 count of 410 cells/mm3, 32% took 3-drug antiretroviral treatment (ART) during pregnancy, 67% took only zidovudine, and 1% took <2 weeks of any antiretrovirals antepartum. Twenty-four-month vital status was available for 888 (99.4%) children. HEU children had a significantly higher risk of death compared with children of HIV- uninfected mothers (5.0% vs 1.8%) (aHR 3.27, 95% CI 1.44–7.40). High collinearity between maternal HIV status and child feeding method precluded analysis of these factors as independent predictors of mortality. Preterm birth, low birth weight, and congenital anomaly were also associated with mortality (in separate analyses), but maternal socioeconomic factors, depression, substance use, and social support were not significant predictors.
Conclusions:
The strongest predictors of 24-month mortality among children in Botswana were HIV exposure and formula feeding, although the relative contribution of these factors to child health could not be separated.
Global scale-up of services to prevent mother-to-child transmission (PMTCT) of HIV-1 has resulted in marked reduction of vertical transmission of HIV-1 from mothers to children. UNAIDS reported a 60% reduction in the number of children newly infected with HIV between 2009 and 2015 in 21 priority countries, with the highest burden in Sub-Saharan Africa [1, 2, 3]. It is expected that the number of new infections among children will further decrease globally as countries in this region continue to expand their PMTCT services. Globally, more than 1 million HIV exposed but uninfected (HEU) children were born in 2014 [4].
Evidence suggests that HIV exposure results in higher rates of morbidity and mortality in HEU children compared with HIV-unexposed (HU) children, even when they are breastfed for some period [5–7]. Several factors are thought to contribute to this increased vulnerability in HEU children, including altered child immunity, maternal HIV-related illness, higher rates of adverse pregnancy outcomes such as preterm delivery, shorter duration of breastfeeding, and psychosocial and socioeconomic stressors among HIV-infected women [8–14]. However, no published studies to date have systematically and concomitantly examined detailed biomedical and psychosocial predictors of mortality in HEU vs. HU children.
Over the last decade, access to 3-drug (combination) antiretroviral therapy (ART) has increased dramatically, including in pregnant women [15]. ART is expected to improve the health of HIV-positive women and potentially improve their quality of life and socioeconomic status. However, it is unknown whether higher morbidity and mortality previously observed in HEU children (compared with HU children) persists with improved maternal health brought about by access to ART.
We therefore compared rates of 2-year morbidity and mortality in HEU vs HU children in Botswana to assess outcomes in an era when maternal ART was available to women based on CD4 and clinical disease stage criteria. We also prospectively assessed detailed social and economic predictors of childhood morbidity and mortality, in an attempt to determine the most important current predictors of morbidity and mortality among HEU children under the age of two in this resource-constrained setting.
Methods
The Tshipidi study was a prospective observational study comparing health and neurodevelopmental outcomes among HEU vs. HU children from birth through 24 months of age. The study design and evaluation of neurodevelopmental outcomes have been presented previously [16].
Study participants included HIV-infected and HIV-uninfected mothers and their liveborn children, enrolled in Gaborone (city) and Mochudi (village) in Botswana between May 2010 and July 2012. Residents of the village (Mochudi) were less likely to have completed tertiary education, be employed, or have a monthly wage of >$100 per month compared with urban residents of Gaborone.
Twins were included; stillborn and HIV-infected infants and their mothers were excluded from this analysis. Mothers met the inclusion criteria of being ≥ 18 years of age, Botswana citizens, able/willing to provide informed consent, and were either pregnant or within 7 days postpartum at the time of enrollment. Maternal HIV status was first assessed with a rapid HIV test. A positive rapid HIV test was confirmed by a second positive HIV test result obtained by trained study staff at the clinic or reference laboratory by rapid HIV test, any licensed enzyme immunoassay (EIA) test kit, a Western blot, or detectable HIV-1 RNA. Plasma HIV-1 RNA was examined to provide final verification of discordant test results.
Study Procedures and Data Collection
Study visits occurred monthly during pregnancy; at delivery; and at 1, 6, 12, 18, and 24 months postpartum. Maternal and child medical care, including the provision of ART and antiretroviral prophylaxis, occurred at government clinics. Study staff provided urgent care and prompt referral as needed, including linkage to care for antiretroviral treatment. Participants also received support and counselling as appropriate from study staff; this included routine pre and post HIV test counselling, and infant feeding counselling. All counselling offered by staff occurred as private individual sessions in the study clinic and in accordance with Botswana policy. Maternal antiretroviral exposure during the current pregnancy was defined as receipt of at least 2 weeks of antiretroviral therapy prior to delivery. Mothers chose infant feeding method after counselling by government and study staff, following the local standard of care. HIV testing occurred at enrollment for all women and again at delivery for women who had tested HIV negative at antenatal enrollment. Women enrolled in the HIV-negative cohort had repeat HIV testing at the 24 months follow up visit.
Data on sociodemographic factors, health, child feeding method where “breastfeeding” represents any breastfeeding, “formula feeding” represents exclusive formula feeding without any breastfeeding and “never fed” represent infant neither breastfed or formula fed before dying. Psychosocial characteristics were collected shortly after enrollment and during follow-up from mothers using structured questionnaires. We administered previously-validated screening instruments to assess for food insecurity using the Household Food Insecurity Access Scale [17], maternal depression with the Beck Depression Inventory Fast Screen for Medical Patients [18], social support with the Duke-UNC Functional Social Support Questionnaire [19], and alcohol use with the Alcohol Use Disorders Identification Test and also asked about use of tobacco and illicit drugs [20]. All assessments were administered within 4 weeks of enrollment; the depression screen was also administered at 1, 12, and 24 months postpartum, and the other assessments were administered at 18 months postpartum.
Child health outcomes were ascertained through caregiver interview, physical assessment of the child, and review of the child’s written medical records. Child HIV status was ascertained by DNA PCR at birth, 4–6 weeks of age, and after the cessation of breastfeeding (for HIV exposed children); and HIV EIA at 18 months in all children, regardless of maternal HIV status. Positive DNA PCR tests were confirmed with a second DNA PCR and further confirmed by EIA at 18 months of age. HIV-infected children and those with unknown HIV status were excluded from this analysis.
Ethical Considerations
The Office of Human Research Administration at the T.H Chan Harvard School of Public Health and the Health Research Development Committee of the Botswana Ministry of Health provided IRB review and approval for the Tshipidi study (approval numbers 18093 and 00524 respectively). All enrolled women provided written informed consent prior to study participation.
Statistical analyses
Our primary, pre-specified outcome for this analysis was child mortality through 24 months of age, among liveborn HEU vs. HU children. We included as a secondary (composite) endpoint; the occurrence of either child hospitalization (for any reason) or death in these two groups of children. We also sought to evaluate (controlling for multiple potential confounders) biologic and social factors that may have been associated with early child morbidity and mortality, such as depression, food insecurity, and social support. Depression and social support scores were put into the models as continuous variables. Food insecurity was categorized as ‘none/mild’ vs. ‘moderate/severe’ insecurity.
Kaplan-Meier curves with a log rank test were used to assess the potential differences between HEU and HU children for both the primary and secondary endpoints. Twins were considered to be the same as singletons, as we did not account for clustering with multiple pregnancies in primary analyses but performed a sensitivity analysis excluding multiplets. Crude and adjusted risk factors for child mortality and morbidity were assessed using Cox proportional hazard models. Covariates were chosen for initial inclusion in the final multivariable model if p≤0.2 in the univariate analysis. Factors such as low birth weight and prematurity that were considered to be potential mediators between HIV exposure and child health outcome were excluded from the main adjusted models but included in subsequent sensitivity analyses. As HIV exposure and child feeding were nearly collinear (99% of HIV-unexposed children breastfed compared with 8% of HIV-exposed children), these factors could not be included simultaneously in adjusted models. Thus, we constructed different multivariable models that included HIV exposure and (separately) child feeding method. Children who had never been fed because they died prior to first feeding were excluded from all models containing child feeding method. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
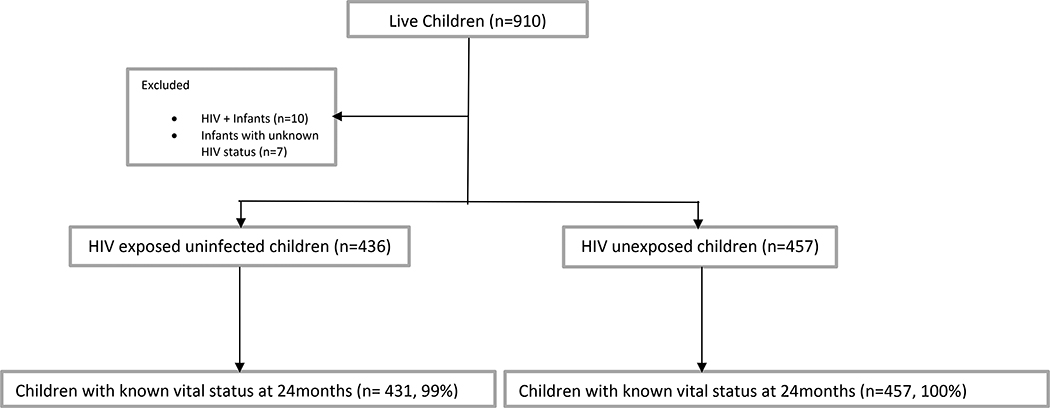
A total of 910 live-born children (453 HIV-exposed, 457 HIV-unexposed) were enrolled. (Figure 1). Seventeen live-born children who were born to HIV-infected mothers were excluded from the mortality analysis: 10 were excluded due to being HIV-infected and 7 were excluded as they had unknown HIV status. Thus, 893 children (436 HEU, 457 HIV-unexposed) and their 878 mothers (15 mothers had twins) were included in the analyses presented here. We had vital status information at 24 months for 888 (99.4%) of these 893 children.
Figure 1:
Flow diagram of participant follow-up
HIV-infected women were older at delivery than HIV-uninfected women (median 29 vs. 25 years, respectively), and had median CD4 count of 410 cells/mm3; 32% took 3-drug ART during pregnancy, 67% took zidovudine, and only 1% of women took fewer than 2 weeks of ARVs antepartum (Table I). HEU and HU children had similar median gestational age at delivery (39 weeks, interquartile range (IQR): 39–42).
Table 1:
Maternal and Child Demographic Characteristics by HIV Exposure Status of Children
| Maternal Demographic Characteristics | |||||
|---|---|---|---|---|---|
| HIV-exposure | |||||
| Total (n= 893*) | HIV-exposed/uninfected (n= 436) | HIV-unexposed (n= 457) | P-value | ||
| Mother’s enrollment cohort (antenatal vs. postnatal) | Antenatal enrollment | 785 (87.9%) | 380 (87.2%) | 405 (88.6%) | 0.50 |
| Post-natal enrollment (within 7 days of delivery) | 108 (12.1%) | 56 (12.8%) | 52 (11.4%) | ||
| Enrollment Site (rural vs urban) | Rural | 393 (44.0%) | 158 (36.2%) | 235 (51.4%) | <0.001 |
| Urban | 500 (56.0%) | 278 (63.8%) | 222 (48.6%) | ||
| Highest education level | None/primary | 90 (1.0%) | 68 (15.6%) | 22 (4.8%) | <0.001 |
| Secondary | 686 (76.8%) | 336 (77.1%) | 350 (76.6%) | ||
| University | 113 (12.7%) | 29 (6.7%) | 84 (18.4%) | ||
| Personal earnings (USD/month) | None | 551 (61.7%) | 233 (53.4%) | 318 (69.6%) | <0.001 |
| <=$50 | 45 (5.0%) | 33 (7.6%) | 12 (2.6%) | ||
| $50 - $100 | 131 (14.7%) | 91 (20.9%) | 40 (8.6%) | ||
| >$100 | 162 (18.1%) | 75 (17.2%) | 87 (19.0%) | ||
| Occupation | Employed | 304 (34.0%) | 177 (40.6%) | 127 (27.8%) | <0.001 |
| Housewife or unemployed | 538 (60.2%) | 248 (56.9%) | 290 (63.5%) | ||
| Student | 48 (5.4%) | 8 (1.8%) | 40 (8.8%) | ||
| Drinking water source | Piped into home | 197 (22.1%) | 82 (18.8%) | 115 (25.2%) | <0.001 |
| Tap in yard | 592 (66.3%) | 282 (64.7%) | 310 (67.8%) | ||
| Communal standpipe | 97(10.9%) | 67(15.4%) | 30(6.6%) | ||
| Borehole, well or other source | 7(0.8%) | 5(1.1%) | 2(0.4%) | ||
| Electricity in the home | Yes | 520 (58.2%) | 217 (49.8%) | 303 (66.3%) | <0.001 |
| No | 370 (41.4%) | 216 (49.5%) | 154 (33.7%) | ||
| Food insecurity by category | None/mild | 521 (58.4%) | 208 (47.7%) | 313 (68.5%) | <0.001 |
| Moderate/severe | 370 (41.4%) | 227 (52.1%) | 143 (31.3%) | ||
| Data not available | 2 (0.2%) | 1 (0.2%) | 1 (0.2%) | ||
| Maternal alcohol use during pregnancy | No | 828 (92.7%) | 400 (91.7%) | 428 (93.7%) | 0.29 |
| Yes | 59 (6.6%) | 34 (7.8%) | 25 (5.5%) | ||
| Unknown | 6 (0.7%) | 2 (0.5%) | 4 (0.8%) | ||
| Mother’s age at randomization (years) | N | 893 | 436 | 457 | <.0001 |
| Min, Max | 18, 46 | 18, 43 | 18, 46 | ||
| Median (IQR) | 26 (20–36) | 28 (21–37) | 24 (19–34) | ||
| Worst (highest) maternal depression score+ | N | 889 | 434 | 455 | <.0001 |
| Min, Max | 0, 20 | 0, 16 | 0, 20 | ||
| Median (IQR) | 2 (0–6) | 2(0–6) | 1(0–5) | ||
| Worst (lowest) maternal social support score# | N | 782 | 381 | 401 | 0.39 |
| Min, Max | 3, 40 | 3, 40 | 10, 40 | ||
| Median (IQR) | 34 (22–40) | 34 (22–40) | 34 (23–40) | ||
| Maternal baseline CD4 >250 | Yes | 375 (86.0%) | |||
| No | 61 (14.0%) | ||||
| Maternal PMTCT regimen | 3-drug ART | 138 (31.7%) | |||
| Zidovudine | 294 (67.4%) | ||||
| No (or <2 weeks) antiretrovirals | 4 (0.9%) | ||||
| Child Characteristics by HIV-exposure Status | |||||
| Sex | Male | 443 (49.6%) | 218 (50.0%) | 225 (49.2%) | 0.82 |
| Female | 450 (50.4%) | 218 (50.0%) | 232 (50.8%) | ||
| Birth weight by category | Very low birth weight (<= 1.5 kg) | 5 (0.6%) | 2 (0.5%) | 3 (0.7%) | <0.001 |
| Low birth weight (1.5–2.5 kg) | 113 (12.7%) | 75 (17.2%) | 38 (8.3%) | ||
| Normal (> 2.5 kg) | 756 (84.6%) | 352 (80.7%) | 404 (88.4%) | ||
| Unknown | 19 (2.1%) | 7 (1.6%) | 12 (2.6%) | ||
| Infant premature (<37 weeks)? | No | 769 (86.1%) | 369 (84.6%) | 400 (87.5%) | 0.21 |
| Yes | 124 (13.9%) | 67 (15.4%) | 57 (12.5%) | ||
| Birth weight (kg) | N | 874 | 429 | 445 | 0.0002 |
| Min, Max | 0.8, 8.2 | 1,5.7 | 0.8, 8.2 | ||
| Median (IQR) | 3.1 (2.4–3.8) | 3 (2.4–3.8) | 3.2 (2.4–3.8) | ||
| Child Feeding | Breastfed | 486 (54%) | 35 (8%) | 451 (99%) | <0.001 |
| Formula Fed only | 401 (45%) | 396 (91%) | 5 (1%) | ||
| Never fed | 6 (<1%) | 5 (1%) | 1 (<1%) | ||
| Duration of breastfeeding, if breastfed (days) | N | 435 | 31 | 404 | <0.0001 |
| Min, Max | 1,934 | 1,243 | 2, 934 | ||
| Median (IQR) | 366 (190–475) | 181 (44–184) | 372 (217–488) | ||
Table 1 presents demographics by Child HIV exposure status and not maternal HIV status.
A total of 893 children who were delivered by 878 mothers were included in the mortality analysis (16 mothers had twins with one mother had just one of her twins enrolled in the study).
higher score indicates worse depression
higher score indicates better social support.
Of the 893 liveborn children included, 30 (3.4%) died by 24 months: 22 (5.0%) HEU and 8 (1.8%) HU. The proportion of children who died through 24 months of follow up by feeding and HIV exposure status are presented in Table 2.
Table 2:
24-Month Mortality, by HIV Exposure Status and Child Feeding Strategy
| HEU | HU | |||
|---|---|---|---|---|
| Feeding Strategy | # HEU Children | # Died (%) | # HU Children | # Died (%) |
| Breastfed | 35 | 1 (2.9%) | 451 | 5 (1.1%) |
| Formula fed | 396 | 16 (4%) | 5 | 2 (40.0%) |
| Never fed | 5 | 5(100%) | 1 | 1 (100%) |
| Total | 436 | 22 (5.0%) | 457 | 8 (1.8%) |
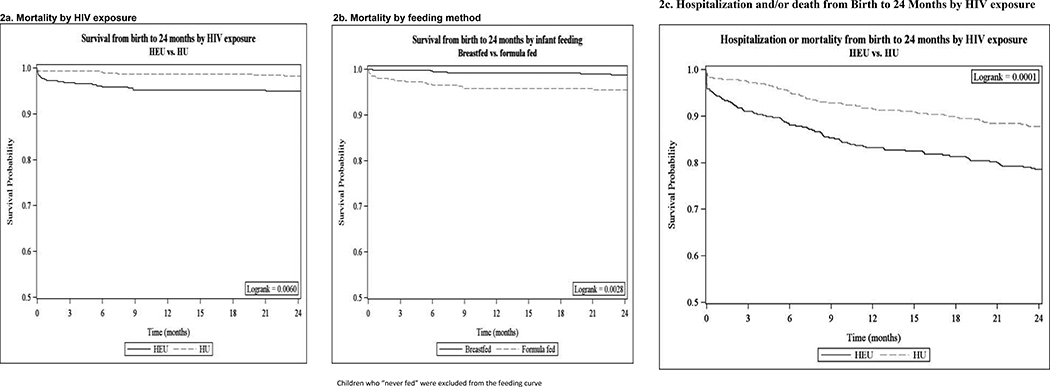
In univariate analyses, HEU children were 3 times more likely to die by 24 months compared with HU children (HR 2.95, 95% CI 1.31–6.62). This increased from 2.9 to 3.6 in sensitivity analyses that included only singletons (HR 3.6, 95% CI 1.46–9.07). Exclusively formula-fed children had higher rates of mortality than breastfed children (4.5% vs 1.2%, HR 3.71 95% CI 1.64–8.40). Mortality among children was highest in the first month of life: 15 (50.0%) deaths occurred within the first month of life (54.6% of deaths in HEU and 37.5% of deaths in HU). Mortality with HIV exposure was higher for deaths occurring during the neonatal period (HR 4.2) vs. deaths occurring after the neonatal period (HR 2.2). In addition, both neonatal deaths and later deaths in babies were associated with being born preterm. The Kaplan Meier curves of time to mortality by HIV exposure group and feeding method are shown in Figure 2 (available at www.jpeds.com).
Figure 2:
Time to Mortality (or Morbidity/Mortality) From Birth to 24 Months, by HIV Exposure Status and Feeding Method
On univariate analysis, predictors of 24-month mortality overall (among HEU and HU children combined) included: being bom to a mother with HIV (as noted above), living in a rural setting (HR 1.68, 95% CI 0.81 −3.46), formula feeding (HR 3.72, 95% CI 1.48–9.38) or preterm birth (HR 3.26, 95% CI 1.53–6.97). Children born with low (≤ 2.5kg) birth weight also had increased risk of dying (HR 3.71, 95% CI 1.64–8.40). These numbers should however be interpreted with caution due to low event rates (Table 3). Maternal depression or alcohol use during pregnancy, lower socioeconomic status, food insecurity, and lower social support were not associated with higher risk of child mortality. When the univariate analysis was restricted to HEUs, lower socioeconomic status (indicated by households with no primary cooking method or those using firewood as their primary cooking source) had a borderline association with higher 24-month infant mortality (HR 1.84, CI 0.77–4.38).
Table 3:
Univariate and Multivariate Predictors of Child Mortality Through 24 Months, Among all Children (and Among HEU Children Only)
| Predictors | All Children (HEU + HU) | HEU Children Only (n= 431) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate including HIV exposure but not child feeding (n=893) | Multivariate including child feeding but not HIV exposure (n=887) | Univariate | Multivariate | |||||||||||
| HR | 95% CI | P | aHR | 95% CI | P | aHR | 95% CI | P | HR | 95% CI | P | aHR | 95% CI | P | |
| HIV-exposure (HIV-unexposed = referent) | 2.95 | 1.31–6.62 | 0.009 | 3.27 | 1.44–7.40 | 0.005 | - | - | - | ||||||
| Formula feeding (Breastfeeding = referent) | 3.72 | 1.48–9.38 | 0.005 | - | 3.83 | 1.52–9.70 | 0.005 | 1.40 | 0.19–10.58 | 0.74 | 1.22 | 0.16–9.45 | 0.85 | ||
| Gender (Female = referent) | 1.02 | 0.50–2.09 | 0.95 | - | - | 1.21 | 0.52–2.80 | 0.65 | - | ||||||
| Low birth weight (⩽2.5kg) * | 3.71 | 1.64–8.40 | 0.002 | 2.51 | 1.03–6.08 | 0.04 | 2.78 | 1.13–6.86 | 0.03 | 2.30 | 0.86–6.14 | 0.10 | 2.52 | 0.97–7.36 | 0.09 |
| Prematurity (<37 weeks) * | 3.20 | 1.50–6.84 | 0.003 | 1.55 | 0.59–4.02 | 0.37 | 1.79 | 0.68–4.70 | 0.24 | 2.69 | 1.10–6.60 | 0.03 | 1.17 | 0.34–4.03 | 0.81 |
| Presence of Congenital anomaly* | 7.10 | 2.15–23.48 | 0.001 | 7.58 | 2.16–26.59 | 0.002 | 4.98 | 1.11–22.34 | 0.04 | 9.98 | 2.94–33.89 | <0.01 | 8.46 | 1.76–40.60 | <0.01 |
| Postnatal enrollment cohort (Antenatal = referent) | 0.52 | 0.12–2.17 | 0.37 | - | - | 0.68 | 0.16–2.90 | 0.60 | - | ||||||
| Rural clinic site (Urban = referent) | 1.68 | 0.81–3.46 | 0.16 | 1.93 | 0.93–4.01 | 0.08 | 1.34 | 0.59–3.05 | 0.39 | 1.48 | 0.64–3.43 | 0.36 | - | ||
| Maternal age | 1.00 | 0.95–1.07 | 0.89 | - | - | 0.98 | 0.91–1.05 | 0.52 | - | ||||||
| None/Primary education level (tertiary = referent) | 1.25 | 0.25–6.19 | 0.79 | - | - | 0.61 | 0.10–3.67 | 0.59 | - | ||||||
| no/< $50 Income / month (> $100 = referent) | 0.81 | 0.32–2.04 | 0.65 | - | - | 0.66 | 0.23–1.87 | 0.43 | - | ||||||
| Use of drinking water source (piped into home = referent) | 1.42 | 0.54–3.72 | 0.47 | - | - | 1.04 | 0.35–3.06 | 0.95 | - | ||||||
| None/Use of fire as primary cooking method (stove = referent) | 1.17 | 0.52–2.64 | 0.70 | - | - | 1.84 | 0.8–4.4 | 0.17 | 1.4 | 0.5–4.1 | 0.52 | ||||
| No electricity in the home | 1.07 | 0.52–2.20 | 0.86 | - | - | 1.2 | 0.5–2.8 | 0.68 | - | ||||||
| No refrigerator in the home | 0.76 | 0.37–1.58 | 0.47 | - | - | 0.8 | 0.3–1.9 | 0.61 | - | ||||||
| Other types of toilet facilities (Indoor toilet =referent) | 1.59 | 0.61–4.16 | 0.34 | - | - | 1.1 | 0.4–3.2 | 0.88 | - | ||||||
| Food insecurity (None/Mild = referent) | 0.70 | 0.33–1.50 | 0.36 | - | - | 0.8 | 0.3–1.8 | 0.52 | - | ||||||
| Maternal depression (No depression = referent) | 0.99 | 0.87–1.14 | 0.92 | - | - | 0.94 | 0.79–1.12 | 0.48 | - | ||||||
| Maternal social support (Presence of social support = referent) | 0.97 | 0.92–1.03 | 0.36 | - | - | 1.00 | 0.93–1.07 | 0.98 | - | ||||||
| Alcohol use during pregnancy (No = referent) | 1.01 | 0.24–4.23 | 0.10 | - | - | 0.55 | 0.07–4.09 | 0.56 | - | ||||||
| Low baseline CD4 count (CD4> 250 = referent) | - | - | - | 0.67 | 0.99–1.00 | 0.58 | - | ||||||||
| None or Zidovudine use only for PMTCT (HAART = referent) | - | - | - | 1.60 | 0.59–4.34 | 0.36 | - | ||||||||
Notes
Adjusted results for causal pathway parameters are from sensitivity models
Excluded from multivariate analysis as only 2 deaths occurred in mothers
HIV exposure and feeding method were collinear in the study (99% of HIV-unexposed children breastfed compared with 8% of HIV-exposed children), and these factors could therefore not be simultaneously included in multivariable models. Instead, two different multivariable models, one with HIV exposure and the other with child feeding, were ran (Table 3). Multivariable analysis of all significant predictors -- excluding covariates on the causal pathway between maternal HIV infection and infant death (i.e., potential mediators such as preterm birth, low birth weight, presence of congenital anomalies) - showed that HIV exposure (aHR 3.27, 95% CI 1.44–7.40), formula feeding (aHR 3.83, 95% CI 1.52–9.70), and rural dwelling (aHR 1.48, 95% CI 0.64–3.43), were independently associated with increased risk for mortality. In primary multivariable analysis among only HEU children, no predictor remained significant.
In secondary multivariable models that included predictors potentially on the causal pathway, both low birth weight and presence of a congenital anomaly remained associated with 24-month mortality among children overall, as well as in HEU children only. Other findings did not differ substantially from the analyses that excluded these potential mediators.
In the secondary analysis of hospitalization and/or mortality in all children by HIV exposure, HEU children had shorter times to first hospitalization or death compared with HU children (log rank p < .0001) as seen in Figure 2. Gastroenteritis, pneumonia and other respiratory illness were the leading cause of hospitalization and death observed (Table 4; available at www.jpeds.com).
Table 4:
Reason for hospitalization and cause of death
| Reason for Hospitalization by HIV exposure | |||
| hivexposure | Total (N=141) | ||
| HIV-exposed (N=89) | HIV-unexposed (N=52) | ||
| Birth asphyxia | 5(5.6%) | 0(0.0%) | 5(3.5%) |
| Congenital anomaly | 3(3.4%) | 2(3.8%) | 5(3.5%) |
| Gastroenteritis | 36(40.4%) | 20(38.5%) | 56(39.7%) |
| Others | 4(4.5%) | 6(11.5%) | 10(7.1%) |
| Pneumonia | 9(10.1%) | 7(13.5%) | 16(11.3%) |
| Complications of Prematurity | 2(2.2%) | 1(1.9%) | 3(2.1%) |
| Pyrexia of unknown origin | 1(1.1%) | 1(1.9%) | 2(1.4%) |
| Respiratory distress syndrome | 2(2.2%) | 0(0.0%) | 2(1.4%) |
| Other respiratory tract infection | 16(18.0%) | 7(13.5%) | 23(16.3%) |
| Sepsis | 8(9.0%) | 4(7.7%) | 12(8.5%) |
| Trauma/poisoning | 3(3.4%) | 4(7.7%) | 7(5.0%) |
| Primary causes of death by HIV exposure status | |||
| hivexposure | Total (N=30) | ||
| HIV-exposed (N=22) | HIV-unexposed (N=8) | ||
| Pneumonia | 6 (27%) | 0 | 6 (20%) |
| Birth Asphyxia | 4 (18%) | 1 (13%) | 5 (17%) |
| Gastroenteritis | 3 (14%) | 2 (25%) | 5 (17%) |
| Complications of Prematurity | 2 (9%) | 2 (25%) | 4 (13%) |
| Sepsis | 3 (14%) | 0 | 3 (10%) |
| Pneumocystis jirovecii pneumonia | 1 (5%) | 0 | 1 (3%) |
| Pyrexia of unknown origin | 0 | 1 (13%) | 1 (3%) |
| Pneumonitis | 1 (5%) | 0 | 1 (3%) |
| Respiratory distress syndrome | 1 (5%) | 0 | 1 (3%) |
| Trauma | 0 | 1 (13%) | 1 (3%) |
| Unknown | 1 (5%) | 1 (13%) | 2 (7%) |
HIV exposure (aHR 1.74, 95% CI 1.23–2.47), formula feeding (aHR 1.65, 95% CI 1.17–2.33), no formal or primary maternal education (aHR 1.38, 95% CI 0.85–2.23), or being a male child (aHR 1.34, CI 0.97–1.85) were each associated with risk of hospitalization and/or death through 24 months in multivariable models constructed in a similar manner as for the mortality-only endpoint. As highlighted above HIV exposure and feeding were collinear in the study, hence both factors were not simultaneously included in multivariable models. When analysis was limited to only HEU children, use of fire as the primary cooking method as compared with stove (aHR 1.46, 95% CI 0.92–2.33) was the only predictor of hospitalization and/or death through 24 months.
Discussion
We found that HIV exposure and formula feeding remain the strongest risk factors for mortality by 24 months of age in Botswana, even after controlling for multiple potential socioeconomic and psychosocial predictors of adverse child outcomes. Despite a healthier population of HIV-infected mothers whose enhanced access to treatment in the modern era has resulted in higher median CD4 counts, HIV-uninfected children who were exposed in utero to HIV were three times more likely than their unexposed peers to die by 2 years after birth. These findings are broadly consistent with prior studies, which have documented 2 to 6-fold higher mortality among HEU children [6, 7, 22–24], and further highlight the need to better understand and mitigate the mechanisms underlying this risk. Formula-fed children were nearly 4 times more likely to die by 24 months than breastfed children; however, the collinearity of HIV status and feeding method unfortunately prevented us from determining the independent contributions of HIV exposure and feeding method to child outcomes. Mortality was also significantly greater among children born preterm or with low birth weight and children born with a congenital anomaly. Finally, half of all observed mortality occurred within the first month of life, which thus represents a particularly vulnerable period that may lend itself to further intervention aimed at improving child health outcomes.
Unique to this study was the richness of the sociodemographic and maternal psychosocial data that we collected. In prior studies, it has not been possible to exclude the role of these potential confounders in the association between HIV exposure and higher HEU mortality. In addition to financial status and maternal education, we examined a range of previously unmeasured factors such as household food insecurity, social support, maternal depression, and alcohol/substance use. We did not find any of these factors to be associated with increased risk for morbidity or mortality. This is an important negative result and suggests that the excess morbidity and mortality observed in HEU children are most likely driven by biologic and immunologic factors related to HIV exposure and replacement feeding. In addition, our study was conducted in the context of a healthier population of HIV-infected pregnant women, with higher ART coverage during pregnancy; nonetheless, the excess mortality among HEU children persisted in this population and was likely exacerbated by formula feeding.
Overall, our findings further highlight the heightened vulnerability of children exposed to HIV. Morbidity and mortality trends observed among HEU children in this and prior studies [25–27] are of public health significance and warrant global attention. To preserve the gains of successful PMTCT programs, it is imperative to recognize HEU infants as a vulnerable group and provide prompt and appropriate medical care and support aimed at reducing their risk of severe illness and mortality. Furthermore, although we cannot disentangle the effects of HIV exposure vs. formula feeding on child morbidity and mortality, both are probably contributing. Considering both the documented beneficial effects of breastfeeding [25, 28–30], the risks of replacement feeding [29, 31], the low risk of HIV MTCT from mothers who are taking 3-drug ART [32,33], and revised feeding guidelines that promote breastfeeding in the region [34,35], it is important for HEU children to breastfeed in regions with substantial child morbidity and mortality, provided their mothers are able to maintain appropriate virologic suppression while receiving antiretroviral therapy.
Our study has limitations. Most HEU children in the study exclusively formula fed and nearly all HIV-unexposed children breastfed, which made separating the effects of HIV exposure from feeding method impossible. Furthermore, information on maternal HIV-1 viral load was not systematically available.
Acknowledgments
The Tshipidi study was supported by funding from the National Institute of Mental Health (RO1MH087344) and by Oak Foundation (OUSA-12–025). G.A., J.L., G.M., E.v.W., M.M., B.K., and S.L. received salary support from the National Institute of Health through funding of the project. R.S. received salary support from the OAK foundation. S.M. received support from the Fogarty International Center and National Institute of Mental Health, of the National Institutes of Health under Award Number D43 TW010543. The content of this manuscript is solely the responsibility of the authors and does not represent the official views of any of the sponsors.
Footnotes
Data statement
Data will be made available on request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS | 2016. Progress report on the Global Plan: On the fast-track to an AIDS-Free Generation. http://www.unaids.org/sites/default/files/media asset/GlobalPlan2016en.pdf
- 2.Idele P, Hayashi C, Porth T, Mamahit A, Mahy M (2017). Prevention of Mother-to-Child Transmission of HIV and Pediatric HIV Care and Treatment Monitoring: From Measuring Process to Impact and Elimination of Mother-to-Child Transmission of HIV. AIDS Behav (2017)21: S23–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Evans C, Jones CE, Prendergast AJ (2016). HIV-exposed, uninfected children: new global challenges in the era of pediatric HIV elimination. Lancet Infect Dis 2016; 16: e92–107 [DOI] [PubMed] [Google Scholar]
- 4.Le Roux SM, Abrams EJ, Myer L (2016). Rethinking the HIV-exposed, uninfected child: epidemiologic perspectives. Future Microbiol. 10.2217/fmb-2016-0055 [DOI] [PubMed] [Google Scholar]
- 5.Naniche D, Bardji A, Lahuerta M, Berenguera A, Mandomando I, Sanz S, et al. (2009) Impact of maternal human immunodeficiency virus infection on birth outcomes and infant survival in rural Mozambique. Am J Trop Med Hyg 80:870–876. [PubMed] [Google Scholar]
- 6.Kurewa ΕΝ, Gumbo FZ, Munjoma MW, Mapingure MP, Chirenje MZ, Rusakaniko S, et al. (2010) Effect of maternal HIV status on infant mortality: evidence from a 9-month follow-up of mothers and their children in Zimbabwe. Journal of Perinatology 30:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shapiro RL, Lockman S, Kim S, Smeaton L, Rahkola JT, Thior I, et al. (2007). Infant Morbidity, Mortality, and Breast Milk Immunologic Profiles among Breast-Feeding HIV- Infected and HIV-Uninfected Women in Botswana. JID 2007:196: 562–569 [DOI] [PubMed] [Google Scholar]
- 8.Boerma RS, Wit F, Orock SO, Schonenberg-Meinema D, Hartdorff CM, Bakia A, et al. (2015). Mortality risk factors among HIV-exposed children in rural and urban Cameroon. Journal of Tropical Medicine and International Health 2015: Volume 20 No 2 pp 170–176 [DOI] [PubMed] [Google Scholar]
- 9.Sugandhi N, Rodrigues J, Kim M, Ahmed S, Amzel A, Tolle M, et al. (2013). HIV- exposed children: rethinking care for a lifelong condition. AIDS 2013, Vol 27: 5187–5195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuhn L, Kasonde P, Sinkala M, Kankasa C, Semrau K, Scott N, et al. (2005). Does Severity of HIV Disease in HIV-Infected Mothers Affect Mortality and Morbidity among their uninfected Children? Clinical Infectious Diseases 2005; 41:1654–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goetghebuer T, Rowland-Jones SL, Kollmann TR (2017). Editorial: Immune Mechanisms Underlying the Increased Morbidity and Mortality of HIV-Exposed Uninfected (HEU) Children. Front. Immunol. 8:1060. doi: 10.3389/fimmu.2017.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Slogrove AL, Archary M, Cotton MF (2016). Optimizing Research Methods to Understand HIV-Exposed Uninfected Infant and Child Morbidity: Report of the Second HEU Infant and Child Workshop. Front. Immunol. 7:576. doi: 10.3389/fimmu.2016.00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Filteau S (2009). The HIV-exposed, uninfected African child. Tropical Medicine and International Health; Vol 14: No 3 pp 276–287 [DOI] [PubMed] [Google Scholar]
- 14.Nicholson L, Chisenga M, Siame J, Kasonka L, Filteau S (2015). Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatrics (2015) 15:66; doi:10.1186/s12887-015-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.UNAIDS GLOBAL AIDS UPDATE | 2016. http://www.unaids.org/sites/default/files/media asset/global-AIDS-update-2016 en.pdf
- 16.Chaudhury S, Williams PL, Mayondi GK, Leidner J, Holding P, Tepper V, et al. Neurodevelopment of HIV-Exposed and HIV-Unexposed Uninfected Children at 24 Months. Paediatrics. 2017. October; 140(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jennifer CJ, Swindale A, and Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for Measurement of Household Food Access: Indicator Guide (v. 3) Washington, D.C: Food and Nutrition Technical Assistance Project, Academy for Educational Development; 2007. [Google Scholar]
- 18.Beck AT, Steer RA, Brown GK. BDI: Fast Screen for medical patient’s manual. San Antonio (TX): The Psychological Corporation; 2000. [Google Scholar]
- 19.Broadhead WE, Gehlbach SH, de Gruy FV, Kaplan BH. The Duke-UNC Functional Social Support Questionnaire. Measurement of social support in family medicine patients. Med Care. 1988;26(7): 709–23 [DOI] [PubMed] [Google Scholar]
- 20.Babor TF., Biddle-Higgins JC, Saunders JB. and Monteiro MG. (2001). AUDIT: The Alcohol Use Disorders Identification Test: Guidelines for Use in Primary Health Care. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 21.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F, et al. (2004). Mortality of infected and uninfected children born to HIV-infected mothers in Africa: a pooled analysis. Lancet 2004: 364(9441): 1236–1243 [DOI] [PubMed] [Google Scholar]
- 22.Marinda E, Humphrey JH, Illiff PJ, Mutasa K, Nathoo KJ, Piwoz EG, et al. (2007). Child mortality according to maternal and infant HIV status in Zimbabwe. Pediatr Infect Dis J. 2007; 26(6): 529–526 [DOI] [PubMed] [Google Scholar]
- 23.Hong R, Banta JE, Kamau JK (2007). Effect of maternal HIV infection on child survival in Ghana. J Community Health. 2007; 32(1): 21–36 [DOI] [PubMed] [Google Scholar]
- 24.Brahmbhatt H, Kigozi G, Wabwire-Mangen F, Serwadda D, Lutalo T, Nalugoda F, et al. (2006). Mortality in HIV-infected and uninfected children of HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2006;41(4):504–508 [DOI] [PubMed] [Google Scholar]
- 25.Thior I, Lockman S, Smeaton L, Shapiro RL, Wester C, Heymann SJ, et al. (2006). Breastfeeding plus infant zidovudine prophylaxis for 6 months’ vs formula feeding plus infant zidovudine for 1 month to reduce mother-to-child HIV transmission in Botswana: a randomized trial: the Mashi study. JAMA, 2006; 296: 794–805 [DOI] [PubMed] [Google Scholar]
- 26.El Arifeen S, Akhter T, Chowdhury HR, Rahman KM, Chowdhury EK, Alam N, et al. (Undated). CAUSES OF DEATH IN CHILDREN UNDER FIVE YEARS OF AGE. http://dhsprogram.com/pubs/pdf/FR165/09Chapter09.pdf
- 27.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. (2012). Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. The Lancet 2012; Volume 379, No. 9832, p2151–2161, 9 June 2012 [DOI] [PubMed] [Google Scholar]
- 28.Kuhn L, Aldrovandi G (2010). Survival and Health Benefits of Breastfeeding Versus Artificial Feeding in Children of HIV-Infected Women: Developing Versus Developed World. Clin Perinatol. 2010 December; 37(4): 843–x. doi: 10.1016/jclp.2010.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taha TE, Hoover DR, Chen S, Kumwenda NI, Mipando L, Nkanaunena K, et al. (2011). Effects of Cessation of Breastfeeding in HIV-1-Exposed, Uninfected Children in Malawi. Clinical Infectious Diseases 2011;53(4):388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young SL, Mbuya MN, Chantry CJ, Geubbels EP, Israel-Ballard K, Cohan D, et al. (2011). Current Knowledge and Future Research on Infant Feeding in the Context of HIV: Basic, Clinical, Behavioral, and Programmatic Perspectives. Adv. Nutr. 2: 225–243, 2011; doi: 10.3945/an.110.000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvarez-Uria G, Midde M, Pakam R, Bachu L, Naik PK.(2012). Effect of Formula Feeding and Breastfeeding on Child Growth, Infant Mortality, and HIV Transmission in Children Born to HIV-Infected Pregnant Women Who Received Triple Antiretroviral Therapy in a Resource-Limited Setting: Data from an HIV Cohort Study in India. ISRN Pediatrics; Vol 2012, 763591, doi: 10.5402/2012/763591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paredes R, Marconi VC, Lockman S, Abrams EJ, Kuhn L (2013). Impact of Antiretroviral Drugs in Pregnant Women and Their Children in Africa: HIV Resistance and Treatment Outcomes. The Journal of Infectious Diseases 2013;207(S2): S93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.UNICEF | 2013. A Case for Options B and B+. https://www.unicef.org/aids/files/CaseforOptionBandBPlus.pdf
- 34.WHO | 2016. Guidelines: Updates on HIV and Infant Feeding. http://apps.who.int/iris/bitstream/10665/246260/1/9789241549707-eng.pdf
- 35.Ministry of Health, Botswana (2016). Guidelines: Handbook of the Botswana 2016 Integrated HIV Clinical Care guidelines.