Abstract
Rats offered 30% sucrose solution plus chow or a sucrose-free diet develop leptin resistance within 4 weeks. This experiment tested whether leptin resistance was associated with the reward of sweet taste or the pre- or post-absorptive effects of consumption of simple carbohydrate. Male Sprague Dawley rats were offered a sucrose-free diet (NS), a diet containing 67% calories as sucrose (HS) or NS diet plus 30% sucrose (LS), 0.03% saccharin (Sacc) or 20% SolCarb® solution for 38 days. SolCarb® is a maltodextrin powder. Sacc rats initially drank more than LS rats, but intakes were the same after Day 20. SolCarb® and LS rats drank the same number of calories from Day 15 to the end of the experiment. SolCarb® and LS rats ate less dry food than other groups, but total energy intake was greater than that of NS, HS and Sacc groups and over 80% of their energy intake was from carbohydrate. Leptin responsiveness was tested on Days 27 and 32 with each rat acting as its own control. An i.p. injection of 2 mg/Kg leptin inhibited food intake of NS, HS and Sacc rats, but had no effect on energy intake of LS or SolCarb® rats or on consumption of Sacc, sucrose or SolCarb® solution. At the end of the experiment all of the rats were insulin sensitive, had the same body composition and serum leptin concentrations. These data indicate that consumption of a calorie containing carbohydrate solution and not sweet taste drives the development of leptin resistance and suggest that there is lower threshold for inhibition of hunger than for inhibition of reward by leptin.
Keywords: Sweet taste, saccharin, maltodextrin, body composition
Introduction
We have previously reported that rats offered a 30% sucrose solution in addition to chow or a purified diet develop leptin resistance within weeks (Harris & Apolzan, 2012; Harris, 2018). Resistance appears to be selective to rats consuming sucrose as a solution because animals eating an equivalent amount of sucrose from a dry diet remain leptin responsive (Harris, 2018). Although the rats offered a 30% sucrose solution consume over 60% of their caloric intake as sucrose, they simultaneously reduce their intake of dry food so that energy intake does not increase significantly compared with that of rats offered a control sucrose-free diet or a dry 66% sucrose diet. Both male and female rats develop leptin resistance when offered sucrose solution (Harris & Apolzan, 2012; Harris, 2018). There are some sex differences in that females develop resistance faster than males and the females offered sucrose solution develop a small, but significant increase in body fat content, whereas male sucrose-drinking rats have the same body composition as their controls (Harris, 2018). It is possible that this difference is due to male rats continuing to grow and deposit both lean and fat mass whereas growth of lean tissue slows down significantly in young adult females and weight gain tends to be composed primarily of fat.
Rats offered sucrose solution and dry high sucrose diet have the same energy intake, but the sucrose solution is consumed in frequent small meals throughout the light and dark period, whereas rats offered only dry diet consume a majority of their food during the dark period (Harris, 2018). This led to the hypothesis that leptin resistance develops in response to the metabolic consequences of the pattern of sucrose consumption and the resulting frequent elevations in blood glucose associated with delivery of glucose and fructose in isolation from other nutrients. However, rats prefer sucrose solution over water and this has been associated both with taste and with post-ingestive consequences (Myers, Taddeo, & Richards, 2013). Either or both of these factors could be responsible for driving the consumption of sucrose and ultimately be responsible for the development of leptin resistance.
The objective of this experiment was to determine whether the development of leptin resistance in rats offered sucrose solution was secondary to the pre- or post-ingestive effects of consuming calories in liquid form or to consumption of a sweet solution. The experiment included rats offered a sucrose free diet or a high sucrose diet containing 66 %kcal as sucrose and groups offered the sucrose free diet plus 30% sucrose solution, 0.03% saccharin solution or 20% Sol-Carb® solution. SolCarb® (Solace Nutrition, Pawcatuck, CT) is a maltodextrin powder with a calorie content of 3.76 kcal/g that is tasteless to humans ((Feigin, Sclafani, & Sunday, 1987) and personal observation). It is the equivalent of Polycose® (Ross Laboratories, Columbus, OH), which is no longer available. Rodents show a high preference for low concentrations of maltodextrin solution (Sclafani & Clyne, 1987) even in the absence of sweet taste receptors (Treesukosol, Smith, & Spector, 2011). A pilot study was conducted to identify a concentration of saccharin that resulted in the rats drinking the same volume of saccharin as 30% sucrose solution and to identify a concentration of SolCarb® that resulted in rats consuming the same amount of energy as from a 30% sucrose solution. Male rats were used in this study to avoid any potential confounds associated with an increase in body fat content of female rats offered 30% sucrose solution (Harris, 2018).
Methods
Forty male Sprague Dawley rats (Envigo, Prattville, AL), weighing 275 – 280 g were individually housed in hanging wire mesh cages with free access to food and water throughout the experiment, unless indicated otherwise. The room was maintained at 72 ± 1°F with lights on for 12 hours a day from 6.00 a.m. Each rat had a Nylabone® in its cage for enrichment. Body weights and food intakes, corrected for spillage, were measured each morning starting at 7.30 a.m. All animal procedures were approved by the Institutional Animal Care and Use Committee of Augusta University and were in compliance with the National Insitutes of Health Guide for the Care and Use of Laboratory Animals (NRC, 2011).
Upon arrival all of the rats were offered a sucrose free (NS) diet (D 11724, Research Diets Inc., New Brunswick, NJ). After 4 days they were divided into five weight matched groups. One group continued to be offered the NS diet. A second group was offered an equicaloric diet containing 66.6% kcal as sucrose (HS diet: DL 11725 Research Diets Inc.). Both diets contained 67.7% kcal as carbohydrate and had a gross energy content of 3.902 kcal/g. In the NS diet, 51.3% kcal came from cornstarch and 15.3% from maltodextrin. The remaining three groups were offered the NS diet, but also were given access to 30% sucrose solution (LS rats), 0.03% saccharin solution (Sac rats) or 10% SolCarb® solution (SolCarb® rats). The rats within each group were subdivided into two cohorts with one cohort starting one day later than the other in order to make tissue harvest at the end of the experiment manageable. A pilot study had been conducted to identify the concentration of saccharin that led the rats to consume the same volume of saccharin solution as they consumed of 30% sucrose. Similarly, different comcentrations of SolCarb® solution were tested to identify a concentration that resulted in the same caloric intake from SolCarb® as was consumed from 30% sucrose. Based on the pilot study we started with a 10% solution of SolCarb®, but the concentration had to be adjusted during the experiment to match calorie intake to that of rats drinking 30% sucrose solution. On Day 6 the concentration was increased to 15%, on Day 9 it was increased to 25% and on Day 15 it was reduced back down to 20%.
On Days 27 and 32 of the experiment the rats were tested for leptin responsiveness. Each animal served as its own control receiving leptin one day and saline on the other day, with treatments assigned randomly. Food and sucrose, saccharin or SolCarb® bottles were removed from the cages at 8.00 a.m. At 5 p.m. each rat received an i.p. injection of 2 mg leptin/kg body weight (1 mg/ml solution, Rat recombinant leptin, R&D Systems, Minneapolis, MN) or an equivalent volume of saline. Food and bottles were returned to the cages at 6.00 p.m. Intakes were recorded 14, 24 and 38 hours after injection and body weights were recroded 14 and 38 hours after injection.
On Day 35 the rats were tested for insulin tolerance. Food and sucrose, saccharin or SolCarb® bottles were removed from the cages at 8.00 a.m. Starting at 12.00 p.m. a small tail blood sample was collected from each rat and used to measure insulin (Rat Insulin RIA Kit, Millipore Corp.) and glucose (EasyGluco Plus, US Diagnostics Inc, New York, NY). The rat was then injected i.p. with 0.75 U/g insulin (Humulin R, Eli Lilly and Company). An additional blood sample was collected to measure insulin 10 minutes after injection and blood glucose was measured at 10, 20, 30 40, 50 and 60 minutes after injection. Food and bottles were returned to the cages after the last measurement of blood glucose.
On Day 38 of the experiment food and bottles were removed from the cages at 8.00 a.m. Starting at 10.00 a.m. rats were decapitated, trunk blood was collected for measurement of serum leptin (Multi-species leptin RIA kit, Millipore Corp.) insulin, glucose, free fatty acids (NEFAC kit, WAKO Chemicals) and total triglycerides (Total Triglyceride kit WAKO Chemicals). Inguinal, epididymal, retroperitoneal and mesenteric fat pads were dissected and weighed. The liver was dissected and weighed and two lobes were snap frozen for subsequent determination of liver lipid and glycogen content as described previously (Harris & Jones, 1991). The rest of the carcass, less gut content, was analyzed for composition, as described previously (Harris, 1991).
Comparisons between groups were determined using Statistica (StatSoft Inc., Tulsa, OK). Daily data were initially compared by repeated measures analysis of variance. Single time point point measures were compared by one-way analysis of variance. Post-hoc comparisions were carried out using Duncan’s Multiple Range test. The data for leptin responsiveness was under powered to test by repeated measures analysis of variance (diet df = 4, injection df = 1, time df = 2, n = 8), however, a post-analysis was carried out for each time point because there was a strong main effect for both diet and leptin on total energy intake, weight gain, and energy from dry diet. P<0.05 was considered statistically significant. The statistical outcomes for comparison of various parameters are listed in Supplemental Table 1.
Results
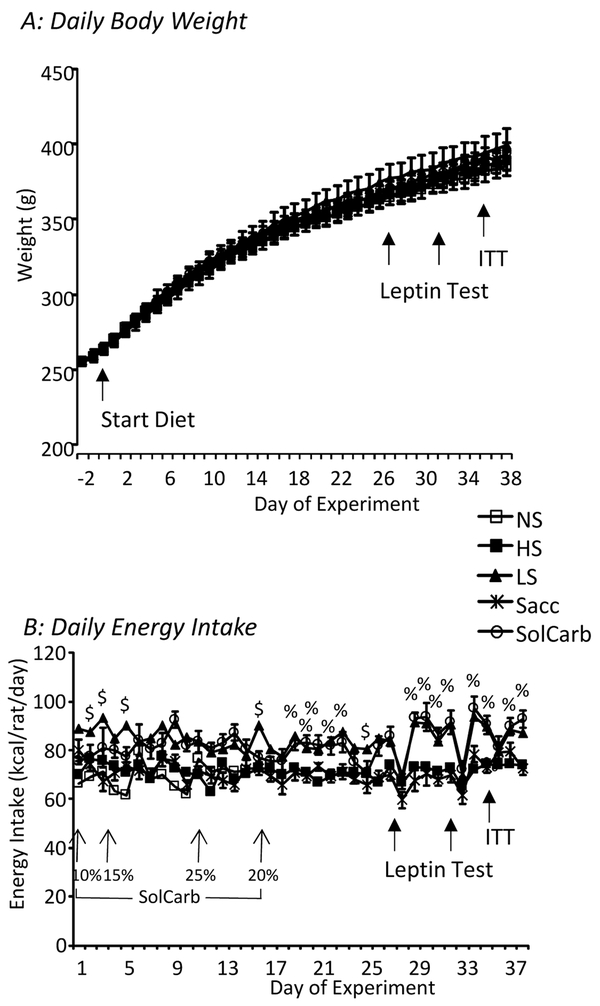
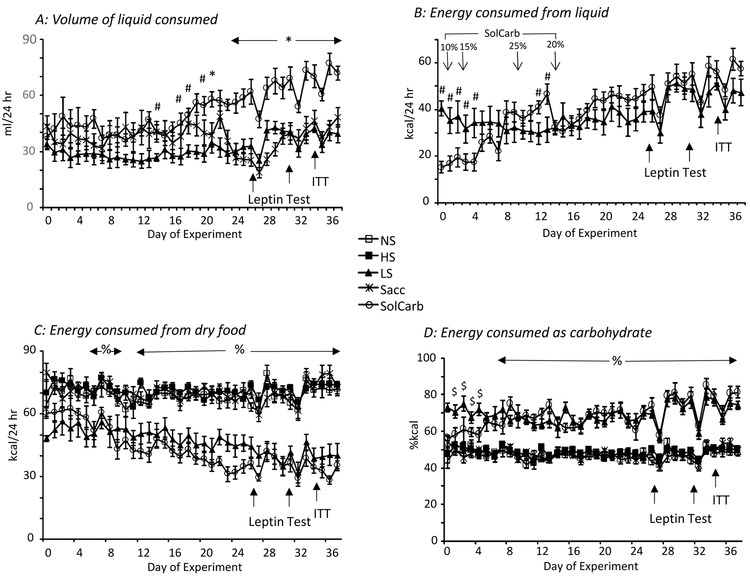
The different diets used in this experiment had no effect on body weight or weight gain of the rats (Figure 1A) which readily consumed the sucrose, saccharin and SolCarb® solutions (Figure 2A). Initially Sacc rats drank a larger volume of solution that did the LS rats, but after Day 20 the two groups of rats had the same intakes. As noted above, the caloric intake from 10% SolCarb® solution was less than that of 30% sucrose and the concentration was adjusted several times during the experiment. The SolCarb® and 30% sucrose rats drank the same number of calories from Day 15 to the end of the experiment when the concentration was held at 20% weight/volume (Figure 2B). SolCarb® and LS rats consumed less dry food than the other groups (Figure 2C), but this did not provide a full compensation for the calories consumed as liquid so that total caloric intake of LS and SolCarb® rats was greater than that of the NS, HS and Sacc rats during the second half of the experiment (Figure 1B). The percent calories consumed as carbohydrate was the same for NS, HS and Sacc groups as both dry diets contained 67.7% kcal in the form of either sucrose or a combination of maltodextrin and cornstarch. The LS and SolCarb® rats had a higher carbohydrate intake because of the large volume of sucrose or SolCarb® solution that was consumed (Figure 2D). The daily intake of rats getting all of their calories from dry food was about 18 g/day whereas the daily intake of LS and SolCarb® rats was reduced to approximately 10 g/day, but because this diet contained 67.7% kcal carbohydrate and the SolCarb® and sucrose solutions consisted of carbohydrate in isolation from other nutrients LS rats consumed 82 ± 1% kcal as carbohydrate and SolCarb® rats consumed 83 ± 1% kcal as carbohydrate during the experiment.
Figure 1:
Daily body weights and energy intakes of the rats during the experiment. There were no differences in body weight of any of the groups at any time during the experiment (Panel A). Daily energy intake (Panel B) of the LS and SolCarb® rats was higher than that of the other groups of rats during the second half of the experiment. % indicates a significant difference between LS and SolCarb® groups and the NS, HS and Sacc groups. $ indicates a significant difference between the LS group and all other animals. Data are means ± SE for groups of 8 rats and were compared by repeated measures analysis of variance and post-hoc Duncan’s Multiple Range test and considered significantly different when P<0.05.
Figure 2:
Source of calories for the different groups during the experiment. Panel A shows the daily intake of sucrose, SolCarb® or saccharin solutions. After day 20 the Sacc rats consumed the same volume of solution as the LS rats. At this time the SolCarb® rats drank a greater volume of solution than either the Sacc or LS rats. The energy consumed from the sucrose and SolCarb® solutions was the same from day 15 to the end of the experiment (Panel B). LS and SolCarb® rats consumed less energy from dry food than the NS, Hs or Sacc rats (Panel C). LS and SolCarb® rats also consumed a greater percentage of their calories from carbohydrate when intake of the solutions plus the dry food was considered (Panel D). # indicates a significant difference between LS and SolCarb® groups. * indicates a significant difference between SolCarb® and LS and Sacc groups. % indicates a significant difference between LS and SolCarb groups and the NS, HS and Sacc groups. $ indicates a difference between the LS groups and the NS, HS and Sacc groups. Data are means ± SE for groups of 8 rats. Statistical significant differences were determined by post-hoc Duncan’s Multiple Range Test and considered significant when P<0.05.
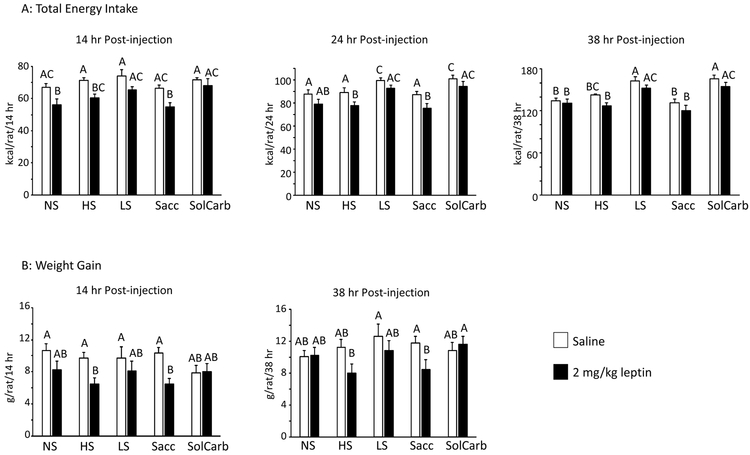
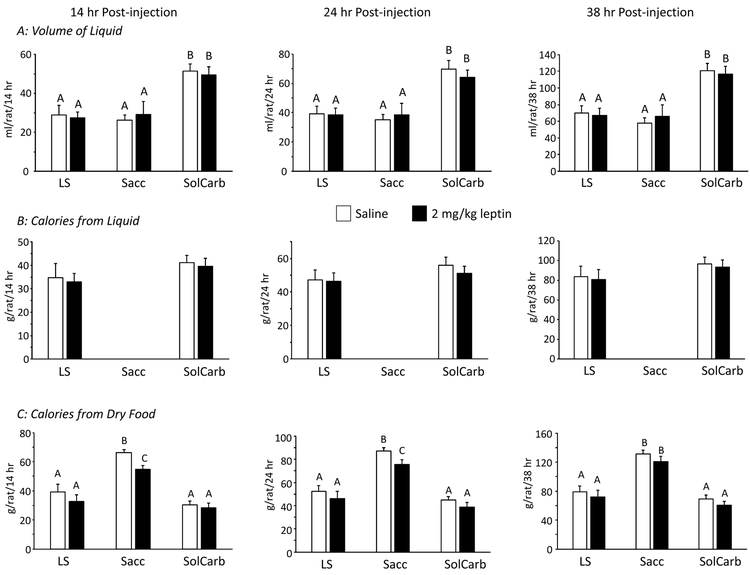
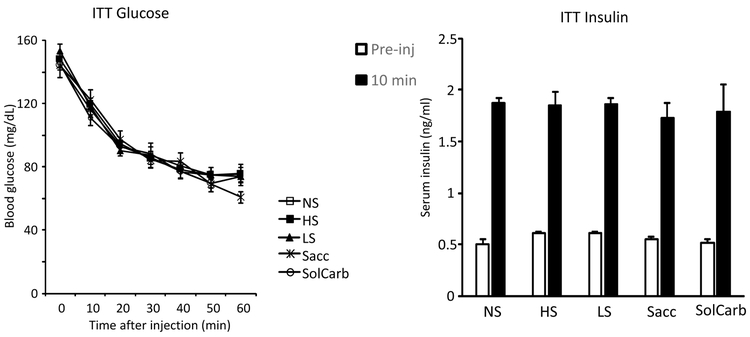
The LS and SolCarb® rats were resistant to the effects of leptin on food intake when tested on Days 27 and 32 of the experiment. All of the other rats reduced their intake of dry food after being treated with leptin (Figure 3A) and this was associated with a reduced weight gain during the 38 hours following injection (Figure 3B). Inhibition of weight gain followed the same pattern as inhibition of food intake in the different groups and it is likely that this was due to a reduction in gut fill, rather than a failure to gain lean or fat tissue. Leptin had no effect on energy intake of LS or SolCarb® rats (Figure 3B) and there was no effect of leptin on weight gain in these animals (Figure 3A). Leptin did not influence the proportion of energy obtained from dry food or from liquid by LS and SolCarb® rats (Figure 4B, C), therefore the resistance was not due to an increased intake of liquid compensating for an inhibition of intake of dry food. Although leptin caused a significant inhibition of food intake, it had no effect on the consumption of saccharin solution by Sacc rats (Figure 4A). There was no effect of diet on insulin responsiveness measured on Day 35 (Figure 5).
Figure 3:
Leptin response of different groups of rats. Cumulative food intake 14, 24 and 38 hours after injection in the different dietary groups (Panel A). Data are means ± SE for groups of 8 rats. Weight gain at 14 hours and 38 hours after an i.p. injection of PBS or 2 mg/kg leptin given on Days 27 and 32 of the experiment with each rat acting as its own control (Panel B). Values on a specific axis that do not share a common superscript are significantly different at P<0.05 determined by Duncan’s Multiple Range test using rat number as covariant. Energy intake was inhibited by leptin in the NS, HS and Sacc groups, but not the LS or SolCarb® groups.
Figure 4:
Consumption of liquid and dry food by Sacc, LS and SolCarb® rats following an i.p. injection of saline or 2mg/kg leptin. There was no effect of leptin on the volume of solution consumed by any of the groups (Panel A) and no effect on the energy intake from the sucrose and SolCarb® solutions (Panel B). Leptin inhibited intake of NS diet in the Sacc rats, but not the LS or SolCarb® rats (Panel C). Data are means ± SE for groups of 8 rats. Values on a specific axis that do not share a ommon superscript are significantly different at P<0.05 determined by Duncan’s Multiple Range test using rat number as a covariant.
Figure 5:
Blood glucose and serum insulin measured during an ITT on Day 35 of the experiment. There was no effect of diet on glucose clearance, basal insulin or insulin measured 10 minutes after the insulin injection.
At the end of the study on Day 38 there were no significant differences in liver weight, liver lipid or liver glycogen content (Table 1). There were no differences in the weights of the fat depots, except the mesenteric fat pad, which was larger in SolCarb® rats than in NS, HS or Sacc rat (Table 1. F(4)= 3.7, P<0.014). There were no differences in total body fat or lean mass and there were no differences in serum leptin, insulin, glucose, free fatty acids or TG (Table 1).
Table 1:
Body composition and serum hormone and metabolites.
| NS | HS | LS | Sacc | SolCarb® | |
|---|---|---|---|---|---|
| Fat Depot weights (g) | |||||
| Inguinal | 7.24 ± 0.50 | 7.41 ± 0.72 | 7.96 ± 0.38 | 7.38 ± 0.57 | 8.16 ± 0.69 |
| Epididymal | 4.44 ± 0.25 | 5.04 ± 0.52 | 5.07 ± 0.39 | 4.75 ± 0.25 | 5.29 ± 0.41 |
| Retroperitoneal | 2.91 ± 0.31 | 2.83 ± 0.29 | 3.49 ± 0.27 | 2.83 ± 0.41 | 3.59 ± 0.41 |
| Mesenteric | 2.18 ± 0.16A | 1.95 ± 0.26A | 2.74 ± 0.29AB | 2.12 ± 0.20A | 3.08 ± 0.30B |
| Liver | |||||
| Weight (g) | 12.23 ± 0.21 | 13.76 ± 0.50 | 14.36 ± 0.48 | 13.08 ± 0.44 | 14.13 ± 0.66 |
| Lipid (mg) | 477 ± 25 | 443 ± 16 | 439 ± 38 | 477 ± 21 | 461 ± 22 |
| Glycogen (mg glucose) | 730 ± 50 | 734 ± 27 | 734 ± 27 | 780 ± 37 | 776 ± 30 |
| Body Composition (g/rat) | |||||
| Carcass wt. | 345 ± 6 | 346 ± 6 | 357 ± 10 | 350 ± 6 | 353 ± 8 |
| Carcass Fat | 30 ± 2 | 32 ± 3 | 36 ± 2 | 29 ± 3 | 37 ± 3 |
| Carcass Ash | 14.7 ± 0.6 | 15.5 ± 0.3 | 14.3 ± 0.8 | 16.6 ± 1.10 | 15.5 ± 0.8 |
| Lean Mass | 300 ± 6 | 298 ± 4 | 307 ± 8 | 304 ± 4 | 301 ± 6 |
| Serum Assays | |||||
| Leptin (ng/ml) | 7.1 ± 1.5 | 6.6 ± 2.0 | 6.3 ± 1.5 | 6.8 ± 1.3 | 8.3 ± 2.2 |
| Insulin (ng/ml) | 0.42 ± 0.11 | 0.62 ± 0.13 | 0.81 ± 0.21 | 0.48 ± 0.15 | 0.36 ± 0.04 |
| Glucose mg/dL) | 164 ± 11 | 154 ± 15 | 161 ± 13 | 155 ± 10 | 137 ± 6 |
| FFA (mEq/L) | 0.53 ± 0.05 | 0.50 ± 0.05 | 0.61 ± 0.03 | 0.49 ± 0.05 | 0.64 ± 0.05 |
| Triglycerides (mg/dL) | 165 ± 24 | 168 ± 15 | 185 ± 19 | 149 ± 16 | 155 ± 21 |
Data are means ± SE for groups of 8 rats fed different diets for 38 days. There were no significant differences in any of the parameters measured when compared by one-way analysis of variance, except for mesenteric fatt which was significantly larger in SolCarb® than NS, HS or Sacc rats (P<0.05, determined by post-hoc Duncan’s Multiple Range test).
Discussion
The objective of this experiment was to test whether the development of leptin resistance in rats consuming 30% sucrose solution was associated with drinking large volumes of a sweet solution or the metabolic effects of the simple sugars delivered by the solution. In this experiment leptin resistance was defined as a failure to suppress food intake following leptin injection, with each animal serving as its own control. Based on these criteria both the LS and SolCarb® rats were leptin resistant after 27 days of access to the diet, a time point that was chosen based on previous studies with LS rats (Harris & Apolzan, 2015). Leptin failed to suppress intake of both dry food and of calorie-containing solutions so that the resistance was not caused by the rats increasing their intake of sucrose or SolCarb® solution and masking an inhibition of intake of dry food. The results indicate that the leptin resistance developed when the rats consumed a preferred solution that contained simple carbohydrate, but not when they consumed a calorie-free sweet solution or a sweet, dry high sucrose diet. This suggests that the development of resistance is associated with the pre- or post-absorptive effects of carbohydrate in the solution, rather than the repeated activation of sweet receptors which are distributed not only in the oral cavity but also in small intestine (Meyer-Gerspach, Wolnerhanssen, & Beglinger, 2014). However, because consumption of sucrose and SolCarb® not only provided liquid carbohydrate calories, but also increased the proportion of carbohydrate in the diet, this experiment did not separate the metabolic effects of carbohydrate consumed as a solution versus those of a very high carbohydrate diet. As with previous studies (Harris, 2018) the leptin resistance of LS and SolCarb® rats was independent of a change in body composition, insulin sensitivity or serum leptin levels, separating the leptin resistance from development of obesity or hyperleptinemia, which has frequently been used as an indirect marker for leptin resistance (Scarpace & Zhang, 2007).
In this study maltodextrin powder (SolCarb®) was used as a source of non-sweet carbohydrate calories. Humans find maltodextrin solutions to be much less sweet than sucrose solutions of equivalent concentration, do not prefer them over sucrose and may report high concentrations to be unpleasant (Feigin et al., 1987). By contrast, rats show a stronger preference for maltodextrin solution than sucrose (Feigin et al., 1987). It has been suggested that this preference can be attributed to a taste receptor that is independent of sweet taste, but responsive to short chain polysaccharides (Sclafani, 2004). The concept of a taste receptor that responds to polysaccharides is supported by evidence that aversive conditioning to sucrose does not generalize to maltodextrin and vice versa (Treesukosol et al., 2011), by observations that mice in which different components of the taste receptor complex have been knocked out show a normal concentration–dependent preference for maltodextrin solution (Treesukosol et al., 2011) and by the consumption of large volumes of Polycose solution by sham-feeding rats (Nissenbaum & Sclafani, 1987). Preference for SolCarb® solution cannot be attributed to taste alone as there is evidence that glucose in the small intestine causes a very rapid positive feed-forward response that promotes consumption within a meal (Myers et al., 2013; Myers & Whitney, 2011; Zukerman, Ackroff, & Sclafani, 2011) and also produces a flavor-nutrient conditioning that contributes to a subsequent preference for that nutrient (Sclafani, 2013). The importance of post-ingestive factors driving consumption of preferred calorie-containing solutions is also supported by the observation that preference for a saccharin sweetened Polycose solution over saccharin solution is blocked if starch digestion is inhibited (Elizalde & Sclafani, 1988).
Although LS and SolCarb® rats consumed a large volume of liquid each day it is unlikely that a shift in fluid balance contributed to leptin resistance in these animals because Sac rats consumed the same volume of liquid as LS rats, but remained leptin responsive. It is much more likely that leptin resistance in rats drinking calorie containing solution has a metabolic basis that has yet to be identified. Interestingly in this study on the three days that the rats were deprived of access to liquid sucrose, SolCarb® or saccharin during the light period in order to perform leptin or insulin sensitivity tests, the amount of liquid consumed was decreased compared with days on which the rats had 24 hour access to the bottles, irrespective of whether the solution was sweet and non-caloric (Sacc), sweet and calorie containing (LS) or non-sweet and calorie containing (SolCarb®). This failure to compensate was unlikely to be associated with gut distention being a limiting factor as the SolCarb® rats regularly drank almost twice as much liquid as the LS and Sacc rats and in the pilot study some rats drank more than 100 ml a day of dilute SolCarb® solution (data not shown). This suggests that there is a drive independent of hunger that initiates frequent meals when a preferred solution is available and that the amount of solution consumed may be less important than the frequency of consumption.
Sucrose-drinking rats consume repeated small meals of solution throughout both the light and dark periods (Harris, 2018). These observations are consistent with those of La Fleur et al (la Fleur, Luijendijk, van der Zwaal, Brans, & Adan, 2014) who found that rats offered chow, 30% sucrose solution and lard consumed a majority of the calories from chow and lard during the dark period, but consumed 40% of their sucrose in meals during the light period. It is known that leptin acts synergistically with peripheral satiety signals, such as amylin (Liberini et al., 2016) and CCK (Emond, Schwartz, Ladenheim, & Moran, 1999) and it is possible that the repeated small meals of sucrose or SolCarb® lead to an inhibition of the synergy between leptin and these satiety signals. Alternatively it is possible that repeated meals of simple carbohydrate cause a sustained elevation of blood glucose that stimulates activity of the hexosamine biosynthetic pathway (Harris & Apolzan, 2015; Zimmerman & Harris, 2015). This would result in increased rates of O-linked beta-N-acetylglucosamine (O-GlcNAc) modification of a multitude of proteins, including STAT3 (Gewinner et al., 2004). The modification is essential for activation of some proteins, but also can compete for phosphorylation sites and inactivate signaling pathways (Hart, Housley, & Slawson, 2007). This hypothesis is based on the observation that rats consuming a sucrose free diet and obtaining approximately 66% of their energy intake from sucrose solution are leptin resistant, whereas other rats consuming a dry formulated diet containing 66% kcal as sucrose remain leptin responsive (Harris & Apolzan, 2015). The additional carbohydrate consumed from the NS diet by the sucrose-drinking rats was not taken into account and in this study it is clear that both sucrose and SolCarb® rats not only drank a large portion of their calories from the carbohydrate solution, but also obtained a significant amount of energy as carbohydrate from the sucrose-free diet. Therefore, future studies will have to determine whether it is the pattern of carbohydrate consumption or the total amount of carbohydrate consumed that leads to leptin resistance.
It was not surprising that development of leptin resistance was associated with the consumption of calorie-containing preffered liquids rather than consumption of a calorie-free sweet solution, but it was an appropriate association to test because there is evidence that leptin can modify both reward and taste responses. Leptin receptors are co-expressed with dopamine in neurons in the ventral tegmental area (VTA) and substantia nigra (Figlewicz, Evans, Murphy, Hoen, & Baskin, 2003; Hommel et al., 2006). Activation of these receptors by central injection of leptin inhibits food intake (Hommel et al., 2006), suppresses motivation to lever press for sucrose solution (Figlewicz 2006) and reduces the reward obtained from voluntary exercise in rats (Fernandes et al., 2015). Although leptin activates the Jak2-STAT3 signaling pathway in the VTA, it appears that the suppression of rewarding aspects of food and exercise are independent of STAT3 activation and may be mediated by an ERK 1/2 dependent pathway (Fernandes et al., 2015; Trinko et al., 2011). In this study, although leptin suppressed food intake in Sacc rats, there was no impact on consumption of saccharin solution, which has no metabolic benefit and is assumed to be consumed due to the reward associated with sweet taste. This implies that the threshold for inhibitory effects of leptin on reward is higher than that required for an inhibition of food intake. Alternative explanations may be that we gave leptin as a peripheral injection, rather than directly into a cerebral ventricle or the VTA, however, serum leptin is in the range of 1500 ng/ml 30 minutes after a peripheral dose of 2 mg/kg in rats (unpublished observations). Thus, it seems likely that central leptin concentrations would have been elevated in the rats in this experiment. Others have shown that firing of dopaminergic neons in the VTA in response to a food reward was suppressed during the hour following an i.p. injection of 1 mg/kg leptin (van der Plasse et al., 2015). In the study described here intake was measured at 12 hour intervals and an early transient response to leptin would not have been recorded, however, a single injection of leptin into the VTA inhibits food intake for at least 24 hours (Hommel et al., 2006) and we would have detected this if it had been present. Another difference between studies is that rats in this experiment had free access to 30% sucrose solution whereas the rats that suppressed sucrose intake following central leptin injection had to lever press to obtain 5% sucrose solution (Figlewicz, Bennett, Naleid, Davis, & Grimm, 2006). It is possible that if animals in this experiment had been required to work to obtain the solutions and motivation was reduced, then the results would have been different. It is also possible that this design would have found differences between Sacc and LS and SolCarb® rats because the pathways mediating the drive to consume caloric and non-caloric solutions are likely to be different. Thus, just as development of leptin resistance in high-fat fed mice is site specific (Munzberg, 2010) it is possible that the effects of leptin on Ingestive behavior are also selective and it cannot be assumed that the central pathways responsible for consumption driven by reward have the same responsiveness as those driven by hunger.
As noted above, both the LS and SolCarb® rats developed leptin resistance without any significant change in body composition and this was consistent with observations in previous experiments with male rats. Total energy intake of the LS and SolCarb® rats was significantly higher than that of the NS, HS and Sacc rats during the second half of the study, and there was a tendency for body fat and individual fat depot weights to be greater in the LS and SolCarb® rats. Therefore, it is likely that there would have been a significant difference in body composition if the study had been extended. However, within the current time frame, leptin resistance could not be attributed to the development of obesity or hyperleptinemia. Sucrose offered with chow does cause weight gain (Harris & Apolzan, 2012; Lindqvist, Baelemans, & Erlanson-Albertsson, 2008), but rats that readily overeat a free choice diet of chow, lard and 30% sucrose solution do not overeat when chow is replaced with a purified low fat diet (Apolzan & Harris, 2012). Therefore, the use of a purified sucrose-free diet may explain why we do not observe an increase in body fat of male LS rats. Although both LS and SolCarb® rats consumed less than 50% of their calories from dry diet it is unlikely that they were leptin resistant because of a deficiency in essential macro- or micronutrients because they had the option to increase consumption of the dry diet. In addition, one of the most sensitive indices of dietary imbalance or deficiency is a failure to gain weight (NRC, 1995) and in this study the LS and SolCarb rats gained weight at the same rate as rats that consumed only dry diet.
Conclusions
This experiment suggests that the development of leptin resistance in rats offered 30% sucrose solution in addition to a sucrose-free diet is due to the consumption of a carbohydrate-containing solution, and is not associated with consumption of equivalent volumes of a sweet, calorie-free solution. Future experiments will have to determine whether leptin resistance is secondary to the metabolic effects of frequent small meals of carbohydrate or to the consumption of over 80% of energy intake as simple carbohydrate. The development of leptin resistance in LS and SolCarb® rats by Day 27 is faster than that found for rats offered a high fat diet. Because neither LS nor SolCarb® rats were significantly fatter than the other groups in this experiment leptin resistance was independent of obesity and hyperleptinemia. Although it has been reported that leptin inhibits reward responses mediated by the VTA and the substantia nigra (Figlewicz et al., 2003; Hommel et al., 2006), a dose of leptin that inhibited 12 hour intake of dry food in NS, HS and Sacc rats had no effect on consumption of saccharin, sucrose or SolCarb® solutions. This suggests that there is a different threshold for the effect of leptin on pathways that mediate hunger and satiety than on those that mediate the reward response to preffered solutions or the pre- or post-absorptive effects of glucose.
Supplementary Material
Acknowledgements
The author thanks Delaney Rauch for assistance with liver analysis.
Funding: This work was supported by the National Institutes of Health (grant R01 DK109997)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apolzan JW, & Harris RB (2012). Differential effects of chow and purified diet on the consumption of sucrose solution and lard and the development of obesity. Physiol Behav, 105(2), 325–331. doi:S0031-9384(11)00418-5 [pii]10.1016/j.physbeh.2011.08.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elizalde G, & Sclafani A (1988). Starch-based conditioned flavor preferences in rats: influence of taste, calories and CS-US delay. Appetite, 11(3), 179–200. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3250334 [DOI] [PubMed] [Google Scholar]
- Emond M, Schwartz GJ, Ladenheim EE, & Moran TH (1999). Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol, 276(5 Pt 2), R1545–1549. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10233050 [DOI] [PubMed] [Google Scholar]
- Feigin MB, Sclafani A, & Sunday SR (1987). Species differences in polysaccharide and sugar taste preferences. Neurosci Biobehav Rev, 11(2), 231–240. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3614791 [DOI] [PubMed] [Google Scholar]
- Fernandes MF, Matthys D, Hryhorczuk C, Sharma S, Mogra S, Alquier T, & Fulton S (2015). Leptin Suppresses the Rewarding Effects of Running via STAT3 Signaling in Dopamine Neurons. Cell Metab, 22(4), 741–749. doi:10.1016/j.cmet.2015.08.003 [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Bennett JL, Naleid AM, Davis C, & Grimm JW (2006). Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav, 89(4), 611–616. doi:10.1016/j.physbeh.2006.07.023 [DOI] [PubMed] [Google Scholar]
- Figlewicz DP, Evans SB, Murphy J, Hoen M, & Baskin DG (2003). Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res, 964(1), 107–115. doi:S0006899302040878 [pii] [DOI] [PubMed] [Google Scholar]
- Gewinner C, Hart G, Zachara N, Cole R, Beisenherz-Huss C, & Groner B (2004). The coactivator of transcription CREB-binding protein interacts preferentially with the glycosylated form of Stat5. J Biol Chem, 279(5), 3563–3572. doi:10.1074/jbc.M306449200M306449200 [pii] [DOI] [PubMed] [Google Scholar]
- Harris RB (1991). Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. Journal of Nutrition, 121(7), 1075–1080. [DOI] [PubMed] [Google Scholar]
- Harris RB, & Apolzan JW (2012). Changes in glucose tolerance and leptin responsiveness of rats offered a choice of lard, sucrose, and chow. Am J Physiol Regul Integr Comp Physiol, 302(11), R1327–1339. doi:ajpregu.00477.2011 [pii]10.1152/ajpregu.00477.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, & Apolzan JW (2015). Hexosamine biosynthetic pathway activity in leptin resistant sucrose-drinking rats. Physiol Behav, 138, 208–218. doi:S0031-9384(14)00507-1 [pii]10.1016/j.physbeh.2014.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RB, & Jones WK (1991). Physiological response of mature rats to replacement of dietary fat with a fat substitute. J Nutr, 121(7), 1109–1116. doi:10.1093/jn/121.7.1109 [DOI] [PubMed] [Google Scholar]
- Harris RBS (2018). Source of dietary sucrose influences development of leptin resistance in male and female rats. Am J Physiol Regul Integr Comp Physiol, 314(4), R598–R610. doi:10.1152/ajpregu.00384.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart GW, Housley MP, & Slawson C (2007). Cycling of O-linked beta-N-acetylglucosamine on nucleocytoplasmic proteins. Nature, 446(7139), 1017–1022. doi:nature05815 [pii]10.1038/nature05815 [DOI] [PubMed] [Google Scholar]
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, … DiLeone RJ (2006). Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron, 51(6), 801–810. doi:S0896-6273(06)00645-3 [pii]10.1016/j.neuron.2006.08.023 [DOI] [PubMed] [Google Scholar]
- la Fleur SE, Luijendijk MC, van der Zwaal EM, Brans MA, & Adan RA (2014). The snacking rat as model of human obesity: effects of a free-choice high-fat high-sugar diet on meal patterns. Int J Obes (Lond), 38(5), 643–649. doi:10.1038/ijo.2013.159 [DOI] [PubMed] [Google Scholar]
- Liberini CG, Boyle CN, Cifani C, Venniro M, Hope BT, & Lutz TA (2016). Amylin receptor components and the leptin receptor are co-expressed in single rat area postrema neurons. Eur J Neurosci, 43(5), 653–661. doi:10.1111/ejn.13163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Baelemans A, & Erlanson-Albertsson C (2008). Effects of sucrose, glucose and fructose on peripheral and central appetite signals. Regul Pept, 150(1-3), 26–32. doi:S0167-0115(08)00114-6 [pii]10.1016/j.regpep.2008.06.008 [DOI] [PubMed] [Google Scholar]
- Meyer-Gerspach AC, Wolnerhanssen B, & Beglinger C (2014). Gut sweet taste receptors and their role in metabolism. Front Horm Res, 42, 123–133. doi:10.1159/000358321 [DOI] [PubMed] [Google Scholar]
- Munzberg H (2010). Leptin-signaling pathways and leptin resistance. Forum Nutr, 63, 123–132. doi:000264400 [pii]10.1159/000264400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KP, Taddeo MS, & Richards EK (2013). Sensory-specific appetition: Postingestive detection of glucose rapidly promotes continued consumption of a recently encountered flavor. Physiol Behav, 121, 125–133. doi:10.1016/j.physbeh.2013.03.021 [DOI] [PubMed] [Google Scholar]
- Myers KP, & Whitney MC (2011). Rats' learned preferences for flavors encountered early or late in a meal paired with the postingestive effects of glucose. Physiol Behav, 102(5), 466–474. doi:10.1016/j.physbeh.2010.12.016 [DOI] [PubMed] [Google Scholar]
- Nissenbaum JW, & Sclafani A (1987). Sham-feeding response of rats to Polycose and sucrose. Neurosci Biobehav Rev, 11(2), 215–222. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3614789 [DOI] [PubMed] [Google Scholar]
- NRC. (1995). Nutrient Requirements of Laboratory Animals: (Fourth Revised Edition ed.). Washington DC: National Academies Press (US). [PubMed] [Google Scholar]
- NRC. (2011). Guide for the Care and Use of Laboratory Animals (8th ed.). Washington, DC: National Academies Press. [PubMed] [Google Scholar]
- Scarpace PJ, & Zhang Y (2007). Elevated leptin: consequence or cause of obesity? Front Biosci, 12, 3531–3544. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17485319 [DOI] [PubMed] [Google Scholar]
- Sclafani A (2004). The sixth taste? Appetite, 43(1), 1–3. doi:10.1016/j.appet.2004.03.007 [DOI] [PubMed] [Google Scholar]
- Sclafani A (2013). Gut-brain nutrient signaling. Appetition vs. satiation. Appetite, 71, 454–458. doi:10.1016/j.appet.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani A, & Clyne AE (1987). Hedonic response of rats to polysaccharide and sugar solutions. Neurosci Biobehav Rev, 11(2), 173–180. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3614783 [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Smith KR, & Spector AC (2011). Behavioral evidence for a glucose polymer taste receptor that is independent of the T1R2+3 heterodimer in a mouse model. J Neurosci, 31(38), 13527–13534. doi:10.1523/JNEUROSCI.2179-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, & DiLeone RJ (2011). Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS One, 6(11), e27180. doi:10.1371/journal.pone.0027180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Plasse G, van Zessen R, Luijendijk MC, Erkan H, Stuber GD, Ramakers GM, & Adan RA (2015). Modulation of cue-induced firing of ventral tegmental area dopamine neurons by leptin and ghrelin. Int J Obes (Lond), 39(12), 1742–1749. doi:10.1038/ijo.2015.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AD, & Harris RB (2015). In vivo and in vitro evidence that chronic activation of the hexosamine biosynthetic pathway interferes with leptin-dependent STAT3 phosphorylation. Am J Physiol Regul Integr Comp Physiol, 308(6), R543–555. doi:ajpregu.00347.2014 [pii]10.1152/ajpregu.00347.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zukerman S, Ackroff K, & Sclafani A (2011). Rapid post-oral stimulation of intake and flavor conditioning by glucose and fat in the mouse. Am J Physiol Regul Integr Comp Physiol, 301(6), R1635–1647. doi:10.1152/ajpregu.00425.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.